Abstract
Bilateral symmetry is a fundamental property of the vertebrate central nervous system. Local deviations from symmetry provide various types of information about the development, evolution and function of elements within the CNS, especially the cerebral hemispheres. Here, we quantify the pattern and extent of asymmetry in cortical folding within the cerebrum of Papio baboons and assess the evolutionary and developmental implications of the findings. Analyses of directional asymmetry show a population-level trend in length measurements indicating that baboons are genetically predisposed to be asymmetrical, with the right side longer than the left in the anterior cerebrum while the left side is longer than the right posteriorly. We also find a corresponding bias to display a right frontal petalia (overgrowth of the anterior pole of the cerebral cortex on the right side). By quantifying fluctuating asymmetry, we assess canalization of brain features and the susceptibility of the baboon brain to developmental perturbations. We find that features are differentially canalized depending on their ontogenetic timing. We further deduce that development of the two hemispheres is to some degree independent. This independence has important implications for the evolution of cerebral hemispheres and their separate specialization. Asymmetry is a major feature of primate brains and is characteristic of both brain structure and function.
Keywords: Gyrification, baboon, primate brain evolution, morphological integration, developmental noise
INTRODUCTION
Primate brains exhibit a number of notable characteristics beyond their large relative size compared with other mammals. Primate brain organization is different from other mammals, with elaboration of specific cortical regions and neural circuits not known in other groups (Schenker et al. 2008; Thiebaut de Schotten et al. 2012; Rilling 2014). Human brains have long been known to be highly asymmetrical, which has been associated with handedness and hemispherical dominance for specific tasks, including language (Broca 1861; Dax 1863; Buxhoeveden et al. 2001; Thompson et al. 2001; Sun and Walsh 2006; Corballis et al. 2012; Rilling et al. 2012; Rilling 2014). Studies characterizing our closest relatives (the great apes) have noted the presence and effects of cortical asymmetries, but much less work has been done on primates beyond the hominoid clade, and with varying findings (Hopkins and Marino 2000; Hopkins and Rilling 2000; Phillips and Sherwood 2007; Hopkins et al. 2008; Balzeau et al. 2011; Fears et al. 2011; Lyn et al. 2011; Bogart et al. 2012; Sweetlove and Verniers 2013). Here we investigate asymmetry in cortical folding in the baboon (genus Papio), an Old World monkey whose neurological features have previously been examined (Rogers et al. 2007; Kochunov et al. 2010; Atkinson et al. 2015) but the asymmetry of which has not been characterized in detail.
Baboons are well situated phylogenetically to provide novel insights into human brain evolution. The genus Papio is evolutionarily close enough to humans that many genetic and developmental similarities exist (Herculano-Houzel 2009).Yet, baboons are distant enough from humans that they may exhibit instructive lineage-specific modifications (Zilles et al. 2013). Our results can thus inform human evolutionary neuroscience by providing an independent evolutionary lineage through which to investigate the relationship between the developmental timing and morphological integration of the cortex, cortical folding asymmetry, and other features relevant to the neurobiology and neuroevolution of primates in general and humans specifically.
DIRECTIONAL ASYMMETRY (DA)
The two human brain hemispheres differ dramatically from each other. Consistent directional anatomical asymmetries have been noted in the length and depth of specific brain sulci, in both the frontal and occipital petalias, and in the anatomical localization of specific tasks to particular brain hemispheres and areas (Thompson et al. 2001; Sun and Walsh 2006; Im et al. 2010; Oleksiak et al. 2011). One well-documented and functionally significant correlate of directional brain morphological asymmetry is language (Sun and Walsh 2006; Corballis 2009; Keller et al. 2009; Schenker et al. 2010). Some hemispherical differences have been linked to asymmetric gene expression during early development (Sun and Walsh 2006; Karlebach and Francks 2015).
Our understanding of human brain evolution will benefit from a broader perspective on the origin and correlates of brain asymmetry across other primate species. As the primate brain expanded, the volume of the cerebral cortex increased much faster than the number of axonal tracts linking the two hemispheres (Rilling and Insel 1999; Olivares et al. 2000, 2001). Consequently, there would be a diminished potential for communication between hemispheres, resulting from fewer direct connections and an increasing delay in signal transmission due to the larger distance between opposite hemispheres of a bigger brain (Ringo et al. 1994; Olivares et al. 2000, 2001; Oleksiak et al. 2011). As evolution would tend to favor the most efficient neural network, it should then theoretically promote a higher degree of intra-hemispherical task completion and, correspondingly, a decrease in cross-hemisphere interaction for accomplishing routine tasks. Such changes may have resulted in an increase in regional asymmetry across the hemispheres (Ringo et al. 1994; Hopkins and Rilling 2000). Detecting significant DA in the cortex of baboons might suggest that like humans and great apes, the two halves of the brain have also developed some degree of independent functionality in this species.
In statistical terms, DA, or signed asymmetry, occurs when the frequency distribution of right-left (R-L) differences is normal but the mean departs significantly from zero (Palmer and Strobeck 1992). This predictability of asymmetry direction results from genetic predisposition and is strongly influenced by developmental mechanisms under genetic control. The phenomenon of handedness in humans, where 90% of the population is right-handed, is an example of DA and indeed has neuroanatomical correlates. Population-level directionality of the frontal petalia is interpreted as an indication of handedness; i.e. a trend towards a right frontal petalia suggests systematic right-handedness and vice versa (Hopkins and Morris 1993; Hopkins 1996; Hopkins and Rilling 2000; Dadda et al. 2006; Sun and Walsh 2006). Systematic hand preferences have been documented in baboons and other non-human primates (Reviewed in Hopkins et al. 2011; Meguerditchian et al. 2011), though these preferences switch for particular tasks in contrast to the pervasive unidirectional handedness of humans. This task-dependency, and debate over whether one can consider non-humans to possess handedness at all, has introduced some uncertainty to the topic. Discovering DA for the frontal petalia in this baboon population would provide an independent neuroanatomical corroboration of behavioral evidence suggesting hand preference in this species.
