Abstract
Objective. Here we aimed to clarify the prognostic significance of perineural invasion (PNI) in esophageal and esophagogastric junction (EGJ) carcinoma. Methods. A comprehensive literature search for relevant reports published up to July 2015 was performed using Pubmed and Embase databases. The pooled HR and 95% CI for overall survival (OS) and disease-free survival (DFS) were used to assess the prognostic value. The association of PNI with pathological characteristics was evaluated by OR and 95% CI. Results. A total of 13 cohorts were retrieved, covering 2770 patients treated by surgery. The cumulative analysis revealed a statistical correlation between PNI and poor OS (HR = 1.76, 95% CI: 1.54–2.20, and P < 0.00001), as well as poor DFS (HR = 1.96, 95% CI: 1.42–2.71, and P < 0.001). Moreover, analysis of 1475 patients showed improved PNI in T3 + T4 (OR = 0.39, 95% CI: 0.21–0.70, and P = 0.002), N+ (OR = 0.52, 95% CI: 0.40–0.69, and P < 0.00001), and G3 + G4 (OR = 0.66, 95% CI: 0.48–0.90, and P = 0.008) patients compared with T1 + T2, N−, and G1 + G2 ones, respectively. No significant heterogeneity was found between the studies. Conclusions. PNI is an adverse prognostic biomarker in esophageal and EGJ carcinoma. Moreover, PNI implies advanced T, N stage and poor cell differentiation.
1. Introduction
Esophageal carcinoma is the 6th most common cause of cancer death worldwide with very aggressive biological behavior [1]. Despite the development in diagnostic modalities and stratified treatment due to TNM classification in recent years, the prognosis of patients remains poor with an overall 5-year survival of 15%–25% [2]. Identification of novel prognostic factors may be helpful to attain more individualized and efficient cancer therapy.
Perineural invasion (PNI) is the process of neoplastic invasion of nerves which facilitates aggressive growth and spread of tumor cells [3]. In multiple malignancies such as head and neck cancer, pancreatic adenocarcinoma, colorectal cancer, gastric and prostate cancer, PNI occurrence is correlated with high recurrence rates, aggressive behavior, and poor survival [4–8]. Given the considerable clinical impact, PNI was added to the 7th UICC/AJCC TNM classification as a new parameter [9]. But the investigations on prognostic role of PNI in esophageal and esophagogastric junction (EGJ) carcinoma have released inconsistent results. Although recognized as independent prognostic factor in several studies [10, 11], PNI was reported by Ochiai et al. not significantly related to overall survival (OS) in esophageal cancer [12]. Moreover, PNI was found to be significant factor for OS in adenocarcinoma of esophagus only but not in squamous cell carcinoma (SCC) by univariate analysis, while multivariate analysis failed to draw the conclusion [13]. The aim of our study was to evaluate the prognostic value of PNI in esophageal and EGJ carcinoma by systematically reviewing the available evidence.
2. Methods
2.1. Search Strategy
We searched Pubmed and Embase for studies correlating the presence of PNI with patients' survival in esophageal and EGJ carcinoma published up to July 30, 2015. The search terms included “perineural invasion”, “neural invasion”, “esophageal carcinoma”, “esophagogastric junction carcinoma”, “gastric cardiac carcinoma”, and “prognosis”. The studies were limited to human articles published in English. In addition, citation lists of retrieved articles were manually screened to identify potentially relevant studies.
2.2. Selection Criteria
Studies were included if they met the following criteria: (1) the diagnoses of esophageal and EGJ carcinoma and PNI were based on pathological examination; (2) the studies reported the outcome of OS or disease-free survival (DFS); (3) they provided hazard ratio (HR) with confidence interval (CI) or original data sufficient for calculating them. Studies published as abstracts only were excluded.
