Abstract
Aims
This analysis evaluated HbA1c-adjusted hypoglycemia risk with glargine versus neutral protamine Hagedorn (NPH) over a 5-year study in patients with Type 2 diabetes mellitus (T2DM). Clinical significance was assessed using number needed to harm (NNH) to demonstrate the risk of one additional patient experiencing at least one hypoglycemic event.
Methods
Individual patient-level data for symptomatic documented hypoglycemia and HbA1c values from a 5-year randomized study comparing once-daily glargine (n = 513) with twice-daily NPH (n = 504) were analyzed. Symptomatic hypoglycemia was categorized according to concurrent self-monitoring blood glucose levels and need for assistance. Hypoglycemic events per patient-year as a function of HbA1c were fitted by negative binomial regression using treatment and HbA1c at endpoint as independent variables. An estimate of NNH was derived from logistic regression models.
Results
The cumulative number of symptomatic hypoglycemia events was consistently lower with glargine compared with NPH over 5 years. Compared with twice-daily NPH, once-daily glargine treatment resulted in significantly lower adjusted odds ratios (OR) for all daytime hypoglycemia (OR 0.74; p = 0.030) and any severe event (OR 0.64; p = 0.035), representing a 26% and 36% reduction in the odds of daytime and severe hypoglycemia, respectively. Our model predicts that, if 25 patients were treated with NPH instead of glargine, then one additional patient would experience at least one severe hypoglycemic event.
Conclusions
This analysis of long-term insulin treatment confirms findings from short-term studies and demonstrates that glargine provides sustained, clinically meaningful reductions in risk of hypoglycemia compared with NPH in patients with T2DM.
Keywords: Clinical science and care, Clinical diabetes, Glargine, NPH, Hypoglycemia
1. Introduction
Hypoglycemia is an important barrier to treatment for many patients with Type 2 diabetes mellitus (T2DM) — in particular, those with an extended duration of disease who receive insulin therapy (Cryer, 2007; Frier, 2008). Fear of hypoglycemia is one of the key factors that prevent good glycemic control because patients and healthcare providers are discouraged from starting or intensifying insulin treatment (Cryer, 1999, 2002; Korytkowski, 2002).
Short-term clinical trials have shown that use of long-acting insulin analogues, such as glargine and insulin detemir, is associated with fewer hypoglycemic events compared with conventional neutral protamine Hagedorn (NPH) insulin therapy (Fritsche, Schweitzer, & Haring, 2003; Massi-Benedetti, Humburg, Dressler, & Ziemen, 2003; Riddle, Rosenstock, & Gerich, 2003; Rosenstock et al., 2001; Yki-Jarvinen, Dressler, & Ziemen, 2000). A meta-analysis of 12 trials comparing glargine with NPH confirmed the benefit of this analogue in reducing the risk of hypoglycemia (Bazzano et al., 2008). A meta-regression analysis that modeled the interaction between hypoglycemia and glycosylated hemoglobin (HbA1c) showed that glargine was also associated with less risk of hypoglycemia than NPH, at any given level of glycemic control (Mullins, Sharplin, Yki-Jarvinen, Riddle, & Haring, 2007).
To date, the advantage of long-acting analogues has not been confirmed in long-term controlled studies under conditions similar to clinical practice. The completion of a 5-year randomized study comparing the effects of glargine versus NPH as basal insulin on progression of retinopathy in patients with T2DM (Rosenstock, Fonseca, McGill, et al., 2009a) provided an opportunity to examine this issue in a long-term setting, as has been done previously for other issues of interest (Rosenstock, Fonseca, McGill, et al., 2009b). The original analysis of the study showed a lower risk of hypoglycemia with glargine compared with NPH, without any differences in the rate of progression of diabetic retinopathy (Rosenstock, Fonseca, McGill, et al., 2009a).
Our present analysis focused on several clinically relevant aspects of hypoglycemia, including: 1) the cumulative time-course of hypoglycemic events; 2) the relationship between hypoglycemic events and HbA1c at endpoint; 3) rates of several categories of hypoglycemia adjusted for HbA1c at endpoint and; 4) an endpoint HbA1c-adjusted computation of the number needed to harm (NNH) for one additional patient to experience at least one hypoglycemic event if NPH is used rather than glargine. NNH is an important metric when comparing medicines, as it directly examines a clinically relevant treatment outcome over a set period of time. NNH compares the outcomes for patients if they were treated with one therapy versus their outcomes if they were treated with an alternative therapy. This enables physicians to make treatment decisions based on evidence of the potential harm of choosing one treatment over another.
2. Research design and methods
The analysis included hypoglycemia and HbA1c data from the 5-year study, which compared randomized treatment with glargine (once daily) or NPH (twice daily), both associated with oral antidiabetic drugs (OADs), in order to assess retinopathy progression (NCT00174824) (Rosenstock, Fonseca, McGill, et al., 2009a). Entry criteria included: T2DM for at least 1 year; age 30–70 years old; HbA1c 6% – 12% at screening; stable OAD and/or insulin treatment; no prior treatment with glargine or other analogues; and no proliferative or severe non-proliferative diabetic retinopathy. Following randomization, patients received open-label glargine once daily (usually at bedtime) or NPH twice daily (usually in the morning and at bedtime). Insulin doses were titrated over the first 3 years of the study in both groups, to achieve standard glycemic control as determined by fasting plasma glucose (FPG) levels of ≤6.7 mmol/L (≤120 mg/dL). This target was reduced to ≤5.5 mmol/L (≤100 mg/dL) for the final 2 years of the study but no systematic titration regimen was enforced. Intensification of conventional therapy was allowed; therefore, in addition to patients receiving basal insulin plus OADs, prandial insulin (regular human insulin but not fast-acting analogues) could be added with meals, at the investigator’s discretion, even if not used at baseline. No specific titration guidelines were provided for preprandial regular insulin dosing. Self-monitoring of blood glucose (SMBG) was to be performed daily in the fasting state before breakfast using an Accu-Chek blood glucose meter (Roche Diagnostics, Indianapolis, IN, USA). All episodes of symptomatic hypoglycemia and all SMBG values were recorded in patient diaries and reviewed by the site personnel at each visit.
