Abstract
Background
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating disorder characterized by severe fatigue and neurocognitive dysfunction. Recent work from our laboratory and others utilizing arterial spin labeling functional magnetic resonance imaging (ASL) indicated that ME/CFS patients have lower resting state regional cerebral blood flow (rCBF) in several brain areas associated with memory, cognitive, affective, and motor function. This hypoperfusion may underlie ME/CFS pathogenesis and may result in alterations of functional relationships between brain regions. The current report used ASL to compare functional connectivity of regions implicated in ME/CFS between patients and healthy controls (HC).
Methods
Participants were 17 ME/CFS patients (Mage=48.88 years, SD=12) fulfilling the 1994 CDC criteria and 17 age/sex matched HC (Mage=49.82 years, SD=11.32). All participants underwent T1-weighted structural MRI as well as a 6-minute pseudo-continuous arterial spin labeling (pCASL) sequence, which quantifies CBF by magnetically labeling blood as it enters the brain. Imaging data were preprocessed using SPM 12 and ASL tbx, and seed-to-voxel functional connectivity analysis was conducted using the CONN toolbox. All effects noted below are significant at p<0.05 with cluster-wise FDR correction for multiple comparisons.
Results
ME/CFS patients demonstrated greater functional connectivity relative to HC in bilateral superior frontal gyrus, ACC, precuneus, and right angular gyrus to regions including precuneus, right postcentral gyrus, supplementary motor area, posterior cingulate gyrus, and thalamus. In contrast, HC patients had greater functional connectivity than ME/CFS in ACC, left parahippocampal gyrus, and bilateral pallidum to regions including right insula, right precentral gyrus, and hippocampus. Connectivity of the left parahippocampal gyrus correlated strongly with overall clinical fatigue of ME/CFS patients.
Conclusion
This is the first ASL based connectivity analysis of patients with ME/CFS. Our results demonstrate altered functional connectivity of several regions associated with cognitive, affective, memory, and higher cognitive function in ME/CFS patients. Connectivity to memory related brain areas (para-hippocampal gyrus) was correlated with clinical fatigue ratings, providing supporting evidence that brain network abnormalities may contribute to ME/CFS pathogenesis.
Keywords: arterial spin labeling, chronic fatigue syndrome, functional connectivity, MRI
1. Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is an illness characterized by persistent and severe fatigue, neurocognitive dysfunction, and sleep abnormalities [1]. Although numerous studies have examined potential causes of ME/CFS, the exact etiology is still undetermined. Increasing evidence suggests that ME/CFS related brain abnormalities, including global and localized cerebral hypo-perfusion of multiple brain areas associated with cognition and memory contribute to the pathogenesis of this illness [2 and 3 and 4 and 5]. Because the key features of ME/CFS include abnormal CNS functioning [6], increased focus has been placed on understanding the neural correlates of ME/CFS.
One important aspect of proper CNS functioning is regional cerebral blood flow (rCBF), which has been used as a surrogate of brain metabolism and neural activity [7]. Arterial spin labeling is a technique that can provide estimates of cerebral blood flow with good spatial and temporal resolution, without the limitations of radioactive tracer studies such as PET and SPECT
Functional connectivity (FC), a measure of the temporal coherence among brain regions, has been used for mapping large-scale brain networks [8]. Additionally, FC has been used to detect alterations in functional neural networks. Given that hypo-perfusion can lead to cerebral metabolic distress resulting in network plasticity, reduced rCBF may be associated with changes in FC [9].
Previous studies have demonstrated regional and global brain hypoperfusion in individuals with ME/CFS [2 and 3 and 4 and 5]. However, the clinical and functional significance of these findings have been questioned [10 and 11]. Understanding the functional network reorganization associated with ME/CFS might provide useful information for interpreting how reduced rCBF contributes to symptoms associated with hypoperfusion.
