Abstract
We conducted a phase 2 study to determine the efficacy of HLA-haploidentical related donor NK cells following cyclophosphamide based lymphodepletion in patients with relapsed or progressive acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS) following allogeneic HCT. Eight patients (2 with MDS, 6 with AML) were treated with cyclophosphamide 50 mg/kg on day −3 and day −2 prior to infusion of NK cells isolated from a haploidentical related donor. One person additionally received fludarabine 25 mg/m2/d × 4. Six doses of 1 million units of interleukin-2 (IL-2) were administered on alternating days beginning on day −1. The median number of infused NK cells was 10.6×106/kg (range 4.3–22.4) and the median number of CD3 cells was 2.1×103/kg (range 1.9–40). NK infusions were well tolerated with a median time to neutrophil recovery of 19 days (range 7-not reached) and no incidence of graft versus host disease after NK infusion. One patient with AML and one patient with MDS achieved a complete response but relapsed at 1.7 and 1.8 months, respectively. One patient with MDS had resolution of dysplastic features but persistence of clonal karyotype abnormalities. This patient remains stable at 65 months post NK cell therapy. The median survival was 12.9 months (range 0.8–65.3 months). Chimerism analysis of CD3−/CD56+ peripheral blood cells did not detect circulating haploidentical NK cells after infusion. NK phenotyping was performed on seven patients during and after IL-2 infusion. We found a slight trend towards greater expression of KIR2DL2/2DL3/2DS2 (5% versus 28%, P = 0.03) at 14 days in patients who survived longer than 6 months from NK cell infusion (N = 4) when compared to those who died within 6 months of NK cell therapy (N=3). In summary, these data support the safety of haploidentical NK cell infusion after allogeneic HCT.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) can result in durable remission of malignancies that arise from myeloid progenitor cells such as myelodysplastic syndrome (MDS), acute myelogenous leukemia (AML), or chronic myelogenous leukemia (CML). This is due to an increasingly recognized graft versus leukemia (GVL) effect mediated by alloreactive donor lymphocytes, including T-cells and natural killer (NK) cells.(1, 2) Unfortunately, relapse occurs in 40% of patients undergoing HCT for myeloid malignancies and there are limited options for these individuals.(3, 4) Chemotherapy may result in a subsequent remission but is poorly tolerated in transplant survivors who typically have a tenuous immune function and poor performance status. A second strategy to address relapse is the infusion of unmanipulated donor lymphocytes (DLI) to increase the potential for GVL. DLI results in remission of CML in approximately 70–90%; however, responses are much less frequent in MDS and occur only rarely in AML.(4–6) DLI may also induce serious graft versus host disease (GVHD), and thus should be used with caution. Available therapies to treat relapsed myeloid malignancy, particularly MDS and AML, therefore have low efficacy and can incur serious adverse effects. Consequently, there are few long-terms survivors of relapse after HCT.
Natural killer (NK) cells play a key role in mediating the GVL effect against myeloid malignancies, particularly AML.(7–11) An array of activating and inhibitory cell surface receptors control NK effector function against target cells, including NKG2A, NKG2D, natural cytotoxicity receptors, and the killer Ig-like receptors (KIR).(12, 13) Among these, the latter have gained considerable attention following reports associating donor KIR genotypes with NK alloreactivity and leukemia control in murine and human HLA mismatched allo HCT.(10, 14–16) NK cells stochastically express inhibitory KIR that interact with specific epitopes in class 1 HLA on target cells. KIR2DL2/3 recognize HLA-C characterized by Lys80 (HLA-C1 allotypes); KIR2DL1 recognizes HLA-C characterized by Asn80 (HLA-C2 allotypes); and KIR3DL1 recognizes HLA-B and HLA-A allotypes with the Bw4 motif.(17, 18) Additionally, the activating KIR2DS1 recognizes HLA-C2.(19) Malignant cells lacking HLA capable of binding inhibitory KIR will induce a net activating signal in NK cells leading to greater effector function in the latter. This effect is pronounced when the inhibitory KIR ligand is expressed in the transplant donor, a phenomenon referred to as activation of licensed NK cells recognizing “missing self” HLA determinants in the host.(14) NK effector function may therefore be enhanced by the intentional use of HLA-mismatched donors.
