Abstract
Adults primarily walk to reach a new location, but why do infants walk? Do infants, like adults, walk to travel to a distant goal? We observed 30 13-month-old and 30 19-month-old infants during natural walking in a laboratory playroom. We characterized the bout structure of walking—when infants start and stop walking—to examine why infants start and stop walking. Locomotor activity was composed largely of brief spurts of walking. Half of 13-month-olds’ bouts and 41% of 19-month-olds’ bouts consisted of three or fewer steps—too few to carry infants to a distant goal. Most bouts ended in the middle of the floor, not at a recognizable goal. Survival analyses of the distribution of steps per bout indicated that the probability of continuing to walk was independent of the length of the ongoing bout; infants were just as likely to stop walking after 5 steps as after 50 steps and they showed no bias toward bouts long enough to carry them across the room to a goal. However, 13-month-olds showed an increased probability of stopping after 1-3 steps, and they did not initiate walking more frequently to compensate for their surfeit of short bouts. We propose that infants’ natural walking is not intentionally directed at distant goals; rather, it is a stochastic process that serves exploratory functions. Relations between the bout structure of walking and other measures of walking suggest that locomotor exploration is constrained by walking skill in younger infants, but not in older infants.
Keywords: infant, locomotion, walking, exploration, survivor analysis
Independent mobility is one of the most important achievements of infancy. Locomotion provides infants with new opportunities to explore the places, surfaces, objects, and people in the larger environment (Gibson, 1988). In fact, exploration is a primary function of spontaneous locomotion in infants. Thus, improvements in independent mobility provide enhanced opportunities for wide-ranging exploration (Gibson, 1988; Gibson & Schmuckler, 1989). As Gibson (1978) said, “Exploratory skills in a human infant begin with looking around the world; everything within the baby’s field of view provides an incentive. When mobility is achieved, the baby first reaches for, then creeps toward and final walks (or runs) for the attractive goal he or she spies (p. 610).”
Indeed, Gibson, Piaget (1954), and other notables (Campos et al., 2000) have assumed that infants’ locomotor exploration is goal-directed: Infants see an attractive object across the room and use their new locomotor abilities to get to it. Although such an idea is not required by theory, it makes good sense. Adults generally initiate a bout of walking to travel to a specific location and stop walking when they arrive at the goal. But is infants’ walking similarly goal directed? It seems reasonable to suppose that infants begin a walking bout because they want to travel to an object, person, or destination that caught their interest or to share an object with their caregiver, and equally reasonable that infants stop walking because they want to play with the target of their interest or because they have reached their destination. However, locomotor exploration might have a less goal-directed quality. Infants might move to burn off energy or for the sheer joy of movement (Harlow & Mears, 1979); they might stop walking because they are tired, lose interest, or fall. To date, no study has described the starting and stopping patterns of infants’ natural locomotion.
Development of Walking and Locomotor Exploration
Rather than characterizing infant locomotion in terms of natural starting and stopping patterns, researchers typically characterize the development of walking in terms of periodic gait—repeated alternating steps in a straight line at a steady pace. Measures of periodic gait require at least four steps to describe movements of both legs, and the initial and final steps in a series are typically lopped off because gait initiation and termination are considered different processes from periodic gait. Thus, the length of each walking sequence is not determined by the infants, but by the practicalities of the testing paradigm: Studies of treadmill walking require infants to walk continuously for minutes at a time (Smith, Kubo, Black, Holt, & Ulrich, 2007) and studies of over-ground walking require children to take a long series of continuous steps through the measurement area (Sutherland, Olshen, Cooper, & Woo, 1980).
In reality, of course, walking sequences do not go on indefinitely. Instead, during natural locomotion moving freely in their homes or in a laboratory playroom, infant walkers spontaneously start and stop at frequent intervals, creating bouts of walking separated by periods when they are not walking (Adolph et al., 2012). Although toddlers accumulate impressive numbers of steps, total distances, and falls over the course of an hour (approximately 2400 steps, 700 m, and 17 falls, on average), they stop for longer periods than they go; infants are only in motion 30% of the time. Yet without knowing how activity is interspersed with periods of rest, we do not have an accurate picture of infants’ locomotor exploration, and can only guess at what drives their decisions to start and stop.
Bouts of Locomotion
Natural behavior is segmented into bouts—periods of action when the behavior is expressed, separated by periods of rest when the behavior is not expressed. In this sense, each action within a bout represents a “decision” about whether to continue the current behavior or to stop. In many domains, the criteria for delimiting a bout are arbitrary. That is, researchers can make a compelling argument to separate bouts based on pauses of a few seconds, several seconds, minutes, and so on, depending on the method used to determine the criterion for separating bouts (Sibly, Nott, & Fletcher, 1990; Slater & Lester, 1982). However, for walking, the beginning and end of a bout are clear: A bout begins when one foot moves and a bout ends when both feet are on the floor for a duration longer than walkers’ typical period of “double support.” A pause between steps that exceeds the typical double support period is no longer walking; it is standing.
Analyses of bout structure have yielded important insights into the organization of behavior in a variety of developmental domains including infant looking time (Messinger, Ekas, Ruvolo, & Fogel, 2013), suckling and feeding (Wolff, 1968), newborn stepping and infant kicking, (Thelen, 1979), fetal and neonatal motility (Hayes, Plante, Kumar, & Delivoria-Papadopoulos, 1993; Robinson, Blumberg, Lane, & Kreber, 2000; Robinson & Smotherman, 1988), and a variety of behaviors in non-human animals, (Blumberg, Seelke, Lowen, & Karlsson, 2005; Fagen & Young, 1978; Hailman, 1974; Martin, Raabe, & Heisenberg, 1999; Machlis, 1977; Sibly et al., 1990; Slater & Lester, 1982). Moreover, the organization of behavior—and how it changes or remains constant across development—can yield insights into the rules governing behavior, including environmental pressures and the underlying status of the nervous system. For example, simple, low level processes such as noise and hysteresis explain the bout structure in neonatal visual exploration (duration of looking at a target and frequency of switching to a new one). But by three months of age, such low-level processes can no longer account for the bout structure in visual exploration; other processes such as attention are at work (Robertson, 2013). Presumably, analyses of bout structure in natural infant locomotion might also yield insight into the real-time and developmental processes guiding locomotor exploration. In particular, the number and length of infants’ walking bouts can provide insight into why infants start and stop walking.
Current Study
The current study was designed to redress gaps in our understanding about the organization of infant walking and exploration. In particular, we focused on the bout structure of natural walking and the number of steps per bout. The number of walking bouts represents the number of times infants chose to initiate walking. The number of steps per bout reflects infants’ propensity to maintain walking by stringing steps together in a continual series. After delineating walking bouts we identified how bouts ended: whether infants stopped walking due to factors beyond their control (fell or were picked up), stopped at a goal (at a toy, at their mother, to climb up or down an elevation), or stopped walking with no apparent goal (after stepping in place, in the middle of the floor).
