Abstract
The relationship between the host and the commensal microbiota regulates physiological functions including inflammation and immunity and it has been scrutinized in the context of cancer. While viruses and bacterial species have been implicated in oncogenesis, commensal microbes also have a beneficial role in the fight against cancer. Therapy efficacy, including adoptive T cell transfer, alkylating agents and immune checkpoint blockers, relies on immunity that receives its education from the gut microbiota. In cancer therapy with immunostimulating oligonucleotides and platinum salts, the microbiota also modulates the response by priming for the release of pro-inflammatory factors and reactive oxygen species, respectively. This new information offers promising clinical possibilities of modulating cancer therapy and its toxic side effects by targeting the microbiota.
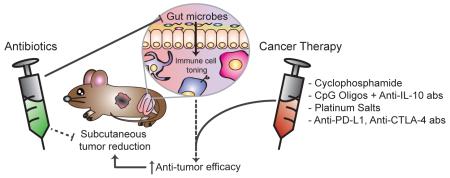
Graphical Abstract
Introduction
Over the last century, inflammation has been shown to affect cancer initiation and progression and approximately 1 out of 6 human cancers originate as a consequence of infection with pathogens [1]. While several oncogenic viruses have been identified, only infection with one bacterial species, Helicobacter pylori, has been clearly associated with an increased incidence of stomach cancer and it is considered a class 1 human carcinogen [2]. A wealth of information has been generated regarding the mechanisms of H. pylori oncogenic potential depending on direct effects on the epithelial cells or alteration of mucosal integrity, functions and associated microbiota contributing to carcinogenesis [3]. Although, guided by the principles set forth by Heinrich H. R. Koch, until recently it has been assumed that pathogenicity is an intrinsic characteristic of a microbial species or strain, new hypotheses have arisen suggesting that commensal microbes may sometimes cause pathology in hosts whose immunological environments deviate from homeostasis. The ‘bad influence’ which turns a symbiont into a disease-causing pathobiont results from genetic deficiencies in the host, often times involving dysregulated inflammation in conjunction with community-wide changes in the microbial composition termed dysbiosis—an altered biota associated with a pathological state.
The advent of high-throughput sequencing of the microbial hyper-variable 16S ribosomal RNA gene and the development of bioinformatic algorithms have allowed investigators to identify these microbes and test their collective contribution to homeostasis and disease without the need to isolate and culture each species. The abundance and diversity of these DNA sequences generate a microbial profile termed the microbiome—the genetic contribution of the microbiota. At the phylum level, the microbes in the gut that belong to the Firmicutes and Bacteriodetes groups form close to 90% of the total ecosystem. Lesser contributions from members of Cyanobacteria, Proteobacteria, Actinobacteria, Fusobacteria and Verrumicrobioa phyla comprise the rest of the community. The microbial mass in the intestine (about 2 pounds of body weight) contains an estimated 3 million genes which provide the metabolic undertaking required for the fitness of both, the microbe and the host [4]. As a result, the relative abundance and proportion of microbial taxa such as Eubacterium, Lactobacillus, Bacteriodes, Clostridium (XIVa and IVa), Faecalibacterium, Bifidobacterium and Roseburia are have been found to be important for maintaining human health [5,6]. On the other hand, investigators pursing an understanding of cancer have unearthed a variety of microbes which may contribute to carcinogenesis. In addition to H. pylori in gastric cancer, other bacterial species such as Escherichia coli, Fusobacterium nucleatum and Bacteroides fragilis have been implicated in the pathogenesis of colon cancer. The mechanism by which these microbes contribute to the pathogenesis of cancer is an area of intense research which has been recently reviewed [7,8]. In addition to the role of bacteria in inducing carcinogenesis in mucosal site on which they reside, commensal bacteria can also have a systemic effect on carcinogenesis in non-mucosal sites. For example, intestinal infection with H. hepaticus allows the development of mammary carcinomas in APCMin/+ mice [9] and commensal bacteria-induced TLR5 signaling is important for malignant progression of tumors with activated K-ras and deleted p53 [10].
