Abstract
Background and Aims
Dysfunction of physiological regulation systems may underlie the disrupted emotional and self-regulatory processes among people with substance use disorder (SUD). This paper reviews evidence as to whether respiratory sinus arrhythmia (RSA), as a psychophysiological index of emotional regulation, could provide useful information in treatment-outcome research to provide insights into recovery processes.
Methods
We reviewed use of RSA in clinical research and studies on SUD treatment. Search terms for the review of RSA in clinical research included respiratory sinus arrhythmia, heart rate variability, vagal, cardiac vagal control, psychophysiology, intervention, treatment, mindfulness, mind-body, mental health, substance use, chemical dependence, regulation, emotion regulation. For the review of RSA in intervention studies, we included only those that provided adequate description of psychophysiological methods, and examined RSA in the context of an intervention study.
Results
RSA appears to be able to provide an index of self-regulatory capacity; however it has been little used in either intervention or treatment research. Of the four intervention studies included in this review, all were mindfulness-based interventions. Two studies were with substance using samples and both showed pre-post increases in RSA and related improved substance use outcomes. Two of the three studies were RCTs and both showed significant increases in RSA in the experimental compared to comparison condition.
Conclusion
Respiratory sinus arrhythmia may be a useful index of emotional regulation in people with substance use disorder, and a potential measure of underlying mechanisms for SUD treatment studies, particularly mindfulness-based interventions.
Introduction
Substance use dependence (SUD) involves a wide range of processes at many levels, including social, psychological and physiological. (1) It can be understood in part to involve a conditioned response associated with aversive physiological and emotional states attributable to poor self-regulation (2). As such, SUD can in certain cases involve a maladaptive means of managing or reducing unpleasant or unwanted sensations (physical and emotional) that are perceived from the body. Some SUD treatments are aimed at facilitating patient self-regulation through education and support for development of cognitive behavioral strategies for managing and coping with negative affect and stress, which can be important factors in relapse (3-6). For example, patients are taught to better attend to their health and emotions, to identify links between behavior and urges/cravings to use, and to seek interactions with people who support rather than undermine their efforts for sobriety. Less often do we see awareness and tolerance of bodily sensations as a focus of treatment or as a means of assessing treatment response.
Theories have been developed that suggest ways in which interoceptive pathways that facilitate awareness and regulation may be disrupted among individuals with SUD.(7-12) Interoception can be defined as receiving, processing, and integrating sensory inputs from inside the body.(13, 14) In addition, these theories propose existence of central nervous system (CNS) processes that underlie interoception among individuals with substance use disorders. For instance, a review of brain imaging studies suggests that regulatory processes involved in interoception may be significantly altered for those with drug dependence relative to those without, although their causal role has yet to be established.(12) Thus, individuals with SUD show greater activation to drug cues within the posterior insula and prefrontal regions that predict relapse vulnerability,(15) but impaired insula activation to comparably evocative social stimuli.(16)
There has been less attention paid to autonomic nervous system differences in this same population, especially psychophysiological changes in response to treatment. This is surprising given that central and peripheral physiological systems likely underlie the disrupted emotional and regulatory processes among those with SUD, contributing to relapse through emotional, physiological, and behavioral mechanisms. Indeed, a better understanding of autonomic nervous system processes could (a) complement existing neurobiological models of central nervous system processes and (b) provide alternative, cost-effective methods for assessing self-regulation improvements in clinical research. For example, within carefully controlled study designs, psychophysiological measures have the potential to provide insight into the body's response to stress, vulnerability to emotion dysregulation, and risk for psychopathology.(17) The purpose of this paper is to describe how one specific physiological measure—respiratory sinus arrhythmia (RSA)—could augment self-report measures and enhance existing physiological models of SUD. Furthermore, we articulate a case for RSA as one physiological measure that could be used in SUD intervention research to elucidate underlying mechanisms of change, especially in treatments that target body awareness and self-regulation.
A Framework for Body-Oriented Treatment and Measurement
There is increased interest in understanding the mechanisms by which mind-body approaches and sensory (physical and emotional) awareness can promote positive health outcomes. Although self-report measures offer one means of assessing self-regulation improvements, there is growing interest in assessing sensory awareness improvements more directly (i.e., in the body). For example, one functional Magnetic Resonance Imaging (fMRI) study reported functional plasticity changes in cortical areas involved in interoceptive representations among those who had received interoceptive awareness training relative to waitlist controls.(18) Specifically, trained participants showed functional differences in the middle and anterior insula, regions that are believed to contribute to present-moment awareness. Importantly, the insula—in coordination with other structures, such as the Anterior Cingulate Cortex (ACC)—also plays a role in regulating the cardiovascular system.(19-22) However, in spite of interesting patterns of covariation between central and autonomic nervous system measures in clinical populations (23) there is a dearth of treatment-outcome studies that incorporate autonomic outcomes relative to imaging research.
Psychophysiological measures have long been used to understand emotions in basic science and clinical research dating back to the late 1800s (see Zisner and Beauchaine, for a review).(24) Since that time, researchers have documented differences between clinical and non-clinical groups on resting state physiology and reactivity to emotional stimuli.(25-27) More recently, cognitive behavioral therapists have also sought to understand psychophysiological processes that may underlie change, as part of an integrated understanding of emotional responses to distress and as a predictor of treatment outcomes.(28, 29) In both capacities, autonomic nervous system (ANS) measures have increased scientific knowledge of rapid physiolgoical responses that often elude conscious awareness but which are reliably involved in emotional responses and regulation.(30)
Although the use of psychophysiological measures in treatment-outcome research is still limited these measures have strong potential for elucidating mechanisms of self-regulation. In particular, RSA—a measure of parasympathetic control over heart rate—could serve as one useful biomarker of improved emotion regulation, particularly in treatments targeting increased sensory and interoceptive awareness. The integration of psychophysiological measures in substance use treatment research is relatively novel but is supported by an increasing body of evidence that:
-
(1)
Respiratory sinus arrhythmia is a valid and reliable psychophysiological index of emotion regulation capacity and is a potentially useful tool for understanding self-regulation improvements during treatment.(31, 32)
-
(2)
Interoception is likely a foundational component of self-regulation, including the ability to identify, process, and regulate emotions. (13, 33)
-
(3)
Interoceptive processes, emotion regulation, and physiological systems are often disrupted among those with vulnerability to psychopathology.(34)
-
(4)
Regulatory processes involving interoception are disrupted among individuals with substance use disorders.(12) These results, along with suppositions based on cognitive neuroscience models,(8, 35, 36) suggest that targeted interventions to improve interoceptive awareness may also improve health outcomes among individuals in substance use disorder treatment.
