Abstract
Donor-to-recipient organ size matching is a critical aspect of thoracic transplantation. In the United States potential recipients for lung transplant and heart transplant are listed with limitations on donor height and weight ranges, respectively. Height is used as a surrogate for lung size and weight is used as a surrogate for heart size. While these measures are important predictors of organ size, they are crude surrogates that fail to incorporate the influence of sex on organ size. Independent of other measures, a man’s thoracic organs are approximately 20% larger than a woman’s. Lung size can be better estimated using the predicted total lung capacity, which is derived from regression equations correcting for height, sex and age. Similarly, heart size can be better estimated using the predicted heart mass, which adjusts for sex, age, height, and weight. These refined organ sizing measures perform better than current sizing practice for the prediction of outcomes after transplantation, and largely explain the outcome differences observed after sex-mismatch transplantation. An undersized allograft is associated with worse outcomes. In this review we examine current data pertaining to size-matching in thoracic transplantation. We advocate for a change in the thoracic allocation mechanism from a height-or-weight-based strategy to a size-matching process that utilizes refined estimates of organ size. We believe that a size-matching approach based on refined estimates of organ size would optimize outcomes in thoracic transplantation without restricting or precluding patients from thoracic transplantation.
Keywords: Lung transplant, Heart transplant, Organ size, Size mismatch, Organ allocation
Core tip: Recipients for lung transplant and heart transplant are listed with acceptable donor height and weight ranges as surrogates for organ size, respectively. While these measures are important predictors of organ size, they are crude surrogates that fail to incorporate the influence of sex on organ size. Lung size can be better estimated using the predicted total lung capacity (derived from height, sex and age). Similarly, heart size can be better estimated using the predicted heart mass (derived from sex, age, height, and weight). These refined organ sizing-measures perform better than current sizing practice for the prediction of outcomes after transplantation.
INTRODUCTION
Donor-to-recipient size matching is a critical issue in thoracic organ transplantation[1-7]. This topic garnered particular attention in June 2013, when a 10-year-old Pennsylvania girl with severe lung damage from cystic fibrosis needed a lung transplant (LTx)[8]. Sarah Murnaghan was not permitted equal access to adult donor lungs because of an age restriction[8]. Children younger than 12 years were not eligible to primarily receive adult lungs, mainly because of lung size mismatch concerns[8].
In the United States height is used as a surrogate for lung size, and potential recipients for LTx are listed with acceptable donor height ranges[1,9]. In heart transplantation body-weight is used as a surrogate for heart size, and recipients for HTx are listed for acceptable donor body-weight ranges[1]. Donors falling outside the specified ranges are excluded automatically in the computerized match run process. Increasingly, evidence indicates the presence of considerable preventable pre- and post-LTx morbidity and mortality attributable to donor-recipient organ size differences that are occult in the current system due to reliance upon height or weight alone as a surrogate for organ size[1-7,10,11]. In this review we advocate for a change in the thoracic allocation mechanism from a height-or-weight-based strategy to a size-matching process that utilizes refined estimates of organ size. We believe that a size-matching approach based on refined estimates of organ size would optimize outcomes in thoracic transplantation without restricting or precluding patients from thoracic transplantation.
LUNG TRANSPLANT OUTCOMES ASSOCIATED WITH SIZE-MATCHING
Primary graft dysfunction
The most prevalent complication observed immediately following LTx is primary graft dysfunction (PGD)[12]. PGD presents with diffuse pulmonary infiltrates and hypoxia within 72 h of transplantation. PGD clinically mirrors the acute respiratory distress syndrome (ARDS) and histologic examination also shows diffuse alveolar damage, as in ARDS[12]. Severe PGD is the primary risk factor for early mortality after LTx, and survivors of PGD are predisposed to the development of chronic rejection (bronchiolitis obliterans), which is the main barrier to long-term survival[13]. Donor-to-recipient lung size mismatch (assessed by the donor-to-recipient predicted total lung capacity (pTLC), as a refined estimate of organ size) modulates the risk for PGD[3,14]. In a study ancillary to the LTx outcome group (LTOG), we found that an undersized allograft was associated with a significantly increased risk of severe PGD after bilateral LTx, Figure 1[14].
Figure 1.
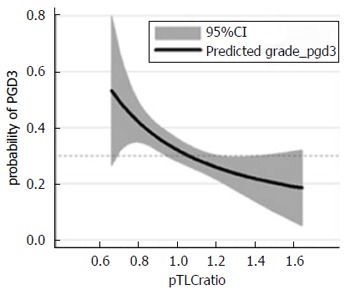
Lung size mismatch (the donor to recipient predicted total lung capacity ratio) is associated with the probability of primary graft dysfunction grade 3. The relationship of pTLCratio (pTLCdonor/pTLCrecipeint) and predicted probability of any grade PGD grade 3 within 72 h is shown using a fractional polynomial fit with 95%CIs (gray area). Adapted with permission from Eberlein et al[14]. pTLC: Predicted total lung capacity; PGD: Primary graft dysfunction.
