Abstract
Objective
Patients with type II diabetes have an increased risk of bladder cancer and are commonly treated with thiazolidinediones and angiotensin receptor blockers (ARBs), which have been linked to cancer risk. We explored the relationship between use of one or both of these medication types and incident bladder cancer among diabetic patients (diabetics) enrolled in Medicare.
Research Design and Methods
We constructed both a prevalent and incident retrospective cohort of pharmacologically treated prevalent diabetics enrolled in a Medicare fee-for-service plan using inpatient, outpatient (2003–2011) and prescription (2006–2011) administrative data. The association of incident bladder cancer with exposure to pioglitazone, rosiglitazone and ARBs was studied using muitivariable Cox’s hazard models with time-dependent covariates in each of the two cohorts.
Results
We identified 1,161,443 prevalent and 320,090 incident pharmacologically treated diabetics, among whom 4433 and 1159, respectively, developed incident bladder cancers. In the prevalent cohort mean age was 75.1 years, mean follow-up time was 38.0 months, 20.2% filled a prescription for pioglitazone during follow-up, 10.4% received rosiglitazone, 31.6% received an ARB and 8.0% received combined therapy with pioglitazone + ARB. We found a positive association between bladder cancer and duration of pioglitazone use in the prevalent cohort (P for trend = 0.008), with ≥24 months of pioglitazone exposure corresponding to a 16% (95% confidence interval 0–35%) increase in the incidence of bladder cancer compared to no use. There was a positive association between bladder cancer and rosiglitazone use for <24 months in the prevalent cohort, but no association with ARB use. There were no significant associations in the incident cohort.
Conclusions
We found that the incidence of bladder cancer increased with duration of pioglitazone use in a prevalent cohort of diabetics aged 65+ years residing in the USA, but not an incident cohort.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-016-0152-4) contains supplementary material, which is available to authorized users.
Keywords: Angiotensin receptor blocker, Pioglitazone, Rosiglitazone, Retrospective cohort
Introduction
Type 2 diabetes mellitus has been associated with an increased risk of diverse cancers [1, 2]. Bladder cancer, the sixth most common cancer in U.S. adults, occurs 10–40% more often in adults with diabetes than in those without diabetes [3]. The etiology of higher cancer incidence among diabetic patients (diabetics) is not fully understood [1]. Common risk factors, such as obesity [4–6] or lack of physical activity [7], may explain part of the increased risk. Hyperglycemia [8, 9] and hyperinsulinemia [6, 10, 11] have been suggested as contributing to the risk of cancer, while the degree of glucose control does not appear to alter the cancer risk [12]. The association between cancer and medications commonly used by diabetics has also been suggested to be associated with an increased cancer risk, but this association remains uncertain. Consequently, medication-associated risk warrants continued exploration given the excess cancer experienced by this population and their relatively intense use of pharmaceuticals.
Researchers assessing the relationship between pharmacotherapies for the treatment of diabetes and cancer risk have reported mixed results. The most common hypoglycemic agent used in the USA, metformin, may reduce the risk of certain cancers [13–16], while sulfonylureas do not appear to modify cancer risk [17]. The effect of exogenous insulin on cancer risk is unclear [18–20]. Angiotensin receptor blockers (ARBs), which are recommended by American Diabetes Association guidelines as either a first choice or alternative to angiotensin-converting enzyme inhibitors (ACE-Is) for hypertension, have been associated with a modest increase in cancer risk [21]. Evidence linking the oral hypoglycemic agent, pioglitazone, to an increased risk of bladder cancer is mixed [22–27]. In 2013 the International Agency for Research on Cancer deemed pioglitazone a possible carcinogen [28]. Less is known about the other thiazolidinedione, rosiglitazone, also in use in the USA [22, 26]. Very little is known about the association of cancer with combination pharmacotherapy among diabetics; however, polypharmacy is common among diabetics, raising concern for additive or synergistic cancer-promoting or cancer-causing effects of such medications.
To advance our understanding of the association between bladder cancer and pharmacotherapies for diabetics, particularly thiazolidinediones (TZD), and the combination of TZD with ARB therapy, we have undertaken a retrospective cohort study using Medicare administrative data. An improved understanding of the relationship between pharmacotherapies for diabetes and bladder cancer could advance clinical care, informing treatment selection and shared decision-making.