FLUCTUATING ASYMMETRY (FA)
The second type of asymmetry, FA or unsigned asymmetry, involves random asymmetrical differences in an organ (Palmer and Strobeck 1992). FA is mathematically defined as the absolute value of the difference between measurements taken on the left and right sides of individuals in a population and quantifies the variability of a trait within an individual. FA is interpreted biologically as a measure of the influence of developmental noise, with the underlying assumption that, in the absence of differential developmental effects, the correlation between the two sides of the same organ should be 1. Since mirror-image sides of an organ share a genome and develop simultaneously in identical uterine environments, deviations from a perfect L-R correlation are considered to gauge the susceptibility of specific traits to developmental perturbations (Palmer and Strobeck 1986, 1992, 1997; Swaddle et al. 1994; Klingenberg and McIntyre 1998,Klingenberg 2003a; Leamy and Klingenberg 2005; Klingenberg et al. 2010).
In addition, FA sheds light on the system’s ability to produce a robust, consistent phenotype despite genetic variance within a population, which dramatically affects natural selection’s ability to shape traits (Palmer and Strobeck 1997; Klingenberg 2008). If traits have little FA, they are strongly canalized and produce a consistent phenotype across genotypic differences. This reduces the quantitative response of a phenotype to a given selection gradient, muting the effects of natural selection. Though constraints are sometimes viewed as negative, canalization can be extremely beneficial to organisms for traits vital for survival. Maintaining a narrow range of possible phenotypes despite developmental disturbances or varying DNA sequences can prevent essential features from wandering into deleterious regions of phenotype space. Furthermore, developmental stability may promote evolutionary divergence by allowing increases in genetic variation in populations. If/when canalization is then disrupted, which can happen via admixture or a change in the selection gradient, there is more genetic diversity on which natural selection can act (Rice 1998).
Significant FA in the brain and endocranium has been documented in a number of non-human primate species (McGrath et al. 1984; Cheverud et al. 1990; Hutchison and Cheverud 1992; Balzeau et al. 2011). However, no study has yet investigated as large a sample size of Old World monkeys as is presented here. As with DA, findings concerning population-level FA can be generalized with implications for the evolutionary history of the cerebral cortex and its development beyond the baboon.
CROSS-HEMISPHERE TRAIT CORRELATIONS
We can assess whether features on opposite sides of the cortex are affected by the same developmental factors by looking at inter-hemispheric covariation (Cheverud 1982,Klingenberg 2003a). Since perturbations of a developmental pathway would affect only those traits influenced by that pathway, variation of two traits would be random with respect to one another if they are produced by separate developmental modules or processes. If corresponding landmarks on opposite brain hemispheres are only weakly correlated, we can infer a difference in the factors operating to direct their development. If corresponding sulci are instead strongly correlated, this implies that perturbations during development are generating related variation in both traits, indicating they are influenced by the same developmental mechanisms (Klingenberg 2003b). Evolutionarily, traits affected by very similar sets of developmental processes would be more difficult to modify independently of one another, as a change in a given pathway would affect both features (Cheverud 1982,Klingenberg 2003a,b, 2005). In this way, FA can reveal the potential for independent evolution of the hemispheres by providing a means to assess whether specific traits in the two hemispheres respond to the same developmental perturbations in similar or different ways.
ANTISYMMETRY
Asymmetry frequency distributions can also exhibit anti-symmetry. This is a bimodal pattern that indicates that one side of an organ is generally larger than the other, but which side is larger is random across a set of individuals. In this case, individuals are predisposed to be asymmetrical but the direction of asymmetry is not specified. Though anti-symmetry is rare in biological systems (Palmer and Strobeck 1986), it is important to rule it out from data, as it can skew the results of FA testing. Anti-symmetry can be detected mathematically by examining the frequency distribution within the sample of departures from normality tending towards platykurtosis.
MATERIALS AND METHODS
STUDY POPULATION
We examined endocranial morphology in 985 pedigreed baboons (genus Papio) from the Southwest National Primate Research Center (SNPRC) in San Antonio, Texas. These animals can be linked into a single multi-generation pedigree, providing outstanding statistical power to detect the influence of genetic variation on phenotypic variation (Atkinson et al. 2015). We use the baboon taxonomic scheme that has become the consensus, in which Papio baboons are divided into six species (Zinner et al. 2013). Under this taxonomy, the SNPRC baboons we examined consist of Papio anubis, P. cynocephalus and their hybrids. Animals are kept in near-identical outdoor enclosures with standard diet and veterinary care in breeding groups of multiple females and a single adult male per enclosure, though some non-breeding males are kept in all male groups. For details about maintenance of the colony, please refer to the SNPRC website at http://txbiomed.org/primate-research-center. This population's pedigree and microsatellite genotypes are available as supplemental files in Atkinson et al. (2015).
TRAIT MEASUREMENT
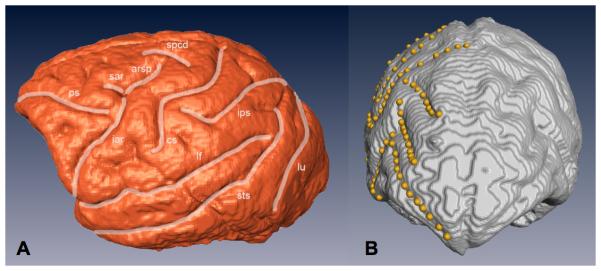
Trait measurements were collected on virtual endocasts created from CT scans of baboon skulls. A detailed description of the protocol for creating virtual endocasts and collecting sulcal length data can be found in Atkinson et al. (2015). For metric traits, points were placed along the entire length of 10 “landmark” sulci on the surface of virtual CT-based endocasts using the Amira 5 program (Stalling et al. 2005). Euclidean distances along each sulcus were calculated separately on the left and right sides. All sulci were double-measured on different days to quantify measurement error. The 10 “landmark” sulci measured were: Arcuate Rectus Spur (arsp), Central Sulcus (cs), Inferior Arcuate Rectus (iar), Intraparietal Sulcus (ips), Lateral Fissure (lf), Lunate Sulcus (lu), Principal Sulcus (ps), Superior Arcuate Rectus (sar), Superior Precentral Dimple (spcd), and the Superior Temporal Sulcus (sts) (Fig. 1A). After employing an extremely conservative data collection standard, the measurements were analyzed from a total of 770 CT scans of the left hemisphere and 800 of the right. Approximately 26% of measured endocasts were from males and 74% from females. Counts for measurements taken on both hemispheres for each trait can be found in Table 1.
