Abstract
Background and Objectives
The time-course when changes in glycemic control and body weight were first manifest in patients with type 2 diabetes mellitus (T2DM) treated with a combination of insulin degludec and liraglutide (IDegLira) was assessed, comparing IDegLira to its individual components.
Methods
Data from weeks 0–12 from two studies were analyzed, one comparing IDegLira to each component (DUAL I), and one comparing IDegLira to insulin degludec titrated to a maximum 50 units (DUAL II). Efficacy endpoints included glycated hemoglobin (HbA1c) and fasting plasma glucose (FPG) reduction, proportion of patients achieving HbA1c [<7.0 % (<53.0 mmol/mol)] and FPG (≤7.2 mmol/L) targets, and proportion achieving HbA1c target without hypoglycemia and without hypoglycemia and weight gain.
Results
Mean HbA1c was lower, and the proportion of patients reaching target HbA1c greater, with IDegLira versus comparators (both studies) at weeks 8 and 12. Proportions of patients reaching target HbA1c without hypoglycemia and without hypoglycemia and weight gain were higher for IDegLira versus insulin degludec, though not versus liraglutide. Mean FPG was lower with IDegLira, and the proportion achieving target FPG higher, versus components (both studies) from weeks 4–12. IDegLira was associated with mean weight reduction from weeks 4–12, although less than with liraglutide alone. Hypoglycemia occurred infrequently in weeks 0–12, with no difference in incidence between IDegLira and insulin degludec in either study.
Conclusions
IDegLira reduces plasma glucose to a greater extent than its components, measurable within the first 12 weeks of therapy, and without weight gain or an increased hypoglycemia risk versus insulin degludec.
Electronic supplementary material
The online version of this article (doi:10.1007/s40261-016-0376-0) contains supplementary material, which is available to authorized users.
Key Points
| This analysis examined data from the early weeks (4, 8, and 12) of the DUAL I and II studies in patients with type 2 diabetes, comparing the fixed-ratio combination product IDegLira to its monocomponents. |
| The results showed lower HbA1c and FPG values at all time points assessed with IDegLira compared with each of its monocomponents, as well as higher percentages of patients reaching the HbA1c target <7 % at weeks 8 and 12 and FPG target ≤7.2 mmol/L at weeks 4, 8, and 12. |
| IDegLira also compared favorably with insulin degludec for the composite endpoint of HbA1c <7 % without hypoglycemia ± weight gain at weeks 8 and 12. |
Introduction
Despite the well accepted benefits of good glycemic control, many patients with type 2 diabetes mellitus (T2DM) have plasma glucose (PG) levels chronically elevated above guideline recommendations because potentially beneficial changes are not made to their treatment regimens [1, 2]. One reason for this “clinical inertia” is anxiety on the part of patients and their clinicians that introduction of insulin therapy will be restrictive and result in hypoglycemic events and weight gain [3–5]. A recent survey of more than 4000 patients showed that even when insulin was introduced, more than 80 % of patients still had a glycated hemoglobin (HbA1c) ≥7.0 % (53 mmol/mol) 6 months later, with 75 % having had no intensification beyond dose titration of their original insulin therapy [1]. Therefore, both insulin-treated patients and those on oral antidiabetic drugs (OADs) are potentially left at increased risk of diabetic complications as a result of clinical inertia [1, 2], although it must be acknowledged that HbA1c targets >7.0 % are appropriate for some individual patients. The prevalence of poor control is perhaps surprising, however, given that patients’ treatment satisfaction is largely influenced by clinical outcomes, especially improvement in glycemic control [6, 7]. Clearly there is a need to address clinical inertia, and given that improving glycemia increases treatment satisfaction, it seems likely that therapies able to tolerably reduce PG without increasing the risk of hypoglycemia within short time intervals could benefit patient engagement with self-management.
In recent years, several new drug classes for T2DM have been introduced providing opportunities to study various combinations with different but complementary mechanisms of action. It is possible that a combination of different agents with different actions could accelerate and enhance the initial PG-lowering response. One such combination is that of basal insulin plus glucagon-like peptide-1 receptor agonist (GLP-1RA) since these agents have pharmacological actions that complement each other [8–10]. The speed with which glycemic control can be established with this combination has hitherto not been studied, but together these agents provide dynamic control of both fasting and postprandial glycemia with low risks of hypoglycemia and weight gain [11]. This is achieved together with, and partly as a consequence of, a minimization of the insulin dose requirement [9]. Many clinical studies have demonstrated such clinical benefits with this regimen [11–22], and the tolerability of this combination relative to intensified insulin therapy is especially appealing and may benefit treatment adherence. In this respect, the improved [15] or relative [22] treatment satisfaction/quality-of-life benefits reported with the incorporation of a GLP-1RA into a basal insulin-based regimen may be relevant.
Following the clinical use of basal insulin plus GLP-1RA therapy given in parallel, and as an approach to simplifying therapy with a goal of improving adherence and convenience, novel, fixed-ratio combination products containing basal insulin and a GLP-1RA are being developed. One such product (IDegLira) consists of insulin degludec and the GLP-1 analog liraglutide. Insulin degludec provides a flat and stable steady-state basal insulin profile [23], and effectively reduces fasting plasma glucose (FPG) levels [24], while liraglutide benefits both FPG and postprandial plasma glucose (PPG) levels [25]. The effects of liraglutide on beta-cell and alpha-cell function are strictly glucose dependent, hence when combined, liraglutide and insulin degludec have the potential to mitigate the risk of hypoglycemia that might otherwise limit intensive glycemic control with insulin therapy [26]. As a GLP-1 analog, liraglutide also reduces hunger, promoting weight loss, hence IDegLira also provides weight advantages when compared with basal insulin-based regimens [26, 27].
