Abstract
Introduction
Hyperkalemia risk is increased in diabetes, particularly in patients with renal impairment or those receiving angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs) or potassium-sparing diuretics. Conversely, other diuretics can increase hypokalemia risk. We assessed the effects of the sodium glucose co-transporter 2 (SGLT2) inhibitor dapagliflozin on serum potassium levels in a pooled analysis of clinical trials in patients with type 2 diabetes mellitus (T2DM).
Methods
Fourteen randomized, placebo-controlled, double-blind T2DM studies were analyzed: pooled data from 13 studies of ≤24 weeks’ duration (dapagliflozin 10 mg, N = 2360; placebo, N = 2295); and one 52-week moderate renal impairment study in patients with baseline eGFR ≥30 to <60 mL/min/1.73 m2 (dapagliflozin 10 mg, N = 85; placebo, N = 84). Central laboratory serum potassium levels were determined at each study visit.
Results
No clinically relevant mean changes from baseline in serum potassium ≤24 weeks were reported for dapagliflozin 10 mg [−0.05 mmol/L; 95% confidence interval (CI) −0.07, −0.03] versus placebo (−0.02 mmol/L; 95% CI −0.04, 0.00) in the pooled population or in the renal impairment study (−0.03 mmol/L; 95% CI −0.14, 0.08 vs. −0.02 mmol/L; 95% CI −0.13, 0.09, respectively). The incidence rate ratio for serum potassium ≥5.5 mmol/L over 24 weeks for dapagliflozin 10 mg versus placebo was 0.90 (95% CI 0.74, 1.10) in the pooled population; with no increased risk in patients receiving ARBs/ACE inhibitors, or potassium-sparing diuretics, or in those with moderate renal impairment. Slightly more patients receiving dapagliflozin 10 mg had serum potassium ≤3.5 mmol/L versus placebo (5.2% vs. 3.6%); however, no instances of serum potassium ≤2.5 mmol/L were reported.
Conclusion
Dapagliflozin is not associated with an increased risk of hyperkalemia or severe hypokalemia in patients with T2DM.
Funding
Bristol-Myers Squibb and AstraZeneca.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-015-0150-y) contains supplementary material, which is available to authorized users.
Keywords: Dapagliflozin, Hyperkalemia, Hypokalemia, Potassium, Sodium glucose co-transporter 2 inhibitor, Type 2 diabetes mellitus
Introduction
Hyperkalemia, defined as a serum potassium level of greater than 5.5 mmol/L [1], is a potentially life-threatening condition that causes increased cardiac depolarization and can lead to rapid electrocardiographic changes and an increased risk of arrhythmias [2]. Conversely, hypokalemia is defined as a serum potassium level of less than 3.5 mmol/L (mild) [3] or less than 2.5 mmol/L (severe) [4] and can lead to an increase in the risk of cardiac arrhythmias [3]. In addition to the risk associated with serum potassium outside the normal range (i.e., below or above normal levels), maintaining stable serum potassium values within physiological levels is important, as high serum potassium fluctuations are associated with increased all-cause and cardiovascular mortality [5].
Patients with diabetes are at increased risk of hyperkalemia [6, 7] and diabetes is an independent risk factor for hyperkalemia [6, 8, 9]. In an outpatient population of patients with diabetes, the prevalence of hyperkalemia has been estimated at 4% [6], whereas in a separate investigation in the general population, it has been estimated to be lower (0.3%) [10]. Additionally, diabetes is associated with a high incidence of renal impairment, which in turn is an independent risk factor for hyperkalemia [9].
Antihypertensive medications, which are frequently prescribed to these patients, can also increase serum potassium [11, 12]. For example, medications that reduce renal potassium excretion by inhibiting the renin-angiotensin-aldosterone system [such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs)], and drugs that inhibit the epithelial sodium channel (known as potassium-sparing diuretics) are associated with a greater hyperkalemia risk [13, 14]. The combination of an ACE inhibitor or ARB and a potassium-sparing diuretic is likely to further increase potassium levels and has been shown to precipitate life-threatening hyperkalemia in patients with diabetes [15–17]. Conversely, other diuretics such as thiazides or loop diuretics are generally associated with an increased risk of hypokalemia [18].
Older patients with diabetes have an increased susceptibility to hyperkalemia due to a number of factors, including an age-related decline in renal function and pharmaceutical side effects, such as those associated with ACE inhibitor or ARB treatment [19].
Sodium glucose co-transporter 2 (SGLT2) inhibitors are a class of glucose-lowering drugs that improve glycemic control, produce favorable effects on body weight and blood pressure, and have a low associated risk of hypoglycemia [20]. Given the renal site of action of SGLT2 inhibitors and the mild diuresis associated with their mode of action [21], there is potential concern about their effects on electrolyte balance, including potassium. Indeed, the SGLT2 inhibitor canagliflozin is associated with an increased risk of hyperkalemia [22].
Dapagliflozin is a highly selective orally available SGLT2 inhibitor for the treatment of type 2 diabetes mellitus (T2DM) [23]. Phase IIb and III clinical studies have demonstrated that dapagliflozin has a favorable safety profile and is well tolerated as monotherapy [24] or in combination with other glucose-lowering agents [25–29]. Here we describe the results of a pooled analysis of dapagliflozin clinical trials in patients with T2DM that aimed to determine whether or not dapagliflozin 10 mg affected the risk of potassium imbalance.