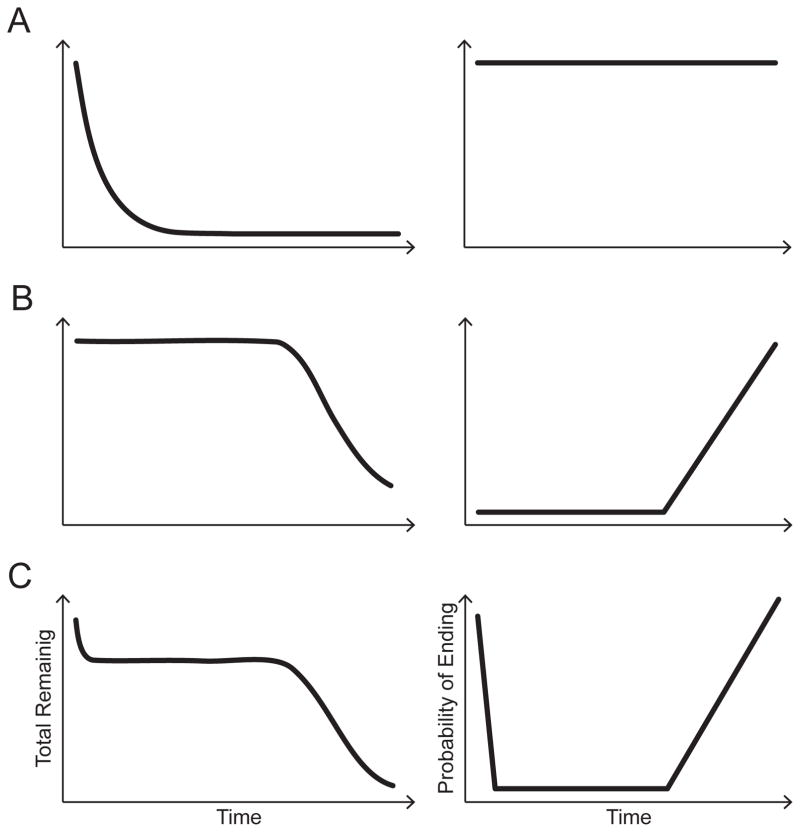
In addition to describing individual bouts, we characterized the distribution of bout lengths and calculated the probability of stopping after each step. We computed cumulative survivor distributions of the number of steps per bout for 13- and 19-month-old walkers. The shape of the resulting distribution can inform on the underlying mechanisms that shape behavior. Survivor analysis underlies actuarial tables, the age-specific mortality listed in life tables that insurance companies use to estimate life expectancy. Indeed, if mortality rates were constant over the life span (right panel of Figure 1A)—if death were no more likely at one age than at any other age—the cumulative survival distribution would closely follow a negative exponential curve (left panel of Figure 1A). But typically, survival rates are not constant. On average, in the US, mortality is low until old age, when it increases more precipitously (Figure 1B). In developing countries, infant mortality is high, so the cumulative survival distribution looks more like the curve illustrated in Figure 1C.
Figure 1.
Sample cumulative survival distributions pictured in the left panel and corresponding probability of stopping (or ending, i.e., dropping out of the distribution) pictured in the right panel. (A) Survivor distribution showing a negative exponential curve (left panel); the probability of stopping is constant (right panel). (B) Survivor distribution that would arise if infants continued walking until they reached a “preferred” bout length (left panel); the probability of stopping is low until infants reach that bout length, then rapidly increases (right panel). (C) Survivor distribution showing a more rapid initial drop-off (left panel); the probability of stopping would be high initially, then plateau before rising again (right panel). Note that the curves in Figure 1B–C are intended as stylized depictions.
By analogy, in the case of natural infant walking, cumulative survivor distributions can tell us whether the probability of continuing a bout (i.e., taking another step) is constant or if a bout is more likely to continue until a certain number of steps. Just as each individual dies once, each walking bout “dies” or ends once when the infant stops; by looking at a sample of many bouts we can describe the rate at which bouts end and drop out of the sample as a function of the number of steps taken. If the probability of continuing a bout is constant, the cumulative survivor distribution will closely follow the negative exponential curve shown in Figure 1A. By necessity, this means that the probability of taking another step is independent of the number of steps already taken. In contrast, if the probability of continuing a bout is not constant, the cumulative survivor distribution would have a different shape. For example, an initially flat distribution as shown in Figure 1B might result if infants typically continue walking long enough to cross the room to reach a distant goal—and then stop after achieving the requisite distance. An initially steep distribution that later plateaus as pictured in Figure 1C might result if infants have trouble initiating walking, but are able to maintain walking after taking several steps.
We focused on the natural bout structure in 13-month-old novice walkers and 19-month-old experienced walkers. Walking in 13- and 19-month-olds differs in important ways. First, natural locomotion differs between novice and experienced walkers: Novices take fewer spontaneous steps, travel shorter overall distances, spend less time in motion, and fall more frequently compared with experienced walkers (Adolph et al., 2012). Second, gait patterns in novice walkers are less mature: Novices take shorter steps with their feet spread wider apart; the time with two feet on the floor between steps is longer and the time with one foot in the air during a step is briefer. With increased walking age (number of days since walking onset), infants take longer, narrower steps, and double-support time decreases while single-support time increases (Adolph, Vereijken, & Shrout, 2003; Hallemans, De Clercq, & Aerts, 2006). Moreover, measures of infants’ natural walking validate measures of periodic gait: Infants who accumulate more steps, spend more time in motion, and fall less frequently during natural walking exhibit more mature gait patterns in standard tests of periodic gait.
By analyzing 13- and 19-month-olds, we could chart developmental changes in the bout structure of infant walking and interrelations among walking variables as walking skill improved. Thus, we conducted integrated analyses of the bout structure of natural walking, global measures of natural walking (total number of steps and falls per hour, cadence), and standard gait measures (step length, width, dynamic base). Using both correlations and hierarchical clustering techniques, we examined interrelations among the various measures of infant walking across development.
Method
Participants
Thirty 13-month-olds (M = 13.05 months, 16 boys and 14 girls) and 30 19-month-olds (M = 19.02 months, 16 boys and 14 girls) from the New York City area participated. Parents were contacted over the phone and asked if their infants were able to walk 10 feet independently; parents who replied that their infants could walk to this criterion were invited to participate in the study. Infants were predominantly white and middle class. In a structured interview, mothers reported the first day that they saw their infant walk 10 feet independently without stopping or falling. The 19-month-olds had more days of walking (M = 192.07 days, range 58–289 days) than the 13-month-olds (M = 45.25 days, range 8–97 days) and the two groups had little overlap, t(55) = 12.63, p < .001. Note, our criterion for walking onset was relatively conservative. Presumably, infants displayed a period prior to the 10-foot onset date when they could take only a step or two without falling. Thus, all infants were capable of producing extended walking sequences in the standard periodic gait paradigm and during natural spontaneous walking.
Procedure and Apparatus
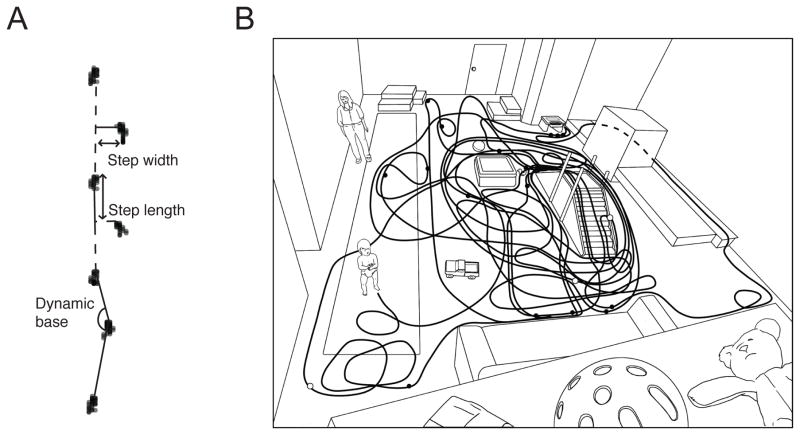
At the start of the session, we collected standard gait measures of infant walking. Infants walked repeatedly (M = 7.31 trials) over a 5.73-m long × 0.92-m wide pressure-sensitive carpet (www.gaitrite.com) that recorded the x and y coordinates of each step (Figure 2A). We aimed to collect at least six trials per infant, and repeated trials as necessary if infants fell or veered off the carpet. Then, we observed 30 minutes of infants’ natural walking in a laboratory playroom filled with elevations (slide, stairs, platforms) and toys. As depicted in Figure 2B, elevations and toys were not evenly distributed across the room; rather, there were large open spaces between them. Infants could move freely through the room and caregivers were instructed to interact with infants normally while minding their safety. Thus, infants could walk or remain stationary as they wished. An experimenter with a hand-held camera recorded infants’ movements and a fixed camera mounted overhead provided an overall view of infants’ movements through the room. The experimenter did not interact with infants or caregivers.