IDegLira is administered as dose steps given once daily, titrated in a similar way to basal insulin. One dose step contains a fixed combination of 1 unit of insulin degludec and 0.036 mg of liraglutide. The pen enables injections in increments of single dose steps (maximum dose: 50 units of degludec and 1.8 mg liraglutide). Two phase III trials (including an extension study) involving IDegLira have currently been completed and published [26–28] and a further five are soon to be published or are still on-going [29–33]. These studies have demonstrated the complementary effects of the components of IDegLira, also demonstrating its good efficacy and tolerability profile. However, data concerning the relative speed of glycemic improvement during IDegLira therapy have not been reported. Increased speed of improvement could potentially translate into enhanced perseverance/adherence with therapy, which could be particularly important with an injectable therapy. This post hoc analysis therefore sought to investigate, in patients with T2DM previously treated with OAD or basal insulin + OAD, the time-course over which differences in glycemic control as well as hypoglycemia risk and changes in body weight occurred. This analysis thus assessed the hypothesis that responses to IDegLira would be faster versus insulin degludec or liraglutide alone.
Methods
Data suitable for assessment had been collected at weeks 4, 8, and 12 in the DUal Action of Liraglutide and Insulin Degludec in Type 2 Diabetes (DUAL) I and II studies [26–28]. In these studies, all procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study. Patients included in DUAL I were 1663 adults (mean age 55 years) with a mean HbA1c of 8.3 % (inclusion criterion 7–10 %) and a mean body mass index (BMI) of 31.2 kg/m2 (inclusion criterion ≤40 kg/m2) who were previously treated with metformin with or without pioglitazone for at least 90 days before screening. Patients were randomized (2:1:1) to daily injections of IDegLira, insulin degludec, or liraglutide [26]. DUAL II included 413 adults (mean age 57–58 years) with a mean HbA1c of 8.7–8.8 % (inclusion criterion 7.5–10 % inclusive) and a mean BMI of 33.7 kg/m2 (inclusion criterion ≥27 kg/m2) who had prior treatment for at least 90 days with basal insulin at a stable dose (20–40 units/day) in combination with metformin with or without sulfonylurea or glinides. In DUAL II, patients were randomized to IDegLira or insulin degludec titrated to a maximum dose of 50 units [27]. In both studies, doses of IDegLira (and insulin degludec) were adjusted twice weekly during a telephone consultation with a study nurse and using a titration algorithm based on the mean self-measured pre-breakfast PG value from 3 consecutive days, aiming for a pre-breakfast glucose concentration target of 4.0–5.0 mmol/L.
Efficacy endpoints assessed in this analysis were change in HbA1c, change in FPG, and change in body weight over time. In addition, responder endpoints were assessed including proportion of patients achieving HbA1c <7.0 % (<53 mmol/mol), and proportion of patients achieving FPG ≤7.2 mmol/L, these values being in line with the current American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) treatment position statement [34]. Two composite responder endpoints were assessed: proportion of patients achieving HbA1c <7.0 % without confirmed hypoglycemia (details below), and proportion of patients achieving HbA1c <7.0 % without confirmed hypoglycemia and without weight gain. For the responder endpoints that included HbA1c <7.0 %, week 4 data were not included in the analysis due to this interval being relatively short compared with the time-scale over which HbA1c changes in response to mean PG exposure, and the timing of increments in dose steps.
Confirmed hypoglycemia rates over the first 12 weeks and the cumulative incidence at weeks 4, 8, and 12 were assessed to investigate the balance between early glycemic control and development of early hypoglycemia risk. Confirmed hypoglycemic episodes were defined as severe events requiring third-party assistance, or non-severe episodes dealt with by the patient and confirmed by a PG value <3.1 mmol/L (<56 mg/dl).
The responder endpoints were analyzed using logistic regression adjusting for treatment, region, and antidiabetic treatment at screening as fixed factors, and baseline values as covariates. Absolute values for HbA1c, FPG, and body weight change were analyzed using analysis of covariance, also adjusting for these fixed factors and baseline values as covariates, and hypoglycemia rates were analyzed using a negative binomial model adjusting for treatment, region, gender, and antidiabetic treatment at screening as fixed factors. DUAL I data were also adjusted for baseline HbA1c stratum and sub-study participation. In the logistic regression a logit-link was used. In the negative binomial model a log link was used and the log of exposure time as offset. Analyses were based on the full analysis set, using the last observation carried forward to impute missing data.
