Abstract
Autophagy is a conserved multistep pathway that degrades and recycles damaged organelles and macromolecules to maintain intracellular homeostasis. The autophagy pathway is upregulated under stress conditions including cell starvation, hypoxia, nutrient and growth-factor deprivation, ER stress, and oxidant injury, most of which are involved in the pathogenesis of acute kidney injury (AKI). Recent studies demonstrate that basal autophagy in the kidney is vital for the normal homeostasis of the proximal tubules. Deletion of key autophagy proteins impaired renal function and increased p62 levels and oxidative stress. In models of AKI, autophagy deletion in proximal tubules worsened tubular injury and renal function, highlighting that autophagy is renoprotective in models of AKI. In addition to nonselective sequestration of autophagic cargo, autophagy can facilitate selective degradation of damaged organelles particularly mitochondrial degradation through the process of mitophagy. Damaged mitochondria accumulate in autophagy-deficient kidneys of mice subjected to ischemia-reperfusion injury, but the precise mechanisms of regulation of mitophagy in AKI are not yet elucidated. Recent progress in identifying the interplay of autophagy, apoptosis, and regulated necrosis has revived interest in examining shared pathways/molecules in this crosstalk during the pathogenesis of AKI. Autophagy and its associated pathways pose potentially unique targets for therapeutic interventions in AKI.
Keywords: acute kidney injury, autophagy, mitophagy, apoptosis, cell death, ischemia reperfusion, cisplatin nephrotoxicity
INTRODUCTION
Basic principle of autophagy and its relevance to acute kidney injury
Christian De Duve (discoverer of lysosomes and peroxisomes in 1955), while studying the structures of lysosome by electron microscopy in the early 1960s, observed double-membrane vesicles containing degraded intracellular organelles and lysosomal enzymes in the cells. In 1963, De Duve coined the term “autophagy” (meaning self-eating in Greek) for the vesicle formation phenomenon, and later on provided evidence that lysosomes are involved in the degradation of sequestered cellular contents enclosed in autophagy vesicles, now known as autophagosomes1-3.
Autophagy is an evolutionarily conserved multi-step process of degradation of intracellular organelles, proteins, and other macromolecules by the hydrolases of lysosomes4-6. The degraded cellular contents are reutilized for the synthesis of new macromolecules and organelles. Many recent studies have documented the pivotal role of autophagy in physiological processes as well as in pathogenesis of a disease. In normal physiological conditions, a low level of basal or constitutive autophagy occurs to maintain cellular homeostasis by controlling the turnover of damaged proteins and organelles. In pathological conditions, a wide range of cellular stresses including cell starvation, hypoxia, nutrient and growth-factor deprivation, oxidant injury, genotoxic agents, and other damaging insults contribute to the induction of autophagy7-9. Among these stresses, autophagy induction in response to starvation is extensively studied, well characterized, and is shown to be mediated through mTOR, AMPK, and sirtuins5, 7, 10.
The pathogenesis of acute kidney injury (AKI) also involves multiple stresses including hypoxia, nutrient and growth-factor deprivation, energy depletion, oxidant injury, genotoxic stress, endoplasmic reticulum (ER) stress, and other damaging insults, all of which are known to drive autophagy induction. In response to numerous stresses, autophagy is a mechanism to promote cellular adaptation with cytoprotective effects by eliminating and recycling of damaged macromolecules and organelles5, 7. In general, autophagy induction in response to multiple stresses including those induced during AKI is cytoprotective (reviewed in later sections). Therefore, autophagy is important in renal injury and is a potential therapeutic target in the pathogenesis of AKI. Dysregulation of autophagy results in pathophysiologies such as cardiomyopathy, infectious diseases, Crohn's disease, and neurodegenerative disorders including Alzheimer's, Huntington's, and Parkinson's diseases11. However, the role and regulation of autophagy induction in AKI is not extensively studied as in other diseases as noted above.
Lysosomal degradation of cellular contents involve three autophagy pathways
Three processes of lysosomal-mediated degradation of intracellular contents have been identified in mammalian cells and classified into three subtypes of autophagy: Macroautophagy, chaperone-mediated autophagy, and microautophagy. The distinct route of delivery of cytoplasmic material to the lysosome distinguishes different types of autophagy. Macroautophagy, generally referred to as “autophagy,” is the main and widely studied pathway to degrade or eliminate damaged cell organelles and proteins. The macroautophagy begins in the cytoplasm by formation of a double membrane structure known as a phagophore or isolation membrane. The studies on the origin or source of this process is under current investigation, and some studies have proposed plasma membrane, Golgi complex, ER or mitochondria as possible sources12-16. Following nucleation, the phagophore membrane sequesters the targeted portion of the cytoplasm containing damaged macromolecules and organelles and then further elongates to form a double-membrane vesicle known as the autophagosome. The autophagosome then fuses with a lysosome and forms an autolysosome. The lysosomal hydrolases degrade cytoplasmic constituents and the resulting breakdown products are recycled to synthesize new proteins, organelles, and energy needs of the cell5, 6. Autophagy plays a vital role in eliminating unwanted damaged macromolecules and organelles in the cells. Chaperone-mediated autophagy (CMA) is a selective form of autophagy that has been described in mammalian cells only. Cytoplasmic proteins containing the KFERQ (Lys-Phe-Glu-Arg-Gln) pentapeptide recognition sequence are recognized by the chaperone complex hsc70 (heat shock cognate 70 of the Hsp70 family). Hsp8 and other co-chaperones target the substrate to the lysosomal surface where they bind to the lysosome-associated membrane protein type 2A (LAMP-2A). Lysosomal hsc73 and hsc70 facilitate subsequent translocation and internalization of the substrate for degradation17. Microautophagy is a process in which cytoplasmic contents are directly engulfed into the lysosome by lysosomal membrane invagination or protrusion. This process is not well studied.
Molecular machinery of autophagy: Sequestration of cytoplasmic contents and their degradation
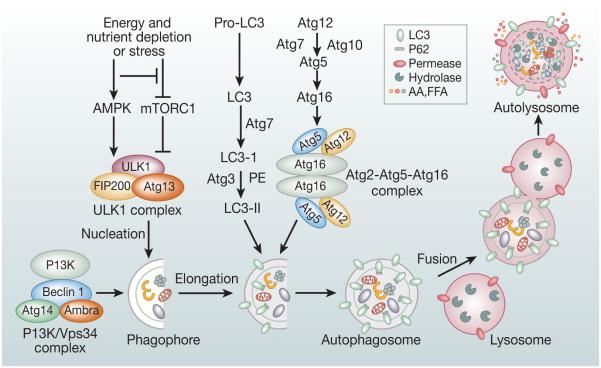
The hallmark of the autophagy process in the mammalian cell is the formation of autophagosomes and autolysosomes. Formation of an autophagosome involves the coordinated action of several Atg protein complexes including (i) the ULK1/ULK2 (uncoordinated (UNC)-51-like kinase ½) complex; (ii) beclin-1/class III phosphatidylinositide-3 kinase (PI3K) complex; (iii) transmembrane proteins Atg9 and vacuole membrane protein 1 (VMP1); and (iv) Atg12-Atg5-Atg16 ubiquitin-like protein conjugation system and lipidation of LC3 with phosphatidylethanolamine (PE) to form LC3-II4, 6, 18 (Figure 1). The LC3-II levels are generally assessed by western blots and LC3-II positive dots in the form of punctate staining by immunostaining. Atg proteins were originally identified by genetic screening in yeast4-6, 19.
Figure 1.
Overview of the autophagy pathway and its molecular machinery: Formation of autophagosome involves a series of steps mediated by functional complexes containing Atg proteins: (i) The ULK1/2 kinase complex composed of ULK1/2, Atg13, RB1CC1/FIP200, and Atg101 is recruited to the phagophore membranes. It is required for initiation of autophagy and is regulated by mTORC1 and AMPK. (ii) PI3K/Vps34 complex composed of Vps34, Vps 15, Beclin-1, Ambra and Atg14L is required for nucleation of the phagophore membrane.(iii) Atg9 vesicles are required for membrane expansion for the autophagosome assembly. (iv) Atg12–Atg5–Atg16 multimeric complex and Atg8/LC3-II are two ubiquitin-like conjugation systems that are involved in the elongation and expansion steps in the autophagosome formation. LC3-II remains present on both the membranes of the autophagosome and can bind to selective substrates including p62. The mature autophagosome then fuses with lysosome to form autolysosome. Upon fusion with lysosome, the cytoplasmic contents sequestered in the autophagosome contents are degraded by the lysosomal hydrolases.
(i) The ULK1/2 complex composed of ULK1/ULK2 (two homologs of yeast Atg1) serine-threonine kinases, ATG13 (mammalian homolog of yeast Atg13), RB1CC1/FIP200 (homolog of yeast Atg17), and ATG10120, 21 function upstream of the autophagy pathway and is recruited to the phagophore membranes. mTOR (mTORC1) and AMPK are two nutrient- and energy-sensitive kinases22-30 involved in regulating activity of the ULK1/2 complex. mTORC1 negatively regulates autophagy, and this function of mTORC1 is conserved in eukaryotes30. AMPK phosphorylates tuberous sclerosis complex (TSC), which inhibits mTORC126, 31. AMPK can also phosphorylate the raptor component of mTORC1 when released from the ULK1 complex, resulting in inactivation of mTORC126.