The mechanisms responsible for this association are likely multiple, but we have hypothesized that the impact of lung size mismatch on mechanical ventilation tidal volumes in the early post-LTx period could be an important factor[14,15]. Conceptually, this is analogous to high-tidal volume ventilation when considered in terms of donor organ size[16,17]. During the period of post-LTx mechanical ventilation hyperinflation of undersized allografts (i.e., donor lungs smaller than recipient thorax) has been reported and has been linked to an increased risk of early allograft failure[18]. In another study of early outcomes undersized allografts similarly were associated with worse outcomes, specifically increased rates of PGD, tracheostomy, and resource utilization[3]. Hyperinflation of significantly undersized allografts by tidal volumes set according to recipient characteristics could increase the risk of ventilator induced lung injury (VILI)[16,17,19,20].
Several lines of evidence confirm differences in ventilator management when considered in terms of donor size. In a survey of the international LTx community, the majority of respondents reported using lung-protective mechanical ventilation after LTx, primarily consisting of low tidal volume (TV) ventilation[21]. Low TVs based on recipient characteristics were frequently chosen[21]. Donor characteristics usually were not taken into consideration and frequently were not even known by the team managing the ventilator after LTx[21]. The relationship between donor-recipient lung size mismatch and postoperative mechanical ventilation TVs was evaluated in a cohort of bilateral LTx patients, Figure 2[15]. TV-settings were expressed as absolute values (in milliliter) and also as fractions of recipient and donor predicted body weight (PBW). Absolute TVs were comparable between subsets of patients with undersized, matched, and oversized allografts. TV-settings according to recipient-PBW were also similar. However, TV-settings according to donor-PBW were significantly different between undersized, matched, and oversized groups (11.4 ± 3.1 mL/kg-DONOR-PBW vs 9.4 ± 1.2 mL/kg-DONOR-PBW vs 8.1 ± 2.1 mL/kg-DONOR-PBW, respectively; P < 0.05)[15]. Thus, during mechanical ventilation after bilateral LTx, patients with undersized allografts received significantly higher TVs compared to those with oversized allografts when TV was considered in terms of donor-PBW (as an estimate of the actual allograft size). This observation was replicated in an ancillary study to the multicenter LTOG study, Figure 3[14].
Figure 2.
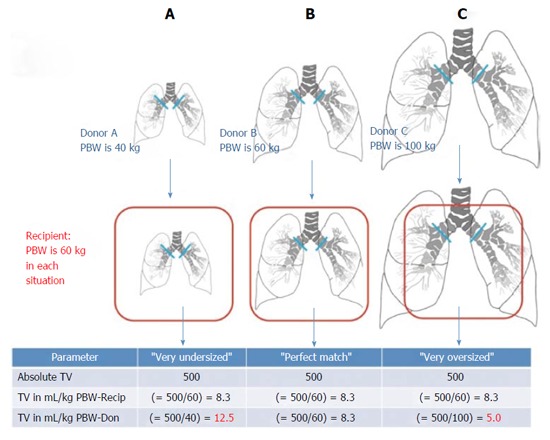
Conceptual graphic on the possible effect of lung size mismatch on mechanical ventilation tidal volumes expressed as mL/kg predicted body weights of the donor. Reproduced with permission from Dezube et al[15]. Recip recipient, Don donor. PBW: Predicted body weight; TV: Tidal volume.
Figure 3.
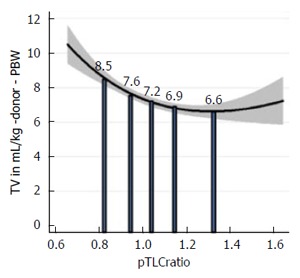
Lung size mismatch (predicted total lung capacity ratio) is associated with the mechanical ventilation tidal volumes at reperfusion, when the tidal volumes is related to the size of the allograft. Fractional polynomial regression of the TV in mL/kg donor-predicted body weight (PBW) plotted against the pTLCratio (pTLCdonor/pTLCrecipeint). The solid vertical bars represent the mean values of the TV in mL/kg donor-PBW according to pTLCratio-quintiles. Adapted with permission from Eberlein et al[14]. TV: Tidal volumes; pTLC: Predicted total lung capacity.
Thus, using a refined estimate of organ size (pTLC) identified an undersized lung allograft as a risk factor for severe PGD. These data suggest that a lung-protective mechanical ventilation strategy based on estimates of the allograft size (i.e., donor-PBW) could lower the risk of PGD, especially for recipients of undersized allografts.
Airway complications
Airway complications (ACs) frequently require multiple invasive interventions and are an important cause of post-LTx morbidity[22]. In a single center study we observed that undersized allografts were associated with a higher incidence and severity of ACs[3]. The association between lung size mismatch and ACs suggests that a mismatch in donor-recipient airway sizes could be a risk factor for ACs. Two other studies reported findings that support the hypothesis that donor-to-recipient airway size mismatch is a risk factor for ACs. The first reported that taller recipients generally experience more frequent ACs[23]. This was attributed to a larger recipient bronchial circumference and not to size mismatch, although neither height nor pTLC mismatch were directly evaluated in that study. The second, a large cohort study from the Cleveland Clinic transplant program, reported that in the setting of a donor-to-recipient size mismatch, obstructive ACs occurred more frequently[24]. Similar to lung size, sex determines airway structure independent of height[25,26]. Thus, while the pTLCratio would better capture donor-recipient lung size mismatch it may yet still underestimate the differences in airway size associated with a sex mismatch. Women tend to have smaller airway diameters than men, even when lung size is the same[25,26]. This effect would not be fully captured in the pTLCratio, which would also not capture the effect of dysanapsis (interindividual differences in airway size in relation to lung size). Computed tomography airway dimension analysis would allow an assessment of the actual airway size mismatch between recipient and donor, but may prove more cumbersome than matching by pTLC.
