Abstract
Introduction
Our main aim was to assess the level of persistence and adherence to therapy with glucagon-like peptide-1 (GLP-1) receptor agonists in type 2 diabetes mellitus (T2DM) patients in the United Kingdom (UK) and Germany, also by comparing once- (OD) with twice-a-day (BID) therapy.
Methods
We used two large retrospective datasets: a German claims dataset and the UK General Practitioner (GP)-based Clinical Practice Research Datalink (CPRD) dataset (2010–2012). All continuously insured T2DM patients with at least one outpatient/inpatient T2DM diagnosis were observed starting with the first prescription of a GLP-1 receptor agonist. Non-persistence (NP) was defined as treatment gap >90 days. Non-adherence (NA) was defined as medication possession ratio <80%, calculated during a period in which a patient continued therapy (no treatment gap >90 days) only.
Results
In the UK sample, 1905 T2DM patients started a treatment with GLP-1 receptor agonists (mean age: 55.5 years, 47.2% female). In the German sample, 1627 T2DM patients started a treatment with GLP-1 receptor agonists (mean age: 56.6 years, 51.4% female). Percentage of NP patients after 12 months was 29.5% in the UK and 36.4% in the German sample. In both countries, a BID treatment was associated with a higher probability to discontinue a treatment with GLP-1 receptor agonists earlier than an OD treatment (hazard ratio [HR] = 1.431 in UK and HR = 1.314 in Germany). The percentages of patients considered NA were 20.2%/20.0%/20.5% (all/OD/BID) for the UK sample, and 19.9%/19.2%/21.8% (all/OD/BID) for the German sample.
Conclusion
NP and NA to treatment with GLP-1 receptor agonists in both UK and Germany appear to be similar. Persistence to OD treatment is higher than to BID treatment in both the UK and Germany.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-015-0149-4) contains supplementary material, which is available to authorized users.
Keywords: GLP-1 receptor agonists, Non-adherence, Non-persistence, Type 2 diabetes mellitus
Introduction
Type 2 diabetes mellitus (T2DM) is amongst the most common chronic diseases and is a growing worldwide epidemic [1]. The primary goal of diabetes treatment is to control blood glucose levels [2, 3]. Treatment guidelines recommend metformin as first-line therapy, followed by several options as second-line agents, including sulfonylureas (SU), thiazolidinediones, alpha-glucosidase inhibitors, dipeptidyl peptidase-4 (DPP-4) inhibitors, basal insulin, sodium/glucose cotransporter 2 inhibitors, and now also, glucagon-like peptide-1 (GLP-1) receptor agonists [2–4]. In Germany and the United Kingdom (UK), there are currently six GLP-1 receptor agonists available, twice-daily (BID) exenatide, once-daily (OD) liraglutide, OD lixisenatide, once-weekly exenatide, once-weekly albiglutide and once-weekly dulaglutide which have all been shown to be both effective and safe options for T2DM treatment after metformin failure [5–7].
However, despite the number and variety of available T2DM treatment options, it is known from several real-world studies that reaching target glucose levels remains a challenge for many patients [8–10]. One of the reasons for this may be non-persistence (NP—discontinuation of a prescribed therapy), and/or non-adherence (NA) (continued use of therapy, but not as prescribed). Several studies have found a high level of both NP and NA with regards to anti-diabetic therapy [11–16] and confirmed worse diabetes-related outcomes associated with NP/NA [17–20]. Much less is known about the level of persistence/adherence to therapy with GLP-1 receptor agonists. Three previous US analyses reported 12-month medication possession ratios (MPRs) of 68% for BID GLP-1 [21], 69.7% for OD GLP-1 and 64.4% for BID GLP-1 [22], or 78.3% for once-weekly GLP-1, compared to 50.0% for BID GLP-1 and 68.3–76.1% for OD GLP-1 [23], the only known European-based study reported a 12-month therapy discontinuation rate of 32.2% for BID therapy with GLP-1 receptor agonists only [24]. Consequently, there is limited real-world data from European T2DM patients about the persistence and adherence to therapy with GLP-1 receptor agonists.
Therefore, our aim was to use two large European datasets, to (1) assess the level of persistence and adherence to therapy with GLP-1 receptor agonists in T2DM patients in UK and Germany, and compare OD with BID therapy and (2) to identify any factors that may explain early discontinuation of therapy with GLP-1 receptor agonists in the first year of therapy as well as to assess outcomes possibly associated with early discontinuation of therapy.
Methods
Our study had access to two large retrospective datasets: a German claims dataset provided by one large sickness fund (AOK PLUS; 2.7 million insured; http://www.aokplus.de) and the UK General Practitioner (GP)-based Clinical Practice Research Datalink (CPRD) database (longitudinal data covering about 4.4 million patients treated by about 500 GPs; http://www.cprd.com; accessed Jan 2015). As far as data available in the two datasets allowed, we used the same methodology for each of the database analyses.
T2DM Samples
This was a retrospective non-interventional cohort analysis based on anonymized data for the calendar years 2010–2012 (separate analyses; no linking of country data). All T2DM patients [at least one outpatient T2DM diagnosis (ICD E11.- or CPRD read codes which are available from the authors upon request) and/or at least one inpatient T2DM diagnosis before index date] who were enrolled continuously in the databases from 01/01/2010 until the end of the observational period were included in the analysis; death during the observational period was the only exception to the continuous enrolment requirement.
We analyzed persistence/adherence to therapy with GLP-1 receptor agonists in common use during the study period [BID exenatide (Anatomical Therapeutic Chemical [ATC] code: A10BX04), OD liraglutide (ATC code: A10BX07)]. We excluded exenatide in its once-weekly formulation, as it was introduced late in the study period and rarely used. In addition, we did not observe therapy with lixisenatide, albiglutide, and dulaglutide because these agents were approved after end of 2012.