In this paper, we highlight emerging research consistent with these tenets as well as areas where further evidence is needed. We argue that RSA ought to be included as a psychophysiological biomarker of regulatory capacity in SUD treatment research, that the inclusion of RSA may be particularly appropriate in interventions that target interoceptive awareness, and that psychophysiological changes may underlie improved clinical outcomes.
Defining Respiratory Sinus Arrhythmia
There is evidence in support of our first tenet that RSA is a valid and reliable psychophysiological index of emotion regulation capacity, although understanding the connection requires a broad understanding of physiological systems. As a brief overview, the autonomic nervous system (ANS) is comprised of two branches—sympathetic (SNS) and parasympathetic (PNS). Each branch subserves different bodily processes and, therefore, measurement of these two branches provides different information about an organism's physical, emotional, and behavioral state. At the most basic level, the SNS facilitates rapid, fight-flight responding whereas the PNS branch is involved in recovery to homeostasis and restoration. Together, these systems influence heart rate, respiration, sweating, pupil dilation, salivation, sexual arousal, urination, defecation, and several other bodily processes.(37) Historically, activity of the SNS and PNS was viewed as reciprocal (i.e., antagonistic) but it is now clear that co-activation and co-inhibition also occur.(38) Indeed, the actions of the SNS and PNS are dynamic and output from these systems includes ongoing homeostatic and allostatic processes as well as fluctuations due to incoming stimuli. Interpreting physiological processes as indices of psychological states requires careful control of the environment, stimuli, and physical state of the participant. When these details are attended to, there is broad evidence that emotional expression and regulation can be observed psychophysiologically.
Because the parasympathetic branch is involved in recovery, restoration, and regulation, researchers have been especially interested in quantifying PNS influences on physiological output, especially RSA.(39-41) Respiratory Sinus Arrhythmia, known alternatively as cardiac vagal control, vagal tone, or high-frequency heart rate variability, is a non-invasive measure of parasympathetic influences on cardiovascular output. It is assessed by extracting the high-frequency component of heart rate (i.e., beat-to-beat variability) that typically corresponds with respiration. Specifically, during inhalation the inhibitory influence of the PNS (via the vagus nerve) decreases and there is a corresponding increase in heart rate. During exhalation, vagal inhibition resumes and heart rate decreases. When RSA is measured during a quiet resting state, higher scores indicate physiological flexibility, the ability to adapt to environmental stressors, and conservation of resources. Therefore, higher resting state RSA is generally considered adaptive and is associated with better emotion regulation abilities.(27, 31) It should be noted, however, that although the range of expected values on RSA has been established,(24) there is no identified clinical cut-off for “healthy” RSA or for the association between RSA and emotion regulation capacity.
Initial interest in RSA as a marker of emotion dysregulation followed from polyvagal theory and has since gained traction in studies with healthy and clinical samples.(40) This is because PNS inputs from the nucleus ambiguus to the heart, which inhibit cardiac output, therefore serve to promote flexible, regulated behavior. In contrast, the SNS has an excitatory effect on heart rate, consistent with other activating functions of the SNS. For example, when a person encounters a challenging or novel social situation, there may be an initial physiological reaction that accompanies a sense of fear. The ability to overcome anxiety and engage socially requires sustained attention and behavioral control over the urge to flee. This is facilitated by increased vagal activity, which slows heart rate and promotes affiliation. If, however, the situation proves to be dangerous or threatening, sympathetically-mediated cardiac output would likely increase and the vagus nerve would release its inhibitory influence on the heart. This would promote the rapid mobilization of resources needed to fight or escape.
As a measure of PNS influences on heart rate, RSA is a strong indicator of a person's self-regulatory capacity.(31) Indeed, there is accumulating evidence that low resting RSA and excessive RSA withdrawal are reliable and valid biomarkers of emotion dysregulation and emotional lability.(32) However, people are unaware of their RSA at rest, unless provided with specific training and visual feedback (e.g., biofeedback.(42) Perhaps because RSA is hard to detect and control, very few treatment providers have sought to integrate RSA measures into SUD treatment. However, attention to one's breath is a common element of mindfulness and body awareness interventions, and paced breathing is known to produce momentary increases in RSA.(43). Further, because RSA is widely understood to index emotion regulation capacity(32) and because it changes with greater respiratory control, it has potential as a mechanism of change in clinical research. This may be especially true for interventions that target interoceptive awareness as a means of achieving better emotion regulation.
Interoceptive Awareness and Self-Regulation in Mindfulness-Based Approaches to Clinical Care and Substance Use Treatment
As articulated in the second tenet, there is compelling clinical and research evidence that interoceptive awareness can be important in self-regulation, and may include the ability to identify, process, and regulate emotions and behavior.(36) Mindfulness-based approaches involve some degree of interoceptive training, as interoceptive awareness is integral to mindfulness strategies (e.g. attention to sensation of the breath; body scans), and have been used to address substance use.(44, 45) In addition, mindfulness interventions are typically focused on the processes by which repeated attention to interoceptive processes can facilitate improved awareness and regulation (e.g. sustained attention to bodily sensations in response to stress to explore response to external events and related internal or behavioral responses).(18) There are also a number of mindfulness-based intervention studies that have examined RSA as a marker of clinical improvements.(46-49) To date, there is considerable variability in the published literature across study designs and the rigor with which psychophysiological methods have been applied. However, based upon our review, four studies merit further mention based upon strong study designs and relatively thorough description of psychophysiological methods and analyses (see Table 1). Of these, three of the four studies found positive changes in RSA in response to a mindfulness based intervention and also demonstrate positive associations between change in RSA and other health-related outcomes.
Table 1.
