Abstract
We initiated a prospective multicenter phase II trial using rituximab and temozolomide in immunocompetent patients with progressive or recurrent primary central nervous system lymphoma (PCNSL) based on activity observed in retrospective studies. Treatment consisted of an induction phase with rituximab (750 mg/m2 ) on days 1, 8, 15 and 22 and temozolomide (150 mg/m2 ) days 1–7 and 15–21, followed by six cycles of consolidation temozolomide (150–200 mg/m2 × 5/28 days), followed by maintenance with methylprednisolone (1 g IV every 28 days) until progression. Sixteen patients were enrolled, and a complete response was seen in 2/14 (14%) evaluable patients. The median progression-free survival was 7 weeks and median overall survival was not reached (median follow-up: 37 months). Treatment was well tolerated, but due to slow accrual and preliminary analysis suggesting futility, the trial was closed early. Given the overall modest activity, this regimen should be reserved for patients who are not candidates for other, more aggressive salvage treatments.
Keywords: CNS lymphoma, rituximab, temozolomide
Introduction
The prognosis of primary central nervous system lymphoma (PCNSL) relapsing after initial treatment with high-dose methotrexate (HD-MTX) based chemotherapy with or without whole brain radiotherapy (WBRT) is poor, and there is no consensus on optimal salvage treatments [1]. Available literature on salvage chemotherapy options is limited, mostly based on small or retrospective studies reporting variable response rates (RRs) ranging from 31 to 86% and 1-year overall survival (OS) of 35–57% (Table I); new alternatives are clearly needed [2–8].
Table I.
Salvage chemotherapy treatments for primary CNS lymphomas without methotrexate.
| Treatment [reference] | n | ORR | 1-year OS |
|---|---|---|---|
| Retrospective studies | |||
| Ara-C, VP-16, ifosfamide [2] | 16 | 37% | 41% |
| Procarbazine, CCNU, vincristine [4] | 7 | 86% | 57% |
| Temozolomide, rituximab [11] | 15 | 53% | 55% |
| Temozolomide [20] | 17 | 59% | 35% |
| Prospective studies | |||
| Topotecan [3] | 27 | 33% | 35% |
| Temozolomide [5] | 23 | 31% | 31% |
| Rituximab [8] | 12 | 36% | NA |
| Temozolomide, rituximab [present study] | 16 | 14% | 71% |
| Ara-C, VP-16 + HDCASCT (patients under age 65 only) [6] | 43 | 47% | 60% |
Ara-C, cytarabine; VP-16, etoposide; CCNU, lomustine; HDCASCT, high dose chemotherapy (busulfan, thiotepa, cyclophosphamide) followed by autologous stem cell transplant; ORR, objective response rate (response criteria and response confirmation requirements varied across studies); OS, overall survival; NA, not available.
Temozolomide is an alkylating agent with excellent blood–brain barrier penetration that is active in PCNSL [5,9]. In a phase II study of 23 patients with recurrent PCNSL treated with single-agent temozolomide, the RR was 31% and 1-year OS was 31% [5].
Rituximab is a chimeric monoclonal antibody against the B-cell specific CD20 antigen and has been incorporated into chemotherapeutic regimens for treatment of PCNSL, which is most often a CD20 + diffuse large B-cell lymphoma [10]. In a study of 12 patients with recurrent PCNSL, confirmed responses were seen in 4/11 patients (36%) treated with single-agent rituximab, with a median OS of 21 months [8].
Given the favorable toxicity profile, two small retrospective studies combined rituximab and temozolomide, and found RRs of 53–100% and 1-year OS of 50–55% [11,12]. To investigate this combination in the prospective setting, we initiated a multicenter phase II trial through the North American Brain Tumor Consortium (NABTC).