Figure 2.
(A) Sample footprint path showing the calculation of standard gait measures obtained on the pressure-sensitive gait carpet (step length, step width, and dynamic base angle). (B) Schematic drawing of the laboratory playroom. The squiggly line represents a typical path in the first 10 minutes of a 13-month-old’s session. The filled circles represent the location of stops that lasted longer than 5 seconds and the open circles represent bouts that ended in a fall. The adult is the infant’s mother.
Data Coding and Reduction
Standard Gait Measures of Walking
As shown in Figure 2A, we calculated standard gait measures based on the raw x-y coordinates from the gait carpet: step length (the distance along the line of progression from the previous heel to the current heel), step width (the distance from the heel to the line of progression), and dynamic base angle (the angle between three consecutive footfalls. Because walking speed is highly correlated with other standard gait measures (Bril & Breniere, 1989), we selected each infant’s two fastest uninterrupted walks for analysis. Test-retest reliability between selected trials was high for all gait measures, rs(54) > .90, ps < .001.
Measures of Natural Walking
Using the Datavyu coding software (www.datavyu.org), a primary coder scored videos of natural walking to identify the onset and offset of each walking bout, the number of steps per bout, and falls. A step was defined as any up-down motion of the foot or any sideways movement that moved the foot across the floor. Steps could be omnidirectional (infants often stepped backward or sideways or in place) and did not have to involve alternating leg movements. A fall was any time infants lost balance and dropped to the floor unsupported. Preliminary analyses suggested a relation between accumulated steps and falls (infants who took more steps had more chances to fall) in the older infants, r(28) = .34, p = .05. We therefore report fall rate—total falls divided by total steps—to avoid unfairly penalizing infants who accumulated more falls not because of poor skill, but because they were more active.
A walking bout reflected a period of walking—either a single step or multiple steps—separated by a rest period of at least half a second. Past research using the standard gait paradigm in infants who have been walking for six months or less indicates that periods of double support (when both feet are on the ground) last approximately 45 to 164 ms (Bril & Breniere, 1989; Hallemans, De Clercq, Otten, & Aerts, 2005). However, double support time decreases with days of walking (Hallemans et al., 2005) and walking speed (Bril & Breniere, 1989). The 500 ms rule was therefore chosen to ensure that sequences of ongoing walking were not erroneously parsed into separate bouts of natural locomotion. This was especially important because in natural locomotion infants sometimes move very slowly—resulting in longer double support times—or take only one or two steps. Moreover, preliminary inspection of the videos revealed that coders could quickly detect half-second pauses in locomotion; these breaks, like longer ones, appeared to the naked eye as a real break in locomotion. We also validated the 500 ms criteria directly by measuring double support times during natural locomotion. We scored double support time in one randomly selected walking bout consisting of three or more steps (the minimum number needed to calculate double support times) from each infant. Double support times during natural walking ranged from 120 to 470 ms (M = 254.8 ms) in 13-month-olds and 80 to 460 ms (M = 233.2 ms) in 19-month-olds. Cadence denoted steps/time per bout. Cadence has been previously measured in periodic gait paradigms, and typically increases during the first six months of independent walking (Bril & Breniere, 1993).
After identifying walking bouts, a primary coder scored how bouts ended in 10% of walking bouts randomly selected from each infant’s data. Some bouts ended at a clear destination: Bouts that ended with a reach toward an initially out-of-reach object were scored as object bouts and bouts that ended within arms’ reach of initially distant mother were scored as mother. Climbing bouts ended when infants arrived at a change in elevation and switched from walking to crawling to climb up or down. Some bouts ended for reasons outside infants’ control: when their mother lifted them, or when they fell. Finally, some bouts had no discernible reason for ending: In-place bouts ended when infants took steps toward or around an object that was within reach at bout initiation and at every step in the bout. Traveling bouts ended when infants stopped walking for no apparent reason, without engaging with an object or mother and without falling or attempting to scale an elevation.
A second coder independently scored 25% of each infant’s walking data and 100% of falls. Inter-rater reliability was high: r = 1.0, p < .001 for number of steps in each bout and r = .97, p < .001 for bout duration; 100% agreement between coders for falls and 90% agreement for how bouts ended.
Bout organization was assessed by computing cumulative survivor distributions of the number of steps per bout; distributions were calculated both for individual infants and for data pooled across all infants within an age group. Group data reflect bout lengths pooled over infants. Tests of relations between the bout-specific probability of stopping and other walking measures used probabilities calculated individually for each infant. The cumulative survivor distributions were constructed by summing the number of bouts containing at least one step (i.e., all bouts), followed by the smaller subset of bouts containing at least two steps, followed by the subset of those bouts containing at least three steps, and so on. The slope of the resulting curve—how quickly it drops off— indicates the probability of an ongoing bout continuing or not, given how many steps were already taken (Hailman, 1974). These distributions then were examined to determine goodness of fit to a negative exponential distribution (which would occur if the likelihood of stopping were unrelated to the number of steps just taken). These distributions also were used to derive the probability of stopping after one, two, three, or four steps.
Results
Walking Bouts
Table 1 summarizes age comparisons for measures of walking bouts. On average, each 13-month-old accumulated 184.8 walking bouts (range 61–252), and each 19-month-old accumulated M = 194.1 walking bouts (range 110–275) over the 30-minute observation period, for a total of 11,368 bouts across the dataset. Infants in both age groups initiated walking the same number of times: We found no significant difference in the number of walking bouts between age groups, t(58) = .93, p > .10, although 19-month-olds averaged 8.3 steps per bout, whereas 13-month-olds averaged only 7.2 steps per bout, t(58) = 2.43, p < .05. But simple averages conceal substantial intra-individual variability in bout length; the largest range in bout length for a 13-month-old was 1-103 steps; the largest range in bout length for a 19-month-old was 1-155 steps. Although the average bout consisted of 7-8 steps, a large percentage of infants’ bouts were extremely short: Half of the younger infants’ bouts and 41% of older infants’ bouts consisted of only one to three steps—too short to calculate standard gait measures for both legs or to count as periodic gait. Indeed, a sizeable percentage of bouts consisted of only a single step: 26.5% of younger infants’ bouts and 18.8% of older infants’ bouts. We checked whether short bouts were due to falling. They were not. In both age groups, bouts of one to three steps were no more likely to end in a fall than longer bouts; M = 3.0% of short bouts ended in a fall, compared to M = 3.8% of longer bouts. Moreover, the larger proportion of short bouts in younger infants was not because they were incapable of stringing several steps together. In fact, every infant in the sample—including the least experienced walkers—exhibited at least one bout consisting of 15 steps or more.
Table 1.