Results
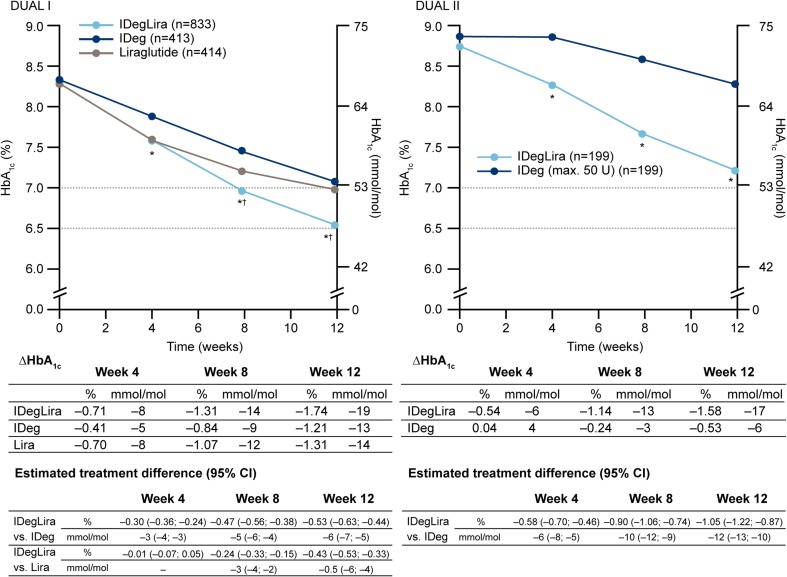
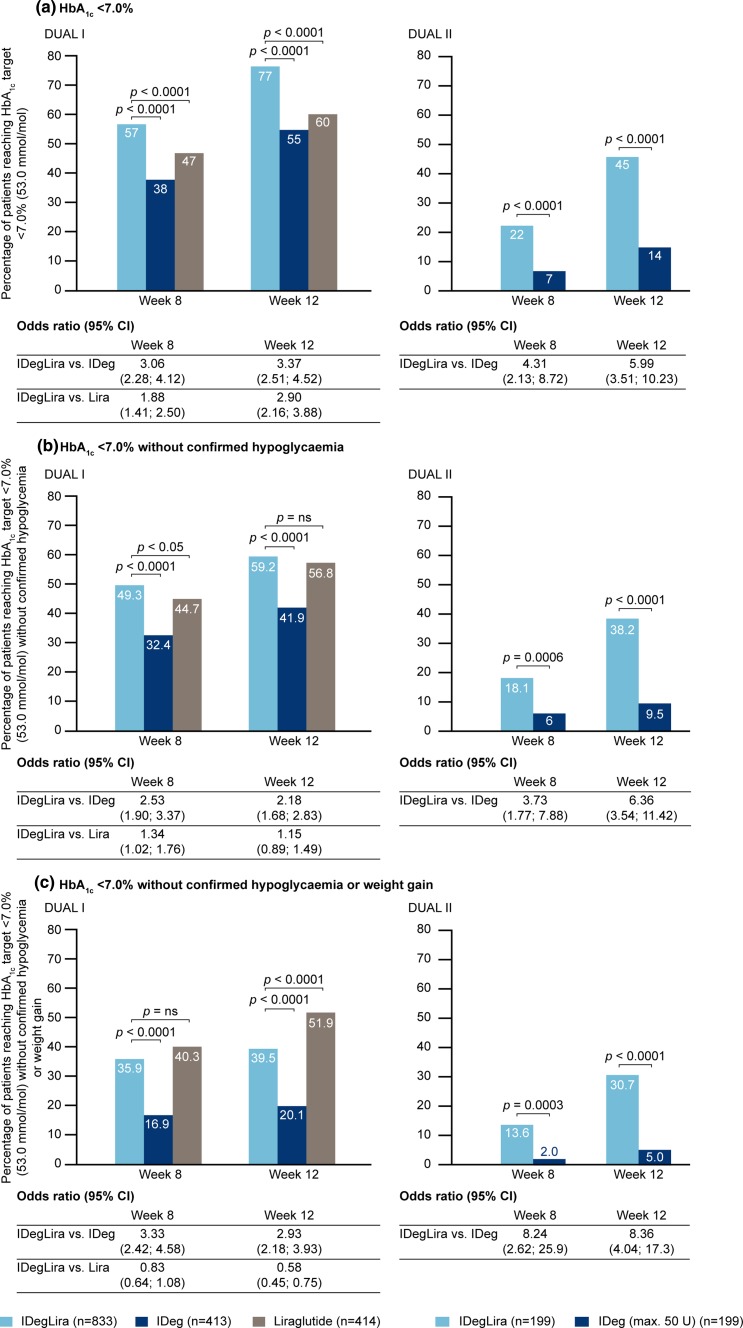
The patient baseline characteristics for the DUAL I and II studies are given in supplementary Table 1; within each study the comparator groups were well matched [26, 27]. Data concerning HbA1c are shown in Figs. 1 and 2. In both DUAL I and II, reduction in mean HbA1c values occurred faster in patients treated with IDegLira, such that significant differences were demonstrated at weeks 4, 8, and 12 versus insulin degludec (both studies) and at weeks 8 and 12 versus liraglutide (DUAL I). The proportion of patients achieving HbA1c <7.0 % at weeks 8 and 12 was significantly higher with IDegLira than comparators (Fig. 2). The proportions of patients achieving this target without hypoglycemia, and without hypoglycemia and weight gain were also higher with IDegLira than with insulin degludec in both studies at weeks 8 and 12. The proportion of patients achieving the target without hypoglycemia were similar for IDegLira and liraglutide at week 12 in DUAL I, but there was a significant difference favoring IDegLira at week 8 (Fig. 2b). The proportion of patients achieving the target without either hypoglycemia or weight gain was higher for liraglutide than IDegLira at week 12 (Fig. 2c).
Fig. 1.
Change in HbA1c in weeks 0–12 in the DUAL I and DUAL II studies. Data given in mmol/mol are calculated values. *p < 0.0001 vs. insulin degludec; † p < 0.0001 vs. liraglutide. CI confidence interval, HbA 1c glycated hemoglobin, IDeg insulin degludec, IDegLira insulin degludec + liraglutide fixed combination, Lira liraglutide
Fig. 2.
