Abstract
We recently shown that α, β, and γ splice variants of neuronal nitric oxide synthase (NOS1) expressed in the macula densa and NOS1β accounts for most of the NO generation. We have also demonstrated that the mice with deletion of NOS1 specifically from the macula densa developed salt-sensitive hypertension. However, the global NOS1KO strain is not hypertensive nor salt-sensitive. This global NOS1KO strain is actually a NOS1αKO model. Consequently, we hypothesized that inhibition of NOS1β in NOS1αKO mice induces salt-sensitive hypertension.
NOS1αKO and C57BL/6 WT mice were implanted with telemetry transmitters and divided into 7-nitroindazole (7-NI) (10mg/kg/day)-treated and non-treated groups. All of the mice were fed a normal salt (0.4% NaCl) diet for 5 days, followed by a high salt diet (4%NaCl). NO generation by the macula densa was inhibited by over 90% in WT and NOS1αKO mice treated with 7-NI. GFR in conscious mice was increased by about 40% following a high salt diet in both NOS1αKO and WT mice. In response to acute volume expansion, GFR, diuretic and natriuretic response were significantly blunted in the WT and KO mice treated with 7-NI. Mean arterial pressure had no significant changes in mice fed a high salt diet, but increased about 15 mmHg similarly in NOS1αKO and WT mice treated with 7-NI.
We conclude that NOS1β, but not NOS1α plays an important role in control of sodium excretion and hemodynamics in response to either an acute or a chronic salt loading.
Keywords: NOS1, splice variant, salt sensitive, hypertension, macula densa
INTRODUCTION
Hypertension affects over 25% of the American adults and is a major risk factor for cardiovascular morbidity and mortality 1,2. More than half of hypertensive patients are salt-sensitive and exhibit a significant rise in blood pressure when salt intake is elevated 3,4. Abundant evidence from numerous studies both in human and experimental animal models indicates the significance of kidney in the development of salt-sensitive hypertension3,5. However, the renal mechanisms for salt-sensitivity have not been fully elucidated. Increases in glomerular filtration rate (GFR) in response to salt loading may play a vital role in rapid elimination of sodium to maintain salt-water balance 6–10. This GFR response is blunted or blocked in human 11,12 and animal models 10,13 with salt-sensitive hypertension, but the underlying mechanism is unclear. GFR is normally regulated by tubuloglomerular feedback (TGF). Increases in tubular flow initiate a TGF response, mediated by increased NaCl delivery to the macula densa. This promotes the release of adenosine and/or ATP, which constricts afferent arterioles and reduces single nephron GFR. Flow and salt delivery in the distal nephron are thus restored 14–18. If the increased flow at the macula densa persists, the TGF curve will shift to right; therefore, TGF functions at a higher operating point (higher flow rate) to permit elevation of GFR 19–21. The mechanisms responsible for TGF modulation remain to be determined.
Nitric oxide (NO) is one of the most important factors that modulate TGF responsiveness. Three isoforms of nitric oxide synthases (NOS), neuronal NOS (nNOS/NOS1), inducible NOS (iNOS/NOS2), and endothelial NOS (eNOS/NOS3), exist in mammals. They are all expressed in the juxtaglomerular apparatus (JGA) of the kidneys. NOS1 is abundantly expressed in the macula densa 15,22. NO generated by NOS1 in the macula densa inhibits TGF response 17,18,23,24. Long-term blockade of NOS1 by 7-nitroindazole (7-NI) leads to hypertension in SD rats 25 and causes salt-sensitive hypertension in Dahl salt-resistant rats26, underlining the significance of NOS1 in controlling salt-water balance and blood pressure. However, studies on global NOS1 knockout (NOS1KO) mice have shown that these animals are normotensive, even on a high-salt diet 27–29. This potential discrepancy can be partially explained by our recent findings 30,31. We have shown that three splice variants of NOS1 exist in the macula densa, namely α, β, and γ; among these, NOS1β is the major splice variant and accounts for most of the NO generated by the macula densa 30,31. We have also demonstrated the significance of TGF responsiveness in long-term control of sodium excretion and blood pressure by using a tissue-specific KO mouse strain, in which NOS1 has been specifically deleted from the macula densa. These KO mice develop salt-sensitive hypertension, associated with enhanced TGF responsiveness and low GFR response in response to an acute salt loading 31. In addition, the global NOS1KO model targets exon-2 and deletes only the NOS1α isoform 32 with an intact NOS1β splice variant 31. Therefore, we will call this strain NOS1αKO in present study. These mice do not develop hypertension, further suggesting that NOS1β plays a dominate role in control of salt sensitivity of blood pressure. Consequently, we hypothesized that inhibition of NOS1β in NOS1αKO mice induces salt-sensitive hypertension. In the present study, we administered 7-NI to NOS1αKO mice and then measured their blood pressure. In addition, we also tested a hypothesis that NOS1α does not play a significant role in response to an acute sodium load. We determined the significance of NOS1α in control of sodium excretion and renal hemodynamics by comparing kidney clearance function between NOS1αKO and wild type (WT) mice in response to acute volume expansion. Current pharmacological study further expanded our understanding of the significance of NOS1 in control of volume homeostasis and blood pressure.