Figure 1.
(A) Fully annotated CT-based virtual endocast displaying the ten landmark metric sulci on the individual’s left hemisphere. White lines represent the sulcal length paths collected, labeled with the sulcus name. (B) Fronto-superior view of an annotated CT-based virtual endocast showing an example of a typical right frontal petalia. Note the protrusion of the right hemisphere beyond the left at the frontal pole. Landmark points used in data collection appear as yellow dots along the sulcus curvature.
Table 1.
Cross-hemisphere trait correlations, the average and difference of sulcus lengths on the left (L) and right (R) hemispheres, and results of paired Student’s t-tests of homologous sulci on opposite hemispheres of the brain. All lengths are shown in millimeters. Significant p values at α=0.05 are signified with bold text. Sulci are arranged from most frontal to most occipital to highlight changes in measurements across this horizontal axis.
| Sulcus | Trait r | Avg. L | Avg. R | Avg. R–L | t-Test p | N |
|---|---|---|---|---|---|---|
| ps | 0.632 | 29.5 | 30.0 | 0.45 | <.001 | 670 |
|
| ||||||
| sar | 0.078 | 12.4 | 15.1 | 2.62 | <.001 | 491 |
|
| ||||||
| iar | 0.518 | 29.3 | 30.9 | 1.68 | <.001 | 621 |
|
| ||||||
| arsp | 0.388 | 7.75 | 8.15 | 0.398 | <.001 | 675 |
|
| ||||||
| spcd | 0.297 | 18.6 | 17.2 | −1.43 | <.001 | 429 |
|
| ||||||
| cs | 0.585 | 47.9 | 48.0 | 0.08 | 0.823 | 360 |
|
| ||||||
| ips | 0.471 | 39.3 | 36.4 | −2.92 | <.001 | 541 |
|
| ||||||
| lf | 0.405 | 50.9 | 48.4 | −2.52 | <.001 | 574 |
|
| ||||||
| sts | 0.301 | 62.6 | 53.3 | −9.26 | <.001 | 507 |
|
| ||||||
| lu | 0.472 | 36.7 | 39.0 | 2.33 | <.001 | 359 |
|
| ||||||
| Avg | 0.415 | 335 | 326 | −8.56 | 0.230 | 522.7 |
The frontal petalia was non-metrically assessed as a separate gross indicator of asymmetry. The frontal petalia was defined in its original sense as the protrusion of one hemisphere of the brain past the other at the frontal pole. Frontal petalias were logged as present (at least 1mm visible difference between frontal lobe protrusion) or absent (hemispheres are even at frontal pole), with those present categorized as left or right. Endocasts in which the frontal pole was poorly resolved were not scored. Cases in which the frontal petalia was omitted from the dataset generally involved situations where the skull was damaged.
All traits were initially screened for potentially confounding covariates: age, sex, age2, the interaction of age and sex, and cranial capacity (CC). Variation due to statistically significant covariates was removed and residual trait values used for all analyses (Atkinson et al. 2015). Age was considered as a covariate because older individuals tend to be larger that smaller ones and thus might have longer cerebral sulci. Age-squared accounts for a potential non-linear relationship between aging and sulcal lengths. CC provides a control for allometry.
Trait distributions were also screened to ensure that they conformed to expectations of normality. This included ruling out anti-symmetry and platykurtosis. Screening was done on trait residuals after centering the distributions to remove DA. No traits exhibited significantly high skewness, but iar showed a statistically significant, though moderate, negative skew (Table 2). Kurtosis excesses were generally not high, with some demonstrating slight leptokurtosis and others platykurtosis. These skewness and kurtosis estimates fall into the range of what has been previously seen for skull and brain traits in primates and do not cause concern for further interpretation of asymmetry results (Hutchison and Cheverud 1992; Balzeau and Gilissen 2010).
Table 2.
Fluctuating asymmetry of cortical sulci. F-test results and their corresponding degrees of freedom (d.f.) are shown followed by the inter-individual trait variance, average percent of phenotypic variation that could be attributed to measurement error, and the FA value. F-test values that are clearly significant are signified with bold text. Values and standard errors (SE) for skewness and kurtosis of the individual R-L differences for metric traits are also provided. An asterisk indicates a significant, moderate skew to the distribution.