In the current study we tested whether HLA-haploidentical purified NK cells can exert a GVL effect without inducing GVHD in patients with relapsed myeloid malignancy after HLA-matched HCT. Adoptive transfer of purified NK cells offers the advantage of leukemotoxicity without the risk of GVHD, a frequent complication of unmodified DLI. Moreover, administration of a purified NK product enables the use of highly HLA-mismatched NK cell donors, promoting the likelihood of greater anti-tumor effect. The primary endpoint of the study is to determine the feasibility and safety of adoptive transfer of haploidentical NK cells for patients with myeloid malignancy that has relapsed following HCT from an HLA-matched sibling or unrelated donor. Such a strategy may capitalize on the inherent strengths of adoptive cell transfer after HCT in that it is well tolerated and has the potential to maximize the relatively lymphodepleted post transplant recipient in order to induce GVL without serious toxicity.
METHODS
Subject eligibility, response assessment, and treatment plan
Patients of any age who had relapsed or persistent AML, MDS, or blastic CML following allogeneic HCT and who were determined to be ineligible for second HCT were eligible for this study. The study was approved by the Institution Review Board and regulatory authorities. All patients gave informed consent. The study was registered at ClinicalTrial.gov with the identifier NCT00526292. Patients were required to have ≥5% bone marrow involvement, determined by morphology, karyotype, or fluorescent in-situ hybridization (FISH). Patients with measurable extramedullary disease were excluded. Patients who received other treatments for relapsed disease were not excluded, as long as patients met bone marrow criteria prior to NK infusion. Patients with GVHD were not excluded provided they did not receive systemic immunosuppression for two weeks prior to enrollment. Toxicities were graded according to the National Cancer Institutes Common Terminology Criteria for Adverse Events, v3.0. Clinical responses were assessed by established criteria.(20, 21) Response was evaluated by bone marrow aspiration/biopsy at days +30, +100, +200, and +365. Neutrophil engraftment is defined as the first day the blood neutrophil count was determined to be >500 for 3 or more consecutive days.
Cytoreductive therapy, IL-2, and supportive care
Cytoreductive chemotherapy included cyclophosphamide 50 mg/kg/day intravenously on days −3 and −2. One patient received cyclophosphamide on days −6 and −5 in addition to fludarabine 25 mg/m2/day on days −6 through −2. In order to promote in vivo expansion of donor NK cells, patients received IL-2 at a dose of 1 million units per m2 every 48 hours for six doses beginning on day −1. NK cells were infused on day 0. Patients were treated with prophylactic antimicrobials for Pneumocystis jiroveci, Herpesviridae, and candida species during therapy according to institutional guidelines.
Donor selection, leukapheresis, and immunomagnetic isolation of donor-derived NK cells for adoptive transfer
Eligible donors were HLA-haploidentical family members who met standard criteria for cell donation based on FACT/NMDP guidelines and who provided informed consent. All donors underwent KIR typing as described below. The selected donor underwent a standard 10-liter apheresis the day prior to the anticipated cell infusion. Isolated peripheral blood mononuclear cells (PBMC) were used to enrich NK cells using a 2-step procedure on the CliniMACS clinical cell selection device (Miltenyi Biotech, Gladbach, Germany) as follows: PBMC were first depleted of CD3+ cells (CD3 Reagent, Miltenyi Biotec, Auburn, CA). The CD3-negative cell product was collected, washed once and underwent a positive selection for CD56+ cells (CD56 Reagent, Miltenyi Biotec, Auburn, CA). Viability was assessed and phenotyping was performed to evaluate the absolute numbers of CD45+, CD3+, and CD56+ cells. Cytokines were not used during in vitro NK cell processing. NK cells were considered acceptable for administration if the total number of CD3+ cells did not exceed 2×105/kg, the CD56 enrichment resulted in ≥ 90% CD3−/CD56+ pure cell product, the viability was ≥70%, endotoxin was ≤5 EU/mL, and mycoplasma, gram stain and bacterial/fungal cultures were negative. Sterility samples from the final infused product were obtained and monitored in culture for 14 days.
