Abstract
Background
The assessment of nociception in preclinical studies is undergoing a transformation from pain-evoked to pain-depressed tests to more closely mimic the effects of clinical pain. Many inflammatory pain-depressed behaviors (reward seeking, locomotion) have been examined, but these tests are limited because of confounds such as stress and difficulties in quantifying behavior.
New Method
The present study evaluates home cage wheel running as an objective method to assess the magnitude and duration of inflammatory pain in male and female rats.
Results
Injection of Complete Freund’s Adjuvant (CFA) into the right hindpaw to induce inflammatory pain almost completely inhibited wheel running for 2 days in males and females. Wheel running gradually returned to baseline levels within 12 days despite persistent mechanical hypersensitivity (von Frey test).
Comparison with Existing Methods
Continuously monitoring home cage wheel running improves on previous studies examining inflammatory pain-depressed wheel running because it is more sensitive to noxious stimuli, avoids the stress of removing the rat from its cage for testing, and provides a complete analysis of the time course for changes in nociception.
Conclusions
The present data indicate that home cage wheel running is a clinically relevant method to assess inflammatory pain in the rat. The decrease in activity caused by inflammatory pain and subsequent gradual recovery mimics the changes in activity caused by pain in humans. The tendency for pain-depressed wheel running to be greater in female than male rats is consistent with the tendency for women to be at greater risk of chronic pain than men.
Keywords: pain-depressed behavior, inflammatory pain, sex differences, wheel running, nociception
1. Introduction
In humans, pain is accompanied by functional impairment and depression of physical behavior. Pain-depressed behaviors can also be measured in rodents as a decrease in rate, frequency, or intensity in response to a noxious stimulus or pain state (Negus et al., 2006; Negus and Altarifi, 2013). Several tests of pain-depressed behaviors in rodents have been developed. These include pain-depressed reductions in feeding/drinking (Kwilasz and Negus, 2012; Stevenson et al., 2006), intracranial self-stimulation (ICSS) (Negus, 2013), overall locomotor activity (Matson et al., 2007; Stevenson et al., 2009), burrowing (Andrews et al., 2012; Rutten et al., 2014), nesting (Negus et al., 2015), and attention (Freitas et al., 2015). Although these tests mimic the effects of clinical pain in many ways, they are limited by assessment of nociception over a constrained time, assessment outside the home cage, and/or difficulty quantifying the duration and magnitude of pain.
Most of these problems can be overcome by examining pain-depressed wheel running. Wheel running is a natural behavior that is both easy to quantify and similar to the reduction in activity that occurs in chronic pain patients (Tierney et al., 2012). Previous studies suggest that depression of wheel running can be used to assess nociception, but that a relatively intense noxious stimulus is needed (i.e., inflammation of both hindpaws) (Cobos et al., 2012, Grace et al., 2014). However, these studies did not mimic the human pain condition because the animals had limited access to the running wheel, whereas human activity is not limited. We hypothesize that allowing continuous access to the running wheel in the rat’s home cage will more accurately mimic the human pain condition and provide a more sensitive measure of nociception.
Female rats and mice have been shown to be more sensitive to noxious chemical, heat, and electrical stimuli than their male counterparts (Wiesenfeld-Hallin, 2005). The consequences of this greater sensitivity to pain on active behaviors are unclear. Despite the greater prevalence of chronic pain in women (Greenspan et al., 2007), we are aware of only one study of pain-depressed behavior that included female rats and this study did not analyze these data separately from male rats (Miller et al., 2011). The present study addresses this problem by assessing pain-depressed wheel running in both male and female rats.
The purpose of the present study was to continuously monitor home cage wheel running to assess inflammatory pain in the same manner that pain disrupts activity in humans. The effects of gender and duration of baseline exposure to the wheel were evaluated to provide a comprehensive analysis of the reliability and validity of home cage wheel running as a clinically relevant method to assess inflammatory pain in male and female rats.
2. Materials and Methods
2.1 Subjects
Data were collected from adult male and female Sprague-Dawley rats bred at Washington State University Vancouver (Vancouver, WA, USA). All rats were 50-90 days old (median = 71 days old) at the start of the study and randomly assigned to treatment groups. Both CFA- and saline-treated rats were included during each week of testing. Each rat was only used in one experiment and was euthanized at the end of the study. Prior to experimentation, rats were housed in pairs in a 22-24 °C colony room on a 12/12-hour light/dark cycle. Each rat was moved to a separate cage with a running wheel in a sound-attenuating booth (2.1 × 2.2 m; Industrial Acoustics Company, Inc., Bronx, NY, USA) to begin data collection. Food and water were available ad libitum except when the rat was removed from the cage to assess mechanical allodynia, habituate to the testing box, or induce inflammation. All procedures were approved by the Washington State University Animal Care and Use Committee and conducted in accordance with the International Association for the Study of Pain’s Policies on the Use of Animals in Research.