Bronchiolitis obliterans
Bronchiolitis obliterans (BO) is a disease that primarily affects small airways and is characterized by progressive obstruction and subsequent loss of small airways[27]. Bronchiolitis obliterans syndrome (BOS) is a standardized term for the clinical presentation in the absence of pathologic confirmation of BO[27]. BOS represents the main cause of long-term mortality after LTx[27].
Undersized allografts have been associated with an increased incidence of BOS, Figure 4[5]. The mechanisms for this association are not clearly elucidated, but it is known that multiple lung immune and non-immune mediated injuries to the small airways are risk factors for BOS. In injured small airways, repetitive opening and closing is associated with accelerated airway epithelial cell damage, inflammation, and ultimately fibrosis.
Figure 4.
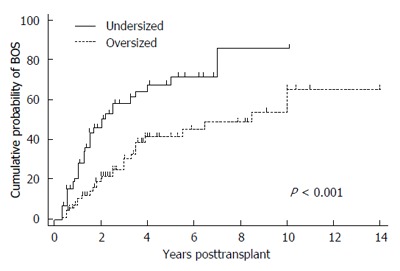
Kaplan Meier estimates of proportion of patients with bronchiolitis obliterans syndrome stratified by recipients of undersized or oversized donor lungs. Oversized was defined as a donor to recipient predicted total lung capacity (pTLC) ratio > 1.0 and undersized as pTLCratio ≤ 1.0. Comparison between over- and undersized cohorts was via log-rank test. Adapted with permission from Eberlein et al[5]. BOS: Bronchiolitis obliterans syndrome.
Chest wall strapping (CWS) is a procedure that involves restricting the thorax and abdomen, forcing the subject to breathe at low lung volumes[28]. It has been utilized to understand basic mechanisms of pulmonary physiology. CWS is conceptually similar to a mismatch between significantly oversized donor lungs transplanted into a recipient with a smaller chest cavity[28]. CWS increases lung elastic recoil, reduces pulmonary compliance, and substantially increases maximal expiratory flows[28]. The interactions between elastic properties of the lung parenchyma and small airways are critical for pulmonary function. CWS reduces the functional residual capacity (FRC) and leads to breathing closer to the residual volume (RV)[28]. This is similar to observations made in donor oversizing[11,28].
The FRC of a LTx recipient is determined by both the recipient’s chest wall mechanics and the properties of the donor lung[5,11]. A patient given an oversized allograft will likely have an FRC that is lower than the donor’s FRC because of the mechanics of the relatively smaller recipient thorax, analogous to the physiology of CWS[5,11,28]. In adults, absolute RV is determined by intrinsic characteristics of the lung (airway closure), rather than the chest wall. Thus the RV of an oversized allograft is likely large relative to the recipient’s thorax. As a consequence, a patient with an oversized allograft will likely breathe at relatively low lung volumes that are closer to the RV of the allograft [that is, the expiratory reserve volume (ERV) is reduced]. This concept was evidenced in a cohort of recipients of oversized lungs in whom the pulmonary function pattern resembled that of CWS[11]. In another group of bilateral LTx patients, an oversized allograft was, again similar to CWS, associated with higher expiratory airflows, higher FEV1/FVC-ratios, and higher flow-volume-loop slope estimates[5]. To evaluate the physiology of the transplanted lung it is helpful to consider post-LTx allograft function in relation to donor predicted function[5]. When flow-volume loops are analyzed in this way, oversized allografts resemble those of CWS, Figure 5[5,28]. There is very limited information on lung compliance and lung elastic recoil pressure after lung transplantation in relation to donor-recipient size matching. In 15 recipients of bilateral LTx whose donor lungs were, on average mildly oversized, elastic recoil of the transplanted lungs was mildly increased[29]. The likely increased elastic recoil of oversized lungs could have a beneficial effect on small airway function from the interdependence between increased elastic recoil and airways leading to greater radial distending forces on small airways and small airway dilation[28].
Figure 5.
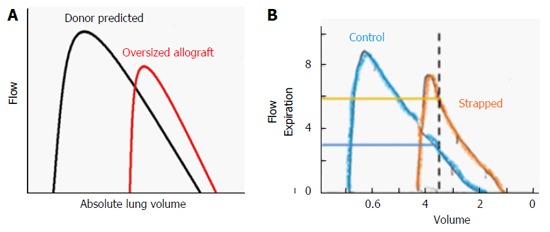
Oversized allograft (A) and chest wall strapping (B) analogy. A: Schematic flow volume loops according to donor predicted values (black line) and measured mean values of recipients of oversized allografts (red line) during the early post-transplant period (1-6 mo). Flows are plotted against absolute lung volume; B: Control (blue) and chest wall strapped (orange) flow volume loops are shown. Adapted with permission from Eberlein et al[5,28].
A possible mechanistic explanation for the described physiology of CWS relates to the surfactant system[5,28]. The associations between the surfactant system and risk factors for BOS are summarized in Table 1. The surfactant system shows adaptive responses to changes in lung compliance. In a model of decreased lung compliance, increases in surfactant protein and phospholipid content mediated a compensatory reduction in surface tension[30]. Furthermore, compared with normal inflation state in the donor chest an oversized allograft would operate at lower lung volumes in the recipient and thus alveolar size would on average be reduced. Surfactant fills in the regions adjacent to infolding of the alveoli as the lung deflates to maintain a spherical inner surface. Thus, a chronically underinflated lung could be expected to accumulate more surfactant.