Selected treatment-outcome studies examining cardiovascular parasympathetic nervous system changes to mindfulness training
| Citation | Research Design | Intervention | Sample | Parasympathetic nervous system measurements | Results |
|---|---|---|---|---|---|
| Delgado-Pastor et al., 2015(46) | Three-group randomized control trial (RCT) | A mindfulness cognitive training group, a mindfulness interoceptive training group, and a non-intervention control group | 45 female college students (n = 15 mindfulness interoceptive training; 12 mindfulness cognitive training; 14 control) | Resting RSA measured across three 5-min periods (baseline, worry, and mindfulness) | Significant increase in RSA for Interoceptive group compared to the Cognitive and Controls groups. Both mindfulness interoceptive training and mindfulness cognitive training showed change in RSA, but only interoceptive training showed a significant change. Significant improvement in depressive symptoms and negative affect for both Mindfulness groups compared to Control. |
| Nyklicek et al., 2013(47) | Two parallel group RCT | Mindfulness-Based Stress Reduction (MBSR) | 88 healthy community-dwelling individuals (n = 44 MBSR; 44 wait list control) | Collected multiple cardiovascular measures during mental arithmetic and speech tasks, including RSA | No within or between group differences in RSA. The Mindfulness group showed significant pre-post decreases in systolic and diastolic blood pressure. |
| Garland et al., 2014(48) | Two-group intervention pre-post study | Mindfulness-Oriented Recovery Enhancement for 8 weeks | 49 chronic pain patients with opioid use problems (n = 20 mindfulness; 29 social control group) | RSA was measured before and after 8 weeks of treatment during a dot probe task | Significant increase in RSA for Mindfulness compared to Social Control group. Decreased opioid craving for Mindfulness group compared to Social Control group. |
| Libby et al., 2012(49) | RCT | Mindfulness meditation | 31 community participants enrolled in a 4-week smoking cessation intervention (participants were a subset of those from the larger RCT) | Resting RSA measured prior to start of treatment (at resting baseline during a meditation task) | Increase in RSA from resting to meditation significantly predicted fewer cigarettes used per day at follow-up. |
Notes: Mindfulness-based treatment-related studies were selected if the researchers included adequate description of psychophysiological methods. Not all studies were ideal in design (e.g., randomized controlled trials with multiple treatment groups and repeated RSA measures). Many studies used alternate terminology for RSA, such as high-frequency heart rate variability. However, upon inspection, the methods used were comparable to those used in RSA studies.
Importantly, body signals that are accessible through interoceptive awareness provide valuable information to potentially guide decisions and behavior, and may be a key conditional precursor for behavior change (50), as the findings from two (48, 49) of these mindfulness-based studies suggest. Feeling comfortable attending to one's body sensations and having the skills to do so are frequently not within an individual's set of self-care tools. Avoidance and lack of comfort with/attending to bodily sensations (emotional or physical) is clinically apparent, often with dissociative symptoms, among individuals with chronic stress(51) or trauma.(52, 53) Related to SUD treatment, the results from a study of Mindful Awareness in Body-oriented Therapy (MABT), an approach that focuses on the development of interoceptive awareness, for women in SUD treatment showed that bodily dissociation was relatively high at baseline and showed significant improvement among those that received MABT compared to those who did not.(54) Furthermore, those who received MABT described increased emotional awareness and ability to cope with stress.(55) These findings suggest that women in SUD treatment are generally disconnected from their bodies and emotions, likely in part because of the high prevalence of interpersonal physical and sexual trauma in this population.(56) Additionally, the results of a focus group study with women one year after receiving interoceptive awareness training through MABT showed that the primary perceived benefit was learning to better identify, accept, and process emotions, which participants considered critical for relapse prevention.(57) Likewise, results from Mindfulness-based Relapse Prevention studies suggest that craving is reduced due to positive changes in present-moment awareness and response to negative affect.(58) Together, these results link interoceptive awareness, emotion regulation, and relapse prevention and point to the need to further understand the associated psychophysiology underlying substance use disorder recovery.
In addition, mindfulness-based intervention research has a growing literature examining CNS changes.(59, 60) The current neuro models specific to emotion regulation in mindfulness meditation(61, 62) are based on the premise that multiple brain regions are likely involved, and that mindfulness meditation may serve to bridge regions (e.g. prefrontal cortex and limbic regions associated with interoception).(63) Positive change in emotion regulation capacity has been largely based on the premise that this process involves prefrontally mediated down-regulation of subcortical structures, such as the amygdala.(32) Specifically, the vagus nerve appears to mediate the link between the prefrontal cortex and cardiovascular output.(32) In support of this theory, studies find that anxiety can be regulated volitionally through prefrontal inhibition of amygdala activity whereas regulation of impulsive behaviors involves top-down prefrontal control over striatal activation. (64-66) Researchers studying mindfulness using fMRI found increased prefrontal activation in expectation of negative stimuli and reduced subcortical activation of the amygdala and parahippocampal gyrus during the presentation of negative stimuli in response to a brief mindfulness instruction.(67) In contrast, mindfulness training (longer term intervention and practice) appears to shift attentional stance from evaluative, cortical midline processing of the prefrontal cortex to an expansive and diffuse form of sensory attention involving the posterior insula and pathways associated with interoceptive awareness.(68, 69) However, the exact mechanisms, as well as modes of self-reference involved, are little understood. Thus, while RSA is linked theoretically to some of the same CNS mechanisms that have been identified in cognitive behavioral and mindfulness research (e.g., prefrontal regulation),(32) mindfulness approaches may utilize various emotion regulation processes(70) and similarly, various brain structures appear to be involved.(59, 63) This is an area for future research to interpret RSA changes in the context of potential CNS underpinnings.
Understanding Emotion Dysregulation in Substance Use
As noted in the third tenet, interoceptive processes, emotion regulation, and physiological systems are often disrupted among those with vulnerability to psychopathology such as SUD.(34) For example, there are several lines of evidence linking interoception with emotion regulation and dysregulation.(71) Emotional awareness is associated with interoceptive ability and is correlated inversely with alexithymia.(72) Many clinical diagnoses are also associated with emotion dysregulation as well as deficits in interoceptive awareness, such as panic disorder and depression.(73) Thus, effective emotion regulation may involve awareness of one's physiological state and an organized response to internal sensations.(63) The ability to cope with negative emotions is especially critical for relapse prevention.(5) For this reason, several studies of SUD have examined emotional processes that increase risk for use.(6, 74-76) Understanding these processes, however, requires a clear definition of emotion, including regulation and dysregulation of emotional systems.
According to one common perspective, emotions can be defined as complex, multisystem responses that involve cognitions, behaviors, and physiological reactions.(77, 78) Emotion regulation and dysregulation, however, have been defined variably in the literature. Broadly, emotion regulation is defined as the ability to modulate the intensity or duration of emotional responses in order to promote goal-directed behavior.(79) In contrast, emotion dysregulation is characterized by intense or labile emotions, poor distress tolerance, experiential avoidance, lack of self-control, and vulnerability for psychopathology.(80, 81) Although many theorists conceptualize emotion dysregulation as the absence of regulation, measurement of these constructs often differs in empirical studies. For example, studies of emotion regulation tend to measure specific regulatory strategies (e.g., suppression, reappraisal), whereas studies of emotion dysregulation typically assess naturally occurring responses to dysregulating stimuli. Researchers studying emotion regulation and dysregulation have utilized a variety of measurement approaches. Most common among these is self-report measures. However, there are an increasing number of studies incorporating psychophysiological and neurobiological assessments.
