Abstract
Bronchiolitis obliterans syndrome (BOS) after allogeneic hematopoietic cell transplantation (HCT) is associated with high mortality. Purpose: We hypothesized that FAM (inhaled Fluticasone, Azithromycin, and Montelukast) with a brief steroid pulse could avert progression of new-onset BOS. Experimental design: We tested this in a phase II, single-arm, open label, multicenter study (NCT01307462). Results: Thirty-six patients were enrolled within 6 months of BOS diagnosis. The primary endpoint was treatment failure, defined as 10% or greater FEV1% decline at 3 months. At 3 months, 6% (2/36, 95% CI 1%–19%) had treatment failure (vs. 40% in historical controls, p<0.001). FAM was well tolerated. Steroid dose was reduced by 50% or more at 3 months in 48% of patients who could be evaluated (n=27). Patient-reported outcomes at 3 months were statistically significantly improved for SF-36 social functioning score and mental component score, FACT emotional well-being, and Lee symptom scores in lung, skin, mouth, and the overall summary score compared to enrollment (n=24). At 6 months, 36% had treatment failure (95% CI 21%–54%, n=13/36, with 6 documented failures, 7 missing pulmonary function tests). Overall survival was 97% (95% CI 84%–100%) at 6 months. These data suggest that FAM was well tolerated and that treatment with FAM and steroid pulse may halt pulmonary decline in new-onset BOS in the majority of patients and permit reductions in systemic steroid exposure, which collectively may improve quality of life. However, additional treatments are needed for progressive BOS despite FAM.
Keywords: bronchiolitis obliterans syndrome, fluticasone, azithromycin, montelukast, hematopoietic cell transplantation, lung chronic graft-versus-host disease, leukotrienes
Introduction
Bronchiolitis obliterans syndrome (BOS) following hematopoietic cell transplantation (HCT), also known as lung chronic graft-versus host disease (GVHD), is an insidious disease with poor outcomes where the donor immune system attacks the small airways in the lungs, leading to obstructive pulmonary disease and air trapping.1,2 While BOS is rare, affecting only 5–12% of HCT recipients, it is a significant problem after HCT because of the high attributed morbidity and mortality.1,3–7 Patients with early BOS are often asymptomatic, with symptoms developing later in the course of the disease such as dyspnea on exertion or a chronic cough, followed by dyspnea at rest, and finally inability to accomplish activities of daily living due to progressive pulmonary compromise and attributed weakness.4 Bronchiolitis obliterans is caused by an immune response to antigens expressed by bronchiolar epithelia, causing inflammation and epithelial disruption, followed by progressive intraluminal fibrosis of the small terminal airways.8–12 Multiple studies have linked decline in lung function to poor survival after chronic GVHD diagnosis, and historically only 44% of BOS patients are expected to survive two years.13–15 Here we present the first prospective study to evaluate the efficacy of the combination therapy of inhaled fluticasone, azithromycin, and montelukast (FAM) to treat new onset BOS after HCT.
Historically, there have been no effective therapies for BOS after HCT. Prior retrospective series have evaluated response of BOS to corticosteroids, cyclosporine, azathioprine, antithymocyte globulin, and extracorporeal photopheresis, and have been limited not only by patient numbers and retrospective evaluations, but also by differences in diagnostic criteria for BOS.15–17 However, consensus criteria developed in 2005 and revised in 2014, have permitted standardization of diagnostic criteria, which were used for this study.1,4,18,19 More recently, prospective studies enrolling patients with restrictive pulmonary disease after HCT have evaluated etanercept20 and azithromycin,21 and included responses in some patients meeting the definition of BOS. A recent study of inhaled budesonide/formoterol in a prospective, randomized, placebo controlled, cross-over trial of 32 patients showed benefit for 62% of patients in the treated arm vs. 25% in the placebo arm with a greater than 12% increase in absolute value of FEV1 at one month. However, only 56% completed the 6 month primary endpoint, and 37% required unblinding due to nonimprovement after 1 month of therapy.22
The FAM regimen was based on encouraging preliminary data with single agent Montelukast reported for the treatment of BOS after HCT, and other prior work that had suggested possible benefit with the use of inhaled Fluticasone and oral Azithromycin for BOS after HCT.1,23–26 Mechanisms of action for these agents could include a decrease in local lung inflammation (inhaled fluticasone), a reduction in local interleukin-8 levels and neutrophilia (azithromycin), and impairment of leukotriene activity (montelukast). Together, these agents may work by interrupting the cellular homing signals of activated cells trafficking to the lungs and decreasing fibroblast activity that results in the fibrotic constriction of the bronchiole lumen.1,25–35 Therefore, we undertook a prospective study to evaluate this novel combination of agents (FAM) for the treatment of newly diagnosed BOS after HCT.24,26,36 Because of the rarity of disease, associated morbidity, and reluctance of investigators to randomize patients to placebo, we conducted an open-label, single arm study.
Materials and Methods
Participants
The trial was limited to patients with new onset BOS, defined as diagnosis within 6 months of enrollment. Inclusion criteria included: (1) BOS, per the NIH modified criteria: FEV1<75% predicted, FEV1/VC <0.7, and an absolute decline of the percent of predicted FEV1 by ≥10% from pre-HCT without evidence of infection, or 2) pathologic diagnosis1,4,18,19. Pulmonary function testing (PFT) was performed with and without bronchodilators at enrollment to confirm that post-bronchodilator values still met diagnostic criteria; and (2) prior or current diagnosis of chronic GVHD per NIH criteria.
