Abstract
Down syndrome (DS) is the most common genetic cause of intellectual disability in children. With aging, DS is associated with an increased risk for Alzheimer's disease (AD). The development of AD neuropathology in individuals with DS can result in further disturbances in cognition and behavior and may significantly exacerbate caregiver burden. Early detection may allow for appropriate preparation by caregivers. Recent literature suggests that declines in gait may serve as an early marker of AD-related cognitive disorders; however, this relationship has not been examined in individuals with DS.
The theory regarding gait dyspraxia and cognitive decline in the general population is reviewed, and potential applications to the population with individuals with DS are highlighted. Challenges and benefits in the line of inquiry are discussed. In particular, it appears that gait declines in aging individuals with DS may be associated with known declines in frontoparietal gray matter, development of AD-related pathology, and white matter losses in tracts critical to motor control. These changes are also potentially related to the cognitive and functional changes often observed during the same chronological period as gait declines in adults with DS. Gait declines may be an early marker of cognitive change, related to the development of underlying AD-related pathology, in individuals with DS. Future investigations in this area may provide insight into the clinical changes associated with development of AD pathology in both the population with DS and the general population, enhancing efforts for optimal patient and caregiver support and propelling investigations regarding safety/quality of life interventions and disease-modifying interventions.
Keywords: Down syndrome, Alzheimer's disease, gait, dyspraxia, cerebrovascular disease, white matter
1. Down Syndrome and Alzheimer's Disease
Down syndrome (DS, or trisomy 21) is the most common genetic cause of intellectual disability in children, which results from triplication of all or part of chromosome 21 (Dierssen, 2012; Lejeune et al., 1959; Millan Sanchez et al., 2012). In addition to the well-established physical phenotype related to DS (Roizen and Patterson, 2003), DS carries increased risk for multiple clinical disorders, including congenital cardiac and gastrointestinal malformations, leukemias and immune disorders, as well as disorders of the endocrine/metabolic systems (Bull, 2011; Cenini et al., 2012; Lott and Dierssen, 2010; Roizen and Patterson, 2003). Individuals with DS also have an elevated risk for developing Alzheimer's disease (AD) as they age (Cosgrave et al., 2000; Holland et al., 2000; Lott and Dierssen, 2010; Lott et al., 2011). AD is the leading known cause of dementia in aging individuals (Reitz, 2012) and has been strongly associated with apolipoprotein E (APOƐ) genotype in the general population (Strittmatter and Roses, 1996). Further, APOƐ genotype appears to interact with the β-amyloid precursor protein (APP) (Bachmeier et al., 2013; Maloney and Lahiri, 2011). The markedly increased susceptibility to AD in DS (or DSAD) is thought to be related to the triplication of all or part of chromosome 21, resulting in overexpression of multiple genes implicated in AD, APP in particular (Lott and Dierssen, 2010; Rumble et al., 1989).
In the general population, the pathological changes associated with AD development, including Aβ production via APP cleavage, likely begin years before the onset of noticeable clinical symptoms (Sperling et al., 2011). Although this process is thought to begin in middle age in the general population, Aβ accumulation in people with DS can begin in childhood (Lott and Dierssen, 2010). This is followed by strikingly accelerated deposition and aggregation into senile plaques beginning at approximately age 30 in DS (Mann and Esiri, 1989). In fact, the accumulation of Aβ plaques and neurofibrillary tangles that follows is so rapid that nearly all individuals with DS have the pathological changes of AD by age 40 (Mann and Esiri, 1989; Wisniewski et al., 1985).
Not all individuals with DS show clinical signs of dementia despite established AD pathology on autopsy (Nieuwenhuis-Mark, 2009; Zigman et al., 1996), but when dementia is present, it is often characterized by disturbances in memory, apraxia, agnosia, and changes in personality with behavioral disorders (Lott and Dierssen, 2010). These cognitive disorders often result in markedly compromised functional skills, including basic activities of self-care such as feeding and bathing (Carr and Collins, 2014; Cosgrave et al., 2000; McKenzie et al., 1998). As such, dementia in intellectually disabled (ID) individuals can significantly increase caregiver burden, as caregivers may not be prepared to meet the needs of loved ones with both ID and dementia (Bittles and Glasson, 2004). This highlights the critical practical value of detecting both cognitive and functional changes as expediently and accurately as possible. Accurate early detection can enhance care efforts as well as furthering understanding of the mechanisms driving such declines. If successful, such efforts can catalyze development of both population-appropriate compensation strategies and disease-based interventions for DSAD.
2. Gait and Cognitive Change
Along with the significant advances in amyloid imaging techniques and spinal fluid biomarkers as tools for early diagnosis of AD (McKhann et al., 2011; Neltner et al., 2012; Sperling et al., 2011), there is a growing body of literature from the general population suggesting that gait declines often emerge before other clinical symptoms of dementia. The association of disorders of gait with the development of dementia over time, suggests that the mechanisms underlying gait change and cognitive change may be closely related (Mielke et al., 2013). Specifically, although declines in gait speed and other motor skills are certainly expected throughout the typical aging process, gait disturbances beyond those expected with age have been associated with multiple types of dementing diseases, including AD (Sheridan et al., 2003) and different subtypes of Mild Cognitive Impairment (MCI) (Pedersen et al., 2014).
Mild Cognitive Impairment (MCI) refers to an early period of cognitive decline during which symptoms do not markedly hinder essential activities of daily living (Albert et al., 2011; Mura et al., 2014; Petersen, 2004). In AD, clinical dementia is often preceded by amnestic MCI, a disorder characterized by largely isolated episodic memory deficits. Subsequent conversion to dementia is determined clinically, when evidence for increasing cognitive and functional impairments are accompanied by objective evidence of a neurocognitive decline (McKhann et al., 2011; Sperling et al., 2011). Burrachio and colleagues demonstrated that individuals in the general population converting to MCI showed more rapid declines in gait compared to participants who did not convert to MCI, and this alteration in gait was observed more than a decade before the usual clinical signs of MCI emerged (Buracchio et al., 2010). In a different analysis involving a 7-year follow-up of 647 autonomously living older women, decreased gait speed was shown to be an independent risk factor for dementia after statistical adjustment for multiple demographic factors, including physical activity levels, comorbidities such as body composition, and self-reported disabilities (van Kan et al., 2012). Some studies have even reported that changes in balance and spatiotemporal gait parameters are more closely connected to cognitive decline in statistical models than chronological age (Achache et al., 2013). Thus, the successful identification of a gait disorder may help predict the onset of dementia in the general population.
To help detect those aging individuals who demonstrate gait decline as a predominant risk factor for cognitive decline, Verghese and colleagues (2012) proposed the construct of Motoric Cognitive Risk syndrome (MCR). The development of the MCR was based on their findings regarding gait, cognition, and functional status from a cohort of 997 community-dwelling adults who were at least 70 years old. Their preliminary criteria for MCR include: (a) the presence of subjective cognitive complaints (b) without the presence of dementia on formal neuropsychological measures, (c) preserved activities of daily living, and (d) slow gait, demonstrated as gait speed one standard deviation or more below other peers in the cohort grouped by age and sex.
Though MCR is young in its conceptualization and validation, initial evidence suggested that individuals meeting criteria for MCR syndrome have more than a threefold greater risk of developing dementia of any type and greater than a twelvefold greater risk of developing vascular dementia (Verghese et al., 2012). A larger-scale investigation of 26,802 adults ages 60 and older from 17 different countries and 5 continents also indicated that individuals meeting MCR criteria (9.7% of the total sample) demonstrated significantly weaker cognitive performances than individuals who did not meet criteria (Verghese et al., 2014). These individuals also were at higher risk for worsened cognitive impairment and frank dementia at follow up, which occurred at least 5.1 years later in these participant groups. Beyond MCR specifically, disordered gait has also been associated with other practical outcomes, including falls (Hausdorff et al., 2001) and overall mortality (Toots et al., 2013). If these patterns of gait and cognitive changes hold true for people with DS, then measurement of gait change may represent a straightforward, noninvasive method for illuminating underlying pathological and clinical changes related to both DSAD and AD.
3. Gait Dyspraxia in Dementia
The term “apraxia” was first used by (Steinthal, 1881) to describe the impairment of a patient's ability to correctly carry out motor programs. Steinthal suggested that apraxia is “...not that the movement ...of the limb is restricted, but the relation of the movement to the object to be handled; the relation of the movement to the purpose is disturbed...”. Praxis requires the adequate operation of and successful integration of multiple cortical and subcortical regions, including the frontal and parietal cortices and their associated white matter tracts. Disruptions in praxis can hinder a variety of basic and complex skills, including volitional movements of the eyelids, mouth, and tongue, speech production, the ability to mime practical movements (like lighting a match or brushing one's teeth), and gait. Dyspraxias, including gait dyspraxias, are often observed in people with dementia in both the general population and in individuals with DS (Chandra et al., 2015; Dalton and Fedor, 1998).
Gait dyspraxia is characterized by diminished capacity to correctly use the legs for ambulation when this deficit cannot be attributed to sensory impairment, motor weakness, poor coordination, or other identifiable causes. It may also extend to other purposeful movements involving the legs and feet such as volitional kicking (Campbell, 2013). Gait dyspraxia has largely been identified and measured via neurological examination, on which individuals may demonstrate difficulties initiating walking movements, executing turns, irregular patterns of raising and lowering the legs, or a shuffling, “magnetic” quality to their steps (Campbell, 2013) and these may occur in the absence of other neurological signs (Liston et al., 2003).