(ii) Beclin-1/class III phosphatidylinositide-3 kinase (PI3K) complex is recruited to the phagophore membrane to further promote autophagosome nucleation. Activated ULK1 complex and transmembrane protein ATG9 facilitate the recruitment of the autophagy-specific class III PI3K complex to the phagophore membranes. VPS34, VPS15, and Beclin-1 are the core components and other accessory proteins such as AMBRA, AtG14L, or UVRAG associate with the complex by binding to Beclin-1.4, 6, 19 VPS34 is the catalytic subunit and other associated components are essential for the catalytic activity of the complex. ULK1/2 complex activates PI3K complex by phosphorylating its components Beclin-1 on Ser 1432 and AMBRA 133, and the active PI3K complex phosphorylates phosphatidyl inositol to form phosphatidyl inositol-3 phosphate (PtdIns3P). PtdIns3P produced at the site of phagophore membranes not only is important for stable phagophore membrane nucleation, further growth, and initial phagophore curvature, but also important to facilitates recruitment of other regulatory PtdIns3P-binding proteins including DFCP1 (double FYVE-containing protein 1) and WIPI (WD-repeat domain phosphoinositide-interacting) proteins34, 35.
(iii) Atg9 vesicles are translocated to the isolation membrane or phagophore sites and play an important role in membrane expansion in the autophagosome assembly both in yeast and mammalian cells36, 37.
(iv) Expansion and elongation of phagophore membranes involve formation of two ubiquitin-like conjugation systems: ATG16L1 complex (ATG12-ATG5-ATG16L1) and lipidation of microtubule-associated protein-1 light chain-3 (LC3), a mammalian homolog of yeast ATG8. For the formation of ATG12-ATG5-ATG16L1, ATG12 is activated by E1-like conjugating enzyme ATG7 and transferred to the E2-like conjugating enzyme ATG10. Formation of an ATG12-ATG10 intermediate facilitates the transfer of ATG12 to Atg5 to form covalently-linked ATG12-ATG5. Further, noncovalent association with ATG16L1 results in the formation of the multimeric complex ATG12-ATG5-ATG16L14, 38. The ATG16L1 complex is recruited to the phagophore membranes and is required for the efficient execution of the second ubiquitin-like conjugation system for lipidation of LC3 (Atg8) by conjugation with phosphatidylethanolamine (PE)38, 39. LC3 is first cleaved by ATG4 to produce cytosolic LC3-I. The E1-like conjugating enzyme ATG7 then activates LC3-I and transfers to the E2-like conjugating enzyme ATG3. Formation of an LC3-1-ATG3 intermediate facilitates the transfer of LC3-I to PE to form covalently linked LC3-PE, also known as LC3-II and it becomes an integral component of the outer and inner membrane of the autophagosome, whereas ATG12-ATG5-ATG16L1, present on the outer membrane, dissociates on completion of the autophagosome19 (Figure 1). A recent study has demonstrated that WIPI2 interacts with ATG16L1 and is involved in the recruitment of ATG12-ATG5-ATG16L1 complex to the expanding phagophore membranes, thus, linking PI3KC3 complex to LC3-lipidation in the process of autophagosome formation40-42.
Fusion of lysosome with autophagosome and cargo degradation
Once the autophagosome formation is complete, it fuses with the lysosome to generate an autolysosome (Figure 1). The process of fusion is not completely understood in mammalian cells, but the Ypt 7 homolog RAB7 is involved43-45. A recent study demonstrated that autophagosome-endolysosome fusion requires oligomeric ATG14 that interacts with STX17–SNAP29 binary t-SNARE complex on autophagosomes and primes it for VAMP8 interaction to promote fusion46. The inner membrane LC3-II of the autolysosome is degraded along with the rest of the sequestered cargo by lysosomal hydolases19.
Selective degradation of cytoplasmic contents by autophagy
The process of autophagy was traditionally viewed as a nonselective sequestration in autophagosomes of the cytoplasmic contents and their degradation by lysosome. Many studies now have demonstrated that autophagy selectively eliminates intracellular molecules as well as damaged organelles and pathogens. Selective autophagy has been reported for the elimination of damaged mitochondria (mitophagy), peroxisomes (pexophagy), lipids (lipophagy), aggregated proteins (aggrephagy), endoplasmic reticulum (ER-phagy), pathogens and microorganisms (xenophagy), and cilia (ciliophagy)47-50.
One of the selective mammalian autophagy cargo receptors first identified is p62/SQSTM, also known as a sequestosome-151, 52. p62 is a well-studied multifunctional protein and among its many structural domains, PB1 domain, ubiquitin-associated domain (UBA), and LC3-interacting region are involved in p62-mediated selective autophagy. The N-terminal PB1 domain enables p62 to self-associate and polymerize in the cytoplasm to form aggregates and cytoplasmic inclusion bodies and these self-aggregates are degraded by autophagy53, 54. The C-terminus ubiquitin binding domain (UBA) enables p62 to recognize and bind to polyubiquitinated proteins. The presence of LC3-interacting region (LIR) in p62 facilitates binding to LC3-II, and targeting p62 and p62-bound polyubiquitinated cargo to the autophagosomes for degradation52, 54, 55. Other related autophagy receptors are NBR1 (neighbor of BRCA1 gene1), NDP52 (nuclear dot protein 52 kDa), optineurin, and TAX1BP1 (Tax1 binding protein 1) and display much similarity to the domain structure of p62 and contain dimerization or polymerization domain, LIR region, and UBA domain48, 55.
Selective degradation of damaged mitochondria (mitophagy) and its induction and role in AKI
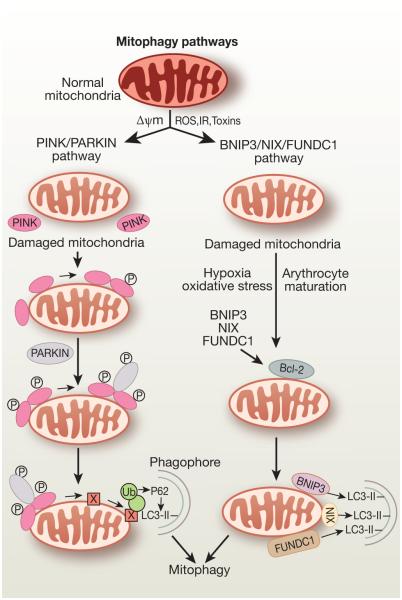
Selective removal of damaged and depolarized mitochondria, known as mitophagy, is necessary to maintain quality control of mitochondria and cellular homeostasis. Although initially identified in neurodegenerative diseases, mitophagy is being recognized as playing important roles during AKI. To meet high energy needs for the processes of electrolyte reabsorption and excretion of waste products, the kidney has the second highest abundance of mitochondria and oxygen consumption next to the heart. Increased oxidative stress, inflammation, and uncoupling of oxygen consumption from ATP production, all of which are associated with AKI56-58, promote mitochondrial depolarization and dysfunction, which can trigger mitophagy59. Parkin, PINK1 (PTEN-induced putative kinase protein 1), NIX, BNIP3, and FUDNC1 are proteins involved in the process of mitophagy in mammalian cells. Mutations in Parkin60 and PINK161 genes were first identified to cause familial Parkinson’s disease, and mitochondrial dysfunction is shown to be associated with this disease62. The underlying mechanism how the PINK1-Parkin pathway results in removal of damaged mitochondria is well characterized (Figure 2). In normal mitochondria, PINK1 is degraded from the mitochondria by the proteasome-mediated pathway but selectively accumulated in the outer membrane of depolarized and damaged mitochondria63. At the mitochondrial surface, PINK1 recruits the E3 ubiquitin ligase Parkin and activates Parkin by phosphorylation62, 64, 65. Active Parkin then promotes ubiquitination of a broad range of outer mitochondrial surface proteins including mitofusins (Mfn1/2), mitochondrial rho GTPase (MIRO), voltage-dependent anion channel (VDAC) TOM70, DRp1, and PARIS (ZNF746)49, 66. The autophagy receptor optineurin and p62 both contain ubiquitin-binding and LIR structural domains that may facilitate autophagic clearance of the damaged mitochondria67. Another mechanism for mitophagy has also been reported. Mitophagy receptors including BNIP3 (BcL-2 and adenovirus E1B 19-kDa-interacting protein 3), BNIP3L (BNIP3-like, also known as NIX), and FUNDCI (FUN 14 domain-containing protein 1) are mitochondrial proteins and play a role in specific autophagic clearance of the damaged mitochondria. BNIP3/Nix and FUNDCI interact with LC3 via their LIR domain and promote autophagic clearance of damaged mitochondria66, 68, 69. BNIP3/Nix may promote mitophagy by directly binding with the mitochondria or through the interaction with Bcl-2/beclin-170. FUNDC1 mediates in hypoxia-induced mitophagy71.
Figure 2.
PINK/PARKIN and BNIP3/NIX/FUNDC1 pathways of mtophagy. In the PINK/PARKIN pathway, mitochondrial deploarization or mitochondrial fission leads to localization of PINK1 in the outer membrane of the depolarized mitochondria. PINK1 then recruits E3 ubiquitin ligase, PARKIN, to the mitochondria and activates PARKIN to facilitate ubiquitination of mitochondrial surface proteins for autophagic clearance of the damaged mitochondria. Polyubiquitinated outer mitochondrial proteins interact with p62 bound to autophagosomal LC3, thereby targeting the mitochondrion for mitophagy In the BNIP3/NIX/FUNDC1 pathway, mitochondrial outer membrane proteins BNIP3, NIX, or FUNDC1 bind to LC3-II via their LIR motifs facing the cytosol and promote selective clearance of mitochondria. NIX is involved in the process of mitochondrial clearance during reticulocytes maturation and BNIP3 is upregulated in renal IR injury and overexpression of BNIP3 in NRK-53E cells selectively induced mitophagy72.