The original primary study outcome was the percentage of patients with ≥3-step progression in Early Treatment Diabetic Retinopathy Study (ETDRS) score after 5 years of treatment. Secondary study outcomes included various assessments of the progression and severity of diabetic retinopathy, as published previously (Rosenstock, Fonseca, McGill, et al., 2009a). Additional secondary study outcomes included HbA1c and FPG change from baseline, incidence and rate of hypoglycemia, and insulin dose.
For the present report, further analyses were performed focusing on HbA1c-adjusted hypoglycemia. Using individual patient-level data from the source trial, the between-treatment comparison of the proportion of patients with at least one hypoglycemic event adjusted for HbA1c values achieved at study end was evaluated. Hypoglycemia was grouped into six non-exclusive categories: (1) all symptomatic hypoglycemia, confirmed or not; (2) symptomatic hypoglycemia confirmed by SMBG <3.9 mmol/L (<70 mg/dL); (3) symptomatic hypoglycemia confirmed by SMBG <2.0 mmol/L (<36 mg/dL); (4) severe hypoglycemia, defined as symptomatic hypoglycemia requiring third-party assistance and either with SMBG levels of ≤3.1 mmol/L (≤56 mg/dL) or prompt recovery after oral carbohydrate, intravenous glucose or glucagon administration; (5) all symptomatic daytime hypoglycemia; (6) all symptomatic nocturnal hypoglycemia. Asymptomatic, non-severe episodes were not included in this analysis.
2.1. Analytical methods for hypoglycemia
The cumulative incidence of symptomatic hypoglycemic events during the study (i.e. comparison of the two types of insulin) was analysed graphically, without formal statistical testing. All hypoglycemic events were included in the analyses; this is different from the previously reported analysis of hypoglycemia in this study (Rosenstock, Fonseca, McGill, et al., 2009a), in which events occurring in the active titration period (first 3 months) were not included owing to the potential for increased rates of hypoglycemia associated with the change in treatment, which may not be representative of long-term therapy with the basal insulin. The treatment effects of glargine compared with NPH, calculated from the logistic regressions, are expressed as odds ratios (ORs) and the corresponding 95% confidence intervals (CIs), with two-sided p values under H0: OR = 1 adjusted for HbA1c achieved at endpoint. Rates of the different categories of hypoglycemia were also adjusted for HbA1c achieved at endpoint. Hypoglycemic events per patient-year were plotted against HbA1c achieved at endpoint and fitted by negative binomial regression using treatment and HbA1c achieved at endpoint as independent variables.
Number needed to harm was defined as the number of patients to be treated with NPH instead of glargine for one additional patient to experience at least one hypoglycemic event. Number needed to harm was calculated as NNH = 1/(pNPH−pglargine), where pNPH and pglargine are the risks of one or more hypoglycemia episodes adjusted for HbA1c at endpoint in a person receiving NPH or glargine, respectively, under the conditions of this study. These risks were derived from the respective logistic regression model and are, therefore, adjusted for endpoint HbA1c.
3. Results
In the original trial, 1017 participants were randomized and received treatment. The treatment groups formed by randomization were generally well balanced in terms of baseline characteristics (Table 1). A total of 498 and 486 patients in the glargine and NPH groups, respectively, had complete information regarding HbA1c and occurrence of hypoglycemia, and were included in this analysis.
Table 1.
Patient baseline characteristics (intention-to-treat population).
| Glargine (n = 513) | NPH (n = 504) | |
|---|---|---|
| Age (years), mean ± SD | 54.9 ± 8.8 | 55.3 ± 8.5 |
| Age <65 years, n (%) | 429 (83.6) | 427 (84.7) |
| Female, n (%) | 235 (45.8) | 234 (46.4) |
| Weight (kg), mean ± SD | 100.2 ± 22.7 | 98.7 ± 22.3 |
| Height (cm), mean ± SD | 170.1 ± 10.1 | 170.1 ± 10.3 |
| Body mass index, kg/m2, mean ± SD | 34.5 ± 7.2 | 34.1 ± 7.2 |
| Duration of diabetes (years), mean ± SD | 10.7 ± 6.9 | 10.8 ± 6.7 |
| Prior use of OAD, n (%) | 494 (96.3) | 476 (94.4) |
| Prior use of insulin, n (%) | 344 (67.1) | 354 (70.2) |
| HbA1c at baseline (%), mean ± SD | 8.4 ± 1.4 | 8.3 ± 1.4 |
NPH = neutral protamine Hagedorn; SD = standard deviation; OAD = oral antidiabetic drug; HbA1c = glycosylated hemoglobin.
3.1. Insulin dosage and glycemic control
Table 2 shows insulin dosages in the two groups at the end of the study. The mean (±standard deviation [SD]) daily doses of once-daily glargine were lower than those of twice-daily NPH, 62.1 ± 39.8 and 73.0 ± 47.9 U, respectively (Table 2). However, of the 283 and 295 patients in the glargine and NPH groups, respectively, requiring prandial insulin (Table 2), the mean daily prandial doses at the end of treatment were greater in the glargine group (mean ± SD: 47.1 ± 42.4 U and 32.9 ± 35.6 U; respectively). The total daily doses of insulin were not significantly different between the treatment groups. At the end of the study, 43.2% of patients in the glargine group and 39.3% in the NPH group were taking basal insulin with OADs but without prandial insulin.
Table 2.
