Abstract
Objective
Exposure therapy in anorexia nervosa has preliminarily been shown to be effective for increasing food intake. d-Cycloserine is a glutamatergic N-methyl-d-aspartate receptor agonist that has been shown to facilitate the benefits of exposure therapy for anxiety disorders by enhancing the emotional learning in the exposures; therefore, we examined d-cycloserine–facilitation of exposure therapy to increase body mass index (BMI) in patients with anorexia nervosa.
Method
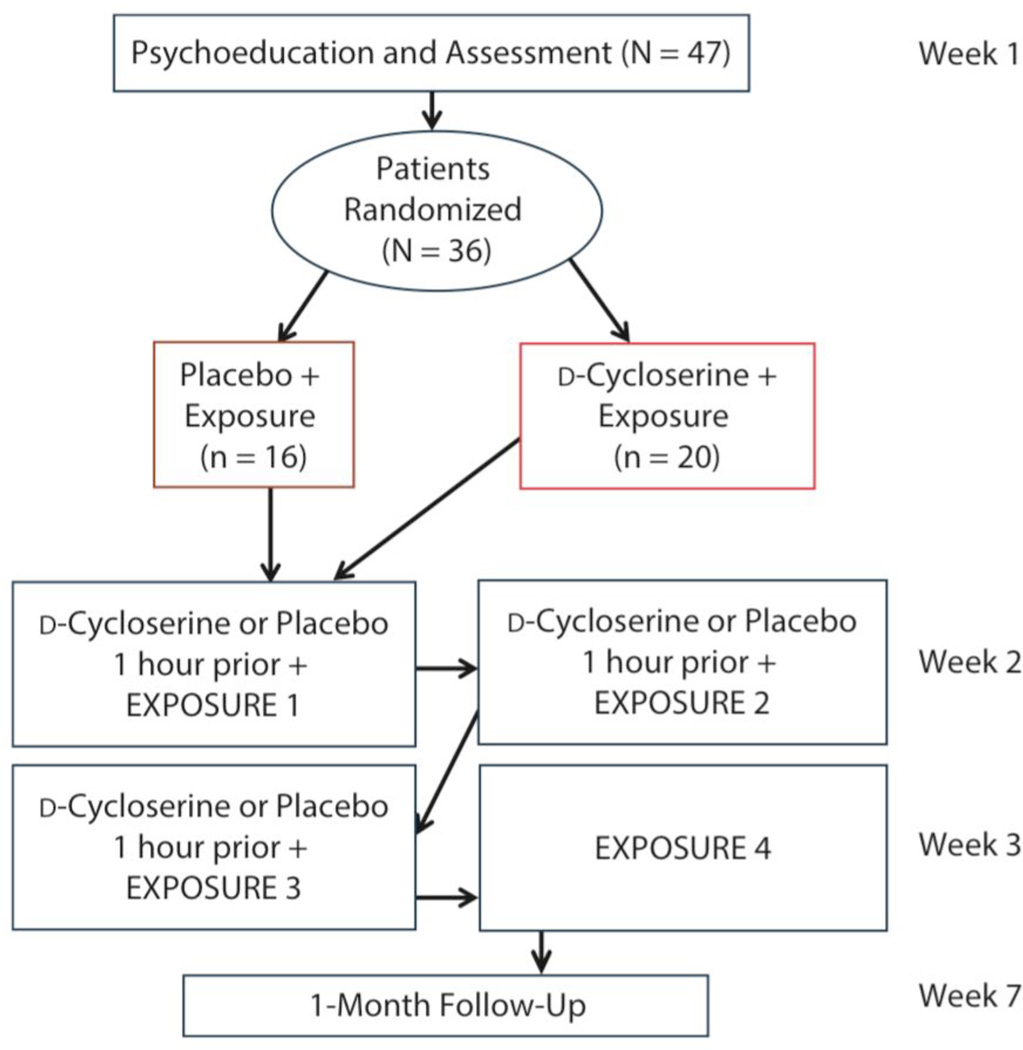
Participants (N = 36) with anorexia nervosa (diagnosed via DSM-IV) were recruited from a partial hospitalization eating disorder clinic between February 2013 and November 2013. Participants were randomly assigned to receive exposure therapy plus d-cycloserine (n = 20) or placebo (n = 16). Participants completed psychoeducation and 4 sessions of exposure therapy, with medication (d-cycloserine vs placebo) given prior to the first 3 exposure sessions. They also completed a 1-month follow-up.
Results
As hypothesized, participants in the d-cycloserine group showed a significantly greater increase in BMI than those in the placebo group (Wilk Λ = 0.86, F3,32 = 2.20, P = .043, ηp2 = 0.12). d-Cycloserine participants gained 3 pounds relative to 0.5 pounds in the placebo group. Both groups experienced significantly decreased anxiety over the course of therapy (Wilk Λ = 0.80, F3,32 = 3.32, P = .023, ηp2 = 0.20).