2.3. Data Extraction and Quality Assessment
Data were extracted independently by two authors (Gao and Wang) using a standard protocol. The following information was collected from each study: first author, year of publication, study design, patients characteristics (country of origin, number, age, sex, duration of follow-up, PNI positive rate, etc.), clinicopathological features (perioperative treatment, TNM stage, pathological grade, cell type, etc.), and survival (OS and DFS), whose data was summarized by HR and its 95% CI. Quality assessment was performed for each eligible study using the Newcastle-Ottawa quality assessment scale (NOS) [14]. Studies with NOS scores ≥6 were considered to be of high quality.
2.4. Statistical Analysis
The meta-analysis was performed using Review Manager V5.3 software (Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration). For studies whose HR and 95% CI were not reported directly, we estimated them according to the method of Tierney [25]. Q statistical test and I 2 value were used to assess the heterogeneity of these studies. P < 0.1 and/or I 2 > 50% was considered as significant heterogeneity and the cause was analyzed [26]. Pooled estimates of the HRs were obtained by fixed-effect model where no significant heterogeneity was found. Otherwise, a random-effect model was used. Publication bias was assessed using a funnel plot. Subgroup analyses were performed by ethnicity and cell type.
3. Results
3.1. Search Results and Study Characteristics
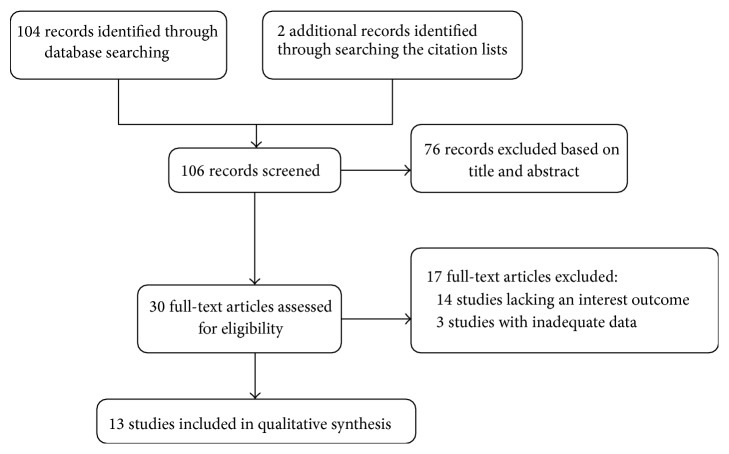
The search strategy retrieved one hundred and four unique articles, of which 76 were excluded after the first screening of titles and abstracts. Hand searching of the citation lists identified two additional articles. After reviewing the full texts of the remaining 30 articles potential to be eligible, 17 studies were excluded for lacking an interest outcome or inadequate data, leaving 13 studies [10, 11, 13, 15–24] comprising 2770 patients treated by surgery with/without perioperative chemo(radio)therapy for final inclusion in the meta-analysis (Figure 1).
Figure 1.
Flow diagram of study selection procedure.
All the included studies were retrospective cohort studies. They were published between 1995 and 2015, with sample size ranging from 26 to 691 (median = 142). Six of these studies were based on Asian population [10, 11, 16, 19, 22, 24], 5 were European [13, 15, 18, 20, 21], and the other two were American [17, 23]. A median of PNI positive rate was 33.3% (5.5% to 61.3%). Three studies investigated patients with SCC [10, 11, 20], six with non-SCC [15, 16, 21–24], and the remaining 4 with mixed pathological types [13, 17, 18, 20]. Among the 13 cohorts, HRs and 95% CIs for OS were directly reported in nine. Four studies [10, 19, 20, 22] simultaneously reported DFS as the outcome in which two HRs were provided directly. For the remaining 4 [13, 16, 17, 21] and 2 [10, 22] studies on OS and DFS, HRs and 95% CIs were calculated using data provided in the original articles. Detailed clinical and pathological characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of included cohorts.