Methods
Patient Population
The analyzed population comprised short-term pooled data from 13 placebo-controlled, double-blind, Phase IIb and III studies of up to 24 weeks’ duration, in which patients with T2DM were treated with dapagliflozin 10 mg (N = 2360) or placebo (N = 2295; Table S1 in the supplementary material). The analysis consisted of three Phase IIb studies with durations of 12 weeks, and 10 Phase III studies with durations of 24 weeks. All patients had a baseline estimated glomerular filtration rate (eGFR) of ≥30 mL/min/1.73 m2, with the exception of 1 patient in each treatment group.
Data from a Phase II/III 52-week dedicated renal impairment study [30], in which all patients had an eGFR at baseline of ≥30 to <60 mL/min/1.73 m2, were analyzed separately [dapagliflozin 10 mg (N = 85); placebo (N = 84)].
This article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Outcomes Measures
Serum potassium levels were determined for each patient by central laboratory measurements at every study visit (at baseline, week 1 and week 4, then every 4 weeks up to week 24). Mean change in serum potassium levels from baseline were determined for dapagliflozin 10 mg versus placebo. This outcome was assessed in the pooled population (overall and in patients treated with ACE inhibitors or ARBs) up to week 24, and in the moderate renal impairment study over 52 weeks [30].
The incidence of marked abnormalities of serum potassium ≥5.5 mmol/L and ≥6.0 mmol/L were determined for dapagliflozin 10 mg compared with placebo. These cut-off points were selected as hyperkalemia is generally defined as a serum potassium level at or above the upper limit of normal (≥5.5 mmol/L) [1], whereas moderate hyperkalemia is generally defined as a serum potassium level of ≥6.0–6.9 mmol/L [31].
Marked abnormalities of serum potassium reported as adverse events (AEs) or serious AEs (SAE; defined as those events meeting the International Conference on Harmonisation/Good Clinical Practice criteria for an SAE [32]) were assessed for dapagliflozin 10 mg versus placebo for the overall pooled population. The incidence of mild and severe hypokalemia (serum potassium ≤3.5 and ≤2.5 mmol/L, respectively) was also determined for dapagliflozin 10 mg versus placebo for the overall pooled population.
Analysis Methods
Presented mean changes from baseline and associated 95% confidence intervals (CI), which were calculated using the t-distribution method, were conducted with SAS/STAT® (SAS Institute Inc. Cary, NC, USA). For the analysis of the incidence of marked abnormalities of serum potassium, incidence rate ratios (IRR) and associated 95% CIs, which were stratified by study, were calculated post hoc using exact methods with StatXact® (Cytel, Cambridge, MA, USA). The IRR was defined as the rate of the event in the dapagliflozin group divided by the rate in the placebo group. Thus, an IRR upper 95% CI of <1 (1 being the null value) would indicate that dapagliflozin was associated with a lower incidence of marked abnormalities of serum potassium than placebo. Conversely, an IRR lower 95% CI of >1 would indicate that dapagliflozin was associated with a higher incidence of marked abnormalities of serum potassium than placebo. No statistical hypothesis testing was conducted and no p values were calculated. All analyses were performed using all available data regardless of the use of rescue therapy. Mean eGFR was determined using the Modification of Diet in Renal Disease formula [33].
Results
Patients
Mean baseline demographic characteristics were well balanced between the dapagliflozin and placebo groups in the pooled population (Table 1). A high proportion of patients received an ACE inhibitor or ARB (65.9% in the dapagliflozin group and 68.2% in the placebo group) whereas only a small proportion of patients were treated with a potassium-sparing diuretic (5.0% with dapagliflozin vs. 5.6% with placebo). The majority of patients treated with a potassium-sparing diuretic also received an ACE inhibitor or ARB (82.4% with dapagliflozin and 80.5% with placebo).
Table 1.
