Abstract
Plasma profiling of patients treated with antiangiogenic agents may identify markers that correlate with toxicity. Objectives were to correlate changes in cytokine and angiogenic factors as potential markers of toxicity to aflibercept. Circulating cytokine and angiogenic factors were measured in 28 patients with recurrent glioblastoma in a single-arm phase II study of aflibercept. Plasma samples were analyzed at baseline, 24 h, and 28 days using multiplex assays or ELISA. We evaluated log-transformed baseline biomarker expressions with Cox proportional hazard regression models to assess the effect of markers on any grade II–IV (Gr II–IV) toxicity, on-target toxicity (hypertension, proteinuria, thromboembolism), and fatigue. All tests were two sided with a statistical significance level of p=0.05. Among 28 pts, there were 116 Gr II–IV events. Changes in IL-13 from baseline to 24 h predicted on-target toxicities. Increases in IL-1b, IL-6, and IL-10 at 24 h were significantly associated with fatigue. Progression-free survival was 14.9 months for patients in the all-toxicity group and 9.0 months for patients in the on-target toxicity group compared to 4.3 months for those who did not develop any Gr II–IV toxicity (p=0.002 and p=0.045, respectively). Toxicity from antiangiogenic therapy remains an important cause of antiangiogenic treatment discontinuation and patient morbidity. Changes in IL6, IL10, and IL13 were repeatedly correlated with toxicity. Profiling of IL-13 as a surrogate for endothelial dysfunction could individualize patients at risk during antiangiogenic therapy, as could identifying those at higher risk for fatigue using IL-6 and IL-10.
Keywords: Glioblastoma, Aflibercept, VEGF, Cytokines, Toxicity
Introduction
The molecular changes that underlie chemotherapeutic toxicity remain unknown. Although studies have begun to focus on identifying molecular markers that correlate with tumor response, data correlating such markers with toxicity are scarce. Baseline and treatment-related changes in cytokines and angiogenic factors may reveal the mechanism of specific toxicities, permit tailored therapy, and potentially predict toxicity to antiangiogenic agents.
Angiogenesis is a prime target for glioblastoma, one of the most vascularized tumors. Several drugs have emerged which target the vascular endothelial growth factor (VEGF) pathway, including cediranib and bevacizumab. However, toxicities caused by these agents can result in severe adverse events and often treatment discontinuation. In the phase II trial of cediranib in patients with recurrent glioblastoma, 48.4 % of patients had drug interruptions due to toxicity [1]. Friedman and colleagues reported that 17.7 % of patients receiving bevacizumab and irinotecan and 4.8 % of patients receiving bevacizumab alone had to discontinue bevacizumab for toxicities [2]. In another study using bevacizumab for recurrent glioblastoma, 12.5 % of patients had a thromboembolic event, one patient had bowel perforation, and another 12.5 % experienced hypertension on treatment. Six of 48 patients (12.5 %) had to be removed from the study for toxicity [3]. Thus, antiangiogenic agents can cause significant morbidity and may lead to discontinuation of an effective therapy. Identifying biomarker-specific toxicities could allow for prophylactic dose modification or early supportive therapy intervention.
Toxicities of vascular-targeted agents are widely recognized, but the mechanisms underlying these events remain elusive. Cytokines induce inflammation and mediate organ toxicity, and therefore may be a surrogate for these processes [4]. Similarly, cytokines have been shown to mediate radiation toxicity in lung [5–8] and prostate cancer [9, 10]. Lung cancer patients experiencing toxicity from radiation were more likely to have increased serum levels of proinflammatory IL-6 [11]. Cytokines and angiogenic factors have also been studied in patients who have had cranial irradiation, showing alterations of fibroblast growth factor (FGF)-2, VEGF, and IL-1 [12, 13], believed to represent early increases of tumor necrosis factor [14, 15]. Similarly, increases in IL-6 levels have been associated with fatigue in both solid and hematological malignancies with a high level of statistical significance (p=0.0005) [16]. Interestingly, there have been several studies suggesting that on-target toxicity is associated with improved outcome on antiangiogenic therapy [3, 17–22]. Together, these findings suggest a possible relationship between adverse events, cytokine expression, and patient outcome.
Aflibercept, or VEGF trap, is a soluble receptor that sequesters VEGF-A, VEGF-B, and placental growth factor (PlGF)-1 and PlGF-2 with an affinity greater than bevacizumab [23]. In phase I clinical trials, on-target toxicities were similar to those seen with bevacizumab including hypertension and proteinuria [24]. We previously reported on the clinical efficacy of aflibercept in recurrent glioblastoma [25] and circulating myeloid chemokines as biomarkers of efficacy [26]. Here, we describe specific toxicities and provide cytokine patterns that are associated with the development of all toxicity, on-target toxicities (hypertension, proteinuria, and bleeding), and fatigue. The association between all toxicity or on-target toxicity and time to progression was determined.
Patients and methods
Study population and design
Thirty-one patients with recurrent glioblastoma received aflibercept in the multi-institutional North American Brain Tumor Consortium (NABTC) 0601 trial. This study was approved by the Cancer Treatment Experimental Program (CTEP) and the Institutional Review Board of each participating NABTC site, and informed consent was obtained from all the subjects prior to study enrollment. One patient withdrew consent prior to treatment, one did not have baseline samples taken, and another did not have any further samples taken after baseline. Those three patients were not included in the final analyses. The supplemental Table 1 shows the patient characteristics.
Eligibility was based on histologically confirmed glioblastoma or gliosarcoma with evidence of progression after chemoradiation and no more than one adjuvant regimen containing temozolomide. Also required was available tissue from primary surgery or at relapse, Karnofsky performance status (KPS) of ≥60, baseline magnetic resonance imaging ≤14 days of registration on a stable steroid dose, and life expectancy >8 weeks. Patients had to be recovered from toxic effects of prior therapy and have adequate bone marrow (ANC≥1,500/mm3; platelets≥100,000/mm3), liver (SGPT/alk phos <2× normal, bilirubin <1.5 mg/dl), and renal (BUN and creatinine <1.5× normal) functions with a urine protein/creatinine ratio <1. They had to be more than 42 days from the completion of either radiation therapy or prior nitrosourea, more than 28 days from cytotoxic chemotherapy or investigational agent, and more than 4 weeks from surgery. Patients were excluded from the study for pregnancy or nursing; history of intracerebral or intratumoral hemorrhage; treatment with prior antiangiogenic therapy; history of significant cardiovascular disease, prior malignancy, severe concurrent illness, and prior venous thromboembolic event; or other reason requiring full dose anticoagulation.