Self-Report of Emotion Dysregulation
In many SUD studies, emotion dysregulation is assessed using self-report measures, such as the Difficulties in Emotion Regulation Scale (DERS).(79) These studies find greater emotion regulation difficulties among individuals in substance use disorder treatment relative to community controls.(82, 83) In addition, difficulties with emotion regulation predict relapse post-treatment.(84) To date, only two studies have examined self-reported changes in emotion regulation following SUD treatment. The first was a single-group study of dialectical behavior therapy for women with comorbid SUD and borderline personality disorder, which found improved pre-post substance use and emotion regulation using the DERS and also that improved emotion dysregulation accounted for reductions in substance use frequency.(85) The second was an initial feasibility study of Mindful Awareness in Body-oriented Therapy (MABT), which found marginally significant DERS improvements in MABT compared to treatment as usual at nine month follow-up among women in substance use disorder treatment.(86)
Although self-report measures of emotion regulation difficulties are used widely in studies of psychopathology, and show expected associations with RSA,(87) this type of measurement has well-established limitations. Self-report measures require some degree of insight and awareness into one's regulation strategies. Answers to emotion regulation questions may also be influenced by the participant's mood at the time of measurement or by recent or very memorable experiences with emotion regulation difficulties. These measures may also be prone to social desirability bias, resulting in inflated estimates of a participant's capabilities. For these reasons, many researchers have incorporated psychophysiological measurements into studies of emotion regulation and dysregulation.
Psychophysiological Measurement of Emotion Dysregulation
Reviews of substance use research provide strong evidence that those with alcohol dependence have lower RSA compared with non-dependent controls, (88, 89) which has also been shown among heroin addicts compared to non-addicted controls.(90) In addition, reviews of SUD provide evidence of alterations in physiology associated with direct drug effects, withdrawal, and cue responsivity.(11, 91, 92) To date, there are only a handful of studies that have examined RSA in SUD intervention research. One was an initial pilot study examining RSA in response to a neutral and a stressful imaging script among individuals in mindfulness compared to cognitive behavior approaches for SUD. Results from this study suggest psychophysiological changes in the expected direction among those who received mindfulness training relative to the cognitive behavior therapy group. However, the sample was small and not all effects were statistically significant.(93) A second study evaluated a smoking cessation treatment. The researchers examined RSA post-treatment to see if the changes in RSA from a resting baseline to meditation were associated with smoking outcomes post treatment.(49) The results showed that individuals with an increase in RSA smoked fewer cigarettes relative to those who showed a decrease in RSA, suggesting psychophysiological measures of emotion regulation improvements are a good statistical predictor of better substance use outcomes. Finally, one pilot study examined whether RSA biofeedback training reduced alcohol and drug craving for men.(94) The findings indicated greater effect size in craving reduction for those receiving RSA training + treatment as usual (TAU) relative to those receiving TAU only. Similarly, a study examining RSA with chronic pain patients who misuse opioids showed significant increase in RSA in response to a mindfulness intervention compared to an active control(48) (see Table 1). Results across these studies are promising but very preliminary and more SUD research including RSA as one measure of treatment effects is needed.
It is important to note that other psychophysiological measures have been used to understand emotional processes in SUD, including sympathetic (i.e., fight-flight) differences. Such measures include electrodermal activity (eccrine sweat gland activation, which is typically measured on the palm of the hand or sole of the foot), cardiac pre-ejection period (a measure of SNS influences on heart rate), and blood pressure. Although these measures are also interesting as potential mechanisms of change in SUD treatment, we are focusing on RSA in this review because (1) it has been linked to emotion dysregulation more clearly than other autonomic measures, such as electrodermal activity or blood pressure;(2) it improves with paced breathing biofeedback(95) and breathing affects RSA.(43) Attention to one's breath, which is a common component of interventions targeting mindful body awareness, may similarly increase RSA; and (3) it is more cost-effective as a measure of physiological change relative to central nervous system (CNS) measurement approaches, such as fMRI.
Applicability of RSA as a Method for SUD Treatment Research
Using RSA as a potential tool for SUD treatment research follows from a growing literature examining RSA as a predictor of treatment response,(96) a mediator of emotion regulation improvements,(97) and a correlate of numerous physical and mental health outcomes.(98) For example, high RSA is associated with social competence resilience among individuals faced with stressors,(99) and the ability to respond flexibly to environmental demands.(41) Alternatively, low levels of resting RSA are associated with several disorders and problems in children and adults, including poor impulse control,(31) and anxiety.(100) This leads to our fourth tenet that treatments for substance dependence, especially those which target improved interoceptive awareness may positively affect health outcomes (emotional, behavioral, and psychophysiological regulation).
In response to stressors, higher RSA has been shown to predict greater self-reported regulatory control and decreased negative emotional arousal.(99) In depression research, greater RSA suppression in response to emotionally arousing video clips has been reported to predict recovery from depression.(101) In a study of women with chronic interpersonal violence, lower RSA was associated with increased PTSD symptoms of avoidance and intrusion.(102) Also, RSA level appears to parallel positive treatment effects, with increases in RSA reported only in patients who exhibited a clinically significant response to treatment.(103)
The examination of the RSA in response to SUD treatment may be most applicable to interventions that involve or target attention to interoceptive awareness. Interoception is important for comfort with sensory stimuli and sensory representation and integration that affect cognitive processes underlying homeostasis and regulation.(14, 33, 35) For individuals who have developed patterns of avoidance or dissociation from the body to cope with chronic pain, extreme stress, trauma, or other mental health challenges, the need for targeted interventions to develop interoceptive awareness may be even greater, particularly relevant to SUD populations given the high prevalence of stress and comorbid conditions.(104-106) Thus interventions that are designed to develop interoceptive awareness such as mindfulness awareness in body-oriented therapy (MABT)(54) or mindfulness-based relapse prevention(58) would be expected to improve regulation of emotions in SUD treatment.
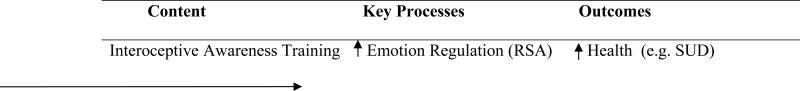
These studies have contributed to the increased interest in using psychophysiological methods to examine emotional processes among those with SUD.(107) To date, there has been little work examining the psychophysiology of emotion and emotion dysregulation in SUD treatment research, an identified gap in the literature.(92) However, consistent with prior research, we theorize that pre-treatment psychophysiological indices of emotion dysregulation are critical to consider in treatment. In particular, treatment approaches that improve psychophysiological and self-reported emotion dysregulation may have more lasting benefits for those with SUD. Indeed, we might expect that improved RSA will mediate positive change in SUD health outcomes in response to a mindfulness approach that targets interoceptive awareness (see Figure 1). RSA as a mediator of health outcomes has not, to our knowledge, been previously addressed in research however this is a research question that we are currently examining in a NIDA-funded study of MABT for women in SUD treatment.(108)
Figure 1.