| Sulcus | F ratio | d.f. | Trait var. | % error | FA | Skew | Skew SE | Kurtosis | Kurtosis SE |
|---|---|---|---|---|---|---|---|---|---|
| arsp | 5.50 | 481 | 3.53 | 11.25 | 2.33 | 0.138 | 0.106 | 0.065 | 0.212 |
|
| |||||||||
| cs | 1.88 | 502 | 3.39 | 2.96 | 2.14 | 0.035 | 0.116 | −0.012 | 0.232 |
|
| |||||||||
| iar | 0.874 | 501 | 3.71 | 4.80 | 3.71 | 0.958* | 0.109 | −0.174 | 0.217 |
|
| |||||||||
| ips | 1.56 | 300 | 9.26 | 3.93 | 2.93 | −0.377 | 0.144 | −1.367 | 0.287 |
|
| |||||||||
| lf | 1.01 | 266 | 18.36 | 3.36 | 3.51 | −0.393 | 0.158 | −1.517 | 0.315 |
|
| |||||||||
| lu | 4.97 | 214 | 7.28 | 4.07 | 3.08 | −0.082 | 0.191 | −0.446 | 0.380 |
|
| |||||||||
| ps | 3.12 | 393 | 1.61 | 2.65 | 2.13 | 0.118 | 0.130 | 0.254 | 0.260 |
|
| |||||||||
| sar | 8.31 | 395 | 27.88 | 7.13 | 4.39 | 0.508 | 0.127 | −1.128 | 0.253 |
|
| |||||||||
| spcd | 3.09 | 253 | 11.79 | 7.57 | 4.14 | 0.015 | 0.156 | −0.011 | 0.310 |
|
| |||||||||
| sts | 2.71 | 208 | 125.99 | 3.479 | 6.21 | −0.454 | 0.194 | −1.483 | 0.386 |
ASYMMETRY CALCULATIONS
For DA, paired two-tailed Student’s t-tests were done on each of the ten metric traits to determine if there was a statistically significant deviation (α=0.05) from the two sides being of equal length. FA was assessed incorporating repeated measurements for each sulcus, since deviation from a side-to-side correlation of 1 could also be due to measurement error. As DA can also skew FA testing, measurements were centered, removing DA, prior to FA analysis. FA was analyzed using a nested mixed-model ANOVA with the factors of Individual (ID), Hemisphere, and Replicate (Hutchison and Cheverud 1992; Swaddle et al. 1994) where repeated measurements are nested within brain hemisphere and hemispheres nested within individuals. The results of this nested analysis provide estimates of the amount of variance that can be explained by differences between individuals, between hemispheres within individuals (FA), and between replicates for individuals (measurement error). The equation specifying the ANOVA is:
The presence of FA is assessed by an F-test of the ratio of the mean square for Hemisphere(ID) divided by the mean square of the error for each trait. This tests if the variation in estimated FA between individuals is significantly greater than what can be explained by measurement error.
Correlations between the same sulcus on opposite sides of the brain were calculated to gauge the potential independence of the two hemispheres. Correlations were also calculated between the amount of FA present for individual traits and their embryonic day of first appearance (Sawada et al. 2012). The day entered was the average between the day of first appearance in gross anatomical dissections and fetal MRI scans. All statistical analyses were conducted using the R statistical package (R Core Team 2012).
RESULTS AND DISCUSSION
FRONTAL PETALIA
We detected a significant population-level trend towards a right frontal petalia in this baboon population. Specifically, ~two-thirds (64.4%) of individuals scored possessed a right frontal petalia, 15% displayed no asymmetry, and 20.6% had a left frontal petalia (Table S1). An example of a typical right frontal petalia can be seen in Figure 1B. Numerous studies have shown that macroscopic brain asymmetries can be associated with functional differences in the brain (Broca 1861; Thompson et al. 2001; Hopkins and Cantalupo 2004; Sun and Walsh 2006). Specifically, the presence of a frontal petalia is an indication of large-scale torque on the brain due to uneven enlargement of regions devoted to specialized tasks and is typically cited as tied to hand preference (Kertesz et al. 1990; Dadda et al. 2006; Balzeau et al. 2011; but see Phillips and Sherwood 2007). In humans, most individuals possess a right frontal petalia, and 90% of us are right-handed. Large-scale behavioral meta-datasets in both captive and wild populations have found that most ape species show population-level trends to be right handed for the bulk of tasks (chimpanzees, bonobos), though hand preference shifts depending on the type of task assigned, and orangutans appear to generally be left-handed (Lonsdorf and Hopkins 2005; Hopkins 2006; Cashmore et al. 2008; Hopkins et al. 2011). The systematic rightward bias in the frontal petalia in these pedigreed baboons is an intriguing piece of neuroanatomical support for behavioral indications of right hand preference among baboons for most tasks (Meguerditchian and Vauclair 2009; Meguerditchian et al. 2011). Intriguingly, baboons have been found to show more pronounced right hand preference for communication-related gestures than other types of movements (Meguerditchian and Vauclair 2009).
DIRECTIONAL ASYMMETRY
Every trait but cs showed statistically significant DA (Table 1). Partitioning the results by brain lobe, sulci are longer on the right anterior and left posterior portions of the brain. The only exception to this pattern is lu, which is notoriously difficult to score (Allen et al. 2006; Iaria and Petrides 2007) and had a limited sample size. Excluding the occipital lobe for lack of sufficient landmarks, there is a clear front-to-back trend over the endocasts with the frontal lobe having a R-L enlargement of 0.745 mm, the parietal lobe −1.42 mm, and the temporal lobe −5.89 mm (Table 1). The central sulcus is the apparent pivot point, its lack of significant DA demarcating the switch from longer to shorter folds. Raw metric trait measurements can be found in Table S2.
This distribution of length measurements across the cortex mirrors our petalia findings. The baboon brain has a characteristic pattern of torque, with lengthened folding patterns on the right side at the frontal pole and on the opposite hemisphere occipitally (Dadda et al. 2006; Hopkins et al. 2008; Balzeau et al. 2011). This means that, at this level of resolution, baboon cerebral organization generally matches the gross organizational pattern noted in humans and most apes.
Our detection of significant, systematic DA has important implications for the underlying control over asymmetry. Asymmetry is not completely random; instead, the baboons are genetically predisposed to be cortically lopsided in a particular direction. Asymmetries in the human brain are a feature of our highly specialized cerebrums, with the two sides functioning in remarkably different ways (Schoenemann 2006; Kanwisher 2010). These significant DA findings are circumstantial evidence that baboons might have evolved some level of lateralized functional partitioning of tasks (Gómez-Robles et al. 2014; Wey et al. 2014). Thus, baboons represent an evolutionary stage subsequent to the origin of primate brain asymmetry, but without the dramatic elaboration of that asymmetry as found in apes and humans. These Old World monkeys offer the opportunity to examine the functional correlates of hemisphere asymmetry without the overlay of the anatomical and functional elaboration underlying human language.