Insulin dosage and HbA1c at study endpoint.
| Glargine (n = 498) |
NPH (n = 486) |
p value | |
|---|---|---|---|
| Final insulin dose, U/day (SD) | |||
| Basal | 62.1 (39.8) | 73.0 (47.9) | 0.0001 |
| Prandiala | 47.1 (42.4) | 32.9 (35.6) | <0.0001 |
| Total | 89.3 (66.5) | 93.2 (66.9) | 0.3646 |
| Final insulin dose, U/kg/day (SD) | |||
| Basal | 0.623 (0.377) | 0.738 (0.465) | <0.0001 |
| Prandiala | 0.483 (0.424) | 0.333 (0.372) | <0.0001 |
| Total | 0.902 (0.660) | 0.942 (0.672) | 0.3495 |
| Mean HbA1c, % (SD) | |||
| Baseline | 8.4 (1.4) | 8.3 (1.4) | – |
| Endpoint | 7.8 (1.3) | 7.6 (1.3) | – |
| Adjusted HbA1c change, % (SE)b | −0.5 (0.1) | −0.7 (0.1) | Δ = −0.19 |
| p = 0.012 |
Intention-to-treat population, patients who have HbA1c values at both baseline and endpoint and data for occurrence of hypoglycemia. HbA1c = glycosylated hemoglobin; NPH = neutral protamine Hagedorn; U = unit; SD = standard deviation; SE = standard error.
Sample size was 283 patients in the glargine group and 295 in the NPH group.
Least squares mean HbA1c calculated using analysis of variance with actual treatment group and pooled center as independent variables.
Titration of basal insulin in both treatment groups was based on FPG targets. Mean ± SD FPG levels decreased from baseline and were similar at study end with glargine (10.5 ± 3.7 to 7.7 ± 3.2 mmol/L [190 ± 66 to 140 ± 58 mg/dL]) and NPH (10.0 ± 3.4 to 7.7 ± 3.2 mmol/L [180 ± 61 to 139 ± 58 mg/dL]). As reported previously, mean HbA1c levels decreased from baseline and remained stable to the end of the study in both insulin treatment groups. The last on-treatment values (mean ± SD) were 7.8% ± 1.3% with glargine and 7.6% ± 1.3% with NPH. The adjusted change (mean ± standard error of the mean) from baseline was −0.5% ± 0.1% with glargine and −0.7% ± 0.1% with NPH, p = 0.012).
The significant difference between groups in the doses of basal and prandial insulins, respectively, at the end of the study, raises the question of whether there may be a subgroup effect of differing treatment with prandial insulin. Investigation of this possible effect found that there is a significant difference between those who received no prandial insulin and those who did receive prandial insulin, for all hypoglycemia rates except for severe hypoglycemia. The treatment effect, however, was homogeneous across the two types of insulin except in the case of evening hypoglycemia, where the treatment effect seemed to be strongly significant for the group with no prandial insulin, whereas the treatment effect for the group that received prandial insulin showed no significant effect. In the context of this being a post-hoc analysis, and the consistent lack of interaction effects for all other hypoglycemia parameters, this result can probably be discounted.
3.2. Unadjusted incidence and event rates for symptomatic hypoglycemia
The total number of symptomatic hypoglycemic events during the 5-year study period was higher with NPH, compared with glargine (15,527 vs 11,995). The cumulative number of symptomatic hypoglycemia events was consistently higher with NPH compared with glargine at all time points (Fig. 1). After approximately 2 years of treatment, the rates (slopes) of cumulative symptomatic hypoglycemic events were constant in both the NPH and insulin glargine groups; before this time point, the curves of cumulative events were steeper in both groups. Throughout the study period, rates of symptomatic hypoglycemia were generally higher in the NPH insulin group than in the insulin glargine group. Of note, the glargine arm had less hypoglycemia despite the fact that during the study it had more subjects on sulfonylureas (20.3% and 15.7% in the glargine and NPH groups; respectively). Unadjusted rates of any symptomatic hypoglycemia event per patient-year were lower with glargine than with NPH (5.3 vs 7.4 events/patient-year; p < 0.001).
Fig. 1.
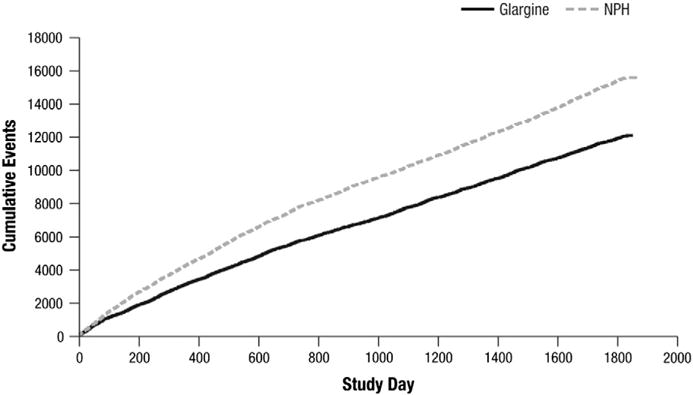
Cumulative number of symptomatic hypoglycemic events.
3.3. HbA1c-adjusted incidence and event rates for categories of hypoglycemia
Table 3 displays incidences and rates for various categories of hypoglycemia without and with adjustment for HbA1c values attained at the end of treatment. In all categories, the adjusted risk of hypoglycemia was lower with glargine treatment than with NPH, with adjusted ORs ranging from 0.64 to 0.86. The difference in risk of experiencing one or more events was statistically significant for all symptomatic events confirmed by SMBG, severe events, and all daytime events. The rates of hypoglycemia expressed as events/patient-year were also lower for all categories with glargine compared with NPH (adjusted rate ratio [RR] ranging from 0.39 to 0.75). The reduction of the event-rate with glargine compared with NPH was statistically significant for all categories except severe hypoglycemia.