As regards gait, the neural mechanisms of the corticospinal tract, basal ganglia, and cerebellum have been well-established, and advances in neuroimaging have shed light on higher cortical activity involved in gait. Studies using single positron emission topography (SPECT) have shown that in addition to expected activations of the basal ganglia, cerebellar vermis, and supplementary motor area (SMA), activations were also observed in the medial primary sensorimotor area (PSM) in the frontal lobes, visual cortex, and small loci in the left medial temporal lobe with walking (Fukuyama et al., 1997). These authors highlighted the key role of frontal structures, including the medial PSM and SMA, in higher-order gait control given their known projections to subcortical structures involved in locomotion, such as the putamen, thalamus, and pons. Later studies using positron emission topography (PET) have confirmed these findings with walking and running (Barthélemy et al., 2011). Additional studies employing both structural and functional imaging of gait-related motor networks in individuals without DS have also implicated a neural network involving the bilateral SMA, superior parietal lobules, pedunculopontine nuclei, and the cerebellum (Allali et al., 2013; Bakker et al., 2008; Malouin et al., 2003; Miyai et al., 2001).
Further studies have suggested more specific roles for cortical structures in gait modulation, especially emphasizing that frontal lobe activation appears to be important in the planning and adaptation of gait. Anterior prefrontal activity has been identified during anticipation of gait movement via functional magnetic resonance imaging (fMRI), with additional activation in the SMA and cingulate motor areas with motor programming (Sahyoun et al., 2004). In addition, premotor cortex and bilateral prefrontal cortices demonstrate increased activation in individuals adapting their locomotor speed, particularly at higher speeds when motor demands were the greatest (Suzuki et al., 2004).
These studies highlight the critical need for multi-modal sensorimotor integration in gait control, with cohesive incorporation of visual, proprioceptive, and vestibular senses as well as higher-order control of the resulting motor programs for successful ambulation. These investigations also highlight frontal involvement in adjusting gait execution to increased environmental demands. This, unsurprisingly, suggests that the frontal lobes play a key role in higher-order gait control, particularly when an individual is ambulating in more demanding, unusual, or difficult conditions.
The areas of the frontal lobes that appear to be particularly critical to gait regulation are the premotor and SMA as well as other supportive structures, including the frontostriatal networks and the corpus callosum (Goldmann Gross and Grossman, 2008; Liston et al., 2003; Meyer and Barron, 1960). Some role for the parietal lobes has also been mentioned (Meyer and Barron, 1960), though lesions in this area may interfere with gait by inducing impairments of sensory processing. The strong emphasis on frontal structures and frontostriatal connectivity suggests that true gait dyspraxia likely arises from difficulties in the higher-order planning, organization, and sequencing of the more automatically programmed movements necessary for ambulation. In fact, the apparently critical role of the frontal lobes in such gait patterns has led some to describe these impaired gait impairments as “higher-level gait disorders” in order to distinguish these gait disorders from gait disturbances related to other known causes, such as cerebellar ataxia or peripheral neuropathy (Briggs and O'Neill, 2014; Liston et al., 2003; Nutt et al., 1993).
Diseases such as cerebrovascular disease that disrupt the frontal structures and frontostriatal pathways induce dyspraxia of gait (Briggs and O'Neill, 2014). Individuals with DS also often demonstrate gait disturbances that become more pronounced during the aging process and during the emergence of DSAD. “Slow and shuffling” gait has been described in the aging DS population (Lai and Williams, 1989), and further decompensation of gait has been described in DSAD (Crapper et al., 1975; Lott and Head, 2001; Lott and Lai, 1982; Menéndez, 2005; Prasher, 1995; Prasher and Filer, 1995). Anecdotally, these individuals often begin to have difficulties navigating obstacles, even those with which they are well familiar (e.g., stairs in the home). They may also demonstrate more fearful and avoidant behaviors when faced with familiar and unfamiliar obstacles alike. Individuals with DS-related cognitive decline may therefore be experiencing declines in gait praxis as part of their disease course, especially considering the pertinent neuropathological changes documented in DSAD that are discussed below.
4. Neuropathological Correlates of Gait Dyspraxia in DS-Related AD
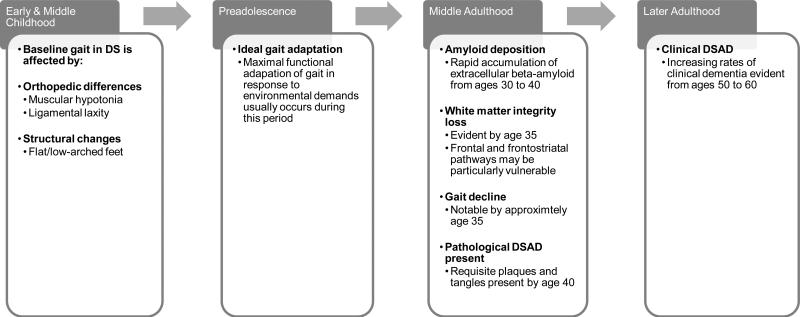
Whereas some of the gait changes that occur during the aging process in DS may be attributed to orthopedic disorders and sensory changes (e.g., cataracts), including elevated rates of ophthalmic disorders, osteoporosis, and osteoarthritis (Krinsky-McHale et al., 2012; Torr et al., 2010), these orthopedic disorders cannot fully explain the declines in higher-order gait control that may be associated with DS. Although the pathological bases for gait dyspraxia have not been fully elucidated in DS, available evidence suggests that these changes may result from the accumulation of DSAD-related pathology, which may induce structural changes in associated cortical areas and in critical white matter pathways (see Figure 1).
Figure 1.
Possible Timeline of Gait Decline as an Early Clinical Sign of Dementia in Down Syndrome
4.1. Development of AD Pathology
Individuals with DS frequently have orthopedic abnormalities from childhood that may affect their gait, including muscular hypotonia, ligamental laxity, and subsequent changes in body structure, including low arched/flat feet (Galli et al., 2014). This has been shown to result in gait patterns that differ between individuals with DS and individuals without DS (Smith et al., 2011). However, it is important to note that individuals with DS also tend to demonstrate gait change beyond these baseline differences during a chronological period in which AD pathology is rapidly accumulating (see Figure 1). First, this pathology may have a predilection for the striatum as demonstrated by Handen and colleagues (2012), which may lead to disruption in motor control at the level of the basal ganglia.
In addition, neurodegenerative change during this chronological period in DS may also hinder higher-order gait control. For instance, younger individuals with DS, particularly preadolescents, often demonstrate a greater capacity to adapt their gait in response to changes in the environment or task demands, such as increased treadmill speed or obstacles in one's path when compared to older adults with DS (Smith et al., 2011; Smith and Ulrich, 2008). Longitudinal examinations of gait in individuals with DS have shown that such occur around the age of 35 years (Smith et al., 2011), a period when people with DS are also undergoing the rapid development of AD pathology (Mann and Esiri, 1989). This confluence of gait changes and known pathological changes related to DSAD suggests that the development of AD pathology may be adversely influencing gait. As such, these changes in ability to adapt gait to environmental demands may be one of the earliest signs of developing dementia in aging adults with DS.
4.2. Frontoparietal Gray Matter Decline
Individuals with DS demonstrate structural variations in cortical regions critical to praxis in general and gait praxis specifically. These differences are evident before the development of dementia with additional change occurring during the aging process. Voxel-based morphometric evaluations have demonstrated that individuals with DS (without dementia) have reduced gray matter volume compared to non-DS controls in the frontal lobes and cerebellum, regions important in praxis and gait coordination (White et al., 2003). During the aging process, individuals with DS experience regional gray matter loss in the bilateral frontal and parietal cortices, including the right precentral gyrus and the left postcentral gyrus (Teipel et al., 2004). As these changes are observed in aging individuals with DS before the onset of clinical dementia, they could be contributing to changes in gait praxis due to declines in primary motor control, motor sequencing, sensory perception, and sensorimotor integration as regulated by these cortical regions.
4.3. White Matter Integrity Decline
This confluence of gait change and the development of pathological cortical degeneration additionally appears to be accompanied by changes in the integrity of white matter pathways involved in gait. Intact frontal-subcortical white matter networks are critical for normal, adaptive gait (Beauchet et al., 2012; Parihar et al., 2013), and a loss of white matter integrity in these frontal-subcortical circuits has been associated with gait impairments in the general population. Studies using fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) have shown that patients with large volume white matter hyperintensities – termed leukoaraiosis (LA) by Hachinski et al. (Hachinski et al., 1987) – often have slower gait and significant declines in gait over time (Willey et al., 2013). Gait disorders are particularly apparent when LA is found in the frontal white matter, demonstrated by lower fractional anisotropy (FA) and higher displacement values on MRI (Willey et al., 2013).
LA involving specific white matter tracts, and especially those important for motor control, may be associated with gait disorders. This includes the corticospinal tract, basal ganglia-thalamic-cortical networks, cortical projections from the superior cerebellar peduncles [28], and intrahemispheric longitudinal fasciculi. The resulting gait impairments are thought to represent the functional consequence of motor network disconnection secondary to lesions in the white matter tracts critical for functional connectivity in the motor system (Allali et al., 2010; Srikanth et al., 2010; Verghese et al., 2002).