2.2 Running wheel
A Kaytee Run-Around Giant Exercise Wheel (Kaytee Products, Inc., Chilton, WI, USA) with a diameter of 27.9 cm was taped to the bottom of the rat’s home cage (Fig. 1A). The floor of the cage was covered with cellulose bedding (BioFresh™, Ferndale, WA, USA). A 0.8 mm thick aluminum plate (5.08 cm × 3.81 cm; K&S Precision Metals, Chicago, IL, USA) was attached to one spoke of the running wheel (Fig. 1B) to interrupt a photobeam projecting across the cage with each rotation. The photobeam was set 18 cm above the floor of the cage so that only the rotation of the wheel, not the normal activity of the rat, would interrupt the beam. The number of wheel revolutions were summed over 5 min bins for 23 hrs each day (1000h to 0900h in Experiment 1; 1700h to 1600h in Experiment 2) using PAS software (San Diego Instruments, San Diego, CA, USA). Recording was suspended for one hour to conduct the pain-evoked test.
Figure 1. Wheel running apparatus.
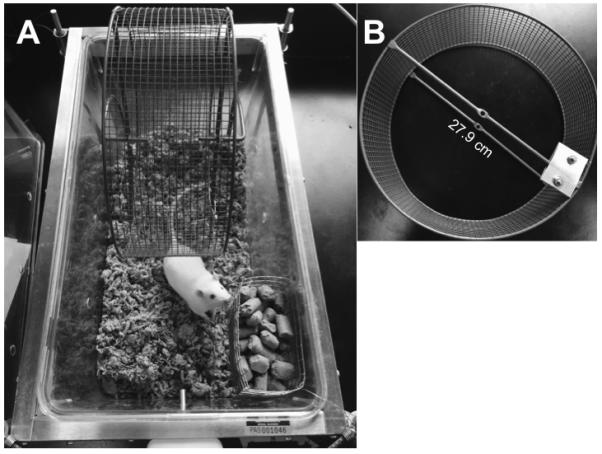
(A) A rodent running wheel (diameter = 27.9 cm) was taped to the bottom of a standard rat cage containing cellulose bedding. Food and water was available ad libitum. The rat had unrestricted access to the wheel with room to move and nest behind, underneath, and to the sides of the wheel. (B) An aluminum plate was attached to the end of one spoke of the wheel to interrupt a photobeam used to count wheel rotations.
2.3 Experiment 1: Three-day acquisition period
A total of 21 male and 12 female rats were used in this experiment. Rats were allowed unrestricted access to the wheel for 23 hr/day (1000 h to 0900 h) for three days prior to induction of inflammation. Rats were removed from their home cage for 50 min each day (0900 h to 0950 h) to test them on pain-evoked tests. Mechanical sensitivity was assessed using an electronic von Frey anesthesiometer (IITC Inc., ALMEMO® 2450, Woodland Hills, CA) because this is the most common test to assess inflammatory pain (Muley et al., 2016). The rat was placed in a Plexiglas chamber (22 cm × 22 cm × 12.8 cm) on an elevated mesh surface and allowed to habituate for approximately 15 min. Baseline von Frey measurements from both hindpaws were obtained after 3 days of baseline running (just before induction of inflammation). The threshold at which a rat withdrew its hindpaw when the von Frey filament was applied to the plantar surface of the hindpaw was recorded in grams. Each paw was tested 3 times with approximately one minute separating each trial. The mean of 3 trials/hindpaw was calculated.
The number of wheel revolutions that occurred during the 23 hrs prior to induction of hindpaw inflammation was used as the baseline measurement. At the end of the third day, the rat was removed from its home cage, briefly anesthetized with isoflurane, and injected with Complete Freund’s Adjuvant (Sigma-Aldrich, St. Louis, MO, USA; CFA; 0.1 mL) into the right hindpaw using a 30-gauge needle. Control animals were anesthetized and injected with saline (Hospira Inc, Lake Forest, IL, USA) into the right hindpaw. The rat was returned to its home cage at 0950 h and wheel running was measured as before (23 hrs/day from 1000 h to 0900 h) for 3 days.
2.4 Experiment 2: 8-day acquisition period
A total of 24 male and 30 female rats were used in this experiment. The methods were identical to Experiment 1 except that rats were allowed unrestricted access to the wheel for 8 days prior to induction of inflammation, and the start of the 23 h recording period was moved from 1000 h to 1700 h so it more closely preceded the active dark phase (1800 h to 0600 h). These modifications were made to optimize assessment of pain depressed wheel running, which takes time to develop and occurs mostly at night. This experiment also included naïve rats that were anesthetized like rats injected with CFA or saline in the paw, but did not receive a hindpaw injection. The von Frey test and the injection of CFA occurred between 1600 h and 1650 h. Both wheel running and von Frey thresholds were measured for three days following injection of CFA or saline into the paw. A subset of rats was monitored for 14 days to provide a more complete time course for recovery.