The goal of the present study was to examine the functional network changes of individuals with ME/CFS using seed-based FC during rest using arterial spin labeling (ASL). A priori regions of interest (ROIs) were chosen based on: 1) previous evidence demonstrating abnormal functional brain activity in ME/CFS patients, and 2) ROIs implicated in fatigue and impaired cognitive/attentional function (i.e., clinical aspects of ME/CFS ) including the: insula [12], inferior frontal gyrus (IFG) [12],, middle frontal gyrus (MFG) [13 and 14], parahippocampal gyrus (PaHcG) [13 and 15], anterior cingulate cortex (ACC) [16 and 17], angular gyrus (AG) [15], posterior cingulate cortex (PCC) [13], hippocampus [12], precuneus [13], caudate nucleus [18], and pallidum [18]. The superior frontal gyrus (SFG) was also selected as an a priori ROI because we have previously identified this region, along with PaHCG and ACC, as being hypo-perfused in ME/CFS patients during the resting state [5]
2. Methods
Participants
ME/CFS subjects had to fulfill the Center for Disease Control criteria for chronic fatigue syndrome (ME/CFS) [6]. ME/CFS subjects could not have a history of heart disease, chronic obstructive pulmonary disease, malignancy, or other systemic disorders including psychiatric illnesses that would confound the diagnosis of ME/CFS [19]. All subjects were recruited from University of Florida outpatient clinics or through advertising. Healthy controls (HC) were excluded if they had a history of chronic fatigue, chronic pain conditions, or mental illness. Individuals meeting entry criteria were asked to get a full night’s sleep (at least 6 hours) and refrain from consuming caffeinated beverages prior to the laboratory session. They were also not allowed to consume alcohol or other psychoactive substances in the 24 hours before the study, or use any medications except vitamins. Additional exclusionary criteria included being a smoker or having any contraindications for MRI, including metal implants. Participants provided written consent prior to the collection of any information. All procedures were approved by the University of Florida Institutional Review Board and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Clinical and Affective Measures
The Florida Fatigue Questionnaire (FFQ) was applied to all study subjects. It has two domains, consisting of ratings of fatigue (VAS) and negative emotions related to chronic fatigue (VAS). The FFQ was only used to characterize the study subjects during the screening phase of the study. Prior to brain scanning, clinical fatigue, pain, depression, and anxiety were assessed using mechanical visual analog scales (VAS; Price et al., 1994). Each scale was anchored on the right by “no fatigue/pain/depression/anxiety at all” and on the left by “the most intense fatigue/pain/depression/anxiety imaginable”. Perceived physical and role function were also assessed using VAS ranging from 0 (“no function”) to 100 (“no impairment in function”). VAS measures ranged from 0–10 and were rescaled to 0–100 by multiplying each value by 10, if necessary. Additionally, each participant completed the Pennebaker Inventory of Limbic Languidness (PILL), which is a 54-item questionnaire designed to measure tendency to notice various physical symptoms and sensations [20].
Image Acquisition
Imaging data were collected using a whole body Phillips Achieva 3T MRI scanner and a 32-channel head coil. All participants were placed in the scanner head first in the supine position. During each scanning session, participants completed a T1-weighted structural MRI scan, as well as a resting scan using a pseudo-continuous ASL protocol (i.e., pCASL). The whole brain structural images were acquired using a three-dimensional (3D) T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE) sequence with a field-of-view (FOV) of 240 mm, in-plane resolution of 1mm × 1mm, 176 contiguous sagittal slices of 1mm thickness, and TR/TE/□= 7.2ms/3.2ms/8°. The total acquisition time was 4 min 34 seconds. The ASL data were acquired using a two-dimensional (2D) pseudo-continuous arterial spin labeling (pCASL) technique [21 and 22]. with a field-of-view (FOV) of 230 mm, in-plane resolution of 3.2mm × 3.2mm, 20 axial slices of 6mm thickness, 1mm inter-slice gap, and TR/TE/□= 4s/11ms/90°. Arterial spin labeling was applied at a plane which was 30.5 mm inferior to the lowest imaging slice with a labeling during of 1500ms, and a post labeling delay time of 1800 ms. The duration of the resting state ASL scan was 6 minutes, producing 45 pairs of control and tag images.
The structural MRI scan required 4 minutes 34 seconds. The duration of the resting ASL scan was 6 minutes 24 seconds, producing 45 pairs of control and tag images.
Preprocessing Protocol
Functional imaging data processing and analyses were performed using MATLAB 2015a (MathWorks, Natick, MA, USA), SPM12 (Wellcome Department of Cognitive Neurology, UK), and ASLtbx [23]. To avoid detection of spurious motion artifacts, label and control images were motion corrected independently [23 and 24 and 24]. The functional images were then co-registered to the T1 images and spatially smoothed with a 6 mm full-width-half-maximum (FWHM) kernel to decrease noise for subsequent image subtraction. Each tag and control pair was subtracted to create 45 perfusion-weighted images. These images were used to create quantified maps of CBF using the software ASLtbx [23]. In the current study, CBF was quantified as ml/100g/min using the equation described in (Equation 1) [23]. Equation 2 contains the parameters utilized for the calculation of CBF values for this study. Four-dimensional CBF images were masked to remove out-of-brain voxels and normalized to the MNI template in SPM12.
| Equation 1 |
| Equation 2 |
where ΔM is the perfusion difference image, λ= 0.9 ml/g is blood/tissue water partition coefficient, R1a = 1/1650 (1/ms) is longitudinal relaxation rate of blood at 3T, α= 0.85 is tagging efficiency, is equilibrium magnetization of the brain, ω= 1800 ms is post-labeling delay, and τ = 1500 ms is duration of the labeling RF pulse train.