Recently, a new field has emerged where the microbiota are not the cause of cancer, but, in fact, agents in the fight against it. Early evidence that gut microbiota benefits cancer treatment was provided by the observation in mice that the success of the adoptive transfer of tumor-targeting T cells depended upon the total body irradiation-induced translocation of the gut microbiota from the intestinal lumen into the mesenteric lymph nodes [11]. The efficacy of tumor-specific T-cell transfer was reduced in TLR4-deficient mice and administration of TLR4 ligand lipopolysaccharide reconstituted the response in mice depleted of commensal microbiota [11]. These data may explain one of the mechanisms by which myeloablative radiation therapy increases the response of patients with metastatic melanoma to adoptive cell therapy using tumor-infiltrating lymphocytes [12].
In this review, we discuss recent experimental findings showing that the microbiota promotes the efficacy of anti-cancer therapy and identify current clinical regimens that may benefit from modulating the microbiota composition. These include cyclophosphamide, platinum salts, as well as immune checkpoint inhibitors. This new paradigm highlights the ensorcelling relationship between host immunity, cancer and the microbiota, paving the way for new avenues of research to unravel their complex interaction.
Cyclophosphamide
Cyclophosphamide (CTX) is a successful anti-cancer alkylating drug that was approved by FDA over fifty years ago. CTX has been commonly used in combination with other therapies to target cancer cells as well as in procedures, such as bone marrow transplants, due to its immunosuppressive properties at high doses. Hence, its uses have expanded to include the treatment of autoimmune disorders including lupus erythematous and rheumatoid arthritis. However, low dose CTX inhibits T regulatory cell functions and enhances immune responses [13]. Also, CTX is one of the drugs that, following anti-tumor therapy, induces immunogenic cell death resulting in the activation of anti-tumor adaptive immunity that contributes to the drug’s efficacy [14].
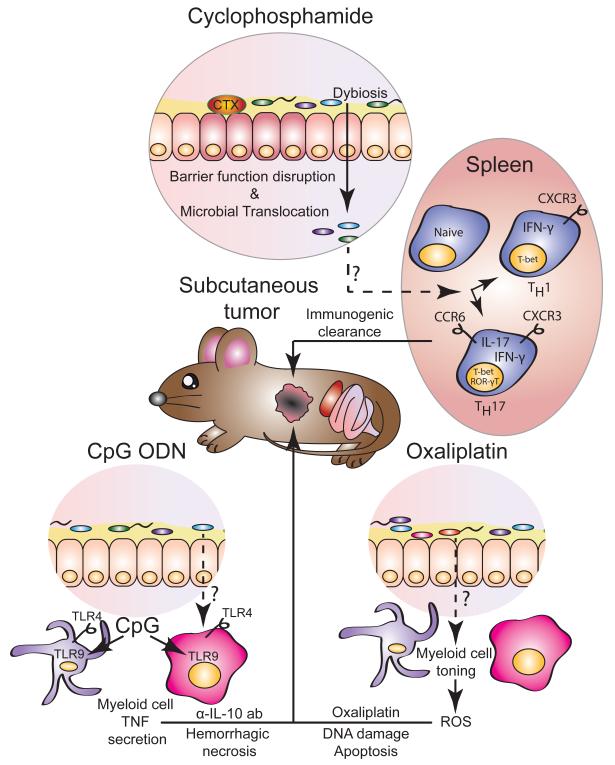
The contribution of the gut microbiota towards chemotherapeutic efficacy, was evaluated by modifying or depleting the commensal microbiota in mice by treatment with antibiotics or by raising the mice in germ-free (GF) condition. When GF mice are transferred to specific pathogen-free (SPF) conditions, they acquire a healthy, diverse biota which serves to promote the development and differentiation of the innate and adaptive immune system. In particular, segmented filamentous bacteria has been shown to be a particularly potent inducer of lamina propria T-helper 17 (Th17) cell differentiation [15]. Viaud and colleagues [16] showed that when GF mice with established transplantable MCA205 sarcomas are treated with CTX, they show poor therapeutic responses compared to SPF mice. A similar reduction in the efficacy of CTX was obtained by the deletion of gram-positive bacteria with the antibiotic vancomycin A, which associated with the accumulation of fewer ‘pathogenic’ Th17 (p Th17) cells in the spleen relative to the untreated controls. Adoptive transfer of pTh17 cells rescued the therapeutic efficacy of CTX. Overall, these findings indicated that CTX-induced gut transmucosal translocation of certain gram-positive bacteria provides the underlying immunological environment, of which pTh17 cells are an integral component, to maximize the therapeutic efficacy of CTX by invoking an anti-tumor adaptive immune response (Figure 1).