Means and standard deviations for measures of walking bouts in 13-month-olds and 19-month-olds
| 13-month-olds | 19-month-olds | |||
|---|---|---|---|---|
|
| ||||
| Mean | SD | Mean | SD | |
|
| ||||
| Total bouts | 184.83 | 36.12 | 194.10 | 40.88 |
| Step per boutc | 7.18 | 2.18 | 8.35 | 1.46 |
| % Bouts < 4 stepsa | 50.49 | 11.26 | 40.75 | 5.78 |
| % Single-step boutsa | 26.76 | 8.47 | 19.03 | 5.49 |
| Probability of stopping | ||||
| After 1 stepa | .27 | .08 | .19 | .05 |
| After 2 stepsb | .21 | .07 | .16 | .03 |
| After 3 stepsc | .16 | .05 | .14 | .03 |
| After 4 steps | .13 | .05 | .12 | .04 |
p < .001;
p < .01,
p < .05
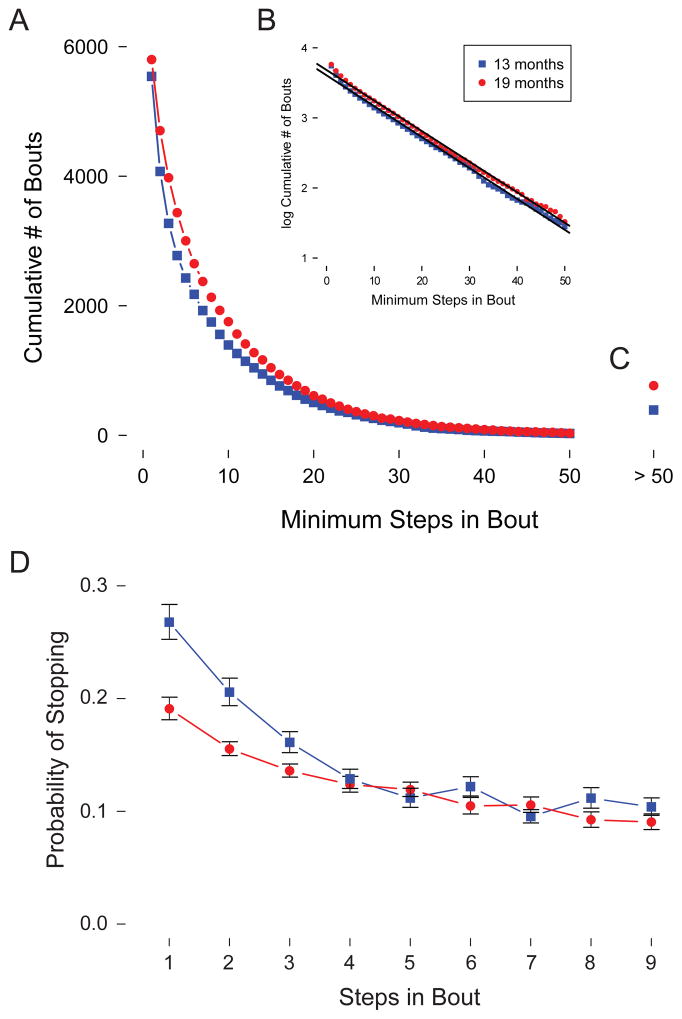
To further compare the tendency for infants in each age group to continue a bout of walking, we examined the cumulative survivor distribution for all bouts (Figure 3A) and discovered that the overall distribution closely followed a negative exponential distribution (r2 = .997). This is especially apparent in the log-normal plot of the data (Figure 3B), where both age groups show a nearly perfect linear trend, Fs > 1272.66, p < .001. (Figure 3C shows all bouts with more than 50 steps). The distributions depicted in Figure 3A & B indicate that the likelihood of adding one more step to an ongoing bout is constant (i.e., the slope of the linear relation does not change as we move along the x-axis) and independent of how many steps have already been taken. Contrary to what might have been expected, infants do not appear to incur a cost for long bouts, such as increased fatigue or waning motivation. That is, infants are just as likely to stop after the fifth step as after the fiftieth step.
Figure 3.
(A) Cumulative survival distribution of steps per bout in 13-month-olds and 19-month-olds. (B) Semi-log plot of the same data. Black lines indicate the linear fit for each age group. (C) Total number of bouts containing more than 50 steps. (D) Probability of ending a bout after taking one to nine steps in 13-month-olds and 19-month-olds. Thirteen-month-olds are represented by blue squares, and 19-month-olds by red circles. Error bars represent the standard error of the mean.
However, close examination of the distribution of short bouts revealed a systematic deviation from the negative exponential fit in the younger age group. A Kolmogorov–Smirnov test confirmed that the distributions in the 13-month-olds and the 19-month-olds were significantly different from each other, D =.02, p < .001. Thirteen-month-olds over-produced short bouts consisting of one to three steps, indicating a reduced probability of continuing the bout. Restricting the Kolmogorov–Smirnov test to the portion of the distribution containing four or more steps confirmed this impression: With one, two, and three step bouts removed, the distributions in the younger and older infants no longer differed, p > .1.
We examined the deviation from the negative exponential fit in short bouts by characterizing the probability that each infant would stop after a given number of steps, creating a “risk plot” for each infant (this is directly analogous to age-specific mortality in actuarial tables). The probability of stopping was calculated as . Dividing the number of bouts of length x + 1 by the number of bouts of length x (e.g., looking at the proportion of bouts with three steps that go on to have a fourth step) yields the probability of adding a step to the bout; the complement of this yields the probability of ending the bout at that point. This parameter is derived from the survivor distribution and describes the slope of the curve at each point, but was calculated separately for each infant and was normally distributed. Figure 3D shows that 13-month-olds were more likely to stop walking after one, two, or three steps, but converge on the same negative exponential function seen in older infants from four steps on. A 2 (age group) x 4 (1, 2, 3, or 4 step-long bout) ANOVA on the probability of stopping confirms this impression: The ANOVA yielded a significant interaction between age group and bout length, F(3,174) = 7.41, p < .001. Follow-up comparisons confirmed age differences in likelihood of stopping after one, two, and three steps, ts(58) > 2.14, ps < .05, and no differences by four steps, t(58) = .22, p > .05.
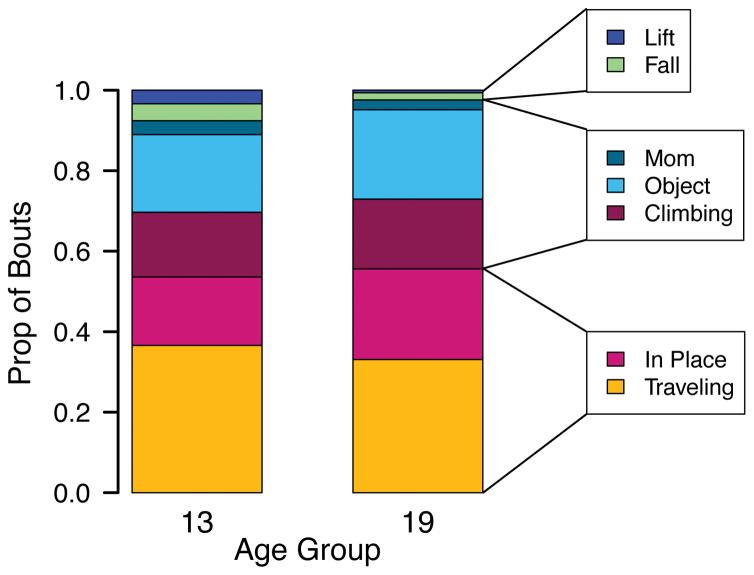
Bout Destination
What caused infants to stop walking? As shown in Figure 4, a substantial percentage of infants’ walking bouts ended at no clear destination or goal: On average, 53% of the bouts for each 13-month-old and 56% of the bouts for each 19-month-old were scored as traveling or in-place. Fewer bouts ended at an elevation, object, or mother: 38% of 13-month-olds’ and 42% of 19-month-olds’ bouts were scored as climbing, object, or mother. Only a small fraction of bouts ended for reasons outside infants’ control: 8% of 13-month-olds’ and 2% of 19-month-olds’ bouts were scored as falls or getting picked up.