Functional Connectivity Analysis
FC analyses were completed using CONN [25]. Prior to correlation analysis, average signals from white matter and the ventricles were removed from the data using linear regression. This step reduces spatial correlations resulting from physiological noise. Specifically, the CBF signal in the ventricles reflects cardiac-induced signal fluctuations while the CBF signal in the white matter reflects the respiratory cycle [26]. CBF volumes were filtered using a low pass (< .07 Hz) filter.
Then seed-to-voxel FC analysis was performed using each a priori ROI as a seed (insula, IFG, MFG, SFG, PaHcG, ACC, AG, PCC, hippocampus, precuneus, caudate nucleus, and pallidum). This analysis produced Fisher’s r-to-z transformed correlation maps for each participant and seeds that subsequently were subjected to independent T-tests. Type I error was controlled through the use of cluster-level false discovery rate (FDR) correction (p<.05). FC values (mean z-scores) for significant clusters were extracted using the REX toolbox.
Statistical Analysis
SPSS 22 was used for all statistical analyses (IBM Corp., Armonk, NY, USA). Following descriptive statistics for demographic variables, characterization of the relationship between individuals’ FC values (i.e., seed-to-cluster z-scores) and relevant clinical and psychosocial factors was conducted using nonparametric (Spearman’s rho) correlation matrices.
3. Results
Demographics and Psychosocial Variables
17 women meeting CDC criteria for chronic fatigue syndrome (CFS; [6] and 17 female HC participated in this study. Participants’ demographic and psychosocial variables are illustrated in Table 1. Independent t-tests indicated HC and ME/CFS were aged matched (t32 = −.24, p = .82). ME/CFS subjects reported significantly higher ratings for fatigue (t23.21 = −9.41, p < .0001), pain (t17.71 = −7.79, p < .0001), anxiety (t17.38 = −5.64, p < .0001), and depression (t25.37 = −4.21, p < .0001) than HC.
Table 1.
Demographic and Psychosocial Characteristics of Participants
|
|
||
|---|---|---|
| HC (n=17) Mean (SD) |
ME/CFS (n=17) Mean (SD) |
|
| Age (years) | 48.88 (12.00) | 49.82 (11.32) |
| Fatigue Symptom Duration (years) | - | 11.78 (9.12) |
| Anxiety (0–100 VAS) | 2.35 (5.79) | 41.29 (27.88)a |
| Fatigue (0–100 VAS) | 6.94 (9.12) | 54.41 (18.70)a |
| Pain (0–100 VAS) | 1.65 (5.15) | 44.71 (22.22)a |
| Depression (0–100 VAS) | 3.94 (14.74) | 34.35 (25.92)a |
| PILL Total Score | 80.12 (18.44) | 139.82 (29.10)a |
| Physical Function (0–100 VAS) | 95.94 (6.67) | 51.76 (22.57)b |
| Role Function (0–100 VAS) | 92.65 (24.63) | 8.82 (26.43)b |
ME/CFS > HC (p<.05);
HC > ME/CFS (p<.05);
PILL: Pennebaker Inventory of Limbid Languidne
Functional Connectivity Analysis
ME/CFS patients demonstrated greater functional connectivity relative to HC in 5 of 21 regions used as seeds in the seed-to-voxel FC analysis (Table 2). These regions were bilateral SFG, ACC, and right AG. Compared to HC, ME/CFS participants had significantly higher FC of bilateral SFG with precuneus and postcentral gyrus, ACC with PCC and left thalamus/hippocampus, right AG with ipsilateral pre/postcentral gyri, and precuneus with bilateral supplementary motor area. HC participants also had greater FC than ME/CFS subjects in 4 of 21 regions, including ACC, left PaHcG, and bilateral pallidum (Table 3). Compared to ME/CFS subjects, HC participants showed significantly higher FC of the ACC with right insula, planum tempolare, temporal pole, putamen, and Heschl’s gyrus. Left PaHcG showed greater FC with right precentral gyrus and IFG. Left pallidum showed significantly greater FC with contralateral lingual gyrus, hippocampus, fusiform cortex, and parahippocampal gyrus. Right pallidum had significantly greater FC with contralateral occipital pole and intracalcerine cortex, as well as bilateral lingual gyri. No significant differences in FC between HC and ME/CFS subjects were detected for right PaHcG, bilateral caudate nuclei, bilateral hippocampus, left AG, bilateral MFG, or PCC. Figures 1–3 represent examples of ROIs and the locations of clusters differing significantly between ME/CFS participants and HC.
Table 2.