Aflibercept was provided by CTEP and administered at 4 mg/kg intravenously on day 1 of every 14-day cycle. Up to a 2-day window on either side of the planned treatment day was allowed. Patients were allowed no more than two dose reductions for grades 3 or 4 toxicity and were allowed to recover from treatment-related toxicities for up to 2 weeks, which could be extended with the permission of the principal investigator and CTEP medical director. The National Cancer Institute Common Terminology Criteria, version 3.0, were utilized to describe and grade treatment-related toxicities. All patients received treatment until tumor progression, toxicity, or withdrawal of consent.
Assay methods: patient blood sampling and processing
Ethylenediaminetetraacetic acid plasma samples were obtained from 28 of 31 enrolled patients at baseline, 24 h, and every 2–4 weeks until progression. Reporting of cytokine measurements was compliant with the guidelines from the National Cancer Institute and European Organization for Research and Treatment of cancer recommendations for reporting tumor marker prognostic studies [27]. Levels of VEGF, PlGF, carbonic anhydrase 9 (CA9), and basic FGF were measured using ELISA assays according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). MMP-9 and e-selectin were measured using a Lincoplex Assay (LINCO Research, Millipore, St. Charles, MO). Plasma samples from baseline and days1 and 28 were analyzed for cytokines and angiogenic factors using commercially available multiplex suspension arrays (BioRad Laboratories, Hercules, CA). Some cytokines and angiogenic factors were missing due to patient-related factors. All 28 patients evaluated had markers at baseline, 26/28 had markers at 24 h, and 21/28 patients had markers at day 28 and/or later.
Statistical analysis
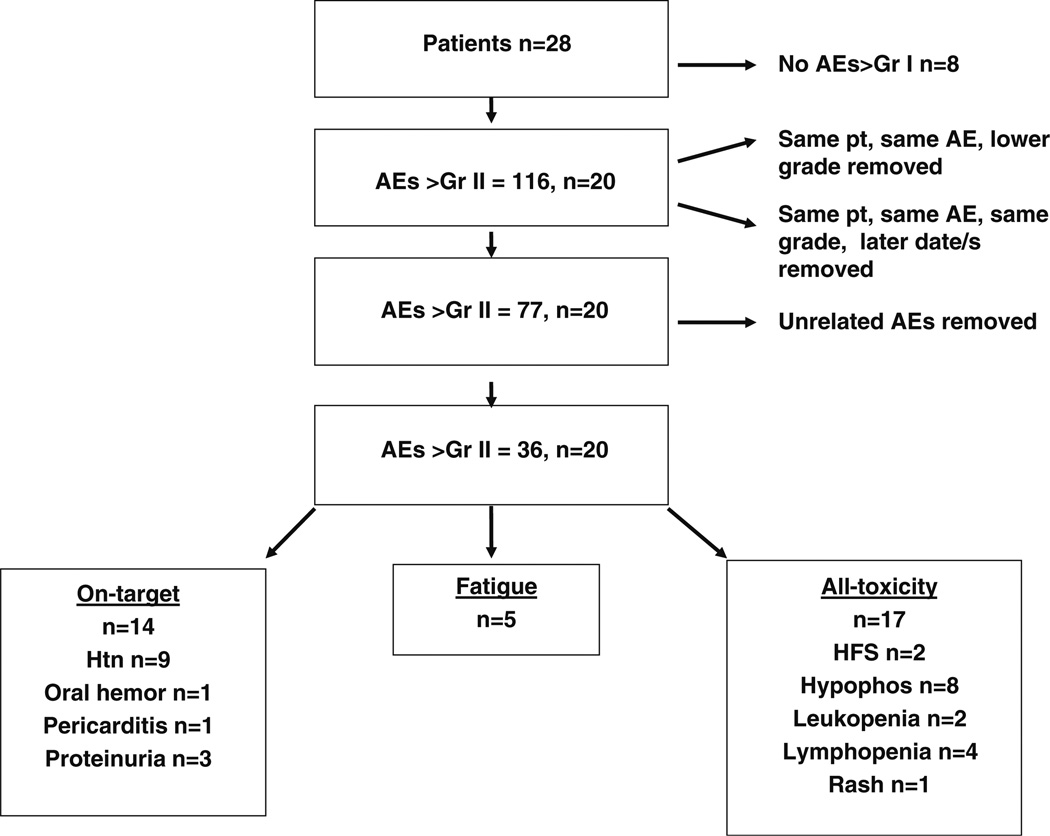
A total of 116 adverse events (AEs) were recorded. The time to toxicity was defined as interval from the date of registration to the date of toxicity. Where duplicate AEs coexisted for a single patient, the highest grade was used for analysis, and where duplicate AEs of the same grade were found, the AE occurring at the earliest point in the trial was assessed. AEs that were felt to be unrelated to treatment were removed using the best clinical judgment of the reviewers and based on data from prior studies of aflibercept. A total of 36 Gr II–IV events remained and were used in the final analyses. Two predominant categories emerged: on-target effects [hypertension, proteinuria, pericarditis (one patient), and bleeding] and fatigue. Those with other toxicities were included in an “all-toxicity” analysis. Figure 1 depicts the selection process of adverse events. Descriptive statistics were used to summarize the baseline measurements and changes in concentration of each biomarker in patients who experienced toxicity and in patients who did not experience toxicity, separately. Log-transformed baseline expressions were used and evaluated with Cox proportional hazard regression models to assess the effect of biomarkers on any grade II–IV (GII–IV) toxicity, GII–IV on-target toxicity, and GII–IV fatigue toxicity. Missing observations were excluded from the analysis, and analytes below the level of detection were conservatively set at zero. Wilcoxon rank sum tests were used to determine the difference of the concentrations between toxicity patients and non-toxicity patients. Time to toxicity was analyzed by using the Kaplan–Meier method. A Cox PH regression model was used to assess the effect of biomarkers on time to develop toxicities. All tests were two sided with a statistical significance level of p=0.05. All analyses were conducted using the SAS (version 9.1; SAS Institute Inc., Cary, NC) and S-PLUS (version 8.0; Insightful Corp., Seattle, WA).