Analysis was done based on treatment-naïve patients only, defined as no prescription of medication of interest in the 6 months before first observed GLP-1 prescription. However, anti-diabetic medication other than the medication of interest was possible. The start of therapy with GLP-1 receptor agonists was between 01/07/2010 and 30/06/2011 with date of the first GLP-1 prescription defining the index date. A 6-month pre-index period was used to determine eligibility for inclusion and a 18-month post-index period was used for the persistence/adherence analysis (Fig. 1).
Fig. 1.
Methodology of analysis. The figure shows how reference period, index date and observational period have been defined. GLP-1 glucagon-like peptide-1, MPR medication possession ratio
Assessment of Treatment Persistence
Our analysis was based on the days’ supply of the observed prescriptions. To enable comparability between databases, and because of incomplete data with regards to prescribed days of supply, we assumed that the prescribed daily dosage was equal to the WHO defined daily dosage per medication [25].
NP was defined as a treatment gap of more than 90 days (sensitivity analysis: 180 days). We reported percentage of patients that could be classified as non-persistent at 3, 6, and 12 months after index date. In the German analysis, hospitalizations periods were taken out from observed days because drug’s supply was assumed to be provided by hospitals during these days. In contrast, in the UK analysis, information about hospitalization periods was not available for all patients. Furthermore, both in the UK and German analyses, stockpiling was included by assuming that, in case there were overlapping medications, the previous supply was taken fully before the new supply was initiated.
Assessment of Treatment Adherence
Treatment adherence was analyzed in two ways. First, for the overall sample which included those patients who may have discontinued therapy during our preset observation period and those continuing their therapy, we analyzed the overall MPR, defined as number of days’ supply received during the whole observational period of 12 months after index date, divided by the number of days in the evaluation period:
In our second NA analysis, we explored adherence only for the period in which a patient continued therapy (no treatment gap >90 days; Fig. 1):
Adherence was reported in three ways, first as mean MPR, second as percentage of patients with a MPR <80% and third, in a sensitivity analysis, as percentage of patients with a MPR <70/90%.
Assessment of Variables Predicting NP to Therapy with GLP-1 Receptor Agonists
Assessment of potential factors predicting 12-month NP to therapy with GLP-1 receptor agonists (treatment gap >90 days) was done using a multivariable Cox regression estimation with time to therapy discontinuation as dependent variable. Only patients receiving either an OD or BID therapy with GLP-1 receptor agonists were included, switchers between different treatment regimes with GLP-1 receptor agonists were excluded. As initial independent variables, age (at 31/12/2009), gender, Charlson Comorbidity Index (CCI) based on diagnoses in the 6 months before the index date and excluding age as factor, OD versus BID treatment regimen with GLP-1 receptor agonists, any anti-diabetic medication in the 180 days before index date (yes/no), number of received anti-diabetic medications (consideration of SU’s, DPP-4 inhibitors, metformin and any insulin) in the 180 days before/after index date, and at least one visit to a diabetologist in the 6 months before the index date (yes/no) were included. In the UK CPRD analysis, a visit to a Diabetologist was assumed to have taken place if a NHS code for “endocrinology” or “diabetic nurse specialist” was included.
Assessment of NP-Related Outcomes
For T2DM patients newly initiating therapy with GLP-1 receptor agonists (no GLP-1 prescriptions in the previous 180 days before index date), we analyzed three diabetes-related outcomes which may be associated with NP (>90 days gap; 6-month NP): (1) insulin initiation in a subgroup of insulin-naïve T2DM patients, (2) occurrence of any acute hospitalizations with T2DM as main diagnosis, and (3) glycated hemoglobin (HbA1c) progression since index date.
In the first analysis, all insulin-naïve patients which initiated a therapy with GLP-1 receptor agonists were observed for 12 months. In a multivariable Cox regression analysis taking into account possible confounding variables available in the database (age, gender, comorbidities and previous/concomitant medications), we analyzed whether 6-month NP with GLP-1 was associated with early insulin therapy initiation (any insulin) in observed patients until end of the 12th month.
In the second analysis, we determined in a multivariable logistic regression analysis whether 6-month NP with therapy with GLP-1 receptor agonists (gap >90 days) was associated with a higher probability of experiencing an acute hospitalization with T2DM as main diagnosis (ICD 10 E11.-/E16.0/E16.1/E16.2) in the second 6 months of the observational period. Please note that in this analysis all patients available in the German dataset were included, whereas only a subsample of CPRD T2DM patients for whom hospitalization data were available (Hospital Episode Statistics) were included in the UK analysis.
In the third analysis, we only included T2DM patients which started a therapy with GLP-1 receptor agonists and had at least three HbA1c values documented: at baseline (last value measured before index date), 6–9 months after index date, and 12–18 months after index date. If more than one value in these periods was available, we used the mean value. Change of HbA1c was compared between patients having continued their therapy with GLP-1 receptor agonists after 6 months versus those having discontinued their therapy at this time.
Statistical Analysis
Discontinuation rates in patient subgroups (GLP-1: OD/BID) were depicted using Kaplan–Meier curves, significance of differences between discontinuation rates was tested using log-rank tests. To assess any factors predicting discontinuation of therapy with GLP-1 receptor agonists and insulin initiation, we did multivariable Cox regression analyses; for assessment of factors associated with T2DM-hospitalization we did a multivariable logistic regression analysis. The models were estimated based on a backward elimination methodology. All factors not reaching a p value <0.1 were excluded in a stepwise procedure (except age, gender and CCI, which remained in the models as fixed independent variables even if they did not reach statistical significance). Finally, factors reaching a p <0.05 were interpreted as statistically significant. All reported p values were two-sided, and 95% confidence intervals (CIs) were calculated for hazard ratios (HRs)/odds ratios (ORs). Patients with missing data were excluded from the dataset.
Descriptive evaluations were done with Microsoft SQL Server 2008 and Microsoft Excel 2010 (Microsoft, Redmond, United States). All other statistical analyses were done with SPSS 17.0 (IBM, Armonk, United States).