2.6 Data analysis
All data are expressed as mean ± SEM except where stated. Total wheel revolutions during the 23 hrs preceding injection of CFA was used as the baseline value. Given individual differences in wheel running, all wheel running data are presented as a percent change from each rat’s baseline value. Analysis of baseline running values revealed a bimodal distribution with most rats significantly over 400 revolutions, and a small subset under 400 revolutions. Given that depression of wheel running cannot be measured unless running levels are relatively high, rats with a baseline wheel running value under 400 revolutions in the 23 hrs preceding injection of CFA were not included in data analysis. The percent change in baseline wheel running following CFA or saline administration was analyzed using a 2-way repeated measures ANOVA (CFA/saline × day). CFA did not alter withdrawal thresholds in the uninjected (left) paw so only data obtained from the injected (right) paw are presented. The mean thresholds for the von Frey test were analyzed using a 2-way ANOVA (CFA/saline × day). Statistical significance was defined as a probability of < 0.05.
3. Results
3.1 Experiment 1: Three-day acquisition period
Wheel running was assessed 23 hrs a day beginning at 1000 h in naïve male and female rats. A burst of running occurred in the hour after the rat was returned to its cage, but most wheel running occurred during the dark phase (1800 h to 0600 h) of the light cycle. This running pattern was consistent across rats, although female rats were consistently more active than male rats (Fig. 2). In fact, 10 of 21 male rats did not reach the criterion of 400 revolutions/day, whereas only 3 of the 12 female rats did not reach the criterion (Table 1). The median revolutions for all male and female rats were 455 (Range = 18 - 1463) and 2094 (Range = 31 - 4629), respectively. Subsequent data analysis focused on the 11 male and 9 female rats that exceeded the 400 revolution criterion on the baseline day.
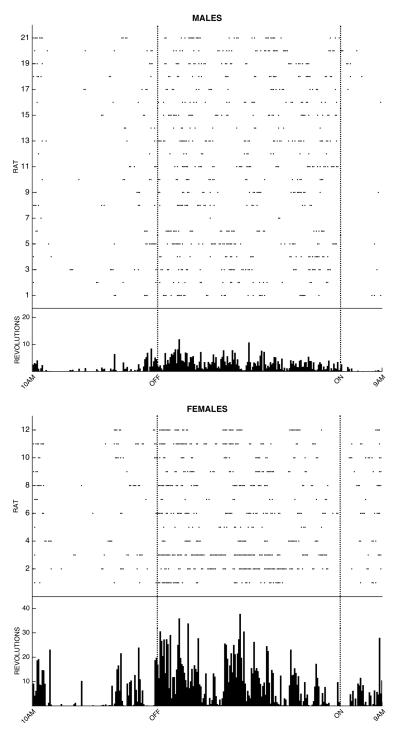
Figure 2. Individual patterns of wheel running for male and female rats on Day 3 of baseline acquisition.
Each dot represents at least one wheel rotation during the 5 min bin for each of the 21 male and 12 female rats. All bins are shown for the 23 hrs preceding injection of CFA. Mean activity for the 21 male and 12 female rats tested (see graphs at the top) is displayed in the histogram at the bottom of the figure. Only 11 of the 21 male rats and 9 of the 12 female rats met the 400 revolution criterion for inclusion in the experiment. Although both male and female rats were most active during the 12 hr dark phase (start of the dark phase is indicated by the dashed line labeled OFF), female rats were consistently more active than male rats.
Table 1.
Percentage of animals that reached the criterion of 400 wheel revolutions following 3- or 8-day acquisition periods
| 3-Day Acquisition Period | 8-Day Acquisition Period | |
|---|---|---|
| Males | 52.4% (11/21) | 100% (24/24) |
| Females | 75.0% (9/12) | 100% (30/30) |
Administration of CFA produced a near complete depression of wheel running in both male and female rats in the 23-hour period post-injection (Fig. 3). Wheel running was significantly lower in both male (F(1,9) = 17.23, p = .002) and female (F(1,7) = 40.01, p < .001) rats with hindpaw inflammation compared to saline-treated control rats. A significant increase in wheel running occurred in male rats with each passing day (F (2,18) = 21.62, p < .001), whereas the modest increase in wheel running in female rats across days was not significant (F(2,14) = 3.07, p = .078). Wheel running in male rats had returned to almost baseline levels on the third day after CFA administration.