Research Design and Methods
Prevalent and Incident Cohorts
We used a 40% random sample of the Medicare Beneficiary Summary (denominator) file and corresponding Medicare administrative data from Parts A (inpatient insurance) and B (outpatient insurance) for the period 2004–2012 and from Part D (prescription insurance) for the period 2006–2012 to identify patients receiving diabetes medications [see Electronic Supplementary Material (ESM) Table 1 for medication codes]. Patients were included in the prevalent cohort if (1) they had filled at least one diabetes-specific prescription between 2006 and 2012, and (2) the prescription receipt was preceded by at least 24 months of continuous enrollment in Parts A and B of the Medicare fee-for-service plan (to permit ascertainment of pre-observation co-morbidities and diabetes complications). Patients were included in the incident cohort if their first fill for a diabetes medication between 2006 and 2012 was preceded by 120 days of enrollment in Part D of the Medicare fee-for-service plan. Patients with a prior cancer diagnoses were excluded (defined as at least one inpatient or two outpatient diagnoses of cancer other than non-melanoma skin) during the 24-month look-back. Patients aged <65, those in a hospice and those originally entitled to Medicare enrollment due to end-stage renal disease or disability were also excluded. See ESM Table 2 for the list of diagnostic codes used for exclusion.
Outcomes
The primary outcome of interest was incident bladder cancer. This was defined as the first inpatient or first of two outpatient diagnoses of bladder cancer (see ESM Table 2 for ICD-9 codes) and a claim for a cytoscopy service within 4 months before or after the first diagnosis date.
Follow-Up and Censoring
Subjects were considered in follow-up from the time they met the definition for entry into the cohort until an occurrence of an initial incident bladder cancer or until right censoring by (1) non-incident non-bladder cancer, (2) end of fee-for-service enrollment in Parts A, B or D of Medicare or (3) death.
Exposures of Interest
The exposures of primary interest were pioglitazone, rosiglitazone and ARBs. In addition, we assessed exposure to metformin, sulfonylureas, insulin glargine, other insulin analogs, human insulin, and ACE-Is (see ESM Table 1 for list) because (1) these medications are common substitutes for the exposures of interest and (2) their use identifies diabetics with similar severity of illness and creates a proxy control for diabetes severity. For each patient we used Part D prescription fill records to identify and quantify drug exposure in a counting process-style format. The exposures are time-dependent covariates defined as ever use or duration of observed use prior to that time.
Covariates
The following covariates were included in the models: age, gender, race (categorized as Black, Hispanic, and other based on the Medicare denominator file variable), and low-income subsidy for Medicare Part D (a marker for income 150% or less of the federally defined poverty level) [29]. The following comorbidities, diagnosed once or more at any time during the 24-month look-back were also included: alcohol abuse, chronic obstructive lung disease and/or tobacco use (combined as “tobacco exposure” proxy variable), obesity, diabetes complications (diabetic retinopathy, nephropathy, neuropathy, vasculopathy) and Charlson comorbidities (see ESM Table 3) not otherwise excluded or included [30].
Statistical Analysis
We evaluated the association of cumulative duration of pharmacotherapy exposures in the prevalent and incident cohorts of treated diabetics with incidence of bladder cancer using cause-specific hazard ratios (HR) estimated using Cox’s proportional hazards models for time-dependent covariates, adjusting for the covariates listed in the “Covariates” section as well as calendar year. This approach assumes that competing risks, such as death, are independent of the occurrence of an incident bladder cancer. The cause-specific HR does not incorporate reduction in the populations at risk due to competing risks as does the sub-distribution HR [31]. We report univariable models (one exposure at a time, controlling for covariates) and multivariable models (association of each exposure controlling for other exposures and covariates). We conducted analyses using cumulative duration of use as a continuous variable and separately cumulative duration categorized using the cut-off points of 1, 12 and 24 months. The time-scale in these models was time since meeting the criteria for cohort entry. We tested for proportionality of hazards using the Schoenfeld’s residual test.
We tested the interaction of ARBs with pioglitazone, with rosiglitazone and with other hypoglycemics, as the product of the cumulative durations as well as a binary indicator of ever having used both (not necessarily concurrently).
The Committee for the Protection of Human Subjects at Dartmouth College approved this study. We used data provided by Medicare and entered a data use agreement with CMS (Center for Medicare and Medicaid Services). Data were stripped of personal health identifiers and are stored behind a secure firewall.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
The prevalent and incident cohorts consisted of 1,161,443 and 320,090 enrollees in the Medicare fee-for service plan filling at least one anti-diabetic prescription, as described in Table 1. The prevalent cohort was 62.5% female, 10.7% black and 9.8% Hispanic. Mean age at the beginning of follow-up was 75.9 years in females and 73.6 years in males; 18.9% had “tobacco exposure” and 4.5% had more than one diabetes complication. The median Charlson score in both cohorts was 1 [interquartile range (IQR) 0–2]. The median follow-up was 38.0 (IQR 19.0–67.0) months.
Table 1.