Fig. 1.
Flow diagram of the selection process of adverse events. AE adverse event, Gr grade, hemor hemorrhage, HFS hand–foot syndrome, HTN hypertension, Hypophos hypophosphatemia, pt patient
Results
Patient characteristics
The median patient age in the cohort of glioblastoma patients receiving aflibercept in the NABTC 0601 trial was 55 years and ranged from 33 to 72 years of age. Of the 28 patients, 16 were men, and the median KPS was 90. Twenty patients experienced grade II or greater toxicity. Eight patients did not develop any toxicity greater than grade I. Adverse events including hypertension, proteinuria, pericarditis, and bleeding were grouped as “on-target” toxicity. Those with fatigue formed a second robust subgroup and were analyzed separately from other on-target toxicities. Other events included hypophosphatemia, lymphopenia, leukopenia, hand–foot syndrome, and rash. One such rash was biopsied and shown in Supplemental Fig. 1. To strengthen our small patient sample, we compared cytokines and angiogenic factors of patients without toxicity with markers in the groups entitled all toxicity, on-target toxicity, and fatigue.
Cytokines and time to develop toxicity
Of the 20 patients who developed any toxicity, 14 were treated for greater than 28 days (range, 42–172). Nine of 13 patients developing on-target toxicity, and three of five patients who experienced fatigue were on treatment for more than 4 weeks (range, 42–161 days). In contrast, only one patient of eight who developed no toxicities during treatment received more than 28 days (56 days) of therapy.
Baseline cytokine and angiogenic factor expression associated with the development of toxicity
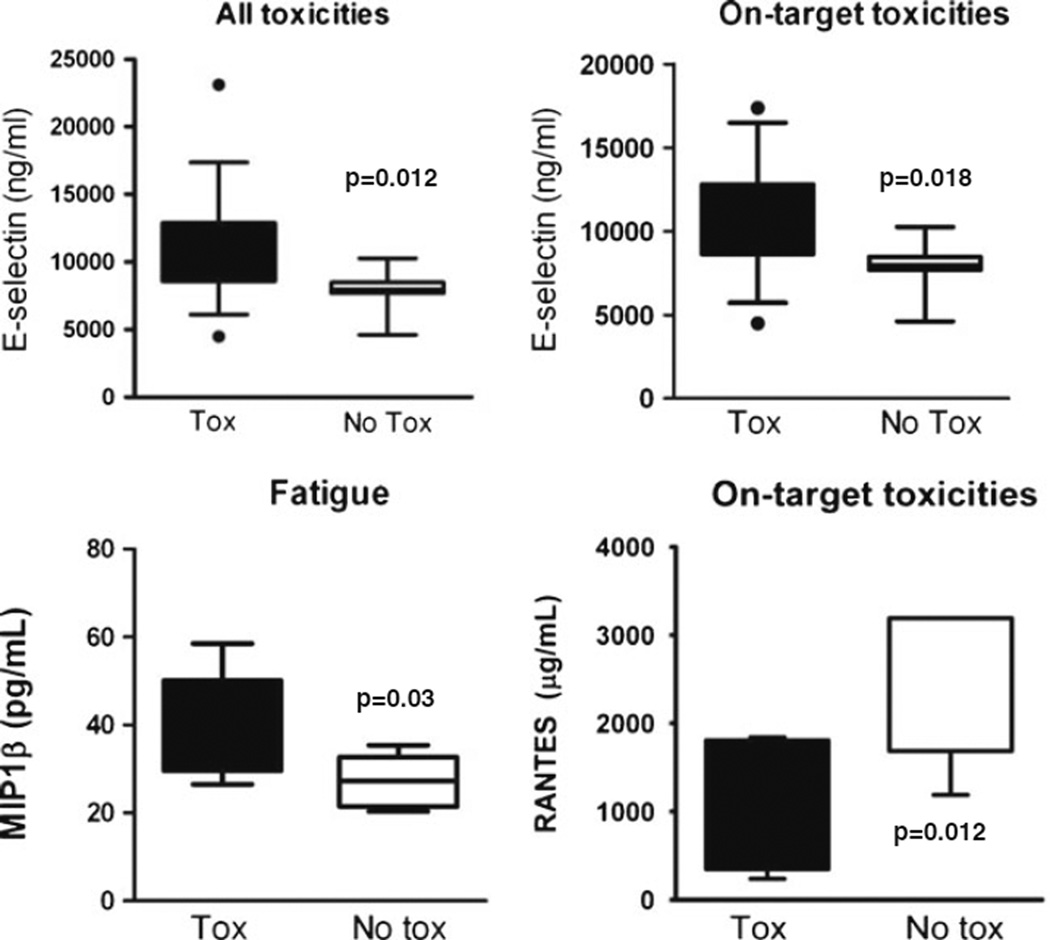
We evaluated cytokines and angiogenic factors at baseline for those without toxicity and compared them to those in our three subgroups. Baseline elevation of e-selectin predicted the development of all toxicity (p=0.012, n=19) as well as on-target toxicity (p=0.008, n=12), while baseline elevation of macrophage inflammatory protein 1b (MIP-1b) correlated with developing fatigue (p=0.030, n=4) (Fig. 2). A lower level of “regulated on activation normal T cell expressed and secreted” (RANTES) at baseline predicted on-target toxicity (p=0.028, n=7). We also found that baseline levels of TIMP2 and CA9 were elevated in patients experiencing all toxicity (p=0.045, n=12, and p=0.014, n=15, respectively); levels of GM-CSF, PDGF, and CA9 (p=0.038, n=12; p=0.020, n=12; and p=0.036, n=10, respectively) were associated with on-target toxicity development, and low levels of GM-CSF (p=0.018, n=4) correlated with the development of fatigue (data not shown).
Fig. 2.