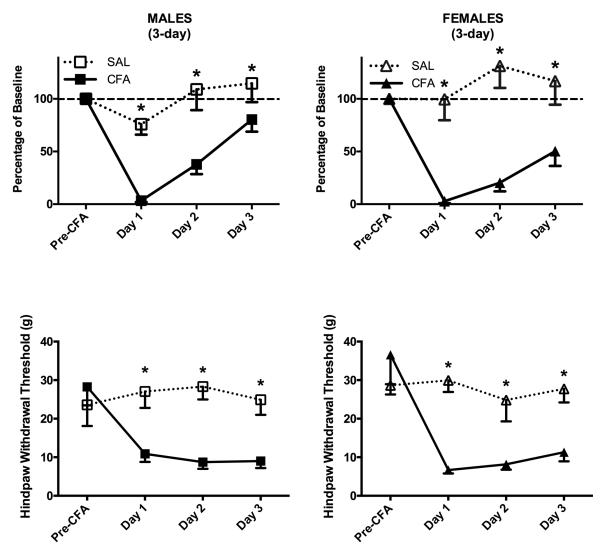
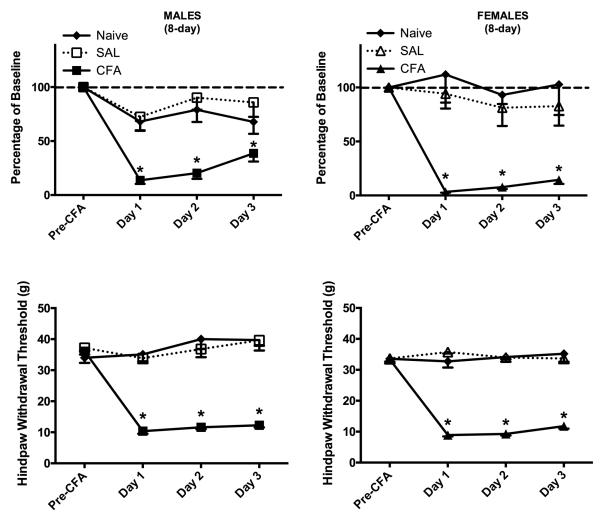
Figure 3. CFA-induced hindpaw inflammation produced depression of wheel running and mechanical allodynia in male and female rats following a 3-day acquisition period.
Top: CFA was injected into the right hindpaw of male and female rats after 3 days of continuous exposure to the wheel in the rat’s home cage. This unilateral inflammation of the hindpaw almost completely inhibited wheel running in male (n = 6) and female (n = 5) rats in the 23 hrs following CFA administration compared to saline-treated control rats (5 male rats; 4 female rats). Wheel running gradually recovered in CFA-treated male and female CFA-treated rats by Day 3. Bottom: Unilateral hindpaw inflammation decreased mechanical withdrawal thresholds in male and female rats compared to non-inflamed control rats. Unlike wheel running, there was no recovery in mechanical thresholds across days. * indicates that groups differ as determined by 95% confidence intervals.
Administration of CFA produced mechanical allodynia in both male (F(1,9) = 14.879, p = 0.004) and female (F(1,7) = 25.536, p = 0.001) rats as demonstrated by a significant decrease in von Frey threshold (Fig. 3). In contrast to wheel running, the decrease in mechanical withdrawal thresholds persisted through all three days of testing.
3.2 Experiment 2: Eight-day acquisition period
Given that many rats did not reach the baseline criterion of 400 revolutions/day, the baseline period was extended from 3 to 8 days. In addition, the CFA injection was moved from 1000 h to 1600 h so that it would directly precede the active running phase that occurs at night. This shift in testing time and baseline acquisition period did not affect the running patterns in male and female rats in that females ran significantly more than males (Fig. 4; F(1,83) = 35.4032, p < .001) and rats receiving 8 days of training ran significantly more than rats receiving 3 days of training (Fig. 4; F(1,83) = 11.795, p = .001). All 24 male and 30 female rats exceeded the 400 revolution criterion (Table 1). As before, all rats ran predominantly during the dark phase (Fig. 5).
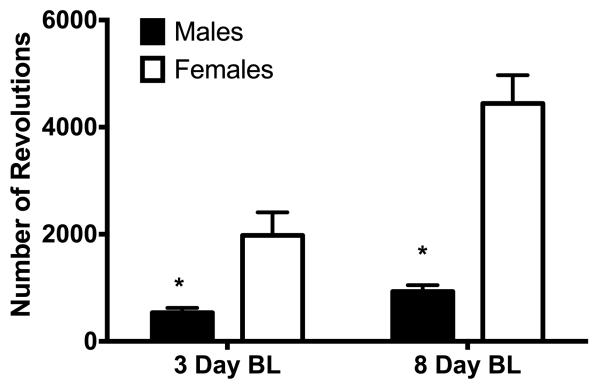
Figure 4. Baseline wheel running in male and female rats following a 3- or 8-day acquisition period.
Mean number of wheel revolutions during the 23 hrs prior to induction of hindpaw inflammation. Increasing the length of the acquisition period from 3 to 8 days caused a slight increase in the amount of baseline running. Female rats (n = 12 and 30 for 3- and 8-day acquisition, respectively) ran considerably more than male rats (n = 21 and 24 for 3- and 8-day acquisition, respectively) regardless of the duration of acquisition period (F(1,83) = 35.402, p < .001*).