Exclusion criteria included: (1) known history of intolerance or allergy to any FAM component; (2) recurrent or progressive malignancy requiring anticancer treatment; (3) serum transaminases concentrations >5X upper limit of normal (ULN) or total bilirubin>3X ULN; (4) treatment with inhaled steroid or montelukast or zafirlukast for greater than one month during the past three months; (5) current treatment with prednisone at >1.2 mg/kg/day (or equivalent steroid) on the enrollment evaluation; (6) treatment with rifampin or phenobarbital, aspirin at doses >325 mg/day, or ibuprofen at doses >1200 mg/day; (7) treatment with any non-FDA approved medication within the past 4 weeks; (8) evidence of any viral, bacterial or fungal infection involving the lung and not responding to appropriate treatment; (9) chronic oxygen therapy; (10) clinical asthma; (11) baseline post-bronchodilator FEV1 < 20% of predicted normal; (12) inability to perform pulmonary function tests reliably; (13) patient age <6 years; (14) life expectancy <6 months at the time of enrollment as judged by the enrolling investigator; (15) any condition that, in the opinion of the enrolling investigator, would interfere with the subject’s ability to comply with the study requirements; (16) pregnancy or nursing.
Central review confirmed pulmonary function test (PFT) eligibility prior to study entry. Patients were typically referred at first PFT that showed BOS, though several months may have elapsed between pulmonary visit and primary HCT visit whereby patients would be diagnosed with BOS and referred for study. The Crapo calculation was used to convert FEV1 volume to percent predicted FEV1.37 All institutions obtained IRB approval, and all participants provided written informed consent. The study is registered at Clinicaltrials.gov as NCT01307462.
Study design
This was a phase II, single arm, open-label study. The primary endpoint was treatment failure by three months, defined as an absolute decline of the percent of predicted FEV1 by ≥10% from pre-enrollment (e.g. 40% to 30% predicted FEV1), confirmed by two PFTs performed at least two weeks apart. The selection of three months for the primary endpoint and six months for study completion was based on data published with similar evaluation periods in lung dysfunction after HCT and preliminary data showing that this timeframe was predictive of changes for the subsequent 1.5 years.22,23,38 If the 3-month PFT was missing, the 2 or 6-month PFT was used for endpoint assessment, with the worse test included in the analysis. If no 2 or 6-month test was available, the patient was considered a treatment failure in the primary analysis. A minimum of a 10% decline was used to define treatment failure because a decline of 8% over a 6–12 month period is clinically important.39 Treatment success was defined as lack of failure, i.e. less than 10% decline in absolute FEV1 percent predicted. Secondary endpoints include the safety of FAM and change in other PFT parameters, non-pulmonary chronic GVHD, six-minute walk test, and patient reported outcomes.
Upon study entry, all participants taking prednisone at <1mg/kg/day increased their dose to 1mg/kg/day orally for 2 weeks, unless contraindicated by other comorbidities. This brief steroid pulse was included due to the prevailing treatment recommendation of prednisone for newly diagnosed BOS; however, the intention of the study was to test the efficacy of FAM, and intolerance of high dose prednisone was not an exclusion criteria. After two weeks, participants started a taper of 0.25 mg/kg/day per week with a goal of attaining an equivalent dose of 0.25 mg/kg/day by 5 weeks after enrollment or the dose of corticosteroids at study entry, whichever was greater, unless higher doses were required by other chronic GVHD manifestations. This tapering schedule is much faster than usually used for BOS to attempt to minimize infectious complications and test the efficacy of FAM. Otherwise, steroids were tapered at the investigator’s discretion, taking into consideration other chronic GVHD manifestation severity. Corticosteroids could be tapered faster in cases of uncontrollable toxicity.
FAM treatment
Fluticasone: Inhaled fluticasone propionate, 440 mcg twice a day (ages 12–99 years) or 220 mcg twice a day (ages 6–11 years) was provided by GlaxoSmithKline to each participant for 6 months under grant 113611. Azithromycin: 250 mg orally for adults (19–99 years) and 5mg/kg orally (max 250mg) for children (6–18 years) taken three days per week was prescribed commercially. Montelukast: 10 mg oral tablet nightly (14–99 years old) or 5 mg oral nightly (6–13 years old) was provided by Merck.
Data Collection
Pulmonary function tests were performed at enrollment then at months 1, 2, 3 and 6 afterwards. If a patient had systemic or pulmonary infection at 3 months, the PFT could be deferred up to 8 weeks to allow recovery before testing since infections may temporarily alter PFT values. The 6-minute walk test was performed at enrollment and at 3 and 6 months. Other chronic GVHD manifestations were assessed at baseline, 3 months, and 6 months, using the 2014 NIH consensus response criteria.19,40 Toxicities were graded using CTCAE version 4.
Patients completed self-administered, validated surveys at enrollment, and at 3 and 6 months. The measures included the Medical Outcomes Study Short Form 36 (SF 36) and the Functional Assessment of Cancer Therapies Bone Marrow Transplant subscale (FACT-BMT).19,41 The SF36 is a 36 item, multi-dimensional, validated instrument that provides 8 subscales and two summary scales, a physical component scale (PCS) and a mental component scale (MCS). The FACT-BMT consists of 39 scored items with four subscales: Physical Well-being, Social/Family Well-being, Emotional Well-being, Functional Well-being, and a BMT-specific subscale. Functional status is measured using the 94-item Human Activity Profile (HAP). The Lee chronic GVHD symptom scale is a 30-item validated instrument that captures symptoms associated with chronic GVHD42. There are seven subscales and one summary scale.
Statistical Analyses
The target sample size was 40 evaluable patients, based on an 84% power to detect a 20% absolute improvement from 40% treatment failure to 20% treatment failure at 3 months, at the 1-sided 0.05 level of significance using an Exact test. The 40% treatment failure rate was estimated from retrospective data, derived from the published studies of BOS with graphs of FEV1 decline permitting evaluation of treatment failure over time. In these retrospective studies, patients were treated with similar immunosuppressive regimens including: steroids alone or with cyclosporine, azathioprine, anti-thymocyte globulin or thalidomide.13,14,43,44 None of these regimens included components of FAM or were deemed to be practice-changing in these retrospective analyses. Furthermore, although the exact inclusion criteria differ between historical studies and in this protocol, the majority of patients would fit criteria used in our trial. To establish a historical treatment failure rate, 111 patients in these 4 studies were reviewed. Of these, 45 (41%) experienced treatment failure defined as an absolute decline of the percent of predicted FEV1 by ≥10% according to criteria used in the current study.13,14,43,44
All participants who completed the baseline assessment and received at least one dose of FAM are included in the analysis. Enrollment was halted after enrollment of 36 patients due to funding limitations.