Conclusions
This study preliminarily demonstrates that d-cycloserine facilitates exposure therapy for anorexia nervosa, leading to increased weight gain. A potential mechanism is that participants who receive d-cycloserine may generalize learning from within-session exposures to food intake during other similar meals, resulting in sustained increases in BMI. Further research is needed to confirm these findings and test the putative mechanism that generalized learning from exposure therapy can increase BMI and stabilize a healthy weight.
Trial Registration
ClinicalTrials.gov identifier: NCT01996644
Anorexia nervosa is a severely impairing disorder characterized by extreme weight loss.1 It typically afflicts young adults and has a chronic course, which is associated with high mortality rates, additional psychiatric comorbidities, and high risk of suicide.2,3 Success rates specifically for the treatment of anorexia nervosa in adults are low, and, for those individuals who seek treatment, the recovery process is costly and lengthy.4,5 Furthermore, in individuals who do improve, relapse (ie, weight loss after regain) is very common, occurring in more than one-third of recovered individuals.6 More efficacious treatments are urgently needed that improve and maintain weight regain in anorexia nervosa.
It has been proposed that exposure therapy (a form of treatment focused on extinguishing fears by enhancing learning) may help facilitate eating in individuals with anorexia nervosa.7–9 In the first clinical trial10 to test exposure therapy for weight-restored patients with anorexia nervosa, exposure and response prevention therapy (ERP) successfully reduced mealtime anxiety such that patients who completed ERP ate significantly more food at a final meal compared to a control group, which, if generalized to similar meals, should relate to increased and sustained weight gain over time. These findings support the use of exposure therapy in anorexia nervosa, but also raise the question of how to improve its effectiveness.
There has been great interest in using learning-enhancement medications, such as d-cycloserine, to facilitate exposure therapy.11 d-Cycloserine is an N-methyl-d-aspartate (NMDA) receptor modulator, and at a single oral dose range of 50–500 mg, d-cycloserine acts as an agonist of the NMDA receptor and facilitates learning.12,13 Thus, d-cycloserine facilitates exposure therapy by augmenting glutamatergic function and increasing the efficiency of fear extinction. Several studies in anxiety disorders have shown that d-cycloserine facilitates exposure therapy and increases behavioral learning,14 enhancing learning that occurs from exposure therapy.15 In regard to anorexia nervosa, d-cycloserine facilitation of exposure could be especially useful if it can lead to weight gain, given that research has shown that patients with a lower rate of weight gain during treatment16 and who are less likely to adhere to their meal plan during treatment are more likely to relapse.17 Further, the best predictor of weight maintenance after treatment is high body mass index (BMI).18 Thus, utilizing d-cycloserine or similar learning-enhancing agents coupled with exposure therapy may be an intervention that could promote weight gain and reduce the high rate of relapse. Additionally, anorexia nervosa is often a treatment-refractory illness, and, therefore, any type of treatment augmentation would be extremely beneficial.
Our objective was to conduct a pilot study to test if d-cycloserine would facilitate exposure therapy in anorexic patients who expressed experiencing anxiety during mealtime. We hypothesized that (1) exposure therapy would be effective in reducing anxiety and increasing percentage of food intake during meals; (2) participants in the d-cycloserine group, relative to placebo, would experience significantly more anxiety reduction over the course of therapy; and (3) participants in the d-cycloserine group would experience greater increases in body mass index relative to participants in the placebo group (perhaps because of the ability to generalize the learning facilitated during exposure meal therapy to subsequent meals). Finally, this study would serve as a test of the feasibility of conducting exposure therapy for anorexic patients in a routine clinical care setting.
METHOD
Participants
Participants were recruited between February 2013 and November 2013 from a community eating disorder treatment center that provides a partial hospitalization program and were currently undergoing treatment in this program. All participants were identified as needing to gain weight. Eligibility required a DSM-IV diagnosis of anorexia nervosa and endorsement of significant mealtime anxiety. All participants met criteria for anorexia nervosa (N = 36). There were participants initially included in the study who met criteria for bulimia nervosa (n = 4) and eating disorder not otherwise specified (n = 1). However, because of the small sample size of these categories, they were not included in the results reported here. Notably, there were no substantive changes to the results when analyzed both with and without participants with bulimia nervosa and eating disorder not otherwise specified. No participants were currently psychotic or manic. All participants endorsed high levels of mealtime or food anxiety determined (1) during the psychoeducation portion (see Procedures for details) of the trial via interview and (2) by a measure19 of fear of food, which assesses trait vulnerability to feel anxious and fearful when eating. These materials are available at request from the first author.
Procedures
This clinical trial was registered at ClinicalTrials.gov (NCT01996644). The Washington University institutional review board approved the study. Participants gave written consent. The complete study protocol is available from the first author. Figure 1 outlines study procedures.
Figure 1.