Patients and methods
This protocol was approved by the institutional review boards of all participating centers. Immunocompetent patients with progressive or recurrent PCNSL with no limit on number of previous recurrences were eligible. Other inclusion criteria consisted of age ≥18, Karnofsky performance status (KPS) ≥60%, histological confirmation of PCNSL (brain biopsy, positive cerebrospinal fluid [CSF] cytology or vitrectomy), presence of measurable disease and radiographic evidence of tumor progression on brain magnetic resonance imaging (MRI), white blood cell count (WBC) ≥3000/μL, absolute neutrophil count (ANC) ≥1500/μL, platelet count ≥100 000/μL, hemoglobin ≥10 g/dL, serum glutamic oxaloacetic transaminase (SGOT) and bilirubin < 2 × upper limit of normal, creatinine < 1.5 mg/dL or creatinine clearance ≥ 60 mL/min. If patients were on corticosteroids, the dose was required to be stable for 5 days; if the dose was increased, a new MRI scan was obtained as baseline. Patients with a history of any other active cancer or history of prior exposure to temozolomide were excluded. Patients had to have recovered from toxic effects of prior therapy (28 days from any investigational drug or cytotoxic therapy, 14 days from methotrexate, 42 days from nitrosoureas, 21 days from procarbazine and 7 days from non-cytotoxic agents). In addition to MRI of the brain, pretreatment evaluation consisted of contrast-enhanced computed tomography (CT) of the chest, abdomen and pelvis, complete ophthalmologic examination including a slit-lamp examination, and a lumbar puncture for CSF cytology. All patients signed an informed consent form.
Treatment
Induction phase
Treatment consisted of one 28-day cycle as follows: rituximab (750 mg/m2 intravenously on days 1, 8, 15 and 22) and temozolomide (150 mg/m2 orally on days 1–7 and 15–21). Following the induction cycle, a MRI scan of the brain was obtained and patients proceeded with consolidation therapy if they had stable disease (SD) or a partial response (PR). Patients with progressive disease (PD) were taken off protocol.
Consolidation phase
Consolidation treatment consisted of six 28-day cycles of temozolomide (150 mg/m2/day on days 1–5). The dose was escalated to 200 mg/m2 after the first cycle if there was no evidence of ≥ grade 2 toxicities. MRI of the brain was obtained every 8 weeks, and patients with PD were removed from the study.
Long-term maintenance
Following the consolidation phase, methylprednisolone was given at a dose of 1 g intravenously every 28 days, indefinitely. MRI of the brain was obtained initially every 8 weeks for the first year of maintenance therapy, and every 3 months thereafter. Patients continued on maintenance until disease progression.
Response criteria
Measurable disease was defined as bi-dimensionally measurable enhancing lesions with clearly defined margins by MRI. A complete response (CR) was defined as complete disappearance of all measurable disease and no new lesions, while off steroids. A PR was defined as ≥50% reduction of all measurable lesions. The corticosteroid dose at the time of scan had to be no greater than the maximum dose used in the first 8 weeks from initiation of therapy. PD was characterized by a 25% increase in the measurable lesions from baseline, clear worsening of any lesion, appearance of a new lesion or clear clinical deterioration. All other cases were characterized as SD.
Statistics
The primary endpoint was RR, with a required sample size of 40 patients. The regimen would be considered successful if ≥14 patients showed a response. The probability of a 35% RR would be 0.10 if the true rate was 25% and 0.92 if the true rate was 45%. Patients in whom no response assessment could be made were considered treatment failures. Progressionfree survival (PFS) and OS were measured from the start of treatment until disease progression or death, respectively. Kaplan–Meier methodology was used for survival analysis.