Cohort characteristics of pharmacotherapeutically treated diabetic patients enrolled in Medicare between 2006 and 2012 overall and by sex
| Cohort characteristics | Prevalent cohort (N = 1,161,443)a | Incident cohort (N = 320,090)a |
|---|---|---|
| Follow-up months | 42.2 (26.4) | 37.3 (23.3) |
| Female | 62.5% | 62.4% |
| Age, years | 75.1 (7.5) | 76.7 (7.2) |
| Race | ||
| Black | 10.7% | 9.4% |
| Hispanic | 9.8% | 9.3% |
| White and other | 79.4% | 81.3% |
| Low-income subsidyb | 42.1% | 41.7% |
| Obese | 7.7% | 7.2% |
| COPD/tobacco usec | 18.9% | 22.7% |
| Charlson comorbiditiesd | 0.9 (1.2) | 1.0 (1.3) |
| Number of diabetes complicationse | ||
| 0–1 | 95.5% | 97.5% |
| 2 | 3.6% | 2.0% |
| ≥3 | 0.9% | 0.5% |
Data in table are presented as the mean with the standard deviation (SD) in parenthesis, or as the percentage
COPD Chronic obstructive pulmonary disease
aPatients were included in the prevalent cohort if (1) they had filled at least one diabetes-specific prescription between 2006 and 2012, and (2) the prescription receipt was preceded by at least 24 months of continuous enrollment in Parts A and B of the Medicare fee-for-service plan. Patients were included in the incident cohort if their first fill for a diabetes medication between 2006 and 2012 was preceded by 120 days of enrollment in Part D of the Medicare fee-for-service plan
bA dichotomous indicator of poverty equals <150% of the federal poverty level
cChronic obstructive pulmonary disease
dCharlson Comorbidities include: human immunodeficiency virus, congestive heart failure, cerebrovascular disease, diabetes, liver diseases, myocardial infarction, peptic ulcer disease, hemiplegia/paralysis, peripheral vascular disease, renal disease, rheumatoid arthritis
eDiabetes complications are: renal, ophthalmologic, neurologic, circulatory, unspecified
We identified 4433 cases of bladder cancer, which is an incidence of 2.2/1000 person years (PY) in men and 0.5/1000 PY in women. As shown in Table 2, 20.1% of the cohort used pioglitazone at least once (mean duration of use 21.1 months). Rosiglitazone was used by 10.4% of the cohort (mean duration of use 16.5 months), ARBs by 31.6% and an ACE-I by 58.9%. A total of 8.0% of the cohort had exposure to both pioglitazone and ARBs, while 4.1% of the cohort had exposure to both rosiglitazone and ARBs.
Table 2.
Frequency of incident bladder cancer and exposure to diabetes medications, overall and by sex
| Bladder cancer cases/diabetes medications | Prevalent cohort | Incident cohort |
|---|---|---|
| Number of bladder cancer cases | 4433 (0.4%) | 1159 (0.4%) |
| Incidence of bladder cancer (cases/1000 PY) | 1.08 | 1.16 |
| Pioglitazone use (N) | 20.1% (233,450) | 11.9% (38,091) |
| Mean exposure, months (SD) | 21.1 (31.8) | 16.6 (27.7) |
| Rosiglitazone use (N) | 10.4% (120,790) | 4.8% (15,364) |
| Mean exposure, months (SD) | 16.5 (17.6) | 11.2 (14.2) |
| ARB use (N) | 31.6% (367,016) | 28.6% (91,546) |
| Mean exposure, months (SD) | 24.5 (25.5) | 20.8 (22.0) |
| ACE-I use (N) | 58.9% (684,090) | 53.0% (169,648) |
| Mean exposure, months (SD) | 28.1 (37.6) | 22.2 (30.7) |
| Metformin use (N) | 61.1% (709,642) | 61.4% (196,535) |
| Mean exposure, months (SD) | 58.3 (59.7) | 37.5 (42.8) |
| Sulfonylurea use (N) | 51.8% (601,627) | 39.6% (126,756) |