Compliance with Ethics Guidelines
Due to the non-interventional, retrospective nature of the present study and the analysis of an anonymized dataset, no ethical review of this study was necessary. However, the study was evaluated by a scientific steering committee to which all the authors belonged as well as by internal scientific committees belonging to the data owners, the AOK PLUS and CPRD (CPRD Protocol Approval Number: 14_022).
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
T2DM Samples
In the UK sample, 1905 T2DM patients started a treatment with GLP-1 receptor agonists during the observation period (mean age: 55.5 years, 47.2% female). In the German sample, 1627 T2DM patients started a treatment with GLP-1 receptor agonists (mean age: 56.6 years, 51.4% female). Out of the total samples, subsets including 1744 UK and 1349 German patients were determined eligible for the adherence analysis. The remaining patients (UK: 8.5%; Germany: 17.1%) discontinued their therapy after only a single prescription. Main patient characteristics are shown in Table 1.
Table 1.
Characteristics of observed type 2 diabetes mellitus patient samples
| Characteristics (baseline period 2010) | UK: CPRD | GER: AOK PLUS | ||
|---|---|---|---|---|
| (Main sample for persistence analysis) | (Main sample for adherence analysis) | (Main sample for persistence analysis) | (Main sample for adherence analysis) | |
| T2DM-prevalent patients having started a therapy with GLP-1 receptor agonists | T2DM-prevalent patients having started a therapy with GLP-1 receptor agonists and having continued this therapy (no gap >90 days) at least until a second prescription | T2DM-prevalent patients having started a therapy with GLP-1 receptor agonists | T2DM-prevalent patients having started therapy with GLP-1 receptor agonists and having continued this therapy (no gap >90 days) at least until a second prescription | |
| N | 1905 | 1744 | 1627 | 1349 |
| Mean age in years (SD) | 55.5 (10.6) | 55.2 (10.4) | 56.6 (10.9) | 56.2 (10.6) |
| Female gender (%) | 900 (47.2%) | 830 (47.6%) | 837 (51.4%) | 677 (50.2%) |
| Mean Charlson Comorbidity Index: CCI (SD) | 2.9 (1.5) | 2.9 (1.5) | 4.2 (2.3) | 4.0 (2.2) |
| Retinopathy (%) | 483 (25.4%) | 432 (24.8%) | 349 (21.5%) | 290 (21.5%) |
| Nephropathy (%) | 64 (3.4%) | 52 (3.0%) | 234 (14.4%) | 178 (13.2%) |
| Neuropathic comorbidities (%) | 283 (14.9%) | 256 (14.7%) | 317 (19.5%) | 255 (18.9%) |
| Cerebrovascular comorbidities (%) | 61 (3.2%) | 50 (2.9%) | 44 (2.7%) | 37 (2.7%) |
| Cardiovascular comorbidities (%) | 358 (18.8%) | 316 (18.1%) | 545 (33.5%) | 440 (32.6%) |
| Peripheral vascular disease (%) | 153 (8.0%) | 133 (7.6%) | 199 (12.2%) | 165 (12.2%) |
| Metabolic comorbidities (%) | 10 (0.5%) | 8 (0.5%) | 24 (1.5%) | 17 (1.3%) |
The table lists characteristics for the observed samples used for persistence and adherence analysis
UK United Kingdom, CPRD Clinical Practice Research Datalink, GER Germany, AOK Allgemeine Ortskrankenkasse, T2DM type 2 diabetes mellitus, GLP-1 glucagon-like peptide-1, SD standard deviation, CCI Charlson Comorbidity Index
Background anti-diabetic medications (6 months prior to index date) of the overall sample of GLP-1 starters included: no anti-diabetic medication in 2.2% of observed UK patients and 10.9% of observed German patients, insulin monotherapy in 3.1% UK and 6.6% German patients, metformin and/or SU and/or DPP-4 inhibitors in 71.2% UK and 57.2% German patients, and combination therapy with Oral Diabetic Drugs (OAD) (metformin and/or SU and/or DPP-4 inhibitors) and any insulin in 23.5% UK and 25.4% German patients.
During the 12-months observational period after start of therapy with GLP-1 receptor agonists (index date), 1.8% (UK) and 5.7% (German) of the patients received no concomitant anti-diabetic medication, 3.9% and 4.8% of the UK and German patients, respectively, received at least one type of insulin, 63.6% of UK and 61.7% of the German patients received metformin and/or SU and/or DPP-4 inhibitors and 30.7% of the UK and 27.8% of German patients were concomitantly treated with a combination therapy of OAD (metformin and/or SU and/or DPP-4 inhibitors) and any insulin.
In the UK, based on the 1905 T2DM patients initiating therapy with GLP-1 receptor agonists, 56.8% received an OD treatment with GLP-1 receptor agonists until end of observational period or observed NP (whichever occurred first), 28.6% received a BID treatment, and 14.5% switched between OD and BID in the observational period (5.8% from OD to BID and 61.4% from BID to OD and 32.9% more than once). In German data, based on 1627 T2DM starters of therapy with GLP-1 receptor agonists, 72.5% received an OD treatment, 18.1% received a BID treatment, and 9.3% switched between OD and BID (40.8% from OD to BID and 35.5% from BID to OD and 23.7% more than once).
