Abstract
Group I metabotropic glutamate receptors (GpI mGluR) including mGluR1 and 5 (mGluR1/5) are coupled to Gq and modulate activity-dependent synaptic plasticity. Direct activation of mGluR1/5 causes protein translation-dependent long-term depression (LTD). Although it has been established that intracellular Ca2+ and the Gq-regulated signaling molecules are required for mGluR1/5-LTD, whether and how Ca2+ regulates Gq signaling and up-regulation of protein expression remain unknown. Through pharmacological inhibition, we tested the function of a Ca2+ sensor calmodulin (CaM) in intracellular signaling triggered by the activation of mGluR1/5. CaM inhibitor W13 (N-[4-Aminobutyl]-5-chloro-2-naphthalenesulfonamide hydrochloride) suppressed the mGluR1/5-stimulated activation of ERK1/2 (extracellular signal-regulated kinase½) and S6K1 (p70-S6 kinase 1) in hippocampal neurons. W13 also blocked the mGluR1/5 agonist-induced synaptic depression in hippocampal slices and in anesthetized mice. Consistent with the function of CaM, inhibiting the downstream targets Ca2+/CaM-dependent protein kinases (CaMK) blocked ERK1/2 and S6K1 activation. Further, disruption of the CaM-CaMK-ERK1/2 signaling cascade suppressed the mGluR1/5-stimulated up-regulation of Arc expression. Together, our data suggest CaM as a new Gq signaling component to couple Ca2+ and protein up-regulation and regulate mGluR1/5-mediated synaptic modification.
Keywords: AB_331647, AB_823494, AB_2265913, AB_823592, AB_887694, Arc, calmodulin, mGluR1/5, synaptic depression, ERK1/2, signal transduction
Graphical abstract
Introduction
The Gq-coupled group I metabotropic glutamate receptors (Gp1 mGluR) consist of mGluR1 and mGluR5 (mGluR1/5), and are involved in modulating numerous neuronal functions including synaptic plasticity and adaptive behaviors (Golubeva et al. 2015; Willard and Koochekpour 2013). Alterations of mGluR1/5 activity are implicated in Fragile X syndrome (Bear et al. 2004), autism (Silverman et al. 2012), addiction (Grueter et al. 2008), and abnormal learning and memory (Sethna and Wang 2014; Xu et al. 2009). At the synapses, the mGluR1/5-mediated long-term depression (mGluR1/5-LTD) represents a major cellular model to understand how mGluR1/5 signaling regulates neuronal function and neurophysiology (Bellone et al. 2008).
It is established that mGluR1/5 stimulates Gq signaling, which triggers the activation of PLC (phospholipase C) and in turn results in IP3 (inositol trisphosphate)-mediated Ca2+ release from intracellular storage (Willard and Koochekpour 2013). In acute hippocampal slices, the expression of low frequency stimulation-induced mGluR1/5-LTD at the Shaffer collateral-CA1 synapse requires intracellular Ca2+ (Oliet et al. 1997). A more recent study further demonstrated that the IP3-mediated Ca2+ release from ER (endoplasmic reticulum) at the dendritic spines is required for agonist-induced mGluR1/5-LTD (Holbro et al. 2009). Other lines of investigation show that mGluR1/5-LTD depends on protein synthesis (Bellone et al. 2008; Huber et al. 2000) and intracellular signaling such as the activation of ERK1/2 (extracellular signal-regulated kinase½) (Gallagher et al. 2004). Additionally, the mGluR1/5-stimulated ERK1/2 activation may impinge on S6K1 (p70-S6 kinase 1), which phosphorylates ribosomal protein S6 and contributes to the enhancement of 5′TOP (terminal 5′ oligopyrimidine tract) mRNA translation (Antion et al. 2008). Because many 5′TOP mRNAs encode ribosomal proteins (such as S6) and elongation factors (such as EF1A), the activation of the ERK1/2-S6K1 signaling cascade may be required for up-regulation of both ribosomal biogenesis and translation elongation. However, whether and how Ca2+ is functionally linked to the mGluR1/5-mediated signal transduction and up-regulation of protein expression remain largely unknown.
Here, we postulate that calmodulin (CaM), which is a major Ca2+ sensor protein (Xia and Storm 2005), connects mGLuR1/5 and the downstream up-regulation of ERK1/2 activity and protein expression. By using a well-known CaM inhibitor W13 (N-[4-Aminobutyl]-5-chloro-2-naphthalenesulfonamide hydrochloride), we found that CaM activity is required for mGluR1/5-mediated up-regulation of the ERK1/2-S6K1 signaling cascade and Arc expression. In both in vivo and in vitro conditions, W13 blocked the agonist-induced mGluR1/5-LTD at the Shaffer collateral-CA1 synapse in hippocampus. Taken together, our data suggest a new function of CaM in mGluR1/5-mediated signal transduction and synaptic modification.
Materials and Methods
Animals
Mice on C57BL/6 background were housed in the University Laboratory Animal Research facility. All manipulations were approved by the Institutional Animal Care and Use Committee at Michigan State University. The mice had ad libitum access to water and food and were housed under 12 h dark/light cycle.