Examination of white matter integrity via diffusion tensor imaging (DTI) indicates that adults with DS indeed show reduced white matter integrity as compared to control participants (Powell et al., 2014), particularly in the frontal and parietal lobes (Head, Powell, et al., 2012; Powell et al., 2014). Furthermore, these declines have been noted in middle age in DS (by the age of 35 years), congruent with the emergence of gait adaptability declines in DS (Head, Powell, et al., 2012; Smith et al., 2011).
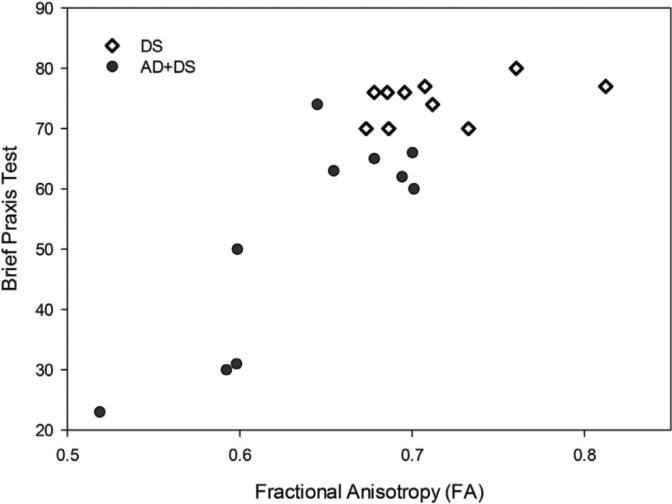
Furthermore, the loss of white matter integrity in DS is associated with a decline in performance on objective tasks of cognitive capacity as well as praxis. Regression analyses have revealed reduced FA in the corpus callosum and key frontoparietal association tracts in individuals with DS. As demonstrated in Figure 2, lower FA values are reliably associated with performance on the Brief Praxis Test (BPT) (Dalton et al., 1999; Powell et al., 2014). This measure includes both purposeful object manipulation (e.g., opening and closing a padlock) and lower body motor control (e.g., standing on one leg). New analysis from our group has also demonstrated that reduced FA is associated with weaker performance on the Severe Impairment Battery (SIB), a measure of overall cognitive performance in significantly impaired individuals (Panisset et al., 1994; Schmitt et al., 1997), and a subscale of the SIB measuring praxis (SIB Praxis). These findings suggest that individuals with DS exhibiting significant compromise of their subcortical white matter demonstrate reductions in cognitive performance and apraxia (with some lower body control elements).
Figure 2.
Linear regression of praxis on white matter integrity in DS in individuals with and without dementia (Powell et al., 2014)
A graph illustrating the association between average brain FA and individual Brief Praxis Test (BPT) scores from all significant voxels determined by diffusion tensor imaging (DTI) in a sample of 20 adults with DS (DS with dementia n = 10, DS without dementia n = 10). FA is strongly correlated with BPT scores in demented individuals, with higher FA scores significantly correlated with higher BPT scores (r = 0.83, n = 20, p < 0.001). Abbreviations: AD = Alzheimer's disease, BPT = Brief Praxis Test, DS = Down syndrome, DTI = diffusion tensor imaging, FA = fractional anisotropy.
Reprinted from Neurobiology of Aging, Vol. 35, Issue 7, Authors David Powell, Allison Caban-Holt, Gregory Jicha, William Robertson, Roberta Davis, Brian T. Gold, Frederick A. Schmitt, & Elizabeth Head. Frontal white matter integrity in adults with Down syndrome with and without dementia, pp. 1562-1569, copyright year 2014, with permission from Elsevier.
5. Mechanisms of White Matter Decline
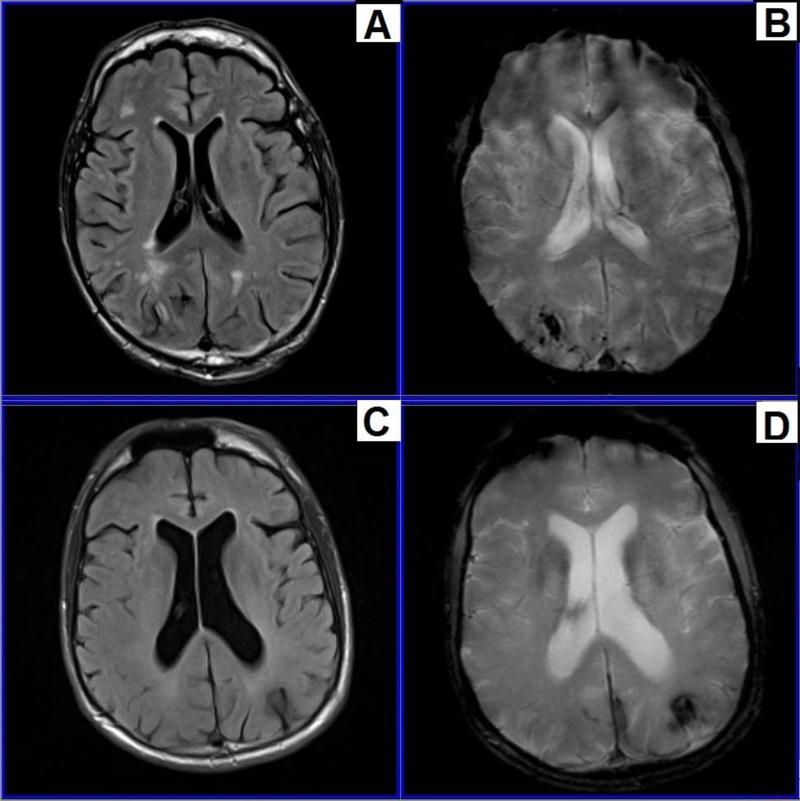
Given these white matter changes, which can be widespread (Figure 3) in aging individuals with DS, as well as their apparent adverse influence on cognition and gait the potential mechanisms, underlying white matter changes may be of particular importance in the understanding of the aging process and the development of dementia in people with DS. Although the pathological processes affecting white matter integrity in individuals with DS require further investigation, some valuable information is available from studies in the general population.
Figure 3.
Vascular change in an individual with Down syndrome.
Magnetic resonance imaging (MRI) images illustrating white matter change in an individual with Down syndrome (DS). Images A & C were obtained via fluid attenuated inversion recovery (FLAIR), with bright white areas of hyperintensity representing white matter lesions. Images B & D were obtained via T2* sequencing, with dark areas indicating hemosiderin deposition from chronic microhemorrhages, often occurring secondary to arterial amyloid deposition. Abbreviations: DS = Down syndrome, FLAIR = fluid attenuated inversion recovery, MRI = magnetic resonance imaging. Images provided by David Powell, Ph.D., from the University of Kentucky.
In the general population, white matter volumes decline with normal aging (Stricker, 2002; Walhovd et al., 2005), and regional white matter declines with normal aging are associated with declines in regional cerebral glucose uptake (Kochunov et al., 2009). Because axons in the white matter arise from cortical neurons, it may be that gray matter loss associated with cortical atrophy may also result in subsequent decline in related white matter regions (O'Sullivan et al., 2001).
White matter changes may also result from pathology more directly involving the white matter itself. As mentioned, LA, a term used to describe hyperintense regions of white matter as visualized via T2- and fluid attenuated inversion recovery (FLAIR)-weighted MRI, likely represents pathology of the white matter. These radiological changes (hyperintensity on T2 or FLAIR imaging) correlate with several pathologic changes, including partial loss of myelin, axons, and oligodendroglial cells (Janota et al., 1989; Kirkpatrick and Hayman, 1987; van Swieten and van Gijn, 1991), which thereby disrupts cortical-subcortical circuits (Filley, 1998).
These changes suggest an underlying cerebrovascular disorder that may be related to neurodegenerative disease (Breteler et al., 1994; Liao et al., 1996; Lindgren et al., 1994; Longstreth et al., 1996; Schmidt et al., 1999; Ylikoski et al., 1993). LA has been associated with a vasculopathy that alters the vessels supplying blood to the white matter. However, this vascular pathology that produces ischemia to the white matter appears to be secondary not only to hyaline fibrosis of arterioles, but also to amyloid deposition (Englund and Brun, 1990; Englund et al., 1988). Congruently, this pathology is often observed in people who have AD without DS, with a threefold greater incidence of LA in patients with AD than in age-matched controls (Almkvist et al., 1992; Bondareff et al., 1988; Erkinjuntti et al., 1987; Fazekas et al., 1989; Fazekas et al., 1987; Fazekas et al., 1996; Hogervorst et al., 2002; Kozachuk et al., 1990; McDonald et al., 1991; Mirsen et al., 1991; Wahlund et al., 1994; Waldemar et al., 1994). However, Chalmers et al. (2005) studied 125 autopsied cases of AD and found that the contribution of vascular disease to LA was relatively minor in most cases. Instead, the severity of frontal white matter damage was more closely related to parenchymal deposition of amyloid.
This concept may be of great salience in adults with DS, since they demonstrate strikingly accelerated rates of amyloid deposition as compared to adults without DS (discussed above). This accelerated deposition of amyloid may be related to factors such as internal jugular vein reflux, which may hinder amyloid clearance and result in increased cerebral amyloid burden (Reed-Cossairt et al., 2012). Together, these factors may promote the particular pathological expression of AD pathology called cerebral amyloid angiopathy (CAA). CAA has been connected to multiple pathological phenotypes of AD (Allen et al., 2014) and interferes with vascular function by limiting vessel dilation and constriction in response to perfusion demands. Indeed, cerebral amyloid angiopathy (CAA) is commonly observed with APP duplication as in DS (Mendel et al., 2010; Sleegers et al., 2006). CAA is not uncommonly observed on autopsy in DS as well as via in vivo imaging studies (see images B & D in Figure 3) using appropriate imaging approaches, such as susceptibility weighted imaging (SWI) (Haacke et al., 2007) and arterial spin labeling (ASL) (Dumas et al., 2012).