Baseline demographic and disease characteristics
| Characteristics | Placebo-controlled poola | Moderate renal impairment study [30] | ||
|---|---|---|---|---|
| Dapagliflozin 10 mg (N = 2360) | Placebo (N = 2295) | Dapagliflozin 10 mg (N = 85) | Placebo (N = 84) | |
| Mean age, years (SD) | 58.4 (10.02) | 58.9 (9.96) | 68 (7.7) | 67 (8.6) |
| Age, n (%) | ||||
| <65 years | 1695 (71.8) | 1584 (69.0) | 29 (34.1) | 36 (42.9) |
| ≥65 years | 665 (28.2) | 711 (31.0) | 56 (65.9) | 48 (57.1) |
| ≥75 years | 98 (4.2) | 81 (3.5) | 16 (18.8) | 19 (22.6) |
| Female, n (%) | 1003 (42.5) | 952 (41.5) | 29 (34.1) | 31 (36.9) |
| Race, n (%) | ||||
| White | 1976 (83.7) | 1930 (84.1) | 77 (90.6) | 69 (82.1) |
| Black | 81 (3.4) | 73 (3.2) | 4 (4.7) | 1 (1.2) |
| Asian | 209 (8.9) | 206 (9.0) | 3 (3.5) | 6 (7.1) |
| Other | 94 (4.0) | 86 (3.7) | 1 (1.2) | 8 (9.5) |
| Region, n (%) | ||||
| North America | 769 (32.6) | 705 (30.7) | 48 (56.5) | 41 (48.8) |
| Latin America | 423 (17.9) | 407 (17.7) | 17 (20.0) | 23 (27.4) |
| Europe | 952 (40.3) | 976 (42.5) | 9 (10.6) | 11 (13.1) |
| Asia/Pacific | 216 (9.2) | 207 (9.0) | 11 (12.9) | 9 (10.7) |
| Mean HbA1c, % (SD) | 8.18 (0.94) | 8.17 (0.94) | 8.22 (0.98) | 8.53 (1.28) |
| Mean FPG, mg/dL (SD) | 164.8 (46.6) | 165.4 (45.3) | 164 (66) | 149 (48) |
| BMI, n (%) | ||||
| ≥25 kg/m2 | 2187 (92.7) | 2086 (90.9) | 80 (94.1) | 75 (89.3) |
| ≥30 kg/m2 | 1478 (62.6) | 1410 (61.4) | 54 (63.5) | 50 (59.5) |
| T2DM duration, years (SD) | 8.9 (8.0) | 8.8 (8.0) | 18.2 (10.1) | 15.7 (9.5) |
| Mean systolic BP, mmHg (SD) | 131.7 (15.3) | 131.6 (14.9) | 133.7 (17.0) | 130.7 (14.1) |
| Systolic BP ≥130 mmHg, n (%) | 1273 (53.9) | 1227 (53.5) | 52 (61.2) | 46 (54.8) |
| eGFR ≥30 to <60 L/min/1.73 m2, n (%) | 265 (11.2) | 268 (11.7) | 80 (94.1) | 75 (89.3) |
| Antihypertensive medication, n (%) | ||||
| ACE inhibitor or ARB | 1555 (65.9) | 1566 (68.2) | 71 (83.5) | 73 (86.9) |
| Potassium-sparing diuretic | 119 (5.0) | 128 (5.6) | 6 (7.1) | 6 (7.1) |
| Loop diuretics | 202 (8.6) | 209 (9.1) | 26 (30.6) | 26 (31.0) |
| Thiazide diuretics | 443 (18.8) | 434 (18.9) | 34 (40.0) | 27 (32.1) |
ACE angiotensin-converting enzyme, ARB angiotensin receptor blocker, BMI body mass index, BP blood pressure, eGFR estimated glomerular filtration rate, FPG fasting plasma glucose, HbA1c glycated hemoglobin, SD standard deviation, T2DM type 2 diabetes mellitus
aPooled data from 13 studies of up to 24 weeks in duration
Patients in the dedicated moderate renal impairment study [30] were older than the pooled population (mean age: dapagliflozin 10 mg, 68 years and placebo, 67 years; vs. 58.4 and 58.9 years in the pooled population, respectively) and had a longer duration of T2DM (mean duration: dapagliflozin 10 mg, 18.2 years and placebo, 15.7; vs. 8.9 and 8.8 years in the pooled population, respectively; Table 1).
Mean Change in Potassium from Baseline up to Week 24
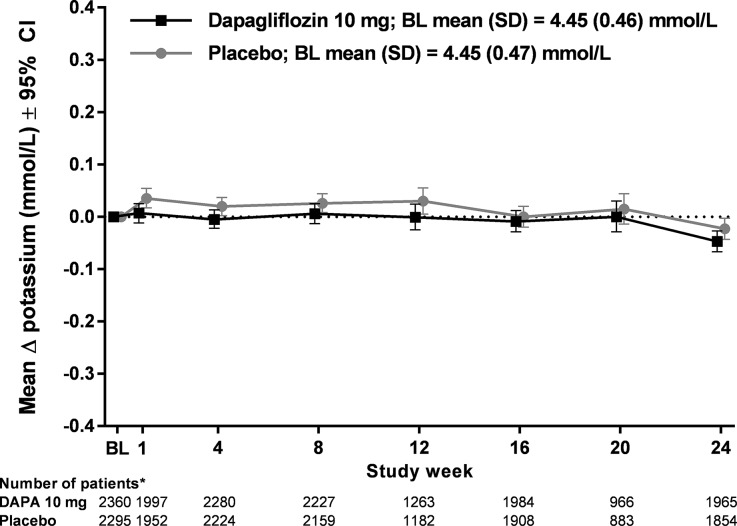
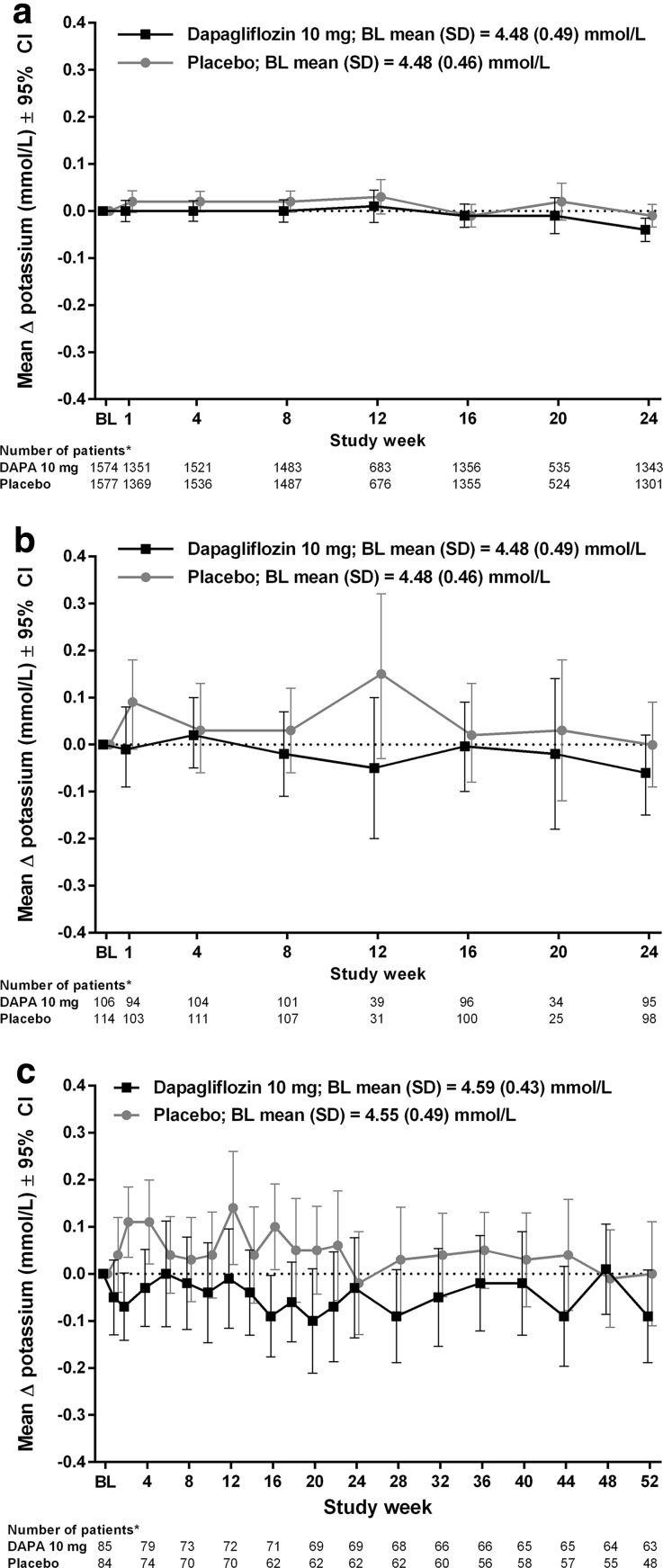