Seed regions with increased functional connectivity in ME/CFS vs. HC
| Seed Region | Cluster Coordinates | Cluster Size | Cluster Regions | Voxels in Region | % Coverage | Cluster p-value (<.05 FDR) | HC Connectivity Mean (SD) | CFS Connectivity Mean (SD) |
|---|---|---|---|---|---|---|---|---|
| Right Superior Frontal Gyrus | −08, −72, 52 | 328 | Precuneus | 178 | 3 | .0008 | −.13 (.16) | .19 (.20) |
| Right Postcentral Gyrus | 36 | 1 | ||||||
| Not assigned or less than 1% coverage | 114 | - | ||||||
| Left Superior Frontal Gyrus | 04, −66, 60 | 195 | Precuneus | 156 | 3 | .04 | −.08 (.19) | .21 (.15) |
| Not assigned or less than 1% coverage | 39 | - | ||||||
| 02, −42, 74 | 172 | Precuneus | 41 | 1 | .04 | −.22 (.20) | .16 (.28) | |
| Left Postcentral Gyrus | 23 | 1 | ||||||
| Right Postcentral Gyrus | 19 | 1 | ||||||
| Not assigned or less than 1% coverage | 89 | - | ||||||
| Anterior Cingulate Cortex | −08, −40, 10 | 259 | Posterior Cingulate Gyrus | 23 | 1 | .01 | −.34 (.17) | .15 (.26) |
| Left Thalamus | 22 | 2 | ||||||
| Left Hippocampus | 7 | 1 | ||||||
| Not assigned or less than 1% coverage | 207 | - | ||||||
| Precuneus | −06, −06, 66 | 465 | Left Supplementary Motor Area | 153 | 24 | .0004 | .01 (.17) | .29 (.12) |
| Right Supplementary Motor Area | 85 | 12 | ||||||
| Left Superior Frontal Gyrus | 49 | 2 | ||||||
| Right Precentral Gyrus | 29 | 1 | ||||||
| Right Superior Frontal Gyrus | 14 | 1 | ||||||
| Not assigned or less than 1% coverage | 125 | - | ||||||
| Right Angular Gyrus | 28, −26, 78 | 361 | Right Precentral Gyrus | 117 | 3 | .004 | −.07 (.18) | .25 (.20) |
| Right Postcentral Gyrus | 69 | 2 | ||||||
| Not assigned or less than 1% coverage | 175 | - |
Table 3.
Seed regions with reduced functional connectivity in ME/CFS vs. HC
| Seed Region | Cluster Coordinates | Cluster Size | Cluster Regions | Voxels in Region | % Coverage | Cluster p-Value (p <.05 FDR) | HC Connectivity Mean (SD) | CFS Connectivity Mean (SD) |
|---|---|---|---|---|---|---|---|---|
| Anterior Cingulate Cortex | 38, −8, −4 | 723 | Right Insula | 303 | 23 | <.0001 | .38 (.11) | .09 (.14) |
| Right Planum Polare | 106 | 28 | ||||||
| Right Temporal Pole | 79 | 3 | ||||||
| Right Putamen | 58 | 7 | ||||||
| Right Heschl’s Gyrus | 19 | 7 | ||||||
| Not assigned or less than 1% coverage | 158 | - | ||||||
| Left Parahippocam pal Gyrus | 54, 6, 44 | 1163 | Right Precentral Gyrus | 373 | 9 | <.00001 | .21 (.11) | −.01 (.10) |
| Right Middle Frontal Gyrus | 365 | 13 | ||||||
| Right Inferior Frontal Gyrus (pars opercularis) | 85 | 12 | ||||||
| Right Inferior Frontal Gyrus (pars triangularis) | 16 | 3 | ||||||
| Not assigned or less than 1% coverage | 324 | - | ||||||
| −54, −30, 52 | 251 | Left Postcentral Gyrus | 154 | 4 | .002 | .18 (.11) | −.02 (.11) | |
| Left Supramarginal Gyrus | 73 | 8 | ||||||
| Not assigned or less than 1% coverage | 24 | 0 | ||||||
| Left Pallidum | 38, −36, −10 | 448 | Right Hippocampus | 82 | 12 | .0006 | .17 (.15) | −.13 (.17) |
| Right Lingual Gyrus | 41 | 2 | ||||||
| Right Fusiform Cortex | 34 | 5 | ||||||
| Right Parahippocam pal Gyrus | 11 | 3 | ||||||
| Not assigned or less than 1% coverage | 280 | - | ||||||
| Right Pallidum | 0, −62, 6 | 286 | Left Lingual Gyrus | 104 | 7 | .03 | .15 (.15) | −.09 (.12) |
| Left Occipital Pole | 45 | 2 | ||||||
| Left Intracalcarine Cortex | 41 | 6 | ||||||
| Right Lingual Gyrus | 25 | 1 | ||||||
| Not assigned or less than 1% coverage | 76 | - |
Figure 1.