6. Structural and Functional Considerations: Executive Functioning and Gait
If AD-associated pathological alterations of white matter promote disconnection of the cortical-subcortical motor networks, resulting in the abnormalities of gait associated with this disorder, then the cognitive functions supported by these and related networks would be expected to decline in concert with the subcortical white matter changes. This premise may have significant implications in the clinical manifestations of dementia in both the general population and in the population with DS.
Specifically, we might anticipate declines in executive functions when frontal-subcortical connections are compromised (Bruce-Keller et al., 2012; Bruce-Keller et al., 2012; Parihar et al., 2013). There appears to be substantial evidence for this, including in other well-established pathologies involving frontostriatal and frontothalamic circuits, including disorders of white matter caused by diseases such as multiple sclerosis and vascular dementia (Bonelli and Cummings, 2008). For example, patients with Tourette's syndrome and Obsessive-Compulsive Disorder (OCD) also reveal sign of frontal executive dysfunction. In addition to reduced volume in key subcortical regions, including the caudate nucleus, children with Tourette's syndrome demonstrate reduced white matter connectivity between the caudate nucleus and prefrontal cortex. This reduced connectivity was further associated with reduced capacities for behavioral inhibition (Makki et al., 2009). Finally, children with OCD demonstrate reduced connectivity in dorsal striatal-thalamic regions to key regions of the anterior cingulate. Greater reductions in connectivity were associated with greater symptom severity in this group (Fitzgerald et al., 2011).
As it does appear that compromise of the frontostriatal and frontothalamic circuits can result in disordered executive functions, it has been suggested that changes in executive functions, including higher-order regulation of personality and behavior, may emerge early in the course of dementia in DS (Ball et al., 2006; Carr and Collins, 2014). If these changes are occurring secondary to the frontal-subcortical pathology demonstrated early in the course of dementia in patients with DS, a decline of executive functions very well may signal clinical decline in aging adults with DS. However, the details of this relationship between executive functioning and DSAD remain unresolved.
Methodological issues can pose a challenge in the study of executive functions and DSAD. as many common neurocognitive measures used to assess executive functions may be inappropriate for the developmental level of individuals with DS (Lott and Dierssen, 2010). Further, declines in executive functioning may be difficult to quantify in individuals with DS given the developmental abnormalities present in the frontal lobes in this group. It has been demonstrated that the frontal lobes demonstrate reduced growth and delayed myelination evident from the first few months postnatally (Pennington et al., 2003). Congruently, younger individuals with DS demonstrate significant deficits in executive functions compared to controls without DS, impairments that are clearly measurable decades before DSAD is generally diagnosed in the 5th decade of life (Lanfranchi et al., 2010). This may render the measurement of purported executive decline difficult in aging adults with DS, particularly those with more pronounced intellectual disabilities resulting in existing, significant executive impairments.
Given these diagnostic difficulties, it is not surprising that there is limited established evidence for the anticipated relationships among white matter decline, executive dysfunction, and gait problems in DS-specific groups. However, studies in the general population have revealed specific associations between some aspects of executive functioning and gait impairments (Allali et al., 2010; Beauchet et al., 2012; IJmker and Lamoth, 2012; Kearney et al., 2013; McGough et al., 2011; Persad et al., 2008; Springer et al., 2006; van Iersel et al., 2008; Watson et al., 2010; Yogev-Seligmann et al., 2008). For example, associations occur between impairments on the Trail Making Test, Part B and impairments of gait, including falls, in elderly individuals [50, 52]. In addition, older individuals with less efficient gait, but without dementia or other known neurological disorders affecting gait such as stroke and/or parkinsonism, have demonstrated larger differences between the times required to complete the Trail Making Test, Part A and the corresponding Part B. Unlike Part A, Part B requires alternating number-letter sequencing, thus placing greater demands on working memory, a function mediated by the frontal lobes (Ble et al., 2005).
Increased stride time variability, an accepted measure of lower limb movement reliability, has been associated with poorer skills in information updating and monitoring (Beauchet et al., 2012). Individuals prone to falls also have weaker performance on tasks correlated with executive functioning, such as the Stroop and go/no-go tasks (a test for response inhibition), and show larger gait changes (specifically, swing time variability) in dual task walking conditions (Springer et al., 2006). Reduced response inhibition (Stroop Interference) and mental flexibility (TMT-B) are associated with to poorer performance on a timed “up & go” test (McGough et al., 2011). In addition, brief reports describe navigation errors and a lack of speed adjustment in elderly individuals prone to falls when faced with a complex, novel navigation task (Foran et al., 2010), suggesting reduced spatial integration and executive control of gait in vulnerable individuals. Evidence also suggests a dose-response relationship between cognitive load and gait: Increasing the difficulty of the task to be performed simultaneously with walking results in greater cognitive load, and thus, a greater decrement in gait performance (Foran et al., 2010).
Discrepancies in practical performance related to age may be related to changes in the networks used to perform these tasks. Functional imaging conducted during gait imagery has noted differences in regional activation in elderly versus young individuals. As mentioned, younger individuals recruit bilateral supplementary cortices, superior parietal lobules, cerebellum, pedunculopontine nuclei, and hippocampal/parahippocampal gyri while completing imagery tasks. In contrast, older individuals rely more heavily on activation of the bilateral prefrontal regions (left BA10 and right BA11), left hippocampus, the right supplementary motor area (BA6), the substantia nigra, and the putamen. These differences in activation may represent compensation for declines in the network normally recruited by younger individuals: however, heavier reliance on this alternate network may also leave elderly individuals vulnerable to network-related declines associated with those compensatory regions, including active spatial navigation, environmental condition monitoring, and higher order, precise gait control (Allali et al., 2013).
7. Challenges and Benefits in the Line of Inquiry
Enhanced understanding of the mechanisms inducing gait changes in aging adults with DS could lead to potential interventions designed to prevent and treat the underlying disorders that lead to impaired gait in this vulnerable population. It may also allow insight into the progression of cognitive decline and its treatment, not only for individuals with DS, but also for the larger population of elders affected by AD. Despite the promise inherent in such investigations, there are significant challenges as noted below.
First, the exact mechanism(s) driving key white matter changes remains undetermined. The connection between white matter decline and the usual conceptualization of vascular cognitive impairment/vascular dementia appears important when examining white matter change in non-DS adults. However, individuals with DS have significantly lower rates of some critical vascular risk factors commonly associated with cerebrovascular disease in the general population, including hypertension, arterial disease, and atherosclerosis. There also appears to be higher rates of obstructive sleep apnea, obesity, type 2 diabetes, and abnormal lipid levels reported for people with DS (Lott and Dierssen, 2010; Reed-Cossairt et al., 2012). Thus, individuals with DS may display a somewhat different constellation of risk factors for white matter decline than aging adults in the general population.
Further, it has been suggested that the white matter changes associated with cognitive and functional declines in DS potentially occur in parallel to white matter pathology often observed in AD (Head, Silverman, et al., 2012). The white matter changes observed in AD patients may be, at least in part, a product of amyloid-induced oligodendrocyte toxicity (Xu, J. et al., 2001). However, there are a myriad of vascular abnormalities common in DS, including gross cardiovascular defects such as ventricular and atrial septal defects (Roizen and Patterson, 2003) and cerebrovascular disorders such as Moyamoya disease (Kainth et al., 2013; Outwater et al., 1989). This evidence argues that the relationship between vascular functioning and white matter decline in DS dementia is highly complex, bearing careful inquiry.
Further investigations of these disorders are needed, including examining changes over time within individuals utilizing longitudinal rather than cross-sectional designs. This examination will more completely determine the relationship between changes in cognitive and functional status and changes in gait over time in the DS population. In addition, knowledge from the general population regarding pathologic gait changes may have limited applicability to the DS population. For instance, increased variability in gait parameters such as stride length and stride time is a sign of motor decline in the general population and non-DS populations with dementia (IJmker and Lamoth, 2012); however, individuals with DS may show more specific and difficult to detect declines specifically in their ability to adapt their gait to environmental demands. This may complicate the identification of the most valuable gait parameters and development of DS-appropriate methodologies to examine such changes in DS over the lifespan. Further, any gait data obtained in DS groups must be interpreted carefully within DS-specific parameters. Motor declines must be demonstrated over and above declines in physical strength and known, commonly occurring orthopedic and sensory disorders. In addition, the expected overlap of motor declines with executive functioning declines may be difficult to demonstrate given the significant floor effects of many commonly-used executive and other neurocognitive measures in this intellectually disabled groups (Lott and Dierssen, 2010).