| Study | Country/area | Number (male/female) |
Tumor sites | PNI positive n (positive rate) |
nCRT1/nCT2
number |
TNM edition | Stage of TNM | Outcome | SQ3 |
|---|---|---|---|---|---|---|---|---|---|
| Castonguay et al., 2014 [15] | Canada | 103 (86/17) | Esophagus | 57 (55.3%) | 5 | AJCC/UICC 7th | I–IV | OS4 | 7 |
| Chen et al., 2014 [10] | China | 433 (321/112) | Esophagus | 209 (48.3%) | 0 | AJCC/UICC 7th | I–III | DFS5, OS | 7 |
| Huang et al., 2013 [16] | China | 42 (33/9) | Esophagus | 14 (33.3%) | 0 | AJCC/UICC 7th | I–IV | OS | 5 |
| Kim et al., 2011 [17] | America | 266 (196/70) | Esophagus and EGJ6 | 29 (10.9%) | 162 | NR7 | NR | OS | 6 |
| Liebl et al., 2014 [18] | Germany | 311 (249/62) | Esophagus and EGJ | 132 (42.4%) | 0 | AJCC/UICC 7th | I–IV | OS | 8 |
| Ning et al., 2015 [19] | China | 243 (194/49) | Esophagus | 54 (22.2%) | NR | AJCC/UICC 7th | II-III | DFS, OS | 7 |
| Noble et al., 2013 [20] | UK | 246 (195/51) | Esophagus and EGJ | 34 (14.0%) | 151 | AJCC/UICC 7th | I–IV | DFS, OS | 7 |
| Paraf et al., 1995 [21] | France | 67 (61/6) | Lower esophagus | 26 (38.2%) | 0 | NR | NR | OS | 5 |
| Sun et al., 2015 [22] | Taiwan | 26 (23/3) | Esophagus and EGJ | 5 (19.2%) | 0 | AJCC/UICC 7th | I–III | DFS, OS | 6 |
| Tachezy et al., 2014 [13] | Germany | 695 (555/140) | Esophagus | 38 (5.0%) | 52 | AJCC/UICC 7th | I–IV | OS | 7 |
| Tanaka et al.,1998 [11] | Japan | 104 (84/20) | Esophagus | 48 (46.2%) | 22 | AJCC/UICC 1993 | NR | OS | 7 |
| Torres et al., 1999 [23] | America | 96 (83/13) | Lower esophagus | 31 (32.3%) | 61 | AJCC/UICC 1993 | I–IV | OS | 5 |
| Zhang et al., 2012 [24] | China | 142 (109/33) | EGJ II-III | 87 (61.3%) | 0 | AJCC/UICC 7th | I–IV | OS | 6 |
1nCRT: neoadjuvant chemoradiotherapy; 2nCT: neoadjuvant chemotherapy; 3SQ: study quality based on the Newcastle-Ottawa scale; 4OS: overall survival; 5DFS: disease-free survival; 6EGJ: esophagogastric junction; 7NR: not reported.
3.2. OS and DFS Related to PNI Status
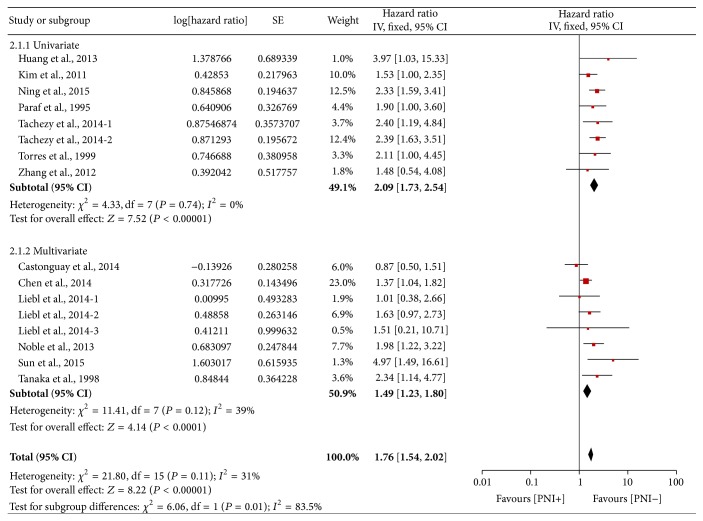
A total of 16 HR values for OS were included because HRs of AEG (adenocarcinoma of esophagogastric junction) I, AEG II/III, SCC in Liebl et al.'s study [18], and HRs of SCC, adenocarcinoma in Tachezy et al.'s study [13], were all reported separately, which were labeled in our analysis as Liebl et al.'s, 2014-1, Liebl et al.'s, 2014-2, Liebl et al.'s, 2014-3, Tachezy et al.'s, 2014-1, and Tachezy et al.'s, 2014-2, respectively. A pooled analysis of 13 cohorts including 16 HRs demonstrated that PNI was associated with poor OS in esophageal and EGJ carcinoma (HR = 1.76, 95% CI: 1.54–2.20, and P < 0.00001; Figure 2). Because no significant heterogeneity was found (P = 0.11, I 2 = 31%), a fixed-effect model was used here. There was no evidence for publication bias (Figure 3).