The proportions experiencing treatment failure at 3 and 6 months were calculated. Other PFT measurements, patient reported outcomes, steroid exposure, and 6 minute walk test were assessed for differences at 3 and 6 months on study compared to baseline using Wilcoxon signed rank tests with p<0.05 considered significant. Baseline steroid exposure was the dose taken after the burst (initiating drug), and was compared with the steroid dose at 3 and 6 months. Survival was estimated by the Kaplan-Meier method.
An interim analysis was conducted after 20 patients had completed 3 months of follow-up to assess for futility. Treatment-related mortality and grade 4 infections were monitored for safety on a continuous basis.
Results
Patient Demographic and Lung Function at Enrollment
Thirty-six patients from 10 institutions were prospectively enrolled in this protocol. The median age was 57 years (range 23–72), 47% were female, and most were Caucasian (92%) (Table 1). Most patients had HCT for treatment of acute leukemia or myelodysplastic syndrome (50%). Patients were enrolled at a median of 1.5 years after HCT (range 0.4–11). Most patients presented with moderate obstruction (median FEV1 46%, range 21%–71%; FEV1/VC 0.5 range 0.28–0.75) at enrollment, suggesting that despite a new diagnosis within the past 6 months, the cohort had already experienced significant loss of lung function at the time of enrollment. This was further characterized by generating a slope of FEV1 loss over time using all available PFTs from enrollment and earlier that showed a median slope of − 2.47 ml/day (range −0.36 to −11.99), which is much more severe than observed in another trial testing the single agent montelukast (NCT00656058) where the median was −1.42 ml/day (range 0 to −3.53). The one patient with a FEV1/VC ratio of 0.75, who would normally not have met diagnostic criteria on the basis of PFTs, had biopsy-confirmed BOS. Patients had a median NIH overall GVHD score of 2 (moderate), excluding consideration of the lung score.
Table.
Patient, Transplant and BOS characteristics (N=36)
| Characteristic | |
|---|---|
|
| |
| Age, median years (range) | 57 (24–72) |
|
| |
| Sex, n | |
| Male | 19 |
| Female | 17 |
|
| |
| Race/ethnicity, n | |
| Caucasian, non-Hispanic | 33 |
| Caucasian, Hispanic | 1 |
| African American | 2 |
| Unknown | 1 |
|
| |
| Disease, n | |
| Acute leukemia | 13 |
| Myelodysplastic syndrome | 5 |
| Chronic leukemia | 7 |
| Lymphoma | 4 |
| Other | 7 |
|
| |
| Time from transplant to BOS diagnosis, median year (range) | 1.5 (0.4–11.0) |
|
| |
| Enrollment FEV1% predicted, median (range) | 46% (21%–75%) |
|
| |
| FEV1/VC, median (range) | 0.5 (0.28–0.75) |
Abbreviations: BOS, bronchiolitis obliterans syndrome; FEV1, forced expiratory volume-first second; VC, vital capacity
Treatment response
Six percent of patients (n=2/36) experienced treatment failure with 10% or more absolute decline in percent predicted FEV1 within the first 3 months. This compares favorably to prior retrospective data (6% vs. 40%, p<0.001) (Figure 1a). In five cases, the worst of 2 or 6 month PFTs were used since the 3 month study was missing; of these, 1 was classified as a treatment failure and 4 were treatment successes. Among the 30 patients who were considered treatment successes based on available 3 month PFTs, 13 had an increase in the absolute percent predicted FEV1 of at least 5% and only six had a decrease of 5% or more in absolute FEV1%, while the remaining had a change of less than 5% (Figure 1b). To allow comparison with studies that defined response as absolute volume changes, our results corresponded to a 10% or more increase in absolute value of FEV1 (in L) of: 51% at 1 month (n=18/35), 34% at 2 months (n=10/29), 37% at 3 months, with less than 16% of patients having 10% or more decline at 1,2, and 3 months. Collectively, these data suggest stabilization of BOS disease progression for at least the first 3 months on study, with a stable FEV1 trajectory for the majority of patients (figure 2).
Figure 1. a and b: FAM stabilizes FEV1 in the majority of patients at 3 months.
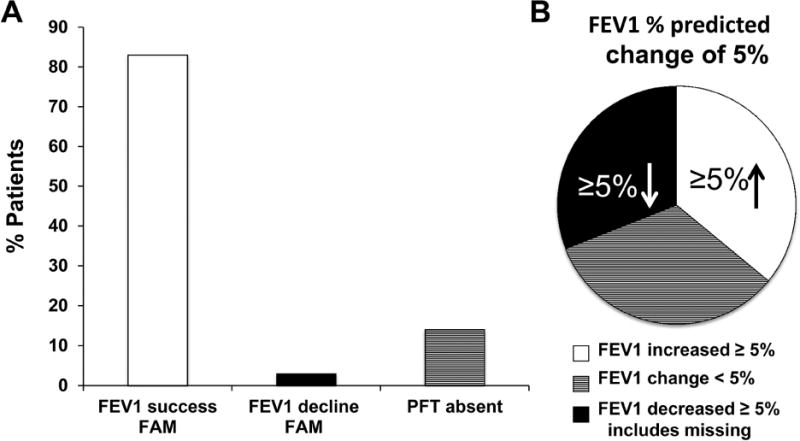
a) 83% of patients had study-defined documented success on 3 month PFTs, 3% percent had documented failure at 3 months (10% or more absolute decline in percent predicted FEV1) and 5 (14%) were missing 3 month PFTs. These 5 patients were later reclassified as 1 failure (no PFTs available at 2,3 or 6 months) and 4 successes (3 based on 2 month PFTs and 1 on the basis of 2 or 6 month PFTs. b) Proportion of patients (n=30 evaluable at 3 months) with greater or equal to 5% improvement, greater than or equal to 5% decline (and including those who were not able to be evaluated as these failures), or less than 5% change (stability).