Immunophenotyping and cell culture conditions
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll density gradient centrifugation. Effector cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 1% sodium pyruvate, and 1% 2-mercaptoethanol, supplemented with human IL-2 (Proleukin, Prometheus Laboratories Inc.; San Diego, CA) at 200 U/ml and incubated at 37°C with 5% CO 2 for 12 to 16 hours prior to assaying function. CD107 mobilization and intracellular IFN-γ production were used as indicators of effector cell activation.(22–24) PBMCs (5 × 105/well) or NK cells (1 × 105/well) were incubated with the human erythroleukemia cell line K562 (ATCC; Manassas, VA) serving as target cells at ratios of 1:5 and 1:1, respectively, for 4 hours in 96-well U-bottom plates with 200 μL medium, described above, per well. APCH7-conjugated anti-CD107a (clone H4A3, BD Biosciences; Franklin Lakes, NJ) was added to each well prior to incubation. Cells were stained with anti-CD3-BV650 (clone SK7, BD Biosciences), anti-CD56-ECD (clone N901, Beckman Coulter; Brea, CA); anti-KIR2DL1/2DS1-PEcy5.5 (clone EB6B, Beckman Coulter), anti-KIR2DL2/2DL3/2DS2-FITC (clone CH-L, BD Biosciences), anti-KIR3DL1/S1-APC (clone Z27, Beckman Coulter), anti-KIR3DL1–Alexa Fluor 700 (clone DX9, Biolegend; San Diego, CA); anti-NKG2A-PEcy7 (clone Z199, Beckman Coulter); anti-LIR-1-PE (clone HP-F1, Beckman Coulter). For IFN-γ evaluation, brefeldin-A (2 μg/ml, Sigma-Aldrich; St. Louis, MO) and GolgiStop (1 μg/ml, BD Biosciences) were added to the mixture 1 hour following incubation, and FIX & PERM (Invitrogen; Carlsbad, CA) was used for staining. Cells were analyzed using multicolor flow cytometry on a FACS LSRFortessa instrument with FACS Diva software (BD Biosciences). Results were interpreted using FlowJo software (FlowJo; Ashland, OR) and GraphPad Prism (GraphPad Software; La Jolla, CA).
RESULTS
Study population
The study population is described in Table 1. The median age at treatment was 19.0 years (range 1.9–55.9). Seven patients underwent transplantation from a HLA-matched sibling and one from a HLA-matched unrelated donor. The median time to relapse after HCT was 3.5 months (range 1–94 months). The median time from HCT to NK infusion was 6.8 months (range 3.9–152 months). Five of eight patients had cytoreductive chemotherapy prior to NK infusion. One patient with extramedullary AML had surgical cytoreduction but had residual disease at the time study enrollment. One patient was treated with azacytidine followed by infusion of donor lymphocytes without response prior study enrollment. Two patients had GVHD of the skin at the time of enrollment but did not require systemic immunosuppressive therapy. The remaining patients had been tapered from systemic immunosuppression prior to enrollment without evidence of GVHD.
TABLE 1.
Characteristics of Study Participants
| Patient | Disease | HCT Donor | NK Donor | Age (y) | Karyotype | Pre-treatment blasts | Time to relapse (m) | Time relapse to NK infusion (m) | Acute GVHD (grade) | Therapy for post HCT relapse |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MDS | Matched sib | Child | 53.8 | del 20q | 0% | 93.6 | 58.0 | None | DLI, azacitidine |
| 2 | AML | Matched sib | Sibling | 55.9 | del 20q | 0% | 33.2 | 10.0 | Skin (2) | Surgical resection |
| 3 | MPAL* | Matched sib | Parent | 29.7 | Complex | 1% | 5.2 | 1.3 | None | Chemotherapy |
| 4 | AML | Matched Sib | Parent | 8.4 | t(9;11), del 20q | 75% | 2.4 | 1.5 | Skin (2) | Chemotherapy |
| 5 | AML | Matched sib | Parent | 2.0 | t(1;5), +20q | 21% | 3.1 | 5.6 | None | Chemotherapy |
| 6 | AML | Matched sib | Parent | 1.9 | t(9p;12p) | 23% | 3.0 | 4.2 | None | Chemotherapy |
| 7 | AML | Matched sib | Parent | 2.5 | t(3q;11p) | 71% | 3.8 | 2.7 | None | Chemotherapy |
| 8 | MDS | URD | Sibling | 32.3 | Complex | 8% | 1.0 | 4.3 | None | None |
Mixed phenotype acute leukemia
NK Cell Enrichment
Leukocytes were successfully collected from all donors. The median number of total nucleated cells (TNC) collected was 130 × 108 (106–147). The CD3−CD56+ cell content ranged from 4.7–10% of the TNC. After immunomagnetic enrichment the number of contaminating CD3+ cells composed <0.1% of the cell product in all instances. The median (range) dose of CD3+ cells/recipient kg was 2.0 × 103 (0.9–40 × 103) and CD3−CD56+ cells/recipient kg was 11 × 106 (4.3–22.4 × 106) Table 2. The CD3−CD56+ viability ranged from 82–100%.