Figure 2.
Forest plot of the combined hazard ratio (HR) for the association of PNI with OS.
Figure 3.
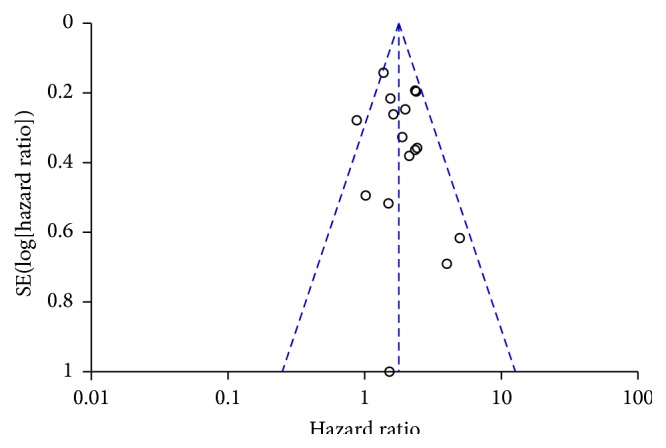
Funnel plot to visualize the potential publication bias in the prognostic assessment of PNI on OS.
Among the 13 studies, seven [13, 16, 17, 19, 21, 23, 24] were investigated by univariate analysis with HRs ranging from 1.48 to 3.97, and six [10, 11, 15, 18, 20, 22] were by multivariate analysis with HRs ranging from 0.87 to 4.97. To further explore the study heterogeneity, we performed subgroup analysis by univariate and multivariate HRs. Again, PNI predicted poor OS in both subgroups (univariate analysis subgroup: HR = 2.09, 95% CI: 1.73–2.54, and P < 0.00001; multivariate analysis subgroup: HR = 1.49, 95% CI: 1.23–1.80, and P < 0.0001; Figure 4). There was no significant heterogeneity in either subgroup (P = 0.74 and 0.12, I 2 = 0 and 39%, resp.)
Figure 4.
Forest plot of the combined HR for the association of PNI with OS in univariate and multivariate subgroups.
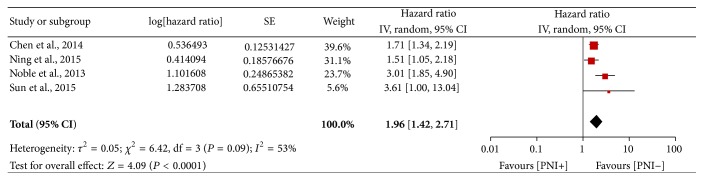
Only four studies [10, 19, 20, 22] comprising 948 patients reported DFS as the outcome simultaneously. A combined analysis revealed a random-effect HR of 1.96 (95% CI: 1.42–2.71, P < 0.0001; Figure 5), suggesting PNI is an unfavourable predictor of DFS. Moderate heterogeneity was observed in the comparison (P = 0.09, I 2 = 53%).
Figure 5.
Forest plot of the combined HR for the association of PNI with DFS.