Assessment of Treatment Persistence
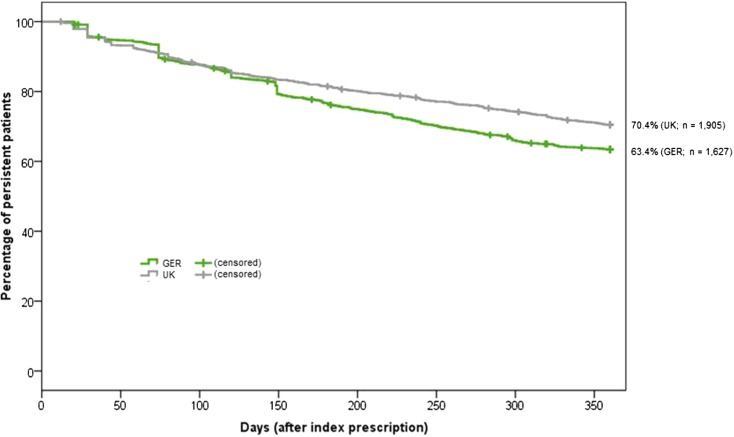
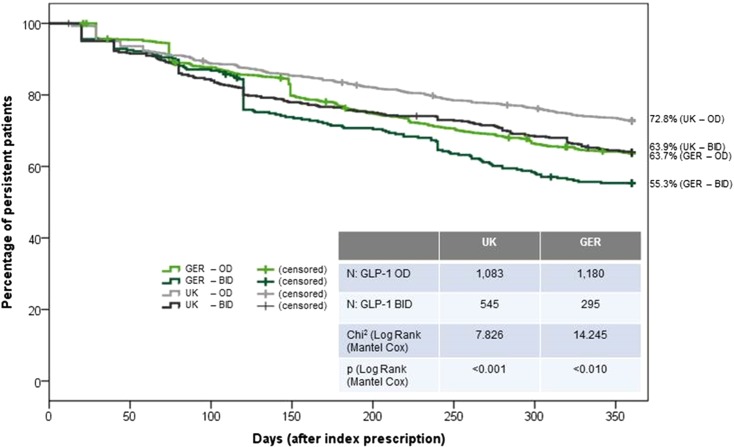
The results of the persistence analysis of treatment with GLP-1 receptor agonists are presented in Figs. 2 and 3 and Table 2. Percentage of NP (gap >90 days) patients after 12 months was 29.5% in the UK and 36.4% in Germany; of these patients, 17.1% (5.0% of all patients) in the UK and 33.9% (12.3% of all patients) of the German patients received only a single GLP-1 prescription. In both countries, persistence was significantly better for OD compared to BID therapy with GLP-1 receptor agonists (Fig. 3): 72.8% (OD) versus 63.9% (BID) in UK (p < 0.001) and 63.7% (OD) versus 55.3% (BID) in Germany (p < 0.010). Table 2 summarizes the results of persistence analysis including a sensitivity analysis using a >180 days treatment gap.
Fig. 2.
Kaplan–Meier curves for percentage of T2DM patients persistent to their therapy with GLP-1 receptor agonists. The figure describes percentage of T2DM still persistent (no gap >90 days) to their therapy with GLP-1 receptor agonists over time (12 months) with regards to UK-T2DM and GER-T2DM patients having started a therapy with GLP-1 receptor agonists. T2DM type 2 diabetes mellitus, GLP-1 glucagon-like peptide-1, UK United Kingdom, GER Germany
Fig. 3.
Kaplan–Meier curves for percentage of persistent GLP-1 patients during 12 months after therapy initiation. The figure describes percentage of T2DM patients having started a therapy with GLP-1 receptor agonists (four groups: UK- and GER-T2DM patients having started either an OD/BID therapy with GLP-1 receptor agonists) being still persistent (no gap >90 days) with therapy over time (12 months). Patients having switched their therapy with GLP-1 receptor agonists between OD and BID were excluded. GLP-1 glucagon-like peptide-1, T2DM type 2 diabetes mellitus, UK United Kingdom, GER Germany, OD once daily, BID twice daily
Table 2.
Results of medication treatment persistence analysis
| Persistence (period: first 12 months after treatment initiation) | UK: CPRD | GER: AOK PLUS | ||||
|---|---|---|---|---|---|---|
| T2DM-prevalent patients having started a therapy with GLP-1 receptor agonists | T2DM-prevalent patients having started a OD therapy with GLP-1 receptor agonists (no switching to BID/once-weekly) | T2DM-prevalent patients having started a BID therapy with GLP-1 receptor agonists (no switching to OD/once-weekly) | T2DM-prevalent patients having started a therapy with GLP-1 receptor agonists | T2DM-prevalent patients having started a OD therapy with GLP-1 receptor agonists (no switching to BID/once-weekly) | T2DM-prevalent patients having started a BID therapy with GLP-1 receptor agonists (no switching to OD/once-weekly) | |
| N | 1905 | 1083 | 545 | 1627 | 1180 | 295 |
| 1. Treatment persistence based on a >90 days treatment gap NP definition | ||||||
| Mean time on therapy (SD) | 298.2 (111.3) | 303.6 (107.2) | 283.0 (121.1) | 284.5 (114.3) | 285.9 (112.9) | 266.8 (120.7) |
| Patients affected by NP after 3 months (%) | 219 (11.5%) | 112 (10.3%) | 83 (15.2%) | 194 (11.9%) | 141 (11.9%) | 38 (12.9%) |
| Patients affected by NP after 6 months (%) | 352 (18.5%) | 179 (16.5%) | 128 (23.5%) | 378 (23.2%) | 272 (23.1%) | 84 (28.5%) |
| Patients affected by NP after 12 months (%) | 562 (29.5%) | 294 (27.1%) | 196 (36.0%) | 593 (36.4%) | 426 (36.1%) | 131 (44.4%) |
| 2. Treatment persistence based on >180 days treatment gap NP definition | ||||||
| Mean time on therapy (SD) | 308.0 (104.6) | 312.1 (100.9) | 292.8 (115.6) | 301.4 (104.4) | 301.9 (103.7) | 283.5 (113.0) |
| Patients affected by NP after 3 months (%) | 185 (9.7%) | 96 (8.9%) | 71 (13.0%) | 141 (8.7%) | 102 (8.6%) | 30 (10.2%) |
| Patients affected by NP after 6 months (%) | 292 (15.3%) | 149 (13.8%) | 109 (20.0%) | 290 (17.8%) | 213 (18.1%) | 66 (22.4%) |
| Patients affected by NP after 12 months (%) | 483 (25.4%) | 256 (23.6%) | 175 (32.1%) | 478 (29.4%) | 345 (29.2%) | 112 (38.0%) |
The table lists results of persistence analysis. Numbers are based on a Kaplan–Meier analysis censoring for death of patients
UK United Kingdom, CPRD Clinical Practice Research Datalink, GER Germany, AOK Allgemeine Ortskrankenkasse, T2DM type 2 diabetes mellitus, GLP-1 glucagon-like peptide-1, OD once daily, BID twice daily, NP non-persistence, SD standard deviation
Assessment of Treatment Adherence
Overall, 12-month MPR for therapy with GLP-1 receptor agonists in the analyzed UK and German T2DM samples was 76.0% and 73.9%, respectively. Table 3 describes the results of the adherence analysis of treatment with GLP-1 receptor agonists which was done during periods of general treatment continuation (no gaps >90 days) only. Mean MPRs in the UK analysis were 88.6% for GLP-1 (all patients), 88.2% for OD GLP-1, and 89.3% for BID GLP-1. Respective numbers for the German analysis were 89.7% for GLP-1 (all patients), 90.0% for OD GLP-1, and 89.4% for BID GLP-1. Adherence observed with OD therapy with GLP-1 receptor agonists was similar to BID therapy with GLP-1 receptor agonists in both countries. Based on a NA definition of MPR <80%, the percentage of patients affected by NA were 20.2%/20.0%/20.5% (all patients/OD/BID) in the UK sample, and 19.9%/19.2%/21.8% (all/OD/BID) in the German sample.