Little information is known on the induction of mitophagy in renal tubular cells in AKI. Ishihara et al.72 demonstrated that BNIP3 and sestrin 2 are upregulated during renal IR in vivo and in NRK-52E cells exposed to hypoxia. Although overexpression of both BNIP3 and sestrin 2 induced autophagy as measured by LC3-II formation, only BNIP3 selectively induced mitophagy as visualized by confocal and electron microscopy72. The expression of PINK, a marker of mitophagy, was increased during renal ischemia-reperfusion injury73. Another study has shown that mitophagy in the kidneys of a rat fed low-calorie diet was markedly increased and ameliorated oxidative damage compared to that of high-calorie fed rats74. Limited information is available on the selective eliminations of other organelles in AKI75. The studies on the role and prevalence of other forms of selective autophagy such as lipophagy, aggrephagy, ER-phagy, and ciliophagy have not been yet investigated during AKI.
Autophagy in AKI
Autophagy in ischemia-reperfusion (IR) injury
Autophagy is induced in response to renal IR injury in in vivo and in vitro models71, 76-81. In most of these studies, autophagy induction was revealed by conversion of LC3-1 to LC3-II or by GFP-LC-II punctate formation using LC3-GFP transgenic mice. In a few studies, induction of autophagy has been demonstrated by formation of autophagosomes when visualized by an electron microscope71, 77, 79. Many studies have reported the role of autophagy during IR-induced AKI. The beneficial effect of autophagy during renal IR was revealed by utilizing conditional kidney proximal tubule-specific Atg5- or Atg7-knockout (KO) mice. Kimura et al.81 used Atg5flox/flox Kap-Cre mice under the control of an inducible promoter KAP (kidney androgen-regulated protein) to specifically delete Atg5 in proximal tubules in response to androgen. The proximal tubule-specific Atg5-KO mice accumulated deformed mitochondria, p62, ubiquitin-positive inclusion bodies, and increased TUNEL-positive cells. These results indicate that basal autophagy is important for the normal homeostasis of proximal tubules. In addition, tubular damage and renal dysfunction worsened in these mice when subjected to IR injury81, suggesting that autophagy is renoprotective in IR injury. Similar results were obtained with proximal tubule-specific Atg7-deficient mice, which were more sensitive to IR injury compared to wild-type mice79. Mice with Atg5 deletion in both proximal and distal tubules when subjected to IR injury also had more severe tubular damage and renal dysfunction, with increased levels of BUN and creatinine for up to 16 days after IR injury71. These mice accumulated damaged mitochondria, p62, and ubiquitinated proteins, and displayed increased apoptosis (caspase-3 activation)71 in kidneys. Mice deficient in Atg5 only in distal tubules did not cause renal dysfunction and tubular damage71. Pharmacological approaches were also considered to examine the role of autophagy in IR injury76-79,82,83. Currently, effective pharmacological inhibitors that specifically target autophagy are yet to be developed. Caloric restriction that stimulates autophagy has also provided evidence for a protective role of autophagy in IR injury as autophagy inhibition abrogated this protection84.
Pitfalls in induction of autophagy by mTORC1 inhibition in IR injury
The underlying mechanisms of how autophagy is regulated in IR injury are not clearly understood. A serine threonine kinase, mTORC1, upon activation negatively regulates autophagy by controlling phosphorylation of ULK1. mTORC1 also participates in multiple cellular processes and promotes cellular growth, proliferation, survival, and metabolism25, 85, 86 and is reported to maintain renal tubular homeostasis. mTORC1 deletion in proximal tubules increased susceptibility to IR injury as reflected in more severe tubular damage and decline in renal function85. However, the beneficial effect of enhanced autophagy in response to inhibition of mTORC1 during IR may be offset by the loss of mTOR1-mediated effects on cellular growth and survival. Along these lines, everolimus, a derivative of rapamycin and inhibitor mTORC1, was able to increase autophagy in rats subjected to IR but was unable to protect from IR-induced renal dysfunction and tubular damage87. Rapamycin, an inhibitor of the mTOR pathway, was reported to impair tubular proliferation and delay recovery of renal function during IR88, 89 in accordance with inhibition of mTOR-mediated delayed graft function in kidney transplant patients and was shown to prevent IR-induced renal injury90. Since multiple cellular processes of cellular growth and survival impinge on the mTORC1 inhibition, caution is needed in the interpretation of the autophagy induction effects by mTORC1 inhibition.
A recent study evaluated autophagic activity (autophagic flux) during both the injury and recovery phase of IR injury. Li et al.91 generated autophagy reporter mice that express a tandem red fluorescent protein (RFP)-EGFP-LC3 fusion protein under the control of chicken β-actin (CAG) and subjected these mice to IR injury. Since RFP is stable in acidic pH while EGFP is quenched in acidic pH, formation of acidic autolysosomes was distinguished from autophagosomes during the course of IR injury. In this study, basal EGFP and RFP fluorescence did not change when examined at the end of 45 minutes of ischemic injury or 4h post-reperfusion period, suggesting that autophagy was not activated during this time. At the later reperfusion periods, both EGFP and RFP fluorescence reached a peak value at 24h after reperfusion, but at 3d after reperfusion, only RFP fluorescence persisted. Additionally, at 7d after reperfusion, RFP fluorescence returned to basal level. These studies suggest that auotophagosome formation is reduced 24 h after reperfusion and thereafter, upon fusion with lysosomes, formation of autolysosomes persist during renal recovery to clear the autophagosomal cargo. However, caution should be exercised in reporting RFP puncta for the autophagosomal vesicles, as RFP protein is reported to accumulate in the lysosome during overexpression, which can lead to incorrect interpretation.
In an in vitro model of ATP-depletion, up-regulation of autophagy protected LLC-PK1 cells via AMPK-mediated down-regulation of phosphorylation of mTOR92. In this model, inhibition of AMPK by small hairpin RNA (shRNA) increased the phosphorylation of mTOR and suppressed autophagy92, suggesting that AMPK-regulated mTOR pathway in an in vitro model of lR. Activation of the AMPK-regulated autophagy pathway in response to quercetin flavonol provided protection from IR injury93.
Autophagy in response to cisplatin
Autophagy induction and its cytoprotective role have been demonstrated in both in vitro and in vivo models of cisplatin-induced AKI. Cisplatin induced autophagy prior to caspase activation and apoptosis in vitro in cultured proximal tubular epithelial cells94, 95 and in vivo in renal tubules from a murine model of cisplatin nephrotoxicity96-100. Inhibition of autophagy either by 3-MA or siRNAs specific to beclin-1 or Atg5 increased cisplatin-induced caspase activation and apoptosis in LLC-PK1 cells94, suggesting that autophagy plays a cytoprotective role against cisplatin injury. A similar pro-survival role of autophagy was observed in cultured rat proximal tubular epithelial cells exposed to cisplatin95. In NRK-52E cells, autophagy provided protection from cisplatin injury at a lower dose (10 µM) of cisplatin, whereas 50 µM cisplatin resulted in very low induction of autophagy and the autophagy inhibitor did not increase cisplatin-induced apoptosis101. In addition, taurine enhanced autophagic protection against cisplatin-induced apoptosis by reducing ER stress in NRK-52E cells101. Another study in NRK-52E cells has shown that suppression of autophagy either by autophagy inhibitors or beclin-1 siRNA also prevented apoptosis96. The discrepancy in this study with other in vitro results could be due to differences in the doses of cisplatin or differences in the cell types used. Primary cultures of proximal tubular epithelial cells prepared from proximal tubule-specific Atg7-KO mice (described below) were more susceptible to cisplatin-induced caspase activation and apoptosis compared to wild-type mice exposed to cisplatin99, further supporting a cytoprotective role of autophagy against cisplatin injury to cultured proximal tubular epithelial cells. Nevertheless, further work in in vivo studies has confirmed the cytoprotective role of autophagy as reported in the in vitro findings.
To evaluate the role of autophagy in vivo in cisplatin nephrotoxicity, both pharmacological and genetic approaches were used. Chloroquine, which blocks autophagy flux and impairs cargo clearance, worsened cisplatin-induced tubular damage and decline in renal function98, 99, suggesting a renoprotective role of cisplatin-induced autophagy. For a genetic approach, proximal tubule-specific autophagy-deficient mice were utilized. Cisplatin treatment caused more severe tubular damage and renal dysfunction in proximal tubule-specific Atg5-KO mice100 as well as in Atg7-KO mice99 compared to cisplatin-administered wild-type mice. Autophagy-deficient mice exhibited enhanced activation of p53, apoptosis (measured by TUNEL assay) and c-Jun terminal kinase signaling pathways known to cause cisplatin-induced AKI. In addition, cisplatin treatment markedly increased damaged mitochondria in immortalized Atg5-deficient proximal tubular cells compared to control cells100.
Autophagy in response to sepsis
Autophagy in rat proximal tubules was transiently induced at 3 h but declined at 9 h until 18 h during cecal ligation and puncture (CLP) model of sepsis102. Augmentation of autophagy either by temsirolimus or an inducer of AMP kinase, 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), protected proximal tubules and improved renal function in a mouse model of endotoxemia103. In these studies, endotoxemia was associated with activation of mTORC1. Therefore, it is likely that mTORC1 inhibition enhances autophagic flux. The inability of older mice to recover from AKI has been attributed to an age-dependent loss of autophagy. In the CLP model, induction of autophagy by AICAR-induced activation of AMPK decreased circulating cytokines, endothelial activation, and improved decline in renal function104. Contrary to the accepted paradigm, a recent study has reported that CaMKIV-dependent preservation of mTORC1 is indispensable in LPS-induced autophagy in renal tubular cells and macrophages both in vitro and in vivo105. However, additional studies are required to investigate the role of mTORC1-mediated induction of autophagy in sepsis-induced AKI. Pexophagy, a form of selective autophagy, was recently shown to be induced in LPS-induced AKI and lysosomal defect accumulated dysfunctional peroxisomes that promoted oxidative injury75.