Cognitive declines must also be conceptualized carefully in light of known, frequent comorbidities experienced in DS, including abnormal thyroid function, obstructive sleep apnea, depression, and APOƐ genotype (Lott and Dierssen, 2010; Verghese et al., 2013). For instance, thyroid dysfunction has been associated with subsequent expression of nerve growth factors, cholinergic activity, and subsequent hippocampal function in DS (Smith et al., 2002). Obstructive sleep apnea can impair cognitive functions secondary to repetitive hypoxias (Gagnon et al., 2014). Presence of depression is associated with more than doubled risk of dementia, perhaps due to associated vascular and metabolic alterations (Byers and Yaffe, 2011). APOƐ-4 genotype also greatly increases risk of incident dementia (Luck et al., 2014). Research suggests that these factors interact in complex ways to affect dementia risk; for instance, individuals with both APOƐ-4 genotype and depression had a higher risk of developing dementia than either factor in isolation (Pink et al., 2014).
These comorbidities may complicate investigation of the potential links among gait praxis and dementia in DS, though the pursuit nonetheless appears promising. If successful, studies of gait dyspraxia in DS dementia could provide significant insight into not only the underlying pathology of this clinical syndrome, but also the challenges in practical functioning resulting from gait declines. Such investigations could provide valuable insight for intervention in AD, in both DS and the general population.
8. Summary
It is proposed that gait decline may serve as one of the earliest signs of developing dementia in aging adults with DS. This gait decline is thought represent a form of gait dyspraxia, as it may largely result from declines in the frontoparietal gray matter and frontostriatal white matter tracts. These neuropathological alterations occur during the aging process in DS and secondary to the rapid deposition of Aβ in this population. The chronological confluence of these changes in middle adulthood (around the age of 35 in DS) suggests that they are pathologically related. As AD pathology is rapidly accumulating, pathological changes in frontal-subcortical white matter become evident, and progressive disorders in the higher-order regulation of gait tend to appear around this time point in adults with DS. Further studies of this potential relationship may provide significant insight into the neuropathological declines experienced by adults in DS. Such future studies could provide better means of clinical identification and intervention for DSAD.
Highlights.
Alzheimer's disease is common in individuals with Down Syndrome (DS).
Gait declines may be the earliest marker of neurodegenerative change.
Adults with DS may experience gait declines differently than non-DS adults.
Neuropathological changes in DS are thought to contribute significantly to gait declines.
Acknowledgements
Thanks are extended to Dr. David Powell for the structural images included in this paper.
Source of Funding: Funding provided by NIH/NICHD R01HD064993.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors do not have conflicts of interest to report.
Contributor Information
Amelia J. Anderson-Mooney, University of Kentucky College of Medicine, Department of Neurology 740 S. Limestone, Suite B-101 Lexington, Kentucky 40536
Frederick A. Schmitt, University of Kentucky College of Medicine, Department of Neurology and Sanders-Brown Center on Aging 800 S. Limestone, Room 312 Lexington, Kentucky 40536, fascom@uky.edu, Phone: 859-218-3850, Fax: 859-323-2866
Elizabeth Head, University of Kentucky, Department of Molecular & Biomedical Pharmacology and Sanders-Brown Center on Aging 800 S. Limestone, Room 203 Lexington, Kentucky 40536, Elizabeth.Head@uky.edu, Phone: 859-218-3172, Fax: 859-323-2866
Ira T. Lott, University of California, Irvine School of Medicine Professor & Chair, Department of Pediatrics Bldg 2 3rd Floor Rt 81 101 The City Drive, Mail Code: 4482 Orange, California 92668, itlott@uci.edu, Phone: 714-456-5333, Fax: 714-456-7658
Kenneth M. Heilman, University of Florida College of Medicine, Department of Neurology Room L3-100, McKnight Brain Institute 1149 Newell Drive Gainesville, Florida 32611, heilman@neurology.ufl.edu, Phone: 352-273-5550, Fax: 352-273-5575
References
- Achache V, Fontaine F, Chadebec V, Quentin V, Pequignot R, Durand E. Evaluation of the relationship between dynamic balance and stance phases during gait in normal ageing. Ann. Phys. Rehabil. Med. 2013;56(Supplement 1) [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allali G, van der Meulen M, Assal F. Gait and cognition: The impact of executive function. Schweiz. Arch. Neurol. Psychiatr. 2010;161(6):195–199. [Google Scholar]
- Allali G, van der Meulen M, Beauchet O, Rieger SW, Vuilleumier P, Assal F. The neural basis of age-related changes in motor imagery of gait: An fMRI study. J. Gerontol. A Biol. Sci. Med. Sci. 2013:glt207. doi: 10.1093/gerona/glt207. [DOI] [PubMed] [Google Scholar]
- Allen N, Robinson A, Snowden J, Davidson Y, Mann D. Patterns of cerebral amyloid angiopathy define histopathological phenotypes in Alzheimer's disease. Neuropathol. Appl. Neurobiol. 2014;40(2):136–148. doi: 10.1111/nan.12070. [DOI] [PubMed] [Google Scholar]
- Almkvist O, Wahlund L-O, Andersson-Lundman G, Basun H, Bäckman L. White-matter hyperintensity and neuropsychological functions in dementia and healthy aging. Arch. Neurol. 1992;49(6):626–632. doi: 10.1001/archneur.1992.00530300062011. [DOI] [PubMed] [Google Scholar]
- Bachmeier C, Paris D, Beaulieu-Abdelahad D, Mouzon B, Mullan M, Crawford F. A multifaceted role for apoE in the clearance of beta-amyloid across the blood-brain barrier. Neurodegener. Dis. 2013;11(1):13–21. doi: 10.1159/000337231. [DOI] [PubMed] [Google Scholar]
- Bakker M, De Lange F, Helmich RC, Scheeringa R, Bloem BR, Toni I. Cerebral correlates of motor imagery of normal and precision gait. Neuroimage. 2008;41(3):998–1010. doi: 10.1016/j.neuroimage.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Ball SL, Holland AJ, Hon J, Huppert FA, Treppner P, Watson PC. Personality and behaviour changes mark the early stages of Alzheimer's disease in adults with Down's syndrome: findings from a prospective population-based study. Int. J. Geriatr. Psychiatry. 2006;21(7):661–673. doi: 10.1002/gps.1545. [DOI] [PubMed] [Google Scholar]
- Barthélemy D, Grey MJ, Nielsen JB, Bouyer L. Involvement of the corticospinal tract in the control of human gait. In: Green A, Chapman CE, Kalaska JF, Lepore F, editors. Progress in Brain Research: Enhancing Performance for Action and Perception. Elsevier; Amsterdam: 2011. pp. 181–197. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Annweiler C, Montero-Odasso M, Fantino B, Herrmann FR, Allali G. Gait control: A specific subdomain of executive function. J. Neuroeng. Rehabil. 2012;9(12):1–5. doi: 10.1186/1743-0003-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittles A, Glasson E. Clinical, social, and ethical implications of changing life expectancy in Down syndrome. Dev. Med. Child Neurol. 2004;46(04):282–286. doi: 10.1017/s0012162204000441. [DOI] [PubMed] [Google Scholar]
- Ble A, Volpato S, Zuliani G, Guralnik JM, Bandinelli S, Lauretani F, et al. Executive function correlates with walking speed in older persons: The InCHIANTI study. J. Am. Geriatr. Soc. 2005;53(3):410–415. doi: 10.1111/j.1532-5415.2005.53157.x. [DOI] [PubMed] [Google Scholar]
- Bondareff W, Raval J, Colletti PM, Hauser DL. Quantitative magnetic resonance imagi ng and the severity of dementia in Alzheimer's disease. Am. J. Psychiatry. 1988;145(7):853–856. doi: 10.1176/ajp.145.7.853. [DOI] [PubMed] [Google Scholar]
- Bonelli RM, Cummings JL. Frontal-subcortical dementias. The Neurologist. 2008;14(2):100–107. doi: 10.1097/NRL.0b013e31815b0de2. [DOI] [PubMed] [Google Scholar]
- Breteler M, van Amerongen NM, van Swieten JC, Claus JJ, Grobbee DE, Van Gijn J, et al. Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging. The Rotterdam Study. Stroke. 1994;25(6):1109–1115. doi: 10.1161/01.str.25.6.1109. [DOI] [PubMed] [Google Scholar]
- Briggs R, O'Neill D. Vascular gait dyspraxia. Clin. Med. (Northfield Il.) 2014;14(2):200–202. doi: 10.7861/clinmedicine.14-2-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Brouillette RM, Tudor-Locke C, Foil HC, Gahan WP, Nye DM, et al. Relationship between cognitive domains, physical performance, and gait in elderly and demented subjects. J. Alzheimers Dis. 2012;30(4):899–908. doi: 10.3233/JAD-2012-120025. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Brouillette RM, Tudor-Locke C, Foil HC, Gahan WP, Correa J, et al. Assessment of cognition, physical performance, and gait in the context of mild cognitive impairment and dementia. J. Am. Geriatr. Soc. 2012;60(1):176–177. doi: 10.1111/j.1532-5415.2011.03762.x. [DOI] [PubMed] [Google Scholar]
- Bull MJ. Health supervision for children with Down syndrome. Pediatrics. 2011;128(2):393–406. doi: 10.1542/peds.2011-1605. [DOI] [PubMed] [Google Scholar]
- Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch. Neurol. 2010;67(8):980–986. doi: 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers AL, Yaffe K. Depression and risk of developing dementia. Nat. Rev. Neurol. 2011;7(6):323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WW. DeJong's The Neurologic Examination. 7th ed. Lippincott, Williams, & Wilkins; Philadephia, PA: 2013. [Google Scholar]
- Carr J, Collins S. Ageing and dementia in a longitudinal study of a cohort with Down syndrome. J. Appl. Res. Intellect. 2014;27(6):555–563. doi: 10.1111/jar.12093. [DOI] [PubMed] [Google Scholar]
- Cenini G, Dowling AL, Beckett TL, Barone E, Mancuso C, Murphy MP, et al. Association between frontal cortex oxidative damage and beta-amyloid as a function of age in Down syndrome. BBA-Mol. Basis Dis. 2012;1822(2):130–138. doi: 10.1016/j.bbadis.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers K, Wilcock G, Love S. Contributors to white matter damage in the frontal lobe in Alzheimer's disease. Neuropathol. Appl. Neurobiol. 2005;31(6):623–631. doi: 10.1111/j.1365-2990.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- Chandra SR, Issac TG, Abbas MM. Apraxias in neurodegenerative dementias. Indian J. Psychol. Med. 2015;37(1):42. doi: 10.4103/0253-7176.150817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrave MP, Tyrrell J, McCarron M, Gill M, Lawlor BA. A five year follow-up study of dementia in persons with Down's syndrome: Early symptoms and patterns of deterioration. Ir. J. Psychol. Med. 2000;17(1):5–11. [Google Scholar]
- Crapper DR, Dalton AJ, Skopitz M, Scott JW, Hachinski VC. Alzheimer degeneration in Down syndrome: Electrophysiologic alterations and histopathologic findings. Arch. Neurol. 1975;32(9):618–623. doi: 10.1001/archneur.1975.00490510074006. [DOI] [PubMed] [Google Scholar]
- Dalton AJ, Fedor BL. Onset of dyspraxia in aging persons with Down syndrome: Longitudinal studies. J. Intellect. Dev. Disabil. 1998;23(1):13–24. [Google Scholar]
- Dalton AJ, Mehta PD, Fedor BL, Patti PJ. Cognitive changes in memory precede those in praxis in aging persons with Down syndrome. J. Intellect. Dev. Disabil. 1999;24(2):169–187. [Google Scholar]
- Dierssen M. Down syndrome: The brain in trisomic mode. Nat. Rev. Neurosci. 2012;13(12):844–858. doi: 10.1038/nrn3314. [DOI] [PubMed] [Google Scholar]
- Dumas A, Dierksen GA, Gurol ME, Halpin A, Martinez-Ramirez S, Schwab K, et al. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann. Neurol. 2012;72(1):76–81. doi: 10.1002/ana.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund E, Brun A. White matter changes in dementia of Alzheimer's type: The difference in vulnerability between cell compartments. Histopathology. 1990;16(5):433–439. doi: 10.1111/j.1365-2559.1990.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Englund E, Brun A, Alling C. White matter changes in dementia of Alzheimer's type biochemical and neuropathological correlates. Brain. 1988;111(6):1425–1439. doi: 10.1093/brain/111.6.1425. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Ketonen L, Sulkava R, Sipponen J, Vuorialho M, Iivanainen M. Do white matter changes on MRI and CT differentiate vascular dementia from Alzheimer's disease? J. Neurol. Neurosurg. Psychiatry. 1987;50(1):37–42. doi: 10.1136/jnnp.50.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Alavi A, Chawluk J, Zimmermann R, Hackney D, Bilaniuk L, et al. A comparison of CT, MRI and PET in Alzheimer's dementia and normal aging. J. Nucl. Med. 1989;30:1067–1615. [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJNR Am. J. Neuroradiol. 1987;8(3):421–426. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Kapeller P, Schmidt R, Offenbacher H, Payer F, Fazekas G. The relation of cerebral magnetic resonance signal hyperintensities to Alzheimer's disease. J. Neurol. Sci. 1996;142(1):121–125. doi: 10.1016/0022-510x(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Filley CM. The behavioral neurology of cerebral white matter. Neurology. 1998;50(6):1535–1540. doi: 10.1212/wnl.50.6.1535. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Stern ER, Angstadt M, Hanna GL, Abelson JL, et al. Developmental Alterations of Frontal-Striatal-Thalamic Connectivity in Obsessive-Compulsive Disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50(9):938–948.e933. doi: 10.1016/j.jaac.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran T, Setti A, Burke K, Fan C, Cogan L, Romero-Ortuno R, et al. The role of aging on efficient spatial navigation and gait velocity. Parkinsonism Relat. Disord. 2010;16:S23. [Google Scholar]
- Fukuyama H, Ouchi Y, Matsuzaki S, Nagahama Y, Yamauchi H, Ogawa M, et al. Brain functional activity during gait in normal subjects: A SPECT study. Neurosci. Lett. 1997;228(3):183–186. doi: 10.1016/s0304-3940(97)00381-9. [DOI] [PubMed] [Google Scholar]
- Gagnon K, Baril A-A, Gagnon J-F, Fortin M, Décary A, Lafond C, et al. Cognitive impairment in obstructive sleep apnea. Pathol. Biol. (Paris) 2014;62(5):233–240. doi: 10.1016/j.patbio.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Galli M, Cimolin V, Rigoldi C, Pau M, Costici P, Albertini G. The effects of low arched feet on foot rotation during gait in children with Down syndrome. J. Intellect. Disabil. Res. 2014;58(8):758–764. doi: 10.1111/jir.12087. [DOI] [PubMed] [Google Scholar]
- Goldmann Gross R, Grossman M. Update on Apraxia. Curr. Neurol. Neurosci. Rep. 2008;8(6):490–496. doi: 10.1007/s11910-008-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke E, DelProposto Z, Chaturvedi S, Sehgal V, Tenzer M, Neelavalli J, et al. Imaging cerebral amyloid angiopathy with susceptibility-weighted imaging. AJNR Am. J. Neuroradiol. 2007;28(2):316–317. [PMC free article] [PubMed] [Google Scholar]
- Hachinski VC, Potter P, Merksey H. Leukoaraiosis. Arch. Neurol. 1987;44(1):21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- Handen BL, Cohen AD, Channamalappa U, Bulova P, Cannon SA, Cohen WI, et al. Imaging brain amyloid in nondemented young adults with Down syndrome using Pittsburgh compound B. Alzheimers Dement. 2012;8(6):496–501. doi: 10.1016/j.jalz.2011.09.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch. Phys. Med. Rehabil. 2001;82(8):1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- Head E, Powell D, Gold BT, Schmitt FA. Alzheimer's Disease in Down Syndrome. Eur. J. Neurodegener. Dis. 2012;1(3):353–364. [PMC free article] [PubMed] [Google Scholar]
- Head E, Silverman W, Patterson D, Lott IT. Aging and Down syndrome. Curr. Gerontol. Geriatr. Res. 2012;2012 doi: 10.1155/2012/412536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E, Ribeiro HM, Molyneux A, Budge M, Smith AD. Plasma homocysteine levels, cerebrovascular risk factors, and cerebral white matter changes (leukoaraiosis) in patients with Alzheimer disease. Arch. Neurol. 2002;59(5):787–793. doi: 10.1001/archneur.59.5.787. [DOI] [PubMed] [Google Scholar]
- Holland A, Hon J, Huppert F, Stevens F. Incidence and course of dementia in people with Down's syndrome: Findings from a population-based study. J. Intellect. Disabil. Res. 2000;44(2):138–146. doi: 10.1046/j.1365-2788.2000.00263.x. [DOI] [PubMed] [Google Scholar]
- IJmker T, Lamoth CJ. Gait and cognition: The relationship between gait stability and variability with executive function in persons with and without dementia. Gait Posture. 2012;35(1):126–130. doi: 10.1016/j.gaitpost.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Janota I, Mirsen TR, Hachinski VC, Lee DH, Merskey H. Neuropathologic correlates of leuko-araiosis. Arch. Neurol. 1989;46(10):1124–1128. doi: 10.1001/archneur.1989.00520460118023. [DOI] [PubMed] [Google Scholar]
- Kainth DS, Chaudhry SA, Kainth HS, Suri FK, Qureshi AI. Prevalence and characteristics of concurrent Down syndrome in patients with moyamoya disease. Neurosurgery. 2013;72(2):210–215. doi: 10.1227/NEU.0b013e31827b9beb. [DOI] [PubMed] [Google Scholar]
- Kearney FC, Harwood RH, Gladman JR, Lincoln N, Masud T. The relationship between executive function and falls and gait abnormalities in older adults: A systematic review. Dement. Geriatr. Cogn. Disord. 2013;36(1-2):20–35. doi: 10.1159/000350031. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick JB, Hayman LA. White-matter lesions in MR imaging of clinically healthy brains of elderly subjects: Possible pathologic basis. Radiology. 1987;162(2):509–511. doi: 10.1148/radiology.162.2.3797666. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Ramage A, Lancaster J, Robin D, Narayana S, Coyle T, et al. Loss of cerebral white matter structural integrity tracks the gray matter metabolic decline in normal aging. Neuroimage. 2009;45(1):17–28. doi: 10.1016/j.neuroimage.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozachuk WE, DeCarli C, Schapiro MB, Wagner EE, Rapoport SI, Horwitz B. White matter hyperintensities in dementia of Alzheimer's type and in healthy subjects without cerebrovascular risk factors: a magnetic resonance imaging study. Arch. Neurol. 1990;47(12):1306–1310. doi: 10.1001/archneur.1990.00530120050009. [DOI] [PubMed] [Google Scholar]