TABLE 2.
Donor Cell Dose and KIR Gene Profile
| Patient | CD3−/CD56+ per kg ×106 | CD3+/kg × 103 | Viability % | CD3−CD56+ % | CD3−CD56+ Yield % | Donor KIR Haplotype | Recipient Missing Ligand |
|---|---|---|---|---|---|---|---|
| 1 | 9.2 | 1.9 | 93 | 98.6 | 64.4 | B,B | None |
| 2 | 4.3 | 0.9 | 82 | 97.8 | 59.0 | A,B | Bw4 |
| 3 | 5.77 | 0.58 | 84 | 99.9 | 40.2 | B,B | C2 |
| 4 | 12 | 7.2 | 92 | 99.7 | 86.8 | A,B | Bw4, C2* |
| 5 | 20 | 3.3 | 89 | 99.8 | 73.0 | A,B | None |
| 6 | 19.9 | 40 | 88 | 99.9 | 41.3 | A,A | Bw4, C2* |
| 7 | 22.4 | 2.0 | 100 | 96.7 | 50.9 | A,B | None |
| 8 | 5.36 | 2.2 | 88 | 98.5 | 41.7 | A,B | Bw4 |
Ligand(s) expressed in donor
Response, neutrophil recovery, and toxicity
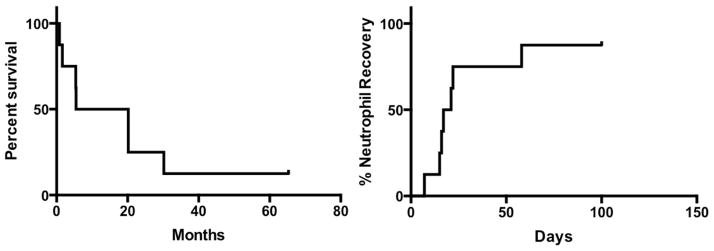
Survival of patients treated is shown in Figure 1A. Three patients achieved responses. Patients 5 and 8 developed CR after treatment, with relapse at 1.7 and 1.8 months post treatment, respectively. Both patients survived 20.2 months after haploidentical NK cell transfer. Patient 1 had donor-derived MDS associated with deletion of chromosome 20q (del20q) after HCT for lymphoma. After haploidentical NK cell transfer the patient had resolution of dysplastic features but persistent del20q. Among the 5 patients without response the median survival was 5.4 months (range 0.8–30.2 months). Patient 2 underwent second allo HCT 18 months after NK infusion. He relapsed 9.0 months from second transplant and died after failed re-induction 30.2 and 12.0 months from NK infusion and second HCT, respectively.
Figure 1.
Overall survival (A) and neutrophil recovery (B) in treated patients.
The median time to neutrophil recovery was 19 days, (Figure 1B). One patient died without neutrophil recovery due to progressive leukemia. No patient developed infusion reaction to the NK cell product. Two patients experienced non-hematologic adverse events: patient 1 developed grade 4 streptococcal lower respiratory tract infection during neutropenia that required intubation and mechanical ventilation, but later resolved. Patient 4 developed grade 2 colitis secondary to Clostridium difficile that resolved with directed therapy. All patients were dependent on blood and platelet transfusions at the time of study enrollment. Among the two patients with cutaneous GVHD, one person developed resolution of symptoms after treatment. There were no cases of GVHD after NK infusion.