3.3. Stratified Analyses
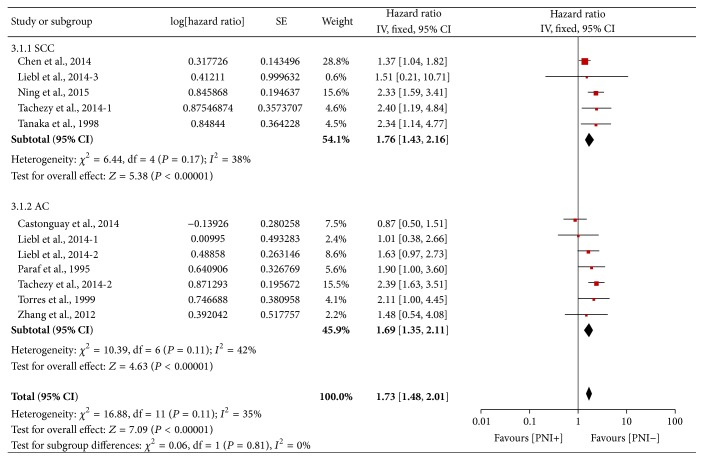
In order to identify the value of PNI on different pathological type of esophageal and EGJ carcinoma, we stratified 12 HRs whose cell type was reported as SCC or adenocarcinoma (AC) only. A total of 1183 patients were included in SCC subgroup and 1007 in AC subgroup. A pooled data showed that PNI was an unfavourable indicator of OS in SCC subgroup with HRs of 1.76 (95% CI: 1.43–2.16, P < 0.00001), as well as in AC subgroup with HR of 1.69 (95% CI: 1.35–2.11, P < 0.00001) (Figure 6). Mild heterogeneity was found in both subgroups (SCC subgroup: P = 0.17, I 2 = 38%; AC subgroup: P = 0.11, I 2 = 42%).
Figure 6.
Forest plot of the combined HR for the association of PNI with OS in SCC and AC subgroups.
The stratified analysis comparing the Asian and non-Asian subgroups yielded similar results. In Asian subgroup comprising 990 subjects, the pooled fixed HR was 1.79 (95% CI 1.45–2.19, P < 0.0001), while in non-Asian subgroup analysis of 1780 subjects yielded a pooled HR of 1.74 (95% CI: 1.46–2.09, P < 0.00001) (Figure 7), indicating that PNI is prognostically important in esophageal and EGJ carcinoma regardless of the geographic area.
Figure 7.
Forest plot of the combined HR for the association of PNI with OS in Asian and non-Asian subgroups.
3.4. Correlation between PNI and Pathological Characteristics
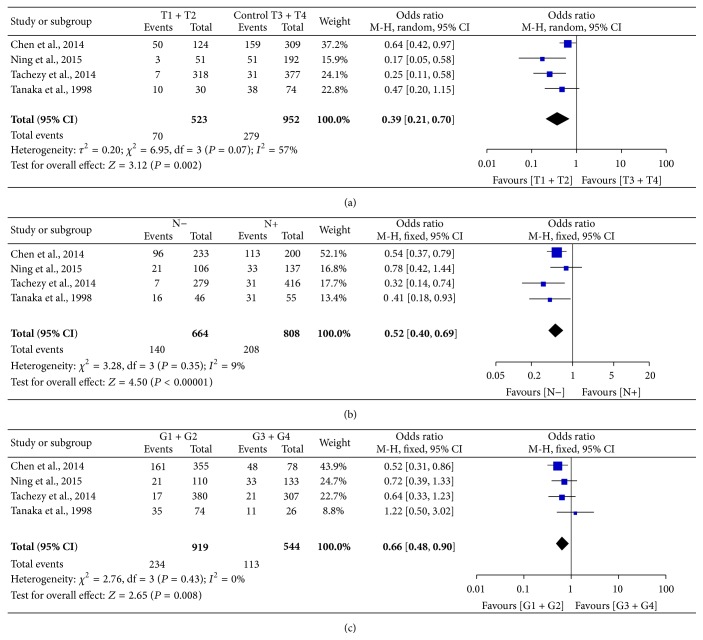
Only four cohorts [10, 11, 13, 19] which included 1475 patients from China, Germany, and Japan reported PNI rate based on different T, N stages and histological grade (G). The combined analysis revealed improved PNI positivity in T3 + T4, N+, and G3 + G4 patients comparing with T1 + T2, N−, and G1 + G2 ones, with ORs of 0.39 (95% CI: 0.21–0.70, P = 0.002, Figure 8(a)), 0.52 (95% CI: 0.40–0.69, P < 0.0001, Figure 8(b)), and 0.66 (95% CI: 0.48–0.90, P = 0.008, Figure 8(c)), respectively, indicating presence of PNI was associated with advanced T, N stage and poor cell differentiation. While no significant heterogeneity was found in the comparison of different N and G status (P = 0.35 and 0.43, I 2 = 9% and 0%, resp.), moderate heterogeneity was found in the comparison of T stage (P = 0.07, I 2 = 57%).