- Krinsky-McHale SJ, Jenkins EC, Zigman WB, Silverman W. Ophthalmic disorders in adults with Down syndrome. Curr. Gerontol. Geriatr. Res. 2012;2012 doi: 10.1155/2012/974253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Williams RS. A prospective study of Alzheimer disease in Down syndrome. Arch. Neurol. 1989;46(8):849. doi: 10.1001/archneur.1989.00520440031017. [DOI] [PubMed] [Google Scholar]
- Lanfranchi S, Jerman O, Dal Pont E, Alberti A, Vianello R. Executive function in adolescents with Down Syndrome. J. Intellect. Disabil. Res. 2010;54(4):308–319. doi: 10.1111/j.1365-2788.2010.01262.x. [DOI] [PubMed] [Google Scholar]
- Lejeune J, Gautier M, Turpin R. Etude des chromosomes somatiques de neuf enfants mongoliens. Comptes Rendus Hebdomadaires des Seances de L'Academie des Sciences. 1959;248:1721–1722. [PubMed] [Google Scholar]
- Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RG, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control: The ARIC study. Stroke. 1996;27(12):2262–2270. doi: 10.1161/01.str.27.12.2262. [DOI] [PubMed] [Google Scholar]
- Lindgren A, Roijer A, Rudling O, Norrving B, Larsson E-M, Eskilsson J, et al. Cerebral lesions on magnetic resonance imaging, heart disease, and vascular risk factors in subjects without stroke: A population-based study. Stroke. 1994;25(5):929–934. doi: 10.1161/01.str.25.5.929. [DOI] [PubMed] [Google Scholar]
- Liston R, Mickelborough J, Bene J, Tallis R. A new classification of higher level gait disorders in patients with cerebral multi-infarct states. Age Ageing. 2003;32(3):252–258. doi: 10.1093/ageing/32.3.252. [DOI] [PubMed] [Google Scholar]
- Longstreth W, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: The Cardiovascular Health Study. Stroke. 1996;27(8):1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- Lott IT, Dierssen M. Cognitive deficits and associated neurological complications in individuals with Down's syndrome. Lancet Neurol. 2010;9(6):623–633. doi: 10.1016/S1474-4422(10)70112-5. [DOI] [PubMed] [Google Scholar]
- Lott IT, Doran E, Nguyen VQ, Tournay A, Head E, Gillen DL. Down syndrome and dementia: A randomized, controlled trial of antioxidant supplementation. Am. J. Med. Genet. A. 2011;155(8):1939–1948. doi: 10.1002/ajmg.a.34114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott IT, Head E. Down syndrome and Alzheimer's disease: A link between development and aging. Ment. Retard. Dev. Disabil. Res. Rev. 2001;7(3):172–178. doi: 10.1002/mrdd.1025. [DOI] [PubMed] [Google Scholar]
- Lott IT, Lai F. Dementia in Down's syndrome: Observations from a neurology clinic. Appl. Res. Ment. Retard. 1982;3(3):233–239. doi: 10.1016/0270-3092(82)90017-0. [DOI] [PubMed] [Google Scholar]
- Luck T, Riedel-Heller S, Luppa M, Wiese B, Bachmann C, Jessen F, et al. A hierarchy of predictors for dementia-free survival in old-age: Results of the AgeCoDe study. Acta Psychiatr. Scand. 2014;129(1):63–72. doi: 10.1111/acps.12129. [DOI] [PubMed] [Google Scholar]
- Makki MI, Munian Govindan R, Wilson BJ, Behen ME, Chugani HT. Altered Fronto-Striato-Thalamic Connectivity in Children with Tourette Syndrome Assessed with Diffusion Tensor MRI and Probabilistic Fiber Tracking. J. Child Neurol. 2009;24(6):669–678. doi: 10.1177/0883073808327838. [DOI] [PubMed] [Google Scholar]
- Maloney B, Lahiri DK. The Alzheimer's amyloid β-peptide (Aβ) binds a specific DNA Aβ-interacting domain (AβID) in the APP, BACE1, and APOE promoters in a sequence-specific manner: Characterizing a new regulatory motif. Gene. 2011;488(1):1–12. doi: 10.1016/j.gene.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: A PET study. Hum. Brain Mapp. 2003;19(1):47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D, Esiri MM. The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down's syndrome. J. Neurol. Sci. 1989;89(2):169–179. doi: 10.1016/0022-510x(89)90019-1. [DOI] [PubMed] [Google Scholar]
- McDonald WM, Ranga K, Krishnan R, Doraiswamy PM, Figiel GS, Husain MM, et al. Magnetic resonance findings in patients with early-onset Alzheimer's disease. Biol. Psychiatry. 1991;29(8):799–810. doi: 10.1016/0006-3223(91)90199-v. [DOI] [PubMed] [Google Scholar]
- McGough EL, Kelly VE, Logsdon RG, McCurry SM, Cochrane BB, Engel JM, et al. Associations between physical performance and executive function in older adults with mild cognitive impairment: gait speed and the timed “up & go” test. Phys. Ther. 2011;91(8):1198–1207. doi: 10.2522/ptj.20100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie K, Murray G, McKenzie S, Muir J. A prospective, longitudinal study of functional decline in individuals with Down's syndrome. J. Intellect. Disabil. 1998;2(2):98–104. [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel T, Bertrand E, Szpak GM, Stępień T, Wierzba-Bobrowicz T. Cerebral amyloid angiopathy as a cause of an extensive brain hemorrhage in adult patient with Down's syndrome–A case report. Folia Neuropathol. 2010;48(3):206–211. [PubMed] [Google Scholar]
- Menéndez M. Down syndrome, Alzheimer's disease and seizures. Brain Dev. 2005;27(4):246–252. doi: 10.1016/j.braindev.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Barron DW. Apraxia of Gait: A Clinico-Physiological Study. Brain. 1960;83(2):261–284. [Google Scholar]
- Mielke MM, Roberts RO, Savica R, Cha R, Drubach DI, Christianson T, et al. Assessing the temporal relationship between cognition and gait: Slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68(8):929–937. doi: 10.1093/gerona/gls256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan Sanchez M, Heyn SN, Das D, Moghadam S, Martin KJ, Salehi A. Neurobiological elements of cognitive dysfunction in Down Syndrome: Exploring the role of APP. Biol. Psychiatry. 2012;71(5):403–409. doi: 10.1016/j.biopsych.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Mirsen TR, Lee DH, Wong CJ, Diaz JF, Fox AJ, Hachinski VC, et al. Clinical correlates of white-matter changes on magnetic resonance imaging scans of the brain. Arch. Neurol. 1991;48(10):1015–1021. doi: 10.1001/archneur.1991.00530220031015. [DOI] [PubMed] [Google Scholar]
- Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, et al. Cortical Mapping of Gait in Humans: A Near-Infrared Spectroscopic Topography Study. Neuroimage. 2001;14(5):1186–1192. doi: 10.1006/nimg.2001.0905. [DOI] [PubMed] [Google Scholar]
- Mura T, Proust-Lima C, Jacqmin-Gadda H, Akbaraly TN, Touchon J, Dubois B, et al. Measuring cognitive change in subjects with prodromal Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2014;85(4):363–370. doi: 10.1136/jnnp-2013-305078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neltner JH, Abner EL, Schmitt FA, Denison SK, Anderson S, Patel E, et al. Digital pathology and image analysis for robust high-throughput quantitative assessment of Alzheimer disease neuropathologic changes. J. Neuropathol. Exp. Neurol. 2012;71(12):1075. doi: 10.1097/NEN.0b013e3182768de4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis-Mark RE. Diagnosing Alzheimer's dementia in Down syndrome: Problems and possible solutions. Res. Dev. Disabil. 2009;30(5):827–838. doi: 10.1016/j.ridd.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Marsden CD, Thompson PD. Human walking and higher-level gait disorders, particularly in the elderly. Neurology. 1993;43(2):268. doi: 10.1212/wnl.43.2.268. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers P, Morris R, Williams S, Markus H. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57(4):632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Outwater EK, Platenberg RC, Wolpert SM. Moyamoya disease in Down syndrome. AJNR Am. J. Neuroradiol. 1989;10(5 suppl):S23–S24. [PMC free article] [PubMed] [Google Scholar]
- Panisset M, Roudier M, Saxton J, Boiler F. Severe Impairment Battery: A neuropsychological test for severely demented patients. Arch. Neurol. 1994;51(1):41–45. doi: 10.1001/archneur.1994.00540130067012. [DOI] [PubMed] [Google Scholar]
- Parihar R, Mahoney JR, Verghese J. Relationship of gait and cognition in the elderly. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2013;2(3):167–173. doi: 10.1007/s13670-013-0052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen MM, Holt NE, Grande L, Kurlinski LA, Beauchamp MK, Kiely DK, et al. Mild Cognitive Impairment status and mobility performance: An analysis From the Boston RISE Study. J. Gerontol. A Biol. Sci. Med. Sci. 2014:glu063. doi: 10.1093/gerona/glu063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF, Moon J, Edgin J, Stedron J, Nadel L. The Neuropsychology of Down Syndrome: Evidence for Hippocampal Dysfunction. Child Dev. 2003;74(1):75–93. doi: 10.1111/1467-8624.00522. [DOI] [PubMed] [Google Scholar]