Figure 2. Trajectory of FEV1 over time after FAM exposure.
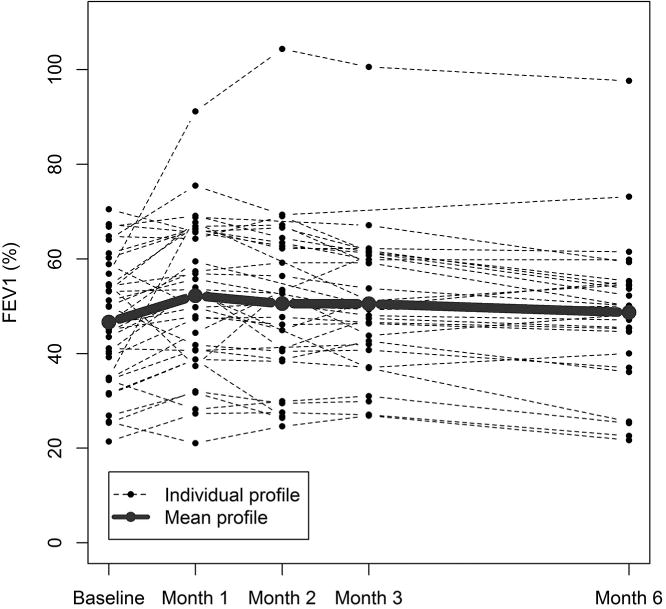
Individual percent predicted FEV1 values are graphed as a function of time for each patient. The bold line represents the median of the cohort.
After 6 months on study therapy, 29/36 patients completed PFTs of which 6/36 (17%) met study-defined criteria for treatment failure, and 7/36 (19%) did not complete PFTs. Of the 23 who had documented treatment success, three (10%) had an absolute improvement of more than 10% in percent predicted FEV1%, 7 (24%) had 5–10% absolute improvement, 5 (17%) had stable to 5% absolute increase, and 8 (28%) had 1–10% absolute deterioration. Again, recalculating our results as a percent change in absolute FEV1 volume, 31% of patients increased their absolute FEV1 (L) by 10% of greater, 41% were stable, and 28% experienced a greater than 10% decline. The 6 patients who declined by greater than 10% at 6 months all experienced an FEV1 decline by greater than 5% at 3 months. When patients who could not be evaluated were considered as treatment failures, treatment failure was 13/36 (36%) at 6 months.
No additional systemic treatments were added for 25 participants during the study, In 11 cases, new systemic treatments were added, 7 within the first 3 months (at 6 months, FEV1 was improved >10% in 2, 2 stable disease, 1 worse, 2 not evaluable), 4 had treatment between 3 and 6 months (at 6 months, FEV1 was worse in 2 and stable in 1). No patient received Etanercept.
Changes in other PFT parameters
We observed no significant changes in other PFT parameters such as FVC, TLC, RV, and DLCO from baseline to 3 or 6 months on study for the cohort.
Systemic steroid exposure
Steroid dose was reduced by at least 50% at 3 months in 13/27 (48%) patients who could be evaluated, with only 19% receiving added immunosuppression for non-pulmonary manifestations during that period (associated with <20% of participants experiencing treatment success). The initial steroid burst to greater than 0.75 mg/kg/day prednisone equivalent occurred in 52% of patients (n=17) and was associated with increase in FEV1 (by greater than 5% predicted at 3 and 6 months, p< 0.01); however, the subsequent rapid steroid taper was not associated with decline of FEV1. Smaller increases in steroids to <0.5 mg/kg/day (36%) or 0.5–0.75mg/kg/day (12%) were not associated with any discernable benefit in FEV1 though numbers were small. At 6 months, steroid dose was reduced by at least 50% in 17/24 (71%) patients who could be evaluated with only 4 additional patients receiving added immunosuppression between 3 and 6 months after enrollment (permitted for other chronic GVHD manifestations).
Other chronic GVHD manifestations
Using the 2014 response criteria but excluding consideration of the lung,40 overall response in all organs at 3 months was 9% CR, 27% PR, 24% stable, 39% PD in 33 patients who could be evaluated. Of the 34 patients who did not have FAM treatment failure at 3 months, overall responses were 9% CR, 28% PR, 22% SD, 41% PD and 2 were not evaluable. At 6 months, overall response was 14% CR, 45% PR, and 41% PD of 29 evaluable patients. Of the 23 who did not have FAM treatment failure at 6 months, overall responses were 18% CR, 41% PR, 41% PD, and 1 not evaluable. There were no clear differences in the overall chronic GVHD responses at 3 and 6 months between FAM treatment failures and treatment successes. In addition, CRs were observed in some organs involved with chronic GVHD but not in the lungs.
Patient-reported and functional outcomes
Patient-reported outcomes for the subgroup that completed the baseline and 3 month questionnaires (n=24, 23 treatment successes, 1 treatment failure) improved for SF-36 social functioning (median 5.45 point improvement, p=0.03), the mental component score (median 4.64 point improvement, p=0.02), and FACT emotional well-being (median 1 point improvement, p=0.03). In addition, patients reported less symptom severity using the Lee symptom scale in lung (median 10 point improvement, p=0.01), skin (median 0, p=0.03), mouth (median 0, p=0.03), and overall summary score (median 6.2 point improvement, p=0.001). The six-minute walk test for 26 patients tested at baseline and 3 months improved by a median of 114 feet at 3 months (p=0.01). Patients who completed 6 month questionnaires (n=24, 19 treatment successes, 4 treatment failures, 1 without 6 month PFTs) reported improved energy (median 7.1 point improvement, p=0.007), eye symptoms (median 8.3 point improvement, p=0.002), mouth symptoms (median 0, p=0.002), overall symptoms (median 7.8 point improvement, p<0.001), and had greater total distance walked (median 188 feet, p=0.02). Other patient-reported outcome parameters were not statistically different comparing baseline and 3 or 6 months.