No clinically relevant mean change from baseline in serum potassium was noted up to 24 weeks in the pooled population: −0.05 mmol/L (95% CI −0.07, −0.03) in the dapagliflozin 10 mg group compared with −0.02 mmol/L (95% CI −0.04, 0.00) in the placebo group (Fig. 1). Similarly, no clinically relevant mean changes from baseline in serum potassium over 24 weeks were noted with dapagliflozin versus placebo in the pooled population of patients treated with ACE inhibitors or ARBs [−0.04 mmol/L (95% CI −0.07, −0.02) vs. −0.01 mmol/L (95% CI −0.03, 0.01), respectively, at week 24; Fig. 2a] or potassium-sparing diuretics [−0.06 mmol/L (95% CI −0.15, 0.02), vs. 0.00 mmol/L (95% CI: −0.09, 0.09), respectively, at week 24; Fig. 2b]. There were no clinically relevant mean changes from baseline in serum potassium in patients with moderate renal impairment [30] at 24 weeks [−0.03 mmol/L (95% CI −0.14, 0.08) vs. −0.02 mmol/L (95% CI −0.13, 0.09) for dapagliflozin 10 mg and placebo groups, respectively] or week 52 [−0.09 mmol/L (95% CI −0.19, 0.01) vs. 0.00 mmol/L (95% CI −0.11, 0.11), respectively; Fig. 2c].
Fig. 1.
Mean change from baseline in serum potassium up to 24 weeks for pooled population. Asterisks number of patients for each visit is the number of treated patients with non-missing values at baseline and at that study visit. Data points are shifted horizontally to prevent overlap of CI bars. BL baseline, CI confidence interval, DAPA dapagliflozin, SD standard deviation
Fig. 2.
Mean change from baseline in serum potassium levels in patients at increased risk of hyperkalemia. Patients receiving a ACE inhibitors or ARBs and b potassium-sparing diuretics in the pooled population (study duration ≤24 weeks); c patients with moderate renal impairment [30] (study duration 52 weeks). Asterisks number of treated patients with non-missing values at baseline and at that study week. ACE angiotensin-converting enzyme, ARBs angiotensin receptor blockers, BL baseline, CI confidence interval, DAPA dapagliflozin, SD standard deviation
Incidence of Marked Abnormalities of Serum Potassium ≥5.5 and ≥6.0 mmol/L
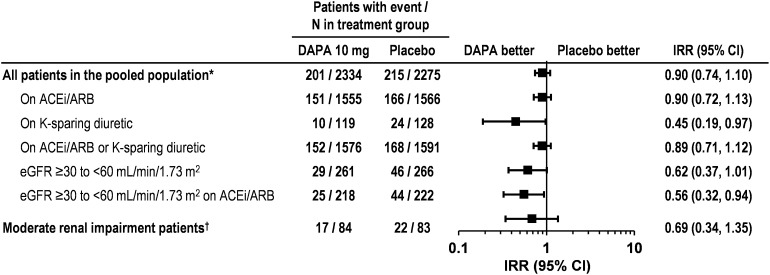
The incidence of serum potassium reported at or above the upper limit of normal (5.5 mmol/L) up to 24 weeks was similar for dapagliflozin 10 mg and placebo overall in the pooled population of patients [8.6% vs. 9.5%, respectively; IRR 0.90 (95% CI 0.74, 1.10)], and for subgroups of patients in the pooled population on ACE inhibitors or ARBs, or with low eGFR (Fig. 3). The IRR upper 95% CI for serum potassium ≥5.5 mmol/L up to 24 weeks was below 1 for dapagliflozin 10 mg versus placebo for patients on potassium-sparing diuretics and for patients with an eGFR of ≥30 to <60 mL/min/1.73 m2 on ACE inhibitors or ARBs (Fig. 3). In the 52 week moderate renal impairment study [30], the incidence of serum potassium ≥5.5 mmol/L was 20.2% with dapagliflozin 10 mg and 26.5% with placebo [IRR 0.69 (95% CI 0.34, 1.35); Fig. 3], with fewer patients treated with dapagliflozin discontinuing due to hyperkalemia (4.7% vs. 9.5%, respectively).