Table 3.
Hypoglycemia adjusted for HbA1c at endpoint.
| n (%) |
Glargine (n = 498) |
NPH (n = 486) |
Unadjusted odds ratio (95% CI) |
p valuea | Odds ratio (95% CI) adjusted for HbA1c at endpoint | p valuea | ||
|---|---|---|---|---|---|---|---|---|
| Incidence of people experiencing at least one hypoglycemia event and odds ratios | ||||||||
| Total (all symptomatic) | 389 (78.1%) | 405 (83.3%) | 0.71 (0.52, 0.98) | 0.039 | 0.74 (0.54, 1.02) | 0.070 | ||
| Symptomatic <2.0 mmol/L (<36 mg/dL) | 153 (30.7%) | 183 (37.7%) | 0.73 (0.56, 0.96) | 0.022 | 0.76 (0.58, 0.99) | 0.038 | ||
| Symptomatic <3.9 mmol/L (<70 mg/dL) | 358 (71.9%) | 380 (78.2%) | 0.71 (0.53, 0.95) | 0.023 | 0.74 (0.55, 1.00) | 0.048 | ||
| Severe | 40 (8.0%) | 60 (12.3%) | 0.62 (0.41, 0.95) | 0.026 | 0.64 (0.42, 0.97) | 0.035 | ||
| All daytime | 326 (65.5%) | 354 (72.8%) | 0.71 (0.54, 0.93) | 0.012 | 0.74 (0.56, 0.97) | 0.030 | ||
| All nocturnal | 269 (54.0%) | 282 (58.0%) | 0.85 (0.66, 1.09) | 0.206 | 0.86 (0.67, 1.11) | 0.259 | ||
| Rates of hypoglycemia per patient-yearb
| ||||||||
| Unadjusted rate ratio (95% CI) |
p valuea | Rate ratio (95% CI) adjusted for HbA1c at endpoint | p valuea | |||||
|
| ||||||||
| Total (all symptomatic)c | 5.346 (0.389) | 7.449 (0.547) | 0.71 (0.58, 0.87) | <0.001 | 0.72 (0.59, 0.88) | 0.001 | ||
| Symptomatic <2.0 mmol/L (<36 mg/dL) | 0.312 (0.039) | 0.793 (0.095) | 0.40 (0.29, 0.56) | <0.001 | 0.39 (0.28, 0.55) | <0.001 | ||
| Symptomatic <3.9 mmol/L (<70 mg/dL) | 4.845 (0.384) | 6.785 (0.543) | 0.70 (0.56, 0.87) | 0.002 | 0.71 (0.57, 0.89) | 0.003 | ||
| Severec | 0.041 (0.008) | 0.065 (0.012) | 0.63 (0.38, 1.07) | 0.087 | 0.63 (0.37, 1.07) | 0.085 | ||
| All daytimec | 3.843 (0.290) | 5.426 (0.413) | 0.69 (0.56, 0.86) | <0.001 | 0.71 (0.57, 0.88) | 0.001 | ||
| All nocturnalc | 1.514 (0.141) | 2.017 (0.189) | 0.75 (0.58, 0.97) | 0.028 | 0.75 (0.58, 0.97) | 0.030 | ||
Intention-to-treat population. HbA1c = glycosylated hemoglobin; NPH = neutral protamine Hagedorn; CI = confidence interval.
Two-sided p value for the null hypothesis: odds ratio = 1 or rate ratio = 1, respectively.
Hypoglycemia rates per patient-year from negative binomial regression.
Standard error shown in parentheses.
Hypoglycemia incidence and event rates were also computed with adjustment for individual patients’ HbA1c change from baseline to endpoint (data not shown). Results of this sensitivity analysis showed a pattern similar to the analysis with adjustment for HbA1c at endpoint. Adjusted odds and rates were significantly lower for glargine compared with NPH for all categories of hypoglycemia, with the exception of the odds of experiencing any symptomatic event and the rate of severe hypoglycemic events.
3.4. Relationships between HbA1c achieved at endpoint and categories of hypoglycemia
Regression curves showing the relationships between the rates of hypoglycemia (events/patient-year) and HbA1c achieved at endpoint for the two treatment groups are shown in Fig. 2. Rates were lower with glargine than NPH at all levels of HbA1c. In these regression analyses, the coefficient for endpoint HbA1c was not significantly different from zero, suggesting that endpoint HbA1c had no significant influence on event rates.
Fig. 2.
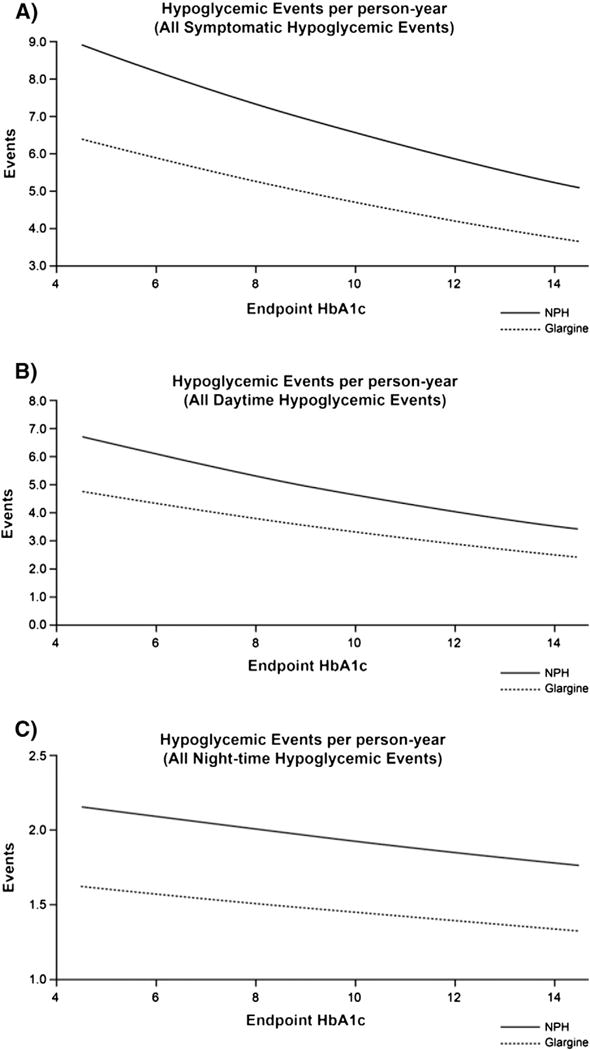
Hypoglycemic events per person-year. (A) All symptomatic events; (B) all daytime events; (C) all nocturnal events. In all three regression analyses, the coefficient for endpoint HbA1c was not significantly different from zero. HbA1c = glycosylated hemoglobin; NPH = neutral protamne Hagedorn.