Baseline cytokines and angiogenic factors and toxicity (Tox) by category. Expression of individual cytokines at baseline for patients with and without the development of all toxicities, on-target toxicities, or fatigue during treatment with aflibercept. Box–whisker plots: horizontal line in the middle portion of the box, mean. Bottom and top boundaries of boxes, 25th and 75th percentiles, respectively. Lower and upper whiskers, 5th and 95th percentiles, respectively. Log-transformed baseline expressions were used and evaluated with Cox proportional hazard regression models to assess the effect of biomarkers on toxicity. All cytokines depicted were statistically significant, p<0.05 between the toxicity and notoxicity groups
Change in cytokine and angiogenic factors during treatment correlated with the development of toxicity
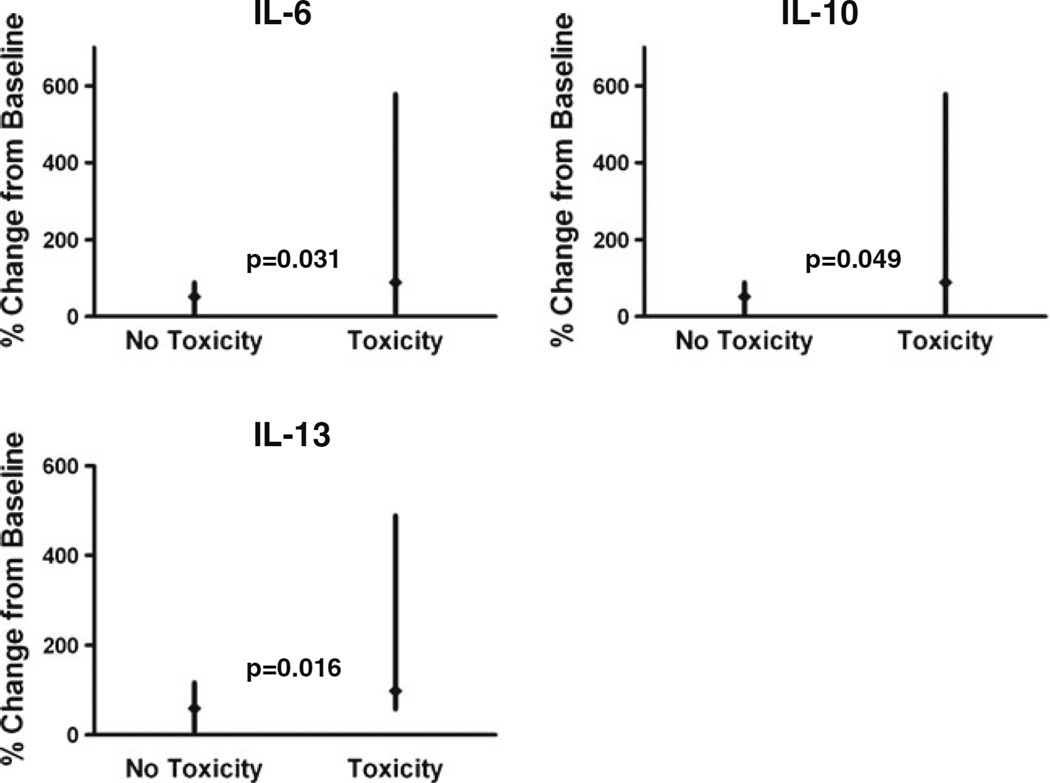
After assessing the relationship between baseline cytokines and angiogenic factors and the development of adverse events, we analyzed the change for each factor during treatment and correlated the change with toxicity. We compared the change in each marker for the no-toxicity group with the marker change in the all-toxicity group. At 24 h, increases in IL-6 (p=0.031, n=17) significantly correlated with all toxicity (Fig. 3). Less decline in IL-10 and IL-13 correlated with all toxicity when compared to reduction of these factors in those without toxicity (p=0.049, 0.016; n=17). At 28 days, there was a trend toward increased toxicity with elevations in IL-13 and pro-inflammatory IL-18, but these were not significant (p=0.126 and 0.082; n=12, respectively). We did not find significant associations between toxicity and VEGF or PlGF, either at baseline or during therapy.
Fig. 3.
Modulation in IL-6, IL-10, and IL-13 related to the development of all toxicities. Relationship between proportional (in percent) cytokine change from baseline to 24 h for patients with and without the development of all toxicity. Data are shown as median change from baseline (solid diamond) with minimum and maximum values (vertical line)
Change in cytokine and angiogenic factors during treatment also correlated with the type of toxicity
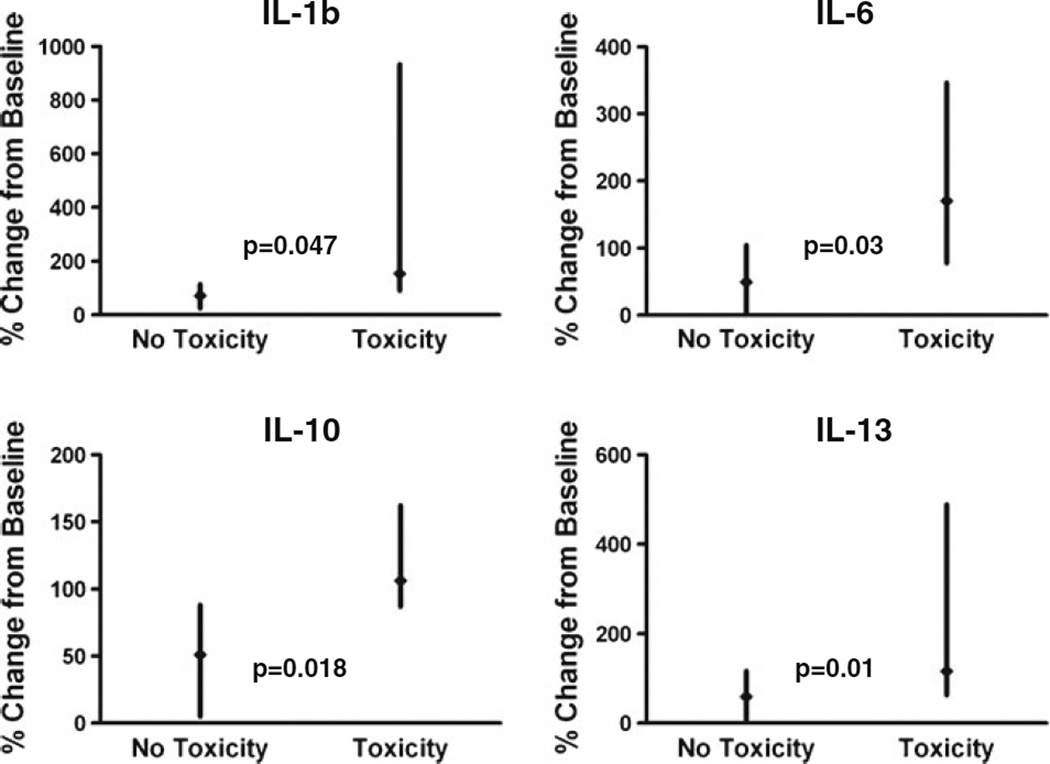
We also analyzed the change for each factor during treatment and correlated the change specifically for the two toxicity subgroups. At 24 h, IL-13 decreased by less than 20 % compared to a 40–60 % reduction from baseline in patients without on-target toxicity (p=0.010, n=7). We also found that a decrease in bFGF at 24 h and an increase in MCP3 at 28 days correlated with the development of on-target toxicities (p=0.016, n=8; p=0.046, n=5). Patients who developed fatigue had higher levels of IL-1b, IL-6, and IL-10 at 24 h (p=0.047, 0.030, and 0.018; n=4) (Fig. 4).