Table 1.
The Surfactant system and its relation to risk factors for bronchiolitis obliterans syndrome
| BOS risk factor | Effect on surfactant system |
| Primary graft dysfunction | Successful treatment with surfactant |
| Acute rejection | Type II pneumocyte destruction and surfactant disruption |
| Rejection is associated with surfactant dysfunction | |
| Immunosuppression preserves Surfactant function | |
| GERD - aspiration | Inactivation of surfactant |
| Pulmonary infection | Inactivation of surfactant |
Adapted with permission from Eberlein et al[5]. GERD: Gastro-esophageal reflux disease; BOS: Bronchiolitis obliterans syndrome.
Survival
We have shown in a series of studies that the pTLC as a more refined estimate of organ sizing performs better than height alone, and is a strong predictor of various meaningful outcomes after LTx[3-7,10,11,31-33]. We have shown that the donor to recipient pTLCratio is an independent predictor of post-LTx survival, by addressing the following: (1) There is a non-linear association between the pTLCratio and post-LTx survival. With the pTLCratio entered as a spline there was a nonlinear association resulting in a declining risk of death with higher pTLCratio from 0.5 to about 1.3, where an inflection occurred with rising risk at higher values, Figure 6[6]; (2) There was no significant interaction with transplant indication[6]. Furthermore, within a single LTx indication [idiopathic pulmonary arterial hypertension (IPAH)], a condition that does not influence the size matching decision, the pTLCratio was a strong independent predictor of survival[4]; and (3) The analysis showed that, after accounting for the pTLCratio, recipient and donor sex matching was not independently associated with death after LTx[6,7,10]. Thus the pTLCratio explains a previously not well understood association between worse survival and a female allograft transplanted into a male recipient (For the same donor height female lungs are on average 20% smaller then male lungs). Furthermore, in a preliminary analysis we find that the effect of race on lung size also explains the previously reported association between donor African American race and higher mortality following LTx. These associations remained significant after adjustment for all known risk factors for post LTx mortality available in the datasets, including centers and center volumes[6].
Figure 6.
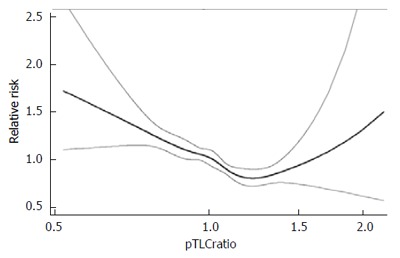
Impact of predicted total lung capacity ratio on the risk of death after lung transplant. Adapted with permission from Eberlein et al[6]. pTLC: Predicted total lung capacity.
Over the period from 1989 to 2010, the mean pTLCratio in US LTxs has decreased progressively from 1.14 to 1.04 (P < 0.0001)[34]. Within diagnoses there has been temporal decline in the pTLCratio by era especially in IPF, IPAH and “Other” indications, Figure 7[34].
Figure 7.
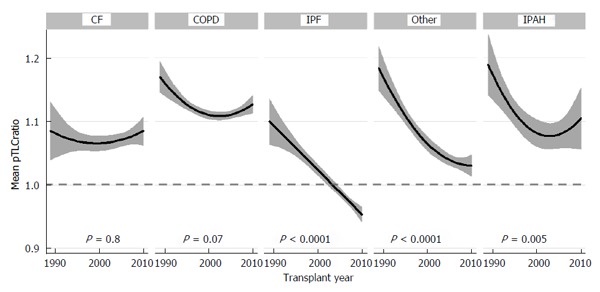
Mean predicted total lung capacity ratio according to transplant year stratified by lung transplant indication. Adapted with permission from Taher et al[34]. pTLC: Predicted total lung capacity; CF: Cystic fibrosis; COPD: Chronic obstructive pulmonary disease; IPF: Idiopathic pulmonary fibrosis; IPAH: Idiopathic pulmonary arterial hypertension.
Our data suggest that the secular trend to favor undersized donor lungs is ill advised. The advantage of using well matched or oversized donor lungs is supported by pathophysiological consideration that link undersized and well matched or oversized allografts to different allograft function and injury patterns.
HEART TRANSPLANT OUTCOMES ASSOCIATED WITH SIZE-MATCHING
In the setting of heart transplantation, a transplant recipient’s heart is often enlarged and dysfunctional such that the size of the explant is dissociated from the workload imposed by the vascular bed. As such, the goal of size matching is to provide a donor organ that is optimally sized to be capable of sustaining the workload needed to perfuse the recipient’s vascular bed - unrelated to the size of the organ removed. Currently, the only surrogate for size used in the allocation process is actual body weight[2,35-40]. The value of the current practice whereby offers are limited to donors within a certain weight range has been questioned in several large studies that have shown no association between outcomes and donor-recipient differences in body weight[2,37]. Heart size varies not only in relation to body weight, but also by other factors including sex in particular[2]. Studies of heart transplantation have consistently observed reduced survival associated with donor organ sex mismatch, particularly for male recipients of female organs[36,40]. The mechanism of this observation has long been unknown, but a recent study examining refined measures of heart size shed considerable light on the issue[2].