3.5. Number needed to harm
Results of HbA1c-adjusted NNH analyses are shown in Table 4 and Fig. 3. The number of patients to be treated with NPH instead of glargine for one additional patient to experience at least one symptomatic hypoglycemic event was 22; although, the difference in incidences between the agents was not significantly different from zero (p = 0.068; Table 4 and Fig. 3). For events confirmed by SMBG <3.9 mmol/L (<70 mg/dL), those confirmed by SMBG <2.0 mmol/L (<36 mg/dL), and severe events, the NNHs were 19, 16 and 25, respectively, and all were statistically significant.
Table 4.
Analysis of HbA1c-adjusted number needed to harm with NPH vs Glargine.
| NPH–Glargine 486/498
|
|||
|---|---|---|---|
| NNH | (95% CI) | p valuea | |
| Total hypoglycemia (all symptomatic)b | 22 | [−∞, − 293)∪(11,+∞]c | 0.0682 |
| Symptomatic b2.0 mmol/L (<36 mg/dL) | 16 | (9, 279) | 0.0377 |
| Symptomatic b3.9 mmol/L (<70 mg/dL) | 19 | (10, 1213) | 0.0466 |
| Severed | 25 | (13, 326) | 0.0340 |
| All daytime | 16 | (9, 152) | 0.0291 |
| All nocturnal | 28 | [−∞, −37)∪(11,+∞]c | 0.2583 |
Increased hypoglycemia with NPH indicated by 1 ≤ NNH b ∞; NNH = number needed to harm; CI = confidence interval; SMBG = self-monitoring of blood glucose.
Two-sided p-value for the null hypothesis NNH = ± ∞.
Irrespective of time of day and SMBG values.
∪ indicates the set union of the disjoint intervals.
Symptomatic hypoglycemia requiring assistance and having either SMBG ≤3.1 mmol/L or prompt recovery after oral carbohydrate, intravenous glucose, or glucagon administration.
Fig. 3.
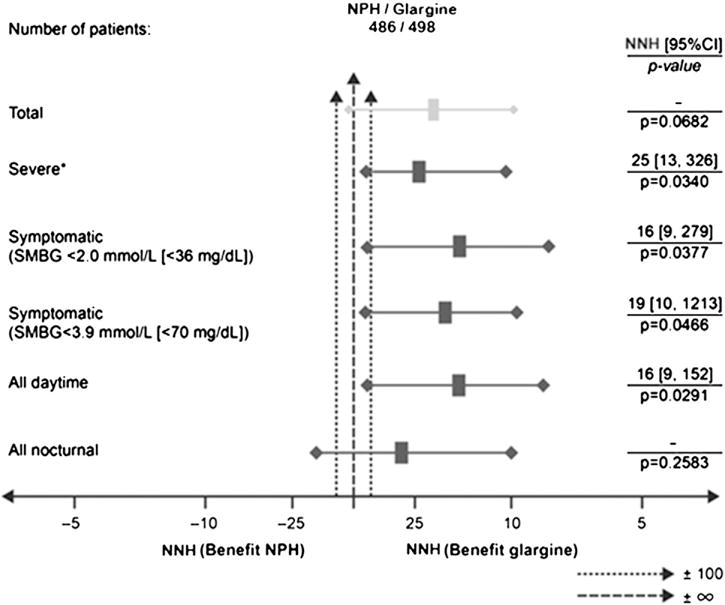
HbA1c-adjusted number needed to harm analysis. *Defined as symptomatic hypoglycemia requiring assistance and having either SMBG ≤3.1 mmol/L or prompt recovery after oral carbohydrate, intravenous glucose, or glucagon administration; HbA1c = glycosylated hemoglobin; NPH = neutral protamine Hagedorn; NNH = number needed to harm; SMBG = self-monitoring of blood glucose; CI = confidence interval.
4. Discussion
The analyses reported here extend our prior observation, outlined in brief previously (Rosenstock, Fonseca, McGill, et al., 2009a), that less hypoglycemia accompanied systematic treatment with glargine than with NPH as basal therapy. These analyses differ from the earlier analyses in several ways. Firstly, in the original report, hypoglycemic events occurring in the first 3 months of treatment were omitted to minimize any possible effect of more active, early, insulin titration with one regimen than the other (Rosenstock, Fonseca, McGill, et al., 2009a). The present analysis included all events recorded throughout the entire 5-year treatment period. Secondly, in the earlier analysis, hypoglycemia was classified as all symptomatic, symptomatic nocturnal, or severe. Here, we have divided hypoglycemic events into additional categories, notably including two categories of hypoglycemia confirmed by SMBG. Thirdly, we have evaluated the relationship between HbA1c levels attained during treatment and the risk of hypoglycemia. This approach was taken because of prior observations that the rate of hypoglycemia in a given clinical situation may be influenced by the intensity of clinical management, and thus the average level of blood glucose and HbA1c achieved during treatment. Without this adjustment, differences in the rate of hypoglycemic events between therapies may be obscured by differences in the clinical efficacy of treatments. Finally, to better describe the potential clinical significance of these findings, an estimate of the NNH was derived from the HbA1c-adjusted incidences of different categories of hypoglycemia. The NNH for one additional patient to experience at least one clinical event is the reciprocal of the absolute risk increase by NPH compared to glargine, and is a commonly used method of describing the findings of controlled trials in a more clinically relevant way (Cook & Sackett, 1995; Tramer & Walder, 2005; Walter, 2001).