Fig. 4.
Modulation in IL-6, IL-10, and IL-1b related to the development of fatigue and modulation in IL-13 related to the development of on-target toxicities. Relationship between proportional (in percent) cytokine change from baseline to 24 h for patients with and without the development of fatigue or on-target toxicity. Data are shown as median change from baseline (solid diamond) with minimum and maximum values (vertical line)
The presence of all toxicity and on-target toxicity correlated with PFS
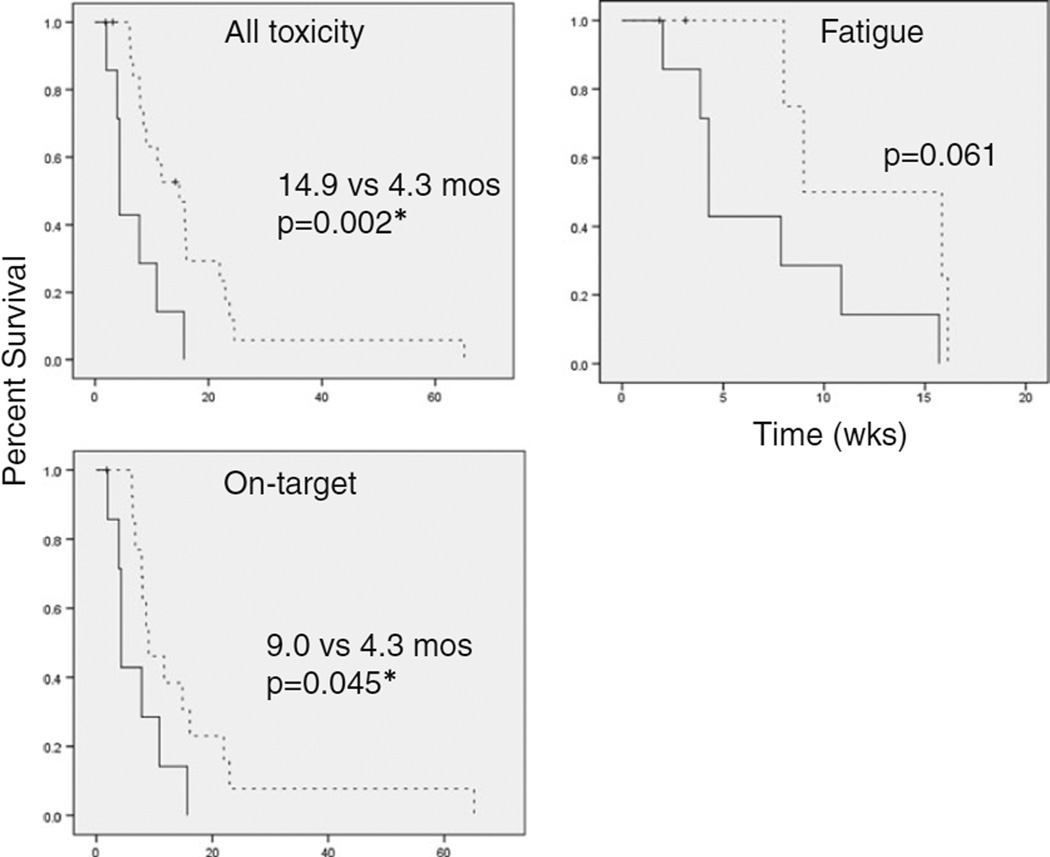
The progression-free survival (PFS) for patients in the all-toxicity group was 14.9 months comparing favorably with 4.3 months in those who did not develop any Gr II–IV toxicity (p=0.002). Patients with toxicity received an average of 2.5 (median, 2.0) cycles prior to the development of toxicity, and those who did not develop any toxicity received an average of 3.125 (median, 2.5) cycles of aflibercept. Of the eight patients without any toxicity, 75 % came off of the study for PD, and among the 20 patients with toxicity, a similar proportion, 70 %, came off of the study for PD (≥25 % increase in the sum of the products of perpendicular diameters of enhancing lesions). Patients with on-target toxicity had a PFS of 9.0 months compared with 4.3 months, which was also statistically significant (Fig. 5, p=0.045). Patients experiencing fatigue also had a PFS of 9.0 months, compared with 4.3 months in the no-toxicity group, but this was not significant (p=0.061), likely due to the limited number of patients in this cohort.
Fig. 5.
Progression-free survival by toxicity group. Kaplan–Meier curves depict the PFS for those in each toxicity group compared with those not experiencing toxicity. Dotted lines indicate toxicity and solid lines indicate no toxicity
Discussion
Cytokines regulate homeostasis by balancing pro-inflammatory Th1-mediated cascades with anti-inflammatory Th2-mediated responses. Antiangiogenic therapy is associated with specific toxicities including fatigue and on-target effects such as hypertension, proteinuria, and bleeding. Hypothesizing that patients who are most likely to develop toxicity to antiangiogenic treatment will have differing levels of pro-inflammatory factors, effectively tipping this balance, levels of cytokines and angiogenic factors were monitored throughout treatment with aflibercept for recurrent glioblastoma. Several factors correlated with the development of toxicity. Elevated levels of e-selectin, RANTES, and MIP1b at baseline correlated with the development of toxicity, whereas changes in IL-6, IL-10, and IL-13 during the course of therapy were associated with general toxicity. More specifically, increases in IL-6, IL-10, and IL-1b correlated with fatigue, and changes in IL-13 and MCP3 were associated with on-target toxicities (hypertension, proteinuria, and bleeding).Many of these significant changes occurred early—within 24 h of treatment initiation—making these factors more useful as predictive tools.