NK Cell Recovery, Licensing, Chimerism and Immunophenotype
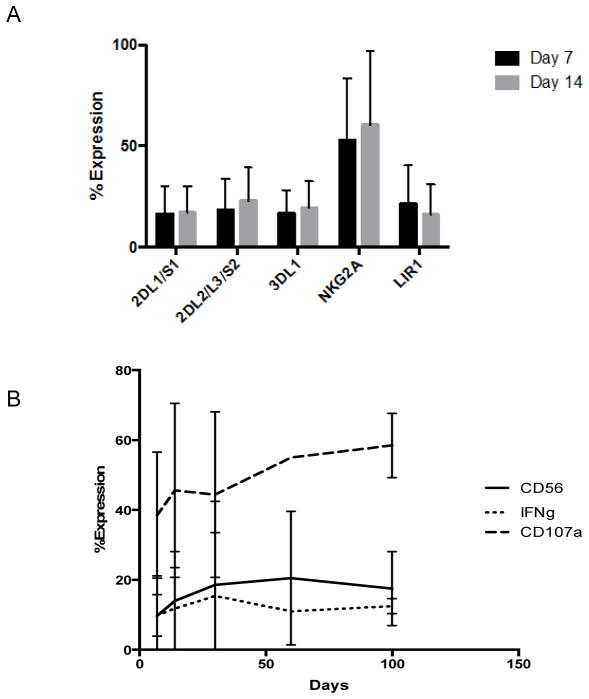
We performed donor/recipient/3rd party chimerism studies on purified CD3−/CD56+ blood cells using polymerase chain reaction amplification of short tandem repeats (STR) at 3, 7, 14, 30, and 60 days post NK cell infusion. We did not detect haploidentical NK cells in any patient post infusion. Four patients had detectable allo HCT donor derived CD3−/CD56+ cells after NK cell infusion. The remaining patient had recipient derived NK cells. In order to examine the effects of IL-2 infusion on NK cell function we examined the phenotype of blood NK cells after infusion in 7 patients (Figure 2). One patient did not undergo NK phenotyping due to early relapse. We found no association between expression of specific NK phenotypes and overall response. Expression of inhibitory KIR was not increased in patients missing respective ligands. We found a slight trend towards greater expression of KIR2DL2/2DL3/2DS2 (5% versus 28%, P = 0.03) and LIR1 (8% versus 29%, P = 0.09) at 14 days in patients who survived longer than 6 months from NK cell infusion (N = 4) when compared to those who died within 6 months of NK cell infusion (N=3). NK effector function at early time points was not associated with response nor was the relative size of the CD56+ peripheral blood population.
Figure 2.
Peripheral blood NK cells were obtained from study subjects after haploidentical cell infusion. Chimerism analysis demonstrated either autologous or original allo HCT donor recovery in all subjects. (A) KIR expression on these cells was consistent during and after IL-2 administration. (B) The fraction of CD3−/CD56+ NK cells as well as their activation state consistent after haploidentical NK cell infusion. NK activation state was assessed via CD107a expression and IFN-γ release after a 24 co-incubation with K562 cells.
DISCUSSION
In the current study we report outcomes in eight patients treated with cyclophosphamide followed by haploidentical NK cells for relapsed AML or MDS after HCT. Here we demonstrate transient responses in 2 individuals and morphologic resolution of dysplasia in a third individual. These results are consistent with other reports of individuals treated with adoptive NK cell infusion after transplantation.(25, 26) One important difference in our study is the use of haploidentical NK cells after HLA-matched HCT. Despite undergoing lymphocyte depleting chemotherapy, we noted no episodes of GVHD after HLA-mismatched NK cell infusion. These results are supportive of the safety of this cell product in a high-risk population of individuals.
The two-step immunomagnetic enrichment of NK cells resulted in a highly pure NK cell product with good viability as described above. We were able to achieve a higher dose of NK cells in the pediatric patients compared to the adult patients. Whether greater numbers of infused NK cells would improve efficacy is unknown. Early reports of adoptive NK cell therapies in children suggest higher overall response rate (27), which may be due to the higher number of infused NK cells per recipient weight in children; however, this was not seen in our smaller series. Several groups are investigating ex vivo NK expansion using platforms both with and without feeder cells.(28–30) Ex vivo expansion may result in a lower activation threshold, but could also decrease mediators of homing necessary to direct NK cells to tumor sites.(31) More importantly, recent evidence suggests that certain ex vivo expansion methods may increase the likelihood of acute GVHD after NK cell infusion.(32) Further work and perhaps comparative studies are warranted in the future to evaluate freshly collected versus cultured NK cell products.