Patient-reported and functional measures were tested for their correlation with changes in FEV1% at 3 months. There was a strong correlation between improvements in FEV1% and improvements in the SF-36 physical component score (p=0.009) and Human Activities Profile maximum (p=0.01) and adjusted (p<0.001) activity score. Reductions in steroid dose were associated with improvement in the Human Activities Profile adjusted activity score (p=0.01) but not with the walk test or any patient-reported outcomes.
We tested whether failure to complete 3 or 6 month surveys correlated with baseline factors. The baseline 6-minute walk test was shorter for patients who did not provide 3 month patient-reported outcomes (p=0.007) but no other differences were observed. Patients who did not complete 6 month patient-reported outcomes had worse baseline FEV1/VC (p=0.006).
FAM safety and tolerability
FAM was well tolerated, and all but one patient continued treatment with all 3 drugs until primary endpoint assessment at 3 months. One patient discontinued inhaled fluticasone and azithromycin prematurely but this was not attributed to adverse events. There was one death during the study period (6 months), which was not attributed to the study intervention, and one patient withdrew from the study. Adverse events were frequent. Those that were possibly, probably, or definitely attributed to study drug included: fever (n=1), pain (n=1), bronchial infection (n=1), lung infection (n=5, events 7), sepsis (n=1), cough (n=1), dyspnea (n=1), pneumonitis (n=1), pneumothorax (n=1), and other respiratory disorders (n=2). All but lung infections were single occurrences per patient. Of these, only 1 was a grade 4 adverse event (sepsis). Grade 3 events included bronchial infection (n=1), lung infection (n=4), dyspnea (n=1), pneumonitis (n=1), respiratory other disorder (n=1), and the remaining adverse events had grade 1 or 2 severity. One patient experienced hyperglycemia definitely related to the prednisone pulse but not attributed to FAM. Survival at 6 months was 97% (n=35/36, 95% CI 84%–100%).
Discussion
We present the results of a prospective multicenter study evaluating a novel regimen for the treatment of new onset BOS after HCT. In most patients, treatment with FAM and steroid burst stabilized lung function for at least the first 3 months, compared favorably to retrospective data, and was durable for 6 months. Therapy was well tolerated and permitted reduction in systemic steroid exposure, a risk factor for life-threatening infections in BOS patients. FAM was safe with adverse events consistent with expected number and severity. Several patient-reported outcomes improved consistent with improvement in functional ability following FAM therapy, including the 6-minute walk test and Lee symptom scale. While these data are hindered by lack of a comparator group and missing data that may enrich for FAM responders, they are consistent with the improvements in objective measurements that have been verified in prior studies of chronic GVHD (PFTs and walk tests).41 While FAM was designed to target lung GVHD, two of the agents may also treat other organs, with global decrease in IL-8 from azithromycin exposure, and decrease in leukotriene production (which is produced in many affected organs) through montelukast suppression, which had been previously published.32 Overall NIH calculated chronic GVHD responses and organ specific responses showed stable disease for more than half of the patients evaluated, using the 2014 scoring criteria, suggesting that this combination of agents did not exacerbate GVHD in other organs and may have even contributed to response.
Few prospective studies have focused on BOS after HCT perhaps due to its rarity and high morbidity.13,45–49 While the optimal evaluation of this treatment regimen would have been a randomized comparison, the low incidence and high morbidity (which meant a control group was not acceptable to treating physicians) precluded this study design. In fact, it was challenging to accrue and complete this prospective study in an acceptable time frame as FAM is easily prescribed in the community. In addition, while ideally these data would be compared to a BOS population not receiving FAM in the modern supportive care era, this was precluded given both the recent modifications in diagnostic criteria (restricting use of CIBMTR registries) and the rarity of disease (limiting single center data). Direct comparisons with prior retrospective single patient and case series data with standard therapies (steroids, calcineurin inhibitors) and novel therapies (e.g. statins, extracorporeal photopheresis) are limited by: differing definitions for BOS (and thus heterogeneous inclusion criteria), small numbers of patients per study with most including fewer than 10 patients per treatment group, differences in outcome variables, differences in disease progression, and confounding by concurrent changes in other immunosuppressive agents.16,17,44 A previous retrospective single institution study by the authors who initiated this study suggested that FAM may provide benefit and lower systemic steroids, though again this was limited by small patient numbers (n=8) and retrospective study design.24 Two recent prospective studies have been published. While the enrollment criteria are different, comparisons may be made using the response criteria of these studies, which is in absolute value of FEV1 in liters. The first trial evaluated etanercept to treat both BOS and restrictive lung disease and showed a 33% response for patients with obstructive lung disease using 10% change in FEV1 absolute value within 1–4 months; our data are similar to this with 31–51% of patients showing 10% improvement within this time frame.38 The second study evaluated FEV1 response to inhaled budesonide/formoterol, in a prospective, randomized, placebo controlled, cross-over design, that showed benefit (12% increase in FEV1) for 62% of patients in the treated arm at 1 month.22 While our data showed only 51% of patients had a 10% improvement at 1 month in FEV1, the median FEV1 percent predicted at enrollment in our study reflects greater severity of BOS disease (median FEV1 46%, range 21%–71%) compared to patients randomized to the treatment arm (median FEV1 66%, range 61–71%).22 In addition, only 56% of those patients completed the 6 month endpoint as compared to 81% of our population, suggesting that FAM was tolerable in terms of toxicity and response rate.22 Finally, our data compare favorably to the placebo arm of the Bergeron trial, in which 25% of patients experienced benefit in the placebo group, which had more mild BOS than our cohort.22 Finally, another single agent, open label study evaluated montelukast (part of FAM) in 25 patients with established BOS and showed stabilization with less than 15% decline in FEV1 for the entire cohort over 6 months of treatment, with similar outcomes.23
Our data compare favorably with these more recent studies, especially in light of the rapidly progressive onset (with median percent predicted FEV1 of 46% and severe slope at baseline), which has been shown to confer the highest mortality and greatest likelihood of rapidly progressive BOS.13 This rate of decline is nearly double the decline of the single agent montelukast study23, documenting the higher severity of pulmonary involvement in our study cohort, which was also true in the budesonide/fometerol trial22. Furthermore, more recent, retrospective data from a larger number of patients has shown improvement in the survival of patients with BOS from 20% to 50–60% at 5 years, which while it includes advances in supportive care, also now incorporates the agents tested in this prospective trial and previously presented in our publications.1,4,6,24
Strengths of our study include the prospective design, central eligibility review, standardized diagnostic and restaging criteria, intervention early in the disease process, and assessment of both objective and subjective outcomes. Limitations of this study include the open-label, single-arm study design, missing patient-reported and PFT data, limited follow-up, and use of retrospective data as a comparator group.50 Randomization was not feasible because the disease is rare and confers high morbidity and mortality causing physicians to be unwilling to enroll on a study with a placebo control. In addition, this study did not attempt to differentiate the effects of the FAM regimen from those achieved with the single agents or steroids alone, rather to determine if the addition of FAM could stabilize disease during steroid taper. Given the small numbers and single arm design, these data would need to be confirmed in a larger study and ideally with randomization, perhaps to a wait control or to other agents, which may be possible as the number of HCTs and patients with chronic GVHD is increasing.