| Mean exposure, months (SD) | 29.1 (23.4) | 22.1 (20.1) |
Data are presented as the number (of enrollees) with the percentage in parenthesis, as the percentage with the number (of enrollees) in parenthesis or as the mean with the SD in parenthesis
PY Person-years, ARB angiotensin II receptor blocker, ACE-I angiotensin converting enzyme inhibitors
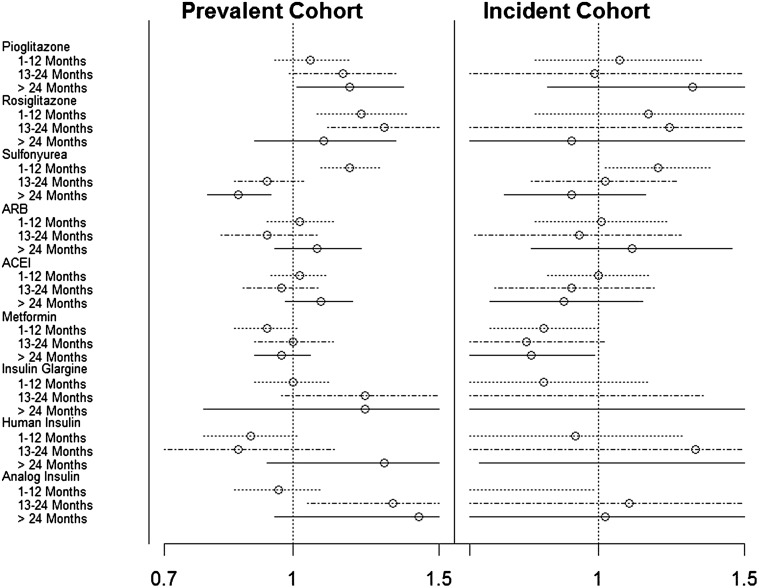
Figure 1 presents the HR relating duration of pharmacotherapy to a subsequent diagnosis of bladder cancer, adjusted for demographics and other patient characteristics at baseline, but not other pharmacotherapies. In the prevalent diabetes cohort, duration of pioglitazone use was associated with increased incidence of bladder cancer (P for trend, adjusted = 0.004), with a 16% (95% CI 0–35%) elevated risk of bladder cancer among those using pioglitazone for ≥24 months. In contrast, there was no association between an increased risk of bladder cancer and pioglitazone use in the incident diabetes cohort (P > 0.10). In the prevalent cohort—but not in the incident cohort—there was a non-monotonic association of rosiglitazone use with bladder cancer. ARB use was found to be unrelated to risk. In the secondary analyses, use of sulfonylureas for 1–11 months was associated with a 17% (95% CI 8–27%) higher risk of bladder cancer in the prevalent cohort and an 18% (95% CI 2–37%) higher risk in the incident cohort, but use for >12 months was not associated with increased risk. Metformin was associated with a significantly lower risk of bladder cancer in the incident cohort.
Fig. 1.
For each of the pharmacotherapies, hazard ratios for bladder cancer occurrence are shown with 95% confidence intervals for duration of use categorized as 1–12, 13–24 and >24 months versus no previous use. Each hazard ratio is adjusted for age, gender, year, race/ethnicity, low income, number of diabetes complications and chronic obstructive pulmonary disease/tobacco use. ARB Angiotensin receptor blocker, ACE-I angiotensin-converting enzyme inhibitors
Table 3 displays the results of multivariable models that include diabetic pharmacotherapies as well as demographic and comorbidity characteristics, for both the prevalent diabetes and incident diabetes cohorts. The monotonic association of pioglitazone duration with bladder cancer persisted (P for trend = 0.008) in the prevalent cohort but was not found in the incident cohort. The incidence of bladder cancer in those who received pioglitazone for ≥24 months was 16% higher than in those who received none. Diabetics in the prevalent cohort who used rosiglitazone for 1–12 and 13–24 months were at a 19% (6–35%) and 28% (9–51%) increased risk of bladder cancer, respectively, compared to those with no use, while those with ≥24 months of pioglitazone use had a 10% (−9 to 34%) higher risk. In contrast, there were no significant findings in the incident diabetes cohort. In the prevalent cohort, duration of sulfonylurea duration had an inconsistent association with bladder cancer risk; similar but non-significant associations were ascertained in the incident cohort. Any metformin use was associated with a 17 (5–27%) lower risk in the incident cohort but not a significantly lower risk in the prevalent cohort.
Table 3.