Fig. 3.
Incidence rate ratio of marked abnormalities of serum potassium ≥5.5 mmol/L. Asterisks short-term pooled data up to 24 weeks; dagger moderate renal impairment study (52 weeks) [30]. ACEi angiotensin-converting enzyme inhibitors, ARBs angiotensin receptor blockers, CI confidence interval, DAPA dapagliflozin, eGFR estimated glomerular filtration rate, IRR incidence rate ratio
Likewise, when the higher serum potassium cut-off point of ≥6.0 mmol/L was used, the incidence of marked abnormalities with dapagliflozin 10 mg was similar to placebo for patients overall (3.0% vs. 2.7%, respectively), with an IRR of 1.08 (95% CI 0.76, 1.55). For all subgroups, IRR analysis of marked abnormalities of serum potassium ≥6.0 mmol/L had 95% CIs that included the null value of 1, suggesting that there were no differences between the dapagliflozin 10 mg and placebo groups [IRR (95% CI) by patient subgroup: ACE inhibitor/ARB, 1.16 (0.78, 1.74); ACE inhibitor/ARB or potassium-sparing diuretic, 1.16 (0.78, 1.75); eGFR ≥30 to <60 mL/min/1.73 m2, 0.60 (0.23, 1.51); eGFR ≥30 to <60 mL/min/1.73 m2 on ACE inhibitor/ARB, 0.54 (0.19, 1.40)]. Furthermore, there was no meaningful difference between dapagliflozin 10 mg and placebo in the incidence of marked abnormalities of serum potassium of ≥6.0 mmol/L in the moderate renal impairment study [30] (IRR 0.34 [95% CI 0.06, 1.41]).
Distribution of Marked Abnormalities of Serum Potassium ≥5.5 mmol/L
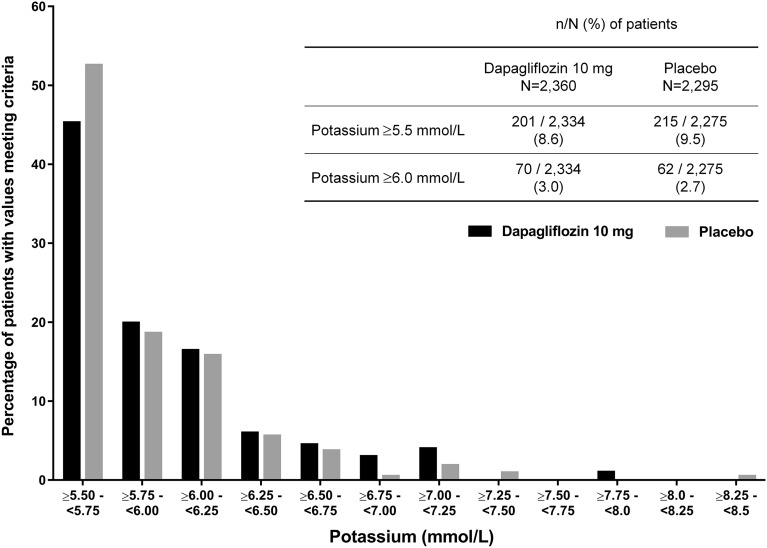
Marked abnormalities of serum potassium for patients in the dapagliflozin 10 mg and placebo groups were most commonly limited to the 5.50–5.75 mmol/L range [91 (45.3%) vs. 113 (52.6%), respectively; Fig. 4].
Fig. 4.
Distribution of marked abnormalities of serum potassium ≥5.5 mmol/L for short-term pooled data up to 24 weeks
Marked Abnormalities of Serum Potassium ≥5.5 mmol/L Reported as Adverse Events
Very few marked abnormalities of increased potassium were reported by the individual study investigators as clinical AEs: 7 (0.3%) in the dapagliflozin 10 mg group versus 0 in the placebo group. One SAE of hyperkalemia (serum potassium of 6.4 mmol/L on day 169 of treatment) was reported in the dapagliflozin 10 mg group. This patient had a history of hyperkalemia and hypertension and was treated with perindopril, which was stopped on day 175. His re-test potassium measurement was 6.1 mmol/L on day 176 and on day 178 this had decreased further to 4.5 mmol/L, at which point the SAE was considered resolved. Dapagliflozin dosing was not interrupted during this time.