METHODS
All procedures and experiments were approved by the Institutional Animal Care and Use Committee at the University of South Florida College of Medicine and the University of Mississippi Medical Center. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except as indicated.
Transmitter implantation and mean arterial pressure (MAP) measurement
Similar methods were used as we previously described33,34 (see the online supplement).
Animal groups and treatment
The C57BL/6 and NOS1αKO mice were divided into 7-NI treated and non-treated groups. MAP was measured for 5 days in all of the mice fed a normal salt diet (0.4% NaCl), followed by 7 days of a high salt diet (4%NaCl; days 6–12). From day 13, in addition to the high salt diet, the mice in 7-NI treated groups were given 7-NI (10mg/kg/day) in drinking water as reported25 for 16 more days. The non-treated groups were maintained on a high salt diet without 7-NI in drinking water.
GFR measurement in conscious mice
We used a single bolus injection of FITC-inulin, similar to a previously published method 35 but with modifications for measurement of GFR in conscious mice (see the online supplement).
Renal clearance in response to isotonic volume expansion
At the end of 7-NI treatment or high salt diet, kidney clearance function was measured as we described recently31 (see the online supplement).
Measurement of NO in isolated perfused macula densa
We measured NO production by the macula densa using a fluorescent NO indicator 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM DA) in isolated perfused JGA as we described previously 36,37 (see online supplement).
Isolation of macula densa cells
Laser capture microdissection (LCM) was used to isolate macula densa cells from frozen kidney slices, as we previously described 31,38,39 (see online supplement).
Real-Time PCR
RNA and quantitative PCR analysis was performed similarly as we previously described 30,31 (see online supplement).
Western blot to measure splice variants of NOS1
Splice variants of NOS1 were measured in renal cortical with Western as we described previously 30,31 (see online supplement).
Statistical analysis
Data are presented as mean ± SEM unless specified. We tested only the effects of interest, using analysis of variance (ANOVA) for repeated measures and a post-hoc Fisher LSD test or a Student’s paired t-test when appropriate. The changes were considered to be significant if p< 0.05.
RESULTS
Expressions of NOS1 splice variants in the macula densa
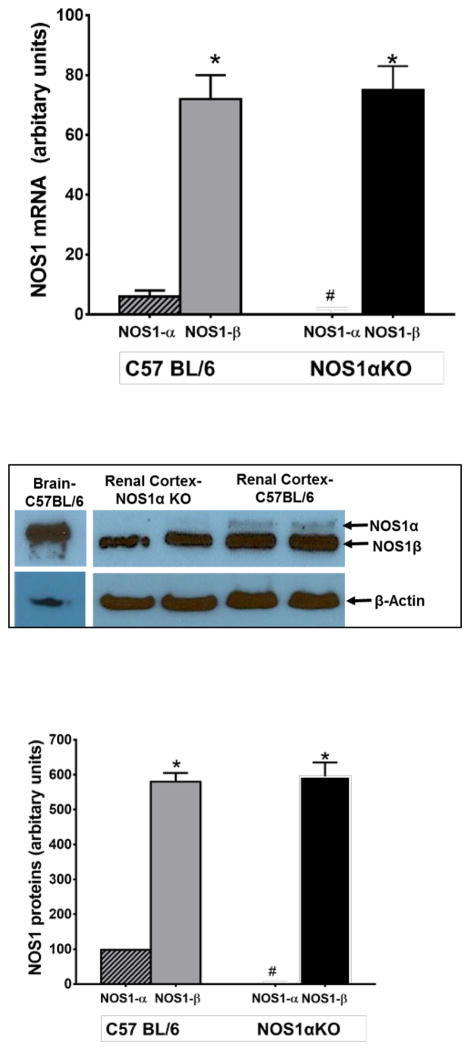
We compared mRNA expressions of NOS1 splice variants in the macula densa using LCM and real-time PCR in normal WT and NOS1αKO mice. We found no significant difference in NOS1β mRNA levels between WT (71.6±4.7 AU) and NOS1αKO mice (75.4±5.9 AU). NOS1β expressions were significantly higher than NOS1α in both strains (p < 0.01), while NOS1α was undetectable in NOS1αKO mice (n=6, Fig 1).