Using these methods, the present analyses confirm the implications of the earlier, simpler, analysis. Although total daily insulin doses were similar with glargine and NPH, there were approximately 29% fewer hypoglycemic events reported with glargine compared with NPH treatment. However, the reduction in HbA1c from baseline was slightly greater with NPH than with glargine treatment, resulting in approximately 0.2% lower mean HbA1c at endpoint. Consequently, it could be argued that the greater frequency of hypoglycemia with NPH may be related to the slightly lower HbA1c, and thus mean daily glucose levels.
When hypoglycemia incidences and event rates were adjusted for individually attained HbA1c levels, most of the categories of hypoglycemia studied still showed significantly lower risk of hypoglycemia with glargine than with NPH. Notably, for events confirmed by SMBG <3.9 and <2.0 mmol/L, the odds ratios for hypoglycemia were 0.74 and 0.76 (odds lower with glargine by 26% and 24%), respectively, with glargine versus NPH (p < 0.05 for both). For severe hypoglycemia, an odds ratio of 0.64 – i.e. 36% lower odds of an event with glargine versus NPH – was observed (p = 0.035). Analysis of event rates, which included multiple events in individuals, also showed significant differences. Risk reduction with glargine versus NPH was 29% (rate ratio = 0.71) for events confirmed by SMBG <3.9 mmol/L (p = 0.003) and 61% (rate ratio = 0.39) for events confirmed by SMBG <2.0 mmol/L (p < 0.001). Taken together, these data demonstrate that adjustment for HbA1c levels during the study support the conclusion that hypoglycemia was less frequent and problematic with glargine compared with NPH. An exploratory subgroup analysis found that the reduced risk for hypoglycemia was consistent in both people receiving regular human insulin, as well as basal insulin and in people only receiving basal insulin.
Converting HbA1c-adjusted data into NNH values demonstrated the potential clinical relevance of these findings. The NNH with NPH rather than glargine in order for one additional patient to experience at least one event of hypoglycemia (all six categories) over 5 years ranged from 16 to 28 patients treated with NPH (depending on the level and timing of hypoglycemia), all in a range that might assist with clinical decision-making. Most notably, the analysis demonstrated that, if 25 patients were treated with NPH rather than glargine over 5 years, then one additional patient would experience at least one episode of severe hypoglycemia.
Strengths of these analyses include prospectively planned hypoglycemia data collection over the course of 5 years in a randomized study, and the consistency in results across the different categories of hypoglycemia (symptomatic, daytime, nocturnal). Persistence of the differences in frequency of hypoglycemia between the treatment groups after adjustment for HbA1c achieved at endpoint strengths the conclusion that a glargine-based regimen is indeed associated with less hypoglycemia.
One limitation of this study is the difference in dosing frequency between the groups. Glargine was dosed once daily at bedtime and NPH twice daily, at bedtime and in the morning. The difference in the risk of hypoglycemia observed between groups could conceivably be a result of this dosing frequency. The mean daily basal insulin dose at endpoint was lower with glargine versus NPH but the prandial dose requirement was greater; as such, there was no significant difference in the mean total daily insulin dose between treatment groups at study endpoint. A meta-analysis comparing once-daily glargine versus once-daily NPH showed that glargine was associated with a significant relative reduction in the risk of symptomatic hypoglycemia, suggesting that dosing frequency does not explain the observed differences in the present study (Home, Fritsche, Schinzel, & Massi-Benedetti, 2010).
The present study represents a robust analysis of the risk of hypoglycemia with basal insulin given the extended duration of the study, which is longer than any previous trials. Previous meta-analyses of short-term clinical trials are also consistent in showing a lower risk of hypoglycemia with the long-acting insulin analogues, glargine and insulin detemir, compared with traditional intermediate-acting insulins, such as NPH (Horvath, Jeitler, Berghold, et al., 2007; Monami, Marchionni, & Mannucci, 2008), with implications for both quality of life and medical outcomes. In addition, hypoglycemia has a negative impact on the resources of healthcare systems (Heaton, Martin, & Brelje, 2003; Leese, Wang, Broomhall, et al., 2003; Lundkvist, Berne, Bolinder, & Jonsson, 2005; Rhoads et al., 2005), with significant additional costs associated with hypoglycemic events. Several studies across the world have demonstrated the high costs of hypoglycemia (Ali, White, Lee, et al., 2008; Allicar et al., 2000; Amiel, Dixon, Mann, & Jameson, 2008; Bullano, Fisher, Grochulski, Menditto, & Willey, 2006; Grima, Thompson, & Sauriol, 2007; Jonsson, Bolinder, & Lundkvist, 2006; Lee, Balu, Cobden, Joshi, & Pashos, 2006; Palmer, Lammert, & Hermansen, 2008; Reviriego et al., 2008). Implementation of therapy with long-acting insulin analogues, such as glargine, has been shown to decrease the rate of hypoglycemic events, as well as the costs associated with their occurrence (Bullano, Al-Zakwani, Fisher, Menditto, & Willey, 2005; Bullano et al., 2006; Leichter, 2008; McEwan, Poole, Tetlow, Holmes, & Currie, 2007; Rhoads et al., 2005; Zhang & Menditto, 2005). Moderate-to-severe hypoglycemia, in particular, is associated with significant expenditure on a per-patient basis and was estimated to incur costs in excess of US$ 3000 per year, or a mean cost per event of US$ 1087 (Bullano et al., 2005; Rhoads et al., 2005). Given the low NNH with NPH in the present analysis, further studies might examine whether this translates into lower treatment costs for glargine relative to NPH, which has a much higher risk of hypoglycemia, during long-term therapy.