Ideally, predictive markers will be identified for which clinicians can screen patients and then intervene to limit toxicity. Baseline elevations of e-selectin, RANTES, and MIP1b predicted the development of toxicity. E-Selectin is a member of a family of carbohydrate-binding proteins and is expressed on cytokine-activated endothelial cells in vivo only when they are activated by pro-inflammatory cytokines in the setting of inflammation or malignancy [28]. RANTES promotes endothelial dysfunction and was elevated at baseline in our patients who developed on-target toxicity. MIP-1b, which was elevated at baseline in those patients who developed fatigue, activates granulocytes leading to acute inflammation. Together, these cytokines may be mechanistically linked to inflammatory-mediated toxicities related to antiangiogenic therapy observed in this trial.
Cytokines and angiogenic factors may mediate injury, and changes in those markers may predict toxicity. Although we cannot exclude that some patients might have an undetected underlying medical condition that made them more sensitive to the drug, the entry criteria should have prevented the enrollment of patients with significant underlying renal or cardiovascular disease onto this trial.
Aflibercept-mediated modulation of circulating factors may have been associated with the development of toxicities. Specifically, we observed that changes in IL-6, IL-10, and IL-13 significantly correlated with all toxicity and aflibercept-specific toxicities. Importantly, increases in IL-6 correlated with fatigue, a well-supported phenomenon in the general oncology literature. In a large meta-analysis of cancer patients, elevated IL-6 correlated with fatigue with a high level of statistical significance (p=0.0005) [16]. We also found that elevations in IL-10, an immune-modulator of both B and T cells, were associated with fatigue. IL-13, a profibrotic cytokine which leads to fibroblast proliferation and decreased prostaglandin E2, was significantly elevated in both the on-target and all-toxicity groups at 24 h compared to those without toxicity [29, 30]. Profiling of IL-6 and IL-10 could identify patients most likely to develop fatigue, while profiling of IL-13 as a surrogate for endothelial dysfunction could individualize patient risk for on-target toxicity.
Cytokines regulate highly complex functions involving immunity, inflammation, and carcinogenesis. Although these markers may directly cause toxicity, it is more probable that these cytokines serve as surrogate markers of multifaceted and complex mechanisms of toxicity, which have yet to be elucidated. Given the exploratory nature of our analysis, we were not able to adjust for other variables. We recognize the possibility that the analysis of multiple markers for this small group may have led to the discovery of false positive markers. For this reason, we focused on markers that were not only significant but also which had plausible biologic explanations. Correlative data, as with all limited-size single-arm clinical trials, is not definitive. The small sample size and lack of a control group not receiving antiangiogenic therapy precludes our ability to confirm the predictive value of our findings. However, several of our findings were confirmed when the data were analyzed for all toxicity as well as specific on-target toxicities which increase our confidence in their importance. Furthermore, some of the specific cytokine changes, such as the relationship between increases in IL-6 and fatigue, have been reported for other malignancies. Additional studies are necessary to validate our findings. Our finding that patients with toxicity had a longer PFS than those without does not appear to be due to increased treatment duration since the median exposure time to develop toxicity was 2.5 cycles which is shorter than the median number of cycles prior to PD. Certainly, those with response were more likely to stay on trial longer to develop toxicity, but those coming off of the study for PD were equally distributed between groups. Extended PFS was not explained by superior rescue: Six patients received bevacizumab after progression without significant response.
Although aflibercept had only minimal efficacy for recurrent glioblastoma (GBM) [25], we believe that: (1) These data may be extrapolated to other VEGF-targeted agents such as bevacizumab, which is FDA approved for recurrent GBM and (2) these data may provide guidance when using aflibercept in other malignancies. Aflibercept has recently been shown to improve overall survival in patients with metastatic colorectal cancer, and early studies of aflibercept in ovarian cancer have also shown efficacy [31–33].
To our knowledge, this is the first study to correlate profiles of cytokines and angiogenic factors and toxicity in patients receiving an anti-VEGF therapy in glioblastoma patients. A recent publication explored the non-hematologic toxicities and cytokine changes associated with survival in patients with hepatocellular cancer treated with sunitinib [34]. Toxicity from antiangiogenic therapy, particularly on-target toxicity and fatigue, remains an important cause of treatment discontinuation and patient morbidity and deserves further investigation.
In this study, almost 20 % of patients had to discontinue treatment due to adverse events, two for hypertension, one for fatigue, one for rash, and another for seizures. Cytokine levels did not consistently correlate with toxicity when we compared levels for patients with grade II toxicity versus grades III/IV toxicity. In other words, patients with grade III/IV toxicity did not necessarily have higher cytokine levels. In addition, the absolute cytokine level did not necessarily correlate with treatment discontinuation.
Of the five patients who came off of the study for toxicity, four had only grade 2 toxicities. This may be due to few grade III/IV events. Although our findings are primarily exploratory, they may be helpful toward elucidating the mechanisms underlying the toxicities of VEGF-targeted agents.
Our data suggest that profiling of specific cytokines, such as IL-13, IL-6, and IL-10, might predict those patients at greatest risk for toxicity or for developing important complications during treatment. Once identified, those patients at higher risk for toxicity could be placed under more stringent surveillance during therapy such as home blood pressure logs or more frequent measurements of urine protein/creatinine ratio. Early detection of toxicity or the identification of patients most likely to develop a specific toxicity could aid in drug dosing and allow for the integration of supportive measures to prevent severe toxicity that might otherwise require treatment discontinuation.
Supplementary Material
Acknowledgments
Funding Funding was received from the National Institutes of Health [U01-CA62399 to NABTC, 1R21A126127 to J. de G.] and the ASCO Career Development Award [to J. de G.].
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11523-013-0254-0) contains supplementary material, which is available to authorized users.
Conflict of interest We report that no funds or benefits of any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Contributor Information
Nicole Shonka, Email: nshonka@unmc.edu, Division of Oncology and Hematology, University of Nebraska Medical Center, 987680, Omaha, NE 68198-7680, USA.
Yuji Piao, M.D. Anderson Cancer Center, University of Texas, 1515 Holcombe Blvd Unit 431, Houston, TX 77030, USA.