Hazard ratios for incident bladder cancer versus medication duration of use from the multivariable model: the multivariable model includes all variables in this table
| Model variables | Prevalent cohort | Incident cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% Confidence interval | P value | Hazard ratio | 95 Confidence interval | P value | |||
| Pioglitazone | ||||||||
| 1–12 months | 1.03 | 0.93 | 1.14 | 0.521 | 1.02 | 0.81 | 1.28 | 0.8845 |
| 13–24 months | 1.14 | 0.98 | 1.31 | 0.091 | 0.95 | 0.62 | 1.44 | 0.8054 |
| >24 months | 1.16 | 1.00 | 1.35 | 0.044 | 1.24 | 0.83 | 1.84 | 0.2969 |
| Rosiglitazone | ||||||||
| 1–12 months | 1.19 | 1.06 | 1.35 | 0.004 | 1.13 | 0.83 | 1.53 | 0.4490 |
| 13–24 months | 1.28 | 1.09 | 1.51 | 0.002 | 1.21 | 0.70 | 2.09 | 0.5011 |
| >24 months | 1.10 | 0.91 | 1.34 | 0.314 | 0.93 | 0.41 | 2.08 | 0.8535 |
| Sulfonylurea | ||||||||
| 1–12 months | 1.15 | 1.07 | 1.25 | 0.000 | 1.14 | 0.98 | 1.32 | 0.0962 |
| 13–24 months | 0.92 | 0.83 | 1.01 | 0.081 | 0.97 | 0.79 | 1.20 | 0.7920 |
| >24 months | 0.84 | 0.77 | 0.92 | 0.000 | 0.88 | 0.72 | 1.07 | 0.2064 |
| ARB | 1.02 | 0.95 | 1.10 | 0.495 | 1.05 | 0.91 | 1.21 | 0.4789 |
| ACE-I | 1.06 | 0.99 | 1.13 | 0.087 | 1.01 | 0.89 | 1.14 | 0.9093 |
| Insulin glargine | 1.00 | 0.91 | 1.10 | 0.994 | 0.99 | 0.79 | 1.26 | 0.9655 |
| Human insulin | 0.90 | 0.82 | 1.00 | 0.049 | 0.99 | 0.79 | 1.24 | 0.9372 |
| Other analog insulin | 1.01 | 0.91 | 1.12 | 0.852 | 0.66 | 0.51 | 0.87 | 0.0032 |
| Metformin | 0.96 | 0.90 | 1.02 | 0.186 | 0.83 | 0.73 | 0.95 | 0.0060 |
| Age (years)a | ||||||||
| 70–74 | 1.38 | 1.27 | 1.51 | 0.000 | 1.21 | 1.01 | 1.45 | 0.0375 |
| 75–79 | 1.75 | 1.60 | 1.91 | 0.000 | 1.41 | 1.17 | 1.70 | 0.0003 |
| 80–84 | 1.81 | 1.64 | 1.99 | 0.000 | 1.81 | 1.49 | 2.20 | 0.0000 |
| 85+ | 1.93 | 1.72 | 2.16 | 0.000 | 1.87 | 1.50 | 2.33 | 0.0000 |
| Year | 1.01 | 0.99 | 1.03 | 0.384 | 1.00 | 0.96 | 1.03 | 0.8086 |
| Racea | ||||||||
| Black | 0.63 | 0.55 | 0.72 | 0.000 | 0.59 | 0.44 | 0.79 | 0.0004 |
| Hispanic | 0.57 | 0.49 | 0.65 | 0.000 | 0.60 | 0.46 | 0.78 | 0.0001 |
| Female | 0.25 | 0.23 | 0.26 | 0.000 | 0.22 | 0.19 | 0.25 | 0.0000 |
| Low income | 0.74 | 0.69 | 0.79 | 0.000 | 0.74 | 0.65 | 0.86 | 0.0000 |
| No. of diabetes complicationsa | ||||||||
| 2 | 0.99 | 0.84 | 1.17 | 0.917 | 1.62 | 1.15 | 2.30 | 0.0060 |
| ≥3 | 0.77 | 0.52 | 1.13 | 0.181 | 0.76 | 0.28 | 2.04 | 0.5910 |
| COPD/tobacco use | 1.48 | 1.38 | 1.59 | 0.000 | 1.40 | 1.23 | 1.60 | 0.0000 |
aReferent groups were (1) Age: <70 years; Race: White; No. of diabetes complications: 0–1
We evaluated the effect of exposure to combined pharmacotherapy with pioglitazone + ARB but found that the interaction was not significant (P = 0.37), nor was there any significant (P > 0.05) association of bladder cancer with pairwise interactions of any of the diabetes medications.
Discussion
Using a Medicare cohort of over 1 million U.S. pharmacologically treated diabetic patients aged ≥67 years, we found that the incidence of bladder cancer increased with duration of pioglitazone use in the prevalent diabetes cohort but not in the incident diabetes one. We also found an association of increased incidence of bladder cancer with duration of rosiglitazone use in the prevalent cohort, but it was non-monotonic, and the association was not present in the incident diabetes cohort. The use of metformin was associated with a decrease in bladder cancer incidence among incident diabetics. Finally, the use of an ARB and the combined therapy with an ARB + TZD was unrelated to bladder cancer incidence.
The TZD, pioglitazone, has been labeled a “probable carcinogen” by the International Agency for Research on Cancer (IARC) [28]. Several meta-analyses have found it to be associated with an increased risk of cancer [22–24, 32–34]. We also found pioglitazone use to be associated with an increased risk of bladder cancer. Our relative risk estimate of 1.16 in the prevalent diabetes cohort and 1.24 in the incident diabetes cohort for ≥24 months of pioglitazone exposure among prevalent diabetics is similar to the value of 1.2 found in a recent meta-analysis of observational studies, but much less than that of 2.5 reported in a meta-analysis of randomized studies [33]. It is possible that our estimate from the prevalent cohort is biased toward the null due to measurement error of the duration of use as a result of unknown treatment histories before the implementation of Part D in 2006 and before patients reached the age of Medicare benefits. Our null finding in the incident cohort may be due to reduced power compared to the prevalent cohort; we did find that individuals with ≥24 months of pioglitazone use had 24% more incident bladder cancers, but this difference was not statistically significant.