In summary, this analysis of hypoglycemia in a large, long-term study contributes to the growing body of evidence and adds translational perspective that, compared with NPH, glargine provides a clinically meaningful reduction in the risk of hypoglycemia in patients with T2DM.
Acknowledgments
This study was supported by Sanofi. Editorial Support was provided by Huw Jones, PhD, and Alexander Jones, PhD, of Medicus International and was funded by Sanofi.
Footnotes
Conflicts of interest: J Rosenstock has served on scientific advisory boards and received honorarium or consulting fees from Roche, Sanofi, Novo Nordisk, Eli Lilly and Company, MannKind, GlaxoSmithKline, Takeda, Daiichi Sankyo, Johnson & Johnson, Novartis and Boehringer Ingelheim, and Amylin Pharmaceuticals. He has also received grants/research support from Merck, Pfizer, Sanofi, Novo Nordisk, Roche, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Takeda, Novartis, AstraZeneca, Amylin Pharmaceuticals, Johnson & Johnson, Daiichi Sankyo, MannKind, Intarcia Therapeutics and Boehringer Ingelheim. V Fonseca has received research support (to Tulane University) with grants from Novo Nordisk, Sanofi, Eli Lilly and Company, Daiichi Sankyo, Pamlabs, Reata Pharmaceuticals and Halozyme, and honoraria for consulting and lectures from GlaxoSmithKline, Takeda, Sanofi, Novo Nordisk, Daiichi Sankyo, Pamlabs, Xoma, AstraZeneca and Eli Lilly and Company. S Schinzel and MP Dain are employees of Sanofi. P Mullins has received grants and/or honoraria for consulting from Astra Zeneca, Boehringer Ingelheim, Merck, Novartis, Roche and Sanofi. M Riddle has received grants for research and/or honoraria for consulting or lectures from Amylin Pharmaceuticals, Eli Lilly and Company, the Amylin Lilly Alliance, Novo Nordisk, Pfizer, Sanofi and Valeritas; this conflict of interest has been reviewed and managed by Oregon Health & Science University.
Author contribution
All authors contributed to the interpretation of data and the drafting and critical revision of the manuscript for intellectual content. All authors provided final approval of this version.
References
- Ali M, White J, Lee CH, Palmer JL, Smith-Palmer J, Fakhoury W, et al. Therapy conversion to biphasic insulin aspart 30 improves long-term outcomes and reduces the costs of type 2 diabetes in Saudi Arabia. Journal of Medical Economics. 2008;11:651–670. doi: 10.3111/13696990802589122. [DOI] [PubMed] [Google Scholar]
- Allicar MP, Megas F, Houzard S, Baroux A, Le Thai F, Augendre-Ferrante B. Frequency and costs of hospital stays for hypoglycemia in France in 1995. Presse Médicale. 2000;29:657–661. [PubMed] [Google Scholar]
- Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in type 2 diabetes. Diabetic Medicine. 2008;25:245–254. doi: 10.1111/j.1464-5491.2007.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzano LA, Lee LJ, Shi L, Reynolds K, Jackson JA, Fonseca V. Safety and efficacy of glargine compared with NPH insulin for the treatment of type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetic Medicine. 2008;25:924–932. doi: 10.1111/j.1464-5491.2008.02517.x. [DOI] [PubMed] [Google Scholar]
- Bullano MF, Al-Zakwani IS, Fisher MD, Menditto L, Willey VJ. Differences in hypoglycemia event rates and associated cost-consequence in patients initiated on long-acting and intermediate-acting insulin products. Current Medical Research and Opinion. 2005;21:291–298. doi: 10.1185/030079905X26234. [DOI] [PubMed] [Google Scholar]
- Bullano MF, Fisher MD, Grochulski WD, Menditto L, Willey VJ. Hypoglycemic events and glycosylated hemoglobin values in patients with type 2 diabetes mellitus newly initiated on insulin glargine or premixed insulin combination products. American Journal of Health-System Pharmacy. 2006;63:2473–2482. doi: 10.2146/ajhp050552. [DOI] [PubMed] [Google Scholar]
- Cook RJ, Sackett DL. The number needed to treat: A clinically useful measure of treatment effect. BMJ. 1995;310:452–454. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes/Metabolism Research and Reviews. 1999;15:42–46. doi: 10.1002/(sici)1520-7560(199901/02)15:1<42::aid-dmrr1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycaemia: The limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45:937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia, functional brain failure, and brain death. Journal of Clinical Investigation. 2007;117:868–870. doi: 10.1172/JCI31669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frier BM. How hypoglycaemia can affect the life of a person with diabetes. Diabetes/Metabolism Research and Reviews. 2008;24:87–92. doi: 10.1002/dmrr.796. [DOI] [PubMed] [Google Scholar]
- Fritsche A, Schweitzer M, Haring H-U. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Annals of Internal Medicine. 2003;138:952–959. doi: 10.7326/0003-4819-138-12-200306170-00006. [DOI] [PubMed] [Google Scholar]
- Grima DT, Thompson MF, Sauriol L. Modelling cost effectiveness of insulin glargine for the treatment of type 1 and 2 diabetes in Canada. PharmacoEconomics. 2007;25:253–266. doi: 10.2165/00019053-200725030-00007. [DOI] [PubMed] [Google Scholar]
- Heaton A, Martin S, Brelje T. The economic effect of hypoglycemia in a health plan. Managed Care Interface. 2003;16:23–27. [PubMed] [Google Scholar]