FLUCTUATING ASYMMETRY
Patterns of FA are informative about a variety of biological processes. From the perspective of developmental canalization, FA reveals how reliably an organism can produce a consistent phenotype in the face of various ontogenetic disturbances.F-values are used to assess the significance of FA. For the range of degrees of freedom here, a value over 1 is suggestive of significance and values approaching 2 are clear indications of significance. In these baboons, we found significant FA for some but not all cortical folding traits (Table 2). Six of the ten metric traits showed significant FA, implying that many cortical features are subject to a non-trivial amount of developmental noise, while four sulci did not show significant FA. Low amounts of population-level FA indicate that features are highly canalized; they do not fluctuate substantially in their final phenotypes, even in the face of inconsistent developmental environments. Taken together, this means that traits were affected in differing degrees by developmental noise, with some traits being more canalized than others.
Upon further examination of FA patterns, a good predictor for trait stability was the embryonic day on which a given sulcus emerges (Fig. 2). The four most canalized sulci are among the first to develop; they all are detectable before fetal day 100 (Sawada et al. 2012; Table S3). Indeed, among the first five sulci to appear, the only trait that has significant FA is sts, for which the F statistic is borderline (Table 2). Generally, baboon sulcal features appearing later in the developmental process are significantly more variable than early-emerging traits, as evidenced by a highly significant correlation between trait FA and the embryonic day of appearance (r2 = 0.638, p = 0.008).
Figure 2.
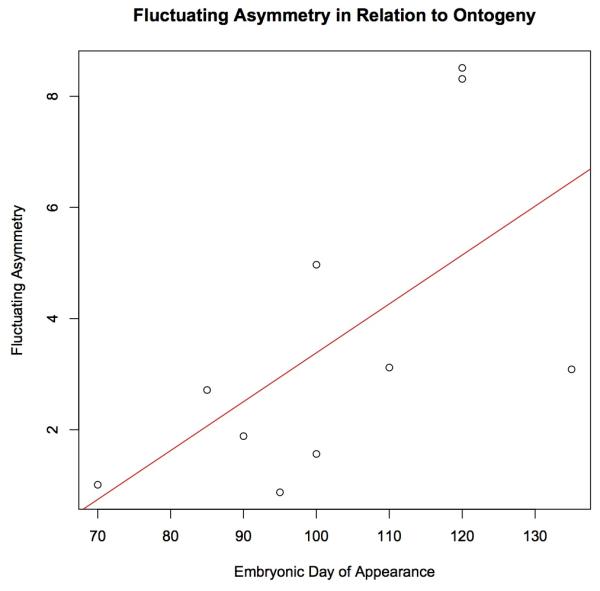
Fluctuating asymmetry is correlated with timing in ontogeny. A high amount of FA implies a large amount of trait variability, and consequently more susceptibility to perturbations during development. Low amounts of FA suggest increased trait canalization. Timing in ontogeny is defined as the first embryonic day on which the sulcus was visible. r2=0.638, p=0.008.
CROSS-HEMISPHERE CORRELATIONS
Patterns of correlation between sulci hint at the pattern of shared developmental influences or mechanisms. Correlated patterns of variation suggest that mirror-image sulci are being affected by the same perturbations during development. Here, we find moderate to low correlations for homologous sulci (average r2=0.172; Table 1), which implies that disruptive developmental effects for at least some cortical traits do not seem to span the midline of the brain.
In humans, increased asymmetry in homologous contra-lateral brain areas corresponds to fewer white matter projections physically linking the sulci (Galaburda et al. 1990). Moderate to low cross-hemisphere correlations, coupled with significant FA and DA for most traits, imply that developmental mechanisms do not generally exert equivalent effects across the midline of the cerebrum. A Mantel’s test comparing the similarity between the two hemisphere’s phenotypic variation resulted in a non-significant, low Monte-Carlo matrix correlation of 0.178 (p = 0.078), further confirming the differential variability of the two halves of the brain in this baboon population (Atkinson et al. 2015). Altogether, this implies that, despite clearly interacting for many tasks and sharing much of their ontogenetic programs, the two hemispheres of the baboon’s cerebral cortex are not tightly constrained with respect to one another in terms of future evolutionary potential. It is possible that natural selection favoring greater asymmetry could drive further specialization and independence between brain hemispheres in baboons. The degree of independence between baboon brain hemispheres also provides another lineage’s perspective on the likely basal condition of hominoid brains.
CONCLUSIONS
We find several significant indications of biologically meaningful asymmetry in the cerebral hemispheres of baboons. This set of animals displayed a population-wide predominance for a right frontal petalia. These neuroanatomical data are consistent with behavioral suggestions of general right hand preference in baboons. Significant DA was found for nearly all traits in this population, indicating a genetic predisposition to cortical asymmetry. Significant FA for some but not all traits implies that anatomical features differ in their susceptibility to developmental noise. The observed differences in FA correlate with ontogenetic timing, with traits that appear earlier in development being more highly canalized and those appearing later more susceptible to environmental effects. Cross-hemisphere sulcal correlations were generally low, implying that traits on opposite hemispheres can be affected by different developmental perturbations or mechanisms, and/or that corresponding traits on opposite hemispheres react differently to the same perturbation. The baboon brain thus seems to be open to the evolution of greater asymmetry. As far as developmental independence between the two hemispheres is concerned, natural selection has the potential to modify the hemispheres separately from one another.
Supplementary Material
Acknowledgements
This work was supported by the National Science Foundation [BCS-1260844, BCS-0725068]. Nonhuman primate resources used in this study were supported by the Southwest National Primate Research Center grant from the National Institutes of Health [P51 OD011133, formerly P51 RR013986]. Advice from Drs. Bruce Carlson, Glenn Conroy, Charles Roseman, Kirk Smith, Alan Templeton, David Van Essen and two anonymous reviewers was pivotal for this work.