Figure 1.
The gut microbiota contributes to the efficacy of cancer therapy with chemotherapy drugs and immunomodulatory oligonucleotides.
Cyclophosphamide (CTX) treatment induces epithelial instability resulting in the breakdown of the barrier function. The translocation of the gut microbes and microbial products induces systemic changes, including the differentiation of naïve T lymphocytes into pathogenic T helper 17 cells (Th17), co-expressing genes characteristic of both Th1 (T-bet, IFN-γ and CXCR3) and Th17 (ROR-γT, IL-17 and CCR6) cells. These cells together with Th1 cells participate in mediating the immunogenic cell death of the tumor. Immunostimulatory CpG oligonucleotides (ODN) engage TLR9 in myeloid cells to induce TNF production. Coupled to the inhibitory effects of anti-IL-10 antibodies (α-IL-10 ab), the ensuing inflammatory response induces the hemorrhagic necrosis of the tumor. The therapeutic efficacy of oxaliplatin relies on reactive oxygen species (ROS) generated by myeloid cells stimulated by microbial signals. Platinum/DNA adducts and ROS induce DNA damage resulting in the apoptosis of tumor cells.
CpG Oligonucleotides and Anti-Interleukin 10 Antibodies
Toll-like receptor 9 (TLR9) is a pattern-recognition receptor used by the innate immune system to detect unmethylated CpG motifs present in microbial DNA. In response to these microbial signals, TLR9 induces the expression of pro-inflammatory cytokines. In human, synthetic CpG oligonucleotides (ODN) are able to bind TLR9 on plasmacytoid dendritic cells and B-cells thereby enhancing the body’s immune response [17]. Recent clinical trials show that administration of CpG-ODN increase the immune response to vaccines against malaria and hepatitis B virus, among others [17]. In cancer patients, CpG-ODN as a standalone therapeutic were not effective but when used in combination with peptide vaccine they enhanced anti-tumor immunity although these responses observed rarely correlated with positive clinical outcomes [17]. Unlike in humans, in the mouse TLR9 is broadly distributed in all myeloid cells. When CpG-ODN are injected intra-tumorally, particularly in combination with anti-interleukin-10 antibodies (α-IL-10 ab), they are effective against established transplantable tumors [18]. CpG-ODN induced a rapid hemorrhagic necrosis in the treated tumors, mediated by release of proinflammatory cytokines including tumor necrosis factor (TNF) followed by the induction of an anti-cancer adaptive immune response that eventually cleared the tumors in most animals [19]. Remarkably, tumors in GF mice and antibiotic-treated mice are refractory to this treatment [20]. In the microbiota-deficient animals treated with CpG ODN/α-IL-10 ab, various subsets of tumor-infiltrating myeloid cells show a reduction in IL-12 and TNF production; cytokines that are required for CpG-ODN induction of hemorrhagic necrosis of the tumor. This response is partially rescued by the administration of the TLR4 ligand, lipopolysaccharide, to microbiota-deficient animals where TLR4-deficiency abrogated the response.