Multiple studies have shown that decline in lung function is the greatest risk factor for death in patients with chronic GVHD after HCT.2,14,49 BOS after HCT confers a dismal prognosis,13–15 both due to progressive lung failure from immune attack and infections secondary to increased immunosuppression.1 The high risk of lung infections and premature death in patients with BOS highlights the need to develop more effective and less toxic treatments. Our results suggest that the progression of BOS can be halted at least temporarily for most patients by using a nontoxic regimen of inhaled fluticasone, azithromycin, and montelukast, while decreasing the dosing of systemic steroid treatment. Our data support future studies to test FAM earlier in the progression to obstruction or in larger studies to evaluate efficacy in conjunction with other promising agents.
Highlights.
Bronchiolitis obliterans syndrome (BOS) after hematopoietic cell transplantation is a rare complication with high morbidity and mortality.
This is a single arm, multicenter phase II study of 36 patients with newly diagnosed BOS treated with fluticasone propionate, azithromycin, and montelukast (FAM)
FAM was well tolerated
FAM with steroid pulse is associated with stable lung function and improved functional and patient-reported outcomes for most newly diagnosed patients with BOS
Acknowledgments
Financial disclosure: The Chronic GVHD Consortium (U54 CA163438) is part of the NCATS Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Disease Research (ORDR), NCATS, funded through a collaboration between NCATS and the National Cancer Institute. GlaxoSmithKline provided fluticasone propionate under grant number 113611, and Merck provided montelukast.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship statement: All authors designed the study, collected and analyzed data, and wrote the manuscript. BS and XC performed the statistical analysis and edited the manuscript. All authors critically revised the manuscript for important intellectual content and approve the manuscript for publication.
Authors’ Conflicts of Interest Disclosure: There are no conflicts of interest to report.
References
- 1.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. Jama. 2009;302(3):306–314. doi: 10.1001/jama.2009.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer J, Williams K, Inamoto Y, et al. Pulmonary symptoms measured by the national institutes of health lung score predict overall survival, nonrelapse mortality, and patient-reported outcomes in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(3):337–344. doi: 10.1016/j.bbmt.2013.11.025. Prepublished on 2013/12/10 as DOI 10.1016/j.bbmt.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sengsayadeth SM, Srivastava S, Jagasia M, Savani BN. Time to explore preventive and novel therapies for bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(10):1479–1487. doi: 10.1016/j.bbmt.2012.03.008. Prepublished on 2012/03/28 as DOI 10.1016/j.bbmt.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation-an increasingly recognized manifestation of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S106–114. doi: 10.1016/j.bbmt.2009.11.002. Prepublished on 2009/11/10 as DOI S1083-8791(09)00519-9 [pii]10.1016/j.bbmt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Au BK, Au MA, Chien JW. Bronchiolitis Obliterans Syndrome Epidemiology After Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2010 doi: 10.1016/j.bbmt.2010.11.018. Prepublished on 2010/12/04 as DOI S1083-8791(10)00517-3 [pii]10.1016/j.bbmt.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergeron A, Godet C, Chevret S, et al. Bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: phenotypes and prognosis. Bone Marrow Transplant. 2013;48(6):819–824. doi: 10.1038/bmt.2012.241. Prepublished on 2012/12/05 as DOI bmt2012241 [pii]10.1038/bmt.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson PA, Lim A, Panek-Hudson Y, et al. Screening with spirometry is a useful predictor of later development of noninfectious pulmonary syndromes in patients undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(6):781–786. doi: 10.1016/j.bbmt.2014.02.011. Prepublished on 2014/02/20 as DOI 10.1016/j.bbmt.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Husain AN, Siddiqui MT, Holmes EW, et al. Analysis of risk factors for the development of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 1999;159(3):829–833. doi: 10.1164/ajrccm.159.3.9607099. Prepublished on 1999/03/02 as DOI 10.1164/ajrccm.159.3.9607099. [DOI] [PubMed] [Google Scholar]
- 9.Nicod LP. Mechanisms of airway obliteration after lung transplantation. Proc Am Thorac Soc. 2006;3(5):444–449. doi: 10.1513/pats.200601-007AW. [DOI] [PubMed] [Google Scholar]