Dose and concentration of DHPG and W13 (N-[4-Aminobutyl]-5-chloro-2-naphthalenesulfonamide hydrochloride, Tocris Cat #0361)
We stimulated the activation of mGluR1/5 with DHPG at 100 uM for hippocampal neuronal culture and in vitro LTD with acute hippocampal slices, as this concentration was used in numerous previous studies (Nosyreva and Huber 2006).
W13 is a well-known CaM inhibitor with IC50 of 68 uM (Hidaka and Tanaka 1983). Thus, we used 70–75 uM W13 (70 for in vitro LTD and 75 for cell culture), which is very close to the IC50 value (i.e. 68 uM).
The examination of in vivo LTD used higher concentration of DHPG (at 2.5 mM) and W13 (at 1.75 mM), as only 0.5 ul was injected locally to the CA1 region and we estimated that reagents will be diffused to the neighboring cells and got diluted by a factor of 25–100.
Neuronal cell culture and Western blotting
Primary hippocampal cultures were established from postnatal day 0 mice as described (Zhou et al. 2009). The mGluR1/5-mediated activation of ERK1/2, S6K1, and Arc expression was determined with neurons at DIV 8 (days in vitro 8). Control and treated neurons were lysed and proteins were extracted in Laemmli buffer. Same amount of cell extract was separated by SDS-PAGE followed by transferring to nitrocellulose membranes. The following antibodies were used to detect the corresponding targets. Arc antibody was obtained from Synaptic Systems (Cat # 156003, 1:1000 dilution). β-actin antibody was from Sigma (Cat #A5441, 1:10,000 dilution). Anti-phospho-ERK1/2 (Cat #9101, 1:1000 dilution), anti-ERK1/2 (Cat #9102, 1:1000 dilution), anti-S6K1 (Cat #9202, 1:1000 dilution), and anti-phospho-S6K1 (at Thr421/Ser424) (Cat #9204, 1:1000 dilution) were from Cell Signaling.
For quantification purpose, the level of Arc was normalized to the level of β-actin. As the phosphorylation of both ERK1 and ERK2 was responsive to mGluR1/5 agonist DHPG and various inhibitors, the level of pERK1 and pERK2 was combined, quantified, and normalized to total ERK1 and ERK2. The level of phosphorylated S6K1 was normalized to the level of total S6K1. The relative intensity of the Western blot signal in the no treatment control group, which was quantified using ImageJ (NIH, MD, USA), was defined as 1. The signal in the treatment samples was normalized to the control group.
Antibody Characterization
All antibodies used in this study were from commercial sources (Table 1). The antibodies against pERK1/2 and total ERK1/2 recognized two bands on Western blots representing ERK1 and ERK2 at 44 KD and 42 KD, respectively. The antibodies against pS6K1 (at Thr421/Ser424) and total S6K1 recognized a major protein band at 70 KD (representing S6K1 and being analyzed in this study) and a minor band at 85 KD (representing p85 S6 kinase) on Western blot. The antibodies against Arc and β-actin recognized protein bands at 45 KD and 42 KD, respectively, on Western blot. The specificity of these antibodies has been verified by the commercial suppliers. Other details on these antibodies are listed in Table 1.
TABLE 1.
Antibody Characterization
| Antigen | Description of Immunogen | Source, Host Species, Cat. No., Lot No., RRID | WB dilution |
|---|---|---|---|
| Phosphorylated ERK1/2 at Thr202/Tyr204 | synthetic phosphopeptide corresponding to residues surrounding Thr202/Tyr204 of human p44 MAP kinase | Cell Signaling, rabbit, polyclonal, catalog No. 9101L, lot No. 28, RRID:AB_331647 | 1:1000 |
| ERK1/2 | synthetic peptide corresponding to a sequence in the C-terminus of rat p44 MAP Kinase | Cell Signaling, rabbit, polyclonal catalog No. 9102L, lot No. 23, RRID:AB_823494 | 1:1000 |
| Phosphorylated | synthetic phosphopeptide corresponding to | Cell Signaling, rabbit, polyclonal, | 1:1000 |
| S6K1 at | residues surrounding Thr421/Ser424 of | catalog No. 9204L, lot No. 7, | |
| Thr421/Ser424 | human p70 S6 kinase | RRID:AB_2265913 | |
| S6K1 | synthetic peptide corresponding to residues surrounding the carboxy-terminus of human p70 S6 kinase | Cell Signaling, rabbit, polyclonal, catalog No. 9202L, lot No. 8, RRID:AB_823592 | 1:1000 |
| Arc | Strep-Tag® fusion protein of full-length mouse Arc | Synaptic Systems, rabbit, polyclonal catalog No. 156003, RRID:AB_887694) | 1:1000 |
| β-actin | synthetic peptide corresponding to the N- terminus of β-cytoplasmic actin (Ac-Asp- Asp-Asp-Ile-Ala-Ala-Leu-Val-Ile-Asp-Asn- Gly-Ser-Gly-Lys, conjugated to KLH). | Sigma, mouse, monoclonal, catalog No. A5441, lot No. 122M4782, | 1:10,000 |
Electrophysiology