Rosiglitazone, the other TZD used in the USA, has been evaluated for its association with bladder cancer in meta-analyses [22, 23, 32–34] and none, including the most recent meta-analysis, detected an association. In the IARC assessment [28], rosiglitazone was unclassifiable with respect to its bladder carcinogenicity. However, an animal study found that it promoted bladder tumor growth [35]. In the prevalent diabetes cohort, rosiglitazone use for >24 months was not associated with an increased risk of bladder cancer, but we did observe a 19% increased risk among those who used this drug for 1–12 months and a 28% increased risk among those using it for 13–23 months. This non-monotonic association is at most weak evidence of an association, or it indicates an unusual mechanism. The association of rosiglitazone and bladder cancer therefore requires further study.
In secondary analyses, we found metformin use was associated with a reduction in the development of bladder cancer in the incident diabetes cohort. Other studies have also reported that metformin is associated with lower cancer incidence [13, 14]. In a claims-based cohort study from Taiwan, patients receiving metformin were at lower risk of bladder cancer [36], and a UK cohort study also found evidence of lower bladder cancer risk, but the results of the latter study were not statistically significant [37]. We examined bladder cancer risk associated with sulfonylureas, another common class of anti-diabetic drugs. A recent meta-analysis reported a 55% increased risk of cancer among sulfonylurea users based on cohort studies, but no association based on data from either randomized or case–control studies [38]. In the prevalent diabetes cohort, we found an increased risk for low to moderate duration of use but a decreased risk for use of >2 years. Thus, the relationship between this class of anti-diabetics and bladder cancer remains unclear.
Most diabetes patients receive an ACE-I or ARB in addition to glucose-lowering therapy. A meta-analysis [39] found the use of ARBs to be associated with a 10% increase in bladder cancer, although a meta-analysis restricted to randomized studies found no difference in risk [40]. We found no association between ARB use and bladder cancer in our two study cohorts. Moreover, we examined the possibility that exposure to a combination of pioglitazone + ARBs or rosiglitazone + ARBs increased bladder cancer risk beyond that expected based on their main effects, but did not find evidence of an interaction.
Our study has several limitations characteristic of claims-based analyses. We lacked information on potential confounders, such as body mass index, physical activity, family history of cancer and environmental exposure. Our measure of tobacco use and past tobacco exposure depended on diagnosis codes. While clinicians are improving their tendency to explicitly diagnose tobacco use, we likely underestimated this important risk factor. Similarly, the diagnosis of chronic obstructive pulmonary disease itself may be incomplete, and even when made likely reflects a broad range of disease and past/current tobacco use. Our co-morbidity assignments and cancer events depend on the coding accuracy in these data sets. Previous studies have found that Medicare administrative data can identify cancer cases with good specificity (≥98%) and acceptable sensitivity (83–90%) [41]. In our analysis, we used a 24-month look-back period to identify prevalent cancer cases, and we assumed that patients with no cancer diagnosis during this period to be cancer free. Some recurrent cancer cases could have been misclassified as incident. Our measurement of duration of treatment and cumulative exposure in the prevalent cohort is truncated at entry into the cohort; we have no records of prescription fills preceding Part D enrollment or preceding the age of 65 years when Medicare benefits begin for elderly beneficiaries. The effect of this measurement error is likely to be non-differential, resulting in estimates biased toward the null. Our definition of an incident cohort is debatable; we define a diabetic as incident if we did not observe them to have a prescription for a diabetes medication for at least 4 months. Furthermore, our list of prescriptions for diabetes medications did not include the newer although less commonly prescribed diabetes drugs (such as GLP-1, DPP-IV, acarbose and glinides). We rely on the dose dispensed, which is an imperfect measure of dose consumed. Prescription fill records have been shown to be a good proxy measure of prescription use, but poor adherence also could result in misclassification of exposure that would bias our findings toward the null [42]. Finally, our findings do not necessarily generalize to individuals with diabetes younger than 65 years of age.
Conclusion
In conclusion, our findings are consistent with those of previous studies revealing a modest elevation in the incidence of bladder cancer associated with pioglitazone use. Associations with rosiglitazone were less clear, and no relation with bladder cancer was detected for the use of ARBs or ARBs combined with either of the TZDs. Clinicians and prescription benefits managers should consider prioritizing alternatives to pioglitazone, when possible, and engaging in shared decision-making when pioglitazone is prescribed. Attention should be paid to the benefits of glycemic control relative to the small but measurable increased risk in bladder cancers. Patients at higher risk of bladder cancer due to other factors (e.g. smoking, male gender, family history or environmental exposures) may warrant more thorough decision-making before initiating pioglitazone and perhaps rosiglitazone.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Funding and support
National Institutes of Health: National Cancer Institute 1R03CA167723-01A1. No funding or support was received for the publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval for the version to be published.