Triggers for induction of autophagy in AKI
A wide range of cellular stresses can trigger autophagy during the pathogenesis acute kidney injury. Production of ROS and subsequent oxidative stress are involved in the development of AKI. ROS are known to trigger autophagy in mammalian cells and tissues106, 107. Mitochondria abundantly present in the kidney are vulnerable to damage by ROS that leads mitochondrial dysfunction, loss of mitochondrial membrane potential initiation of mitophagy108. ER stress contributes to the development of AKI in humans and in animal models of AKI109-111. ER stress is known to induce autophagy in mammalian cells111, 112 and in the kidney113. Tunicamycin-induced ER stress and pharmacological compound Bix that activates UPR afforded protection against renal ischemia-reperfusion (IR) injury114, 115 and ER-induced autophagy was shown to be involved in this protection113. Energy sensitive kinases mTORC1 and AMPK that are important components of AKI, are known to regulate autophagy24, 26, 28, 29, 31. mTORC1, phosphorylates ULK1/2 and Atg13 that inhibit ULK1/2 kinase activity23, 28 and therefore negatively regulates autophagy. AMPK acts as an appositive regulator of autophagy by inactivating mTORC1 and activating ULK1/2 kinase26, 31. Other molecules including antiapoptotic members of the Bcl-2 family, BNIP3, HIF, and p53 that play important roles in AKI are known to induce autophagy116. The specific roles of induction of autophagy in response to these triggers are yet to be precisely elucidated in models of AKI.
Interplay of autophagy, apoptosis, and caspases and their interactions in AKI
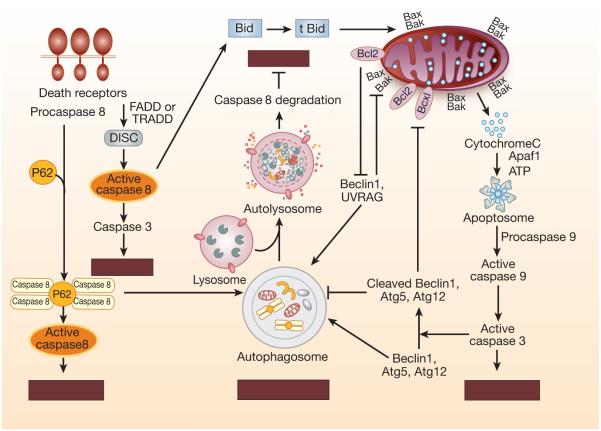
Both autophagy and apoptosis are generally induced in response to a common stimulus. The regulation of shared pathways between autophagy and apoptosis determines the outcome of cell fate for either cell survival or cell death. The cross-talk between autophagy and apoptosis is mediated by several key molecules including members of Bcl-2 family, autophagy proteins, and apoptosis related proteins including caspases (Figure 3, Table 1). Levine and colleagues first identified that beclin-1 interacts with the Bcl-2 antiapoptotic protein and inhibits autophagy117, 118. Further studies demonstrated that beclin-1, through its BH3-only domain, is able to bind to other Bcl-2 antiapoptotic proteins including Bcl-2, Bcl-xL, Bcl-x, Bcl-B, and, to a lesser extent, Mcl-1119, 120. Bcl-2 bound to beclin-1 retains its antiapoptotic function121. Under nutrient-rich conditions, beclin-1 is bound to antiapoptotic Bcl-2 proteins and thereby inhibits autophagy118. Under starvation conditions, JNK1-mediated phosphorylation of Bcl-2 prevents its interaction with beclin-1, enabling free beclin-1 to promote autophagy by binding to the class III PI3K complex122. In addition, other proteins that disrupt interaction between the beclin-1 and antiapoptotic Bcl-2 family to promote positive regulation of autophagy are BH3-only proteins including BNIP3, NIX, NOXA, PUMA, BID, and BAD123, 124. Similarly, BH3 mimetics can displace Bcl-2 from beclin-1 and activate a pro-autophagic pathway125, 126. In a renal ischemia-reperfusion model, although Adv-Bcl-xL administration significantly reduced beclin-1 expression76 and Bcl-2 augmentation suppressed autophagy78, the interaction between Bcl-xL and beclin-1 in this model of AKI has not been elucidated.
Figure 3.
Cross-talk between apoptosis and autophagy. Common proteins in the autophagy and apoptotic pathways are shared and intimately linked in cross-talk between apoptosis and autophagy regulating cell death in mammalian cells. Degradation of key autophagy proteins such as Atg5, Atg3, Atg4D, Beclin-1 and Atg12 by active caspases represents one mechanism that limits continuous autophagosome formation. Antiapoptic Bcl-2 family members bind to Beclin-1 and restrict availability Beclin-1 for autophagosome formation and suppress autophagy. Autophagy substrate P62 participates in recruitment of caspase-8 that facilitates self-oligomerization of caspase-8 and subsequent activation. Caspase-8 once recruited to the autophagosome can also be degraded that suppresses apoptosis.
Table 1.
Molecular interactions between apoptosis and autophagy
| Apoptosis-related proteins |
Autophagy-related proteins |
Effects of interaction | References |
|---|---|---|---|
| Bcl-2, BclxL, Bcl-x, Mcl-1 (antiapoptotic proteins) |
Beclin-1 | Beclin-1 binding to antiapoptotic proteins suppresses autophagy |
104,105,106, 107 |
| Bcl-2 and Mcl-1 | Atg12 | Atg12 enhances mitochondrial-targeted apoptosis by binding and antagonizing Bcl-2 and Mcl-1 |
178 |
| Caspase-3, -7, -8 | Beclin-1, PI3KC3/Vps 34 |
Cleavage of Beclin-1 and PI3KC3/Vps 34 by caspases suppresses autophagy and cleaved Beclin-1 fragments enhance mitochondrial apoptosis |
120,122,124 |
| Caspase-8 | Atg5 | Caspase-8-mediated cleavage of Atg5 enhances apoptosis |
123 |
| Caspase-8-FADD complex |
Atg5-Atg12 complex | Caspase-8 activation results in apoptosis |
114, 115, 116 |
| Caspase-3 | Atg4D | Atg4D cleaved by caspase-3 increases autophagy and mitochondrial-targeted apoptosis |
126 |
| Caspase-3, -7 | Atg16L1 | Caspase degradation of Atg16L1 suppresses autophagy |
127 |
| Caspases | Ambra1 | Suppression of autophagy | 128 |
| Bnip3, Nix, Noxa, Puma, bid, and Bad |
Bcl-2 - Beclin-1 | Promote autophagy by disruption of interaction between Bcl-2 and Beclin-1 |
110,111 |
| Bax | Beclin-1 interacting UV radiation-associated gene (UVRAG) |
UVRAG prevents translocation of Bax to mitochondria and prevents apoptosis |
179 |
| Bim | Beclin-1 | Bim sequesters Beclin-1 and suppresses autophagy |
180 |
| DAP kinase | Beclin-1 | DAPK Phosphorylate BH3 domain of Beclin-1 and promotes dissociation of Beclin-1-BclxL and induction of autophagy |
181 |
| DAP kinase | VPS34 | DAP kinase activates VPS34 via PKD and upregulates autophagy |
182 |
| DAP kinase 2 | mTORC1 | Interacts with mTORC1 and its partners, raptor and ULK1 and negatively regulates mTORC1 |
183 |
| cFLIP | Atg3 | cFLIP prevents Atg3 conjugation to LC3 and suppresses autophagy |
174, 175 |
| Cytoplasmic p53 | FIP200 | Cytoplasmic p53 suppresses autophagy by interacting with FIP200 |
184 |
| Nuclear p53 | ULK1, sestrin1/2, and DRAM |
Nuclear p53 induces autophagy by tranactivation of ULK1, sestrin1/2, and DRAM |
185-187 |
Caspases also play important roles in the regulation of autophagy, in addition to their role in apoptosis. Caspase-8 has been shown to interact as a caspase-8-FADD complex with the Atg5-Atg12 complex127-129, be recruited to the autophagosome130 and activated, leading to autophagy-mediated apoptosis (Figure 3, Table 1). p62 has also been shown to participate in recruitment of caspase-8 to the autophagosome. Ubiquitinated caspase-8 binds to p62 through its ubiquitin binding domain (UBD) and is recruited to the autophagosome by binding to LC3-II through the LC3-interacting region (LIR) of p62130, 131.The recruitment to the autophagosome by p62 facilitates self-oligomerization of caspase-8 and subsequent activation.
Both autophagy and caspases are activated in response to cisplatin in the renal tubular cell in vitro and in vivo. Autophagy induction is an immediate response, whereas caspases are activated following a pre-apoptotic lag phase in response to cisplatin94, 95, 132. It has been demonstrated that an initial induction of autophagy was responsible for the pre-apoptotic lag phase in cisplatin injury94, 95, 132. Inhibition of cisplatin-induced autophagy by pharmacological inhibitors 3-MA or Wortmannin or bafilomycin A and by siRNA specific to Atg5 or beclin-1 enhanced caspase-3/7 and -6 activation94, 132 and apoptosis94, 95, 132, and overexpression of Atg5 and beclin-1 proteins prevented cisplatin-induced caspase activation and apoptosis98 in cultured renal proximal tubular epithelial cells. Cisplatin administration following inhibition of autophagy by chloroquine or by utilizing proximal tubule-specific Atg5-KO or Atg7-KO mice enhanced apoptosis and caspase activation4, 98, 99.
