Major unmet needs in managing patients with atrial fibrillation (AF) are to track AF propensity, monitor therapeutic response, and ultimately predict AF episodes. We are disappointingly far from these goals because our basic tools in AF—atrial electrograms and their classic interpretation—provide limited actionable data on substrates, their progression, AF initiation, or critical sites that maintain AF.1
A major limitation of clinical AF studies is that classic interpretation2 is challenging, focusing on unipolar and bipolar electrogram activation timing and qS-type deflections but largely ignoring repolarization.3 However, AF operates at the limits of dynamic activation and recovery. Thus, defining beat-to-beat repolarization is critical to identify whether AF electrograms are local or far-field (ie, within refractoriness of prior cycles) (Figure 1),4 when activity encroaches on recovery to cause block, reentry, and AF,4,5 and to compute phase6 to reveal sources that drive human AF in recent clinical7 or human atrial optical mapping8 studies.
Figure 1.
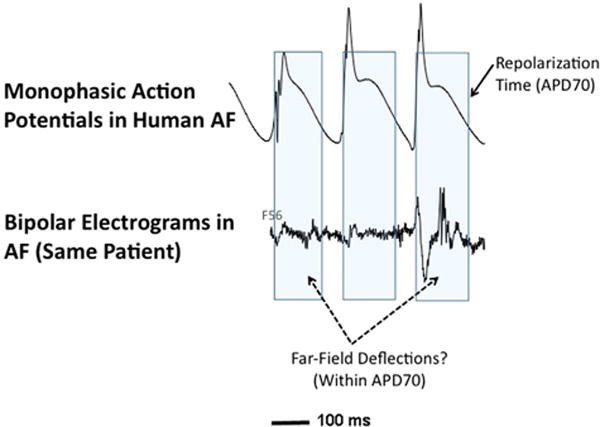
Repolarization information simplifies interpretation of atrial electrophysiology. Simultaneous monophasic action potentials (top) and bipolar electrograms (bottom) in human left atrium during atrial fibrillation (AF). Top: Beat-to-beat tracking of repolarization time, indicated by time to ≈70% repolarization (APD70) in the monophasic action potential,3 shows that clinical electrograms in human AF may poorly indicate true local activation. Several deflections (nonfractionated and fractionated) fall well within APD70 and likely are far-field (see text and Narayan et al9).
Human atrial activation and recovery in AF have traditionally been measured by monophasic action potentials (MAPs)4 that record local activity.3 Unipolar2 and bipolar electrograms in AF, on the other hand, summate far-field events that are often asynchronous wavelets. This challenges conclusive interpretation of “qS” or “rR” shapes in AF,9 unlike organized rhythms such as flutter in which local and far-field signals indicate the same wavefront. Moreover, atrial refractoriness is seen in MAPs3 yet has been obscure in bipolar and unipolar signals (Figure 1). The unipolar activation-recovery interval may approximate refractoriness in the ventricles10 but is undemonstrated in the atria.
Identifying atrial repolarization from standard electrodes
Into this field comes the timely work of Verrier et al,11 who set out to detect atrial repolarization from standard electrograms in order to determine AF susceptibility. Using standard clinical catheters, the authors recorded atrial repolarization (Ta) waves in anesthetized pigs and studied the impact of vagus nerve stimulation and of atrial ischemia or acetylcholine plus epinephrine on AF inducibility. Ta waves correlated with MAP recordings, and PTa intervals (the “atrial QT”) shortened during right or left vagus nerve stimulation. Ischemia or autonomic stimulation caused alternans (TWAa) and spatiotemporal heterogeneity (TWHa) of Ta, which preceded AF onset from a trigger. The authors concluded that standard catheters can measure Ta waves and indices of vulnerability (TWAa and TWHa) that precede AF.
AF prediction
This study advances the science of AF prediction. There is clear interest in magnetic resonance imaging as a tool to identify AF substrates, yet structural indices may not fully identify dynamic risk for AF. The authors’ findings are in agreement with clinical studies in which indices of atrial MAPs12,13 predict AF, potentially via conduction slowing5 that interacts with MAP alternans4 to cause block/reentry and initiate AF. The findings of Verrier et al11 may enable such prediction from standard electrodes, such as pacemaker leads.
Novel detection of atrial repolarization
The means by which Verrier et al11 revealed atrial repolarization from standard catheters is of interest and previously had been challenging. First, catheters were placed in the lateral right atrium, which minimizes the ventricular QRS that often overshadows atrial repolarization because the PR interval is inconveniently similar to the human atrial action potential duration (~200–300 ms in sinus rhythm, 100–160 ms at fast rates).4,12 Whether this method applies throughout both atria must be determined. Second, atrial action potentials often are triangular (Figure 1, first complex), with the flat slope from “plateau” to final repolarization causing a more gradual gradient (dV/dt) and flat Ta (much like mouse and rat ventricles that lack clear T waves). In their Figures, Verrier et al11 show well-developed Ta waves, even in bipolar recordings, which may reflect specific atrial regions or may be dynamic. Again, this should be tested widely in the atria. Third, the authors’ analysis residuae may have facilitated detection of TWAa and TWHa despite the limitations of unipolar and bipolar signals for detecting repolarization.