Author contributions
MK, NM and TM conceived the study. JS, RZ, NM and TM developed the analytic dataset. JS conducted the analysis. NM and TM guided the statistical analysis. NM, RZ and TM wrote the manuscript. JS and MK reviewed/edited the manuscript.
Disclosures
The authors declare that they have no conflict of interests to report.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care. 2015;38:264–270. doi: 10.2337/dc14-1996. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia. 2006;49:2819–2823. doi: 10.1007/s00125-006-0468-0. [DOI] [PubMed] [Google Scholar]
- 4.Garg SK, Maurer H, Reed K, Selagamsetty R. Diabetes and cancer: two diseases with obesity as a common risk factor. Diabetes Obes Metab. 2014;16:97–110. doi: 10.1111/dom.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin Q, Xu X, Wang X, Zheng XY. Obesity and risk of bladder cancer: a meta-analysis of cohort studies. Asian Pac J Cancer Prev. 2013;14:3117–3121. doi: 10.7314/APJCP.2013.14.5.3117. [DOI] [PubMed] [Google Scholar]
- 6.Westley RL, May FE. A twenty-first century cancer epidemic caused by obesity: the involvement of insulin, diabetes, and insulin-like growth factors. Int J Endocrinol. 2013;2013:632461. doi: 10.1155/2013/632461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keimling M, Behrens G, Schmid D, Jochem C, Leitzmann MF. The association between physical activity and bladder cancer: systematic review and meta-analysis. Br J Cancer. 2014;110:1862–1870. doi: 10.1038/bjc.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryu TY, Park J, Scherer PE. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab J. 2014;38:330–336. doi: 10.4093/dmj.2014.38.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stattin P, Bjor O, Ferrari P, et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care. 2007;30:561–567. doi: 10.2337/dc06-0922. [DOI] [PubMed] [Google Scholar]
- 10.Novosyadlyy R, LeRoith D. Hyperinsulinemia and type 2 diabetes: impact on cancer. Cell Cycle. 2010;9:1449–1450. doi: 10.4161/cc.9.8.11512. [DOI] [PubMed] [Google Scholar]
- 11.Dankner R, Shanik MH, Keinan-Boker L, Cohen C, Chetrit A. Effect of elevated basal insulin on cancer incidence and mortality in cancer incident patients: the Israel GOH 29-year follow-up study. Diabetes Care. 2012;35:1538–1543. doi: 10.2337/dc11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson JA, Bowker SL. Intensive glycaemic control and cancer risk in type 2 diabetes: a meta-analysis of major trials. Diabetologia. 2011;54:25–31. doi: 10.1007/s00125-010-1933-3. [DOI] [PubMed] [Google Scholar]
- 13.Gandini S, Puntoni M, Heckman-Stoddard BM, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prevent Res. 2014;7:867–885. doi: 10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS ONE. 2013;8:e71583. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aldea M, Craciun L, Tomuleasa C, et al. Repositioning metformin in cancer: genetics, drug targets, and new ways of delivery. Tumour Biol. 2014;35:5101–5110. doi: 10.1007/s13277-014-1676-8. [DOI] [PubMed] [Google Scholar]
- 16.Pernicova I, Korbonits M. Metformin-mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143–156. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 17.Soranna D, Scotti L, Zambon A, et al. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist. 2012;17:813–822. doi: 10.1634/theoncologist.2011-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlstad O, Starup-Linde J, Vestergaard P, et al. Use of insulin and insulin analogs and risk of cancer—systematic review and meta-analysis of observational studies. Curr Drug Saf. 2013;8:333–348. doi: 10.2174/15680266113136660067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colmers IN, Bowker SL, Tjosvold LA, Johnson JA. Insulin use and cancer risk in patients with type 2 diabetes: a systematic review and meta-analysis of observational studies. Diabetes Metab. 2012;38:485–506. doi: 10.1016/j.diabet.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Morden Nancy E, Liu Stephen K, Smith J, Mackenzie TA, Skinner J, Korc M. Further exploration of the relationship between insulin glargine and incident cancer: a retrospective cohort study of older medicare patients. Diabetes Care. 2011;34:1965–71. [DOI] [PMC free article] [PubMed]
- 21.Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 2010;11:627–636. doi: 10.1016/S1470-2045(10)70106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colmers IN, Bowker SL, Majumdar SR, Johnson JA. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: a meta-analysis. CMAJ. 2012;184:E675–83. [DOI] [PMC free article] [PubMed]