Assessment and Intervention Procedures for d-Cycloserine– vs Placebo-Facilitated Exposure Therapy for Mealtime Anxiety in Patients With Anorexia
Measures
Body mass index was our primary outcome variable, as a major goal of treatment in an eating disorder center is to increase and stabilize a healthy weight. We also assessed anxiety and percentage of meal completed (the amount of the meal consumed) as process variables. Body mass index was assessed by either a licensed nurse or approved staff and was measured by a medical grade Detecto precision scale and height tool. All participants were weighed in light clothing without shoes and were not informed of their weight.
Anxiety was measured with the Subjective Units of Distress Scale (SUDS),20 which is a behavioral measure often used during exposure treatment and behavioral assessment to measure anxiety. The SUDS has been shown to be a valid and reliable measure of state anxiety for research outcomes.21 Subjective Units of Distress Scale ratings can range from 0 (completely calm) to 100 (highest anxiety) and were measured before, during, and after the meal. We tested both a composite of mean SUDS ratings across all time points within a given exposure (total average SUDS) and SUDS ratings at each time point individually. Internal consistency for the SUDS across all time points was excellent (α levels ≥ .96).
Food percentage was measured by the kitchen staff who recorded total caloric intake of meals based on grams before the meal and then calculated the percentage of the meal eaten after the exposure. Eating disorder symptoms were measured by the Eating Disorder Inventory, 2nd revision (EDI-2),22 and depression levels were measured by the Beck Depression Inventory, 2nd revision (BDI-II)23 (see Table 1).
Table 1.
Demographic and Clinical Characteristics at Study Entry Across Group
| Characteristic |
d-Cycloserine (n = 20), Mean (SD) |
Placebo (n = 16), Mean (SD) |
Combined (N = 36), Mean (SD) |
Statistica | Pa |
|---|---|---|---|---|---|
| Age, y | 26.10 (10.53) | 24.63 (8.96) | 25.44 (9.75) | t = 0.44 | .659 |
| BMI | 20.10 (2.13) | 20.44 (1.91) | 20.25 (2.09) | t = 0.28 | .782 |
| Scores on EDI-2 | |||||
| Drive for thinness | 16.85 (7.00) | 21.19 (6.25) | 18.78 (6.93) | t = 1.93 | .071 |
| Bulimic symptoms | 6.95 (6.95) | 6.12 (5.80) | 6.58 (6.38) | t = 0.38 | .706 |
| Body dissatisfaction | 18.10 (7.73) | 20.88 (4.98) | 19.33 (6.71) | t = 1.24 | .223 |
| Scores on BDI-II | |||||
| Depression | 33.10 (10.02) | 34.50 (9.10) | 33.74 (9.50) | t = 0.43 | .672 |
| Diagnoses | n (%) | n (%) | n (%) | ||
| BMI ≥ 18.5 | 15 (75.0) | 14 (87.5) | 29 (80.6) | χ2 = 0.887 | .346 |
| Anxiety disorder | 15 (75.0) | 15 (93.8) | 30 (83.3) | χ2 = 1.55 | .213 |
| Depressive disorder | 15 (75.0) | 13 (81.3) | 28 (77.8) | χ2 = 0.83 | .662 |
There are no significant differences across condition. However, we should note that there are slight differences (though nonsignificant) in drive for thinness, body dissatisfaction, and in the numbers of participants whose BMI equaled or exceeded 18.5 and who were diagnosed with a comorbid anxiety disorder.
Abbreviations: BDI-II = Beck Depression Inventory, 2nd revision, BMI = body mass index, EDI-2 = Eating Disorder Inventory, 2nd revision.
Participants were given a formal assessment using 2 structured clinical interviews: the Mini-International Neuropsychiatric Inventory24 and the Structured Clinical Interview for DSM-IV Eating Disorder Module25 to determine eligibility.
Treatment
Participants were given structured psychoeducation on the purpose of anxiety and exposure therapy. Specifically, participants were educated on the purpose and nature of anxiety, the tendency for anxiety to reduce with sustained exposure to a feared stimulus, and the purpose of exposure therapy. All psychoeducation materials are available at request from the first author. Participants were explicitly instructed to allow themselves to feel anxiety during the meal exposure (explained below) instead of avoiding anxiety via distraction or by using compulsive behaviors (ie, cutting the food into tiny pieces). Participants were given an explanation of the SUDS, asked for their current SUDS level to assess understanding of the scale, and asked what their SUDS level would be during a meal.
Participants completed 4 exposure sessions consisting of mealtime exposures across 2 weeks, similar to 1 previous small trial26 of d-cycloserine–facilitated exposure. All participants were given the same combination of a sandwich, fruit, yogurt, and pretzels to control for differences that differing foods across exposures could produce in anxiety levels. Exposure sessions were carried out in a group format with 2 to 5 participants during 45-minute lunchtime meals that were led by a trained cognitive behavioral therapist (C.A.L.). During these exposure sessions, participants were reminded periodically to experience and not avoid any anxiety that they felt. Any anxiety-reducing behaviors (ie, tearing food) were redirected, and patients were reminded not to engage in any anxiety-reducing compulsions, but rather to allow themselves to experience the anxiety. At the end of the meal, participants completed a short postmeal therapy group focusing on anxiety.