Figure 4.
Average proportion of walking bouts that ended with no clear goal (traveling or in-place bouts), at a potential goal (an object, mother, or climbing), or due to factors outside the infant’s control (falling or being picked up).
A 2 (age group) x 3 (bout type) ANOVA on the proportion of bouts ending with a goal, no goal, or due to external factors confirmed the predominance of walking without a discernible goal in both age groups. The ANOVA yielded a main effect of bout type, F(2,116) = 214.24, p < .001, but no main effect of age group and no interaction between age group and bout type. Follow-up comparisons indicated that bouts without a clear goal (traveling or in-place bouts) were significantly more common than bouts with a goal (an object, mother, or climbing), p < .001. Bouts with a goal were, in turn, significantly more common than bouts that ended due to factors outside the infants’ control (falling or being picked up), p < .001.
Relations Among Bouts, Natural Walking, and Standard Gait Measures
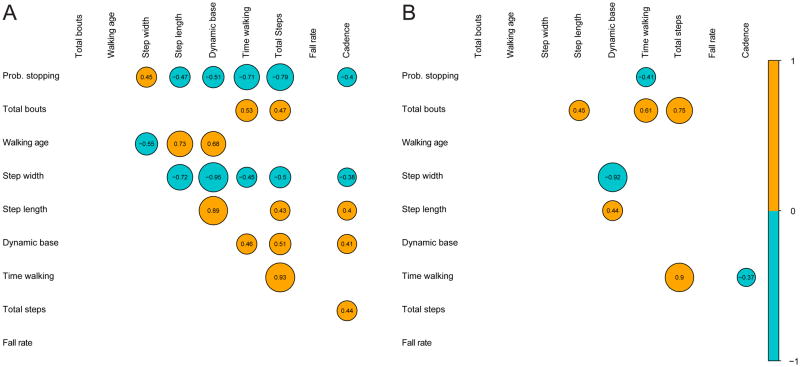
Does the greater propensity to stop in younger children relate to other measures of walking? We examined relations between the bout structure of infant walking (total number of bouts, probability of ending a bout after two steps calculated for each infant), walking age (days since onset), standard gait measures (step length, width, dynamic base), and measures of natural walking (total time walking, total number of steps, rate of falling, and cadence). We chose the probability of ending a bout after two steps because once infants take a third step it becomes possible to calculate standard gait measures for one leg. A two-step bout, in contrast, is not gait by definition. We also performed these analyses using the probability of ending a bout after only one or three steps and found similar results. Figure 5A depicts inter-correlations for the younger infants and Figure 5B depicts inter-correlations for the older infants. We report correlations separately to illustrate the different patterns of inter-correlations in novice and experienced infant walkers.
Figure 5.
Graphical depiction of inter-correlations between measures of walking for (A) 13-month-olds and (B) 19-month-olds. A teal circle denotes a positive correlation, and a gold circle denotes a negative correlation. The diameter of the circle is scaled to the strength of the correlation; correlations not significant at the p < .05 level are not shown.
In both age groups, measures of the same type tended to inter-correlate. That is, standard gait measures were correlated with each other, and measures of accumulated natural walking were correlated with each other. However, examination of Figure 5 shows that there were clear differences in the patterns of correlation in each age group. In the 13-month-olds, many measures of walking were strongly inter-correlated; 24 of the possible 45 correlations reached significance (Figure 5A). In addition to inter-correlations among measures of the same category, standard gait measures were related to measures of natural walking. Most relevant, the probability of stopping after two steps was related to all three standard gait measures and to total steps, time walking, and cadence during natural walking. Total walking bouts—our other measure of bout structure—correlated only with time walking and total steps; infants who initiated walking more often accumulated more steps and more time in motion.
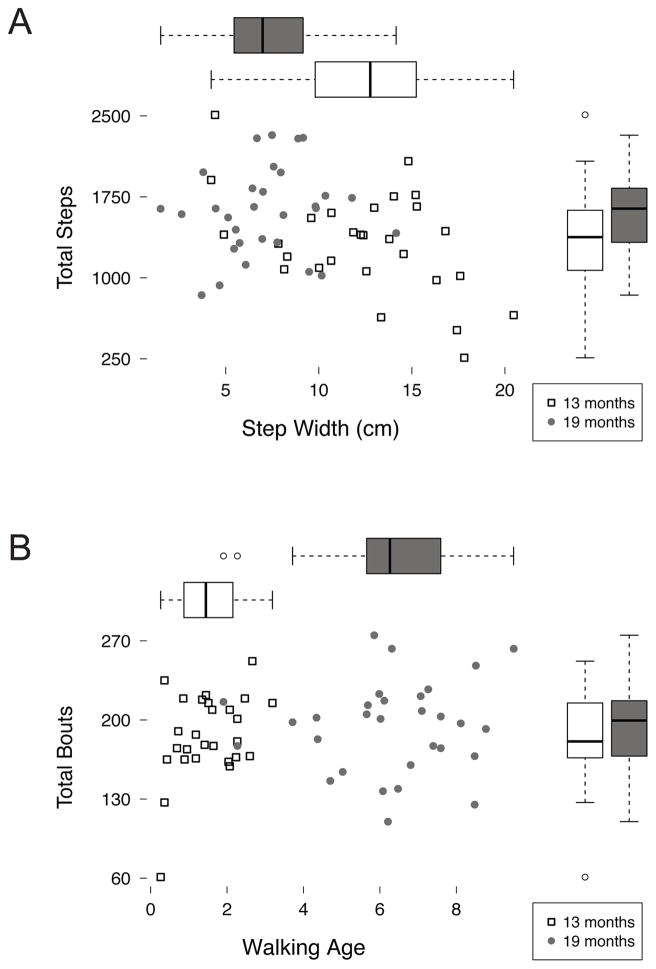
In contrast, only 7 of the 45 possible correlations reached significance in the 19-month-olds (Figure 5B). Although measures of the same type inter-correlated, measures of standard gait were poor predictors of natural walking. Likewise, measures of the bout structure of walking showed little relation with measures of gait or accumulated amount of walking. This lack of correlations in 19-month-olds cannot be attributed to reduced variability. Figure 6 illustrates two example correlations and the ranges of the variables being tested. Figure 6A depicts step width and total steps. The box plots show substantial variability in total steps in both age groups (right hand side) and in step width in both age groups (top). However, only 13-month-olds—shown in square symbols—show a significant correlation between step width and total steps. In 19-month-olds (round symbols) no such relation exists between standard skill measures and measures of natural walking. Similarly, Figure 6B shows the spread of walking bouts (right hand side) and walking age (top). Despite substantial between-subject variability on both measures, there is no relation between walking age and total walking bouts at either age.
Figure 6.
Scatterplots and box plots of (A) step width by total steps and (B) walking age by total bouts. Open squares denote 13-month-olds, filled circles denote 19-month-olds. Boxplots along the top depict the distribution of the x-axis variable and boxplots along the right depict the distribution of the y-axis variable separately for 13-month-olds (white boxes) and 19-month-olds (grey boxes). Solid lines in the box plots denote medians; circles denote outliers beyond 1.5 times the interquartile range.