- Persad CC, Jones JL, Ashton-Miller JA, Alexander NB, Giordani B. Executive function and gait in older adults with cognitive impairment. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63(12):1350–1355. doi: 10.1093/gerona/63.12.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Pink A, Acosta J, Roberts R, Mielke M, Christianson T, Pankratz V, et al. Neuropsychiatric Symptoms, ApoE4 and the Risk Of Incident Dementia: The Mayo Clinic Study of Aging (P3. 215). Neurology. 2014;82(10 Supplement):P3. 215. [Google Scholar]
- Powell D, Caban-Holt A, Jicha G, Robertson W, Davis R, Gold BT, et al. Frontal white matter integrity in adults with Down syndrome with and without dementia. Neurobiol. Aging. 2014;35(7):1562–1569. doi: 10.1016/j.neurobiolaging.2014.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher V. End-stage dementia in adults with Down syndrome. Int. J. Geriatr. Psychiatry. 1995;10(12):1067–1069. [Google Scholar]
- Prasher V, Filer A. Behavioural disturbance in people with Down's syndrome and dementia. J. Intellect. Disabil. Res. 1995;39(5):432–436. doi: 10.1111/j.1365-2788.1995.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Reed-Cossairt A, Zhu X, Lee H-G, Reed C, Perry G, Petersen RB. Alzheimer's disease and vascular deficiency: Lessons from imaging studies and Down syndrome. Curr. Gerontol. Geriatr. Res. 2012;2012:1–5. doi: 10.1155/2012/929734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C. Alzheimer's disease and the amyloid cascade hypothesis: A critical review. Int. J. Alzheimers Dis. 2012;2012 doi: 10.1155/2012/369808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizen NJ, Patterson D. Down's syndrome. Lancet. 2003;361(9365):1281–1289. doi: 10.1016/S0140-6736(03)12987-X. [DOI] [PubMed] [Google Scholar]
- Rumble B, Retallack R, Hilbich C, Simms G, Multhaup G, Martins R, et al. Amyloid A4 protein and its precursor in Down's syndrome and Alzheimer's disease. N. Engl. J. Med. 1989;320(22):1446–1452. doi: 10.1056/NEJM198906013202203. [DOI] [PubMed] [Google Scholar]
- Sahyoun C, Floyer-Lea A, Johansen-Berg H, Matthews PM. Towards an understanding of gait control: Brain activation during the anticipation, preparation and execution of foot movements. Neuroimage. 2004;21(2):568–575. doi: 10.1016/j.neuroimage.2003.09.065. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung H-P. MRI white matter hyperintensities: Three-year follow-up of the Austrian Stroke Prevention Study. Neurology. 1999;53(1):132–132. doi: 10.1212/wnl.53.1.132. [DOI] [PubMed] [Google Scholar]
- Schmitt FA, Ashford W, Ernesto C, Saxton J, Schneider LS, Clark CM, et al. The Severe Impairment Battery: Concurrent validity and the assessment of longitudinal change in Alzheimer's disease. Alzheimer Dis. Assoc. Disord. 1997;11:51–56. [PubMed] [Google Scholar]
- Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: Divided attention increases gait variability in Alzheimer's disease. J. Am. Geriatr. Soc. 2003;51(11):1633–1637. doi: 10.1046/j.1532-5415.2003.51516.x. [DOI] [PubMed] [Google Scholar]
- Sleegers K, Brouwers N, Gijselinck I, Theuns J, Goossens D, Wauters J, et al. APP duplication is sufficient to cause early onset Alzheimer's dementia with cerebral amyloid angiopathy. Brain. 2006;129(11):2977–2983. doi: 10.1093/brain/awl203. [DOI] [PubMed] [Google Scholar]
- Smith BA, Stergiou N, Ulrich BD. Patterns of gait variability across the lifespan in persons with and without Down syndrome. J. Neurol. Phys. Ther. 2011;35(4):170. doi: 10.1097/NPT.0b013e3182386de1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BA, Ulrich BD. Early onset of stabilizing strategies for gait and obstacles: Older adults with Down syndrome. Gait Posture. 2008;28(3):448–455. doi: 10.1016/j.gaitpost.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Evans AT, Costall B, Smythe JW. Thyroid hormones, brain function and cognition: A brief review. Neurosci. Biobehav. Rev. 2002;26(1):45–60. doi: 10.1016/s0149-7634(01)00037-9. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: The role of aging, falls, and executive function. Mov. Disord. 2006;21(7):950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- Srikanth V, Phan TG, Chen J, Beare R, Stapleton JM, Reutens DC. The location of white matter lesions and gait—A voxel-based study. Ann. Neurol. 2010;67(2):265–269. doi: 10.1002/ana.21826. [DOI] [PubMed] [Google Scholar]
- Steinthal H. Einleitung in die Psychologie und Sprachwissenschaft. F. Dummlers; Berlin: 1881. Abriss der Sprachwissenschaft. pp. 167–171. [Google Scholar]
- Stricker G. What is a scientist-practitioner anyway? J. Clin. Psychol. 2002;58(10):1277–1283. doi: 10.1002/jclp.10111. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer's Disease. Annu. Rev. Neurosci. 1996;19(1):53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Miyai I, Ono T, Oda I, Konishi I, Kochiyama T, et al. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: An optical imaging study. Neuroimage. 2004;23(3):1020–1026. doi: 10.1016/j.neuroimage.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Alexander GE, Schapiro MB, Möller HJ, Rapoport SI, Hampel H. Age-related cortical grey matter reductions in non-demented Down's syndrome adults determined by MRI with voxel-based morphometry. Brain. 2004;127(4):811–824. doi: 10.1093/brain/awh101. [DOI] [PubMed] [Google Scholar]
- Toots A, Rosendahl E, Lundin-Olsson L, Nordström P, Gustafson Y, Littbrand H. Usual gait speed independently predicts mortality in very old people: A population-based study. J. Am. Med. Dir. Assoc. 2013;14(7):529. e521–529. e526. doi: 10.1016/j.jamda.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Torr J, Strydom A, Patti P, Jokinen N. Aging in Down Syndrome: Morbidity and Mortality. Journal of Policy and Practice in Intellectual Disabilities. 2010;7(1):70–81. [Google Scholar]
- van Iersel MB, Kessels RP, Bloem BR, Verbeek AL, Rikkert MGO. Executive functions are associated with gait and balance in community-living elderly people. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63(12):1344–1349. doi: 10.1093/gerona/63.12.1344. [DOI] [PubMed] [Google Scholar]
- van Kan GA, Rolland Y, Gillette-Guyonnet S, Gardette V, Annweiler C, Beauchet O, et al. Gait speed, body composition, and dementia. The EPIDOS-Toulouse cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67(4):425–432. doi: 10.1093/gerona/glr177. [DOI] [PubMed] [Google Scholar]
- van Swieten J, van Gijn J. Leuko-araiosis: Arteriosclerotic dementia revived. Clin. Neurol. Neurosurg. 1991;93(2):174. [Google Scholar]
- Verghese J, Annweiler C, Ayers E, Barzilai N, Beauchet O, Bennett DA, et al. Motoric cognitive risk syndrome: Multicountry prevalence and dementia risk. Neurology. 2014;83(8):718–726. doi: 10.1212/WNL.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Wang C, Katz MJ, Barzilai N, Lipton RB. Role of APOE genotype in gait decline and disability in aging. J. Gerontol. A Biol. Sci. Med. Sci. 2013:glt115. doi: 10.1093/gerona/glt115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer's dementia. N. Engl. J. Med. 2002;347(22):1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2012;68:412–418. doi: 10.1093/gerona/gls191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlund L-O, Basun H, Almkvist O, Andersson-Lundman G, Julin P, Sääf J. White matter hyperintensities in dementia: Does it matter? Magn. Reson. Imaging. 1994;12(3):387–394. doi: 10.1016/0730-725x(94)92531-3. [DOI] [PubMed] [Google Scholar]
- Waldemar G, Christiansen P, Larsson H, Høgh P, Laursen H, Lassen N, et al. White matter magnetic resonance hyperintensities in dementia of the Alzheimer type: Morphological and regional cerebral blood flow correlates. J. Neurol. Neurosurg. Psychiatry. 1994;57(12):1458–1465. doi: 10.1136/jnnp.57.12.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol. Aging. 2005;26(9):1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Watson N, Rosano C, Boudreau R, Simonsick E, Ferrucci L, Sutton-Tyrrell K, et al. Executive function, memory, and gait speed decline in well-functioning older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65(10):1093–1100. doi: 10.1093/gerona/glq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NS, Alkire MT, Haier RJ. A voxel-based morphometric study of nondemented adults with Down Syndrome. Neuroimage. 2003;20(1):393–403. doi: 10.1016/s1053-8119(03)00273-8. [DOI] [PubMed] [Google Scholar]
- Willey JZ, Scarmeas N, Provenzano FA, Luchsinger JA, Mayeux R, Brickman AM. White matter hyperintensity volume and impaired mobility among older adults. J. Neurol. 2013;260(3):884–890. doi: 10.1007/s00415-012-6731-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann. Neurol. 1985;17(3):278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Ylikoski R, Ylikoski A, Erkinjuntti T, Sulkava R, Raininko R, Tilvis R. White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Arch. Neurol. 1993;50(8):818–824. doi: 10.1001/archneur.1993.00540080029009. [DOI] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov. Disord. 2008;23(3):329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman WB, Schupf N, Sersen E, Silverman W. Prevalence of dementia in adults with and without Down syndrome. American Journal on Mental Retardation. 1996 [PubMed] [Google Scholar]