In vivo field excitatory postsynaptic potentials (fEPSP) in the CA1 region of the hippocampus were measured with anaesthetized mice (2- to 3-month old males) (100 mg/kg Nembutal sodium solution for the initial dose, another dose of 10 mg/kg was given 30 min later) as described in our previous study (Zhang et al. 2011). To induce synaptic depression, 0.5 ul of 2.5 mM mGluR1/5 agonist DHPG ([RS]-3,5-Dihydroxyphenylglycine, Tocris Cat #0342) was infused at 0.05 ul/min to the CA1 region of the anaesthetized mice. To determine the effects of calmodulin inhibition on mGluR1/5-mediated synaptic depression, 0.5 ul 1.75 mM W13 (N-[4-Aminobutyl]-5-chloro-2-naphthalenesulfonamide hydrochloride, Tocris Cat #0361) plus 2.5 mM DHPG (with the infusion rate of 0.05 ul/min) was delivered to the CA1 region. To determine the mGluR1/5-LTD in vitro, acute hippocampal slices (from 1-month old male mice) were obtained as described in our previous study (Bhattacharya et al. 2012). Following cervical dislocation, 400-micron transverse hippocampal slices were sectioned in cold oxygenated cutting solution (CS; in mM 110 sucrose, 60 NaCl, 3 KCl, 1.25 NaH2PO4, 28 NaHCO3, 0.5 CaCl2, 7 MgCl2, 5 glucose, and 0.6 ascorbate). The slices were then incubated in a 50:50 solution of CS: artificial cerebrospinal fluid (ACSF) (in mM: 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 25 D-glucose, 2 CaCl2, and 1 MgCl2) for 30 min followed by a 30 min-recovery in ACSF at room temperature. In an interface chamber, fEPSP at the CA1 synapses in the stratum radiatum of area was evoked by stimulating the Schaffer collateral pathway. When baseline fEPSP was stabilized for at least 30 min, DHPG (100 uM) or DHPG (100 uM) plus W13 (70 uM) was perfused to the recording chamber. The ratio of fEPSP after drug administration to baseline fEPSP was used to determine the degree of synaptic depression.
Statistic analysis
All data are presented as mean +/− SEM. One-way ANOVA followed by post-hoc test was used to compare data from multiple groups. Two-way repeated measures ANOVA were used to analyze the electrophysiology data. Differences with p values less than 0.05 were considered significant. SPSS 11.5 for Windows (IBM) was used for all data analysis.
Results
Calmodulin activity is required for mGluR1/5-mediated intracellular signaling
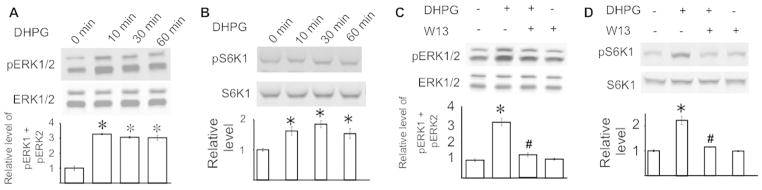
ERK1/2 activity is required for LTD induced by multiple types of Gq-coupled receptors including mGluR1/5 (Gallagher et al. 2004; Volk et al. 2007). Activation of ERK1/2 may stimulate S6K1 (p70-S6 kinase 1), which up-regulates translation machinery and in turn increases protein synthesis. Here, we first confirmed that stimulating cultured hippocampal neurons with the mGluR1/5 agonist DHPG ([RS]-3,5-Dihydroxyphenylglycine) caused significant phosphorylation of ERK1/2 in hippocampal neurons (Fig. 1A) (F3, 20 = 5.21, p < 0.05). The activity of S6K1 is positively regulated by phosphorylation and involved in regulating ribosomal biogenesis. In vitro studies have shown that the phosphorylation of S6K1 at Thr421/Ser424 is mediated by MEK-ERK1/2 (Lehman et al. 2003). Consistently, activation of mGluR1/5 stimulated phosphorylation of S6K1 at Thr421/Ser424 (F3, 20 = 3.98, p < 0.05) (Fig. 1B).
Figure 1.
Calmodulin activity is required for mGluR1/5 signaling in primary hippocampal neurons. DIV (days in vitro) 8 mouse hippocampal neurons were treated with 100 uM DHPG for 10, 30 and 60 min (A and B). In C and D, hippocampal neurons were pre-treated with 75 uM W13 or vehicle control for 30 min, after which the cells were treated with 100 uM DHPG or vehicle for 30 min. The levels of pERK1/2 (A and C) and pS6K1 (at Thr421/Ser424) (B and D) were determined by Western blot. Representative images are shown in the upper panels and quantifications are shown in the lower panels. The level of pERK1/2 and pS6K1 was normalized to the level of total ERK1/2 and total S6K1, respectively. All data were collected from 6 independent samples. *: p < 0.05 between control and the indicated group. #: p < 0.05 between the indicated group and the DHPG-treated group. The p value was determined by one-way ANOVA followed by post hoc analysis.
Because increased Ca2+ release from intracellular storage is one of the major downstream cellular events following Gq activation, we expected that inhibiting the Ca2+ effector molecule CaM might block the propagation of mGluR1/5 signaling. We pre-treated neurons with a CaM inhibitor W13 followed by DHPG stimulation. W13 blocked DHPG-stimulated phosphorylation of ERK1/2 (F3, 20 = 5.24, p < 0.05) (Fig. 1C) and S6K1 (F3, 20 = 4.39, p < 0.05) (Fig. 1D).