Cluster Analysis
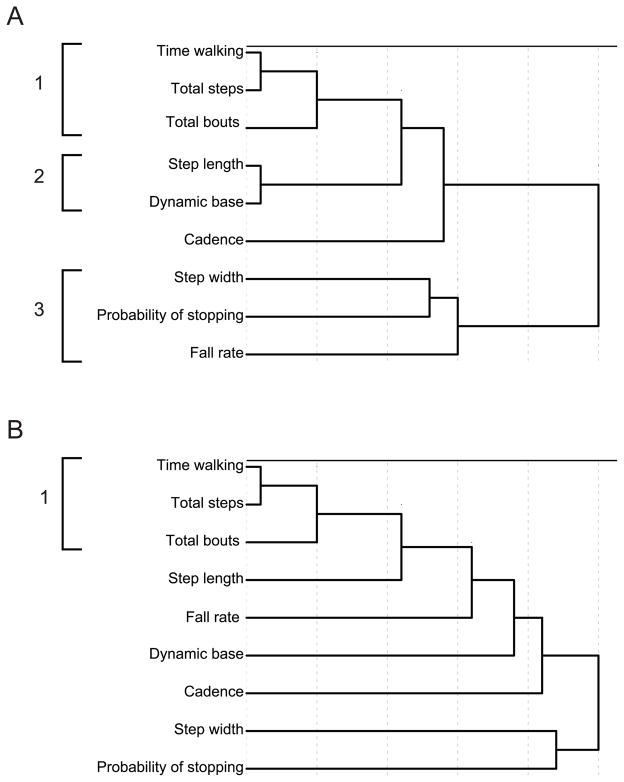
We used hierarchical cluster analysis to analyze inter-relations among variables in a different way by revealing which variables cohere most strongly. Figure 7 depicts the resulting dendrograms from the cluster analysis using an average linkage algorithm (complete and single linkage algorithms yielded similar results). We used Squared Euclidean distance as the measure of similarity, and all variables were standardized using a z-score transformation. The clustering algorithm works in stages, progressively merging related variables and clusters to form a hierarchy. Variables that are closely related link together early in the procedure, creating tight clusters; variables that are added late in the procedure are more distantly related.
Figure 7.
Dendrograms illustrating the results of cluster analyses for (A) 13-month-olds and (B) 19-month-olds. Variables that join early in the procedure (further to the left) are more closely related than variables that join late in the procedure (further to the right). Resulting clusters are highlighted by the brackets on the left side.
In the 13-month-olds, a clear structure emerges with three primary clusters, Figure 7A. Gait measures of walking proficiency (step length and dynamic base angle) form a tight cluster, and measures of how much infants walk (total steps, total time walking, and total walking bouts) form a second cluster. The cluster of standard gait measures and the natural walking cluster are more distantly related, and link later in the procedure. Step width—often used as an indicator of balance—is grouped with fall rate and the probability of stopping after two steps, forming a third “balance” cluster. In other words, for 13-month-olds, the variables are intercorrelated as shown in Figure 5, and in addition, the variables cohere into conceptually meaningful clusters.
For 19-month-olds, the cluster analysis did not reveal a clear tree structure (Figure 7B). Total steps and total time walking are initially grouped together, with total walking bouts being added at the next stage. These variables are necessarily related: Infants who spend more time walking accumulate more steps. However, instead of forming the basis of clear clusters as in the younger infants, the remaining variables are added to these clusters one at a time later in the procedure; this further indicates that various measures of walking are no longer mutually dependent in the older infants in the way that they are in younger infants
Discussion
During natural infant walking, approximately 50% of bouts consisted of only a few steps. The impressive numbers of accumulated steps previously reported in walking infants (Adolph et al., 2012) are largely the result of hundreds of brief spurts of walking. Survival analyses of the distribution of steps per bout revealed that the probability of continuing an ongoing bout is constant and independent of how many steps infants have already taken. However, novice infant walkers deviate from this distribution by over-producing bouts of one to three steps, and they do not compensate for their surfeit of short bouts by initiating walking more often. For both novice and experienced walkers, most bouts ended at no clear destination or goal. Correlational and cluster analyses indicated that the bout structure of walking, standard gait measures, and measures of natural walking were closely related in younger novice walkers, but not in older more experienced walkers.
Why do infants initiate walking?
Researchers have long assumed that infant locomotor exploration is goal-directed: Infants spy an attractive goal across the room and travel to it (Campos et al., 2000; Gibson, 1988; Gibson & Schmuckler, 1989). The ability to crawl first gives infants the opportunity for locomotor exploration; the onset of walking grants greater visual and physical access to the world, allowing infants to see farther and travel longer distances to the objects they see (Adolph et al., 2012; Kretch, Franchak, & Adolph, 2014). However, a new line of work indicates that the traditional assumptions about goal-directed, visually motivated locomotor exploration are incorrect. While crawling, infants primarily see the floor beneath their hands (Kretch et al., 2014). When crawlers sit up to look around, the whole room swoops into view, but the transition from quadruped to sitting turns their bodies 90°–180° away from the direction of original heading (Soska, Robinson, & Adolph, in press). When infants revert back to crawling, the transition in posture now turns their bodies in a completely different direction from what they were facing while sitting. Thus, exploration in crawling infants has a far more random character than what researchers had assumed. Similarly, the current study suggests that traveling to a distant goal—an enticing object, an elevation to climb, or mother—is not the driving force in locomotor exploration in walking infants.
Bouts ending in a goal
One line of evidence against goal-directed locomotor exploration is that most walking bouts had no clear goal at all. The most common way that infants ended a walking bout was to stop in the middle of the floor. Another substantial proportion of walking bouts took place with an object in reach the entire time; these bouts cannot be said to be goal-directed, because there was no need to locomote to reach the “goal.” In fact, our coding criterion erred on the side of giving infants credit for having had a goal: Because we could not infer infants’ intentions when they initiated walking, we coded any bout that ended with object engagement, an approach to mother, or climbing up or down a change in elevation as a goal-directed bout. But in many cases, infants seemed to happen upon objects by chance while already in motion, or to traverse a change in elevation en route to another location. In fact, infants occasionally looped around the room in bouts of over a hundred steps, passing an object several times before stopping to engage with it. Our estimates thus represent the most generous estimate of goal-directed exploration; infants got credit simply for stopping at a potential goal. Nevertheless, even while granting the generous assumption that all object, climbing, or mother bouts were goal-directed, only 40% of walking bouts had a potential goal compared to 60% that did not. Of course, infants are capable of walking directly from point A to point B, and they sometimes do so. But more often, they do not.
Our assertion that locomotor exploration is not geared specifically toward distant goals does not contradict previous findings that walking infants are more likely to engage with distal objects (Karasik, Adolph, Tamis-LeMonda, & Zuckerman, 2012). Rather, it suggests that walkers may interact with distal objects not because they preferentially seek them out across the room—walking from one distant object to another—but because in covering more ground as walkers, they come upon interesting objects along the way. Our data are also compatible with previous reports that infants frequently do not end bouts at any object at all; they simply stop walking with no clear goal in sight (Karasik et al., 2012).
Our findings do not imply that the environment is irrelevant to infant locomotor exploration. In the current study, we observed infants playing freely with mobile caregivers in a novel, target-rich environment replete with slides, staircases, and toys separated by wide, open spaces. Each of these factors, alone and in combination could have affected the frequency of goal-directed locomotion, and alternate layouts could have resulted in different patterns of locomotor activity. The novelty of the lab playroom, for example, might have increased the likelihood that infants seek out or approach new objects and places; however, infants’ natural walking in a novel laboratory playroom is similar to their walking produced in the home (Adolph, et a., 2012). The rich layout and availability of toys in our playroom likely increased the frequency of goal-directed exploration; a more impoverished environment may have yielded longer walking bouts and even fewer bouts ending at a goal. The large open spaces between potential goals in the current study allowed for long walking bouts to travel directly from one object to another, but most bouts were short and did not end at a potential goal. The non-random distribution of potential goals could have led to a non-random structure in walking, but it did not. An empty room with no potential goals or a highly familiar home environment might induce more or less goal-directed behavior. A stationary caregiver might have reduced locomotor exploration—as in using mother as a “secure base,” however few bouts ended at mother. Further research testing infants in different environments is underway to determine how environmental layout, available objects, and the location of the caregiver affects patterns of locomotor activity and exploration.