Caspases are able to cleave several key autophagy proteins including beclin-1133-135, Atg5136, VPS34137, ATG3138, ATG4D139, Atg16 L140, and AMBRA1141 that result in suppression of autophagy (Table 1). The cleaved fragments of autophagy proteins produced have been shown to have a pro-apoptotic function. The protein fragments produced upon cleavage of beclin-1 by caspase-3, -6, or -9, and caspase-3 cleaved fragments of Atg4D, can localize to the mitochondria, resulting in mitochondrial permeabilization leading to cytochrome c release137, 139. In cisplatin nephrotoxicity, autophagy proteins beclin-1, Atg5, and Atg12 were cleaved and degraded during the course of in RTEC in vitro and in vivo and the pancaspase inhibitor zVAD-fmk prevented cleavage of autophagy proteins98. Thus, autophagy proteins are targets of cisplatin-induced caspase activation and the degradation of autophagy proteins is responsible for the decrease in autophagy during the course of cisplatin injury.
These studies suggest a cross-talk between autophagy, caspases, and apoptosis in renal injury in in vitro and in vivo models of AKI. Although not understood completely, some information on the signaling pathways involved in cross-talk between autophagy and apoptosis has been obtained from studies in non-renal cells. The signaling mechanisms in interactions between autophagy and apoptosis in AKI have not been yet elucidated.
Autophagy and necroptosis
Necroptosis is regulated necrosis or a programmed form of necrotic cell death that is mediated by receptor-interacting protein kinase-1 (RIPK1), RIPK3, and mixed-lineage kinase domain-like protein (MLKL). Necroptosis has morphological features similar to that of unregulated necrotic cell death and was initially observed in response to inhibition of caspase-8 in vitro142, 143 and in caspase-8-deficient mice in vivo144, 145. Diverse stimuli including death receptors of the TNF-α superfamily143, 146, genotoxic stress147, 148, Toll-like receptors149, 150, interferons151, oxidative stress152, 153, and virus-induced activation of DNA-dependent activator of IFN regulatory factors (DAI)133, 154. The process of necroptosis is very well studied, initially in the TNF-α signaling pathway.
Upon activation of the death receptor TNFR1 by binding with a TNF-α ligand, the active receptor recruits several proteins including TRADD (TNF receptor-associated death domain protein), RIP1 (receptor-interacting protein 1), TRAFs (TNF receptor-associated factor proteins), cIAP1 or cIAP2 (cellular inhibitors of apoptosis) and forms complex 1155. In the complex 1, RIP1 is polyubiquitinated by cIAP1 and cIAP2, and it prevents the activation of caspase-8, and promotes cell survival156, 157. The deubiquitination of RIPK1 by cylindromatosis (CYLD)158 promotes the formation of the pro-apoptotic complex IIa comprised of TNFR1, TRADD, FADD, RIPK1, RIPK3, and procaspase-8, This complex, also known as death inducing signaling complex (DISC), facilitates activation of caspase-8, which subsequently activates the executioner caspases to promote cell apoptosis159. When caspase-8 is not fully active or inhibited by viral or chemical inhibitors or the levels of RIPK3 and MLKL are high, a necrosome composed of RIPK1, RIPK2, and MLKL is formed that results in necroptosis160-162. Active caspase-8 has been reported to inhibit necroptosis by cleaving RIPK1, RIPK3, and CYLD163-165. RIPK1 associate with RIPK3 through their unique interacting domain known as RIP homotypic interaction motif (RHIM), leading to the formation of supramolecular complex necrosome. RIPK3 in the complex is activated by autophosphorylation that enables RIPK3 to phosphorylate MLKL166. The phosphorylation of MLKL leads to the formation of MLKL oligomers that translocate from the cytosol to the plasma membrane. It has been suggested that binding of MLKL oligomers to phosphatidylinositol phosphates in plasma membranes disrupt membrane integrity167, 168 and induce influx of Ca+2 and Na+, causing membrane rupture or necrosis168-170.
Necrostatin-1 (an inhibitor of RIPK1, RIPK3-KO, and MLKL-KO) mice were recently used to demonstrate the role of necroptosis in IR- and cisplatin-induced AKI. Inhibition of necroptosis by necrostatin-1 provided protection from tubular damage and prevented a decline in renal function during IR-171 and cisplatin-induced172 AKI. RIPK3-KO mice were protected from tubular necrosis and renal dysfunction during renal IR173. Both RIPK3-KO and MLKL-KO mice subjected to cisplatin nephrotoxicity were resistant to cisplatin-induced necroptosis and renal dysfunction172. Inhibition of apoptosis was unable to prevent a cisplatin-induced decline in renal function98, 172, 174, and it was suggested that cisplatin-induced cell death is predominantly due to necroptosis172.
Autophagy induction has been shown to regulate necroptosis in some models128, 175, 176. Pancaspase-inhibitor zVAD induced autophagy, as well as caspase-independent cell death that involved RIPKI175 but autophagy provided a protective role in zVAD-induced cell death177. Inhibition of autophagy has been shown to prevent necroptosis178 and conversely, inhibition of necroptosis suppressed not only necrosis but also autophagy179. While insufficient production of bioenergetics or ATP depletion results in induction of both autophagy10, 180 and necrosis/necroptosis181-183, autophagy induction promotes production of ATP and meets energy needs to ensure cell viability177, 180, 184, 185. Some molecular mechanisms that link autophagy with RIPK1-mediated necroptosis have been reported128. A recent study has identified that RIPK3 interacts with the selective autophagy substrate p62 and regulates the p62-LC3 complex via caspase-8-dependent cleavage of p62186. Another study has shown that Atg3 and cFLIP participate in a cross-talk between autophagy and necroptosis. cFLIP mediates the initiation of necroptosis by inhibiting caspase-8, binds to Atg3 and prevents its conjugation to LC3, and blocks the formation of LC3-II and autophagy187, 188. Since autophagy plays a protective role in acute kidney injury, more studies are required to elucidate whether autophagy influences AKI-induced necroptosis48.
Conclusions
There is a growing interest in studying the role and regulation of the autophagy pathway in AKI partly because of the notion that autophagy is cytoprotective in response to various stresses. The development of kidney-specific KO mice has provided evidence that deletion of autophagy in proximal tubules worsens tubular injury and renal function. However, direct evidence of the role of overexpression of autophagy in AKI is not yet known. There is a need to specifically induce autophagy in proximal tubules to examine the effect in models of AKI. Also, there is a need to develop effective pharmacological inducers of autophagy that are specifically able to induce autophagy without having side effects on other metabolic pathways. Although pharmacological inhibition of mTOR may upregulate autophagy, the mTOR pathway is also an important regulator of cell growth and proliferation and cellular homeostasis. The pathogenesis of AKI may result in multiple stresses that induce autophagy. Whether signaling pathways transduced from these stresses converge into suppression of the mTOR pathway for the induction of autophagy is yet to be elucidated.
At present, the efficiency of the autophagic flux of the autophagy pathway in AKI is not known. The precise kinetics of autophagy activation during the course of development of AKI in experimental models is not yet established. It is not known whether autophagy is insufficient or the flux is impaired during AKI. Since efficient flux is critical for survival, more careful studies are required for measurement of autophagic flux in vivo during AKI. Since lysosomes are involved in autophagy cargo clearance it may be necessary to critically assess lysosomal dysfunction in the pathogenesis of AKI. Recent studies have recognized that autophagy is selective in degrading specific targets including damaged organelles. However, more studies are required to understand precise mechanisms of selective autophagy including mitophagy in AKI.
Finally, both in animal models and humans severity of AKI is linked with the progression of CKD. About 15-20% patients with AKI progress to CKD stage IV. Considering tubulointerstitial fibrosis as the hallmark of end stage renal disease and the basic degradative potential of normal autophagic flux, there is a need to explore whether autophagy defect plays a role in the progression of AKI to CKD.