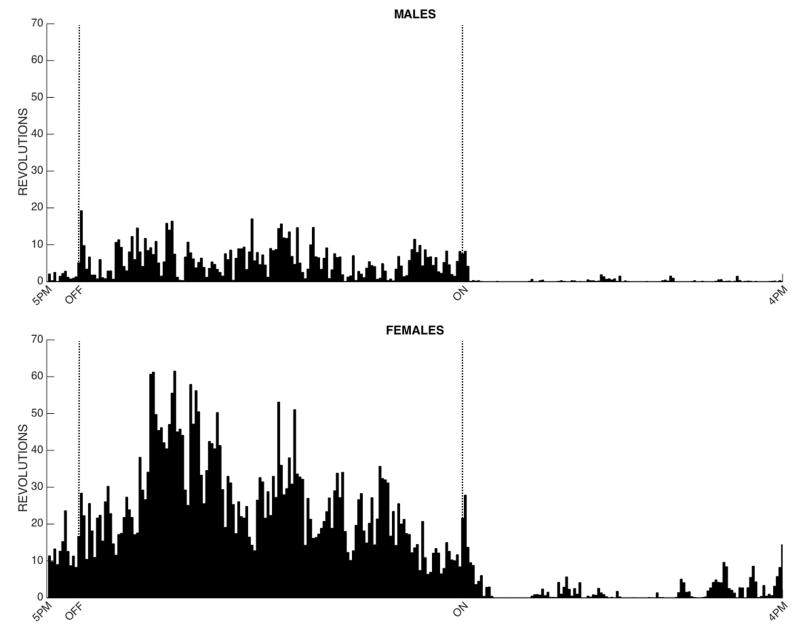
Figure 5. Patterns of wheel running for male and female rats on Day 8 of baseline acquisition.
All bins are shown for the 23 hrs preceding injection of CFA. Mean activity for the 24 male and 30 female rats is displayed in the histogram. All rats met the 400 revolution criterion for inclusion in the experiment (see Table 1). Both male and female rats were most active during the 12 hr dark phase (start of the dark phase is indicated by the dashed line labeled OFF). Female rats were consistently more active than male rats.
The duration of the baseline acquisition period did not alter the effect of CFA on wheel running. Administration of CFA significantly depressed wheel running in male (F(2,21) = 16.765, p < .001) and female (F(2,27) = 26.098, p < .001) rats compared to saline-treated or naive controls. Wheel running was almost nonexistent in CFA-treated rats in the 23 hours post-injection (Fig. 6). As before, wheel running gradually increased over the 3 days of testing in both male and female rats, although this recovery was faster in male compared to female rats (F(1,19) = 13.796, p = .001). Administration of CFA also produced a consistent mechanical allodynia over the three test days in both male (F(2,21) = 226.462, p < .001) and female rats (F(2,27) = 446.551, p < .001) (Fig. 6).
Figure 6. CFA-induced hindpaw inflammation reduced wheel running and mechanical withdrawal thresholds in male and female rats following an 8-day acquisition period.
Top: CFA was injected into the right hindpaw of male and female rats after 8 days of exposure to the wheel in the rat’s home cage. Unilateral inflammation of the hindpaw almost completely inhibited wheel running in male (n = 9) and female (n = 12) rats compared to naïve (7 male rats; 8 female rats) and saline-treated control rats (8 male rats; 10 female rats). A gradual increase in wheel running occurred from Days 1 to 3 following induction of inflammation. Bottom: Unilateral hindpaw inflammation decreased mechanical withdrawal thresholds in male and female rats compared to naïve rats and saline-treated controls. Unlike wheel running, there was no recovery in mechanical thresholds across days. * indicates that groups differ as determined by 95% confidence intervals.
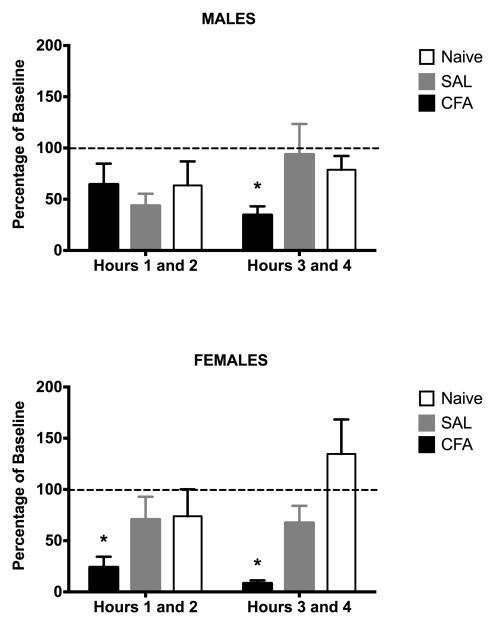
Wheel running did not differ in the first two hours following hindpaw injection of saline or CFA in male rats (Fig. 7; F(2, 21) = 2.768, p = .086). The depression of wheel running caused by CFA administration was evident 3 – 4 h after CFA administration compared to saline treated or naïve rats. In contrast, administration of CFA to female rats caused a significant reduction in wheel running compared to saline-treated and naïve rats that was evident in the first two hours (Fig. 7; F(2, 27) = 5.382, p = .011).
Figure 7. Initial effects of CFA are greater in female than male rats.