Predominance of 1- to 3-step bouts
A second line of evidence against the traditional story is the predominance of extremely short walking bouts, with no compensatory increase in the frequency of bout initiation. As Figure 3A shows, infants’ natural walking was skewed toward short bouts despite every infant demonstrating the ability to produce long bouts. This predominance of short bouts was not an artifact of our scoring criterion. To the contrary, our definition of a pause in walking was conservative relative to established norms for infant walking (Bril & Breniere, 1989; Hallemans et al., 2005). Thus, our half-second criterion was biased toward parsing behavior into fewer, longer bouts. Due to infants’ short step lengths, bouts of one to three steps cannot cover much ground. In principle, infants could reach a distant goal by stringing together several short walking bouts, but they did not. Moreover, given that all infants were capable of long walking bouts, they had no reason to stop every few steps rather than proceed directly to the goal. The younger infants showed even less ability to cover ground: 13-month-olds took shorter steps and were more likely to end a walking bout after only a few steps. If infants initiated locomotion to travel to a distant goal, the younger infants would have been forced to compensate for their short bouts by initiating walking more often. But they did not. In most cases, traversing the room—whether to reach a goal or simply to walk—is not what motivates infants to initiate walking.
Survival analysis
The survival analysis provides a third line of evidence against the traditional story of goal-directed exploration. In experienced infant walkers, the probability of adding another step to an ongoing bout was constant. Novice walkers’ bouts followed the same distribution after they had taken at least four steps. The constant probability of ending a bout indicates that, at least within the 30-minute timescale of the current study, infants’ decision to stop a bout is not affected by growing fatigue or a bias to cover particular distances: Infants are just as likely to end a bout having gone nowhere (after only a step or two) as they are having crossed the room (after dozens of steps) or having done laps around the room without stopping anywhere along the way (after 100 steps). If walking were predominantly goal-directed, the survival analysis would not have revealed a constant probability of stopping (i.e., more closely resembled Figure 1B than Figure 1A).
Notably, the assumption of goal-directed walking may not even hold true for adults. The bout structure of natural walking in infants—frequent short bouts with a constant probability of stopping—aligns well with similar data on natural walking in adults. Orendurff and colleagues (2008) found that most natural walking in adults consists of relatively short bouts: 60% of bouts last 30 seconds or less and 40% consist of 12 or fewer steps. In fact, Orendurff et al likely underestimated the frequency of short bouts because their criterion used to define a pause in adult walking was extremely conservative and biased towards long bouts (periods of inactivity less than 10 seconds long were not considered a break in walking, and single steps were discarded from analysis as measurement errors). Likewise, although the adult data were not reported in terms of survival analyses, inspection of the data suggests that the cumulative survivor distribution would closely follow a negative exponential up to bouts of about 60 steps.
Thus, like infants, adults do not appear to show a bias to continue walking long enough to travel to a distant goal. At first, goal-less walking in adults may seem counterintuitive: People’s subjective impression may be that they initiate walking to travel to a specific destination. However, adult walking can also be aimless. We stroll leisurely through the park; we pace in circles while talking on the phone; we take unnecessary steps in place while preparing dinner in front of the counter. However, at least some of adults’ walking must be goal-directed. As such, adult walking will be influenced by the distribution of desirable objects in the environment. In the current study, objects were not evenly distributed through the space; rather, they clustered together, leaving large areas of empty space (see Figure 2B). Without evaluating the distribution of adult walking under similar controlled conditions, we cannot accurately address similarities and differences between infant and adult walking.
What does guide locomotor exploration?
If walking to reach a distant goal is not a driving force in infants’ early locomotor exploration, what does guide infants’ movements through a room? One possibility is that infants’ body dimensions may shape or constrain walking. For example, older infants with more adult-like body proportions may find it easier to maintain walking because their center of mass is lower. However, this was not the case. After accounting for the effects of age and walking age, body dimensions such as weight-for-height percentile did not explain additional variance, R2 change < .04, Fs < 2.04, ps > .05.
Based on the current data, we find evidence of two processes shaping early locomotor exploration. The first, already discussed, is a constant background probability of ending a walking bout, regardless of how long the infant has been walking. The second process—found primarily in younger infants—results in an increased probability of ending bouts early. Why do 13-month-olds infants frequently end their bouts immediately after initiating walking? Our findings regarding the relations between measures of bout structure and other walking variables provide some suggestions.
Specifically, younger infants are initially constrained by poor walking skill. Younger infants showed strong inter-correlations among all measures of walking: bout measures, standard gait measures, and measures of natural walking. More important, bout structure (the probability of ending a bout early) and accumulated amounts of natural walking (total steps, time walking, and cadence) were predicted by one or more standard measures of walking gait. Infants who showed less mature gait patterns were more likely to end a walking bout early and they accumulated less walking overall. Likewise, the cluster analysis for 13-month-olds showed a clear pattern of relations with three separate clusters. The third cluster that emerged in the younger infants specifically supports the idea that poor skill and postural control were related to difficulties in maintaining balance during natural walking. The rate of falling, the probability of ending a bout after two steps, and step width—commonly taken as an indicator of infants’ developing balance (Bril & Breniere, 1993)—grouped together, despite being strongly correlated with measures grouped into other clusters. This resulting cluster can be thought of as a “balance factor”: Infants who walked with wide steps to compensate for poor balance also fell more frequently during the session and were more likely to end their bouts after only a few steps. In contrast, 19-month-olds showed little relation between standard gait measures and bout structure or accumulated walking, and did not show evidence of clear clusters of related variables. Taken together, these results indicate that 13-month-olds’ walking patterns—and by extension their locomotor exploration—are heavily shaped by their limited walking experience and low levels of walking skill, whereas 19-month-olds show no such constraint.
Bouts of one, two, or three steps could result from errors in standing or errors in initiating walking. That is, infants may lose balance while standing and take a step to catch themselves, or they may lose balance after they initiate walking and then stop to regain equilibrium. Both scenarios fit with results from standardized skill paradigms. Even when provided with an external support, infants have larger postural sway while standing than adults (Barela, Jeka, & Clark, 1999). Likewise, adults make compensatory postural adjustments prior to initiating walking—allowing them to control their movements before gravity takes over during the first step—but children do not show this ability until 6 to 8 years of age (Breniere & Do, 1986; Ledebt, Bril, & Breniere, 1998). In either case, the challenge for young infants is to maintain balance long enough to take several steps. It would have been reasonable to expect that maintaining balance in the context of ongoing movement becomes progressively harder for longer bouts, like building a tower of blocks taller and taller until it topples and falls. Instead, it appears that maintaining balance is more like riding a bike or pushing a stalled car: The greatest difficulty is at the outset. Once younger infants have taken several steps, the greatest danger is past, and they exhibit the same constant probability of stopping as older infants.
Conclusions
The ability to walk provides infant with new opportunities for exploration and learning. Poor walking skill makes new walkers walk less and stop more frequently. Improved walking skill allows more experienced walkers to walk more and take more steps per bout. Although both novice and experienced walkers can explore the environment in a deliberate and goal-directed way, our findings indicate that frequently they do not. Infants can spy an attractive target and walk toward it. But more often, infant locomotor exploration follows a stochastic process, in which they just walk and arrive at attractive targets more serendipitously.