Results
After the first 16 patients were enrolled, the study was terminated early due to slow accrual and preliminary analysis suggesting futility. The study remained open from 2005 to 2008. Among the enrolled patients, 12 (75%) were women and 4 (25%) were men. The median age was 63 years (range, 42–79 y) and median KPS was 90% (range, 60–100%). Based on the Memorial Sloan-Kettering Cancer Center prognostic model, 3 patients were classified as class 1 (Age <50 years), 8 patients as class 2 (Age ≥50, KPS ≥70), and 2 patients as class 3 (Age ≥50, KPS <70). The KPS on the remaining 3 patients was not available; they were ≥50 years of age. The number of previous relapses was 1 in 11 patients, 2 in 2 patients and 3 in 3 patients. Data on prior chemotherapy was available for 14/16 patients, of whom 13 had received a HDMTX based regimen (multidrug regimen: 9, single agent HDMTX: 4). Four patients had received prior WBRT in addition to HD-MTX. Three patients had disease refractory to first line treatment; all others had recurrent disease after response to initial treatment. The median time from initial diagnosis to enrollment on this trial was 1 year (range, 0.2–7.5 y).
Response
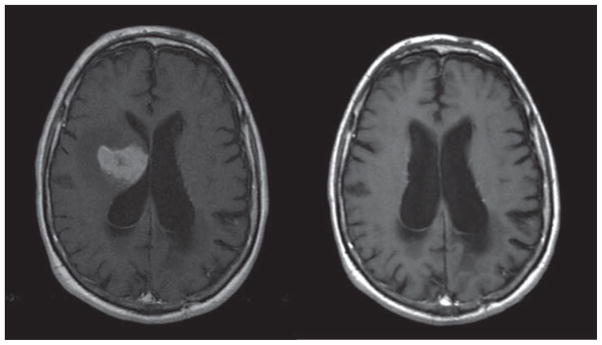
Fourteen patients were evaluable for response; one died prematurely and one withdrew consent before completion of the induction cycle. On the first MRI following the induction phase, five patients (two CR, three PR; 36%) showed a radiographic response. However, three of those patients showed progression of disease on subsequent MRI. The other two patients had their response confirmed on subsequent MRI, both of whom achieved a CR. Therefore, the objective RR was 14% (95% confidence interval [CI], 17–43; Figure 1). Thirteen patients came off study for PD.
Figure 1.
T1 post-contrast MRI showing initial lesion and sustained complete response 5 years after treatment.
Survival
Median PFS was 7 weeks (95% CI 4–13) and 6-month PFS was 13% (95% CI 2–35) (Figure 2). Five patients died, four from disease progression and one from an unrelated infection. Median OS was not reached (median follow-up: 37 months) (Figure 2). The 1-year OS rate was 71% (95% CI, 40–88).
Figure 2.
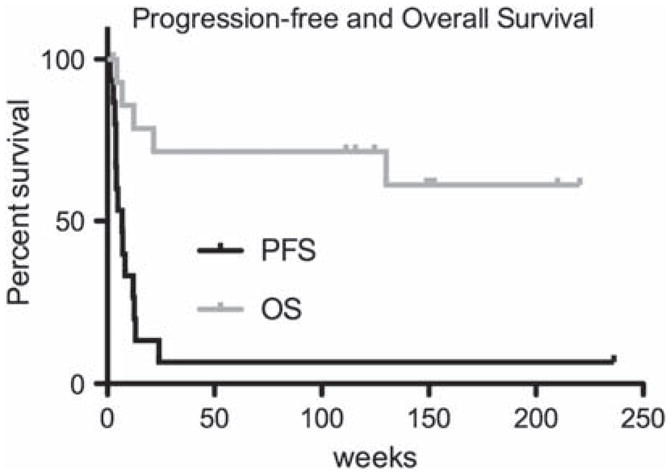
Progression-free survival (PFS) and overall survival (OS) Kaplan–Meier curves. Median PFS: 7 weeks (CI 4–13), 6-month PFS: 13% (CI 2–35); median OS: not reached, 1-year OS: 71% (CI 40–88).
Toxicity
No serious adverse events or grade 3–5 toxicities were seen on this treatment. Grade 1–2 toxicities consisted of hematotoxicity (n = 8), fatigue (n = 3), liver enzyme elevation (n = 2) and skin rash (n = 1).