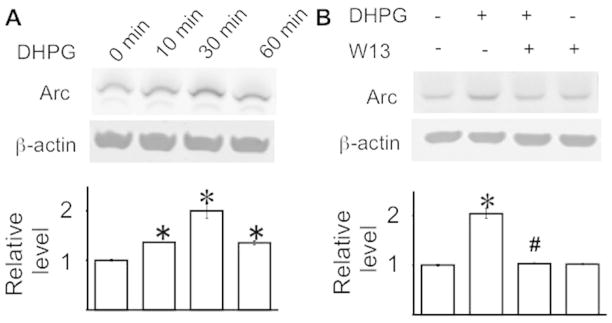
Calmodulin activity is required for mGluR1/5-mediated Arc up-regulation
It has been demonstrated that activity-dependent protein synthesis is required for mGluR1/5-mediated LTD (Huber et al. 2000). Activation of mGluR1/5 in cultured hippocampal neurons by DHPG caused significant up-regulation of Arc protein (Fig. 2A) (F3, 20 = 3.71, p < 0.05), whose function is implicated in mGluR1/5-mediated LTD (Park et al. 2008). The DHPG-induced Arc protein expression has also been demonstrated to be required for mGluR1/5-mediated LTD (Waung et al. 2008). W13 abolished the DHPG-induced up-regulation of Arc protein (F3, 20 = 5.64, p < 0.05) (Fig. 2B).
Figure 2.
Calmodulin activity is required for mGluR1/5-mediated Arc up-regulation in primary hippocampal neurons. DIV 8 mouse hippocampal neurons were treated by DHPG with or without W13 as described in Figure 1. The level of Arc was determined by Western blot and normalized to β-actin. All data were collected from 6 independent samples. *: p < 0.05 between control and the indicated group. #: p < 0.05 between the indicated group and the DHPG-treated group. The p value was determined by one-way ANOVA followed by post hoc analysis.
Functional relevance of CaM-regulated ERK1/2 activity in mGluR1/2-mediated Arc up-regulation
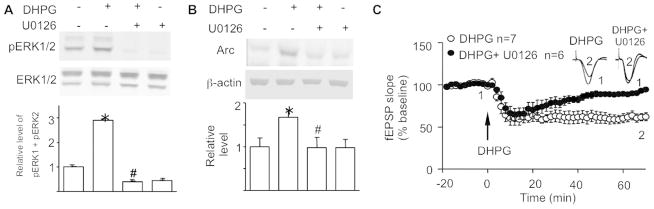
We found that CaM activity is required for both ERK1/2 activation and Arc up-regulation following mGluR1/5 stimulation. To determine whether W13 inhibits Arc up-regulation through suppressing ERK1/2 activity, we pre-treated hippocampal neurons with the MEK inhibitor U0126. Because MEK directly regulates ERK1/2 phosphorylation, U0126 blocked the DHPG-induced up-regulation of phospho-ERK1/2. U0126 also suppressed the basal ERK/12 phosphorylation in un-stimulated neurons (Fig. 3A) (F3, 20 = 4.85, p<0.05). We next found that U0126 significantly blocked DHPG-stimulated Arc up-regulation in hippocampal neurons (Fig. 3B) (F3, 20 = 3.22, p<0.05). A previous study using hippocampal slices showed that U0126 suppresses mGluR1/5-LTD (Gallagher et al. 2004). Here, we observed that U0126 inhibited DHPG-induced synaptic depression in vivo in anesthetized mice (61.8 ± 4.7% for the DHPG-treated group; 91.1 ± 2.2%; for the DHPG ± U0126 group; F44,484 = 5.0, p < 0.001 for the interaction of treatment and time; Fig. 3C). These data imply that CaM inhibition attenuates ERK1/2 activity and in turn suppresses DHPG-stimulated up-regulation of Arc protein and synaptic depression.
Figure 3.
Inhibition of ERK1/2 suppresses mGluR1/5-mediated Arc up-regulation and synaptic depression. (A and B) Primary hippocampal neurons were treated with 10 uM MEK inhibitor U0126 for 30 min, following which the cells were stimulated with 100 uM DHPG for 30min. Protein samples were analyzed by Western blot and are presented as normalized protein levels relative to the no treatment control. (A) U0126 suppressed ERK1/2 phosphorylation in both control and DHPG-stimulated neurons. (B) U0126 inhibited DHPG-stimulated up-regulation of Arc protein expression. All data were collected from 6 independent samples. *: p < 0.05 between control and the indicated group. #: p < 0.05 between the indicated group and the DHPG-treated group. The p value was determined by one-way ANOVA followed by post hoc analysis. (C) U0126 suppressed DHPG-induced in vivo synaptic depression at CA1 synapses in anesthetized mice. 0.5 ul DHPG (2.5 mM, n=7) or 0.5 ul DHPG (2.5 mM) plus U0126 (2 mM) (n=6) was infused to the CA1 region. The relative fEPSP value was normalized to the baseline level.