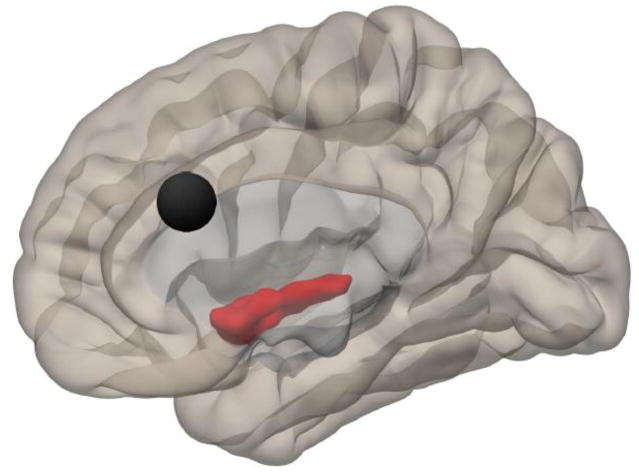
Right medial view illustrating the ACC (black circle) and the location of Cluster 1 (38, −8, −4), for which connectivity was higher in HC than ME/CFS. Regions included in the cluster include right insula, right planum polare, right temporal pole, right putamen, and right Heschl’s gyrus.
Figure 3.
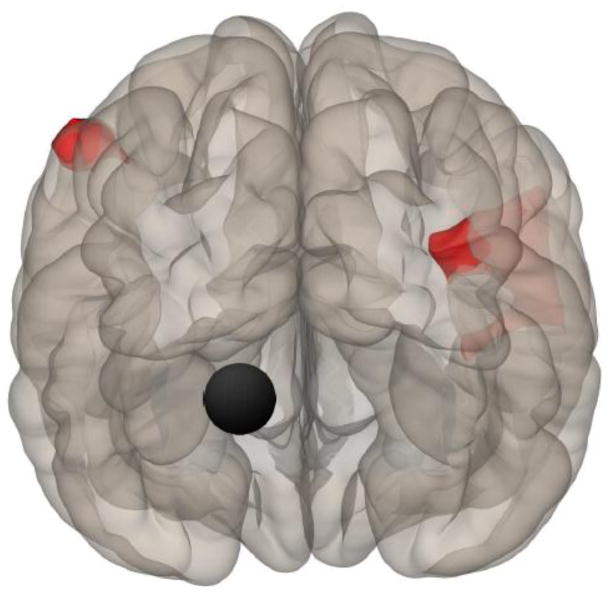
Right view showing location of the right SFG (black circle) and a significant cluster (0, −72, 52) where ME/CFS showed greater connectivity than HC to a 328 voxel cluster including precuneus and right postcentral gyrus (i.e., primary somatosensory cortex).
Relationship between Connectivity Measures and Clinical/Affective Measures
For brain regions (clusters) identified as different between HC and ME/CFS groups, the correlations between FC and clinical/affective measures were all significant (p<.01). For clusters where HC had greater FC than ME/CFS subjects, higher connectivity was associated with lower anxiety, fatigue, pain, and depression, and higher physical and role function. Where ME/CFS participants had greater FC than HC, the opposite pattern was apparent. This is likely because selection criteria (clinical symptoms), by design, differed between HC and ME/CFS subjects. The connectivity measures for the significant clusters also differed between ME/CFS participants and HC. Therefore, these correlations likely simply recapitulate the diagnosis of ME/CFS as well as differences between HC and ME/CFS subjects for the previously noted clusters.
Exploratory Spearman’s correlational analyses conducted for ME/CFS participants alone revealed a significant negative correlation between left PaHcG connectivity to left postcentral gyrus and left supra-marginal gyrus and fatigue ratings (r = −.71, p = .001). In addition, right pallidum connectivity to left lingual gyrus, intracalcarine cortex, and right lingual gyrus was significantly correlated with depression ratings (r = .50, p = .04). Precuneus connectivity was also negatively correlated with depression ratings (r = −.61, p = .01). Finally, ACC connectivity to PCC, left thalamus, and left hippocampus was significantly correlated with both fatigue (r = .56, p = 0.02) and pain (r = .52, p = .03) ratings. No other correlations reached significance (p < .05).
4. Discussion
To our best knowledge, this is the first resting state FC study of ME/CFS patients in general and the first one using ASL. Previous investigations of CBF using different blood flow measurements, including ASL have repeatedly demonstrated global or regional hypoperfusion in ME/CFS patients [3 and 4 and 5 and 27]. We have recently reported decreased rCBF in ME/CFS patients at rest and during an exhaustive cognitive task [5]. Because the significance of this hypoperfusion for ME/CFS is unclear [10], additional studies appeared to be indicated to determine the functional relationships between abnormal rCBF and cerebral function Therefore the present study examined the functional neural networks of ME/CFS patients and their association with brain regions demonstrating abnormal rCBF during ASL, as well as with regions relevant for neurocognitive, motor, and affective functioning.