Table 3.
Results of adherence analysis
| Adherence (period: first 12 months after treatment initiation) | UK: CPRD | GER: AOK PLUS | ||||
|---|---|---|---|---|---|---|
| T2DM-prevalent patients having started a therapy with GLP-1 receptor agonists | T2DM-prevalent patients having started a OD therapy with GLP-1 receptor agonists (no switching to BID/once-weekly) | T2DM-prevalent patients having started a BID therapy with GLP-1 receptor agonists (no switching to OD/once-weekly) | T2DM-prevalent patients having started a therapy with GLP-1 receptor agonists | T2DM-prevalent patients having started a OD therapy with GLP-1 receptor agonists (no switching to BID/once-weekly) | T2DM-prevalent patients having started a BID therapy with GLP-1 receptor agonists (no switching to OD/once-weekly) | |
| 1. Overall MPR for 12 months observational period since therapy starta | ||||||
| N | 1905 | 1083 | 545 | 1627 | 1180 | 295 |
| Mean MPR in % (SD) | 76.0 (29.0) | 76.5 (28.2) | 73.2 (31.7) | 73.9 (29.5) | 74.1 (29.1) | 69.7 (32.1) |
| Patients affected by NA: MPR <80%, (%) | 711 (37.3%) | 391 (36.1%) | 226 (41.5%) | 685 (42.1%) | 488 (41.4%) | 148 (50.2%) |
| 2. Adherence between first and last observed prescription during therapy persistenceb | ||||||
| N | 1744 | 998 | 484 | 1349 | 975 | 234 |
| Mean observational period in days (SD) | 288.6 (96.5) | 295.1 (90.1) | 272.2 (109.0) | 253.6 (98.6) | 255.4 (97.7) | 233.9 (104.9) |
| Mean MPR in % (SD) | 88.6 (14.9) | 88.2 (15.0) | 89.3 (15.6) | 89.7 (15.0) | 90.0 (14.7) | 89.4 (15.9) |
| Patients affected by NA: MPR <70%, (%) | 222 (12.7%) | 133 (13.3%) | 60 (12.4%) | 164 (12.2%) | 115 (11.8%) | 33 (14.1%) |
| Patients affected by NA: MPR <80%, (%) | 353 (20.2%) | 200 (20.0%) | 99 (20.5%) | 269 (19.9%) | 187 (19.2%) | 51 (21.8%) |
| Patients affected by NA: MPR <90%, (%) | 635 (36.4%) | 375 (37.6%) | 161 (33.3%) | 443 (32.8%) | 315 (32.3%) | 77 (32.9%) |
The table lists results of adherence analysis
UK United Kingdom, CPRD Clinical Practice Research Datalink, GER Germany, AOK Allgemeine Ortskrankenkasse, T2DM type 2 diabetes mellitus, GLP-1 glucagon-like peptide-1, OD once daily, BID twice daily, NP non-persistence, MPR medication possession ratio, SD standard deviation, NA non-adherence
aMPR calculation was done for a fixed period of 360 days (or, in case of death within this period, for the time until the date of death)
bMPR calculation for the time between the first and last prescription during persistent treatment periods
Assessment of Predictors of Discontinuation of Therapy with GLP-1 Receptor Agonists
To be able to assess the influence of the specific treatment regimen with GLP-1 receptor agonists (OD versus BID), only patients who did not switch between BID and OD were included in this analysis (UK: 1628 patients; German: 1475 patients).
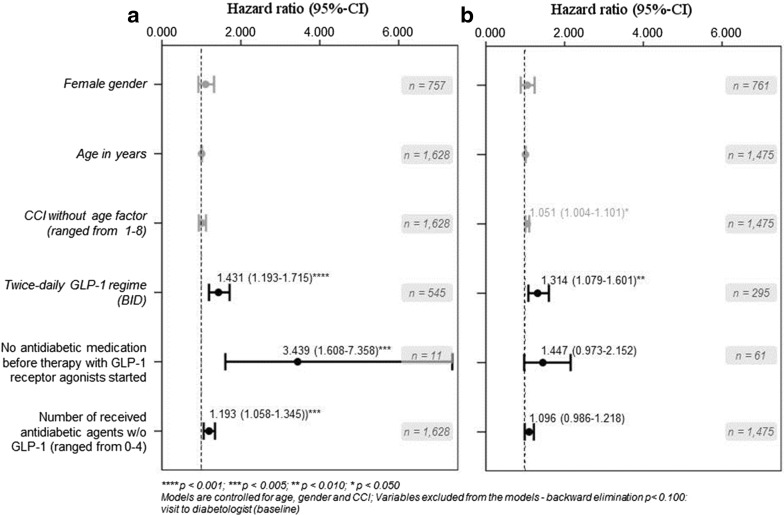
In the Cox regression analysis, age or gender of patients as well as their CCI were not significantly associated with discontinuation of therapy with GLP-1 receptor agonists in the UK T2DM sample (Fig. 4). Predictors of early NP were injection frequency (HR = 1.431, related to BID versus OD; 95% CI 1.19–1.71), no antidiabetic medication before index date (HR = 3.439; 95% CI 1.61–7.36), and number of received anti-diabetic agents in the 180 days pre-/post index date (HR = 1.193, referring to values between 0 and 4; 95% CI 1.06–1.34).