Hypothesized Mediation Model for Mindfulness-based Approach
Conclusion and Implications for Future SUD Research
In summary, RSA is a potentially important psychophysiological measure in treatment research. As a marker of at least some aspects of self-regulation capacity, the use of RSA in SUD research is relevant since treatment is typically focused on increasing regulatory capacity as a means of achieving behavior change. For SUD treatment studies involving mindfulness-based approaches, the inclusion of RSA may be of particular interest and relevance due to the focus on interoceptive awareness training, the deficits in regulatory processes involved in interoception among substance users, and the theorized link between interoceptive awareness for emotion regulation and behavior change.
It is important to note several limitations of this review and of the literature to date. Although there are multiple methods that can be used to examine psychophysiological biomarkers, we focused exclusively on RSA and thus do not address other approaches (e.g. electrodermal activity) that have been used in treatment research. Furthermore, our argument in favor of RSA is not a systematic review of RSA findings in clinical treatment studies. However, we did look extensively at the research literature in this regard. In spite of a rich literature examining psychological correlates of RSA, there is a significant dearth of high-quality treatment-outcome research examining RSA as a potential mechanism underlying symptom change. In our examination of the existing treatment-outcome literature, this appears to be due to a number of factors, including the use of idiosyncratic psychophysiological techniques that don't map well onto the wealth of basic science studies (e.g., failing to log transform high frequency RSA), poor study design (e.g., small sample sizes, lack of randomization, no control group), confounding low frequency and high frequency RSA (LF-RSA includes both sympathetic and parasympathetic influences), failure to assess, report, or control for physical health factors or medication use, and, most often, an insufficient description of methods for acquiring, cleaning, and analyzing psychophysiological data. Unfortunately, these shortcomings make it difficult to interpret contradictory or non-significant findings, which may be artifacts of study design or methodology rather than a true indication of null results. The need for expertise in psychophysiological methods may also help explain why few treatment studies that have incorporated RSA to examine underlying intervention mechanisms or biomarkers of emotion regulation processes in SUD research. Finally, this review focuses heavily on cognitive-behavioral, mindfulness, and interoceptive approaches and their underlying theories. This review neglects other approaches to understanding SUD that may impact regulation and recovery such as disease models of addiction.(109)
Nonetheless, the inclusion of RSA in future SUD treatment studies is recommended as one important biomarker for the examination of intervention mechanisms. The relatively low cost of psychophysiological research reduces barriers to entry into this interesting but complex field. As such, one of the benefits of RSA is that it can be gathered at the clinical site, which is important for community-based clinical trials. As many clinical researchers are likely aware, it is helpful if all aspects of the research occur within the clinic environment to ensure accessibility and engagement; this is particularly true in substance use treatment research due to the inherent challenges of attendance, attrition, etc.(110) However, given the limitations of the existing literature, researchers interested in incorporating psychophysiological methods into treatment-outcome research should be thoughtful in their approach. We recommend that researchers receive expert training or consultation prior to extending their work to include RSA.
Acknowledgement
Research reported in this publication was supported by the National Institute for Drug Abuse of the National Institutes of Health under award number R01DA033324. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no competing interests to declare
References
- 1.West R. Publications Office of the European Union. European Monitoring Centre for Drugs and Drug Addiction; Luxembourg: 2013. Models of Addiction. pp. 1–161. [Google Scholar]
- 2.Le Moal M, Koob GF. Drug addiction: Pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol. 2007;17(6-7):377–93. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Lowman C, Allen J, Stout RL. Replication and extension of Marlatt's taxonomy of relapse precipitants: overview of procedures and results. The Relapse Research Group. Addiction. 1996;91(Suppl):S51–71. [PubMed] [Google Scholar]
- 4.Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, et al. Stress enhancement of craving during sobriety: a risk for relapse. Alcoholism, clinical and experimental research. 2005;29(2):185–95. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha R. The role of stress in addiction relapse. Current psychiatry reports. 2007;9(5):388–95. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- 6.Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced Negative Emotion and Alcohol Craving, and Altered Physiological Responses Following Stress and Cue Exposure in Alcohol Dependent Individuals. Neuropsychopharmacology. 2009;34(5):1198–208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein R, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, et al. The Neurocircuity of Impaired Insight. Trends Cogn Sci. 2010;13(9):372–8. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulus MP, Tapert SF, Schulteis G. The role of interoception and alliesthesia in addiction. Pharmacology, biochemistry, and behavior. 2009;94(1):1–7. doi: 10.1016/j.pbb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neuroscience and biobehavioral reviews. 2012;36(8):1857–69. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Noel X, Brevers D, Bechara A. A neurocognitive approach to understanding the neurobiology of addiction. Current opinion in neurobiology. 2013;23(4):632–8. doi: 10.1016/j.conb.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garland EL, Froeliger B, Howard MO. Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Frontiers in psychiatry. 2014;4:173. doi: 10.3389/fpsyt.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76(Pt B):342–50. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature reviews Neuroscience. 2002;3(8):655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 14.Craig AD. How do you feel--now? The anterior insula and human awareness. Nature reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 15.Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 16.Kim YT, Song HJ, Seo JH, Lee JJ, Lee J, Kwon DH, et al. The differences in neural network activity between methamphetamine abusers and healthy subjects performing an emotion - matching task: functional MRI study. NMR in Biomedicine. 2011;24(10):1392–400. doi: 10.1002/nbm.1702. [DOI] [PubMed] [Google Scholar]