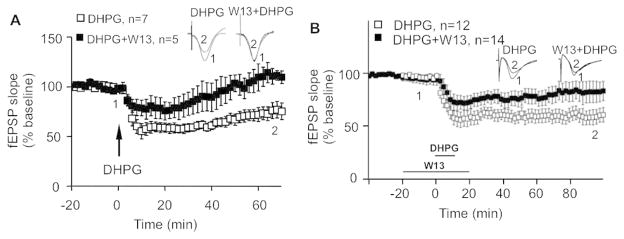
Calmodulin activity is required for mGluR1/5-mediated synaptic depression in vivo and in vitro
As CaM activity is required for mGluR1/5-mediated ERK1/2 activation and protein up-regulation, we further examined the effects of CaM inhibition on synaptic depression. After infusion of mGluR1/5 agonist DHPG to the CA1 region of the hippocampus in anesthetized mice, we observed significant synaptic depression that lasted for at least 60 min (68.4 ± 4.6%). Infusion of DHPG along with W13 only caused transient synaptic depression (Fig. 4A). Two-way repeated measures ANOVA indicated significant effects of W13 on DHPG-induced synaptic depression (F44,440=3.6, p < 0.001 for the interaction of treatment and time)
Figure 4.
Calmodulin activity is required for mGluR1/5-mediated synaptic LTD in vitro and in vivo. (A) DHPG (n=7) or W13 plus DHPG (n=5) was infused to the CA1 region of the anesthetized mice. The changes of fEPSP during the whole recording period are presented as mean +/− SEM. (B) DHPG (n=12) or W13 plus DHPG (n=14) was applied to acute hippocampal slices, and changes of fEPSP were recorded and presented as mean +/− SEM. Representative fEPSP traces at time points 1 and 2 (as indicated) following different drug treatment are shown.
In acute hippocampal slices, application of DHPG induced significant long-term depression, which was significantly suppressed by W13 (F59,1416=2.56, p < 0.001 for the interaction of treatment and time) (Fig. 4B). These data implicate CaM as a physiologically functional component in the mGluR1/5-mediated signaling cascade.
CaM-dependent protein kinases are required for mGluR1/5-mediated up-regulation of ERK1/2 and Arc
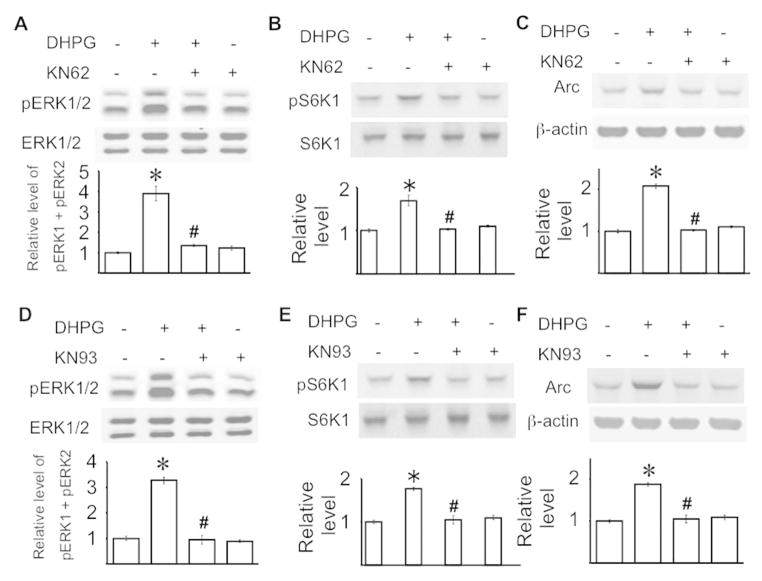
To determine how CaM inhibition suppresses mGluR1/5-mediated intracellular signaling, we tested the function of the major Ca2+/CaM-dependent protein kinases (CaMK) (Wayman et al. 2008). We examined the effects of two well-known inhibitors (i.e. KN62 and KN93) for CaMKI, II, and IV (Zheng et al. 2011) on mGluR1/5-mediated signaling in cultured hippocampal neurons. Both KN62 and KN93 prevented DHPG-induced phosphorylation of ERK1/2 (F3, 20 = 5.38, p < 0.01 for KN62, F3, 20 = 5.77, p < 0.01 for KN93) (Fig. 5A and 5D) and S6K1 at Thr421/Ser424 (F3, 20 = 3.99, p < 0.05 for KN62, F3, 20 = 4.27, p < 0.05 for KN93) (Fig. 5B and 5E). The DHPG-stimulated Arc up-regulation was also blocked by KN62 and KN93 (F3, 20 = 5.65, p < 0.01 for KN62, F3, 20 = 5.88, p < 0.01 for KN93) (Fig. 5C and 5F). These data suggest that CaM may regulate mGluR1/5-mediated signaling through CaMK.
Figure 5.
Ca2+/CaM-dependent protein kinase activity is required for mGluR1/5 signaling in primary hippocampal neurons. DIV 8 mouse hippocampal neurons were pre-treated with 10 uM KN62 (A to C), 5 uM KN93 (D to F), or vehicle control for 30 min, after which the cells were treated with 100 uM DHPG or vehicle for 30 min. The levels of pERK1/2 (A, D), pS6K1 (at Thr421/Ser424) (B, E), and Arc (C, F) were determined by Western blot. Representative images are shown in the upper panels and quantifications are shown in the lower panels. The level of pERK1/2 and pS6K1 was normalized to the level of total ERK1/2 and total S6K1, respectively. The level of Arc was normalized to the level of β-actin. The relative protein level in the control group was defined as 1, and all samples were normalized to the control group. All data were collected from 6 independent samples. *: p < 0.05 between control and the DHPG-treated samples. #: p < 0.05 between the indicated group and the DHPG-treated group. The p value was determined by one-way ANOVA and post hoc LSD test.