Proportion of patients achieving HbA1c and composite endpoint targets in weeks 8 and 12 in the DUAL I and DUAL II studies. Data given in mmol/mol are calculated values. CI confidence interval, HbA 1c glycated hemoglobin, IDeg insulin degludec, IDegLira insulin degludec + liraglutide fixed combination, Lira liraglutide
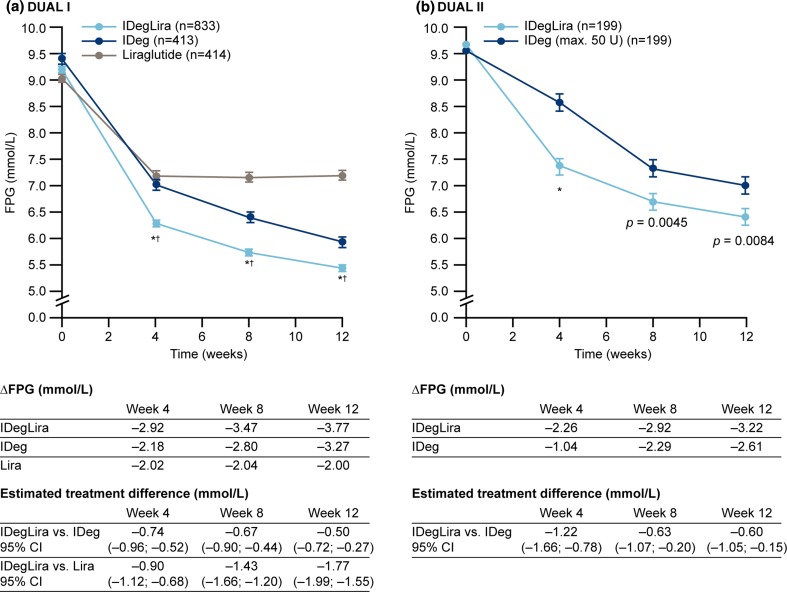
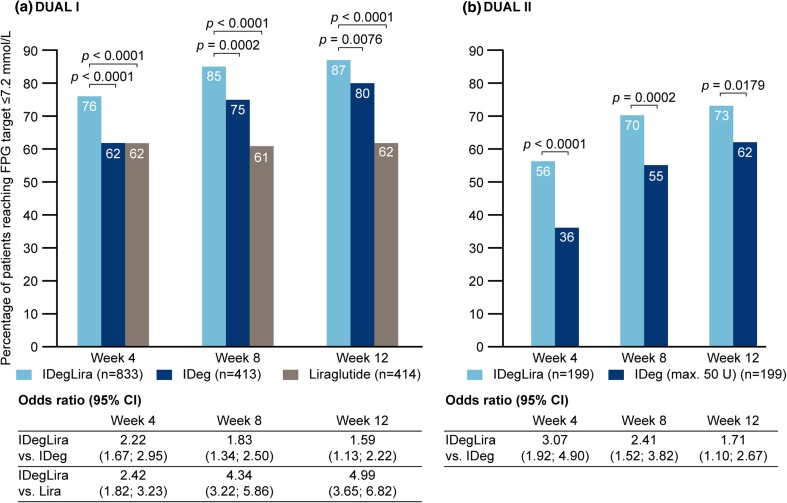
FPG data are shown in Figs. 3 and 4. In DUAL I, mean FPG was lower with IDegLira versus individual components at weeks 4, 8, and 12 (all p < 0.0001) (Fig. 3a). In DUAL II, mean FPG was also lower with IDegLira versus insulin degludec from week 4, continuing through weeks 8 and 12 (Fig. 3b). In DUAL I, the proportion of patients who reached FPG ≤7.2 mmol/L at week 4 was greater with IDegLira versus its individual components, and this was also observed at week 8 (Fig. 4a). In DUAL II, the proportions of patients reaching FPG ≤7.2 mmol/L at weeks 4 and 8 were higher with IDegLira than with insulin degludec, and a diminished but statistically significant between-treatment difference was still evident at week 12 (Fig. 4b).
Fig. 3.
Change in fasting plasma glucose in weeks 0–12 in the DUAL I and DUAL II studies. *p < 0.0001 vs. insulin degludec, † p < 0.0001 vs. liraglutide. CI confidence interval, FPG fasting plasma glucose, IDeg insulin degludec, Lira liraglutide, IDegLira insulin degludec + liraglutide fixed combination
Fig. 4.