Footnotes
Data Archival Location
Tables S1 and S2 contain the raw metric and non-metric phenotypic measurements for all individuals on which data was collected. Table S3 summarizes the embryonic data gleaned from Sawada et al (2012).
Conflict of Interest
The authors declare no conflicts of interest.
LITERATURE CITED
- Allen JS, Bruss J, Damasio H. Looking for the lunate sulcus: a magnetic resonance imaging study in modern humans. Anat. Rec. Part A, Discov. Mol. Cell. Evol. Biol. 2006;288:867–876. doi: 10.1002/ar.a.20362. [DOI] [PubMed] [Google Scholar]
- Atkinson EG, Rogers J, Mahaney MC, Cox LA, Cheverud JM. Cortical Folding of the Primate Brain: An Interdisciplinary Examination of the Genetic Architecture, Modularity, and Evolvability of a Significant Neurological Trait in Pedigreed Baboons. Genus Papio. 2015;200:651–665. doi: 10.1534/genetics.114.173443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzeau A, Gilissen E. J. Hum. Evol. Vol. 59. Elsevier Ltd; 2010. Endocranial shape asymmetries in Pan paniscus, Pan troglodytes and Gorilla gorilla assessed via skull based landmark analysis; pp. 54–69. [DOI] [PubMed] [Google Scholar]
- Balzeau A, Gilissen E, Grimaud-Hervé D. Shared pattern of endocranial shape asymmetries among great apes, anatomically modern humans, and fossil hominins. PLoS One. 2011;7:e29581. doi: 10.1371/journal.pone.0029581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogart SL, Mangin J-F, Schapiro SJ, Reamer L, Bennett AJ, Pierre PJ, Hopkins WD. Neuroimage. Vol. 61. Elsevier Inc; 2012. Cortical sulci asymmetries in chimpanzees and macaques: a new look at an old idea; pp. 533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca P. Perte de la parole, ramollissement chronique et destruction partielle du lobe antérieur gauche du cerveau. Bull Soc Anthr. 1861;2:235–238. [Google Scholar]
- Buxhoeveden DP, Switala AE, Litaker M, Roy E, Casanova MF. Lateralization of minicolumns in human planum temporale is absent in nonhuman primate cortex. Brain. Behav. Evol. 2001;57:349–358. doi: 10.1159/000047253. [DOI] [PubMed] [Google Scholar]
- Cashmore L, Uomini N, Chapelain A. The evolution of handedness in humans and great apes: A review and current issues. J. Anthropol. Sci. 2008;86:7–35. [PubMed] [Google Scholar]
- Cheverud J. Phenotypic, genetic, and environmental morphological integration in the cranium. Evolution (N. Y) 1982;36:499–516. doi: 10.1111/j.1558-5646.1982.tb05070.x. [DOI] [PubMed] [Google Scholar]
- Cheverud JM, Falk D, Vannier M, Konigsberg L, Helmkamp RC, Hildebolt C. Heritability of Brain Size and Surface Features in Rhesus Macaques (Macaca mulatta) J. Hered. 1990;81:51–57. doi: 10.1093/oxfordjournals.jhered.a110924. [DOI] [PubMed] [Google Scholar]
- Corballis MC. Philos. Trans. R. Soc. Lond. B. Biol. Sci. Vol. 364. The Royal Society; 2009. The evolution and genetics of cerebral asymmetry; pp. 867–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corballis MC, Badzakova-Trajkov G, Häberling IS. Right hand, left brain: Genetic and evolutionary bases of cerebral asymmetries for language and manual action. Wiley Interdiscip. Rev. Cogn. Sci. 2012;3:1–17. doi: 10.1002/wcs.158. [DOI] [PubMed] [Google Scholar]
- Dadda M, Cantalupo C, Hopkins WD. Further evidence of an association between handedness and neuroanatomical asymmetries in the primary motor cortex of chimpanzees (Pan troglodytes) Neuropsychologia. 2006;44:2582–6. doi: 10.1016/j.neuropsychologia.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dax G. Observations tendant à prouver la coïncidence constante des dérangements de la parole avec une lésion de l’hémisphère gauche du cerveau. Comptes rendus Hebd. des séances l’Académie des Sci. 1863;61:534. [Google Scholar]
- Fears SC, Scheibel K, Abaryan Z, Lee C, Service SK, Jorgensen MJ, Fairbanks L. a, Cantor RM, Freimer NB, Woods RP. Anatomic brain asymmetry in vervet monkeys. PLoS One. 2011;6:e28243. doi: 10.1371/journal.pone.0028243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM, Rosen GD, Sherman GF. Individual variability in cortical organization: its relationship to brain laterality and implications to function. Neuropsychologia. 1990;28:529–46. doi: 10.1016/0028-3932(90)90032-j. [DOI] [PubMed] [Google Scholar]
- Gómez-Robles A, Hopkins WD, Sherwood CC. Modular structure facilitates mosaic evolution of the brain in chimpanzees and humans. Nat. Commun. 2014;5:4469. doi: 10.1038/ncomms5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front. Hum. Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W. Chimpanzee handedness revisited: 55 years since Finch (1941) Psychon. Bull. Rev. 1996;3:449–457. doi: 10.3758/BF03214548. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Comparative and familial analysis of handedness in great apes. Psychol. Bull. 2006;132:538–59. doi: 10.1037/0033-2909.132.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Handedness in chimpanzees (Pan troglodytes) is associated with asymmetries of the primary motor cortex but not with homologous language areas. Behav. Neurosci. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Marino L. Asymmetries in cerebral width in nonhuman primate brains as revealed by magnetic resonance imaging (MRI) Neuropsychologia. 2000;38:493–9. doi: 10.1016/s0028-3932(99)00090-1. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Morris RD. Handedness in great apes: A review of findings. Int. J. Primatol. 1993;14:1–25. [Google Scholar]