The analysis of the microbial composition of animals treated with CpG-ODN/α-IL-10 ab allowed the identification of various bacterial species of the perturbed ecosystem that positively or negatively correlated with the anti-tumor response. For example, the abundance of Gram-negative Alistipes genera in the feces positively correlated with TNF production in the tumor whereas the abundance of Lactobacillus genera negatively correlated with it. In fact, when antibiotic-treated mice are reconstituted with A. shahii, the number of TNF-producing myeloid cells in the tumor is comparable to that observed in untreated mice and is significantly higher than the number observed in mice treated with antibiotics alone. Conversely, gavage instillation of conventional mice with L. fermentum reduced the TNF response. In summary, the authors’ findings demonstrate that the microbiota primes the tumor-infiltrating myeloid cells through TLR4, thereby potentiating their TNF production in response to TLR9 ligands at the site of the tumor culminating in hemorrhagic necrosis and immunogenic cell death that recruit tumor-specific CD8+ T-cells able to eradicate the tumor (Figure 1).
Platinum Salts
Oxaliplatin and cisplatin induce apoptosis of tumor-forming cells by binding DNA and causing intrastrand cross-link adducts, followed by DNA damage that results in the activation of pro-apoptotic pathways [21]. Similar to the findings with CpG-ODN treatment, Iida et al. demonstrated that antibiotics reduce the therapeutic efficacy of platinum compounds against subcutaneous transplanted tumors [20]. The anti-tumor response is also dampened in GF mice relative to SPF-reared controls. The authors presented evidence that the release of reactive-oxygen species (ROS) from myeloid cells significantly contributes to oxaliplatin-induced DNA damage. Remarkably, antibiotic treatment of mice developing subcutaneous EL4 lymphomas attenuated oxaliplatin-induced DNA damage and reduced the expression of ROS-responsive genes. The resistance to oxaliplatin was also observed in Cybb−/− mice, deficient for the myeloid NADPH-oxidase NOX2, which produce a dampened ROS response. Therefore, the authors concluded that the efficacy of oxaliplatin depended upon the priming of the myeloid cells by the gut microbiota for the release of ROS that contribute to genotoxicity and tumor reduction (Figure 1).
Immune checkpoint inhibitors
Immune checkpoints inhibitors and, in particular, monoclonal antibodies against lymphocyte-associated antigen 4 (CLTA-4) and the programmed cell death protein 1 (PD1) or its ligand PDL-1 have shown significant clinical efficacy in patients with advanced melanoma, renal cell cancer and lung cancer [22]. The combination of the two classes of antibodies has shown, in some tumors, an even more favorable clinical response, but the molecular mechanism by which these antibodies elicit their anti-tumor response is an area of intense investigation [23,24]. Antibodies against CTLA-4 block the interaction of CTLA-4 with its ligands, thereby releasing the checkpoint inhibition and favoring T cell proliferation. Depletion of regulatory T lymphocytes (Tregs) from the tumor microenvironment is also a major consequence of anti-CLTA-4 treatment that helps drive the infiltration and clearance of the tumor by cytotoxic T-lymphocytes [22,25]. PD-L1 is up-regulated in many cells in the tumor microenvironment including the tumor cells, in part due to the influx of T cells that produce IFN-γ, which up-regulates PD-L1. PD-L1 binds to PD-1 on activated T cells resulting in exhausted T cells with decreased ability to kill tumor cells; a phenomenon that it is prevented by anti-PD1 and anti-PD-L1 antibodies [22]. The immunostimulating effect of checkpoint inhibitors naturally result in immune-related adverse effects, particularly colitis and hypophysitis which are frequently observed in response to antibodies to CLTA-4 and thyroid dysfunctions and pneumonitis which are result from blocking the PD1/PD-L1 pathway [26].