Studies utilizing cardiac MRI have provided prediction models of cardiac mass that incorporate height, weight, age, and gender. These prediction models provide estimates of heart size that differ significantly from estimates using body weight alone. For example, the predicted cardiac mass of a man and a woman both 55-year-old, 80 kg in weight, and 1.75 m tall yields a difference in predicted cardiac mass of 19%[2]. Applying these measures again, a man would have to weigh 20 kg (25%) less than an otherwise similar woman to yield an equivalent predicted heart size[2]. It is therefore likely that the current practice of matching donor organs to recipients based on body weight differences fails to discriminate substantial size mismatches[2].
To evaluate whether worsened outcomes in sex mismatching are related to mismatch of organ size in heart transplantation, we performed a retrospective cohort study of 31634 donor-recipient adult HTx pairings from the United Network for Organ Sharing transplant registry[2]. We used predictive models to calculate the predicted total heart mass (pHM) for recipient and donor pairs. By assessing organ size mismatch by calculating the percent difference between the donor and recipient pHM [= pHM recipient - pHM donor)/(pHM recipient)] × 100, we found that the most undersized pHM septile experienced higher mortality during the first year post transplant (HR 1.27, P < 0.001)[2]. This remained robust with very little change in the point estimate (suggesting absence of confounding) in adjusted models (HR 1.25, P = 0.03), Figure 8[2]. Supporting the assertion that weight differences provide no clinically useful information, survival did not vary across septiles of weight differences, Figure 8[2]. In univariate analysis, gender mismatch was associated with higher mortality in males. Controlling for differences in pHM eliminated this association (1 year HR, 1.00, P = 1). We concluded that differences in donor-recipient predicted heart sizes modulate the survival associated with donor-recipient gender mismatch and identifies donor heart undersizing as an otherwise occult and potentially preventable cause of excess mortality following orthotopic heart transplantation[2,39].
Figure 8.
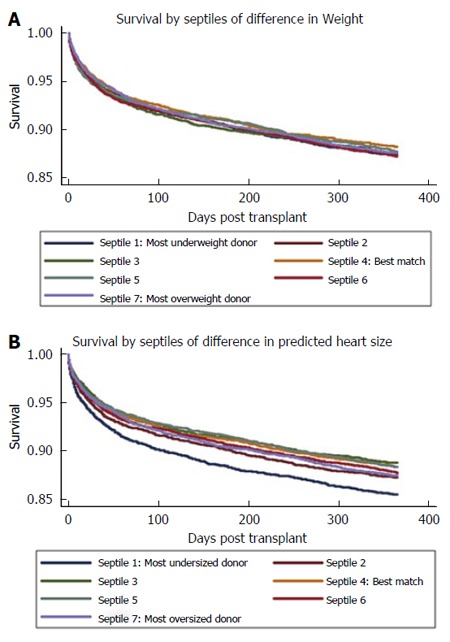
Unadjusted Kaplan-Meier graphs of survival, by septiles of matching by body weight (A) vs predicted total heart mass (B). Adapted with permission from Reed et al[2].
WAIT-LIST CONSIDERATIONS
We have made the argument for both lung and HTx, that the current method for listing size preferences sub-optimally predicts outcomes after thoracic transplantation[1]. In addition to those issues already described, the practice of limiting donor-recipient matches based on current size surrogates conceptually conveys further added morbidity and mortality based on both suboptimal matches as well as missed allocation opportunities. As mentioned previously, potential recipients for LTx are listed with acceptable donor height ranges, and recipients for HTx are listed with acceptable donor weight ranges. While these measures crudely correlate with organ size, they function particularly poorly in the setting of sex mismatch in particular. This is because a man’s thoracic organs are approximately 20% larger than a woman’s, Figure 9[7].
Figure 9.
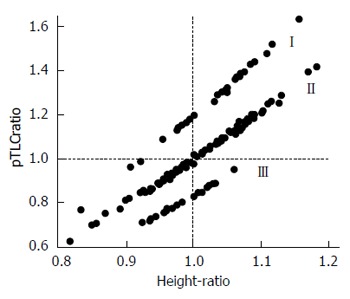
Relationship between predicted total lung capacity ratio and height ratio. The separation between clusters I (male donor-female recipient), II (sex matched) and III (female to male) is due to effects of sex on lung size. Adapted with permission from Eberlein et al[7]. pTLC: Predicted total lung capacity.
In order to exemplify the concepts of occult suboptimal organ allocation that occur in the current system, we will present a lung recipient and three theoretical potential donors. The concept would apply similarly in the setting of HTx.
For this example, the listed transplant candidate is a 55 year old man with end stage lung disease from idiopathic pulmonary fibrosis (IPF) who is listed for LTx. Candidates for LTx with IPF are often listed for height ranges below or up to their own height, as there has traditionally been a preference towards under-sizing[34]. For this example we consider a candidate with IPF who is 170 cm tall (and has a pTLC of 6.54 L) and is listed for an acceptable donor height range from 147-170 cm, Table 2[34].
Table 2.