- Hopkins WD, Phillips K. a, Bania A, Calcutt SE, Gardner M, Russell J, Schaeffer J, Lonsdorf EV, Ross SR, Schapiro SJ. J. Hum. Evol. Vol. 60. Elsevier Ltd; 2011. Hand preferences for coordinated bimanual actions in 777 great apes: implications for the evolution of handedness in hominins; pp. 605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Rilling JK. A comparative MRI study of the relationship between neuroanatomical asymmetry and interhemispheric connectivity in primates: Implication for the evolution of functional asymmetries. Behav. Neurosci. 2000;114:739–748. doi: 10.1037//0735-7044.114.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Taglialatela JP, Meguerditchian A, Nir T, Schenker NM, Sherwood CC. Gray matter asymmetries in chimpanzees as revealed by voxel-based morphometry. Neuroimage. 2008;42:491–7. doi: 10.1016/j.neuroimage.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison DW, Cheverud JM. Fluctuating asymmetry in tamarin (Saguinus) cranial morphology: intra- and interspecific comparisons between taxa with varying levels of genetic heterozygosity. J. Hered. 1992;86:280–8. doi: 10.1093/oxfordjournals.jhered.a111582. [DOI] [PubMed] [Google Scholar]
- Iaria G, Petrides M. Occipital sulci of the human brain: variability and probability maps. J. Comp. Neurol. 2007;501:243–259. doi: 10.1002/cne.21254. [DOI] [PubMed] [Google Scholar]
- Im K, Jo HJ, Mangin J-F, Evans AC, Kim SI, Lee J-M. Spatial distribution of deep sulcal landmarks and hemispherical asymmetry on the cortical surface. Cereb. Cortex. 2010;20:602–11. doi: 10.1093/cercor/bhp127. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Functional specificity in the human brain: a window into the functional architecture of the mind. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11163–70. doi: 10.1073/pnas.1005062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlebach G, Francks C. Lateralization of gene expression in human language cortex. Cortex. 2015;67:30–36. doi: 10.1016/j.cortex.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Keller SS, Roberts N, Hopkins W. A comparative magnetic resonance imaging study of the anatomy, variability, and asymmetry of Broca’s area in the human and chimpanzee brain. J. Neurosci. 2009;29:14607–16. doi: 10.1523/JNEUROSCI.2892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, Polk M, Black SE, Howell J. Sex, handedness, and the morphometry of cerebral asymmetries on magnetic resonance imaging. Brain Res. 1990;530:40–48. doi: 10.1016/0006-8993(90)90655-u. [DOI] [PubMed] [Google Scholar]
- Klingenberg C. Developmental instability: causes and consequences. Oxford University Press; New York: 2003a. Developmental instability as a research tool: using patterns of fluctuating asymmetry to infer the developmental origins of morphological integration; pp. 427–442. [Google Scholar]
- Klingenberg C, McIntyre G. Geometric morphometrics of developmental instability: analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution (N. Y) 1998;52:1362–1375. doi: 10.1111/j.1558-5646.1998.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP. A developmental perspective on developmental instability: theory, models and mechanisms. Developmental instability: causes and consequences. 2003b:14–34. [Google Scholar]
- Klingenberg CP. Developmental constraints, modules, and evolvability. In: Hallgrímsson B, Hall BK, editors. Variation. San Diego Academic Press; 2005. pp. 219–247. [Google Scholar]
- Klingenberg CP. Morphological Integration and Developmental Modularity. Annu. Rev. Ecol. Evol. Syst. 2008;39:115–132. [Google Scholar]
- Klingenberg CP, Debat V, a Roff D. Quantitative genetics of shape in cricket wings: developmental integration in a functional structure. Evolution. 2010;64:2935–51. doi: 10.1111/j.1558-5646.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Glahn DC, Fox PT, Lancaster JL, Saleem K, Shelledy W, Zilles K, Thompson PM, Coulon O, Mangin JF, Blangero J, Rogers J. Neuroimage. Vol. 53. Elsevier Inc; 2010. Genetics of primary cerebral gyrification: Heritability of length, depth and area of primary sulci in an extended pedigree of Papio baboons; pp. 1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamy LJ, Klingenberg CP. The Genetics and Evolution of Fluctuating Asymmetry. Annu. Rev. Ecol. Evol. Syst. 2005;36:1–21. [Google Scholar]
- Lonsdorf EV, Hopkins WD. Wild chimpanzees show population-level handedness for tool use. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12634–8. doi: 10.1073/pnas.0505806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyn H, Pierre P, Bennett AJ, Fears S, Woods R, Hopkins WD. Neuropsychologia. Vol. 49. Elsevier Ltd; 2011. Planum temporale grey matter asymmetries in chimpanzees (Pan troglodytes), vervet (Chlorocebus aethiops sabaeus), rhesus (Macaca mulatta) and bonnet (Macaca radiata) monkeys; pp. 2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JW, Cheverud JM, Buikstra JE. Genetic correlations between sides and heritability of asymmetry for nonmetric traits in rhesus macaques on Cayo Santiago. Am. J. Phys. Anthropol. 1984;64:401–11. doi: 10.1002/ajpa.1330640405. [DOI] [PubMed] [Google Scholar]
- Meguerditchian A, Molesti S, Vauclair J. Right-handedness predominance in 162 baboons (Papio anubis) for gestural communication: consistency across time and groups. Behav. Neurosci. 2011;125:653–660. doi: 10.1037/a0023823. [DOI] [PubMed] [Google Scholar]
- Meguerditchian A, Vauclair J. Brain Lang. Vol. 108. Elsevier Inc; 2009. Contrast of hand preferences between communicative gestures and non-communicative actions in baboons: implications for the origins of hemispheric specialization for language; pp. 167–74. [DOI] [PubMed] [Google Scholar]