Mark Gilbert, M.D. Anderson Cancer Center, University of Texas, 1515 Holcombe Blvd Unit 431, Houston, TX 77030, USA.
Alfred Yung, M.D. Anderson Cancer Center, University of Texas, 1515 Holcombe Blvd Unit 431, Houston, TX 77030, USA.
Susan Chang, University of California San Francisco, 400 Parnassus Ave A808, San Francisco, CA 94143, USA.
Lisa M. DeAngelis, Memorial Sloan Kettering Cancer Center, 1275 York Avenue C732, New York, NY 10021, USA
Andrew B. Lassman, Department of Neurology and Herbert Irving Comprehensive Cancer Center, New York-Presbyterian/Columbia University Medical Center, 710 West 168th Street, New York, NY 10032, USA
Jun Liu, M.D. Anderson Cancer Center, University of Texas, 1515 Holcombe Blvd Unit 431, Houston, TX 77030, USA.
Timothy Cloughesy, University of California Los Angeles, 710 Westwood Plaza Room 1-230, Los Angeles, CA 90095, USA.
H. Ian Robins, University of Wisconsin Medical School, 600 Highland Ave K4/534, Madison, WI 53792-6164, USA.
Rita Lloyd, University of Wisconsin Medical School, 600 Highland Ave K4/534, Madison, WI 53792-6164, USA.
Alice Chen, NIH, NCI and CTEP, Bethesda, MD, USA.
Michael Prados, University of California San Francisco, 400 Parnassus Ave A808, San Francisco, CA 94143, USA.
Patrick Y. Wen, Dana Farber Cancer Institute, 44 Binney St. SW430D, Boston, MA 02115, USA
John Heymach, M.D. Anderson Cancer Center, University of Texas, 1515 Holcombe Blvd Unit 431, Houston, TX 77030, USA.
John de Groot, M.D. Anderson Cancer Center, University of Texas, 1515 Holcombe Blvd Unit 431, Houston, TX 77030, USA.
References
- 1.Batchelor TT, Duda DG, di Tomaso E, Ancukiewicz M, Plotkin SR, Gerstner E, Eichler AF, Drappatz J, Hochberg FH, Benner T, Louis DN, Cohen KS, Chea H, Exarhopoulos A, Loeffler JS, Moses MA, Ivy P, Sorensen AG, Wen PY, Jain RK. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 3.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu AX, Duda DG, Ancukiewicz M, di Tomaso E, Clark JW, Miksad R, Fuchs CS, Ryan DP, Jain RK. Exploratory analysis of early toxicity of sunitinib in advanced hepatocellular carcinoma patients: kinetics and potential biomarker value. Clin Cancer Res. 2011;17(4):918–927. doi: 10.1158/1078-0432.CCR-10-0515. doi: 10.1158/1078-0432.CCR-10-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okunieff P, Chen Y, Maguire DJ, Huser AK. Molecular markers of radiation-related normal tissue toxicity. Cancer Metastasis Rev. 2008;27(3):363–374. doi: 10.1007/s10555-008-9138-7. doi: 10.1007/s10555-008-9138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33(1):99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 7.De Jaeger K, Seppenwoolde Y, Kampinga HH, Boersma LJ, Belderbos JS, Lebesque JV. Significance of plasma transforming growth factor-beta levels in radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58(5):1378–1387. doi: 10.1016/j.ijrobp.2003.09.078. doi: 10.1016/j.ijrobp.2003.09.078. [DOI] [PubMed] [Google Scholar]
- 8.Menard C, Johann D, Lowenthal M, Muanza T, Sproull M, Ross S, Gulley J, Petricoin E, Coleman CN, Whiteley G, Liotta L, Camphausen K. Discovering clinical biomarkers of ionizing radiation exposure with serum proteomic analysis. Cancer Res. 2006;66(3):1844–1850. doi: 10.1158/0008-5472.CAN-05-3466. doi: 10.1158/0008-5472.CAN-05-3466. [DOI] [PubMed] [Google Scholar]
- 9.Kovacs CJ, Daly BM, Evans MJ, Johnke RM, Lee TK, Karlsson UL, Allison R, Eaves GS, Biggs LM. Cytokine profiles in patients receiving wide-field+prostate boost radiotherapy (xRT) for adenocarcinoma of the prostate. Cytokine. 2003;23(6):151–163. doi: 10.1016/s1043-4666(03)00185-6. [DOI] [PubMed] [Google Scholar]
- 10.Christensen E, Pintilie M, Evans KR, Lenarduzzi M, Menard C, Catton CN, Diamandis EP, Bristow RG. Longitudinal cytokine expression during IMRT for prostate cancer and acute treatment toxicity. Clin Cancer Res. 2009;15(17):5576–5583. doi: 10.1158/1078-0432.CCR-09-0245. doi: 10.1158/1078-0432.CCR-09-0245. [DOI] [PubMed] [Google Scholar]
- 11.Hartsell WF, Scott CB, Dundas GS, Mohiuddin M, Meredith RF, Rubin P, Weigensberg IJ. Can serum markers be used to predict acute and late toxicity in patients with lung cancer? Analysis of RTOG 91-03. Am J Clin Oncol. 2007;30(4):368–376. doi: 10.1097/01.coc.0000260950.44761.74. doi: 10.1097/01.coc.0000260950.44761.74. [DOI] [PubMed] [Google Scholar]
- 12.Wichers M, Maes M. The psychoneuroimmunopathophysiology of cytokine-induced depression in humans. Int J Neuropsychopharmacol. 2002;5(4):375–388. doi: 10.1017/S1461145702003103. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- 13.Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(Suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 14.Souhami L, Seiferheld W, Brachman D, Podgorsak EB, Werner-Wasik M, Lustig R, Schultz CJ, Sause W, Okunieff P, Buckner J, Zamorano L, Mehta MP, Curran WJ., Jr Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60(3):853–860. doi: 10.1016/j.ijrobp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Daigle JL, Hong JH, Chiang CS, McBride WH. The role of tumor necrosis factor signaling pathways in the response of murine brain to irradiation. Cancer Res. 2001;61(24):8859–8865. [PubMed] [Google Scholar]