All other AEs of hyperkalemia reported for patients in the dapagliflozin 10 mg group were reported as mild or moderate and had resolved by the next laboratory measurement (7 to 25 days later). No other patients had an AE that required treatment and no patients discontinued due to an AE of hyperkalemia.
Marked Abnormalities of Serum Potassium ≤2.5 and ≤3.5 mmol/L
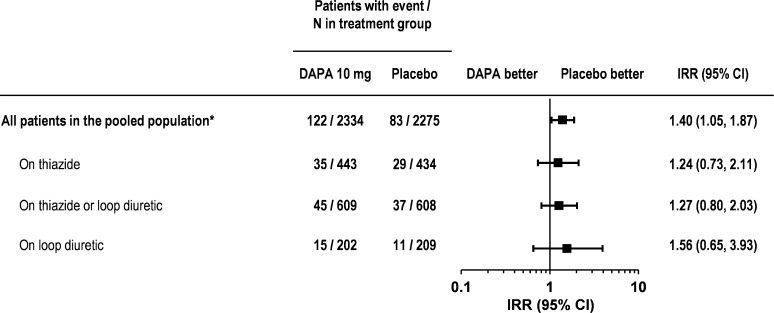
The proportion of patients with a serum potassium level of ≤3.5 mmol/L was slightly higher with dapagliflozin 10 mg than placebo in the overall pooled population [5.2% vs. 3.6%, respectively; IRR 1.40 (95% CI 1.05, 1.87); Fig. 5], and for subgroups of patients in the pooled population receiving thiazide diuretics [7.9% vs. 6.7%; IRR 1.24 (95% CI 0.73, 2.11)] or loop diuretics [7.4% vs. 5.3%; IRR 1.56 (95% CI 0.65, 3.93)].
Fig. 5.
Incidence rate ratio of marked abnormalities of serum potassium ≤3.5 mmol/L. Asterisks short-term pooled data up to 24 weeks. CI confidence interval, DAPA dapagliflozin, IRR incidence rate ratio
Few marked abnormalities of potassium ≤3.5 mmol/L were reported as AEs [9 (0.4%) vs. 4 (0.2%) with dapagliflozin 10 mg vs. placebo, respectively]. None of these AEs resulted in discontinuation and the majority were mild in intensity [8 (0.3%) with dapagliflozin 10 mg vs. 2 (0.1%) with placebo], with no severe events reported. In total, 6 (0.3%) patients in the dapagliflozin group and 1 (<0.1%) patient in the placebo group had an AE of hypokalemia that required intervention.
There were no reports of a serum potassium level of ≤2.5 mmol/L in the pooled population.
Discussion
The effect of dapagliflozin on serum potassium levels in over 4000 patients with T2DM across the dapagliflozin clinical trials program, and for a duration of up to 24 weeks was explored. No clinically relevant mean changes from baseline in serum potassium were noted in patients receiving dapagliflozin 10 mg or placebo, and few marked abnormalities of serum potassium were reported in either group; all cases resolved without treatment and did not lead to discontinuation. Furthermore, no clinically relevant changes from baseline in serum potassium and no differences in marked abnormalities in serum potassium were reported with dapagliflozin 10 mg compared with placebo in patients at higher risk of hyperkalemia, such as those with moderate renal impairment or those receiving treatment with ACE inhibitors, ARBs or potassium-sparing diuretics. While this analysis indicated that dapagliflozin did not increase hyperkalemia risk in patients with moderate renal impairment, dapagliflozin is not indicated for use in patients with moderate or low renal function (eGFR <60 mL/min/1.73 m2) [34]. The frequency of any hyperkalemia episode (i.e., serum potassium level ≥5.5 mmol/L) in the overall pooled population was approximately 9% for both the dapagliflozin 10 mg and placebo groups, whereas moderate or severe episodes of hyperkalemia (i.e., patients with a serum potassium level ≥6.0 mmol/L) occurred in 3% of patients in both groups. The majority of these episodes were asymptomatic and few AEs of potassium ≥5.5 mmol/L were reported (0.3% vs. 0.0% with dapagliflozin and placebo, respectively).
Episodes of mild hypokalemia (serum potassium ≤3.5 mmol/L) in the pooled population were slightly more frequent with dapagliflozin than with placebo. As expected, these episodes were more common in patients receiving loop diuretics or thiazide diuretics both in patients taking dapagliflozin and placebo, with no relevant difference between the groups. Few AEs of hypokalemia were reported in either group (0.4% with dapagliflozin vs. 0.2% with placebo), and did not lead to discontinuation. There were no reports of severe hypokalemia (serum potassium ≤2.5 mmol/L) in the pooled population.
A trend towards increased hypokalemia risk could be consistent with dapagliflozin’s mild diuretic effect [35]; however, a lack of any difference between the groups when used with typical diuretics is both reassuring and somewhat unexpected. Renal potassium excretion is a complex process that is primarily determined by the serum aldosterone concentration and driven by the sodium concentration in the distal nephron [31, 36]. Due to the co-transportation of sodium and glucose, transient increases in urinary sodium excretion are seen for a few days upon initiation of dapagliflozin, after which levels tend to normalize [23]. Thus, unlike typical diuretics, long-term treatment with dapagliflozin should not affect sodium delivery to the distal nephron. However, it should be acknowledged that in a previous small exploratory study [35], dapagliflozin treatment increased median plasma renin activity relative to placebo, to approximately a quarter of the hydrochlorothiazide 25 mg effect, and increased median serum aldosterone activity to a similar extent as hydrochlorothiazide. A potential trend for hypokalemia does not seem to be explained by dapagliflozin’s mechanism of action, which is not reported to promote kaliuresis [34], neither is it consistent across the SGLT2 inhibitor class, as the SGLT2 inhibitor canagliflozin has been associated with an increased risk of hyperkalemia. Hyperkalemia risk with canagliflozin was greater in patients with moderate renal impairment or receiving ACE inhibitors, ARBs or potassium-sparing diuretics [22], and was more pronounced with the canagliflozin 300 mg dose than the 100 mg dose [37].