- 17.Beauchaine TP, Neuhaus E, Brenner SL, Gatzke-Kopp L. Ten good reasons to consider biological processes in prevention and intervention research. Dev Psychopathol. 2008;20(3):745–74. doi: 10.1017/S0954579408000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farb NA, Segal ZV, Anderson AK. Mindfulness meditation training alters cortical representations of interoceptive attention. Social cognitive and affective neuroscience. 2013;8(1):15–26. doi: 10.1093/scan/nss066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol-London. 2000;523(1):259–70. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianaros PJ, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41(4):521–30. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianaros PJ, Derbyshire SW, May JC, Siegle GJ, Gamalo MA, Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005;42(6):627–35. doi: 10.1111/j.1469-8986.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Critchley HD, Rotshtein P, Nagai Y, O'Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. NeuroImage. 2005;24(3):751–62. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Woodward SH, Kaloupek DG, Schaer M, Martinez C, Eliez S. Right anterior cingulate cortical volume covaries with respiratory sinus arrhythmia magnitude in combat veterans. J Rehabil Res Dev. 2008;45(3):451–63. doi: 10.1682/jrrd.2007.06.0082. [DOI] [PubMed] [Google Scholar]
- 24.Zisner A, Beauchaine TP. In: Physiological methods and developmental psychopathology. 3 ed. Cicchetti D, editor. Wiley; Hoboken, NJ: Forthcoming. [Google Scholar]
- 25.Pu J, Schmeichel BJ, Demaree HA. Cardiac vagal control predicts spontaneous regulation of negative emotional expression and subsequent cognitive performance. Biol Psychol. 2010;84(3):531–40. [Google Scholar]
- 26.Crowell SE, Beauchaine TP, McCauley E, Smith CJ, Stevens AL, Sylvers P. Psychological, autonomic, and serotonergic correlates of parasuicide among adolescent girls. Dev Psychopathol. 2005;17(04):1105–27. doi: 10.1017/s0954579405050522. [DOI] [PubMed] [Google Scholar]
- 27.Porges SW, Doussard-Roosevelt JA, Maiti AK. Vagal tone and the physiological regulation of emotion. Monogr Soc Res Child Dev. 1994;59(2-3):167–86. [PubMed] [Google Scholar]
- 28.Delgado LC, Guerra P, Perakakis P, Vera MN, Reyes del Paso G, Vila J. Treating chronic worry: Psychological and physiological effects of a training programme based on mindfulness. Behav Res Ther. 2010;48(9):873–82. doi: 10.1016/j.brat.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Bagner DM, Graziano PA, Jaccard J, Sheinkopf SJ, Vohr BR, Lester BM. An Initial Investigation of Baseline Respiratory Sinus Arrhythmia as a Moderator of Treatment Outcome for Young Children Born Premature With Externalizing Behavior Problems. Behav Ther. 2012;43(3):652–65. doi: 10.1016/j.beth.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemeny ME, Foltz C, Cavanagh JF, Cullen M, Giese-Davis J, Jennings P, et al. Contemplative/emotion training reduces negative emotional behavior and promotes prosocial responses. Emotion. 2012;12(2):338–50. doi: 10.1037/a0026118. [DOI] [PubMed] [Google Scholar]
- 31.Beauchaine T. Vagal tone, development, and Gray's motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- 32.Beauchaine TP. Respiratory sinus arrhythmia: a transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in Psychology. 2015;3(0):43–7. doi: 10.1016/j.copsyc.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron OG. Interoception: the inside story--a model for psychosomatic processes. Psychosom Med. 2001;63(5):697–710. doi: 10.1097/00006842-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Schulz A, Vogele C. Interoception and stress. Front Psychol. 2015;6:993. doi: 10.3389/fpsyg.2015.00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain structure & function. 2010;214(5-6):435–50. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farb NA, Daubenmier J, Price C, Gard T, Kerr CE, Dunn BD, et al. Interoception, Contemplative Practice and Health. Frontiers in Psychology. 2015;6:e763. doi: 10.3389/fpsyg.2015.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cacioppo JT, Tassinary LG, Berntson GG. Handbook of psychophysiology. 3 ed. Cambridge University Press; New York, NY, U.S.: 2007. [Google Scholar]
- 38.Berntson GG, Cacioppo JT, Quigley KS, Fabro VT. Autonomic space and psychophysiological response. Psychophysiology. 1994;31(1):44–61. doi: 10.1111/j.1469-8986.1994.tb01024.x. [DOI] [PubMed] [Google Scholar]
- 39.Berntson GG, Cacioppo JT, Grossman P. Whither vagal tone. Biological psychology. 2007;74(2):295–300. doi: 10.1016/j.biopsycho.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Porges SW. Cardiac vagal tone: a physiological index of stress. Neuroscience and biobehavioral reviews. 1995;19(2):225–33. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- 41.Porges SW. The polyvagal perspective. Biological psychology. 2007;74(2):116–43. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nolan RP, Kamath MV, Floras JS, Stanley J, Pang C, Picton P, et al. Heart rate variability biofeedback as a behavioral neurocardiac intervention to enhance vagal heart rate control. Am Heart J. 2005;149(6):1137, e1–.e7. doi: 10.1016/j.ahj.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Berntson GG, Quigley KS, Lozano D. Cardiovascular Psychophysiology. In: Cacioppo JTT,LG, Berntson GG, editors. Handbook of psychophysiology. 3 ed Cambridge University Press; New York, NY, U.S.: 2007. pp. 182–210. [Google Scholar]
- 44.Black DS. Mindfulness-Based Interventions: An Antidote to Suffering in the Context of Substance Use, Misuse, and Addiction. Subst Use Misuse. 2014;49(5):487–91. doi: 10.3109/10826084.2014.860749. [DOI] [PubMed] [Google Scholar]
- 45.Zgierska A, Marcus MT. Mindfulness-based therapies for substance use disorders: part 2. Subst Abus. 2010;31(2):77–8. doi: 10.1080/08897071003641248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delgado-Pastor LC, Ciria LF, Blanca B, Mata JL, Vera MN, Vila J. Dissociation between the cognitive and interoceptive components of mindfulness in the treatment of chronic worry. J Behav Ther Exp Psychiatry. 2015;48:192–9. doi: 10.1016/j.jbtep.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Nyklicek I, Mommersteeg PM, Van Beugen S, Ramakers C, Van Boxtel GJ. Mindfulness-based stress reduction and physiological activity during acute stress: a randomized controlled trial. Health Psychol. 2013;32(10):1110–3. doi: 10.1037/a0032200. [DOI] [PubMed] [Google Scholar]