Discussion
It is well established that the activation of mGluR1/5 stimulates the Gq signaling cascade (Willard and Koochekpour 2013). Consistent with the function of Gq in PLC activation and the subsequent IP3-trigered Ca2+ release from intracellular storage, chelation of Ca2+ blocks mGluR1/5-LTD [(Bolshakov and Siegelbaum 1994; Oliet et al. 1997) but also see (Fitzjohn et al. 2001)]. Interestingly, general inhibition of PLC has no significant effect on mGluR1/5-LTD in hippocampal slices (Mockett et al. 2011), suggesting possible involvement of other PLC-independent Ca2+ sources following mGluR1/5 activation (Awad et al. 2000; Chavis et al. 1995; Choe et al. 2006; Gee et al. 2003). However, Holbro at al. found that the IP3 receptor antagonist heparin blocked mGluR1/5-dependent LTD at the ER+ spines, which are the major sub-cellular location of this specific synaptic depression (Holbro et al. 2009). Nevertheless, how the Gq-mediated Ca2+ increase impinges on signal molecules that are required for mGluR1/5-LTD remains unknown. The present study demonstrates that the activity of a Ca2+ sensor CaM may link the mGluR1/5-stimulated Ca2+ increase and the down stream signaling molecules and regulate synaptic depression.
Following the stimulation of mGluR1/5, both ERK1/2 and S6K1 are activated (Antion et al. 2008; Gallagher et al. 2004). It has been further demonstrated that ERK1/2 is required for mGluR1/5-mediated synaptic depression in vitro (Gallagher et al. 2004) and in vivo (this study). As CaM inhibitor W13 blocked mGluR1/5-stimulated ERK1/2 and S6K1 activation, our data demonstrate how Gq activates ERK1/2 and identify a new functional component to link mGluR1/5 and ERK1/2 activation. Mechanistically, the effects of CaM inhibition on mGluR1/5-LTD may be attributed to that W13 blocked ERK1/2 and S6K1 activation.
CaMKs are the major signaling molecules related to synaptic functions and directly regulated by Ca2+ and CaM (Wayman et al. 2008; Xia and Storm 2005). There have been controversial conclusions on the function of CaMKs in regulating mGluR1/5-LTD. While Schnabel et al. showed that inhibition of CaMK by KN62 enhances LTD (Schnabel et al. 1999), a more recent study found that CaMK inhibitor KN62 and CaMKII (CaM-dependent protein kinase II) inhibitor AIP (autocamtide-2-related inhibitory peptide) both block mGluR1/5-LTD at the CA1 synapse (Mockett et al. 2011). Nevertheless, neither of these studies examined whether and how CaMK activity regulates the mGluR1/5-mediated signaling cascade. Here, we demonstrate that, consistent with the effects of CaM inhibition, CaMK inhibitors also blocked mGluR1/5-mediated ERK1/2 activation. It is evident that CaMKs not only regulate ERK1/2 activity in hippocampal neurons (this study) but also in striatal neurons (Choe and Wang 2001) following mGluR1/5 activation. Consequently, the activity of the ERK1/2 downstream target S6K1 (Lehman et al. 2003) was also suppressed by CaMK inhibitors. We measured phosphorylation at the Thr202/Tyr204 residues in ERK1 and ERK2, which have been demonstrated as the main targets of MEK1 (Rubinfeld and Seger 2005). It remains unknown whether CaMKs can directly activate ERK1/2 through specific phosphorylation at Thr202/Tyr204.
Activation of mGluR1/5 triggers new protein synthesis. Functionally, protein synthesis inhibitors block mGluR1/5-LTD (Huber et al. 2000; Mockett et al. 2011). Consistent with their function in regulating mGluR1/5-LTD, the Gq-triggered signaling molecules are also required for activity-dependent protein translation. By using pharmacological inhibition and radioactive amino acid, a recent study has shown that CaMK activity is required for the overall mGluR1/5-stimulated new protein synthesis (Mockett et al. 2011). In neurons or cells with overactivated mGluR1/5 signaling, inhibition of ERK1/2 or S6K1 dampens the aberrantly elevated protein synthesis (Bhattacharya et al. 2012; Kumari et al. 2014; Osterweil et al. 2010). Although it is hypothesized that up-regulation of certain “LTD proteins” can facilitate the internalization of AMPA receptors leading to the reduction/depression of synaptic response, few functional “LTD proteins” have been identified. Among them, translational up-regulation of Arc is coupled to mGluR1/5 activation, and required for AMPA receptor internalization (Park et al. 2008; Waung et al. 2008; Zhang et al. 2008). However, whether and how Gq-mediated signaling regulates mGluR1/5-induced Arc up-regulation remain unknown. Here, our data demonstrate that the CaM-CaMK-ERK1/2 signaling cascade is required for mGluR1/5-induced Arc up-regulation. Interestingly, the basal level of Arc was not affected by inhibition of the CaM-CaMKII-ERK1/2 cascade. This is consistent with that suppression of mGluR5, ERK1/2, and S6K1 does not affect the overall protein translation level in untreated control neurons (Bhattacharya et al. 2012; Dolen et al. 2007; Osterweil et al. 2010). One limitation of our data is that neuronal stimulation may lead to up-regulation of both Arc mRNA transcription and translation (Park et al. 2008; Taylor et al. 2010; Waung et al. 2008) and our measurement does not discriminate translation from the up-regulation of transcription-coupled translation. However, previous studies have emphasized that the protein rather than mRNA level of Arc is important for mGluR1/5-mediated LTD, and we found that the up-regulation of Arc protein following DHPG stimulation is sensitive to inhibition of the CaM-CaMKII-ERK1/2 cascade.