Medication and randomization
Participants were randomly assigned by a random number generator to 250 mg of d-cycloserine or placebo in identical-appearing sequentially numbered capsules by a data manager unaffiliated with the treatment center and who had no interaction with study participants. We decided to use 250 mg based on scientific and pragmatic considerations: it is in the middle of the range of well-tolerated and efficacious doses for learning facilitation,11 it is a dose that has previously been shown to facilitate learning in humans, and it is a dose that has been previously used in a study of biological target engagement for d-cycloserine.27 Further, data suggest that the minimum dosage of 50 mg might be too low, given some failures to find target engagement at that dosage in conjunction with target engagement being found at higher dosages.27,28 All individuals who had any interaction with patients were blinded to treatment assignment until study completion. Adequacy of the blind was assessed by having both participants and experimenters guess treatment assignment after session 1 of the study; neither participants nor experimenters were able to discern assignment (P values > .75). Participants ingested their assigned pill 1 hour prior to the session.
The following was our completion rate: 28 participants completed 4 sessions, 4 participants completed only 3 sessions, 2 participants completed only 2 sessions, and 2 participants completed only 1 session. Sessions were missed because insurance no longer supported participant treatment (n = 5) or a scheduled outing conflicted with the session date (n = 2). Completers did not differ by group (P = .61). We also conducted a 1-month follow-up assessment in which participants were asked to imagine they were eating a meal, and we collected SUDS levels before, during, and after the imagined meal, as well as recorded weight for BMI in person (n = 28).
Statistical analyses
All analyses were conducted in SPSS Version 19 or in R (Version 2.15). Missing data were imputed using the Amelia package in R.29 Group differences at baseline were examined using independent t tests for continuous variables and the χ2 test for categorical variables. Repeated measures analyses of variance (ANOVAs) were conducted to assess whether there was a significant effect of group, time, and time-by-group interaction on the outcome and process variables (anxiety and meal percentage). Time was a within-participant variable, whereas group (d-cycloserine vs placebo) was a between-participant variable. Body mass index; total average anxiety (SUDS); anxiety before, during, and after exposure; and percentage of food intake at each visit were entered as dependent variables.
RESULTS
Demographic and Clinical Characteristics
Participants (N = 36) were diagnosed with anorexia nervosa (n= 10, binge-purge type; n=26, restricting type). Participants were mostly female (n = 35; 97.2%). The mean age of participants was 25.44 years (range, 14–49 years) and participants were mostly white (n = 35; 97.2%). Participants exhibited high levels of comorbidity for at least 1 anxiety (n = 30, 83.3%) or depressive disorder (n = 28, 77.7%). Participants’ mean BMI at Exposure 1 was 20.24 (SD = 2.02; range, 15.34–24.27). Most participants (n = 29) were nearing a healthy weight, but a substantial percentage (n = 7; 19.4%) were underweight as defined by a BMI < 18.5. The participants ≥ 18.5 BMI cutoff for being underweight were not at their target healthy weight via recommendation by their primary dietician and clinical team (ie, it was recommended that they gain additional weight). Table 1 shows clinical and demographic characteristics (means and standard deviations and number of participants) across groups. There were no significant differences on any pretreatment variable across groups.
d-Cycloserine Facilitation of Exposure for Weight Regain
We first evaluated the effect of the intervention on change in BMI. We conducted a repeated measures ANOVA with time as the within-subject variable and BMI as the dependent variable. There was a significant time (Wilk Λ = 0.59, F3,32 = 7.55, P < .001, ηp2 = 0.40) and time-by-group interaction (Wilk Λ = 0.86, F3,32 = 2.20, P = .043, ηp2 = 0.12), such that participants in the d-cycloserine group had a greater increase in BMI relative to participants in the placebo group. Participants in the d-cycloserine group increased their BMI on average by 0.385 points, whereas participants in the placebo condition increased their BMI on average by 0.098 points. In other words, the clinical impact of these results showed that d-cycloserine–facilitated participants gained and maintained a 3-pound weight change, versus only 0.5 pounds in the placebo group. When we included 1-month follow-up as a fifth time point, there was a nonsignificant, moderate effect size for a time-by-group interaction (Wilk Λ = 0.88, F3,32=1.03, P = .387, ηp2 = 0.12), such that at 1-month follow-up, BMI continued to increase for participants in the d-cycloserine–facilitated exposure group, whereas in the placebo group, participants’ BMI began to decrease (Figure 2). These effects held when initial BMI was included as a covariate. These effects held when age was included as a covariate, which was a nonsignificant predictor of BMI (F3,32 = 0.72, P = .404). These results also held when adolescent participants (n = 4) were removed from analyses.