Proportion of patients achieving fasting glucose ≤7.2 mmol/L in weeks 4, 8, and 12 in the DUAL I and DUAL II studies. CI confidence interval, FPG fasting plasma glucose, IDeg insulin degludec, Lira liraglutide, IDegLira insulin degludec + liraglutide fixed combination
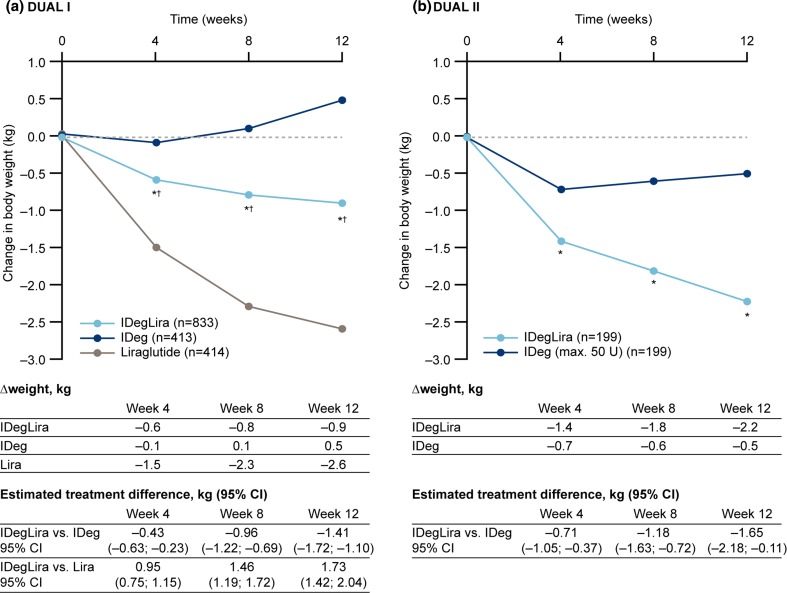
Trajectories for body weight change are shown in Fig. 5. In DUAL I, mean weight reduction from baseline to 4 weeks was greater with IDegLira versus insulin degludec, but lower with IDegLira versus liraglutide, and these differences in trajectories continued over weeks 8 and 12 (Fig. 5a). In DUAL II, mean weight reduction from baseline was greater with IDegLira versus insulin degludec after 4, 8, and 12 weeks (Fig. 5b).
Fig. 5.
Change in weight in weeks 0–12 in the DUAL I and DUAL II studies. *p < 0.0001 vs. IDeg; † p < 0.0001 vs. liraglutide. CI confidence interval, IDeg insulin degludec, Lira liraglutide, IDegLira insulin degludec + liraglutide fixed combination
Hypoglycemia data during the initial 12 weeks are presented in Table 1. The absolute event rates and cumulative incidence were low in the initial weeks with all treatments. There was no difference in hypoglycemia rate between IDegLira and insulin degludec in either study, but hypoglycemia was more frequent with IDegLira than with liraglutide in DUAL I.
Table 1.
Confirmed hypoglycemia incidence and cumulative incidence during initial 12 weeks of the DUAL I and II studies
| Study/week of study | IDegLira | Insulin degludec | Liraglutide | |||
|---|---|---|---|---|---|---|
| Hypoglycemic events per patient year | Cumulative % of patients reporting a hypoglycemic event | Hypoglycemic events per patient year | Cumulative % of patients reporting a hypoglycemic event | Hypoglycemic events per patient year | Cumulative % of patients reporting a hypoglycemic event | |
| DUAL I study | ||||||
| 4 | 1.18 | 6.4 | 0.87 | 5.8 | 0.54* | 3.2 |
| 8 | 1.18 | 11.6 | 1.21 | 11.9 | 0.34** | 3.6 |
| 12 | 1.48 | 19.6 | 1.62 | 19.7 | 0.26** | 4.4 |
| DUAL II study | ||||||
| 4 | 0.77 | 4.0 | 0.78 | 3.5 | – | – |
| 8 | 1.08 | 9.5 | 1.67 | 10.6 | – | – |
| 12 | 1.33 | 15.1 | 2.24 | 16.1 | – | – |
p values are for rate ratio for the observed mean number of events per patient per year, for which there were no statistically significant differences between IDegLira and insulin degludec
Cumulative % percent of cohort having recorded at least one confirmed hypoglycemic event by the time-points of week 4, 8, and 12, IDegLira insulin degludec + liraglutide fixed combination
* p < 0.02 vs. IDegLira, ** p < 0.0001 vs. IDegLira
Discussion
In DUAL I, baseline mean HbA1c was 8.3 % (67 mmol/mol) and this was reduced at 26 weeks by 1.9 % (21 mmol/mol) with IDegLira [final mean dose, 38 dose steps (38 units insulin, 1.37 mg liraglutide)], versus 1.4 % (15 mmol/mol) with insulin degludec (final mean dose 53 units), and 1.3 % (14 mmol/mol) with liraglutide (final dose 1.8 mg), estimated treatment differences p < 0.0001 for IDegLira versus each comparator. A higher proportion of patients reached HbA1c <7.0 % (53 mmol/mol) with IDegLira (80.6 %) than with insulin degludec (65.1 %) or liraglutide (60.4 %; p < 0.0001 both comparisons) [26]. These advantages for IDegLira were preserved through to 52 weeks in an extension study [28]. In DUAL II, mean HbA1c improvement from baseline was greater with IDegLira than with insulin degludec, with respective mean reductions of 1.9 % (21 mmol/mol) and 0.9 % (10 mmol/mol; p < 0.0001) from respective baseline values of 8.7 and 8.8 % (72 and 73 mmol/mol) at equal final mean insulin doses of 45 units. Again, more patients achieved HbA1c <7.0 % with IDegLira (60 %) than with insulin degludec (23 %; p < 0.0001). Data collected from the first few weeks of these studies and analyzed here clearly show that much of the improvement with IDegLira occurred in the early weeks, with relatively rapid improvement of FPG and HbA1c, resulting in lower mean values and higher proportions of patients achieving target values early on therapy when compared with insulin degludec or liraglutide given alone. A relative mean body weight reduction versus insulin degludec was apparent from 4 weeks in both studies, although body weight reduction was not as substantial with IDegLira as with liraglutide alone in the initial weeks of treatment in the DUAL I study. Importantly, these early clinical benefits are likely to be meaningful to patients [6, 7].