Hypothetical donor offers for a subject with idiopathic pulmonary fibrosis listed for lung transplantation
| Listed subject with IPF | Donor listing | Offer A | Offer B | Offer C | |
| Age (yr) | 55 | 12-60 | 42 | 45 | 42 |
| Gender | Male | Either | Female | Male | Female |
| Height (cm) | 170 | 147-170 | 147 | 170 | 175 |
| pTLC (L) | 6.54 | 3.98-6.54 | 3.98 | 6.54 | 5.76 |
| pTLCratio | 0.61 | 1.00 | 0.88 |
Adapted from Taher et al[34]. IPF: Idiopathic pulmonary fibrosis; pTLC: Predicted total lung capacity.
Offer B: Appropriately identified size match
If we consider a 45-year-old male donor, who is 170 cm (and has a pTLC of 6.54 L), this would represent an appropriately identified size match and would be appropriately included in the match run for allocation to our hypothetical recipient (Table 2).
Offer C: Missed opportunity
If we then consider a 42-year-old female donor, who is 175 cm tall, this would fall outside the upper limits of the height listing range and be identified in the current system as oversized. As such, this donor would be automatically eliminated and would not appear in the match run for our hypothetical recipient. This example represents an incorrect assessment of size as the pTLC of the donor is actually 5.76 L - which is a smaller pTLC than the 170 cm male donor, Table 2[34]. Furthermore, this match would represent a pTLCratio of 0.88, which although undersized, would likely represent an acceptable match (Table 2).
Offer A: Inappropriately undersized
If we finally consider an offer from a donor who is a 147 cm tall female, we can see that in the current system this would fall within acceptable parameters and would enter into the match run and potentially be allocated to our hypothetical recipient. While the height difference falls within the lower limit of the acceptable height range listed, the pTLCratio of 0.61 reveals the organ to be markedly undersized with outcomes predictably suboptimal. This would represent a failure of the current system to identify and possibly avoid an inappropriately undersized match (Table 2).
Not only is this hypothetical candidate not receiving by lung size (pTLC) well matched donor lung offers; but in addition we have shown in a series of studies, that it is not necessary to avoid oversizing. On the contrary, we have shown that a higher donor to recipient pTLCratio, suggestive of an oversized allograft, is associated with improved survival after LTx, irrespective of indication. Thus oversizing, up to a point, should not be avoided and is an important additional means of increasing the chance of receiving an appropriately sized donor offer[6].
However it has been shown that short stature is associated with increasing wait list times and increased risk of death on the wait list. Since the implementation of the Lung Allocation Scoring (LAS) system, characteristics of candidates on the wait list have changed to include a sicker group of patients with a greater proportion of LAS diagnoses group D (restrictive lung diseases)[9]. As a consequence, wait-list mortality rates are again rising despite higher wait-list transplant rates compared to the pre-LAS era. Potential LTx-recipients with short stature and small thoracic cavities have longer waiting times on the LTx list, as donor lungs considered to be size-appropriate are particularly limited[41]. This often affects patients with cystic fibrosis and pulmonary fibrosis. In both groups, LTx can become an urgent issue when significant disease exacerbations occur, and in this setting in particular patients are at high risk for wait list mortality. Higher acuity at the time of LTx is in turn associated with decreased survival. It would thus seem logical to consider a change to thoracic organ allocation to incorporate better estimates of organ size. Rather than relying on a donor height range for lung allocation, it would be logical to express sizing preferences in terms of an acceptable donor pTLC range.
CONCLUSION
Donor-to-recipient organ size matching is a critical aspect in thoracic transplantation. We advocate for a change in the thoracic allocation mechanism from a height-or-weight-based strategy to a size-matching process that utilizes refined estimates of organ size. Studies examining the impact of refined estimates of organ size suggest that there is considerable preventable pre- and post-LTx morbidity and mortality attributable to organ size differences that are occult in the current system due to reliance upon height (in LTx) and weight (in HTx) alone as a surrogate for organ size. The current allocation system also misclassifies a proportion of well-matched organs as inappropriately sized, and thus fails to optimally match available organs to the highest-priority appropriate recipients. Further studies simulating the impact of this proposed organ allocation change will hopefully provide the foundation for a change in the United States (UNOS/OPTN), and consequently improve donor lung utilization with resulting reductions in post-LTx complications and graft failure rates.
Footnotes
Supported by Flight Attendants Medical Research Institute in part (to Robert M Reed); Michael Eberlein is supported by a PILOT grant from the Institute for Clinical and Translational Science (ICTS) at the University of Iowa via the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, grant 2 UL1 TR000442-06.