- 48.Garland EL, Froeliger B, Howard MO. Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology. 2014;231(16):3229–38. doi: 10.1007/s00213-014-3504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Libby DJ, Worhunsky PD, Pilver CE, Brewer JA. Meditation-induced changes in high-frequency heart rate variability predict smoking outcomes. Front Hum Neurosci. 2012;6:54. doi: 10.3389/fnhum.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mehling WE, Wrubel J, Daubenmier JJ, Price CJ, Kerr CE, Silow T, et al. Body Awareness: a phenomenological inquiry into the common ground of mind-body therapies. Philosophy, ethics, and humanities in medicine : PEHM. 2011;6:6. doi: 10.1186/1747-5341-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aderibigbe YA, Bloch RM, Walker WR. Prevalence of depersonalization and derealization experiences in a rural population. Soc Psychiatry Psychiatr Epidemiol. 2001;36(2):63–9. doi: 10.1007/s001270050291. [DOI] [PubMed] [Google Scholar]
- 52.Herman J. Trauma and Recovery: The Aftermath of Violence - From Domestic Abuse to Political Terror. HarperCollins; New York: 1997. [Google Scholar]
- 53.Timms R, Connors P. Embodying healing: integrating bodywork and psychotherapy in recovery from childhood sexual abuse. The Safer Society Press; Orwell, VT: 1992. [Google Scholar]
- 54.Price CJ, Wells EA, Donovan DM, Rue T. Mindful awareness in body-oriented therapy as an adjunct to women's substance use disorder treatment: a pilot feasibility study. Journal of substance abuse treatment. 2012;43(1):94–107. doi: 10.1016/j.jsat.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Price CJ, Wells E, Donovan D, Brooks M. Implementation and Acceptability of Mindful Awareness in Body-oriented Therapy in Women's Substance Use Disorder Treatment. J Altern Complement Med. 2012;18(5):1–9. doi: 10.1089/acm.2011.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, et al. Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug Alcohol Depend. 2007;86(1):1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price C, Smith-DiJulio K. Interoceptive Awareness is Important for Relapse Prevention: Perceptions of Women who Received Mindful Body Awareness in Substance Use Disorder Treatment. J Addict Nurs. doi: 10.1097/JAN.0000000000000109. forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Witkiewitz K, Lustyk MK, Bowen S. Retraining the addicted brain: a review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol Addict Behav. 2013;27(2):351–65. doi: 10.1037/a0029258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang YY, Holzel BK, Posner MI. The neuroscience of mindfulness meditation. Nature reviews Neuroscience. 2015;16(4):213–25. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- 60.Fox KC, Nijeboer S, Dixon ML, Floman JL, Ellamil M, Rumak SP, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neuroscience and biobehavioral reviews. 2014;43:48–73. doi: 10.1016/j.neubiorev.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Holzel BK, Lazar SW, Gard T, Schumann-Olivier Z, Vago DR, Ott U. How does mindfulness meditation work? Proposing mechanisms of actions from a conceptual and nueral perspective. Perspect Psychol Sci. 2011;6(6):537–59. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- 62.Teper R, Segal ZV, Inzlicht M. Inside the Mindful Mind: How Mindfulness Enhances Emotion Regulation Through Improvements in Executive Control. Curr Dir Psychol Sci. 2013;22(6):449–54. [Google Scholar]
- 63.Farb NA, Anderson AK, Segal ZV. The mindful brain and emotion regulation in mood disorders. Canadian journal of psychiatry Revue canadienne de psychiatrie. 2012;57(2):70–7. doi: 10.1177/070674371205700203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biological psychiatry. 2002;51(1):68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 65.Heatherton TF, Wagner DD. Cognitive Neuroscience of Self-Regulation Failure. Trends Cogn Sci. 2011;15(3):132–9. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heatherton TF. Neuroscience of Self and Self-Regulation. Annu Rev Psychol. 2011;62:363–90. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lutz J, Herwig U, Opialla S, Hittmeyer A, Jancke L, Rufer M, et al. Mindfulness and emotion regulation--an fMRI study. Soc Cogn Affect Neurosci. 2014;9(6):776–85. doi: 10.1093/scan/nst043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farb NA, Anderson AK, Mayberg H, Bean J, McKeon D, Segal ZV. Minding one's emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10(1):25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farb NA, Segal ZV, Anderson AK. Attentional modulation of primary interoceptive and exteroceptive cortices. Cerebral cortex. 2013;23(1):114–26. doi: 10.1093/cercor/bhr385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gross JJ. In: Handbook of Emotion Regulation. 2nd edn. Gross JJ, editor. Guildford Press; 2014. pp. 3–20. [Google Scholar]
- 71.Barrett LF, Quigley KS, Bliss-Moreau E, Aronson KR. Interoceptive Sensitivity and Self-Reports of Emotional Experience. J Pers Soc Psychol. 2004;87(5):684–97. doi: 10.1037/0022-3514.87.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herbert BM, Herbert C, Pollatos O. On the Relationship Between Interoceptive Awareness and Alexithymia: Is Interoceptive Awareness Related to Emotional Awareness? J Pers. 2011;79(5):1149–75. doi: 10.1111/j.1467-6494.2011.00717.x. [DOI] [PubMed] [Google Scholar]
- 73.Dunn BD, Dalgleish T, Ogilvie AD, Lawrence AD. Heartbeat perception in depression. Behaviour research and therapy. 2007;45(8):1921–30. doi: 10.1016/j.brat.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Ostafin BD, Marlatt GA, Greenwald AG. Drinking without thinking: An implicit measure of alcohol motivation predicts failure to control alcohol use. Behav Res Ther. 2008;46(11):1210–9. doi: 10.1016/j.brat.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 75.Tate SR, Wu J, McQuaid JR, Cummins K, Shriver C, Krenek M, et al. Comorbidity of substance dependence and depression: Role of life stress and self-efficacy in sustaining abstinence. Psychol Addict Behav. 2008;22(1):47–57. doi: 10.1037/0893-164X.22.1.47. [DOI] [PubMed] [Google Scholar]
- 76.Garland EL, Carter K, Ropes K, Howard MO. Thought suppression, impaired regulation of urges, and Addiction-Stroop predict affect-modulated cue-reactivity among alcohol dependent adults. Biological psychology. 2012;89(1):87–93. doi: 10.1016/j.biopsycho.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–90. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- 78.Crowell SE, Baucom BR, Yaptangco M, Bride D, Hsiao R, McCauley E, et al. Emotion dysregulation and dyadic conflict in depressed and typical adolescents: Evaluating concordance across psychophysiological and observational measures. Biol Psychol. 2014;98(0):50–8. doi: 10.1016/j.biopsycho.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment. 2004;26(1):41–54. [Google Scholar]