Illustrative of the relative differences in the early improvement in glycemic control is the observation that the proportion of patients achieving the HbA1c target at week 8 with IDegLira was similar to the proportion achieving this target at week 12 with insulin degludec in both studies, and the proportion of liraglutide-treated patients achieving this target at week 12 in DUAL I (not statistically tested) (Fig. 2). Similar observations apply to FPG (Figs. 3, 4), where greater reductions early in treatment were again seen for IDegLira in both studies. Fasting glycemia is likely to be especially important for early establishment of treatment satisfaction since immediate improvements are seen for this clinical endpoint, and since titration of IDegLira was directed to an FPG target, patients are likely to view FPG as a direct measure of treatment success.
Hypoglycemia rate and weight gain with IDegLira were relatively reduced compared with insulin degludec alone in the DUAL I and II studies [26, 27], although not when compared with liraglutide alone [26]. Each of these potential insulin therapy side effects may be particularly important in treatment initiation and the early stages of insulin therapy since their early occurrence might discourage subsequent dose titration to appropriate levels and affect future adherence behaviors, and they have been recognized as psychological barriers to insulin initiation and adherence [3, 5, 35]. Hypoglycemia and weight gain were therefore included in the composite endpoints, and the proportions of patients achieving the HbA1c target without these adverse events remained higher with IDegLira than with insulin degludec alone; indeed, the relative advantage became larger. In patients previously treated with OADs (DUAL I), a higher percentage of patients achieved the HbA1c target without hypoglycemia and without weight gain with liraglutide than with IDegLira at week 12. One question of interest is whether patients with different degrees of disease progression will all respond equally well to IDegLira. Post hoc analyses have demonstrated that the efficacy of IDegLira in the DUAL I and II studies was independent of several parameters that can be considered markers of disease progression including, baseline HbA1c, disease duration, and BMI [28, 36]. However, further research is merited to assess the efficacy of IDegLira in advanced T2DM.
Another consideration with the use of GLP-1RAs is gastrointestinal (GI) side effects, with transient nausea being the most frequently reported event. The results from DUAL I showed a lower rate of nausea with IDegLira compared to liraglutide alone, probably attributable to the much slower titration of the liraglutide dose when using the IDegLira combination [26]. In blinded comparison in DUAL II, the difference between IDegLira and insulin degludec was very small [27]. Our analysis, together with these previous data, therefore suggests that patients treated with IDegLira can expect to observe relatively early therapeutic benefits in terms of glycemic control, but with a relatively low risk of the adverse reactions that might be expected from the components.
It should be noted, however, that the maximum dose of liraglutide was not reached with IDegLira in either the DUAL I or DUAL II study, hence the therapeutic potential of the GLP-1RA component of the regimen may not have been realized to the full extent that is possible with a free combination regimen. Nevertheless, clinically relevant efficacy and safety outcomes were achieved with the convenient fixed combination regimen in these studies. It can be hypothesized that a therapeutic profile characterized by the prospect of clinical benefits combined with good tolerability might help patients and their healthcare professionals overcome clinical inertia as well as enhancing treatment adherence. It should be noted, however, that treatment satisfaction and adherence to IDegLira relative to comparators was not studied in the DUAL I and II studies, although treatment satisfaction was included as an endpoint in other recently completed IDegLira trials [29, 30]. Within a strictly monitored, controlled clinical trial, adherence is likely to be better than in everyday clinical practice. In fact, the subject of how the speed of clinical improvement in response to pharmacological intervention affects patient self-management behaviors appears to have been little studied in any field of medicine, including diabetes. The gold standard used for judging success in glycemic control has long been HbA1c, and improvement in this endpoint is typically assessed after a period of at least 16 weeks of treatment or longer. A patient’s attitude to their therapy, however, may be shaped more by their experiences of side effects and the clinical responses they can observe (e.g., self-measured blood glucose-determined FPG) in the earlier stages of therapy. Diabetes is a condition where self-management behavior and good glycemic control are critical determinants for long-term prognosis, but where suboptimal control and adherence do not produce immediate unpleasant consequences for the patient. This is therefore an area worthy of further study.
One caveat in this assessment is that to achieve parity in insulin dosing in DUAL II (which was designed to assess the clinical contribution of liraglutide in IDegLira), both IDegLira and insulin degludec were initiated at 16 dose-steps/units. This basal insulin dose reduction (from a mean of 29 units) in patients randomized to insulin degludec would have compromised the early efficacy that might have been achieved had patients applied the same titration algorithm to their pre-trial insulin dose. Therefore, the relative differences in speed of improvement may not reflect what might be expected where the choice is to switch patients from their basal insulin regimen to IDegLira, or to continue and optimize their basal insulin regimen. Nevertheless, mean HbA1c had decreased by approximately 1 % (and FPG by more than 2.5 mmol/L) by week 8 (Figs. 1, 3) in patients commencing IDegLira, despite the reduction in insulin dose, hence this transfer was made without any immediate loss in glycemic control. It is also important to note that, depending on the patient’s HbA1c, a reduction in basal insulin dose is appropriate when GLP-1RA therapy is introduced to the regimen of a patient on basal insulin [9]. A further consideration with DUAL II is that the maximum dose of insulin degludec was limited to 50 units. This should not have impacted early between-treatment differences during titration, but might have affected outcomes by week 12 when doses were approaching final mean values [27]. Another limitation of our analysis is that the greater speed of glucose reduction might merely reflect the more potent total glucose-lowering effect of IDegLira versus its comparators, with both the rates and final totals of glucose reduction largely determined by the titration algorithms. The same 2–0–2 titration algorithm was used for both IDegLira and insulin degludec, and while IDegLira contains two agents in one injection, these findings indicate that glycemic target could be achieved more quickly with IDegLira versus insulin degludec, with a similar number of injections and effort in terms of titration, and without an increased risk of hypoglycemia and weight gain. The titration algorithms used in the trials are those recommended for everyday clinical practice, although we are aware that this may be challenging in a regular (non-trial) out-patient setting.