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 19, 2015
First decision: September 22, 2015
Article in press: December 8, 2015
P- Reviewer: Janicki PK, Mahapatra S S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
References
- 1.Reed RM, Eberlein M. Sizing considerations in lobar lung transplantation. Transpl Int. 2014;27:e132–e133. doi: 10.1111/tri.12391. [DOI] [PubMed] [Google Scholar]
- 2.Reed RM, Netzer G, Hunsicker L, Mitchell BD, Rajagopal K, Scharf S, Eberlein M. Cardiac size and sex-matching in heart transplantation: size matters in matters of sex and the heart. JACC Heart Fail. 2014;2:73–83. doi: 10.1016/j.jchf.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eberlein M, Arnaoutakis GJ, Yarmus L, Feller-Kopman D, Dezube R, Chahla MF, Bolukbas S, Reed RM, Klesney-Tait J, Parekh KR, et al. The effect of lung size mismatch on complications and resource utilization after bilateral lung transplantation. J Heart Lung Transplant. 2012;31:492–500. doi: 10.1016/j.healun.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Eberlein M, Diehl E, Bolukbas S, Merlo CA, Reed RM. An oversized allograft is associated with improved survival after lung transplantation for idiopathic pulmonary arterial hypertension. J Heart Lung Transplant. 2013;32:1172–1178. doi: 10.1016/j.healun.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Eberlein M, Permutt S, Chahla MF, Bolukbas S, Nathan SD, Shlobin OA, Shelhamer JH, Reed RM, Pearse DB, Orens JB, et al. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest. 2012;141:451–460. doi: 10.1378/chest.11-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberlein M, Reed RM, Maidaa M, Bolukbas S, Arnaoutakis GJ, Orens JB, Brower RG, Merlo CA, Hunsicker LG. Donor-recipient size matching and survival after lung transplantation. A cohort study. Ann Am Thorac Soc. 2013;10:418–425. doi: 10.1513/AnnalsATS.201301-008OC. [DOI] [PubMed] [Google Scholar]
- 7.Eberlein M, Reed RM, Permutt S, Chahla MF, Bolukbas S, Nathan SD, Iacono A, Pearse DB, Fessler HE, Shah AS, et al. Parameters of donor-recipient size mismatch and survival after bilateral lung transplantation. J Heart Lung Transplant. 2012;31:1207–1213.e7. doi: 10.1016/j.healun.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Sweet SC, Barr ML. Pediatric lung allocation: the rest of the story. Am J Transplant. 2014;14:11–12. doi: 10.1111/ajt.12546. [DOI] [PubMed] [Google Scholar]
- 9.Eberlein M, Garrity ER, Orens JB. Lung allocation in the United States. Clin Chest Med. 2011;32:213–222. doi: 10.1016/j.ccm.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Eberlein M, Reed RM, Bolukbas S, Parekh KR, Arnaoutakis GJ, Orens JB, Brower RG, Shah AS, Hunsicker L, Merlo CA. Lung size mismatch and survival after single and bilateral lung transplantation. Ann Thorac Surg. 2013;96:457–463. doi: 10.1016/j.athoracsur.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 11.Eberlein M, Permutt S, Brown RH, Brooker A, Chahla MF, Bolukbas S, Nathan SD, Pearse DB, Orens JB, Brower RG. Supranormal expiratory airflow after bilateral lung transplantation is associated with improved survival. Am J Respir Crit Care Med. 2011;183:79–87. doi: 10.1164/rccm.201004-0593OC. [DOI] [PubMed] [Google Scholar]
- 12.Lee JC, Christie JD. Primary graft dysfunction. Clin Chest Med. 2011;32:279–293. doi: 10.1016/j.ccm.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, Patterson GA, Trulock EP, Hachem RR. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 14.Eberlein M, Reed RM, Bolukbas S, Diamond JM, Wille KM, Orens JB, Brower RG, Christie JD. Lung size mismatch and primary graft dysfunction after bilateral lung transplantation. J Heart Lung Transplant. 2015;34:233–240. doi: 10.1016/j.healun.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dezube R, Arnaoutakis GJ, Reed RM, Bolukbas S, Shah AS, Orens JB, Brower RG, Eberlein M. The effect of lung-size mismatch on mechanical ventilation tidal volumes after bilateral lung transplantation. Interact Cardiovasc Thorac Surg. 2013;16:275–281. doi: 10.1093/icvts/ivs493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 17.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2014;370:980. doi: 10.1056/NEJMc1400293. [DOI] [PubMed] [Google Scholar]
- 18.Kozower BD, Meyers BF, Ciccone AM, Guthrie TJ, Patterson GA. Potential for detrimental hyperinflation after lung transplantation with application of negative pleural pressure to undersized lung grafts. J Thorac Cardiovasc Surg. 2003;125:430–432. doi: 10.1067/mtc.2003.139. [DOI] [PubMed] [Google Scholar]
- 19.Serpa Neto A, Simonis FD, Barbas CS, Biehl M, Determann RM, Elmer J, Friedman G, Gajic O, Goldstein JN, Horn J, et al. Association between tidal volume size, duration of ventilation, and sedation needs in patients without acute respiratory distress syndrome: an individual patient data meta-analysis. Intensive Care Med. 2014;40:950–957. doi: 10.1007/s00134-014-3318-4. [DOI] [PubMed] [Google Scholar]
- 20.Gajic O, Frutos-Vivar F, Esteban A, Hubmayr RD, Anzueto A. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med. 2005;31:922–926. doi: 10.1007/s00134-005-2625-1. [DOI] [PubMed] [Google Scholar]