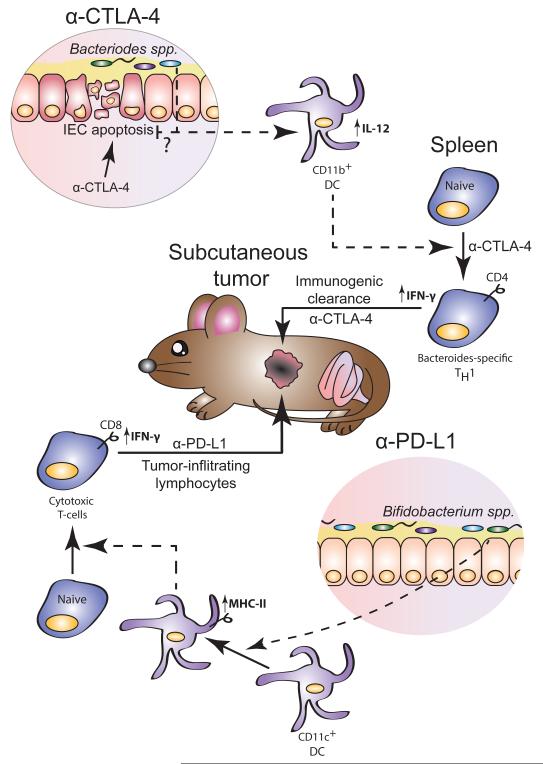
Considering the well-defined role of the microbiota in modulating the immune response both at barrier level and systemically, there was considerable interest in evaluating the role of the microbiota composition in cancer therapy using immune checkpoint inhibitors. Recently Vetizou et al. [27] reported that anti-CTLA-4 therapy of subcutaneous tumors is not effective in GF mice or in mice treated with antibiotics. They showed that the microbiota controlled the treatment efficacy in part by modulating the functions of dendritic cells that were responsible both for an anti-bacterial and anti-tumor responses amplified by anti-CTLA-4 treatment. Anti-CTLA-4 induced mucosal damage resulting in the modification of the microbiota composition both in experimental mice and in patients. The anti-tumor effect of CTLA-4 blockade depended on the abundance in the intestinal microbiota of Bacteriodes species such as B. thetaiotaomicron and B. fragilis and the proteobacteria Burkholderia cepacia. The frequency of these bacterial species was enhanced in anti-CTLA-4 treated patients. The defective response to anti-CTLA-4 in GF mice could be rescued by gavage with the three species mentioned above, by colonization with the microbiota of anti-CTLA-4 treated patients enriched with the Bacteriodes spp. associated with a positive response, by immunization with B. fragilis polysaccharides and by adoptive transfer of the B. fragilis-specific Th-1 cells elicited in conventional mice by the anti-CTLA-4 treatment. Interestingly, gavage of GF mice with a combination of B. fragilis and B. cepacia rescued, in part, the anti-tumor efficacy of anti-CTLA-4 treatment while ameliorated the mucosal toxicity with reduced colitis, thus indicating that modification of the microbiota composition could improve therapy effectiveness while preventing some of the treatment adverse reactions (Figure 2).
Figure 2.
Role of the gut microbiota in modulating immune checkpoint blocker cancer therapy. Anti-CTLA-4 treatment induces an immune activation dependent mucosal damage with dysbiosis and transmucosal microbial translocation of certain Bacteriodes spp. that are required for therapy effectiveness by inducing activation of IL-12 producing dendritic cells and activation of Bacteriodes-specific Th1 cells that together are creating a favorable immune environment for anti-CTLA-4 to stimulate a protective anti-tumor immune response. The gut microbiota is not strictly necessary for the anti-tumor effect of anti-PD-L1 but Bifibacterium spp. constitutively present in the microbiota of mice from certain mouse colonies induce an activation of CD11c+ dendritic cells that results in elevated anti-tumor response and slower tumor growth, allowing anti-PD-L1 treatment a more complete arrest of tumor growth than observed in mice missing these bacterial species.