Figure 2.
d-Cycloserine vs Placebo Facilitation of Exposure Therapy Effects on Body Mass Index Across 4 Exposure Sessions and at 1 -Month Follow-Up
Process Variables
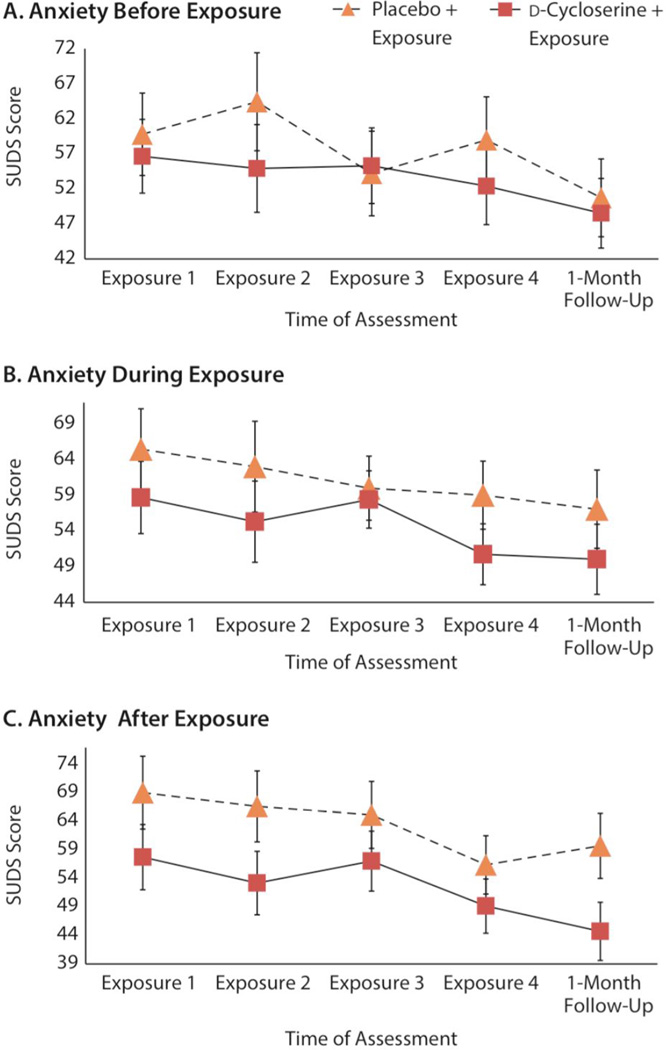
We then conducted this analysis with total average SUDS scores as the dependent variable and group as the between-subject variable. There was a significant multivariate effect for time (Wilk Λ = 0.80, F3,32 = 3.32, P = .023, ηp2 = 0.20), meaning that participants’ subjective anxiety decreased over the course of the intervention. The interaction between group and time was nonsignificant (P = .575), showing that anxiety levels significantly decreased over the course of the 4 exposure sessions, regardless of group (d-cycloserine vs placebo). The same analysis was conducted with food percentage as the dependent variable. There was a significant multivariate effect for time (Wilk Λ = 0.63, F3,37 = 6.03, P = .002, ηp2 = 0.36). The interaction between group and time was nonsignificant (P = .878), showing that all participants’ food intake increased over the course of the intervention, regardless of group. The effect of time for anxiety held at 1-month follow-up when 1-month follow-up was included as the fifth time point (Wilk Λ = 0.67, F3,32=4.95, P = .001, ηp2 = 0.26). This effect held for total average anxiety and anxiety before, during, and after exposure (Figure 3).
Figure 3.
Process Variables Assessed During Interventiona
aEstimated marginal means of mealtime anxiety across 4 d-cycloserine–facilitated exposure sessions and at 1-month follow-up.
Abbreviation: SUDS = Subjective Units of Distress Scale
Post Hoc Analysis
Finally, we conducted a post hoc analysis to determine if the interaction between time and group for BMI was driven by starting weight, such that those with lower weight were gaining more weight more rapidly. We predicted the standardized residual of Exposure 4 BMI controlling for Exposure 1 BMI by group, Exposure 1 BMI, and the interaction between group and Exposure 1 BMI. Group held as a significant predictor (partial r = 0.35, (β* = 0.35, P = .042). There was no significant interaction between group and Exposure 1 BMI (P = .259), showing that the increase in BMI was not dependent on or moderated by starting BMI.
DISCUSSION
In this randomized controlled trial, we found that d-cycloserine may facilitate exposure therapy, thereby increasing weight gain in patients diagnosed with anorexia nervosa, within a partial hospitalization setting. Participants who received d-cycloserine to facilitate exposure therapy, relative to placebo, were more likely to gain weight during the course of intervention. Further, at 1-month follow-up, for participants in the d-cycloserine group, there was some indication that participants continued to gain weight, whereas participants in the placebo group did not gain significant weight; their weight began to lapse. This finding is promising because it suggests that d-cycloserine may facilitate weight regain, and it is even more important given the recent research that implicates weight gain and high BMI as an important marker of symptom reduction in anorexia nervosa.18,30 However, we need future research with larger sample sizes to replicate this finding. Furthermore, this study is also the first to show that exposure therapy can be implemented in an anorexic population within a routine clinical care setting.