A major limitation to NK cell infusion is the difficulty detecting donor NK cells after transfer, presumed to reflect poor in vivo persistence. (33) Haploidentical donor NK cells were not detected in the blood of any patient in this series. Lack of persistence of transferred NK cells may be attributed to either an inadequate number of infused cells, inhibition from cell populations such as T-regulatory or myeloid suppressor cells, or competition for cytokines from other lymphocyte populations. We did not examine blood cytokines or T-regulatory cell populations in the patients in this series, however, IL-2 infusion here may have increased their number and function in study subjects, paradoxically inhibiting NK cell function. Bachanova and colleagues recently reported that T-regulatory-directed therapy with IL-2/diphtheria toxin recombinant protein increased the persistence of donor NK cells after transfer.(34) While feasible, such an approach requires caution in the post-HCT patient due to the potential to induce GVHD. The use of activating cytokines may induce GVHD in the context of allogeneic NK cell products.(32) Therefore, caution is warranted, particularly with the use of activated, HLA-mismatched cell products. Here we were able to demonstrate safety but not efficacy in this setting. To address this problem, NK cells may be more effective as prophylaxis against overt relapse, an approach that has recently yielded promising results in 16 individuals who received donor NK cells after T-cell depleted haploidentical transplantation.(26)
We examined the phenotype and function of blood NK cells after haploidentical NK cell infusion. Chimerism studies demonstrate that the NK cells studied here were recipient or original alloHCT donor, therefore, these results are relevant to the activation state during IL-2 therapy but do not reflect function of the infused cell product. We did not note any trends in NK phenotyping, including for KIR, in patients with or without a response. Effector function was relatively stable over time and was not associated with clinical response. Interestingly, lack of a KIR ligand (HLA-C1, C2, or Bw4) in the recipient was not associated with detectable expansion of cognate KIR+ NK cells. Our sample size is too small to draw conclusions with respect to the role of missing ligand in haploidentical NK cell transfer. Curti and colleagues treated 13 AML patients with haploidentical NK cells following cyclophosphamide and fludarabine, using recipient HLA and donor KIR gene expression to select recipients lacking KIR-ligands for donor KIR. One individual with active disease experienced a transient response and 3/6 individuals treated in CR remained so in long-term follow up. These findings require larger scale studies to confirm whether donor KIR profile may be used to increase efficacy of adoptive NK cell products.
In summary these data are supportive of the safety of haploidentical NK cells for myeloid malignancy after HCT. Further efforts to improve the efficacy of this product will incorporate donor selection to maximize NK alloreactivity, improved timing of NK cell infusion, and ex vivo manipulations to improve NK numbers and activation state.
Highlights.
Haploidentical NK cells were administered to allogeneic transplant recipients
Responses were seen in 3 of 8 treated patients with relapsed malignancy
No patient developed graft versus host disease
Haploidentical NK cells did not persistent despite immunocompromised recipients
Footnotes
Financial Disclosure Statement:
The authors declare no competing financial interests relevant to this material.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stern M, de Wreede LC, Brand R, van Biezen A, Dreger P, Mohty M, et al. Sensitivity of hematological malignancies to graft-versus-host effects: an EBMT megafile analysis. Leukemia. 2014;28(11):2235–40. doi: 10.1038/leu.2014.145. [DOI] [PubMed] [Google Scholar]
- 2.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–83. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 3.Barrett AJ, Battiwalla M. Relapse after allogeneic stem cell transplantation. Expert review of hematology. 2010;3(4):429–41. doi: 10.1586/ehm.10.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter DL, Alyea EP, Antin JH, DeLima M, Estey E, Falkenburg JHF, et al. NCI First International Workshop on the Biology, Prevention and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(11):1467–503. doi: 10.1016/j.bbmt.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(31):4938–45. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 6.Campregher PV, Gooley T, Scott BL, Moravec C, Sandmaier B, Martin PJ, et al. Results of donor lymphocyte infusions for relapsed myelodysplastic syndrome after hematopoietic cell transplantation. Bone marrow transplantation. 2007;40(10):965–71. doi: 10.1038/sj.bmt.1705840. [DOI] [PubMed] [Google Scholar]