Hyperkalemia risk is greater in patients with diabetes, which may be partly attributed to hyperosmolarity caused by hyperglycemia, resulting in a shift in potassium out of cells [38]. Impaired elimination of potassium can also increase the risk of hyperkalemia [31, 36], particularly in patients with reduced renal function or those treated with ACE inhibitors, ARBs or potassium-sparing diuretics [36].
Monitoring potassium levels is an important consideration in patients with diabetes, particularly as these patients often have hypertension and may be treated with ACE inhibitors, ARBs and/or potassium-sparing diuretics, making them more susceptible to hyperkalemia [13, 14]. Likewise, potassium monitoring may be important in patients with renal impairment [12], who are also considered to be at high risk of hyperkalemia [9]. Clinicians should balance the opposing risks of avoiding hyperkalemia and causing hypokalemia, or vice versa. For example, potassium-sparing diuretics have been shown to prevent diuretic-induced hypokalemia but are associated with an increased risk of hyperkalemia [39]. Furthermore, combinations of drugs with an associated risk of hyperkalemia (e.g., ACE inhibitor, ARBs, potassium-sparing diuretics [15–17]) or hypokalemia (e.g., thiazides or loop diuretics [18]) may rapidly precipitate serious serum electrolyte abnormalities. Therefore, knowledge of the presence or absence of an effect on potassium homeostasis with antidiabetic drugs is important. Previous studies indicate that dapagliflozin does not affect potassium levels when taken in combination with ACE inhibitors, ARBs, thiazides or loop diuretics [40–42].
A limitation of this analysis was the low number of patients in some of the subgroups (i.e., patients on potassium-sparing diuretics or with low eGFR), restricting the reliability of the assessment of risk for the AEs or marked abnormalities in these patients.
Conclusions
Dapagliflozin does not appear to increase serum potassium levels in patients with T2DM, including patients at a higher risk of hyperkalemia, such as those with moderate renal impairment or treated with ACE inhibitors, ARBs or potassium-sparing diuretics. Furthermore, although there were more episodes of hypokalemia overall in the dapagliflozin group, dapagliflozin was not associated with an increased risk of hypokalemia in any of the patient categories associated with increased hypokalemia risk.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by Bristol-Myers Squibb and AstraZeneca. The article processing charges for this publication were funded by AstraZeneca. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Editorial assistance in the preparation of this manuscript was provided by Helen Brereton and Julian Martins of inScience Communications, Springer Healthcare Ltd, London, UK. Support for this assistance was funded by AstraZeneca. The data in this manuscript were previously presented as a poster at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, 13–17 June 2014.
Disclosures
Yshai Yavin and Agata Ptaszynska are employees of Bristol-Myers Squibb. Traci A. Mansfield, Kristina Johnsson, Eva Johnsson, and Shamik Parikh are employees of AstraZeneca.
Compliance with Ethics Guidelines
This article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.Nyirenda MJ, Tang JI, Padfield PL, Seckl JR. Hyperkalaemia. BMJ. 2009;339:b4114. doi: 10.1136/bmj.b4114. [DOI] [PubMed] [Google Scholar]
- 2.Mattu A, Brady WJ, Robinson DA. Electrocardiographic manifestations of hyperkalemia. Am J Emerg Med. 2000;18:721–729. doi: 10.1053/ajem.2000.7344. [DOI] [PubMed] [Google Scholar]
- 3.Rastegar A, Soleimani M. Hypokalaemia and hyperkalaemia. Postgrad Med J. 2001;77:759–764. doi: 10.1136/pmj.77.914.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brophy DF. Disorders of potassium and magnesium homeostasis. In: DiPiro JT, Talbert RL, Yee GC, et al., editors. Pharmacotherapy: a pathophysiologic approach. 9th edn. New York: McGraw-Hill; 2014.