Why would 3 doses of a drug, given across 2 weeks, facilitate weight gain? d-Cycloserine is believed to be a learning-facilitation medication that arguably helps to enhance learning gained during exposure therapy.31 Thus, in our study, the putative mechanism could be that d-cycloserine increases positive learning during exposure sessions by disruption of the connection between fear and eating, which may then generalize to similar meals completed outside of the exposure sessions (between-exposure sessions). In other words, the learning that occurs within the exposure-facilitated session is reflected between sessions by an overall increase in food intake during other situations when food is present. This learning facilitated during exposure therapy may increase calorie consumption and weight gain for those in the d-cycloserine–facilitated group only. This finding is similar to other research finding that d-cycloserine facilitates between-session learning.32 These hypotheses need to be confirmed by additional research that explicitly tests the mechanisms behind d-cycloserine–facilitated exposure for weight regain.
In terms of behavior during the exposure sessions, we found that self-reported anxiety decreased similarly for both groups and was no different in the d-cycloserine versus placebo group. Given that participants in the d-cycloserine group were gaining weight more rapidly, they may have experienced the same level of anxiety as participants in the placebo group because of an overall larger intake of food outside of the therapy session, which would create a more challenging and anxiety-provoking exposure. This finding is consistent with other positive studies of d-cycloserine facilitation of exposure therapy, which found no differences in within-session anxiety33,34 and with current theories of exposure therapy for anxiety, which suggest that the goal of exposure therapy should be to promote behavioral learning and increase fear tolerance in spite of experienced anxiety.35,36 This result is also consistent with findings suggesting that most learning that occurs from exposure therapy is not done within session, but from the habituation that occurs between therapy sessions.37,38 In this case, we hypothesize that increased BMI was reflective of learning that was initiated during the exposure session and applied outside of the defined treatment session.
These results are promising for further development of d-cycloserine and other pharmacologic or nonpharmacologic facilitation of exposure therapy in anorexia nervosa. Anorexia nervosa is often chronic, treatment-resistant, and characterized by lack of motivation and high levels of relapse. Facilitation of exposure may be a mechanism that can decrease the negative effects of lower rate of weight gain during treatment16 and may help increase adherence to meal plans, which could prevent long-term relapse17 and decreased negative symptoms.18,30 Future researchers should attempt to replicate these results with a population that is entirely underweight and include additional potentially important covariates, such as time in treatment or history of treatment receipt. Future researchers should also consider utilizing alternate measures of weight, such as the body shape index or expected BMI for age and gender, given that these may be more suitable measures of body weight.39 Finally, future researchers should test potential mechanisms driving these effects, as well as test the optimal dosage of d-cycloserine and whether d-cycloserine affects appetite or metabolism.
Limitations of our study include a small sample size, short follow-up period, and limited outcome measures, including a limited measure of food intake. The small sample size increases concerns about the interpretability of the results and limits our power to detect small or medium size effects, which is often the case when conducting research with anorexia nervosa.1 For example, a larger sample size would provide greater power to detect any significant pretreatment differences on variables such as body dissatisfaction and presence of a comorbid anxiety disorder. Future research should replicate the results found here using a larger sample. However, a strength of this design was that we found 4 sessions of d-cycloserine–facilitated exposure to be easily administrated in a routine clinical setting. Unfortunately, we did not include a control group for the exposure-plus-placebo group. We hope that researchers will test if a similar type of intervention is effective in this type of setting regardless of d-cycloserine administration. Additionally, it is acknowledged that there still remain overarching questions on the optimal dose of d-cycloserine, for which patients d-cycloserine might be most effective,40–42 and whether other learning-facilitation methods might be more efficacious.43 However, our results suggest that treatment of anorexia nervosa with d-cycloserine augmentation may be a novel and valuable usage of d-cycloserine. Finally, our sample was primarily weight-restored and in a partial hospitalization program. Future researchers should test if these results generalize to additional populations of patients with anorexia nervosa, such as non–weight-restored individuals in residential treatments. Nevertheless, this is the first study to show that exposure therapy can be successfully implemented in an anorexic population undergoing treatment in a routine clinical care setting.
In summary, this study builds upon literature that suggests that exposure therapy may be an effective treatment for anorexia nervosa10 and adds to research showing that d-cycloserine augments learning from exposure therapy for anxiety disorders.14,32,44 We found that d-cycloserine–facilitated exposure can increase weight gain in patients with anorexia nervosa, which may be a step toward alleviating the extreme impairment and suffering associated with this disorder.
Clinical Points.
-
■
This study tested d-cycloserine facilitation of exposure therapy for anorexia nervosa in a routine clinical care setting.
-
■
Exposure therapy during mealtime was effective for decreasing anxiety experienced during meals and was feasible to implement in a partial hospitalization program.
-
■
d-Cycloserine–facilitated exposure therapy enhanced weight gain in patients with anorexia nervosa.