Administration of CFA into the right hindpaw of female rats (n = 12) caused a significant reduction in wheel running in the first two hours following injection, which persisted into the next two hours. In contrast, CFA did not depress wheel running in male rats (n = 9) until 3 to 4 hours after administration. There is no significant difference between naïve and saline-treated rats indicating that injection of saline into the hindpaw does not disrupt wheel running. Baseline is the average hourly revolutions during the dark phase. * indicates p < .05 for CFA compared to naïve and SAL-treated rats.
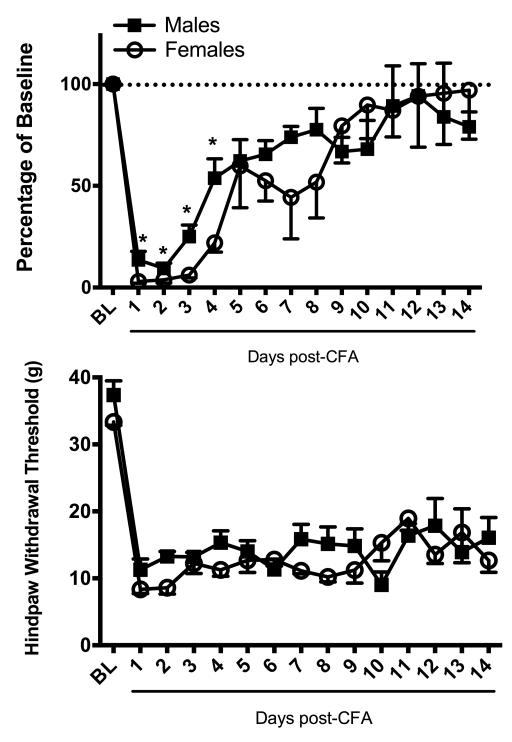
A subset of rats injected with CFA were monitored for 14 days to determine the full time course to return to baseline running levels. Wheel running recovered rapidly on Days 4 and 5 and then showed a more gradual return to baseline levels by Day 12. During this recovery, rats tended to run on the toes of the right paw (see supplementary video). There was no overall difference in the recovery of wheel running between male or female rats when compared across these 14 days (Fig. 8; F(1,8) = .171, p = .69). Mechanical allodynia persisted throughout all fourteen days of testing in both sexes (Fig. 8; F(1, 8) = 2.768, p = .035).
Figure 8. Time course for recovery of CFA-induced depression of wheel running.
Top: CFA was injected into the right hindpaw of male and female rats after 8 days of exposure to the wheel in the rat’s home cage. Unilateral inflammation of the hindpaw almost completely inhibited wheel running in male (n = 4) and female (n = 6) rats. Wheel running gradually returned to baseline levels following induction of inflammation. Bottom: Unilateral hindpaw inflammation decreased mechanical withdrawal thresholds in male and female rats. Unlike wheel running, there was no recovery in mechanical withdrawal thresholds within 14 days. * indicates that groups differ as determined by 95% confidence intervals.
4. Discussion
The present study indicates that depression of wheel running in rats may be an appropriate model of inflammatory pain-depressed activity in humans. Although baseline wheel running is much greater in female compared to male rats, the induction of unilateral hindpaw inflammation almost completely inhibited running in male and female rats. Both male and female rats showed a day-by-day recovery of wheel running. In contrast, mechanical withdrawal thresholds remained low for both male and female rats throughout the entire testing period.
The patterns of baseline running shown here are consistent with previous studies showing that female rats run more than male rats and that most running occurs during the dark phase (Eikelboom and Mills, 1988). Higher rates of running can be achieved in male rats with an acquisition period of 21 days (Stevenson et al., 2011); however, our data show that male rats reach stable levels of running and meet the criterion of 400 revolutions/day within 8 days. A short acquisition period (i.e., 3 days) results in greater variability with fewer rats reaching stable running levels. Although there are many factors that impact acquisition and rate of wheel running in rodents (Sherwin, 1998), an acquisition period of 8 days provides stable data.
Results from our pain-evoked experiments are consistent with previous studies showing that depression of wheel running recovers faster than reversal of evoked hypersensitivity (Cobos et al., 2012; Grace et al., 2014). This difference between pain-depressed and pain-evoked measures of nociception highlights one of the advantages of wheel running as a measure of nociception, namely that it reveals whether allodynia and hyperalgesia produce a functional deficit in behavior. Although CFA-induced allodynia persists at least 14 days post-injection, wheel running is completely restored by 12 days in males and females (see Fig. 8). Video analysis two days after CFA administration reveals that rats will run even while protecting the injured paw (see supplementary video). These data indicate that the von Frey and wheel running tests measure different aspects of nociception. This is not surprising in that it is common for active behaviors in humans (e.g., walking, working, socializing) to recover despite the fact that directly stimulating (e.g., poking, pressing) an injury is painful. Rather than developing treatments to completely eliminate hypersensitivity, a more realistic treatment goal may be to enhance and restore daily function. Thus, pain-depressed wheel-running complements assessment of evoked hypersensitivity to provide a more complete analysis of the effects of inflammatory pain in laboratory rats.