Fig. 4.
Factors associated with early GLP-1 discontinuation. The figure shows the results of the multivariable Cox regression analyses with regards to independent factors influencing early GLP-1 discontinuation (4a GER, 4b UK). GLP-1 glucagon-like peptide-1, UK United Kingdom, GER Germany, CI confidence interval, CCI Charlson Comorbidity Index, BID twice daily
In German patients, results for age and gender were similar to UK, however, a higher CCI was associated with a higher probability of early GLP-1 NP (HR = 1.051; 95% CI 1.00–1.10). As in the UK, a BID treatment was associated with a higher probability to discontinue a treatment with GLP-1 receptor agonists earlier than an OD treatment (HR = 1.314; 95% CI 1.08-1.60).
Assessment of NP-Related Outcomes
We identified 1398 (73.4%) UK and 1107 (68.0%) German insulin-naïve patients having started a therapy with GLP-1 receptor agonists during our inclusion period. In multivariable Cox regression analyses using time until insulin initiation as dependent variable, among other variables (number of additionally received anti-diabetic agents, visit to diabetologists, retinopathy, neuropathy, diuretics use, female gender), NP to treatment with GLP-1 receptor agonists after 6 months (treatment gap >90 days) was significantly associated with earlier insulin initiation (HR: 2.664 for UK (95% CI 2.11–3.35) and 2.755 for German patients (95% CI 2.25–3.37).
In the multivariable logistic regression analysis using T2DM-related hospitalizations during the last 6 months of the observational period as dependent variable, all German GLP-1 starters (1627 patients) could be considered, whereas in the UK only 320 patients with availability of hospital data could be analyzed. In both countries, NP to therapy with GLP-1 receptor agonists after 6 months (treatment gap >90 days) was not significantly associated with the probability to experience a diabetes-related hospitalization.
For the third analysis describing progression of HbA1c values since initiation of treatment with GLP-1 receptor agonists, 520 UK patients and 252 German patients newly initiating a therapy with GLP-1 receptor agonists and having all required HbA1c data available were analyzed. Patients persistent to therapy with GLP-1 receptor agonists showed a higher mean relative decrease in HbA1c after 6–9 months compared to NP patients (UK: −6.5% versus −4.7%; German: −4.4% versus −1.0%); however, these differences were not statistically significant. Initial differences in HbA1c were smaller (more moderate) or even changed direction after 12–18 months (UK: −5.4% versus −7.8%; German: −4.7% versus −2.9%).
Discussion
Study Objectives and Main Results
We aimed to assess the level of persistence and adherence to therapy with GLP-1 receptor agonists in T2DM patients and to identify predictors/outcomes of GLP-1 discontinuation. One of the main advantages of this study is use of two different databases from two European countries, but using the same methodology to calculate treatment persistence/adherence in the analyses.
Our data show that patients receiving therapy with GLP-1 receptor agonists are broadly similar in their demographic characteristics in the UK and Germany. The main difference was the CCI, with German GLP-1-patients having greater morbidity at a similar age. This is likely related to the existing therapy advice of the German Federal Joint Committee, which restricts reimbursement for GLP-1s in healthier patients, recommending GLP-1 use mainly in T2DM patients with insulin resistance and a Body Mass Index (BMI) >30 [26].
However, pre-index anti-diabetic therapy differed between the countries. Generally, therapy with GLP-1 receptor agonists seems to be prescribed as second or third-line therapy in UK patients whereas it is chosen as first-line therapy in at least 10% of the German T2DM patients. Concomitant anti-diabetic medication patterns were similar across countries.
Our analysis identified a large percentage of patients having discontinued their therapy with GLP-1 receptor agonists after 12 months. In the UK, this percentage was slightly lower than in Germany (29.6% versus 36.6%). In a sensitivity analysis using a treatment gap of >180 days instead of 90 days, NP rates were lower, as expected, but still with a slightly higher NP rate in Germany. This corresponded with a slightly higher overall 12-month MPR in the UK compared to Germany in the overall sample of persistent and non-persistent patients. Finally, percentage of patients affected by NA during general continuation of treatment with GLP-1 receptor agonists was very similar across the countries (20.2% in UK versus 19.9% in Germany). The observed persistence differences between UK and German patients may be related to different patient characteristics, different medication patterns before therapy with GLP-1 receptor agonists started, different concomitant medication patterns, or the fact that we could not exclude hospitalization days from analysis in the UK dataset. Moreover, probably more important, the UK CPRD database documents all prescriptions issued by physicians whereas the German claims database documents issued prescriptions having been filled in pharmacies only. Finally, in the UK “repeated prescriptions” which can be supplied for a certain period of time on a regular basis without having to be seen by a physician each time can be normally authorized for a period of 6–12 months. It can be assumed that repeated prescriptions lead to a certain over-statement of treatment persistence because a refill practice like repeated prescriptions is not known in Germany. Therefore, taking all these factors into account, we conclude that GLP-1 related persistence/adherence is similar across the UK and Germany.