Figure 8.
Forest plot of the pooled OR for the association of PNI with T stage (a), N stage (b), and histological grade (c).
4. Discussion
Although PNI is considered as a distinct route for dissemination and metastasis of tumor cells [27], there are conflicting reports on its prognostic significance in esophageal and EGJ carcinoma. Therefore, we performed the meta-analysis and demonstrated that PNI was an adverse factor of OS and DFS in patients treated with surgery. The pooled HR from multivariate analyses indicated that its prognostic effect on OS was independent of depth of invasion, lymph node status, and tumor grade as well as other clinicopathological features. In head and neck cancers, the great majority of patients with leptomeningeal carcinomatosis originating from PNI have no evidence of lymph node metastasis, confirming that the process of PNI is a distinct way of metastasis [28, 29]. We advocate that PNI might be included in the TNM staging system of esophageal and EGJ carcinoma as a new prognostic parameter, helping to identify patients who might benefit from additional treatment or intensified follow-up.
In the NCCN guideline for esophageal and EGJ cancers (version 3, 2015), PNI is recommended as a high-risk feature to guide the postoperative treatment of lower-esophagus and EGJ adenocarcinoma patients. However, our stratified analysis by cell type showed that positivity of PNI predicted poor OS in both SCC and AC subgroups, which would probably extend its importance in clinical practice.
Asia, often referred to as “esophageal cancer belt,” is a high-risk area with distinct epidemiological and histological properties from non-Asian area [1]. However, our analysis based on Asian and non-Asian population did not affect the prognostic value of PNI, suggesting the predictive significance is independent of ethnicity.
We also evaluated the relationship of PNI with the well-known and established prognostic criteria, including T, N stage and histological grade, which constitute the basis for the AJCC TNM classification of esophageal and EGJ cancer [30]. As indicated by the pooled analyses, PNI is statistically significantly related to infiltration depth (described as T stage) and lymph node metastasis, suggesting that PNI is an important biological marker of tumor invasion. Furthermore, improved PNI positivity in poorly differentiated tumors implied that PNI indicated more malignant behavior of tumor cells. The underlying mechanism may be explained by the presence of cross talk between tumor cells and nerve terminations around, which is supported by substantial experimental researches [31–33]. In this biological process, neurotransmitters and neuropeptides secreted by nerve terminations act as molecule determinants and promote tumor invasion and metastasis [27, 34].
This meta-analysis, to our knowledge, is the first study to systematically evaluate the prognostic significance of PNI in esophageal and EGJ carcinoma. Only mild to moderate heterogeneity was detected between these studies, which can be attributed mainly to inconsistent definitions and detection methods of PNI, different geographic areas, editions of TNM classification, tumor sites, histological types, and therapy strategies. Notably, the included cohorts were all retrospective researches which may cause heterogeneity themselves. In spite of this discrepancy, the prognostic significance of PNI was not affected. Standardized definition and detection method are desirable for getting accurate PNI positive rate in the future.
In conclusion, PNI is an independent and adverse prognostic indicator which implies aggressive and metastatic behaviors of tumor cells in esophageal and EGJ carcinoma patients treated with surgery. Introduction of PNI as a novel prognostic parameter may provide additional information to guide clinical therapy and follow-up. Furthermore, prospective cohort studies with large samples are expected to validate our findings in the future.