Regular prolonged exercise (3-5 weeks) has been shown to attenuate some types of pain in rodents (Stagg et al., 2011; Kuphal et al., 2007). Exercise-induced antinociception was not evident in our rats when assessed for mechanical hypersensitivity (Fig. 3, 6, and 8). However, our rats were only subjected to 3 or 8 days of baseline running prior to induction of hindpaw inflammation and running was almost completely eliminated in the days following CFA administration. Thus, it is unlikely that exercise-induced changes in nociception confounded our findings.
The depression of wheel running produced by a noxious stimulus as shown in the present study is consistent with other studies examining wheel running. However, there are two main differences between our data and prior publications. First, our data show that a single unilateral injection of CFA into the hindpaw produces a pronounced and prolonged depression of wheel running (Fig. 8), such that wheel running is almost completely inhibited in the first day after CFA administration and slowly recovers over the next 14 days. Previous studies in which access to the running wheel was limited needed bilateral injections of CFA into the hindpaws to produce a reduction in wheel running (Cobos et al., 2012; Grace et al., 2014). The sensitivity of our test is probably related to continuous access to the wheel in the rat’s home cage as opposed to assessing wheel running for 1 hr a day. Limiting access to the wheel for 1 hr a day via locking the wheel (Grace et al., 2014) or moving the animal to a chamber with a wheel (Cobos et al., 2012) may increase motivation to run. Moreover, testing rats outside the home cage may induce stress-induced antinociception. Either of approaches could mask the effects of a milder noxious stimulus (i.e., unilateral vs. bilateral CFA administration). Our data show that recording wheel running for 23 hrs a day in the rat’s home cage allows nociception to be assessed throughout the day in a stress-free setting.
Our data also differ from prior publications in that only one other study has examined pain-depressed behavior in female rodents, and that study found no differences between male and female rats in wheel running following induction of visceral pain (Miller et al., 2011). Our data show that hindpaw inflammation produces a comparable depression of wheel running in male and female rats, but the initial recovery of running tends to be slower in female compared to male rats (Fig. 3, 6, and 8). Furthermore, we show that the immediate effects of CFA on physical activity are greater in females than in males (Fig. 7). However, even though female rats were only running at 14% of their baseline levels three days after administration of CFA into the paw (males were at 38%), the number of revolutions by female rats (median = 400) was greater than in male rats (median = 289 revolutions) (see Experiment 2). Figure 8 suggests that absolute running distance is less relevant than percent change from baseline in that both male and female rats return to baseline levels of running given enough time. Previous studies report increases in paw edema relative to the uninjected paw in male (~2.2-fold in thickness) compared to female (~1.8-fold in thickness) rats (Craft et al., 2013). Despite greater inflammation in male rats, female rats tended to show a greater reduction in wheel running. Thus, hindpaw inflammation had a greater impact on wheel running in female rats relative to their baseline activity, which is consistent with the higher incidence of chronic pain in women than men (Mogil, 2012).
One limitation of wheel running as a measure of nociception is that it is a complex behavior that can be influenced by subtle environmental or experimental changes. However, this complexity is also what makes wheel running a good model for the many psychological and environmental factors that influence the response to pain in humans. Wheel running is a natural and rewarding behavior in rodents much as going to work, socializing, and exercising are natural rewarding activities in humans. Our data demonstrate that inflammatory pain reduces wheel running in the same way that pain limits going to work, socializing, and exercising in humans. Another similarity, and a key advantage of home cage wheel running over other nociceptive assays, is the ability to assess nociception in a relatively stress-free environment. Continuous access to home cage wheel running also allows both the duration and magnitude of nociception to be assessed in a completely objective manner. Data can be analyzed to reveal the global impact of pain in addition to conducting analyses at specific times such as following drug treatments. No scientist is in the room during data collection which prevents experimenter bias or other confounds. Finally, the differences in recovery of wheel running and mechanical hypersensitivity indicates that depression of wheel running may be useful in assessing the affective and/or motivational components of pain – an important aspect of pain affecting human behavior that is rarely assessed in rodents (Price, 2000; Spuz and Borszcz, 2012).
This study focused on CFA-induced inflammatory pain because it is a well-characterized model. Given the clinical relevance of assessing pain-depressed behavior in the home cage, almost any pain condition could be studied. We have preliminary data showing that neuropathic and migraine pain also cause reductions in wheel running. Wheel running could be an especially useful tool in drug development because a treatment that is effective in restoring pain-depressed wheel running must produce antinociception without disabling side effects such as dysphoria or sedation. The high levels of running reported here and by others (Eikelboom and Mills, 1988) suggests that the limited movement available to most laboratory rats may interfere with normal recovery processes. Finally, unlike pain-evoked behavioral tests, wheel running allows for the physical, motivational, and affective effects of pain to be evaluated.
5. Conclusions
In summary, this is the first study to monitor pain-induced changes continuously for 3 days and longer, to examine differences in pain-depressed behavior in male and female rats, and to provide a method to assess nociception that closely mimics the effects of pain in humans. Taken together, continuous home cage monitoring of wheel running is a simple, objective, and clinically relevant method to assess nociception in the rat.