- 23.He S, Tang YH, Zhao G, Yang X, Wang D, Zhang Y. Pioglitazone prescription increases risk of bladder cancer in patients with type 2 diabetes: an updated meta-analysis. Tumour Biol. 2014;35:2095–2102. doi: 10.1007/s13277-013-1278-x. [DOI] [PubMed] [Google Scholar]
- 24.Ferwana M, Firwana B, Hasan R, et al. Pioglitazone and risk of bladder cancer: a meta-analysis of controlled studies. Diabet Med. 2013;30:1026–1032. doi: 10.1111/dme.12144. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Z, Shen Z, Lu Y, Zhong S, Xu C. Increased risk of bladder cancer with pioglitazone therapy in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract. 2012;98:159–163. doi: 10.1016/j.diabres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Levin D, Bell S, Sund R, et al. Pioglitazone and bladder cancer risk: a multipopulation pooled, cumulative exposure analysis. Diabetologia. 2015;58:493–504. doi: 10.1007/s00125-014-3456-9. [DOI] [PubMed] [Google Scholar]
- 27.Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA. 2015;314:265–277. doi: 10.1001/jama.2015.7996. [DOI] [PubMed] [Google Scholar]
- 28.Grosse Y, Loomis D, Lauby-Secretan B, et al. Carcinogenicity of some drugs and herbal products. Lancet Oncol. 2013;14:807–808. doi: 10.1016/S1470-2045(13)70329-2. [DOI] [PubMed] [Google Scholar]
- 29.US Department of Health and Human Resources. Creation of new race–ethnicity codes and socioeconomic status (SES) indicators for Medicare beneficiaries. 2008. Available at: http://www.ahrq.gov/qual/medicareindicators/medicareindicators2.htm. Accessed 23 Apr 2010.
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Wolbers M, Koller MT, Stel VS, et al. Competing risks analyses: objectives and approaches. Eur Heart J. 2014;35:2936–2941. doi: 10.1093/eurheartj/ehu131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosetti C, Rosato V, Buniato D, Zambon A, La Vecchia C, Corrao G. Cancer risk for patients using thiazolidinediones for type 2 diabetes: a meta-analysis. Oncologist. 2013;18:148–156. doi: 10.1634/theoncologist.2012-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner RM, Kwok CS, Chen-Turner C, Maduakor CA, Singh S, Loke YK. Thiazolidinediones and associated risk of bladder cancer: a systematic review and meta-analysis. Br J Clin Pharmacol. 2014;78:258–273. doi: 10.1111/bcp.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monami M, Dicembrini I, Mannucci E. Thiazolidinediones and cancer: results of a meta-analysis of randomized clinical trials. Acta Diabetol. 2014;51:91–101. doi: 10.1007/s00592-013-0504-8. [DOI] [PubMed] [Google Scholar]
- 35.Lubet RA, Fischer SM, Steele VE, Juliana MM, Desmond R, Grubbs CJ. Rosiglitazone, a PPAR gamma agonist: potent promoter of hydroxybutyl(butyl)nitrosamine-induced urinary bladder cancers. Int J Cancer. 2008;123:2254–2259. doi: 10.1002/ijc.23765. [DOI] [PubMed] [Google Scholar]
- 36.Tseng CH. Metformin may reduce bladder cancer risk in Taiwanese patients with type 2 diabetes. Acta Diabetol. 2014;51:295–303. doi: 10.1007/s00592-014-0562-6. [DOI] [PubMed] [Google Scholar]
- 37.Mamtani R, Pfanzelter N, Haynes K, et al. Incidence of bladder cancer in patients with type 2 diabetes treated with metformin or sulfonylureas. Diabetes Care. 2014;37:1910–1917. doi: 10.2337/dc13-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thakkar B, Aronis KN, Vamvini MT, Shields K, Mantzoros CS. Metformin and sulfonylureas in relation to cancer risk in type II diabetes patients: a meta-analysis using primary data of published studies. Metab Clin Exp. 2013;62:922–934. doi: 10.1016/j.metabol.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 39.Hallas J, Christensen R, Andersen M, Friis S, Bjerrum L. Long term use of drugs affecting the renin–angiotensin system and the risk of cancer: a population-based case-control study. Br J Clin Pharmacol. 2012;74:180–188. doi: 10.1111/j.1365-2125.2012.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collaboration ARBT. Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J Hypertens. 2011;29:623–635. doi: 10.1097/HJH.0b013e328344a7de. [DOI] [PubMed] [Google Scholar]
- 41.Setoguchi S, Solomon DH, Glynn RJ, Cook EF, Levin R, Schneeweiss S. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between medicare claims and cancer registry data. Cancer Causes Control. 2007;18:561–569. doi: 10.1007/s10552-007-0131-1. [DOI] [PubMed] [Google Scholar]
- 42.Lau HS, de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50:619–625. doi: 10.1016/S0895-4356(97)00040-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.