Acknowledgments
This meta-analysis was supported in part by grants from Science and Technology Development Plan of Jinan (Grant no. 201401071), Natural Science Foundation of Shandong Province (Grant no. 2014BSA01002), and the project of the National Natural Science Foundation of China (Grant no. 81402299).
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A., Gibson M. K., Jobe B. A., Luketich J. D. Oesophageal carcinoma. The Lancet. 2013;381(9864):400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 3.Liebig C., Ayala G., Wilks J. A., Berger D. H., Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115(15):3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 4.Cozzi G., Rocco B. M., Grasso A., et al. Perineural invasion as a predictor of extraprostatic extension of prostate cancer: a systematic review and meta-analysis. Scandinavian Journal of Urology. 2013;47(6):443–448. doi: 10.3109/21681805.2013.776106. [DOI] [PubMed] [Google Scholar]
- 5.Deng J., You Q., Gao Y., et al. Prognostic value of perineural invasion in gastric cancer: a systematic review and meta-analysis. PLoS ONE. 2014;9(2) doi: 10.1371/journal.pone.0088907.e88907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A., Veness M., De'Ambrosis B., Selva D., Huilgol S. C. Management of squamous cell and basal cell carcinomas of the head and neck with perineural invasion. Australasian Journal of Dermatology. 2016;57(1):3–13. doi: 10.1111/ajd.12314. [DOI] [PubMed] [Google Scholar]
- 7.Liebig C., Ayala G., Wilks J., et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. Journal of Clinical Oncology. 2009;27(31):5131–5137. doi: 10.1200/JCO.2009.22.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J.-F., Hua R., Sun Y.-W., et al. Influence of perineural invasion on survival and recurrence in patients with resected pancreatic cancer. Asian Pacific Journal of Cancer Prevention. 2013;14(9):5133–5139. doi: 10.7314/APJCP.2013.14.9.5133. [DOI] [PubMed] [Google Scholar]
- 9.Sobin L. H. TNM Classification of Malignant Tumours. 7th. Oxford, UK: Wiley-Blackwell; 2009. [Google Scholar]
- 10.Chen J.-W., Xie J.-D., Ling Y.-H., et al. The prognostic effect of perineural invasion in esophageal squamous cell carcinoma. BMC Cancer. 2014;14(1, article 313) doi: 10.1186/1471-2407-14-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka A., Matsumura E., Yosikawa H., et al. An evaluation of neural invasion in esophageal cancer. Surgery Today. 1998;28(9):873–878. doi: 10.1007/s005950050245. [DOI] [PubMed] [Google Scholar]
- 12.Ochiai M., Arai K., Funabiki T., et al. Local spread of carcinoma of the esophagus by perineural invasion. Nihon Geka Gakkai Zasshi. 1995;96(3):137–144. [PubMed] [Google Scholar]
- 13.Tachezy M., Tiebel A. K., Gebauer F., et al. Prognostic impact of perineural, blood and lymph vessel invasion for esophageal cancer. Histology and Histopathology. 2014;29(11):1467–1475. doi: 10.14670/HH-29.1467. [DOI] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 15.Castonguay M. C., Li-Chang H. H., Driman D. K. Venous invasion in oesophageal adenocarcinoma: enhanced detection using elastic stain and association with adverse histological features and clinical outcomes. Histopathology. 2014;64(5):693–700. doi: 10.1111/his.12308. [DOI] [PubMed] [Google Scholar]
- 16.Huang Q., Wu H., Nie L., et al. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. The American Journal of Surgical Pathology. 2013;37(4):467–483. doi: 10.1097/pas.0b013e31826d2639. [DOI] [PubMed] [Google Scholar]
- 17.Kim T., Grobmyer S. R., Smith R., et al. Esophageal cancer—the five year survivors. Journal of Surgical Oncology. 2011;103(2):179–183. doi: 10.1002/jso.21784. [DOI] [PubMed] [Google Scholar]
- 18.Liebl F., Demir I. E., Mayer K., et al. The impact of neural invasion severity in gastrointestinal malignancies: a clinicopathological study. Annals of Surgery. 2014;260(5):900–908. doi: 10.1097/sla.0000000000000968. [DOI] [PubMed] [Google Scholar]