- 5.Xu Q, Xu F, Fan L, et al. Serum potassium levels and its variability in incident peritoneal dialysis patients: associations with mortality. PLoS One. 2014;9:e86750. doi: 10.1371/journal.pone.0086750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarman PR, Kehely AM, Mather HM. Hyperkalaemia in diabetes: prevalence and associations. Postgrad Med J. 1995;71:551–552. doi: 10.1136/pgmj.71.839.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med. 2015;373:548–559. doi: 10.1056/NEJMra1503102. [DOI] [PubMed] [Google Scholar]
- 8.Jarman PR, Mather HM. Diabetes may be independent risk factor for hyperkalaemia. BMJ. 2003;327:812. doi: 10.1136/bmj.327.7418.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramadan FH, Masoodi N, El-Solh AA. Clinical factors associated with hyperkalemia in patients with congestive heart failure. J Clin Pharm Ther. 2005;30:233–239. doi: 10.1111/j.1365-2710.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- 10.Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med. 2013;126:256–263. doi: 10.1016/j.amjmed.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 11.Perazella MA. Drug-induced hyperkalemia: old culprits and new offenders. Am J Med. 2000;109:307–314. doi: 10.1016/S0002-9343(00)00496-4. [DOI] [PubMed] [Google Scholar]
- 12.Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med. 2010;25:326–333. doi: 10.1007/s11606-009-1228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollander-Rodriguez JC, Calvert JF., Jr Hyperkalemia. Am Fam Physician. 2006;73:283–290. [PubMed] [Google Scholar]
- 14.Salem CB, Badreddine A, Fathallah N, Slim R, Hmouda H. Drug-induced hyperkalemia. Drug Saf. 2014;37:677–692. doi: 10.1007/s40264-014-0196-1. [DOI] [PubMed] [Google Scholar]
- 15.Burnakis TG, Mioduch HJ. Combined therapy with captopril and potassium supplementation. A potential for hyperkalemia. Arch Intern Med. 1984;144:2371–2372. doi: 10.1001/archinte.1984.00350220091020. [DOI] [PubMed] [Google Scholar]
- 16.Wrenger E, Muller R, Moesenthin M, Welte T, Frolich JC, Neumann KH. Interaction of spironolactone with ACE inhibitors or angiotensin receptor blockers: analysis of 44 cases. BMJ. 2003;327:147–149. doi: 10.1136/bmj.327.7407.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston RT, de Bono DP, Nyman CR. Preventable sudden death in patients receiving angiotensin converting enzyme inhibitors and loop/potassium sparing diuretic combinations. Int J Cardiol. 1992;34:213–215. doi: 10.1016/0167-5273(92)90159-Z. [DOI] [PubMed] [Google Scholar]
- 18.Knochel JP. Diuretic-induced hypokalemia. Am J Med. 1984;77:18–27. doi: 10.1016/S0002-9343(84)80004-2. [DOI] [PubMed] [Google Scholar]
- 19.Perazella MA, Mahnensmith RL. Hyperkalemia in the elderly: drugs exacerbate impaired potassium homeostasis. J Gen Intern Med. 1997;12:646–656. doi: 10.1046/j.1525-1497.1997.07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey CJ, Day C. SGLT2 inhibitors: glucuretic treatment for type 2 diabetes. Br J Diabetes Vasc Dis. 2010;10:193–199. doi: 10.1177/1474651410377832. [DOI] [Google Scholar]
- 21.Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502. doi: 10.1038/nrendo.2011.243. [DOI] [PubMed] [Google Scholar]
- 22.FDA. Invokana (canagliflozin): Highlights of prescribing information. 2014. https://www.invokanahcp.com/prescribing-information.pdf. Accessed October 20, 2015.
- 23.Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85:513–519. doi: 10.1038/clpt.2008.250. [DOI] [PubMed] [Google Scholar]
- 24.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217–2224. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223–2233. doi: 10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- 26.Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34:2015–2022. doi: 10.2337/dc11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473–1478. doi: 10.2337/dc11-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928–938. doi: 10.1111/j.1463-1326.2011.01434.x. [DOI] [PubMed] [Google Scholar]
- 29.Wilding JP, Woo V, Soler NG, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405–415. doi: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- 30.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–971. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandal AK. Hypokalemia and hyperkalemia. Med Clin N Am. 1997;81:611–639. doi: 10.1016/S0025-7125(05)70536-8. [DOI] [PubMed] [Google Scholar]
- 32.International Conference on Harmonisation. Guideline for good clinical practice. 1996. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed October 20, 2015.
- 33.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 34.FDA. Farxiga (dapagliflozin): Highlights of prescribing information. 2014. http://www1.astrazeneca-us.com/pi/pi_farxiga.pdf. Accessed October 20, 2015.
- 35.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–62. [DOI] [PMC free article] [PubMed]
- 36.Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351:585–592. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
- 37.FDA. Invokana (canagliflozin): Briefing Document. 2013. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM334550.pdf. Accessed October 20, 2015.
- 38.Lehnhardt A, Kemper MJ. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. 2011;26:377–384. doi: 10.1007/s00467-010-1699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Widmer P, Maibach R, Kunzi UP, et al. Diuretic-related hypokalaemia: the role of diuretics, potassium supplements, glucocorticoids and beta 2-adrenoceptor agonists. Results from the comprehensive hospital drug monitoring programme, berne (CHDM) Eur J Clin Pharmacol. 1995;49:31–36. doi: 10.1007/BF00192355. [DOI] [PubMed] [Google Scholar]
- 40.Cefalu WT, Leiter L, Johnsson E, Sugg J, Gause-Nilsson I. Safety and efficacy of dapagliflozin in patients with T2DM and cardiovascular disease receiving loop diuretics. Diabetes. 2015;64(Suppl 1):1216-P. [Google Scholar]
- 41.Weber MA, Mansfield TA, Alessi F, Iqbal N, Parikh S, Ptaszynska A. Dapagliflozin effects on blood pressure in hypertensive diabetic patients on renin-angiotensin system blockade. Blood Press. 2015. doi:10.3109/08037051.2015.1116258 [DOI] [PubMed]
- 42.Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh SJ, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, placebo-controlled study. Lancet Diabetes Endocrinol. 2015. doi:10.1016/S2213-8587(15)00417-9 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.