- 10.Girnita AL, Duquesnoy R, Yousem SA, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005;5(1):131–138. doi: 10.1111/j.1600-6143.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- 11.Panoskaltsis-Mortari A, Tram KV, Price AP, Wendt CH, Blazar BR. A new murine model for bronchiolitis obliterans post-bone marrow transplant. Am J Respir Crit Care Med. 2007;176(7):713–723. doi: 10.1164/rccm.200702-335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivasan M, Flynn R, Price A, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119(6):1570–1580. doi: 10.1182/blood-2011-07-364414. Prepublished on 2011/11/11 as DOI blood-2011-07-364414 [pii]10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark JG, Crawford SW, Madtes DK, Sullivan KM. Obstructive lung disease after allogeneic marrow transplantation. Clinical presentation and course. Ann Intern Med. 1989;111(5):368–376. doi: 10.7326/0003-4819-111-5-368. [DOI] [PubMed] [Google Scholar]
- 14.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biology of Blood & Marrow Transplantation. 2003;9(10):657–666. doi: 10.1016/s1083-8791(03)00242-8. [DOI] [PubMed] [Google Scholar]
- 15.Nishio N, Yagasaki H, Takahashi Y, et al. Late-onset non-infectious pulmonary complications following allogeneic hematopoietic stem cell transplantation in children. Bone Marrow Transplant. 2009;44(5):303–308. doi: 10.1038/bmt.2009.33. Prepublished on 2009/04/08 as DOI bmt200933 [pii]10.1038/bmt.2009.33. [DOI] [PubMed] [Google Scholar]
- 16.Lucid CE, Savani BN, Engelhardt BG, et al. Extracorporeal photopheresis in patients with refractory bronchiolitis obliterans developing after allo-SCT. Bone Marrow Transplant. 2011;46(3):426–429. doi: 10.1038/bmt.2010.152. Prepublished on 2010/06/29 as DOI 10.1038/bmt.2010.152. [DOI] [PubMed] [Google Scholar]
- 17.Hildebrandt GC, Fazekas T, Lawitschka A, et al. Diagnosis and treatment of pulmonary chronic GVHD: report from the consensus conference on clinical practice in chronic GVHD. Bone Marrow Transplant. 2011;46(10):1283–1295. doi: 10.1038/bmt.2011.35. Prepublished on 2011/03/29 as DOI bmt201135 [pii]10.1038/bmt.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.12.001. Prepublished on 2014/12/23 as DOI 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Yanik GA, Horowitz MM, Weisdorf DJ, et al. Randomized, double-blind, placebo-controlled trial of soluble tumor necrosis factor receptor: enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: blood and marrow transplant clinical trials network protocol. Biol Blood Marrow Transplant. 2014;20(6):858–864. doi: 10.1016/j.bbmt.2014.02.026. Prepublished on 2014/03/13 as DOI 10.1016/j.bbmt.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam DC, Lam B, Wong MK, et al. Effects of azithromycin in bronchiolitis obliterans syndrome after hematopoietic SCT–a randomized double-blinded placebo-controlled study. Bone Marrow Transplant. 2011;46(12):1551–1556. doi: 10.1038/bmt.2011.1. Prepublished on 2011/02/15 as DOI bmt20111 [pii]10.1038/bmt.2011.1. [DOI] [PubMed] [Google Scholar]
- 22.Bergeron A, Chevret S, Chagnon K, et al. Budesonide/Formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2015;191(11):1242–1249. doi: 10.1164/rccm.201410-1818OC. Prepublished on 2015/04/04 as DOI 10.1164/rccm.201410-1818OC. [DOI] [PubMed] [Google Scholar]
- 23.Kirsten M, Williams SZP, Stephanie Lee, Paul Martin, Candice Cottle-Delisle, Frances T Hakim, Beryl Manning-Geiss, Sandra A Mitchell, Juan C Gea-Banacloche, Leora Comis, Edward W Cowen, Kristin Baird, James H Shelhamer, Daniel Fowler, Bazetta AJ Blacklock-Schuver, Daniele Avila, Ronald E Gress. Interim Analysis of a Phase II Trial of Montelukast for the Treatment of Bronchiolitis Obliterans Syndrome after HSCT reveal Immunobiology of Disease. Biology of Blood & Marrow Transplantation. 2013:S109–S392. [Google Scholar]
- 24.Norman BC, Jacobsohn DA, Williams KM, et al. Fluticasone, azithromycin and montelukast therapy in reducing corticosteroid exposure in bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: a case series of eight patients. Bone Marrow Transplant. 2010 doi: 10.1038/bmt.2010.311. Prepublished on 2010/12/07 as DOI bmt2010311 [pii]10.1038/bmt.2010.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khalid M, Al Saghir A, Saleemi S, et al. Azithromycin in bronchiolitis obliterans complicating bone marrow transplantation: a preliminary study. Eur Respir J. 2005;25(3):490–493. doi: 10.1183/09031936.05.00020804. [DOI] [PubMed] [Google Scholar]
- 26.Bashoura L, Gupta S, Jain A, et al. Inhaled corticosteroids stabilize constrictive bronchiolitis after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41(1):63–67. doi: 10.1038/sj.bmt.1705877. [DOI] [PubMed] [Google Scholar]
- 27.Gerhardt SG, McDyer JF, Girgis RE, Conte JV, Yang SC, Orens JB. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Respir Crit Care Med. 2003;168(1):121–125. doi: 10.1164/rccm.200212-1424BC. [DOI] [PubMed] [Google Scholar]
- 28.Gottlieb J, Szangolies J, Koehnlein T, Golpon H, Simon A, Welte T. Long-term azithromycin for bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2008;85(1):36–41. doi: 10.1097/01.tp.0000295981.84633.bc. [DOI] [PubMed] [Google Scholar]
- 29.Yates B, Murphy DM, Forrest IA, et al. Azithromycin reverses airflow obstruction in established bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2005;172(6):772–775. doi: 10.1164/rccm.200411-1537OC. [DOI] [PubMed] [Google Scholar]