ACKNOWLEDGMENTS
This work was supported by grants from by the Veterans Administration (VA Merit Review BX000444 and Translational Research Institute grant UL1TR000039 through the NIH National Center for Advancing Translational Sciences to GPK and VA Merit Review BX001519 to SVS. The authors would like to thank Cindy Reid for technical editing support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.De Duve C. The lysosome. Sci Am. 1963;208:64–72. doi: 10.1038/scientificamerican0563-64. [DOI] [PubMed] [Google Scholar]
- 2.Deter RL, De Duve C. Participation of lysosomes in cellular autophagy induced in rat liver by glucagon. J Cell Biol. 1967;35:C11–16. doi: 10.1083/jcb.35.2.c11. P. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klionsky DJ. Autophagy revisited: a conversation with Christian de Duve. Autophagy. 2008;4:740–743. doi: 10.4161/auto.6398. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroemer G, Marino G. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. B. L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kundu M, Thompson CB. Autophagy: basic principles and relevance to disease. Annu Rev Pathol. 2008;3:427–455. doi: 10.1146/annurev.pathmechdis.2.010506.091842. [DOI] [PubMed] [Google Scholar]
- 9.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11:709–730. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell RC, Yuan HX, Guan KL. Autophagy regulation by nutrient signaling. Cell Res. 2014;24:42–45. doi: 10.1038/cr.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19:983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- 12.Mari M, Griffith J, Rieter E, Krishnappa L, et al. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J Cell Biol. 2010;190:1005–1022. doi: 10.1083/jcb.200912089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yen WL, Shintani T, Nair U, Cao Y, et al. The conserved oligomeric Golgi complex is involved in double-membrane vesicle formation during autophagy. J Cell Biol. 2010;188:101–114. doi: 10.1083/jcb.200904075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Axe EL, Walker SA, Manifava M, Chandra P, et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tooze SA, Jefferies HB, Kalie E, Longatti A, et al. Trafficking and signaling in mammalian autophagy. IUBMB Life. 2010;62:503–508. doi: 10.1002/iub.334. [DOI] [PubMed] [Google Scholar]
- 16.Ge L, Schekman R. The ER-Golgi intermediate compartment feeds the phagophore membrane. Autophagy. 2014;10:170–172. doi: 10.4161/auto.26787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaushik S, Cuervo AM. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 2012;22:407–417. doi: 10.1016/j.tcb.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tooze SA, Yoshimori T. The origin of the autophagosomal membrane. Nat Cell Biol. 2010;12:831–835. doi: 10.1038/ncb0910-831. [DOI] [PubMed] [Google Scholar]
- 19.Rubinsztein DC, Shpilka T, Elazar Z. Mechanisms of autophagosome biogenesis. Curr Biol. 2012;22:R29–34. doi: 10.1016/j.cub.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14:759–774. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- 21.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Jung CH, Jun CB, Ro SH, Kim YM, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosokawa N, Hara T, Kaizuka T, Kishi C, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara T, Takamura A, Kishi C, Iemura S, et al. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol. 2008;181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan EY, Longatti A, McKnight NC, Tooze SA. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol Cell Biol. 2009;29:157–171. doi: 10.1128/MCB.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunlop EA, Tee AR. mTOR and autophagy: a dynamic relationship governed by nutrients and energy. Semin Cell Dev Biol. 2014;36:121–129. doi: 10.1016/j.semcdb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Russell RC, Tian Y, Yuan H, Park HW, et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol. 2010;191:155–168. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koyama-Honda I, Itakura E, Fujiwara TK, Mizushima N. Temporal analysis of recruitment of mammalian ATG proteins to the autophagosome formation site. Autophagy. 2013;9:1491–1499. doi: 10.4161/auto.25529. [DOI] [PubMed] [Google Scholar]
- 35.Carlsson SR, Simonsen A. Membrane dynamics in autophagosome biogenesis. J Cell Sci. 2015;128:193–205. doi: 10.1242/jcs.141036. [DOI] [PubMed] [Google Scholar]
- 36.Reggiori F, Tooze SA. Autophagy regulation through Atg9 traffic. J Cell Biol. 2012;198:151–153. doi: 10.1083/jcb.201206119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puri C, Renna M, Bento CF, Moreau K, et al. ATG16L1 meets ATG9 in recycling endosomes: additional roles for the plasma membrane and endocytosis in autophagosome biogenesis. Autophagy. 2014;10:182–184. doi: 10.4161/auto.27174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, et al. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci. 2003;116:1679–1688. doi: 10.1242/jcs.00381. [DOI] [PubMed] [Google Scholar]
- 39.Romanov J, Walczak M, Ibiricu I, Schüchner S, et al. Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation. EMBO J. 2012;31:4304–4317. doi: 10.1038/emboj.2012.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dooley HC, Razi M, Polson HE, Girardin SE, et al. WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol Cell. 2014;55:238–252. doi: 10.1016/j.molcel.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dooley HC, Wilson MI, Tooze SA. WIPI2B links PtdIns3P to LC3 lipidation through binding ATG16L1. Autophagy. 2015;11:190–191. doi: 10.1080/15548627.2014.996029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vergne I, Deretic V. The role of PI3P phosphatases in the regulation of autophagy. FEBS Lett. 2010;584:1313–1318. doi: 10.1016/j.febslet.2010.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutierrez MG, Munafó DB, Berón W, Colombo MI. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci. 2004;117:2687–2697. doi: 10.1242/jcs.01114. [DOI] [PubMed] [Google Scholar]
- 44.Jäger S, Bucci C, Tanida I, Ueno T, et al. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117:4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 45.Carroll B, Mohd-Naim N, Maximiano F, Frasa MA, et al. The TBC/RabGAP Armus coordinates Rac1 and Rab7 functions during autophagy. Dev Cell. 2013;25:15–28. doi: 10.1016/j.devcel.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diao J, Liu R, Rong Y, Zhao M, et al. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature. 2015;520:563–566. doi: 10.1038/nature14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshii SR, Mizushima N. Autophagy machinery in the context of mammalian mitophagy. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbamcr.2015.01.013. [Epub ahead of print] Review. [DOI] [PubMed] [Google Scholar]
- 48.Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol. 2014;16:495–501. doi: 10.1038/ncb2979. [DOI] [PubMed] [Google Scholar]
- 49.Mizumura K, Choi AM, Ryter SW. Emerging role of selective autophagy in human diseases. Front Pharmacol. 2014;5:1–8. doi: 10.3389/fphar.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khaminets A, Heinrich T, Mari M, Grumati P, et al. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature. 2015;522:354–358. doi: 10.1038/nature14498. [DOI] [PubMed] [Google Scholar]
- 51.Bjørkøy G, Lamark T, Brech A, Outzen H, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pankiv S, Clausen TH, Lamark T, Brech A, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 53.Komatsu M, Waguri S, Koike M, Sou YS, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 54.Ichimura Y, Kumanomidou T, Sou YS, Mizushima T, et al. Structural basis for sorting mechanism of p62 in selective autophagy. J Biol Chem. 2008;283:22847–22857. doi: 10.1074/jbc.M802182200. [DOI] [PubMed] [Google Scholar]
- 55.Birgisdottir ÅB, Lamark T, Johansen T. The LIR motif - crucial for selective autophagy. J Cell Sci. 2013;126:3237–3247. doi: 10.1242/jcs.126128. [DOI] [PubMed] [Google Scholar]
- 56.Stallons LJ, Funk JA, Schnellmann RG. Mitochondrial homeostasis in acute organ failure. Curr Pathobiol Rep. 2013;2013:3. doi: 10.1007/s40139-013-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhan M, Brooks C, Liu F, Sun L, et al. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. 2013;83:568–581. doi: 10.1038/ki.2012.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parikh SM, Yang Y, He L, Tang C, et al. Mitochondrial function and disturbances in the septic kidney. Semin Nephrol. 2015;35:108–119. doi: 10.1016/j.semnephrol.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kitada T, Asakawa S, Hattori N, Matsumine H, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 61.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 62.Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koyano F, Okatsu K, Kosako H, Tamura Y, et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510:162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- 65.Okatsu K, Koyano F, Kimura M, Kosako H, et al. Phosphorylated ubiquitin chain is the genuine Parkin receptor. J Cell Biol. 2015;209:111–128. doi: 10.1083/jcb.201410050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei H, Liu L, Chen Q. Selective removal of mitochondria via mitophagy: distinct pathways for different mitochondrial stresses. Biochim Biophys Acta. 2015;S0167-4889:00114–00117. doi: 10.1016/j.bbamcr.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 67.Wong YC, Holzbaur EL. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci U S A. 2014;111:E4439–4448. doi: 10.1073/pnas.1405752111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Novak I, Kirkin V, McEwan DG, Zhang J, et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16:939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu L, Feng D, Chen G, Chen M, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14:177–185. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 72.Ishihara M, Urushido M, Hamada K, Matsumoto T, et al. Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am J Physiol Renal Physiol. 2013;305:F495–509. doi: 10.1152/ajprenal.00642.2012. [DOI] [PubMed] [Google Scholar]