Supplementary Material
Highlights.
Hindpaw inflammation depressed home cage wheel running in male and female rats.
Wheel running recovered within 12 days in both male and female rats.
Mechanical withdrawal thresholds remained low for all testing days.
This is a sensitive, objective, clinically relevant measure of inflammatory pain.
Acknowledgments
The authors would like to thank Courtney Miskell, Arthur Serkov, Andrea Lee, and Shauna Schoo for technical assistance. This investigation was supported in part by funds provided by medical and biological research by the State of Washington Initiative Measure No. 171 to R.K. and by NIH/NIDA DA027625 to M.M.M.
Footnotes
None of the authors declare a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, Pheby T, Huang W, Burgess G, Machin I, Rice AS. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur J Pain. 2012;16:485–495. doi: 10.1016/j.ejpain.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. PAIN. 2012;153:876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Kandasamy R, Davis SM. Sex differences in anti-allodynic, anti-hyperalgesic and anti-edema effects of Δ9-tetrahydrocannabinol in the rat. PAIN. 2013;154:1709–1717. doi: 10.1016/j.pain.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol. Behav. 1988;43:625–630. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- Freitas KC, Hillhouse TM, Leitl MD, Negus SS. Effects of acute and sustained pain manipulations on performance in a visual-signal detection task of attention in rats. Drug Dev. Res. 2015;76:194–203. doi: 10.1002/ddr.21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Maier SF, Watkins LR. Suppression of voluntary wheel running in rats is dependent on the site of inflammation: evidence for voluntary running as a measure of hind paw-evoked pain. J Pain. 2014;15:121–128. doi: 10.1016/j.jpain.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Sex and gender differences in pain and analgesia: a consensus report. PAIN. 2007;132(Suppl 1):S25–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuphal KE, Fibuch EE, Taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J Pain. 2007;8:989–997. doi: 10.1016/j.jpain.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS. Dissociable effects of the cannabinoid receptor agonists Δ9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. Journal of Pharmacology and Experimental Therapeutics. 2012;343:389–400. doi: 10.1124/jpet.112.197780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. Inflammation-induced reduction of spontaneous activity by adjuvant: A novel model to study the effect of analgesics in rats. J. Pharmacol. Exp. Ther. 2007;320:194–201. doi: 10.1124/jpet.106.109736. [DOI] [PubMed] [Google Scholar]
- Miller LL, Picker MJ, Schmidt KT, Dykstra LA. Effects of morphine on pain-elicited and pain-suppressed behavior in CB1 knockout and wildtype mice. Psychopharmacology (Berl.) 2011;215:455–465. doi: 10.1007/s00213-011-2232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012;13:859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- Muley MM. Preclinical assessment of inflammatory pain. CNS Neurosci. Ther. 2016;22:88–101. doi: 10.1111/cns.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Expression and treatment of pain-related behavioral depression. Lab Anim (NY) 2013;42:292–300. doi: 10.1038/laban.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Altarifi AA. Mu, Delta and Kappa Opioid Agonist Effects In Novel Assays of Pain-Depressed Behavior. In: Ko M-C, Husbands SM, editors. Research and Development of Opioid-Related Ligands. American Chemical Society; 2013. pp. 163–176. [Google Scholar]
- Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL. Effects of Ketoprofen, Morphine, and Kappa Opioids On Pain-Related Depression of Nesting in Mice. PAIN. 2015;156:1153–1160. doi: 10.1097/j.pain.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J. Pharmacol. Exp. Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Rutten K, Schiene K, Robens A, Leipelt A, Pasqualon T, Read SJ, Christoph T. Burrowing as a non-reflex behavioural readout for analgesic action in a rat model of sub-chronic knee joint inflammation. Eur J Pain. 2014;18:204–212. doi: 10.1002/j.1532-2149.2013.00358.x. [DOI] [PubMed] [Google Scholar]
- Sherwin C. Voluntary wheel running: a review and novel interpretation. Anim Behav. 1998;56:11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- Spuz CA, Borszcz GS. NMDA or non-NMDA receptor antagonism within the amygdaloid central nucleus suppresses the affective dimension of pain in rats: evidence for hemispheric synergy. J Pain. 2012;13:328–337. doi: 10.1016/j.jpain.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Philip Malan T. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114:940–948. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson GW, Bilsky EJ, Negus SS. Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J Pain. 2006;7:408–416. doi: 10.1016/j.jpain.2006.01.447. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Cormier J, Mercer H, Adams C, Dunbar C, Negus SS, Bilsky EJ. Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sciences. 2009;85:309–315. doi: 10.1016/j.lfs.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, Jezierski J, Luginbuhl A, Bilsky EJ. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain. Pharmacol. Biochem. Behav. 2011;98:35–42. doi: 10.1016/j.pbb.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney M, Fraser A, Kennedy N. Physical activity in rheumatoid arthritis: a systematic review. J Phys Act Health. 2012;9:1036–1048. doi: 10.1123/jpah.9.7.1036. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2:137–145. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.