We acknowledge certain technical limitation in linking mGluR1/5-mediated signaling and LTD. In this study, in vitro LTD was performed with in acute hippocampal slices collected from 1-month old mice, and in vivo LTD in intact hippocampus was performed with anaesthetized 2- to 3-month old mice. It is important to note that mGluR1/5-mediated LTD in slices was mostly performed with juvenile (i.e. <4- to 6-week old) mice and not detected in adult slices (e.g. >6-week old) in many labs. Here, we, for the first time, report that DHPG can induce synaptic depression in vivo in anaesthetized young adult mice (2- to 3-month old). This is possibly due to that some important circuitry is disrupted in brain slices following sectioning. To avoid contribution from glial cells (if brain slices were used), we used cultured hippocampal neurons under conditions that minimize glial cell growth (Zhou et al. 2009) to study the function of CaM in regulating mGluR1/5-mediated neuronal signaling. As only young slices show mGluR1/5-LTD in in vitro conditions, we used young cultured neurons at DIV 8 (days in vitro 8). Interestingly, fully developed older neurons (such as at DIV 14–21) showed significantly less and inconsistent activation of the ERK1/2 signaling following DHPG. This is consistent with the significant less and inconsistent LTD outcome in mature slices.
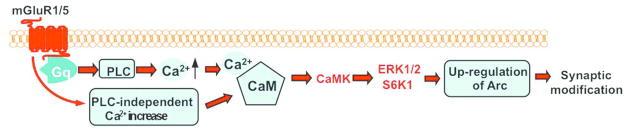
Together with reports from previous studies, our data suggest a working model to show that CaM senses the mGluR1/5-triggered Ca2+ increase and activates the CaMKs-ERK1/2-S6K1 signaling cascade leading to up-regulation of the LTD-related proteins and synaptic depression (Fig. 6).
Figure 6.
Role of CaM in mGluR1/5-mediated intracellular signaling and synaptic modification.
Significance Statement.
Long-term synaptic depression (LTD) reflects how brain cells react to stimulus, and is considered as a cellular substrate underlying certain aspects of adaptive behavior such as learning and memory. The mGluR1/5-dependent LTD requires Gq-mediated signaling cascade, the alteration of which is implicated in abnormal brain function and neurological disorders. This study demonstrates a previously unknown function of calmodulin (CaM) in regulating the ERK1/2-S6K1-Arc cascade and mGluR1/5 LTD.
Acknowledgments
Support and grant information: This study was supported by FRAXA Research Foundation (HW) and NIH grants R01MH093445 (HW), and R01NS034007 and R01NS047384 (EK).
Footnotes
Conflict of interest statement
The authors declare no competing financial interest.
Role of authors
HW initiated the research. HW, EK, and CC designed the research. FS, MZ, HK, and DA performed the research and data analysis. HW and FS wrote the manuscript.
Literature Cited
- Antion MD, Hou L, Wong H, Hoeffer CA, Klann E. mGluR-dependent long-term depression is associated with increased phosphorylation of S6 and synthesis of elongation factor 1A but remains expressed in S6K-deficient mice. Mol Cell Biol. 2008;28(9):2996–3007. doi: 10.1128/MCB.00201-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20(21):7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27(7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bellone C, Luscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell Mol Life Sci. 2008;65(18):2913–2923. doi: 10.1007/s00018-008-8263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Kaphzan H, Alvarez-Dieppa AC, Murphy JP, Pierre P, Klann E. Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice. Neuron. 2012;76(2):325–337. doi: 10.1016/j.neuron.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov VY, Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994;264(5162):1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- Chavis P, Fagni L, Bockaert J, Lansman JB. Modulation of calcium channels by metabotropic glutamate receptors in cerebellar granule cells. Neuropharmacology. 1995;34(8):929–937. doi: 10.1016/0028-3908(95)00082-h. [DOI] [PubMed] [Google Scholar]
- Choe ES, Shin EH, Wang JQ. Regulation of phosphorylation of NMDA receptor NR1 subunits in the rat neostriatum by group I metabotropic glutamate receptors in vivo. Neurosci Lett. 2006;394(3):246–251. doi: 10.1016/j.neulet.2005.10.072. [DOI] [PubMed] [Google Scholar]
- Choe ES, Wang JQ. Group I metabotropic glutamate receptors control phosphorylation of CREB, Elk-1 and ERK via a CaMKII-dependent pathway in rat striatum. Neurosci Lett. 2001;313(3):129–132. doi: 10.1016/s0304-3940(01)02258-3. [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56(6):955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzjohn SM, Palmer MJ, May JE, Neeson A, Morris SA, Collingridge GL. A characterisation of long-term depression induced by metabotropic glutamate receptor activation in the rat hippocampus in vitro. J Physiol. 2001;537(Pt 2):421–430. doi: 10.1111/j.1469-7793.2001.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher SM, Daly CA, Bear MF, Huber KM. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci. 2004;24(20):4859–4864. doi: 10.1523/JNEUROSCI.5407-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee CE, Benquet P, Gerber U. Group I metabotropic glutamate receptors activate a calcium-sensitive transient receptor potential-like conductance in rat hippocampus. J Physiol. 2003;546(Pt 3):655–664. doi: 10.1113/jphysiol.2002.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubeva AV, Moloney RD, O’Connor RM, Dinan TG, Cryan JF. Metabotropic Glutamate Receptors in Central Nervous System Diseases. Curr Drug Targets. 2015 doi: 10.2174/1389450116666150316224011. [DOI] [PubMed] [Google Scholar]