Conclusions
In summary, the present analysis complements the original DUAL I and II study publications by providing data from the early phases of IDegLira therapy in patients previously treated with OADs with or without basal insulin. These data are of clinical importance as they show that the improved balance between glycemic control, hypoglycemia, and weight change, comparing IDegLira with its individual components, manifested early during therapy (remaining thereafter throughout the duration of the studies). Indeed, IDegLira produced marked improvements in glycemic control, with beneficial effects on body weight, as early as 4 weeks after initiation, not achieved at the expense of early side effects such as hypoglycemia. These findings support the hypothesis that drug combinations with complementary actions could accelerate the onset of therapeutic benefits in T2DM patients. Early improvement in glycemic control could enhance patient perception of progress and hence their satisfaction and perseverance with treatment, and may also help overcome clinical inertia in the drive for improved glycemic control.
Electronic supplementary material
Acknowledgments
The authors are grateful to Watermeadow Medical, UK (Murray Edmunds and Adele Buss for help in the drafting of this manuscript and Gabrielle Parker for help with submission). This assistance was funded by Novo Nordisk.
Compliance with Ethical Standards
Funding
Novo Nordisk funded the study, participated in study design, data analysis, and manuscript preparation and review. The decision to publish was the authors’.
Financial and competing interests disclosures
Tina Vilsbøll has received lecture fees from AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, Novartis, Sanofi, and Zealand Pharma, and is a member of the Advisory Boards of AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, and Sanofi.
Jiten Vora has served on advisory boards and received support for research and attendance for national and international educational meetings and honoraria for lecturing from Novo Nordisk, Eli Lilly and Company, Sanofi, Merck Sharp & Dohme, Takeda, Novartis, and Abbott.
Lawrence Blonde has received lecture honoraria from AstraZeneca, Janssen Pharmaceuticals Inc., Merck & Co, Novo Nordisk and Sanofi, consultancy honoraria from AstraZeneca, GlaxoSmithKline, Intarcia Therapeutics Inc, Janssen Pharmaceuticals Inc., Merck & Co, Novo Nordisk, Quest Diagnostics, and Sanofi and research grants to his institution from Eli Lilly and Company, Novo Nordisk, and Sanofi.
Henrik Jarlov and Kajsa Kvist are employees and shareholders of Novo Nordisk.
Compliance with ethical guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study.
References
- 1.Blak BT, Smith HT, Hards M, Maguire A, Gimeno V. A retrospective database study of insulin initiation in patients with Type 2 diabetes in UK primary care. Diabet Med. 2012;29:e191–e198. doi: 10.1111/j.1464-5491.2012.03694.x. [DOI] [PubMed] [Google Scholar]
- 2.Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411–3417. doi: 10.2337/dc13-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brod M, Kongsø JH, Lessard S, Christensen TL. Psychological insulin resistance: patient beliefs and implications for diabetes management. Qual Life Res. 2009;18:23–32. doi: 10.1007/s11136-008-9419-1. [DOI] [PubMed] [Google Scholar]
- 4.Peyrot M, Rubin RR, Khunti K. Addressing barriers to initiation of insulin in patients with type 2 diabetes. Prim Care Diabetes. 2010;4(Suppl. 1):S11–18. [DOI] [PubMed]
- 5.Petrak F, Herpertz S, Stridde E, Pfützner A. Psychological insulin resistance in type 2 diabetes patients regarding oral antidiabetes treatment, subcutaneous insulin injections, or inhaled insulin. Diabetes Technol Ther. 2013;15:703–711. doi: 10.1089/dia.2012.0257. [DOI] [PubMed] [Google Scholar]
- 6.Rubin RR, Peyrot M. Assessing treatment satisfaction in patients treated with pramlintide as an adjunct to insulin therapy. Curr Med Res Opin. 2007;23:1919–1929. doi: 10.1185/030079907X210804. [DOI] [PubMed] [Google Scholar]
- 7.Peyrot M, Rubin RR. How does treatment satisfaction work? Modeling determinants of treatment satisfaction and preference. Diabetes Care. 2009;32:1411–17. [DOI] [PMC free article] [PubMed]
- 8.Jendle J, Martin SA, Milicevic Z. Insulin and GLP-1 analog combinations in type 2 diabetes mellitus: a critical review. Expert Opin Investig Drugs. 2012;21:1463–1474. doi: 10.1517/13543784.2012.707190. [DOI] [PubMed] [Google Scholar]
- 9.Vora J, Bain SC, Damci T, Dzida G, Hollander P, Meneghini LF, Ross SA. Incretin-based therapy in combination with basal insulin: a promising tactic for the treatment of type 2 diabetes. Diabetes Metab. 2013;39:6–15. doi: 10.1016/j.diabet.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Ahrén B. Insulin plus incretin: a glucose-lowering strategy for type 2-diabetes. World J Diabetes. 2014;5:40–51. doi: 10.4239/wjd.v5.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228–2234. doi: 10.1016/S0140-6736(14)61335-0. [DOI] [PubMed] [Google Scholar]
- 12.Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31:1511–1523. doi: 10.1016/j.clinthera.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther. 2011;13:592–595. doi: 10.1089/dia.2010.0221. [DOI] [PubMed] [Google Scholar]