Unlike anti-CTLA-4 treatment, a role of the gut microbiota in sustaining the mechanism of action of anti-PD-L1 treatment was not observed. Similar to the observed clinical adverse effect, anti-PD-L1 did not induce the type of mucosal toxicity and bacterial translocation observed in anti-CTLA-4 treated mice [27,28]. However, difference in the microbiota composition in mice from different vendors was observed to affect the basal level of anti-tumor immunity that result in differential tumor growth rate [28]. In particular, mice from Jackson Laboratories showed higher immunity to B16 transplantable melanoma tumors and slower tumor growth than mice obtained from Taconic Farms [28]. Mice from these two sources presented many differences in their intestinal microbiota composition but only Bifidobacterium spp. present in the Jackson mice were identified as responsible for the differential tumor growth regulation [28]. Anti-PD-L1 treatment further amplified the heightened anti-tumor immune response of Jackson mice. In Taconic mice, the antitumor effect of anti-PD-L1 was significant even though the therapeutic efficacy was lower than that observed in Jackson mice. However, when these mice were supplemented with a cocktail of Bifidobacterium spp. in addition to anti-PD-L1, tumor growth was almost completely abrogated comparable to that observed in Jackson mice.
Conclusions
The microbiota has been implicated in the promotion and resolution of cancer, demonstrating the complexity of immune system-microbe-tumor axis [29]. At homeostasis, the gut microbiota provides toning of the immune system, necessary to potentiate the effects of anti-cancer drugs. Other functions of the microbiota, such as nutrient metabolism, pathogen exclusion, and biofilm formation, have yet to be addressed in the context of cancer therapy efficacy. Also, many of the findings in experimental animals remain to be fully validated in the clinic, where little is yet known about the presence of dysbiotic microbial communities in the context of extra-intestinal cancers [30]. In the cancer therapy models in which the role of the microbiome has been analyzed, the effects were, in part, mediated by the ability of gut commensals to systemically prime various myeloid cell subsets. This is the case for early pro-inflammatory anti-tumor mechanisms (e.g. CpG-ODN therapy [20]) or genotoxic effects (e.g. platinum salts [20]) in which myeloid cells are primed to produce pro-inflammatory cytokines and tumor-damaging ROS, respectively, as well as for immune mediated effects (T cell adoptive therapy, cyclophosphamide, immune checkpoint inhibitors [11,16,27,28]) in which the microbiota appears to affect the tone of immunomodulatory and antigen-presenting myeloid cell subsets [31].
The molecular mechanisms of this priming of systemic and tumor infiltrating myeloid cells as well as how the signal is transmitted from the mucosal barrier to the distant tumor cells remain poorly characterized although the possibility has been suggested that the heightened responsiveness of myeloid cells to therapy-induced activating signals may involve microbiota-controlled epigenetic changes [32]. However, the experimental results indicate the importance to further extend the study of the role of the microbiota to regulate anti-cancer immune response and therapy in the clinical settings. Profiling the microbial community of patients, the presence of the ‘essential’ microbes could be assessed in order to potentiate clinical outcomes. Various procedures are known or could be established to modify the composition of the microbiota, including the use of selected antibiotics and bacterial species administration. The recent granting by FDA of breakthrough therapy designation to Ser-109, an orally administrated composition of purified eubacterial spores for re-establishing intestinal microbial ecology in the treatment of Clostridium difficile infections, indicated that new effective technologies will be available soon for targeted perturbation of the microbiota composition. The recent report regarding CTLA-4 blockade therapy demonstrates the possibility of dissociating the effect of the microbiota on therapeutic efficacy from its effect on the drug’s adverse reactions, thereby generating optimism that it could be possible to target the microbiota for enhancing the drug’s mechanism of action while controlling collateral toxicity [27]. The outcomes of these studies will foster the development of personalized therapeutics and novel paradigms that will elucidate the complex relationship between cancer the gut microbiota.
Highlights.