Results of our FC analyses showed abnormal connectivity patterns of ME/CFS patients for several brain regions, suggestive of functional network reorganization, including areas involved in memory (left parahippocampal gyrus), motor (bilateral pallidum), mood (ACC), and higher-order neurocognitive functions (ACC, AG, and SFG). An area of particular interest that showed altered connectivity in ME/CFS was ACC. The ACC has been a substantial focus of interest and is known to play a critical role in higher-order neurocognitive functions, especially attention (Petersen and Posner, 2012). Furthermore, previous studies have demonstrated abnormal patterns of activation in ACC in ME/CFS patients [17]. Along with the anterior insula, the ACC has been described as a key hub of the salience network, which is associated with high-level neurocognitive control and attention [28]. Our data indicated that ME/CFS patients had significantly reduced FC between ACC and right insula. Such reduced resting-state connectivity between these regions has been associated with neuropsychological deficits [29 and 30], similar to those seen in patients with ME/CFS. Furthermore, ME/CFS patients in the present study showed increased connectivity between the ACC and hippocampus/PCC. Altered resting-state FC between these regions has been associated with Alzheimer’s disease [31], and may also be relative for memory deficits of ME/CFS patients. Alternatively, increased resting-state FC between the ACC and thalamus has been previously demonstrated in patients with major depressive disorder [32]. Taken together, these findings suggest that hypoperfusion of the ACC [5] results in functional network reorganization associated with decreased neurocognitive functioning and increased affective disturbance in ME/CFS. Although the present study did not collect neuropsychological data that would confirm neurocognitive impairments in these participants, our results showed that increased connectivity of ACC with the thalamus, hippocampus, and PCC was associated with greater fatigue and pain ratings of ME/CFS patients.
Another potentially important finding was significantly lower connectivity in ME/CFS participants between left PaHCG and two distinct clusters. The PaHCG, which includes the entorhinal cortex, is involved in aspects of limbic function as well as memory retrieval and storage [33]. The first cluster encompassed broad right frontal areas including precentral gyrus, middle frontal gyrus, and inferior frontal gyrus. Notably, these anatomical regions as a whole include primary and secondary motor cortex, suggesting perturbation of the link between limbic and motor function in ME/CFS. Notably, the middle and inferior frontal gyri include structures of significant importance to higher-order cognitive function, including dorsolateral prefrontal cortex and orbitofrontal cortex. The second cluster encompassed left postcentral gyrus (i.e., primary sensory cortex) and supramarginal gyrus. Reduced connectivity between PaHCG and regions in the second cluster suggests abnormality in the link between memory functions subserved by the PaHCG and bodily sensation associated with the postcentral gyrus. Indeed, lower connectivity to this cluster was strongly correlated to higher fatigue ratings of ME/CFS participants, accounting for approximately 50% of the variance in this measure.
Finally, ME/CFS patients showed altered connectivity for both right and left SFG. Lesion studies indicate that the SFG serves a critical role in working memory function [34]. Our data suggest SFG connectivity to the precuneus is significantly higher in patients with ME/CFS than in HC. Similarly, connectivity between the precuneus and supplementary motor area, an area that contributes to the planning of movement, was also higher in ME/CFS patients than in HC. The precuneus is a medial posterior parietal structure of increasing interest in cognitive and behavioral neuroscience which has extensive cortical and subcortical connections to areas including PCC, ACC, dorsolateral prefrontal cortex, supplementary motor area, thalamus, among others [35]. The precuneus is also an important part of the brain’s default mode network [36]. Evidence suggests the precuneus is activated in a wide variety of higher-order neurocognitive processes, including motor coordination, attention, motor imagery, mental rotation, mental navigation, episodic memory retrieval, social cognition, perspective taking, and self-reflection (see Cavanna and Trimble, 2006 for review). Thus, altered connectivity between the precuneus, SFG, and supplementary motor area suggest perturbations in networks mediating motor function, working memory, and higher-order neurocognitive functions, including self-monitoring, in ME/CFS patients.
Study Strengths and Limitations
A primary strength of this study is the use of ASL, which provides a non-invasive method of directly quantifying blood flow in whole brain or in specific ROIs [26 and 26 and 37 and 38]. Furthermore, temporal fluctuation in CBF (assessed in this case using ASL) appear to correspond to underlying patterns of neuronal activity [26 and 39]. ASL has frequency characteristics that make it especially suitable for FC analyses (specifically, no significant increase in noise power at low frequencies), and has similar statistical power and reliability for FC analysis using blood-oxygenation level dependent (BOLD) contrast [26],
Although our data provide useful information regarding the functional neural correlates of global and regional hypoperfusion characteristic of ME/CFS, they are limited by the cross-sectional design of the study. Specifically, it is unclear whether the network abnormalities suggested by our data predated the diagnosis or were the result of ME/CFS. Longitudinal analysis of ASL fMRI in individuals at risk for ME/CFS may provide relevant information related to this question.