Acknowledgments
The project described was supported by Award Number R37HD033486 from NICHD to KEA. Additional support was provided by Procter and Gamble International Relations.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or P&G.
References
- Adolph KE, Cole WG, Komati M, Garciaguirre JS, Badaly D, Lingeman JM, … Sotsky RB. How do you learn to walk? Thousands of steps and dozens of falls per day. Psychological Science. 2012;23:1387–1394. doi: 10.1177/0956797612446346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Vereijken B, Shrout PE. What changes in infant walking and why. Child Development. 2003;74:474–497. doi: 10.1111/1467-8624.7402011. [DOI] [PubMed] [Google Scholar]
- Barela JA, Jeka JJ, Clark JE. The use of somatosensory information during the acquisition of independent upright stance. Infant Behavior and Development. 1999;22:87–102. doi: 10.1016/S0163-6383(99)80007-1. [DOI] [Google Scholar]
- Blumberg MS, Seelke AMH, Lowen SB, Karlsson KE. Dynamics of sleep-wake cyclicity in development rats. Proceedings of the National Academy of Sciences. 2005;102:14860–14864. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breniere Y, Do MC. When and how does steady state gait movement induced from upright posture begin? Journal of Biomechanics. 1986;19:1035–1040. doi: 10.1016/0021-9290(86)90120-x. [DOI] [PubMed] [Google Scholar]
- Bril B, Breniere Y. Steady-state velocity and temporal structure of gait during the first six months of autonomous walking. Human Movement Science. 1989;8:99–122. [Google Scholar]
- Bril B, Breniere Y. Posture and independent locomotion in early childhood: Learning to walk or learning dynamic postural control? In: Savelsbergh GJP, editor. The development of coordination in infancy. North-Holland, The Netherlands: Elsevier; 1993. pp. 337–358. [Google Scholar]
- Campos JJ, Anderson DI, Barbu-Roth MA, Hubbard EM, Hertenstein MJ, Witherington DC. Travel broadens the mind. Infancy. 2000;1:149–219. doi: 10.1207/S15327078IN0102_1. [DOI] [PubMed] [Google Scholar]
- Fagen RM, Young DM. Temporal patterns of behavior: durations, intervals, latencies, and sequences. In: Colgan PW, editor. Quantitative ethology. Wily; New York: 1978. pp. 79–114. [Google Scholar]
- Gibson EJ. C’est moi. Contemporary Psychology: A Journal of Reviews. 1978;23:609–614. [Google Scholar]
- Gibson EJ. Exploratory behavior in the development of perceiving, acting, and the acquiring of knowledge. Annual Review of Psychology. 1988;39:1–41. [Google Scholar]
- Gibson EJ, Schmuckler MA. Going somewhere: An ecological and experimental approach to development of mobility. Ecological Psychology. 1989;1:3–25. [Google Scholar]
- Hailman JP. A stochastic model of leaf-scratching bouts in two emberizine species. The Wilson Bulletin. 1974;86:296–298. [Google Scholar]
- Hallemans A, De Clercq D, Aerts P. Changes in 3D joint dynamics during the first 5 months after the onset of independent walking: A longitudinal follow-up study. Gait and Posture. 2006;24:270–279. doi: 10.1016/j.gaitpost.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Hallemans A, De Clercq D, Otten B, Aerts P. 3D joint dynamics of walking in toddlers: A cross-sectional study spanning the first rapid development phase of walking. Gait and Posture. 2005;22:107–118. doi: 10.1016/j.gaitpost.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Mears C. The human model: Primate perspectives. New York: Wiley; 1979. [Google Scholar]
- Hayes MJ, Plante L, Kumar SP, Delivoria-Papadopoulos M. Spontaneous motility in premature infants: Features of behavioral activity and rhythmic organization. Developmental Psychobiology. 1993;26:279–291. doi: 10.1002/dev.420260505. [DOI] [PubMed] [Google Scholar]
- Karasik LB, Adolph KE, Tamis-LeMonda CS, Zuckerman A. Carry on: Spontaneous object carrying in 13-month-old crawling and walking infants. Developmental Psychology. 2012;48:389–397. doi: 10.1037/a0026040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretch KS, Franchak JM, Adolph KE. Crawling and walking infants see the world differently. Child Development. 2014 doi: 10.1111/cdev.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledebt A, Bril B, Breniere Y. The build-up of anticipatory behavior: An analysis of the development of gait initiation in children. Experimental Brain Research. 1998;120:9–17. doi: 10.1007/s002210050372. [DOI] [PubMed] [Google Scholar]
- Machlis L. An analysis of the temporal patterning of pecking in chicks. Behaviour. 1977;63:1–70. [Google Scholar]
- Martin JR, Raabe T, Heisenberg M. Central complex substructures are required for the maintenance of locomotor activity in Drosophila melanogaster. Journal of Comparative Physiology A. 1999;185:277–288. doi: 10.1007/s003590050387. [DOI] [PubMed] [Google Scholar]
- Messinger DS, Ekas NV, Ruvolo P, Fogel AD. ‘Are you interested, baby?’ young infants exhibit stable patterns of attention during interaction. Infancy. 2013;17:233–244. doi: 10.1111/j.1532-7078.2011.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J. The construction of reality in the child. New York: Basic Books; 1954. [Google Scholar]
- Orendurff MS, Schoen JA, Bernatz GC, Segal AD, Klute GK. How humans walk: Bout duration, steps per bout, and rest duration. Journal of Rehabilitation Research & Development. 2008;45:1077–1090. doi: 10.1682/jrrd.2007.11.0197. [DOI] [PubMed] [Google Scholar]
- Robertson SS. Empty-headed dynamical model of infant visual foraging. Developmental Psychobiology. 2013:1–5. doi: 10.1002/dev.21165. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Blumberg MS, Lane MS, Kreber LA. Spontaneous motor activity in fetal and infant rats is organized into discrete multilimb bouts. Behavioral Neuroscience. 2000;114(2):328–336. doi: 10.1037//0735-7044.114.2.328. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Chance and chunks in the ontogeny of fetal behavior. In: Smotherman WP, Robinson SR, editors. Behavior of the fetus. Caldwell, NJ: Telford Press; 1988. pp. 95–115. [Google Scholar]
- Sibly RM, Nott HMR, Fletcher DJ. Splitting behavior into bouts. Animal Behavior. 1990:63–69. [Google Scholar]
- Slater PJB, Lester NP. Minimizing errors in splitting behavior into bouts. Behaviour. 1982;79:153–161. [Google Scholar]
- Smith BA, Kubo M, Black DP, Holt KG, Ulrich BD. Effect of practice on a novel task--walking on a treadmill: Preadolescents with and without Down syndrome. Physical Therapy. 2007;87:766–777. doi: 10.2522/ptj.20060289. [DOI] [PubMed] [Google Scholar]
- Soska KC, Robinson SR, Adolph KE. A new twist on old ideas: How sitting reorients crawlers. Developmental Science. doi: 10.1111/desc.12205. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland DH, Olshen R, Cooper L, Woo S. The development of mature gait. Journal of Bone and Joint Surgery. 1980;62:336–353. [PubMed] [Google Scholar]
- Thelen E. Rhythmical stereotypies in normal human infants. Animal Behavior. 1979;27:699–715. doi: 10.1016/0003-3472(79)90006-X. [DOI] [PubMed] [Google Scholar]
- Wolff PH. The serial organization of suckling in the young infant. Pediatrics. 1968;42:943–956. [PubMed] [Google Scholar]