- 30.Medoff BD, Seung E, Wain JC, et al. BLT1-mediated T cell trafficking is critical for rejection and obliterative bronchiolitis after lung transplantation. J Exp Med. 2005;202(1):97–110. doi: 10.1084/jem.20042481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilborn J, Bailie M, Coffey M, Burdick M, Strieter R, Peters-Golden M. Constitutive activation of 5-lipoxygenase in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1996;97(8):1827–1836. doi: 10.1172/JCI118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Or R, Gesundheit B, Resnick I, et al. Sparing effect by montelukast treatment for chronic graft versus host disease: a pilot study. Transplantation. 2007;83(5):577–581. doi: 10.1097/01.tp.0000255575.03795.df. [DOI] [PubMed] [Google Scholar]
- 33.Bergeron A, Belle A, Chevret S, et al. Combined inhaled steroids and bronchodilatators in obstructive airway disease after allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39(9):547–553. doi: 10.1038/sj.bmt.1705637. Prepublished on 2007/03/14 as DOI 10.1038/sj.bmt.1705637. [DOI] [PubMed] [Google Scholar]
- 34.Whitford H, Orsida B, Kotsimbos T, et al. Bronchoalveolar lavage cellular profiles in lung transplantation: the effect of inhaled corticosteroids. Ann Transplant. 2000;5(3):31–37. Prepublished on 2001/01/09 as DOI. [PubMed] [Google Scholar]
- 35.Whitford H, Walters EH, Levvey B, et al. Addition of inhaled corticosteroids to systemic immunosuppression after lung transplantation: a double-blind, placebo-controlled trial. Transplantation. 2002;73(11):1793–1799. doi: 10.1097/00007890-200206150-00016. Prepublished on 2002/06/27 as DOI. [DOI] [PubMed] [Google Scholar]
- 36.Maimon N, Lipton JH, Chan CK, Marras TK. Macrolides in the treatment of bronchiolitis obliterans in allograft recipients. Bone Marrow Transplant. 2009;44(2):69–73. doi: 10.1038/bmt.2009.106. Prepublished on 2009/05/12 as DOI 10.1038/bmt.2009.106. [DOI] [PubMed] [Google Scholar]
- 37.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123(6):659–664. doi: 10.1164/arrd.1981.123.6.659. Prepublished on 1981/06/01 as DOI. [DOI] [PubMed] [Google Scholar]
- 38.Yanik GA, Mineishi S, Levine JE, et al. Soluble tumor necrosis factor receptor: enbrel (etanercept) for subacute pulmonary dysfunction following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(7):1044–1054. doi: 10.1016/j.bbmt.2011.11.031. Prepublished on 2011/12/14 as DOI 10.1016/j.bbmt.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang ML, Petsonk EL. Repeated measures of FEV1 over six to twelve months: what change is abnormal? J Occup Environ Med. 2004;46(6):591–595. doi: 10.1097/01.jom.0000128159.09520.2a. Prepublished on 2004/06/24 as DOI. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, Wolff D, Kitko C, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant. 2015;21(6):984–999. doi: 10.1016/j.bbmt.2015.02.025. Prepublished on 2015/03/23 as DOI 10.1016/j.bbmt.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee SJ, Kim HT, Ho VT, et al. Quality of life associated with acute and chronic graft-versus-host disease. Bone Marrow Transplant. 2006;38(4):305–310. doi: 10.1038/sj.bmt.1705434. Prepublished on 2006/07/05 as DOI 10.1038/sj.bmt.1705434. [DOI] [PubMed] [Google Scholar]
- 42.Lee S, Cook EF, Soiffer R, Antin JH. Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8(8):444–452. doi: 10.1053/bbmt.2002.v8.pm12234170. [DOI] [PubMed] [Google Scholar]
- 43.Philit F, Wiesendanger T, Archimbaud E, Mornex JF, Brune J, Cordier JF. Post-transplant obstructive lung disease (“bronchiolitis obliterans”): a clinical comparative study of bone marrow and lung transplant patients. Eur Respir J. 1995;8(4):551–558. [PubMed] [Google Scholar]
- 44.Sanchez J, Torres A, Serrano J, et al. Long-term follow-up of immunosuppressive treatment for obstructive airways disease after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997;20(5):403–408. doi: 10.1038/sj.bmt.1700894. [DOI] [PubMed] [Google Scholar]
- 45.Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168(2):208–214. doi: 10.1164/rccm.200212-1468OC. [DOI] [PubMed] [Google Scholar]
- 46.Holland HK, Wingard JR, Beschorner WE, Saral R, Santos GW. Bronchiolitis obliterans in bone marrow transplantation and its relationship to chronic graft-v-host disease and low serum IgG. Blood. 1988;72(2):621–627. [PubMed] [Google Scholar]
- 47.Duell T, van Lint MT, Ljungman P, et al. Health and functional status of long-term survivors of bone marrow transplantation. EBMT Working Party on Late Effects and EULEP Study Group on Late Effects. European Group for Blood and Marrow Transplantation. Ann Intern Med. 1997;126(3):184–192. doi: 10.7326/0003-4819-126-3-199702010-00002. Prepublished on 1997/02/01 as DOI. [DOI] [PubMed] [Google Scholar]
- 48.Curtis DJ, Smale A, Thien F, Schwarer AP, Szer J. Chronic airflow obstruction in long-term survivors of allogeneic bone marrow transplantation. Bone Marrow Transplant. 1995;16(1):169–173. [PubMed] [Google Scholar]
- 49.Marras TK, Chan CK, Lipton JH, Messner HA, Szalai JP, Laupacis A. Long-term pulmonary function abnormalities and survival after allogeneic marrow transplantation. Bone Marrow Transplantation. 2004;33(5):509–517. doi: 10.1038/sj.bmt.1704377. [DOI] [PubMed] [Google Scholar]
- 50.Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant. 2006;12(3):252–266. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]