Future Directions
Results of our study suggest several promising directions for future research. The generalizability of the role of FC between regions identified in this report in the experience of fatigue should be investigated in the context of fatigue induction in both ME/CFS patients and HC. Findings that fatigue induction modulates FC measures between these regions would support the supposition that they are robust neural correlates of fatigue. In addition, investigations regarding the effect of behavioral and pharmacological treatments on FC of ME/CFS patients between seed regions and those within significant clusters are warranted. Because ME/CFS patients experience a wide range of symptoms (fatigue, pain, affective disturbance) the specificity of the FC abnormalities to ME/CFS will need additional investigations to determine the unique contributions of ME/CFS to FC.
5. Conclusions
This is the first study using ASL perfusion data to demonstrate that ME/CFS patients have abnormal FC between several brain regions subserving neurocognitive, motor, and affective-related networks, including ACC, precuneus, SFG, AG, pallidum, and PaHCG. Furthermore, the abnormal FC of ME/CFS patients correlates significantly with their symptoms, including fatigue, depression, and pain. Future studies examining the link between FC characteristics for these regions and ME/CFS are needed. In addition, the relevance of connections between the identified regions for fatigue-related symptoms in patients suffering from other conditions (e.g., multiple sclerosis) should be investigated.
Figure 2.
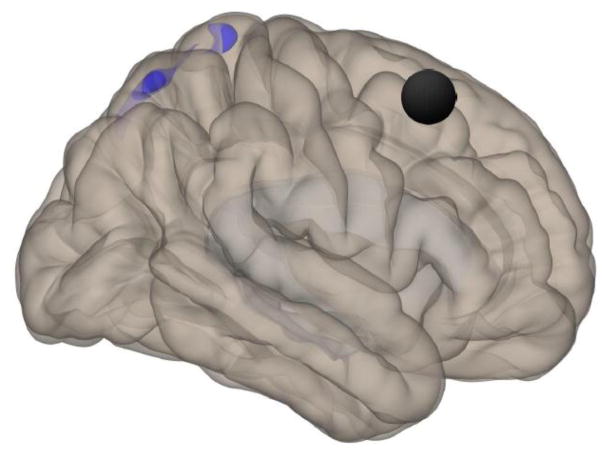
Posterior view showing location of the left PaHCG (black circle) and clusters 1 (56, 6, 44) and 2 (−54, −30, 52), for which HC had higher connectivity than ME/CFS. Cluster 1 regions include right precentral gyrus, right middle frontal gyrus, and right inferior frontal gyrus (pars opercularis and pars triangularis). Cluster 2 regions include left postcentral gyrus and left supramarginal gyrus
Footnotes
Disclosure: This study was supported by NIH grant R01 NR014049-01 and NIH/NCATS Clinical and Translational Science grants UL1 TR000064. None of the authors have any financial or other relationships that might result in a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holgate ST, Komaroff AL, Mangan D, Wessely S. Chronic fatigue syndrome: understanding a complex illness. Nature Reviews Neuroscience. 2011;12(9):539–44. doi: 10.1038/nrn3087. [DOI] [PubMed] [Google Scholar]
- 2.Biswal B, Kunwar P, Natelson BH. Cerebral blood flow is reduced in chronic fatigue syndrome as assessed by arterial spin labeling. J Neurol Sci. 2011;301(1–2):9–11. doi: 10.1016/j.jns.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa DC, Tannock C, Brostoff J. Brainstem perfusion is impaired in chronic fatigue syndrome. QJM. 1995;88(11):767–73. [PubMed] [Google Scholar]
- 4.Yoshiuchi K, Farkas J, Natelson BH. Patients with chronic fatigue syndrome have reduced absolute cortical blood flow. Clin Physiol Funct Imaging. 2006;26(2):83–6. doi: 10.1111/j.1475-097X.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 5.Staud R, Craggs JG, Lai S, Price DD, Robinson ME. Fatiguing Task is Associated with Decreased Cerebral Blood Flow in Patients with Chronic Fatigue Syndrome but not in Healthy Controls. Neuroimage Clin. 2015 in press. [Google Scholar]
- 6.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121(12):953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Liang X, Zou Q, He Y, Yang Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci U S A. 2013;110(5):1929–34. doi: 10.1073/pnas.1214900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van E, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jann K, Gee DG, Kilroy E, Schwab S, Smith RX, Cannon TD, Wang DJ. Functional connectivity in BOLD and CBF data: similarity and reliability of resting brain networks. Neuroimage. 2015;106:111–22. doi: 10.1016/j.neuroimage.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cope H, David AS. Neuroimaging in chronic fatigue syndrome. J Neurol Neurosurg Psychiatry. 1996;60(5):471–3. doi: 10.1136/jnnp.60.5.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afari N, Buchwald D. Chronic Fatigue Syndrome: A Review. Am J Psychiatry. 2003;160(2):221–36. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 12.Cook DB, O’Connor PJ, Lange G, Steffener J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage. 2007;36(1):108–22. doi: 10.1016/j.neuroimage.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 13.Caseras X, Mataix-Cols D, Rimes KA, Giampietro V, Brammer M, Zelaya F, Chalder T, Godfrey E. The neural correlates of fatigue: an exploratory imaginal fatigue provocation study in chronic fatigue syndrome. Psychological Medicine. 2008;38(7):941–51. doi: 10.1017/S0033291708003450. [DOI] [PubMed] [Google Scholar]