Our estimates describing persistence/adherence of starters of therapy with GLP-1 receptor agonists in UK and Germany are generally consistent with numbers presented in earlier studies. In a US analysis, a 12-month MPR of 68% for BID therapy with GLP-1 receptor agonists was reported, related to patients having received at least 2 prescriptions [21]. Another US claims analysis found that, after adjusting for confounding factors, patients receiving OD liraglutide were 11% more adherent than patients receiving BID exenatide; mean MPRs were 69.7% OD GLP-1 and 64.4% for BID GLP-1 [22]. A third US claims data analysis compared adherence between BID, OD and once-weekly therapy with GLP-1 receptor agonists. It reported a higher unadjusted MPR for once-weekly therapy (78.3%), compared to BID (50.0%) or OD therapy (68.3–76.1% depending on dosage) [23]. The only known European-based study which analyzed adherence/persistence to therapy with GLP-1 receptor agonists reported a 12-month therapy discontinuation rate of 32.2% to BID treatment [27]. So, generally, our numbers indicate a slightly higher persistence/adherence than shown in previous studies. We confirm the conclusion of earlier studies that a BID treatment with GLP-1 receptor agonists is discontinued sooner than an OD treatment with GLP-1 receptor agonists. Moreover, our high numbers of patients (5.0% of all observed patients in the UK and 12.3% of all observed German patients) not having received a second GLP-1 prescription indicate that there seems to be an initial “trial period” in treatment with GLP-1 receptor agonists, which may be due to the common gastrointestinal adverse events of these drugs [28].
A specific characteristic of our study is that it differentiated between treatment adherence and treatment persistence. We think that persistence and adherence to treatment are different real-world phenomena which may be caused by different factors and which may lead to different conclusions. However, in most of the publications known to the authors of this study, adherence and persistence are analyzed jointly so that reported percentage of NA patients still includes patients who discontinued their therapy during a pre-defined observational period. To allow comparison of our reported numbers with those in known publications, we both reported the overall MPR as well as specific persistence/adherence measures.
In terms of outcomes related to GLP-1 discontinuation, our analysis shows that delayed HbA1c decrease and early insulin initiation are probably associated with NP to therapy with GLP-1 receptor agonists. However, because insulin therapy is a recommended follow-up treatment after failure/discontinuation of therapy with GLP-1 receptor agonists, it was very difficult to assess the association between GLP-1 continuation and HbA1c decrease. Furthermore, we did not find any association between T2DM-related hospitalizations and discontinuation of therapy with GLP-1 receptor agonists which may be explained by the comparatively young age of GLP-1 starters in our samples, but could also be due to effective and safe follow-up treatments and a short observational period we had available.
Limitations
We acknowledge several limitations of our analysis. Due to the longitudinal limitations of our dataset at the time of these analyses, we could only observe a period of 12 months after start of therapy with GLP-1 receptor agonists.
We defined NP as treatment gap >90 days and NA as MPR <80%. While these thresholds are widely used in adherence/persistence literature; they have hardly been clinically validated so far [15]. We dealt with this weakness by reporting results of a sensitivity analysis using a >180 days gap as NP definition and using different MPR-thresholds of 70%, 80%, and 90%.
In our multivariable analyses, we could not include all variables of interest. Patient characteristics not associated with specific diagnoses and/or prescription patterns like smoking/non-smoking behavior, specific GFR values [29] or level of physical activity [30] were not available.
In our analysis of HbA1c progression over time and T2DM-related hospitalizations, we could not include all patients because of data limitations. Moreover, time to insulin initiation may be a biased clinical outcome in the German analysis because, based on the mentioned treatment recommendations in Germany, only patients with insulin resistance and a BMI >30 are recommended for GLP-1 use.
We concluded that OD treatment is associated with a higher persistence than BID treatment with GLP-1 receptor agonists. However, since exenatide is known to evoke adverse gastrointestinal symptoms more frequently than liraglutide, these persistence differences could also be due to the medicine instead of the intake frequency.
Finally, due to our large sample size, some independent variables may have exerted a statistical influence but, due to low hazards/odds ratios, not in a clinically meaningful way.
Conclusions and Implications for Practice
NP to treatment with GLP-1 receptor agonists in both UK and Germany seems to be comparable. Persistence to OD treatment with GLP-1 receptor agonists is higher than to BID treatment with GLP-1 receptor agonists across the UK and Germany. Discontinuation of treatment with GLP-1 receptor agonists may warrant that physicians closely follow patients on GLP-1s to ensure continuity of anti-diabetic treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Glaxo Smith Kline, London, UK. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Thomas Wilke has received honoraria from several pharmaceutical/consultancy companies (Novo Nordisk, GSK, BMS, LEO Pharma, Astra Zeneca, Bayer, Boehringer Ingelheim, Pharmerit). Sabrina Mueller participated in this study as a staff member of IPAM; IPAM work in this study was sponsored by GSK. Antje Groth participated in this study as a staff member of IPAM; IPAM work in this study was sponsored by GSK. Bjoern Berg participated in this study as a staff member of IPAM; IPAM work in this study was sponsored by GSK. Mirko Sikirica is an employee of GlaxoSmithKline. John Logie is an employee of GlaxoSmithKline. Alan Martin is an employee of GlaxoSmithKline. Ulf Maywald is an employee of AOK PLUS. Andreas Fuchs is an employee of AOK PLUS.
Compliance with ethics guidelines
Due to the non-interventional, retrospective nature of the present study and the analysis of an anonymized dataset, no ethical review of this study was necessary. However, the study was evaluated by a scientific steering committee to which all the authors belong as well as by internal scientific committees belonging to the data owners, the AOK PLUS and CPRD (CPRD Protocol Approval Number: 14_022). This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2011;8(4):228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation Guideline Development Group Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104(1):1–52. doi: 10.1016/j.diabres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). Nationale VersorgungsLeitlinie Therapie des Typ-2-Diabetes—Langfassung, 1. Auflage. Version 4. 2013, zuletzt geändert: November 2014. Available from http://www.dm-therapie.versorgungsleitlinien.de.