Cancer therapy efficacy is often reduced in antibiotics-treated and germ-free mice
Alkylating agents rely on microbial signals to induce anti-tumor immunity
The microbiota primes myeloid cells to produce TNF in response to CpG ODN
The microbiota primes for oxygen species production in response to platinum salts
Bacteria differentially regulate efficacy and toxicity of anti-CTLA-4 therapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. The lancet oncology. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 2.Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 3.Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10:324–335. doi: 10.1016/j.chom.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommer F, Backhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 5.Hollister EB, Gao C, Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology. 2014;146:1449–1458. doi: 10.1053/j.gastro.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Human_Microbiome_Project_Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15:317–328. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9 *.Lakritz JR, Poutahidis T, Mirabal S, Varian BJ, Levkovich T, Ibrahim YM, Ward JM, Teng EC, Fisher B, Parry N, et al. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget. 2015;6:9387–9396. doi: 10.18632/oncotarget.3328. This study provides evidence of a role of intestinal microbiota in modulating carcinogenesis at distant sites.
- 10 *.Rutkowski MR, Stephen TL, Svoronos N, Allegrezza MJ, Tesone AJ, Perales-Puchalt A, Brencicova E, Escovar-Fadul X, Nguyen JM, Cadungog MG, et al. Microbially Driven TLR5-Dependent Signaling Governs Distal Malignant Progression through Tumor-Promoting Inflammation. Cancer Cell. 2015;27:27–40. doi: 10.1016/j.ccell.2014.11.009. This study analyze th eimmunological mechanism by which the intestinal microbiota modulates carcinogenesis at distant sites.
- 11 **.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. This study provided the earliest description of intestinal microbiota translocation enhancing role of immunotherapy.
- 12.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madondo MT, Quinn M, Plebanski M. Low dose cyclophosphamide: Mechanisms of T cell modulation. Cancer Treat Rev. 2016;42:3–9. doi: 10.1016/j.ctrv.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, et al. Focused specificity of intestinal T17 cells towards commensal bacterial antigens. Nature. 2014 doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16 **.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. This study reported the role of the intestinal microbiota in modulating the anti-tumor immune response induced by cylophosphamide therapy
- 17.Scheiermann J, Klinman DM. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine. 2014;32:6377–6389. doi: 10.1016/j.vaccine.2014.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vicari AP, Chiodoni C, Vaure C, Ait-Yahia S, Dercamp C, Matsos F, Reynard O, Taverne C, Merle P, Colombo MP, et al. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J Exp Med. 2002;196:541–549. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 20 **.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. This study describes the role of the microbiota in licensing intratumoral myeloid cells that are required for the efficacy the anti-tumor therapy with immunostimulatory oligonucleotides and platinum salts
- 21.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 22.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 23.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teply BA, Lipson EJ. Identification and management of toxicities from immune checkpoint-blocking drugs. Oncology (Williston Park) 2014;28(Suppl 3):30–38. [PubMed] [Google Scholar]
- 27 **.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. This study describes the requirement of intestinal microbiota and certain Bacteriodes spp in particular for anti-CTLA-4 anti-tumor therapy by licensing antigen presenting myeloid cells to effectively induce anti-microbial and anti-tumoral immunity
- 28.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. This study that certain bacterial species and in particular Bifidobacterium spp. promotes anti-tumor immunity that is important for full effectiveness of anti-PD-L1 therapy
- 29.Dzutsev A, Goldszmid RS, Viaud S, Zitvogel L, Trinchieri G. The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur J Immunol. 2015;45:17–31. doi: 10.1002/eji.201444972. [DOI] [PubMed] [Google Scholar]
- 30.Xuan C, Shamonki JM, Chung A, Dinome ML, Chung M, Sieling PA, Lee DJ. Microbial dysbiosis is associated with human breast cancer. PLoS One. 2014;9:e83744. doi: 10.1371/journal.pone.0083744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldszmid RS, Dzutsev A, Viaud S, Zitvogel L, Restifo NP, Trinchieri G. Microbiota modulation of myeloid cells in cancer therapy. Cancer Immunol Res. 2015;3:103–109. doi: 10.1158/2326-6066.CIR-14-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, Lienenklaus S, Weiss S, Staeheli P, Aichele P, et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37:171–186. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]