- Oleksiak A, Postma A, van der Ham IJM, Klink PC, van Wezel R. J. a. A review of lateralization of spatial functioning in nonhuman primates. Brain Res. Rev. 2011;67:56–72. doi: 10.1016/j.brainresrev.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Olivares R, Michalland S, Aboitiz F. Cross-species and intraspecies morphometric analysis of the corpus callosum. Brain. Behav. Evol. 2000;55:37–43. doi: 10.1159/000006640. [DOI] [PubMed] [Google Scholar]
- Olivares R, Montiel J, Aboitiz F. Species differences and similarities in the fine structure of the mammalian corpus callosum. Brain. Behav. Evol. 2001;57:98–105. doi: 10.1159/000047229. [DOI] [PubMed] [Google Scholar]
- Palmer AR, Strobeck C. Fluctuating asymmetry and developmental stability: heritability of observable variation vs. heritability of inferred cause. J. Evol. Biol. 1997;10:39–49. [Google Scholar]
- Palmer AR, Strobeck C. Fluctuating asymmetry as a measure of developmental stability: implications of non-normal distributions and power of statistical tests. Acta Zool. Fenn. 1992;191:57–72. [Google Scholar]
- Palmer A, Strobeck C. Fluctuating asymmetry: measurement, analysis, patterns. Annu. Rev. Ecol. Syst. 1986;17:391–421. [Google Scholar]
- Phillips KA, Sherwood CC. Cerebral Petalias and Their Relationship to Handedness in Capuchin Monkeys (Cebus apella) Neuropsychologia. 2007;45:2398–2401. doi: 10.1016/j.neuropsychologia.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing. Vienna, Austria: 2012. R: A language and environment for statistical computing. [Google Scholar]
- Rice S. The evolution of canalization and the breaking of von Baer’s laws: modeling the evolution of development with epistasis. Evolution (N. Y) 1998;52:647–656. doi: 10.1111/j.1558-5646.1998.tb03690.x. [DOI] [PubMed] [Google Scholar]
- Rilling JK. Curr. Opin. Neurobiol. Vol. 28. Elsevier Ltd; 2014. Comparative primate neurobiology and the evolution of brain language systems; pp. 10–14. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Jbabdi S, Andersson J, Preuss TM. Continuity, Divergence, and the Evolution of Brain Language Pathways. Front. Evol. Neurosci. 2012;3:1–6. doi: 10.3389/fnevo.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. J. Hum. Evol. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Ringo JL, Doty RW, Demeter S, Simard PY. Time Is of the Essence: A Conjecture that Hemispheric Specialization Arises from Interhemispheric Conduction Delay. Cereb. Cortex. 1994;4:331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- Rogers J, Kochunov P, Lancaster J, Shelledy W, Glahn D, Blangero J, Fox P. Heritability of brain volume, surface area and shape: an MRI study in an extended pedigree of baboons. Hum. Brain Mapp. 2007;28:576–83. doi: 10.1002/hbm.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K, Fukunishi K, Kashima M, Saito S, Sakata-Haga H, Aoki I, Fukui Y. Fetal gyrification in cynomolgus monkeys: a concept of developmental stages of gyrification. Anat. Rec. (Hoboken) 2012;295:1065–74. doi: 10.1002/ar.22478. [DOI] [PubMed] [Google Scholar]
- Schenker NM, Buxhoeveden DP, Blackmon WL, Amunts K, Zilles K, Semendeferi K. A comparative quantitative analysis of cytoarchitecture and minicolumnar organization in Broca’s area in humans and great apes. J. Comp. Neurol. 2008;510:117–128. doi: 10.1002/cne.21792. [DOI] [PubMed] [Google Scholar]
- Schenker NM, Hopkins WD, Spocter M. a, Garrison AR, Stimpson CD, Erwin JM, Hof PR, Sherwood CC. Broca’s area homologue in chimpanzees (Pan troglodytes): probabilistic mapping, asymmetry, and comparison to humans. Cereb. Cortex. 2010;20:730–42. doi: 10.1093/cercor/bhp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenemann PT. Evolution of the Size and Functional Areas of the Human Brain. Annu. Rev. Anthropol. 2006;35:379–406. [Google Scholar]
- Stalling D, Westerhoff M, Hege H-C. Amira - A highly interactive system for visual data analysis. In: Hansen CD, Johnson CR, editors. The Visualization Handbook. Elsevier; 2005. pp. 749–767. [Google Scholar]
- Sun T, Walsh C. Molecular approaches to brain asymmetry and handedness. Nat. Rev. Neurosci. 2006;7:655–62. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- Swaddle J, Witter M, Cuthill I. The analysis of fluctuating asymmetry. Anim. Behav. 1994;48:986–989. [Google Scholar]
- Sweetlove M, Verniers J. Anatomical Asymmetries In the Baboon Brain. 2013 [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, Catani M. Cortex. Vol. 48. Elsevier Srl; 2012. Monkey to human comparative anatomy of the frontal lobe association tracts; pp. 82–96. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen V-P, Huttunen M, Lönnqvist J, Standertskjöld-Nordenstam C-G, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW. Genetic influences on brain structure. Nat. Neurosci. 2001;4:1253–8. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Wey H-Y, a Phillips K, McKay DR, Laird AR, Kochunov P, Davis MD, Glahn DC, Duong TQ, Fox PT. Multi-region hemispheric specialization differentiates human from nonhuman primate brain function. Brain Struct. Funct. 2014;219:2187–2194. doi: 10.1007/s00429-013-0620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher N, Amunts K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013;36:275–84. doi: 10.1016/j.tins.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Zinner D, Wertheimer J, Liedigk R, Groeneveld LF, Roos C. Baboon phylogeny as inferred from complete mitochondrial genomes. Am. J. Phys. Anthropol. 2013;150:133–140. doi: 10.1002/ajpa.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.