- 21.Beer A, Reed RM, Bölükbas S, Budev M, Chaux G, Zamora MR, Snell G, Orens JB, Klesney-Tait JA, Schmidt GA, et al. Mechanical ventilation after lung transplantation. An international survey of practices and preferences. Ann Am Thorac Soc. 2014;11:546–553. doi: 10.1513/AnnalsATS.201312-419OC. [DOI] [PubMed] [Google Scholar]
- 22.Machuzak M, Santacruz JF, Gildea T, Murthy SC. Airway complications after lung transplantation. Thorac Surg Clin. 2015;25:55–75. doi: 10.1016/j.thorsurg.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Van De Wauwer C, Van Raemdonck D, Verleden GM, Dupont L, De Leyn P, Coosemans W, Nafteux P, Lerut T. Risk factors for airway complications within the first year after lung transplantation. Eur J Cardiothorac Surg. 2007;31:703–710. doi: 10.1016/j.ejcts.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Murthy SC, Blackstone EH, Gildea TR, Gonzalez-Stawinski GV, Feng J, Budev M, Mason DP, Pettersson GB, Mehta AC. Impact of anastomotic airway complications after lung transplantation. Ann Thorac Surg. 2007;84:401–409, 409.e1-4. doi: 10.1016/j.athoracsur.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Sheel AW, Guenette JA, Yuan R, Holy L, Mayo JR, McWilliams AM, Lam S, Coxson HO. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J Appl Physiol (1985) 2009;107:1622–1628. doi: 10.1152/japplphysiol.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis. 1980;121:339–342. doi: 10.1164/arrd.1980.121.2.339. [DOI] [PubMed] [Google Scholar]
- 27.Meyer KC, Raghu G, Verleden GM, Corris PA, Aurora P, Wilson KC, Brozek J, Glanville AR. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014;44:1479–1503. doi: 10.1183/09031936.00107514. [DOI] [PubMed] [Google Scholar]
- 28.Eberlein M, Schmidt GA, Brower RG. Chest wall strapping. An old physiology experiment with new relevance to small airways diseases. Ann Am Thorac Soc. 2014;11:1258–1266. doi: 10.1513/AnnalsATS.201312-465OI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glanville AR, Theodore J, Harvey J, Robin ED. Elastic behavior of the transplanted lung. Exponential analysis of static pressure-volume relationships. Am Rev Respir Dis. 1988;137:308–312. doi: 10.1164/ajrccm/137.2.308. [DOI] [PubMed] [Google Scholar]
- 30.Dixon DL, De Pasquale CG, De Smet HR, Klebe S, Orgeig S, Bersten AD. Reduced surface tension normalizes static lung mechanics in a rodent chronic heart failure model. Am J Respir Crit Care Med. 2009;180:181–187. doi: 10.1164/rccm.200809-1506OC. [DOI] [PubMed] [Google Scholar]
- 31.Eberlein M, Bolukbas S, Reed RM. eComment. Gender mismatching in lung transplantation: lung size mismatch is the issue! Interact Cardiovasc Thorac Surg. 2013;16:435–436. doi: 10.1093/icvts/ivt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eberlein M, Bolukbas S, Reed RM. Bilateral lobar lung transplantation and size mismatch by pTLC-ratio. Eur J Cardiothorac Surg. 2013;44:394–395. doi: 10.1093/ejcts/ezt004. [DOI] [PubMed] [Google Scholar]
- 33.Eberlein M, Reed RM. Letter by Eberlein and Reed regarding article, “transplantation for idiopathic pulmonary arterial hypertension: improvement in the lung allocation score era”. Circulation. 2014;129:e457. doi: 10.1161/CIRCULATIONAHA.113.004888. [DOI] [PubMed] [Google Scholar]
- 34.Taher H, Robert RM, Eberlein M. Characterization of Donor to Recipient Size Matching in Lung Transplantation. Austin J Pulm Respir Med. 2014;1:1014. [Google Scholar]
- 35.Jayarajan SN, Taghavi S, Komaroff E, Mangi AA. Impact of low donor to recipient weight ratios on cardiac transplantation. J Thorac Cardiovasc Surg. 2013;146:1538–1543. doi: 10.1016/j.jtcvs.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Kittleson MM, Shemin R, Patel JK, Ardehali A, Kawano M, Davis S, Moriguchi JD, Kobashigawa JA. Donor-recipient sex mismatch portends poor 10-year outcomes in a single-center experience. J Heart Lung Transplant. 2011;30:1018–1022. doi: 10.1016/j.healun.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Patel ND, Weiss ES, Nwakanma LU, Russell SD, Baumgartner WA, Shah AS, Conte JV. Impact of donor-to-recipient weight ratio on survival after heart transplantation: analysis of the United Network for Organ Sharing Database. Circulation. 2008;118:S83–S88. doi: 10.1161/CIRCULATIONAHA.107.756866. [DOI] [PubMed] [Google Scholar]
- 38.Reed RM, Eberlein M. Donor/recipient sex mismatch and survival after heart transplantation: only an issue in female recipients? Transpl Int. 2015;28:622. doi: 10.1111/tri.12502. [DOI] [PubMed] [Google Scholar]
- 39.Reed RM, Eberlein M. Heart donation and the Grinch effect. J Heart Lung Transplant. 2015;34:137. doi: 10.1016/j.healun.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Weiss ES, Allen JG, Patel ND, Russell SD, Baumgartner WA, Shah AS, Conte JV. The impact of donor-recipient sex matching on survival after orthotopic heart transplantation: analysis of 18 000 transplants in the modern era. Circ Heart Fail. 2009;2:401–408. doi: 10.1161/CIRCHEARTFAILURE.108.844183. [DOI] [PubMed] [Google Scholar]
- 41.Keeshan BC, Rossano JW, Beck N, Hammond R, Kreindler J, Spray TL, Fuller S, Goldfarb S. Lung transplant waitlist mortality: height as a predictor of poor outcomes. Pediatr Transplant. 2015;19:294–300. doi: 10.1111/petr.12390. [DOI] [PubMed] [Google Scholar]