- 19.Ning Z. H., Zhao W., Li X. D., et al. The status of perineural invasion predicts the outcomes of postoperative radiotherapy in locally advanced esophageal squamous cell carcinoma. International Journal of Clinical and Experimental Pathology. 2015;8(6):6881–6890. [PMC free article] [PubMed] [Google Scholar]
- 20.Noble F., Hopkins J., Curtis N., et al. The role of systemic inflammatory and nutritional blood-borne markers in predicting response to neoadjuvant chemotherapy and survival in oesophagogastric cancer. Medical Oncology. 2013;30(3, article 596) doi: 10.1007/s12032-013-0596-6. [DOI] [PubMed] [Google Scholar]
- 21.Paraf F. F., Pignon J. P., Fékété F., Potet F. Surgical pathology of adenocarcinoma arising in Barrett's esophagus. Analysis of 67 cases. American Journal of Surgical Pathology. 1995;19(2):183–191. doi: 10.1097/00000478-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y. H., Lin S. W., Chen C. H., Liang W. Y., Hsieh C. C. Adenosquamous carcinoma of the esophagus and esophagogastric junction: clinical manifestations and treatment outcomes. Journal of Gastrointestinal Surgery. 2015;19(7):1216–1222. doi: 10.1007/s11605-015-2852-x. [DOI] [PubMed] [Google Scholar]
- 23.Torres C. W., Turner J. R., Richards W., Sugarbaker D., Shahsafaei A., Odze R. D. Pathologic prognostic factors in esophageal squamous cell carcinoma: a follow-up study of 74 patients with or without preoperative chemoradiation therapy. Modern Pathology. 1999;12:961–968. [PubMed] [Google Scholar]
- 24.Zhang Y.-F., Shi J., Yu H.-P., et al. Factors predicting survival in patients with proximal gastric carcinoma involving the esophagus. World Journal of Gastroenterology. 2012;18(27):3602–3609. doi: 10.3748/wjg.v18.i27.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tierney J. F., Stewart L. A., Ghersi D., Burdett S., Sydes M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8, article 16 doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ioannidis J. P. A., Patsopoulos N. A., Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. The British Medical Journal. 2007;335(7626):914–916. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancino M., Ametller E., Gascón P., Almendro V. The neuronal influence on tumor progression. Biochimica et Biophysica Acta (BBA)—Reviews on Cancer. 2011;1816(2):105–118. doi: 10.1016/j.bbcan.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Padhya T. A., Cornelius R. S., Athavale S. M., Gluckman J. L. Perineural extension to the skull base from early cutaneous malignancies of the midface. Otolaryngology—Head and Neck Surgery. 2007;137(5):742–746. doi: 10.1016/j.otohns.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan L. M., Smee R. Leptomeningeal carcinomatosis from perineural invasion of a lip squamous cell carcinoma. Australasian Radiology. 2006;50(3):262–266. doi: 10.1111/j.1440-1673.2006.01577.x. [DOI] [PubMed] [Google Scholar]
- 30.Rice T. W., Blackstone E. H., Rusch V. W. 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Annals of Surgical Oncology. 2010;17(7):1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 31.Ding Y., He D., Florentin D., et al. Semaphorin 4F as a critical regulator of neuroepithelial interactions and a biomarker of aggressive prostate cancer. Clinical Cancer Research. 2013;19(22):6101–6111. doi: 10.1158/1078-0432.ccr-12-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magnon C., Hall S. J., Lin J., et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142) doi: 10.1126/science.1236361.1236361 [DOI] [PubMed] [Google Scholar]
- 33.Scanlon C. S., Banerjee R., Inglehart R. C., et al. Galanin modulates the neural niche to favour perineural invasion in head and neck cancer. Nature Communications. 2015;6, article 6885 doi: 10.1038/ncomms7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchesi F., Piemonti L., Mantovani A., Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine & Growth Factor Reviews. 2010;21(1):77–82. doi: 10.1016/j.cytogfr.2009.11.001. [DOI] [PubMed] [Google Scholar]