Acknowledgments
Dr Lenze receives support from Lundbeck, Roche, and Johnson & Johnson. Dr McCallum is a stock shareholder at McCallum Place.
Funding/support: This project was supported by the National Institute of Mental Health F31-MH096433-01 to Ms Levinson by providing training and materials for the study and the Taylor Family Institute for Innovative Psychiatric Research, which provided salary to Dr Lenze.
Role of the sponsors: The sponsors had no role in the design, analysis, or writing and publication of the study.
We thank Angela Basgall, AA, Department of Psychiatry, Washington University School of Medicine in St. Louis; Alice Kassinger, BA, and Emily Ness, BA, Department of Psychology, Washington University in St Louis, Missouri; and all the staff at McCallum Place, St Louis, Missouri for their work on this project. Ms Basgall provided randomization and medication support. Ms Kassinger and Ms Ness provided assistance with material preparation and data entry.
Footnotes
Potential conflicts of interest: Drs Rodebaugh, McCallum, and Lenze and Mss Levinson, Fewell, Kass, Riley, and Stark have no other conflicts of interest.
They have no conflicts of interest to report
REFERENCES
- 1.Fairburn CG. Evidence-based treatment of anorexia nervosa. lnt J Eat Disord. 2005;37(suppl):S26–S30. doi: 10.1002/eat.20112. discussion S41–S42. [DOI] [PubMed] [Google Scholar]
- 2.Beumont PJV, Russell JD, Touyz SW. Treatment of anorexia nervosa. Lancet. 1993;341(8861):1635–1640. doi: 10.1016/0140-6736(93)90769-d. [DOI] [PubMed] [Google Scholar]
- 3.Berkman ND, Lohr KN, Bulik CM. Outcomes of eating disorders: a systematic review of the literature. lnt J Eat Disord. 2007;40(4):293–309. doi: 10.1002/eat.20369. [DOI] [PubMed] [Google Scholar]
- 4.Finfgeld DL. Anorexia nervosa: analysis of long-term outcomes and clinical implications. Arch Psychiatr Nurs. 2002;16(4):176–186. doi: 10.1053/apnu.2002.34390. [DOI] [PubMed] [Google Scholar]
- 5.Herzog W, Schellberg D, Deter HC. First recovery in anorexia nervosa patients in the long-term course: a discrete-time survival analysis. J Consult Clin Psychol. 1997;65(1):169–177. doi: 10.1037//0022-006x.65.1.169. [DOI] [PubMed] [Google Scholar]
- 6.Herzog DB, Dorer DJ, Keel PK, et al. Recovery and relapse in anorexia and bulimia nervosa: a 7.5-year follow-up study. J Am Acad Child Adolesc Psychiatry. 1999;38(7):829–837. doi: 10.1097/00004583-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Koskina A, Campbell IC, Schmidt U. Exposure therapy in eating disorders revisited. Neurosci Biobehav Rev. 2013;37(2):193–208. doi: 10.1016/j.neubiorev.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Steinglass JE, Sysko R, Glasofer D, et al. Rationale for the application of exposure and response prevention to the treatment of anorexia nervosa. lnt J Eat Disord. 2011;44(2):134–141. doi: 10.1002/eat.20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh BT. The importance of eating behavior in eating disorders. Physiol Behav. 2011;104(4):525–529. doi: 10.1016/j.physbeh.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinglass JE, Albano AM, Simpson HB, et al. Confronting fear using exposure and response prevention for anorexia nervosa: a randomized controlled pilot study. lnt J Eat Disord. 2014;47(2):174–180. doi: 10.1002/eat.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of d-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Walker DL, Ressler KJ, Lu KT, et al. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of d-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22(6):2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis M, Ressler K, Rothbaum BO, et al. Effects of d-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60(4):369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann SG, Smits JAJ, Rosenfield D, et al. d-Cycloserine as an augmentation strategy with cognitive-behavioral therapy for social anxiety disorder. Am J Psychiatry. 2013;170(7):751–758. doi: 10.1176/appi.ajp.2013.12070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegmund A, Golfels F, Finck C, et al. d-Cycloserine does not improve but might slightly speed up the outcome of in-vivo exposure therapy in patients with severe agoraphobia and panic disorder in a randomized double blind clinical trial. J Psychiatr Res. 2011;45(8):1042–1047. doi: 10.1016/j.jpsychires.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Castro J, Gila A, Puig J, et al. Predictors of rehospitalization after total weight recovery in adolescents with anorexia nervosa. lnt J Eat Disord. 2004;36(1):22–30. doi: 10.1002/eat.20009. [DOI] [PubMed] [Google Scholar]
- 17.McFarlane T, Olmsted MP, Trottier K. Timing and prediction of relapse in a transdiagnostic eating disorder sample. lnt J Eat Disord. 2008;41(7):587–593. doi: 10.1002/eat.20550. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan AS, Walsh BT, Olmsted M, et al. The slippery slope: prediction of successful weight maintenance in anorexia nervosa. Psychol Med. 2009;39(6):1037–1045. doi: 10.1017/S003329170800442X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levinson C, Byrne M. The Fear of Food Measure: a novel measure for use in exposure therapy for eating disorders. lnt J Eat Disord. 2015;48(3):271–283. doi: 10.1002/eat.22344. [DOI] [PubMed] [Google Scholar]