- Grueter BA, McElligott ZA, Robison AJ, Mathews GC, Winder DG. In vivo metabotropic glutamate receptor 5 (mGluR5) antagonism prevents cocaine-induced disruption of postsynaptically maintained mGluR5-dependent long-term depression. J Neurosci. 2008;28(37):9261–9270. doi: 10.1523/JNEUROSCI.2886-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H, Tanaka T. Naphthalenesulfonamides as calmodulin antagonists. Methods Enzymol. 1983;102:185–194. doi: 10.1016/s0076-6879(83)02019-4. [DOI] [PubMed] [Google Scholar]
- Holbro N, Grunditz A, Oertner TG. Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc Natl Acad Sci U S A. 2009;106(35):15055–15060. doi: 10.1073/pnas.0905110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288(5469):1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Kumari D, Bhattacharya A, Nadel J, Moulton K, Zeak NM, Glicksman A, Dobkin C, Brick DJ, Schwartz PH, Smith CB, Klann E, Usdin K. Identification of fragile X syndrome specific molecular markers in human fibroblasts: a useful model to test the efficacy of therapeutic drugs. Hum Mutat. 2014;35(12):1485–1494. doi: 10.1002/humu.22699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JA, Calvo V, Gomez-Cambronero J. Mechanism of ribosomal p70S6 kinase activation by granulocyte macrophage colony-stimulating factor in neutrophils: cooperation of a MEK-related, THR421/SER424 kinase and a rapamycin-sensitive, m-TOR-related THR389 kinase. J Biol Chem. 2003;278(30):28130–28138. doi: 10.1074/jbc.M300376200. [DOI] [PubMed] [Google Scholar]
- Mockett BG, Guevremont D, Wutte M, Hulme SR, Williams JM, Abraham WC. Calcium/calmodulin-dependent protein kinase II mediates group I metabotropic glutamate receptor-dependent protein synthesis and long-term depression in rat hippocampus. J Neurosci. 2011;31(20):7380–7391. doi: 10.1523/JNEUROSCI.6656-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95(5):3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18(6):969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 2010;30(46):15616–15627. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59(1):70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Molecular biotechnology. 2005;31(2):151–174. doi: 10.1385/MB:31:2:151. [DOI] [PubMed] [Google Scholar]
- Schnabel R, Palmer MJ, Kilpatrick IC, Collingridge GL. A CaMKII inhibitor, KN-62, facilitates DHPG-induced LTD in the CA1 region of the hippocampus. Neuropharmacology. 1999;38(4):605–608. doi: 10.1016/s0028-3908(98)00229-9. [DOI] [PubMed] [Google Scholar]
- Sethna F, Wang H. Pharmacological enhancement of mGluR5 facilitates contextual fear memory extinction. Learn Mem. 2014;21(12):647–650. doi: 10.1101/lm.035857.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Smith DG, Rizzo SJ, Karras MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH, Crawley JN. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci Transl Med. 2012;4(131):131ra151. doi: 10.1126/scitranslmed.3003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Dieterich DC, Ito HT, Kim SA, Schuman EM. Microfluidic local perfusion chambers for the visualization and manipulation of synapses. Neuron. 2010;66(1):57–68. doi: 10.1016/j.neuron.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci. 2007;27(43):11624–11634. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59(1):84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Lee YS, Tokumitsu H, Silva AJ, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59(6):914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard SS, Koochekpour S. Glutamate, glutamate receptors, and downstream signaling pathways. Int J Biol Sci. 2013;9(9):948–959. doi: 10.7150/ijbs.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nature reviews Neuroscience. 2005;6(4):267–276. doi: 10.1038/nrn1647. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29(12):3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Storm DR, Wang H. Bidirectional synaptic plasticity and spatial memory flexibility require Ca2+-stimulated adenylyl cyclases. J Neurosci. 2011;31(28):10174–10183. doi: 10.1523/JNEUROSCI.0009-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28(42):10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Zhou X, Luo Y, Xiao H, Wayman G, Wang H. Regulation of brain-derived neurotrophic factor exon IV transcription through calcium responsive elements in cortical neurons. PLoS One. 2011;6(12):e28441. doi: 10.1371/journal.pone.0028441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Moon C, Zheng F, Luo Y, Soellner D, Nunez JL, Wang H. N-methyl-D-aspartate-stimulated ERK1/2 signaling and the transcriptional up-regulation of plasticity-related genes are developmentally regulated following in vitro neuronal maturation. J Neurosci Res. 2009;87(12):2632–2644. doi: 10.1002/jnr.22103. [DOI] [PMC free article] [PubMed] [Google Scholar]