- 73.Declèves AE, Sharma K, Satriano J. Beneficial effects of AMP-activated protein kinase agonists in kidney ischemia-reperfusion: autophagy and cellular stress markers. Nephron Exp Nephrol. 2014 doi: 10.1159/000368932. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cui J, Shi S, Sun X, Cai G, et al. Mitochondrial autophagy involving renal injury and aging is modulated by caloric intake in aged rat kidneys. PLoS One. 2013;8:e69720. doi: 10.1371/journal.pone.0069720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vasko R, Ratliff BB, Bohr S, Nadel E, et al. Endothelial peroxisomal dysfunction and impaired pexophagy promotes oxidative damage in lipopolysaccharide-induced acute kidney injury. Antioxid Redox Signal. 2013;19:211–230. doi: 10.1089/ars.2012.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chien CT, Shyue SK, Lai MK. Bcl-xL augmentation potentially reduces ischemia/reperfusion induced proximal and distal tubular apoptosis and autophagy. Transplantation. 2007;84:1183–1190. doi: 10.1097/01.tp.0000287334.38933.e3. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki C, Isaka Y, Takabatake Y, Tanaka H, et al. Participation of autophagy in renal ischemia/reperfusion injury. Biochem Biophys Res Commun. 2008;368:100–106. doi: 10.1016/j.bbrc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 78.Isaka Y, Suzuki C, Abe T, Okumi M, et al. Bcl-2 protects tubular epithelial cells from ischemia/reperfusion injury by dual mechanisms. Transplant Proc. 2009;41:52–54. doi: 10.1016/j.transproceed.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 79.Jiang M, Liu K, Luo J, Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol. 2010;176:1181–1192. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turkmen K, Martin J, Akcay A, Nguyen Q, et al. Apoptosis and autophagy in cold preservation ischemia. Transplantation. 2011;91:1192–1197. doi: 10.1097/TP.0b013e31821ab9c8. [DOI] [PubMed] [Google Scholar]
- 81.Kimura T, Takabatake Y, Takahashi A, Kaimori J, et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol. 2011;22:902–913. doi: 10.1681/ASN.2010070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang YL, Zhang J, Cui LY, Yang S. Autophagy activation attenuates renal ischemia-reperfusion injury in rats. Exp Biol Med (Maywood) 2015 doi: 10.1177/1535370215581306. pii: 1535370215581306. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lempiäinen J, Finckenberg P, Mervaala EE, Sankari S, et al. Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1α-eNOS pathway and enhanced autophagy. Acta Physiol (Oxf) 2013;208:410–421. doi: 10.1111/apha.12120. [DOI] [PubMed] [Google Scholar]
- 85.Grahammer F, Haenisch N, Steinhardt F, Sander L, et al. mTORC1 maintains renal tubular homeostasis and is essential in response to ischemic stress. Proc Natl Acad Sci U S A. 2014;111:E2817–2826. doi: 10.1073/pnas.1402352111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wanner N, Hartleben B, Herbach N, Goedel M, et al. Unraveling the role of podocyte turnover in glomerular aging and injury. J Am Soc Nephrol. 2014;25:707–716. doi: 10.1681/ASN.2013050452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakagawa S, Nishihara K, Inui K, Masuda S. Involvement of autophagy in the pharmacological effects of the mTOR inhibitor everolimus in acute kidney injury. Eur J Pharmacol. 2012;696:143–154. doi: 10.1016/j.ejphar.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 88.Lieberthal W, Fuhro R, Andry CC, Rennke H, et al. Rapamycin impairs recovery from acute renal failure: role of cell-cycle arrest and apoptosis of tubular cells. Am J Physiol Renal Physiol. 2001;281:F693–706. doi: 10.1152/ajprenal.2001.281.4.F693. [DOI] [PubMed] [Google Scholar]
- 89.Lieberthal W, Fuhro R, Andry CC, Patel V, et al. Rapamycin delays but does not prevent recovery from acute renal failure: role of acquired tubular resistance. Transplantation. 2006;82:17–22. doi: 10.1097/01.tp.0000225772.22757.5e. [DOI] [PubMed] [Google Scholar]
- 90.Esposito C, Grosjean F, Torreggiani M, Esposito V, et al. Sirolimus prevents short-term renal changes induced by ischemia-reperfusion injury in rats. Am J Nephrol. 2011;33:239–249. doi: 10.1159/000324577. [DOI] [PubMed] [Google Scholar]
- 91.Li L, Wang ZV, Hill JA, Lin F. New autophagy reporter mice reveal dynamics of proximal tubular autophagy. J Am Soc Nephrol. 2014;25:305–315. doi: 10.1681/ASN.2013040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang LT, Chen BL, Wu CT, Huang KH, et al. Protective role of AMP-activated protein kinase-evoked autophagy on an in vitro model of ischemia/reperfusion-induced renal tubular cell injury. PLoS One. 2013;8:e79814. doi: 10.1371/journal.pone.0079814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen BL, Wang LT, Huang KH, Wang CC, et al. Quercetin attenuates renal ischemia/reperfusion injury via an activation of AMP-activated protein kinase-regulated autophagy pathway. J Nutr Biochem. 2014;25:1226–1234. doi: 10.1016/j.jnutbio.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 94.Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008;294:F777–787. doi: 10.1152/ajprenal.00590.2007. [DOI] [PubMed] [Google Scholar]
- 95.Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, et al. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 2008;74:631–640. doi: 10.1038/ki.2008.214. [DOI] [PubMed] [Google Scholar]
- 96.Inoue K, Kuwana H, Shimamura Y, Ogata K, et al. Cisplatin-induced macroautophagy occurs prior to apoptosis in proximal tubules in vivo. Clin Exp Nephrol. 2010;14:112–122. doi: 10.1007/s10157-009-0254-7. [DOI] [PubMed] [Google Scholar]
- 97.Bolisetty S, Traylor AM, Kim J, Joseph R, et al. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol. 2010;21:1702–1712. doi: 10.1681/ASN.2010030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Herzog C, Yang C, Holmes A, Kaushal GP. z-VAD-fmk prevents cisplatin-induced cleavage of autophagy proteins but impairs autophagic flux and worsens renal function. Am J Physiol Renal Physiol. 2012;303:F1239–F1250. doi: 10.1152/ajprenal.00659.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang M, Wei Q, Dong G, Komatsu M, et al. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takahashi A, Kimura T, Takabatake Y, Namba T, et al. Autophagy guards against cisplatin-induced acute kidney injury. Am J Pathol. 2012;180:517–525. doi: 10.1016/j.ajpath.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 101.Rovetta F, Stacchiotti A, Consiglio A, Cadei M, et al. ER signaling regulation drives the switch between autophagy and apoptosis in NRK-52E cells exposed to cisplatin. Exp Cell Res. 2012;318:238–250. doi: 10.1016/j.yexcr.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 102.Hsiao HW, Tsai K, Wang L, Chen Y, et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock. 2012;37:289–296. doi: 10.1097/SHK.0b013e318240b52a. [DOI] [PubMed] [Google Scholar]
- 103.Howell GM, Gomez H, Collage RD, Loughran P, et al. Augmenting autophagy to treat acute kidney injury during endotoxemia in mice. PLoS One. 2013;8:e69520. doi: 10.1371/journal.pone.0069520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Escobar DA, Botero-Quintero AM, Kautza BC, Luciano J, et al. Adenosine monophosphate-activated protein kinase activation protects against sepsis-induced organ injury and inflammation. J Surg Res. 2015;194:262–272. doi: 10.1016/j.jss.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang X, Howell GM, Guo L, Collage RD, et al. CaMKIV-dependent preservation of mTOR expression is required for autophagy during lipopolysaccharide-induced inflammation and acute kidney injury. J Immunol. 2014;193:2405–2415. doi: 10.4049/jimmunol.1302798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16:1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- 107.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 108.Wang Y, Nartiss Y, Steipe B, McQuibban GA, et al. ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy. 2012;8:1462–1476. doi: 10.4161/auto.21211. [DOI] [PubMed] [Google Scholar]
- 109.Peyrou M, Hanna PE, Cribb AE. Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol Sci. 2007;99:346–353. doi: 10.1093/toxsci/kfm152. [DOI] [PubMed] [Google Scholar]
- 110.Kitamura M. Endoplasmic reticulum stress and unfolded protein response in renal pathophysiology: Janus faces. Am J Physiol Renal Physiol. 2008;295:F323–334. doi: 10.1152/ajprenal.00050.2008. [DOI] [PubMed] [Google Scholar]
- 111.Kawakami T, Inagi R, Takano H, Sato S, et al. Endoplasmic reticulum stress induces autophagy in renal proximal tubular cells. Nephrol Dial Transplant. 2009;24:2665–2672. doi: 10.1093/ndt/gfp215. [DOI] [PubMed] [Google Scholar]
- 112.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress trigger autophagy. J Biol Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chandrika BB, Yang C, Ou Y, Feng X, et al. Endoplasmic reticulum stress-induced autophagy provides cytoprotection from chemical hypoxia and oxidant injury and ameliorates renal ischemia-reperfusion injury. PLoS One. 2015;10:e0140025. doi: 10.1371/journal.pone.0140025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prachasilchai W, Sonoda H, Yokota-Ikeda N, Ito K, et al. The protective effect of a newly developed molecular chaperone-inducer against mouse ischemic acute kidney injury. J Pharmacol Sci. 2009;109:311–314. doi: 10.1254/jphs.08272sc. [DOI] [PubMed] [Google Scholar]
- 115.Prachasilchai W, Sonoda H, Yokota-Ikeda N, Oshikawa S, et al. A protective role of unfolded protein response in mouse ischemic acute kidney injury. Eur J Pharmacol. 2008;592:138–145. doi: 10.1016/j.ejphar.2008.06.108. [DOI] [PubMed] [Google Scholar]
- 116.Livingston MJ, Dong Z. Autophagy in acute kidney injury. Semin Nephrol. 2014;34:17–26. doi: 10.1016/j.semnephrol.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liang XH, Kleeman LK, Jiang HH, Gordon G, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pattingre S, Tassa A, Qu X, Garuti R, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 119.Erlich S, Mizrachy L, Segev O, Lindenboim L, et al. Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy. 2007;3:561–568. doi: 10.4161/auto.4713. [DOI] [PubMed] [Google Scholar]
- 120.Robert G, Gastaldi C, Puissant A, Hamouda A, et al. The anti-apoptotic Bcl-B protein inhibits BECN1-dependent autophagic cell death. Autophagy. 2012;8:637–649. doi: 10.4161/auto.19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ciechomska IA, Goemans GC, Skepper JN, Tolkovsky AM. Bcl-2 complexed with Beclin-1 maintains full anti-apoptotic function. Oncogene. 2009;28:2128–2141. doi: 10.1038/onc.2009.60. [DOI] [PubMed] [Google Scholar]
- 122.Wei Y, Pattingre S, Sinha S, Bassik M, et al. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chang NC, Nguyen M, Germain M, Shore GC. Antagonism of Beclin 1-dependent autophagy by BCL-2 at the endoplasmic reticulum requires NAF-1. EMBO J. 2010;29:606–618. doi: 10.1038/emboj.2009.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mazure NM, Pouysségur J. Atypical BH3-domains of BNIP3 and BNIP3L lead to autophagy in hypoxia. Autophagy. 2009;5:868–869. doi: 10.4161/auto.9042. [DOI] [PubMed] [Google Scholar]
- 125.Malik S, Orhon I, Morselli E, Criollo A, et al. BH3 mimetics activate multiple pro-autophagic pathways. Oncogene. 2011;30:3918–3929. doi: 10.1038/onc.2011.104. [DOI] [PubMed] [Google Scholar]