- 14.Okada T, Tanaka M, Kuratsune H, Watanabe Y, Sadato N. Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol. 2004;4(1):14. doi: 10.1186/1471-2377-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puri BK, Jakeman PM, Agour M, Gunatilake KDR, Fernando KAC, Gurusinghe AI, Treasaden IH, Waldman AD, Gishen P. Regional grey and white matter volumetric changes in myalgic encephalomyelitis (chronic fatigue syndrome): a voxel-based morphometry 3 T MRI study. Br J Radiol. 2012;85(1015):E270–E273. doi: 10.1259/bjr/93889091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorist MM, Boksem MA, Ridderinkhof KR. Impaired cognitive control and reduced cingulate activity during mental fatigue. Brain Res Cogn Brain Res. 2005;24(2):199–205. doi: 10.1016/j.cogbrainres.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Caseras X, Mataix-Cols D, Giampietro V, Rimes KA, Brammer M, Zelaya F, Chalder T, Godfrey EL. Probing the working memory system in chronic fatigue syndrome: A functional magnetic resonance imaging study using the n-back task. Psychosom Med. 2006;68(6):947–55. doi: 10.1097/01.psy.0000242770.50979.5f. [DOI] [PubMed] [Google Scholar]
- 18.Miller AH, Jones JF, Drake DF, Tian H, Unger ER, Pagnoni G. Decreased Basal Ganglia Activation in Subjects with Chronic Fatigue Syndrome: Association with Symptoms of Fatigue. PLoS ONE. 2014;9(5) doi: 10.1371/journal.pone.0098156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves WC, Lloyd A, Vernon SD, Klimas N, Jason LA, Bleijenberg G, Evengard B, White PD, Nisenbaum R, Unger ER. Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Serv Res. 2003;3(1):25. doi: 10.1186/1472-6963-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pennebaker J. The psychology of physical symptoms. New York: Springer Verlag; 1983. [Google Scholar]
- 21.Dai W, Garcia D, deBazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60(6):1488–97. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2007;58(5):1020–7. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Aguirre GK, Rao H, Wang J, Fernandez-Seara MA, Childress AR, Detre JA. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26(2):261–9. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z. Improving cerebral blood flow quantification for arterial spin labeled perfusion MRI by removing residual motion artifacts and global signal fluctuations. Magn Reson Imaging. 2012;30(10):1409–15. doi: 10.1016/j.mri.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–41. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Seara MA, Mengual E, Vidorreta M, Castellanos G, Irigoyen J, Erro E, Pastor MA. Resting state functional connectivity of the subthalamic nucleus in Parkinson’s disease assessed using arterial spin-labeled perfusion fMRI. Hum Brain Mapp. 2015;36(5):1937–50. doi: 10.1002/hbm.22747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswal B, Kunwar P, Natelson BH. Cerebral blood flow is reduced in chronic fatigue syndrome as assessed by arterial spin labeling. J Neurol Sci. 2011;301(1–2):9–11. doi: 10.1016/j.jns.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16(1):55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 30.Day GS, Farb NA, Tang-Wai DF, Masellis M, Black SE, Freedman M, Pollock BG, Chow TW. Salience network resting-state activity: prediction of frontotemporal dementia progression. JAMA Neurol. 2013;70(10):1249–53. doi: 10.1001/jamaneurol.2013.3258. [DOI] [PubMed] [Google Scholar]
- 31.Wang K, Liang M, Wang L, Tian L, Zhang X, Li K, Jiang T. Altered functional connectivity in early Alzheimer’s disease: a resting-state fMRI study. Hum Brain Mapp. 2007;28(10):967–78. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10(4):272–82. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- 34.du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006;129(Pt 12):3315–28. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- 35.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 36.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–84. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 37.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23(1):37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 38.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89(1):212–6. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci U S A. 1998;95(3):765–72. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]