Salvage therapy
One patient remains in remission; he received monthly prednisolone for a period of 3 years, after which treatment was discontinued at the investigator’s decision. Information on salvage treatment is available for 11 patients. Four patients were treated with a HD-MTX based regimen, two with pemetrexed followed by high dose chemotherapy and autologous stem cell rescue, three with WBRT, one received focal RT to the spine and etoposide, and one received no treatment.
Discussion
In this prospective, multicenter phase II study in progressive/relapsed PCNSL, the combination of rituximab and temozolomide was associated with only modest efficacy. Although some activity was observed following the induction treatment, responses were short-lived, which may reflect poor efficacy of single-agent temozolomide in the consolidation phase. Unfortunately, the promising results seen in the retrospective studies were not replicated in this trial, and a decision was made to discontinue accrual. While the discrepancy between our study and previous retrospective studies on temozolomide and rituximab may be explained by the inherent selection bias in retrospective series, we were unable to reproduce results from prospective studies on single agent temozolomide or single agent rituximab (Table 1). This may reflect the fact that patients in our study had received more intensive, multi-drug methotrexate regimens whereas many patients in the other studies had failed single-agent methotrexate; our patients were therefore more likely to harbor chemoresistant tumors. Moreover, three patients in our study had HD-MTX refractory disease. Another potential explanation is the utilization of more stringent response criteria, which required confirmation of responses. In our study, the initial MRI showed a radiographic response in 31% of patients, similar to other studies, but the following MRI performed two months later only confirmed responses in 14% of patients.
In addition to the studies of single-agent temozolomide and rituximab, there have been few prospective chemotherapy trials in recurrent PCNSL (Table I). To date, the best results have been reported by Soussain et al., who utilized induction chemotherapy with cytarabine and etoposide followed by high-dose chemotherapy with stem-cell rescue [13]. The median PFS was 12 months in the intent-to-treat population, and 41.1 months in patients who were transplanted; the median OS was 18.3 and 58.6 months, respectively. However, the induction chemotherapy was highly toxic, and this approach was restricted to high-performing patients, all younger than 65 years. A prospective trial using topotecan monotherapy achieved a RR of 33% with a median PFS of 2 months [14]. The combination of etoposide, ifosfamide and cytarabine demonstrated a RR of 37% and a median PFS of 4 months; toxicity was significant [15].
The favorable OS in our study suggests that subsequent salvage treatments were effective; these consisted mainly of either re-introduction of HD-MTX or WBRT in those who had not received it previously. Retrospective studies have suggested that HD-MTX based regimens remain the most effective salvage therapy for patients with PCNSL who previously responded to HD-MTX, with reported RRs of 71–91% and OS of 23–61 months [16,17]; however, this is not applicable to patients with primary refractory disease. Salvage WBRT has also been an effective option in patients who have not received this treatment, but neurotoxicity remains a concern [18,19]. Although the rituximab–temozolomide combination did not show great efficacy, it was effective for at least two patients, one of whom remains in a sustained remission after 6 years. The high variability of response to this and other regimens suggests that unknown aspects of the biology of this disease are critical in determining the appropriate choice of salvage therapy.