- 4.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 5.Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19–28. doi: 10.1177/2042018814559725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34(Suppl 2):S279–S284. doi: 10.2337/dc11-s231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papaetis GS. Incretin-based therapies in prediabetes: current evidence and future perspectives. World J Diabetes. 2014;5(6):817–834. doi: 10.4239/wjd.v5.i6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Pablos-Velasco P, Parhofer KG, Bradley C, et al. Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: data from the PANORAMA study. Clin Endocrinol (Oxf). 2014;80(1):47–56. doi: 10.1111/cen.12119. [DOI] [PubMed] [Google Scholar]
- 9.Slabaugh SL, Xu Y, Stacy JN et al. Antidiabetic treatment patterns in a medicare advantage population in the United States. Drugs Aging. 2015. [Epub ahead of print]. [DOI] [PubMed]
- 10.Miller BR, Nguyen H, Hu CJ, Lin C, Nguyen QT. New and emerging drugs and targets for type 2 diabetes: reviewing the evidence. Am Health Drug Benefits. 2014;7(8):452–463. [PMC free article] [PubMed] [Google Scholar]
- 11.Krass I, Schieback P, Dhippayom T. Adherence to diabetes medication: a systematic review. Diabet Med. 2014. [Epub ahead of print]. [DOI] [PubMed]
- 12.Kirkman MS, Rowan-Martin MT, Levin R, et al. Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes Care. 2015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 13.Farr AM, Sheehan JJ, Curkendall SM, Smith DM, Johnston SS, Kalsekar I. Retrospective analysis of long-term adherence to and persistence with DPP-4 inhibitors in US adults with type 2 diabetes mellitus. Adv Ther. 2014;31(12):1287–1305. doi: 10.1007/s12325-014-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cramer JA, Benedict A, Muszbek N, Keskinaslan A, Khan ZM. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62(1):76–87. doi: 10.1111/j.1742-1241.2007.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimes RT, Bennett K, Tilson L, Usher C, Smith SM, Henman MC. Initial therapy, persistence and regimen change in a cohort of newly treated type 2 diabetes patients. Br J Clin Pharmacol. 2014. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 16.Wilke T, Groth A, Mueller S, et al. How to use pharmacy claims data to measure patient nonadherence? The example of oral diabetics in therapy of type 2 diabetes mellitus. Eur J Health Econ. 2013;14(3):551–568. doi: 10.1007/s10198-012-0410-y. [DOI] [PubMed] [Google Scholar]
- 17.Dirmaier J, Watzke B, Koch U, et al. Diabetes in primary care: prospective associations between depression, nonadherence and glycemic control. Psychother Psychosom. 2010;79(3):172–178. doi: 10.1159/000296135. [DOI] [PubMed] [Google Scholar]
- 18.Doggrell SA, Warot S. The association between the measurement of adherence to anti-diabetes medicine and the HbA1c. Int J Clin Pharm. 2014;36(3):488–497. doi: 10.1007/s11096-014-9929-6. [DOI] [PubMed] [Google Scholar]
- 19.An J, Nichol MB. Multiple medication adherence and its effect on clinical outcomes among patients with comorbid type 2 diabetes and hypertension. Med Care. 2013;51(10):879–887. doi: 10.1097/MLR.0b013e31829fa8ed. [DOI] [PubMed] [Google Scholar]
- 20.Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther. 2011;33(1):74–109. doi: 10.1016/j.clinthera.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Fabunmi R, Nielsen LL, Quimbo R, et al. Patient characteristics, drug adherence patterns, and hypoglycemia costs for patients with type 2 diabetes mellitus newly initiated on exenatide or insulin glargine. Curr Med Res Opin. 2009;25(3):777–786. doi: 10.1185/03007990802715199. [DOI] [PubMed] [Google Scholar]
- 22.Malmenäs M, Bouchard JR, Langer J. Retrospective real-world adherence in patients with type 2 diabetes initiating once-daily liraglutide 1.8 mg or twice-daily exenatide 10 μg. Clin Ther. 2013;35(6):795–807. doi: 10.1016/j.clinthera.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Johnston SS, Nguyen H, Felber E, et al. Retrospective study of adherence to glucagon-like peptide-1 receptor agonist therapy in patients with type 2 diabetes mellitus in the United States. Adv Ther. 2014;31(11):1119–1133. doi: 10.1007/s12325-014-0166-0. [DOI] [PubMed] [Google Scholar]
- 24.Ostenson CG, Matthaei S, Reaney M, et al. Treatment outcomes after initiation of exenatide twice daily or insulin in clinical practice: 12-month results from CHOICE in six European countries. Diabetes Metab Syndr Obes. 2014;6:171–185. doi: 10.2147/DMSO.S41827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Collaborating Centre for Drug Statistics Methodology. (2013) Guidelines for ATC classification and DDD assignment. Oslo: 2014.
- 26.BAnz. Bekanntmachung eines Beschlusses des Gemeinsamen Bundesausschusses über eine Änderung der Arzneimittel-Richtlinie in Anlage 4: Therapiehinweis zu Exenatide. 2008. Available from https://www.g-ba.de/downloads/39-261-736/2008-10-16-AMR4-Exenatide_BAnz.pdf.
- 27.Ostenson CG, Matthaei S, Reaney M, et al. Treatment outcomes after initiation of exenatide twice daily or insulin in clinical practice: 12-month results from CHOICE in six European countries. Diabetes Metab Syndr Obes. 2013;6:171–185. doi: 10.2147/DMSO.S41827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lund A, Knop FK, Visboll T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: differences and similarities. Eur J Intern Med. 2014;25(5):407–414. doi: 10.1016/j.ejim.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Fabbian F, De Giorgi A, Monesi M, et al. All-cause mortality and estimated renal function in type 2 diabetes mellitus outpatients: Is there a relationship with the equation used? Diab Vasc Dis Res. 2014. [Epub ahead of print]. [DOI] [PubMed]
- 30.Zethelius B, Gudbjörnsdottir S, Eliasson B, Eeg-Olofsson K, Cederholm J. Level of physical activity associated with risk of cardiovascular diseases and mortality in patients with type-2 diabetes: report from the Swedish National Diabetes Register. Eur J Prev Cardiol. 2014;21(2):244–251. doi: 10.1177/2047487313510893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.