- 16.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21(4):413–427. doi: 10.1016/j.bbi.2006.11.004. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Drevs J, Siegert P, Medinger M, Mross K, Strecker R, Zirrgiebel U, Harder J, Blum H, Robertson J, Jurgensmeier JM, Puchalski TA, Young H, Saunders O, Unger C. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25(21):3045–3054. doi: 10.1200/JCO.2006.07.2066. doi: 10.1200/JCO.2006.07.2066. [DOI] [PubMed] [Google Scholar]
- 18.Rixe O, Billemont B, Izzedine H. Hypertension as a predictive factor of sunitinib activity. Ann Oncol. 2007;18(6):1117. doi: 10.1093/annonc/mdm184. [DOI] [PubMed] [Google Scholar]
- 19.Rixe O, Dutcher J, Motzer R, Wilding G, Stadler W, Kim S, Tarazi J, Rosbrook B, Rini B. Association between diastolic blood pressure >90 mmHg and efficacy in patients with metastatic renal cell carcinoma receiving axitinib. Ann Oncol. 2008;19(8):189. [Google Scholar]
- 20.Rini BI, Wilding G, Hudes G, Stadler WM, Kim S, Tarazi J, Rosbrook B, Trask PC, Wood L, Dutcher JP. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27(27):4462–4468. doi: 10.1200/JCO.2008.21.7034. doi: 10.1200/JCO.2008.21.7034. [DOI] [PubMed] [Google Scholar]
- 21.Rini B, Schiller J, Fruehauf J, Cohen E, Tarazi J, Rosbrook B, Ricart A, Olszanski A, Kim S, Spano J. Association of diastolic blood pressure (dBP) >=90 mmHg with overall survival (OS) in patients treated with axitinib (AG-013736) J Clin Oncol. 2008;26(15):3543. [Google Scholar]
- 22.Fruehauf J, Lutzky J, McDermott D, Brown CK,Meric JB, Rosbrook B, Shalinsky DR, Liau KF, Niethammer AG, Kim S, Rixe O. Multicenter, phase II study of axitinib, a selective second-generation inhibitor of vascular endothelial growth factor receptors 1, 2, and 3, in patients with metastatic melanoma. Clin Cancer Res. 2011;17(23):7462–7469. doi: 10.1158/1078-0432.CCR-11-0534. doi: 10.1158/1078-0432.CCR-11-0534. [DOI] [PubMed] [Google Scholar]
- 23.Riely G, Miller V. Vascular endothelial growth factor trap in non small cell lung cancer. Clin Cancer Res. 2007;15(2):s4623–s4627. doi: 10.1158/1078-0432.CCR-07-0544. [DOI] [PubMed] [Google Scholar]
- 24.Lockhart AC, Rothenberg ML, Dupont J, Cooper W, Chevalier P, Sternas L, Buzenet G, Koehler E, Sosman JA, Schwartz LH, Gultekin DH, Koutcher JA, Donnelly EF, Andal R, Dancy I, Spriggs DR, Tew WP. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol. 2010;28(2):207–214. doi: 10.1200/JCO.2009.22.9237. doi: 10.1200/JCO.2009.22.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Groot JF, Lamborn KR, Chang SM, Gilbert MR, Cloughesy TF, Aldape K, Yao J, Jackson EF, Lieberman F, Robins HI, Mehta MP, Lassman AB, Deangelis LM, Yung WK, Chen A, Prados MD, Wen PY. Phase II study of aflibercept in recurrent malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol. 2011;29(19):2689–2695. doi: 10.1200/JCO.2010.34.1636. doi: 10.1200/JCO.2010.34.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Groot JF, Piao Y, Tran H, Gilbert M, Wu HK, Liu J, Bekele BN, Cloughesy T, Mehta M, Robins HI, Lassman A, DeAngelis L, Camphausen K, Chen A, Yung WK, Prados M, Wen PY, Heymach JV. Myeloid biomarkers associated with glioblastoma response to anti-VEGF therapy with aflibercept. Clin Cancer Res. 2011;17(14):4872–4881. doi: 10.1158/1078-0432.CCR-11-0271. doi: 10.1158/1078-0432.CCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (remark) Exp Oncol. 2006;28(2):99–105. [PubMed] [Google Scholar]
- 28.Shamay Y, Paulin D, Ashkenasy G, David A. E-selectin binding peptide-polymer-drug conjugates and their selective cytotoxicity against vascular endothelial cells. Biomaterials. 2009;30(32):6460–6468. doi: 10.1016/j.biomaterials.2009.08.013. doi: 10.1016/j.biomaterials.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 30.Saito A, Okazaki H, Sugawara I, Yamamoto K, Takizawa H. Potential action of IL-4 and IL-13 as fibrogenic factors on lung fibroblasts in vitro. Int Arch Allergy Immunol. 2003;132(2):168–176. doi: 10.1159/000073718. doi: 73718. [DOI] [PubMed] [Google Scholar]
- 31.Van Cutsem ETJ, Lakomy R. Intravenous aflibercept versus placebo in combination with irinotecan/5-FU (FOLFIRI) for second-line treatment of metastatic colorectal cancer (MCRC): results of a multinational phase III trial (EFC10262-VELOUR) Ann Oncol. 2011;22 [Google Scholar]
- 32.Coleman RL, Duska LR, Ramirez PT, Heymach JV, Kamat AA, Modesitt SC, Schmeler KM, Iyer RB, Garcia ME, Miller DL, Jackson EF, Ng CS, Kundra V, Jaffe R, Sood AK. Phase 1–2 study of docetaxel plus aflibercept in patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer. Lancet Oncol. 2011;12(12):1109–1117. doi: 10.1016/S1470-2045(11)70244-3. doi: 10.1016/S1470-2045(11)70244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colombo N, Mangili G, Mammoliti S, Kalling M, Tholander B, Sternas L, Buzenet G, Chamberlain D. A phase II study of aflibercept in patients with advanced epithelial ovarian cancer and symptomatic malignant ascites. Gynecol Oncol. 2012;125(1):42–47. doi: 10.1016/j.ygyno.2011.11.021. doi: 10.1016/j.ygyno.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Zhu AX, Duda DG, Sahani DV, Jain RK. Development of sunitinib in hepatocellular carcinoma: rationale, early clinical experience, and correlative studies. Cancer J. 2009;15(4):263–268. doi: 10.1097/PPO.0b013e3181af5e35. doi: 10.1097/PPO.0b013e3181af5e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.