Limitations and future directions
All models of AF have limitations. Verrier et al11 did not test their measures of atrial repolarization clinically, which is required to ensure that this approach is not specific to their porcine model. In terms of prediction, AF induction is a poor predictor of clinical AF, so alternative endpoints are needed. Clinically, AF often follows sympathetic rather than vagal stimulation and is uncommonly associated with ischemia,14 so these data must be tested in clinical AF scenarios.
If repolarization metrics can be obtained routinely during clinical AF, then the impact would be substantial. Accurate repolarization data may clarify the debate on complex fractionated atrial electrograms by identifying which signal components are far-field vs “pivot points.”1 Repolarization data also may help resolve the dichotomy that optical mapping—the gold standard—mostly shows that rotors sustain AF (including in human atria8), yet most classic electrogram analyses of AF do not15. Finally, repolarization data may enable tracking of response to therapy.
Concluding statements
Verrier et al11 should be congratulated for demonstrating that dynamic atrial repolarization may be detected from routine clinical catheters. Although their results require further validation, they hold promise both as predictive tools and as aids in improving AF mapping and resolving the mechanistic debate. We look forward to exciting future developments in this area.
Acknowledgments
Dr. Narayan has received research grants from the National Institutes of Health (HL83359, HL103800); reports being co-inventor on intellectual property owned by the University of California and licensed to Topera Inc, in which he has held equity; and reports having received consulting fees from the American College of Cardiology and Uptodate, and speaking fees from St. Jude Medical and Medtronic.
References
- 1.Chugh A. Complex fractionated atrial electrograms in catheter ablation of atrial fibrillation: dead and buried? Circ Arrhythm Electrophysiol. 2015;8:999–1001. doi: 10.1161/CIRCEP.115.003276. [DOI] [PubMed] [Google Scholar]
- 2.de Groot NM, Houben RP, Smeets JL, Boersma E, Schotten U, Schalij MJ, Crijns H, Allessie MA. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation. 2010;122:1674–1682. doi: 10.1161/CIRCULATIONAHA.109.910901. [DOI] [PubMed] [Google Scholar]
- 3.Bode F, Kilborn M, Karasik P, Franz MR. The repolarization-excitability relationship in the human right atrium is unaffected by cycle length, recording site and prior arrhythmias. J Am Coll Cardiol. 2001;37:920–925. doi: 10.1016/s0735-1097(00)01189-x. [DOI] [PubMed] [Google Scholar]
- 4.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011;123:2922–2930. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schricker A, Rostamian A, Lalani G, Krummen DE, Narayan SM. Human atrial fibrillation initiates by organized not disorganized mechanisms. Circ Arrhythm Electrophysiol. 2014;7:816–824. doi: 10.1161/CIRCEP.113.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–78. doi: 10.1038/32164. [DOI] [PubMed] [Google Scholar]
- 7.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W-J, Miller J. Treatment of atrial fibrillation by the ablation of localized sources: the Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation: CONFIRM trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen BJ, Zhao J, Csepe TA, et al. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J. 2015;36(35):2390–2401. doi: 10.1093/eurheartj/ehv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayan SM, Wright M, Derval N, et al. Classifying fractionated electrograms in human AF using monophasic action potentials and activation mapping: evidence for localized drivers, rate acceleration and non-local signal etiologies. Heart Rhythm. 2011;8:244–253. doi: 10.1016/j.hrthm.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yue AM, Paisey JR, Robinson S, Betts TR, Roberts PR, Morgan JM. Determination of human ventricular repolarization by noncontact mapping: validation with MAP recordings. Circulation. 2004;110:1343–1350. doi: 10.1161/01.CIR.0000141734.43393.BE. [DOI] [PubMed] [Google Scholar]
- 11.Verrier RL, Fuller H, Justo F, Nearing BD, Rajamani S, Belardinelli L. Unmasking atrial repolarization to assess alternans, spatiotemporal heterogeneity, and susceptibility to atrial fibrillation. Heart Rhythm. 2016;13:XXX–XXX. doi: 10.1016/j.hrthm.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Kim B-S, Kim Y-H, Hwang GS, Pak H-N, Lee SC, Shim WJ, Oh DJ, Ro YM. Action potential duration restitution kinetics in human AF. J Am Coll Cardiol. 2002;39:1329–1336. doi: 10.1016/s0735-1097(02)01760-6. [DOI] [PubMed] [Google Scholar]
- 13.Lalani GG, Schricker AA, Clopton P, Krummen DE, Narayan SM. Frequency analysis of atrial action potential alternans: a sensitive clinical index of individual propensity to AF. Circ Arrhythm Electrophysiol. 2013;6:859–867. doi: 10.1161/CIRCEP.113.000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong KC, Lim C, Sadarmin PP, Jones M, Qureshi N, De Bono J, Rajappan K, Bashir Y, Betts TR. High incidence of acute sub-clinical circumflex artery “injury” following mitral isthmus ablation. Eur Heart J. 2011;32:1881–1890. doi: 10.1093/eurheartj/ehr117. [DOI] [PubMed] [Google Scholar]
- 15.Narayan SM, Zaman JA. Mechanistically-based mapping of human cardiac fibrillation. J Physiol. 2015 doi: 10.1113/JP270513. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