- 80.Moore SA, Zoellner LA, Mollenholt N. Are expressive suppression and cognitive reappraisal associated with stress-related symptoms? Behav Res Ther. 2008;46(9):993–1000. doi: 10.1016/j.brat.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eftekhari A, Zoellner LA, Vigil SA. Patterns of emotion regulation and psychopathology. Anxiety, stress, and coping. 2009;22(5):571–86. doi: 10.1080/10615800802179860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fox HC, Axelrod SR, Paliwal P, Sleeper J, Sinha R. Difficulties in emotion regulation and impulse control during cocaine abstinence. Drug Alcohol Depend. 2007;89(2-3):298–301. doi: 10.1016/j.drugalcdep.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 83.Fox HC, Hong KA, Sinha R. Difficulties in emotion regulation and impulse control in recently abstinent alcoholics compared with social drinkers. Addict Behav. 2008;33(2):388–94. doi: 10.1016/j.addbeh.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 84.Berking M, Poppe C, Luhmann M, Wupperman P, Jaggi V, Seifritz E. Is the association between various emotion-regulation skills and mental health mediated by the ability to modify emotions? Results from two cross-sectional studies. Journal of behavior therapy and experimental psychiatry. 2012;43(3):931–7. doi: 10.1016/j.jbtep.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 85.Axelrod SR, Perepletchikova F, Holtzman K, Sinha R. Emotion regulation and substance use frequency in women with substance dependence and borderline personality disorder receiving dialectical behavior therapy. Am J Drug Alcohol Abuse. 2011;37(1):37–42. doi: 10.3109/00952990.2010.535582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Price CJ, Donovan D, Wells E, Rue T. Mindful Awareness in Body-oriented Therapy as an Adjunct to Women's Substance Use Disoder Treatment: A Pilot Feasibility Study. J Subst Abuse Treat. 2012;94:94–107. doi: 10.1016/j.jsat.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vasilev CA, Crowell SE, Beauchaine TP, Mead HK, Gatzke-Kopp LM. Correspondence between physiological and self-report measures of emotion dysregulation: A longitudinal investigation of youth with and without psychopathology. J Child Psychol Psyc. 2009;50(11):1357–64. doi: 10.1111/j.1469-7610.2009.02172.x. [DOI] [PubMed] [Google Scholar]
- 88.Quintana DS, McGregor IS, Guastella AJ, Malhi GS, Kemp AH. A meta-analysis on the impact of alcohol dependence on short-term resting-state heart rate variability: implications for cardiovascular risk. Alcoholism, clinical and experimental research. 2013;37(Suppl 1):E23–9. doi: 10.1111/j.1530-0277.2012.01913.x. [DOI] [PubMed] [Google Scholar]
- 89.Karpyak VM, Romanowicz M, Schmidt JE, Lewis KA, Bostwick JM. Characteristics of heart rate variability in alcohol-dependent subjects and nondependent chronic alcohol users. Alcoholism, clinical and experimental research. 2014;38(1):9–26. doi: 10.1111/acer.12270. [DOI] [PubMed] [Google Scholar]
- 90.Chang LR, Lin YH, Kuo TB, Ho YC, Chen SH, Wu Chang HC, et al. Cardiac autonomic modulation during methadone therapy among heroin users: a pilot study. Progress in neuro psychopharmacology & biological psychiatry. 2012;37(1):188–93. doi: 10.1016/j.pnpbp.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 91.Sinha R. Chronic Stress, Drug Use, and Vulnerability to Addiction. Addiction Reviews 2008. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheetham A, Allen NB, Yucel M, Lubman DI. The role of affective dysregulation in drug addiction. Clin Psychol Rev. 2010;30(6):621–34. doi: 10.1016/j.cpr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 93.Brewer JA, Sinha R, Chen JA, Michalsen RN, Babuscio TA, Nich C, et al. Mindfulness training and stress reactivity in substance abuse: results from a randomized, controlled stage I pilot study. Subst Abus. 2009;30(4):306–17. doi: 10.1080/08897070903250241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eddie D, Kim C, Lehrer P, Deneke E, Bates ME. A pilot study of brief heart rate variability biofeedback to reduce craving in young adult men receiving inpatient treatment for substance use disorders. Appl Psychophysiol Biofeedback. 2014;39(3-4):181–92. doi: 10.1007/s10484-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zucker TL, Samuelson KW, Muench F, Greenberg MA, Gevirtz RN. The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: a pilot study. Appl Psychophysiol Biofeedback. 2009;34(2):135–43. doi: 10.1007/s10484-009-9085-2. [DOI] [PubMed] [Google Scholar]
- 96.Mathewson KJ, Schmidt LA, Miskovic V, Santesso DL, Duku E, McCabe RE, et al. Does respiratory sinus arrhythmia (RSA) predict anxiety reduction during cognitive behavioral therapy (CBT) for social anxiety disorder (SAD)? Int J Psychophysiol. 2013;88(2):171–81. doi: 10.1016/j.ijpsycho.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 97.Patron E, Messerotti Benvenuti S, Favretto G, Gasparotto R, Palomba D. Depression and reduced heart rate variability after cardiac surgery: the mediating role of emotion regulation. Auton Neurosci. 2014;180:53–8. doi: 10.1016/j.autneu.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 98.Harte CB, Meston CM. Association between cigarette smoking and erectile tumescence: the mediating role of heart rate variability. Int J Impot Res. 2013;25(4):155–9. doi: 10.1038/ijir.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fabes RA, Eisenberg N. Regulatory control and adults' stress-related responses to daily life events. Journal of personality and social psychology. 1997;73(5):1107–17. doi: 10.1037//0022-3514.73.5.1107. [DOI] [PubMed] [Google Scholar]
- 100.Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biological psychiatry. 1996;39(4):255–66. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 101.Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts subsequent recovery from depression. Psychophysiology. 2005;42(3):277–81. doi: 10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 102.Depierro J, D'Andrea W, Pole N. Attention biases in female survivors of chronic interpersonal violence: relationship to trauma-related symptoms and physiology. European journal of psychotraumatology. 2013:4. doi: 10.3402/ejpt.v4i0.19135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chambers AS, Allen JJB. Vagal tone as an indicator of treatment response in major depression. Psychophysiology. 2002;39(6):861–4. doi: 10.1111/1469-8986.3960861. [DOI] [PubMed] [Google Scholar]
- 104.Wu LT, Blazer DG. Substance use disorders and psychiatric comorbidity in mid and later life: a review. Int J Epidemiol. 2014;43(2):304–17. doi: 10.1093/ije/dyt173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lamps CA, Sood AB, Sood R. Youth with substance abuse and comorbid mental health disorders. Current psychiatry reports. 2008;10(3):265–71. doi: 10.1007/s11920-008-0043-0. [DOI] [PubMed] [Google Scholar]
- 106.Mannelli P, Pae CU. Medical comorbidity and alcohol dependence. Current psychiatry reports. 2007;9(3):217–24. doi: 10.1007/s11920-007-0022-x. [DOI] [PubMed] [Google Scholar]
- 107.Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clin Psychol Rev. 2010;30(2):217–37. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 108.Abuse NIoD Washington Uo. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda, MD: [2015 Oct 05]. Body-oriented Therapy for Women in SUD Treatment.ClinicalTrials.gov Available from: https://clinicaltrials.gov/show/NCT01960036. [Google Scholar]
- 109.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 110.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. The American journal of psychiatry. 2008;165(2):179–87. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]