- Home PD, Fritsche A, Schinzel S, Massi-Benedetti M. Meta-analysis of individual patient data to assess the risk of hypoglycaemia in people with type 2 diabetes using NPH insulin or insulin glargine. Diabetes, Obesity & Metabolism. 2010;12:772–779. doi: 10.1111/j.1463-1326.2010.01232.x. [DOI] [PubMed] [Google Scholar]
- Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. 2007:CD005613. doi: 10.1002/14651858.CD005613.pub3. [DOI] [PubMed] [Google Scholar]
- Jonsson L, Bolinder B, Lundkvist J. Cost of hypoglycemia in patients with type 2 diabetes in Sweden. Value in Health. 2006;9:193–198. doi: 10.1111/j.1524-4733.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- Korytkowski M. When oral agents fail: Practical barriers to starting insulin. International Journal of Obesity and Related Metabolic Disorders. 2002;26:S18–S24. doi: 10.1038/sj.ijo.0802173. [DOI] [PubMed] [Google Scholar]
- Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: An analysis of third-party managed care claims data. Clinical Therapeutics. 2006;28:1712–1725. doi: 10.1016/j.clinthera.2006.10.004. discussion 1710–1711. [DOI] [PubMed] [Google Scholar]
- Leese GP, Wang J, Broomhall J, et al. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: A population-based study of health service resource use. Diabetes Care. 2003;26:1176–1180. doi: 10.2337/diacare.26.4.1176. [DOI] [PubMed] [Google Scholar]
- Leichter S. Is the use of insulin analogues cost-effective? Advances in Therapy. 2008;25:285–299. doi: 10.1007/s12325-008-0043-9. [DOI] [PubMed] [Google Scholar]
- Lundkvist J, Berne C, Bolinder B, Jonsson L. The economic and quality of life impact of hypoglycemia. The European Journal of Health Economics. 2005;6:197–202. doi: 10.1007/s10198-005-0276-3. [DOI] [PubMed] [Google Scholar]
- Massi-Benedetti M, Humburg E, Dressler A, Ziemen M. A one-year, randomised, multicentre trial comparing insulin glargine with NPH insulin in combination with oral agents in patients with type 2 diabetes. Hormone and Metabolic Research. 2003;35:189–196. doi: 10.1055/s-2003-39080. [DOI] [PubMed] [Google Scholar]
- McEwan P, Poole CD, Tetlow T, Holmes P, Currie CJ. Evaluation of the cost-effectiveness of insulin glargine versus NPH insulin for the treatment of type 2 diabetes in the UK. Current Medical Research and Opinion. 2007;23(Suppl 1):21–31. [Google Scholar]
- Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues versus NPH human insulin in type 2 diabetes: A meta-analysis. Diabetes Research and Clinical Practice. 2008;81:184–189. doi: 10.1016/j.diabres.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Mullins P, Sharplin P, Yki-Jarvinen H, Riddle MC, Haring HU. Negative binomial meta-regression analysis of combined glycosylated hemoglobin and hypoglycemia outcomes across eleven phase III and IV studies of insulin glargine compared with neutral protamine Hagedorn insulin in type 1 and type 2 diabetes mellitus. Clinical Therapeutics. 2007;29:1607–1619. doi: 10.1016/j.clinthera.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Palmer AJ, Lammert M, Hermansen K. Health economic consequences of insulin analogues in the treatment of type 1 diabetes in Denmark. Ugeskrift for Laeger. 2008;170:1250–1254. [PubMed] [Google Scholar]
- Reviriego J, Gomis R, Maranes JP, Ricart W, Hudson P, Sacristan JA. Cost of severe hypoglycaemia in patients with type 1 diabetes in Spain and the cost-effectiveness of insulin lispro compared with regular human insulin in preventing severe hypoglycaemia. International Journal of Clinical Practice. 2008;62:1026–1032. doi: 10.1111/j.1742-1241.2008.01783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads GG, Orsini LS, Crown W, Wang S, Getahun D, Zhang Q. Contribution of hypoglycemia to medical care expenditures and short-term disability in employees with diabetes. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2005;47:447–452. doi: 10.1097/01.jom.0000161727.03431.3e. [DOI] [PubMed] [Google Scholar]
- Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: Randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Fonseca V, McGill JB, et al. Similar progression of diabetic retinopathy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: A long-term, randomised, open-label study. Diabetologia. 2009a;52:1778–1788. doi: 10.1007/s00125-009-1415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock J, Fonseca V, McGill JB, et al. Similar risk of malignancy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: Findings from a 5 year randomised, open-label study. Diabetologia. 2009b;52:1971–1973. doi: 10.1007/s00125-009-1452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock J, Schwartz SL, Clark CM, Jr, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631–636. doi: 10.2337/diacare.24.4.631. [DOI] [PubMed] [Google Scholar]
- Tramer MR, Walder B. Number needed to treat (or harm) World Journal of Surgery. 2005;29:576–581. doi: 10.1007/s00268-005-7916-8. [DOI] [PubMed] [Google Scholar]
- Walter SD. Number needed to treat (NNT): Estimation of a measure of clinical benefit. Statistics in Medicine. 2001;20:3947–3962. doi: 10.1002/sim.1173. [DOI] [PubMed] [Google Scholar]
- Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130–1136. doi: 10.2337/diacare.23.8.1130. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Menditto L. Incremental cost savings 6 months following initiation of insulin glargine in a Medicaid fee-for-service sample. American Journal of Therapeutics. 2005;12:337–343. doi: 10.1097/01.mjt.0000160934.55260.d5. [DOI] [PubMed] [Google Scholar]