- 14.Buse JB, Bergenstal RM, Glass LC, Heilmann CR, Lewis MS, Kwan AY, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154:103–112. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- 15.Lind M, Jendle J, Torffvit O, Lager I. Glucagon-like peptide 1 (GLP-1) analogue combined with insulin reduces HbA1c and weight with low risk of hypoglycemia and high treatment satisfaction. Prim Care Diabetes. 2012;6:41–46. doi: 10.1016/j.pcd.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Seino Y, Min KW, Niemoeller E, Takami A, EFC10887 GETGOAL-L Asia Study Investigators Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia) Diabetes Obes Metab. 2012;14:910–917. doi: 10.1111/j.1463-1326.2012.01618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeVries JH, Bain SC, Rodbard HW, Seufert J, D’Alessio D, Thomsen AB, et al. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care. 2012;35:1446–1454. doi: 10.2337/dc11-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riddle MC, Aronson R, Home P, Marre M, Niemoeller E, Miossec P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L) Diabetes Care. 2013;36:2489–2496. doi: 10.2337/dc12-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddle MC, Forst T, Aronson R, Sauque-Reyna L, Souhami E, Silvestre L, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1) Diabetes Care. 2013;36:2497–2503. doi: 10.2337/dc12-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathieu C, Rodbard HW, Cariou B, Handelsman Y, Philis-Tsimikas A, Ocampo Francisco AM, et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON). Diabetes Obes Metab. 2014;16 636-44. [DOI] [PubMed]
- 21.Rosenstock J, Fonseca VA, Gross JL, Ratner RE, Ahrén B, Chow FC, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37:2317–2325. doi: 10.2337/dc14-0001. [DOI] [PubMed] [Google Scholar]
- 22.Diamant M, Nauck MA, Shaginian R, Malone JK, Cleall S, Reaney M, et al. Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37:2763–2773. doi: 10.2337/dc14-0876. [DOI] [PubMed] [Google Scholar]
- 23.Haahr H, Heise T. A review of the pharmacological properties of insulin degludec and their clinical relevance. Clin Pharmacokinet. 2014;53:787–800. doi: 10.1007/s40262-014-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinman B, Philis-Tsimikas A, Cariou B, Handelsman Y, Rodbard HW, Johansen T, et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long). Diabetes Care. 2012;35:2464–71. [DOI] [PMC free article] [PubMed]
- 25.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 26.Gough SC, Bode B, Woo V, Rodbard HW, Linjawi S, Poulsen P, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885–893. doi: 10.1016/S2213-8587(14)70174-3. [DOI] [PubMed] [Google Scholar]
- 27.Buse JB, Vilsbøll T, Thurman J, Blevins TC, Langbakke IH, Bøttcher SG, Rodbard HW, NN9068-3912 (DUAL-II) Trial Investigators Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira) Diabetes Care. 2014;37:2926–2933. doi: 10.2337/dc14-0785. [DOI] [PubMed] [Google Scholar]
- 28.Gough SC, Bode B, Woo VC, Rodbard HW, Linjawi S, Zacho M, Reiter PD, Buse JB. One-year efficacy and safety of a fixed combination of insulin degludec and liraglutide in patients with type 2 diabetes: results of a 26-week extension to a 26-week main trial. Diabetes Obes Metab. 2015;17:965–973. doi: 10.1111/dom.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.A trial comparing the efficacy and safety of insulin degludec/liraglutide versus insulin glargine in subjects with type 2 diabetes mellitus (DUAL™ V). https://clinicaltrials.gov/ct2/show/NCT01952145. Accessed 25 Nov 2015.
- 30.The efficacy of insulin degludec/liraglutide in controlling glycaemia in adults with type 2 diabetes inadequately controlled on GLP-1 receptor agonist and OAD therapy (DUAL™ III). https://clinicaltrials.gov/ct2/show/NCT01676116. Accessed 25 Nov 2015.
- 31.The efficacy of insulin degludec/liraglutide as add-on therapy in controlling glycaemia in adults with type 2 diabetes inadequately controlled on sulphonylurea with or without metformin therapy (DUAL™ IV). https://clinicaltrials.gov/ct2/show/NCT01618162. Accessed 25 Nov 2015.
- 32.A clinical trial comparing efficacy and safety of insulin degludec/liraglutide (IDegLira) in subjects with type 2 diabetes mellitus using two different titration algorithms (DUAL™ VI). https://clinicaltrials.gov/ct2/show/NCT02298192. Accessed 25 Nov 2015.
- 33.A trial comparing sequential addition of insulin aspart versus further dose increase with insulin degludec/liraglutide in subjects with type 2 diabetes mellitus, previously treated with insulin degludec/liraglutide and metformin and in need of further intensification (DUAL™). https://www.clinicaltrials.gov/ct2/show/NCT02100475. Accessed 25 Nov 2015.
- 34.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 35.Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29:682–689. doi: 10.1111/j.1464-5491.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodbard HW, Buse JB, Woo V, Vilsbøll T, Langbakke IH, Kvist K, Gough SC. Benefits of combination of insulin degludec and liraglutide are independent of baseline glycated haemoglobin level and duration of type 2 diabetes. Diabetes Obes Metab. 2016;18:40–8. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.