Acknowledgments
This study was funded by the National Cancer Institute, NIH grant # U01-CA062399. Temozolomide was provided by Schering-Plough/Merck. This trial is registered at the ClinicalTrials. gov registry (# NCT00248534), and was presented in part at the American Society of Clinical Oncology Annual Meeting, 2011.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Graber JJ, Omuro A. Pharmacotherapy for primary CNS lymphoma: progress beyond methotrexate? CNS Drugs. 2011;25:447–457. doi: 10.2165/11589030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Arellano-Rodrigo E, Lopez-Guillermo A, Bessell EM, et al. Salvage treatment with etoposide (VP-16), ifosfamide and cytarabine (Ara-C) for patients with recurrent primary central nervous system lymphoma. Eur J Haematol. 2003;70:219–224. doi: 10.1034/j.1600-0609.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 3.Fischer L, Thiel E, Klasen H-A, et al. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol. 2006;17:1141–1145. doi: 10.1093/annonc/mdl070. [DOI] [PubMed] [Google Scholar]
- 4.Herrlinger U, Brugger W, Bamberg M, et al. PCV salvage chemotherapy for recurrent primary CNS lymphoma. Neurology. 2000;54:1707–1708. doi: 10.1212/wnl.54.8.1707. [DOI] [PubMed] [Google Scholar]
- 5.Reni M, Zaja F, Mason W, et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer. 2007;96:864–867. doi: 10.1038/sj.bjc.6603660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soussain C, Hoang-Xuan K, Taillandier L, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol. 2008;26:2512–2518. doi: 10.1200/JCO.2007.13.5533. [DOI] [PubMed] [Google Scholar]
- 7.Kasenda B, Schorb E, Fritsch K, et al. Primary CNS lymphoma–radiation-free salvage therapy by second autologous stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:281–283. doi: 10.1016/j.bbmt.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Batchelor TT, Grossman SA, Mikkelsen T, et al. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology. 2011;76:929–930. doi: 10.1212/WNL.0b013e31820f2d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omuro AM, Taillandier L, Chinot O, et al. Temozolomide and methotrexate for primary central nervous system lymphoma in the elderly. J Neurooncol. 2007;85:207–211. doi: 10.1007/s11060-007-9397-0. [DOI] [PubMed] [Google Scholar]
- 10.Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25:4730–4735. doi: 10.1200/JCO.2007.12.5062. [DOI] [PubMed] [Google Scholar]
- 11.Enting RH, Demopoulos A, DeAngelis LM, et al. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology. 2004;63:901–903. doi: 10.1212/01.wnl.0000137050.43114.42. [DOI] [PubMed] [Google Scholar]
- 12.Wong ET, Tishler R, Barron L, et al. Immunochemotherapy with rituximab and temozolomide for central nervous system lymphomas. Cancer. 2004;101:139–145. doi: 10.1002/cncr.20339. [DOI] [PubMed] [Google Scholar]
- 13.Soussain C, Hoang-Xuan K, Taillandier L, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol. 2008;26:2512–2518. doi: 10.1200/JCO.2007.13.5533. [DOI] [PubMed] [Google Scholar]
- 14.Fischer L, Thiel E, Klasen HA, et al. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol. 2006;17:1141–1145. doi: 10.1093/annonc/mdl070. [DOI] [PubMed] [Google Scholar]
- 15.Arellano-Rodrigo E, Lopez-Guillermo A, Bessell EM, et al. Salvage treatment with etoposide (VP-16), ifosfamide and cytarabine (Ara-C) for patients with recurrent primary central nervous system lymphoma. Eur J Haematol. 2003;70:219–224. doi: 10.1034/j.1600-0609.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 16.Pentsova E, DeAngelis LM, Omuro A. Methotrexate (MTX) rechallenge for recurrent primary CNS lymphoma (PCNSL) J Clin Oncol. 2011;29(15 Suppl):Abstract 2025. [Google Scholar]
- 17.Plotkin SR, Betensky RA, Hochberg FH, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res. 2004;10:5643–5646. doi: 10.1158/1078-0432.CCR-04-0159. [DOI] [PubMed] [Google Scholar]
- 18.Hottinger AF, DeAngelis LM, Yahalom J, et al. Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology. 2007;69:1178–1182. doi: 10.1212/01.wnl.0000276986.19602.c1. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen PL, Chakravarti A, Finkelstein DM, et al. Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma. J Clin Oncol. 2005;23:1507–1513. doi: 10.1200/JCO.2005.01.161. [DOI] [PubMed] [Google Scholar]
- 20.Makino K, Nakamura H, Hide T, et al. Salvage treatment with temozolomide in refractory or relapsed primary central nervous system lymphoma and assessment of the MGMT status. J Neurooncol. 2012;106:155–160. doi: 10.1007/s11060-011-0652-z. [DOI] [PubMed] [Google Scholar]