- 126.Pedro JM, Wei Y, Sica V, Maiuri MC, et al. BAX and BAK1 are dispensable for ABT-737-induced dissociation of the BCL2-BECN1 complex and autophagy. Autophagy. 2015;11:452–459. doi: 10.1080/15548627.2015.1017191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pyo JO, Jang MH, Kwon YK, Lee HJ, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2005;280:20722–20729. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- 128.Bell BD, Leverrier S, Weist BM, Newton RH, et al. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sci U S A. 2008;105:16677–16682. doi: 10.1073/pnas.0808597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Laussmann MA, Passante E, Düssmann H, Rauen JA, et al. Proteasome inhibition can induce an autophagy-dependent apical activation of caspase-8. Cell Death Differ. 2011;18:1584–1597. doi: 10.1038/cdd.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Young MM, Takahashi Y, Khan O, Park S, et al. Autophagosomal membrane serves as platform for intracellular death-inducing signaling complex (iDISC)-mediated caspase-8 activation and apoptosis. J Biol Chem. 2012;287:12455–12468. doi: 10.1074/jbc.M111.309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jin Z, Li Y, Pitti R, Lawrence D, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–735. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 132.Kaushal GP, Kaushal V, Herzog C, Yang CA. Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity. Autophagy. 2008;4:710–712. doi: 10.4161/auto.6309. [DOI] [PubMed] [Google Scholar]
- 133.Cho DH, Jo YK, Hwang JJ, Lee YM, et al. Caspase-mediated cleavage of ATG6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Lett. 2009;274:95–100. doi: 10.1016/j.canlet.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 134.Luo S, Rubinsztein DC. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 2010;17:268–277. doi: 10.1038/cdd.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu Y, Zhao L, Liu L, Gao P, et al. Beclin 1 cleavage by caspase-3 inactivates autophagy and promotes apoptosis. Protein Cell. 2010;1:468–477. doi: 10.1007/s13238-010-0048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.You M, Savaraj N, Kuo MT, Wangpaichitr M, et al. TRAIL induces autophagic protein cleavage through caspase activation in melanoma cell lines under arginine deprivation. Mol Cell Biochem. 2012;374:181–190. doi: 10.1007/s11010-012-1518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wirawan E, Vande Walle L, Kersse K, Cornelis S, et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010;1:e18. doi: 10.1038/cddis.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oral O, Oz-Arslan D, Itah Z, Naghavi A, et al. Cleavage of Atg3 protein by caspase-8 regulates autophagy during receptor-activated cell death. Apoptosis. 2012;17:810–820. doi: 10.1007/s10495-012-0735-0. [DOI] [PubMed] [Google Scholar]
- 139.Betin VM, Lane JD. Caspase cleavage of Atg4D stimulates GABARAP-L1 processing and triggers mitochondrial targeting and apoptosis. J Cell Sci. 2009;122:2554–2566. doi: 10.1242/jcs.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lassen KG, Kuballa P, Conway KL, Patel KK, et al. Atg16L1 T300A variant decreases selective autophagy resulting in altered cytokine signaling and decreased antibacterial defense. Proc Natl Acad Sci U S A. 2014;111:7741–7746. doi: 10.1073/pnas.1407001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pagliarini V, Wirawan E, Romagnoli A, Ciccosanti F, et al. Proteolysis of Ambra1 during apoptosis has a role in the inhibition of the autophagic pro-survival response. Cell Death Differ. 2012;19:1495–1504. doi: 10.1038/cdd.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vercammen D, Beyaert R, Denecker G, Goossens V, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Holler N, Zaru R, Micheau O, Thome M, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 144.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oberst A, Dillon CP, Weinlich R, McCormick LL, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wilson CA, Browning JL. Death of HT29 adenocarcinoma cells induced by TNF family receptor activation is caspase-independent and displays features of both apoptosis and necrosis. Cell Death Differ. 2002;9:1321–1333. doi: 10.1038/sj.cdd.4401107. [DOI] [PubMed] [Google Scholar]
- 147.Tenev T, Bianchi K, Darding M, Broemer M, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 148.Feoktistova M, Geserick P, Kellert B, Dimitrova D, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kaiser WJ, Sridharan H, Huang C, Mandal P, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Thapa RJ, Nogusa S, Chen P, Maki JL, et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A. 2013;110:E3109–3118. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shindo R, Kakehashi H, Okumura K, Kumagai Y, et al. Critical contribution of oxidative stress to TNFα-induced necroptosis downstream of RIPK1 activation. Biochem Biophys Res Commun. 2013;436:212–216. doi: 10.1016/j.bbrc.2013.05.075. [DOI] [PubMed] [Google Scholar]
- 153.Davis CW, Hawkins BJ, Ramasamy S, Irrinki KM, et al. Nitration of the mitochondrial complex I subunit NDUFB8 elicits RIP1- and RIP3-mediated necrosis. Free Radic Biol Med. 2010;48:306–317. doi: 10.1016/j.freeradbiomed.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sridharan H, Upton JW. Programmed necrosis in microbial pathogenesis. Trends Microbiol. 2014;22:199–207. doi: 10.1016/j.tim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 155.Micheau O. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. TJ. [DOI] [PubMed] [Google Scholar]
- 156.O'Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, et al. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr Biol. 2007;17:418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vince JE, Wong WW, Khan N, Feltham R, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 158.Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ. 2010;17:25–34. doi: 10.1038/cdd.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 160.He S, Wang L, Miao L, Wang T, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 161.Cho YS, Challa S, Moquin D, Genga R, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang DW, Shao J, Lin J, Zhang N, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 163.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Feng S, Yang Y, Mei Y, Ma L, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 165.O'Donnell MA, Perez-Jimenez E, Oberst A, Ng A, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sun L, Wang H, Wang Z, He S, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 167.Dondelinger Y, Declercq W, Montessuit S, Roelandt R, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 168.Wang H, Sun L, Su L, Rizo J, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 169.Chen X, Li W, Ren J, Huang D, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cai Z, Jitkaew S, Zhao J, Chiang HC, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Linkermann A, Bräsen JH, Himmerkus N, Liu S, et al. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81:751–761. doi: 10.1038/ki.2011.450. [DOI] [PubMed] [Google Scholar]
- 172.Xu Y, Ma H, Shao J, Wu J, et al. A role for tubular necroptosis in cisplatin-induced AKI. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014080741. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Linkermann A, Heller JO, Prókai A, Weinberg JM, et al. The RIP1-kinase inhibitor necrostatin-1 prevents osmotic nephrosis and contrast-induced AKI in mice. J Am Soc Nephrol. 2013;24:1545–1557. doi: 10.1681/ASN.2012121169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sridevi P, Nhiayi MK, Wang JY. Genetic disruption of Abl nuclear import reduces renal apoptosis in a mouse model of cisplatin-induced nephrotoxicity. Cell Death Differ. 2013;20:953–962. doi: 10.1038/cdd.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yu L, Alva A, Su H, Dutt P, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 176.Bonapace L, Bornhauser BC, Schmitz M, Cario G, et al. Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest. 2010;120:1310–1323. doi: 10.1172/JCI39987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wu YT, Tan HL, Huang Q, Kim YS, et al. Autophagy plays a protective role during zVAD-induced necrotic cell death. Autophagy. 2008;4:457–466. doi: 10.4161/auto.5662. [DOI] [PubMed] [Google Scholar]
- 178.Khan M, Rizwan Alam M, Waldeck-Weiermair M, Karsten F, et al. Inhibition of autophagy rescues palmitic acid-induced necroptosis of endothelial cells. J Biol Chem. 2012;287:21110–21120. doi: 10.1074/jbc.M111.319129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang YQ, Wang L, Zhang MY, Wang T, et al. Necrostatin-1 suppresses autophagy and apoptosis in mice traumatic brain injury model. Neurochem Res. 2012;37:1849–1858. doi: 10.1007/s11064-012-0791-4. [DOI] [PubMed] [Google Scholar]
- 180.Loos B, Engelbrecht AM, Lockshin RA, Klionsky DJ, et al. The variability of autophagy and cell death susceptibility: Unanswered questions. Autophagy. 2013;9:1270–1285. doi: 10.4161/auto.25560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lemasters JJ. The mitochondrial permeability transition: from biochemical curiosity to pathophysiological mechanism. Gastroenterology. 1998;115:783–786. doi: 10.1016/s0016-5085(98)70160-x. [DOI] [PubMed] [Google Scholar]
- 182.Jin S, DiPaola RS, Mathew R, White E. Metabolic catastrophe as a means to cancer cell death. J Cell Sci. 2007;120:379–383. doi: 10.1242/jcs.03349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liang X, Chen Y, Zhang L, Jiang F, et al. Necroptosis, a novel form of caspase-independent cell death, contributes to renal epithelial cell damage in an ATP-depleted renal ischemia model. Mol Med Rep. 2014;10:719–724. doi: 10.3892/mmr.2014.2234. [DOI] [PubMed] [Google Scholar]
- 184.Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13:7271–7279. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]
- 185.Wu YT, Tan HL, Huang Q, Ong CN, et al. Activation of the PI3K-Akt-mTOR signaling pathway promotes necrotic cell death via suppression of autophagy. Autophagy. 2009;5:824–834. doi: 10.4161/auto.9099. [DOI] [PubMed] [Google Scholar]
- 186.Matsuzawa Y, Oshima S, Nibe Y, Kobayashi M, et al. RIPK3 regulates p62-LC3 complex formation via the caspase-8-dependent cleavage of p62. Biochem Biophys Res Commun. 2015;456:298–304. doi: 10.1016/j.bbrc.2014.11.075. [DOI] [PubMed] [Google Scholar]
- 187.Lee JS, Li Q, Lee JY, Lee SH, et al. FLIP-mediated autophagy regulation in cell death control. Nat Cell Biol. 2009;11:1355–1362. doi: 10.1038/ncb1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.He MX, He YW. CFLAR/c-FLIPL: a star in the autophagy, apoptosis and necroptosis alliance. Autophagy. 2013;9:791–793. doi: 10.4161/auto.23785. [DOI] [PMC free article] [PubMed] [Google Scholar]