- 7.Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105(12):4878–84. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. The New England journal of medicine. 2012;367(9):805–16. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109(11):5058–61. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94(1):333–9. [PubMed] [Google Scholar]
- 11.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science (New York, NY) 2002;295(5562):2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 12.Dupont B, Hsu KC. Inhibitory killer Ig-like receptor genes and human leukocyte antigen class I ligands in haematopoietic stem cell transplantation. Current opinion in immunology. 2004;16(5):634–43. doi: 10.1016/j.coi.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Kruse PH, Matta J, Ugolini S, Vivier E. Natural cytotoxicity receptors and their ligands. Immunology and cell biology. 2014;92(3):221–9. doi: 10.1038/icb.2013.98. [DOI] [PubMed] [Google Scholar]
- 14.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110(1):433–40. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bix M, Liao NS, Zijlstra M, Loring J, Jaenisch R, Raulet D. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349(6307):329–31. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 16.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 17.Hsu KC, Chida S, Geraghty DE, Dupont B. The killer cell immunoglobulin-like receptor (KIR) genomic region: gene-order, haplotypes and allelic polymorphism. Immunological reviews. 2002;190:40–52. doi: 10.1034/j.1600-065x.2002.19004.x. [DOI] [PubMed] [Google Scholar]
- 18.Raulet DH, Vance RE, McMahon CW. Regulation of the natural killer cell receptor repertoire. Annual review of immunology. 2001;19:291–330. doi: 10.1146/annurev.immunol.19.1.291. [DOI] [PubMed] [Google Scholar]
- 19.Chewning JH, Gudme CN, Hsu KC, Selvakumar A, Dupont B. KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. Journal of immunology (Baltimore, Md : 1950) 2007;179(2):854–68. doi: 10.4049/jimmunol.179.2.854. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(24):4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96(12):3671–4. [PubMed] [Google Scholar]
- 22.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. The Journal of experimental medicine. 2005;202(7):1001–12. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nature medicine. 2003;9(11):1377–82. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 24.Wolint P, Betts MR, Koup RA, Oxenius A. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. The Journal of experimental medicine. 2004;199(7):925–36. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kuhne T, et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18(11):1835–8. doi: 10.1038/sj.leu.2403524. [DOI] [PubMed] [Google Scholar]
- 26.Stern M, Passweg JR, Meyer-Monard S, Esser R, Tonn T, Soerensen J, et al. Pre-emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone marrow transplantation. 2013;48(3):433–8. doi: 10.1038/bmt.2012.162. [DOI] [PubMed] [Google Scholar]
- 27.Koehl U, Sorensen J, Esser R, Zimmermann S, Gruttner HP, Tonn T, et al. IL-2 activated NK cell immunotherapy of three children after haploidentical stem cell transplantation. Blood cells, molecules & diseases. 2004;33(3):261–6. doi: 10.1016/j.bcmd.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer research. 2009;69(9):4010–7. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PloS one. 2012;7(1):e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim SA, Kim TJ, Lee JE, Sonn CH, Kim K, Kim J, et al. Ex vivo expansion of highly cytotoxic human NK cells by cocultivation with irradiated tumor cells for adoptive immunotherapy. Cancer research. 2013;73(8):2598–607. doi: 10.1158/0008-5472.CAN-12-2893. [DOI] [PubMed] [Google Scholar]
- 31.Berg M, Lundqvist A, McCoy P, Jr, Samsel L, Fan Y, Tawab A, et al. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy. 2009;11(3):341–55. doi: 10.1080/14653240902807034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah NN, Baird K, Delbrook CP, Fleisher TA, Kohler ME, Rampertaap S, et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood. 2015;125(5):784–92. doi: 10.1182/blood-2014-07-592881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer immunology, immunotherapy : CII. 2010;59(11):1739–44. doi: 10.1007/s00262-010-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein 2014. 2014 Jun 19;:3855–63. doi: 10.1182/blood-2013-10-532531. 00:00:00. [DOI] [PMC free article] [PubMed] [Google Scholar]