- 20.Wolpe J. Subjective anxiety scale. In: Hersen M, Bellack AS, editors. Dictionary of Behavioral Assessment Techniques. New York, NY: Pergamon; 1998. pp. 455–457. [Google Scholar]
- 21.Kaplan DM, Smith T, Coons J. A validity study of the Subjective Unit of Discomfort (SUD) score. Meas Eval Couns Dev. 1995;27(4):195–199. [Google Scholar]
- 22.Garner DM, Olmstead MP, Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexia-nervosa and bulimia. lnt J Eat Disord. 1983;2(2):15–34. [Google Scholar]
- 23.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression lnventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 24.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 26.Steinglass J, Sysko R, Schebendach J, et al. The application of exposure therapy and d-cycloserine to the treatment of anorexia nervosa: a preliminary trial. J Psychiatr Pract. 2007;13(4):238–245. doi: 10.1097/01.pra.0000281484.89075.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onur OA, Schlaepfer TE, Kukolja J, et al. The N-methyl- d-aspartate receptor co-agonist d-cycloserine facilitates declarative learning and hippocampal activity in humans. Biol Psychiatry. 2010;67(12):1205–1211. doi: 10.1016/j.biopsych.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Santa Ana EJ, Rounsaville BJ, Frankforter TL, et al. d-Cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: a pilot investigation. Drug Alcohol Depend. 2009;104(3):220–227. doi: 10.1016/j.drugalcdep.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honaker J, King G, Blackwell M, et al. A program for missing data. J Stat Softw. 2011;45(7):1–47. [Google Scholar]
- 30.Accurso EC, Ciao AC, Fitzsimmons-Craft EE, et al. Is weight gain really a catalyst for broader recovery? the impact of weight gain on psychological symptoms in the treatment of adolescent anorexia nervosa. Behav Res Ther. 2014;56:1–6. doi: 10.1016/j.brat.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann SG, Meuret AE, Smits JA, et al. Augmentation of exposure therapy with d-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63(3):298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 32.Rothbaum BO, Price M, Jovanovic T, et al. A randomized, double-blind evaluation of d-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. Am J Psychiatry. 2014;171(6):640–648. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guastella AJ, Howard AL, Dadds MR, et al. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34(6):917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Rodebaugh TL, Levinson CA, Lenze EJ. A high-throughput clinical assay for testing drug facilitation of exposure therapy. Depress Anxiety. 2013;30(7):631–637. doi: 10.1002/da.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouton ME, King DA. Contextual control of the extinction of conditioned fear: tests for the associative value of the context. J Exp Psychol Anim Behav Process. 1983;9(3):248–265. [PubMed] [Google Scholar]
- 36.Craske MG, Kircanski K, Zelikowsky M, et al. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46(1):5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Abramowitz JS. The practice of exposure therapy: relevance of cognitive-behavioral theory and extinction theory. Behav Ther. 2013;44(4):548–558. doi: 10.1016/j.beth.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Plendl W, Wotjak CT. Dissociation of within- and between-session extinction of conditioned fear. J Neurosci. 2010;30(14):4990–4998. doi: 10.1523/JNEUROSCI.6038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. 2012;7(7):e39504. doi: 10.1371/journal.pone.0039504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Javitt DC. Harnessing N-methyl-d-aspartate receptors for new treatment development in psychiatry: positive lessons from negative studies. Am J Psychiatry. 2013;170(7):699–702. doi: 10.1176/appi.ajp.2013.13040503. [DOI] [PubMed] [Google Scholar]
- 41.Rodebaugh TL, Lenze EJ. Lessons learned from d-cycloserine: the promise and limits of drug facilitation of exposure therapy. J Clin Psychiatry. 2013;74(4):415–416. doi: 10.4088/JCP.13ac08464. [DOI] [PubMed] [Google Scholar]
- 42.Hofmann SG, Wu JQ, Boettcher H. d-Cycloserine as an augmentation strategy for cognitive behavioral therapy of anxiety disorders. Biol Mood Anxiety Disord. 2013;3(1):11. doi: 10.1186/2045-5380-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smits JA, Rosenfield D, Davis ML, et al. Yohimbine enhancement of exposure therapy for social anxiety disorder: a randomized controlled trial. Biol Psychiatry. 2014;75(11):840–846. doi: 10.1016/j.biopsych.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Difede J, Cukor J, Wyka K, et al. d-cycloserine augmentation of exposure therapy for post-traumatic stress disorder: a pilot randomized clinical trial. Neuropsychopharmacology. 2014;39(5):1052–1058. doi: 10.1038/npp.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]