Figure 1. Splice variants of NOS1.
mRNA of splice variants of NOS1 were measured by real-time PCR in the macula densa isolated with laser capture microdissection (A). Splice variants of NOS1 protein were measured with a C-terminal antibody in renal cortex and compared with that in brain (B). Bands of Western blot were semi-quantified by densitometry (C). * p<0.01 vs NOS1α, # p<0.01 vs C57BL/6
The protein levels of NOS1 splice variants in renal cortex were measured by Western blot using a C-terminal NOS1 antibody that can detect all the splice variants of NOS1 30,40. As shown in Fig 1B and C, NOS1α was the primary splice variant expressed in the brain while NOS1β was the major splice variant in renal cortex. NOS1β protein levels in the renal cortex were 580±25 AU in WT mice and 596±34 AU in NOS1αKO mice (p < 0.01 vs NOS1α). There was no significant difference between them. NOS1α was undetectable in NOS1αKO mice (n=5/group).
NO generation by the macula densa
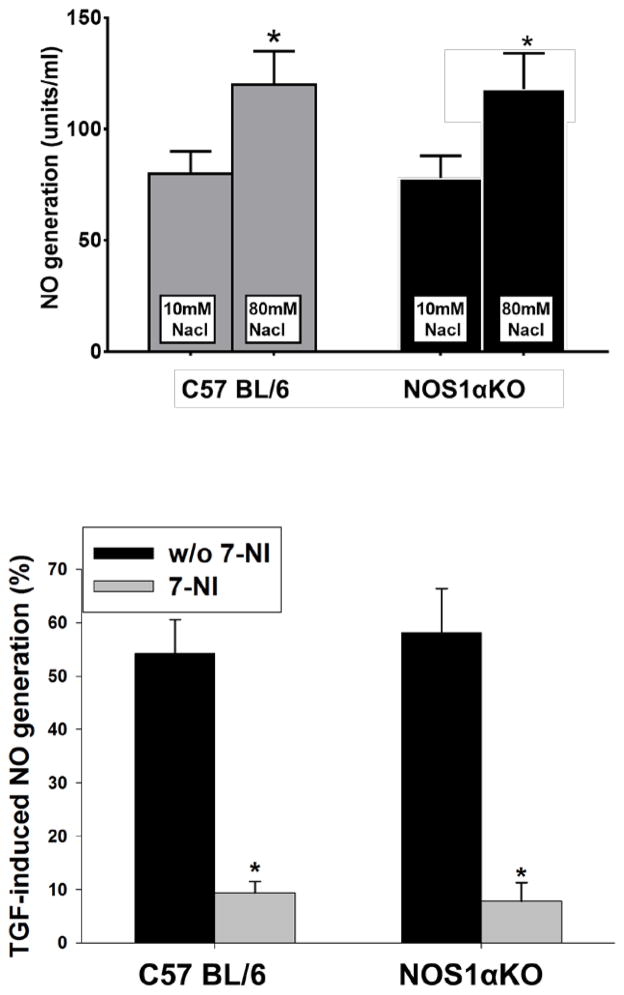
To determine whether there was any difference in NO generation by the macula densa between NOS1αKO and WT mice, we compared TGF-induced NO generation by the macula densa in isolated perfused JGA in normal WT and NOS1αKO mice fed a normal salt diet. The NO generation by the macula densa in WT mice increased by 40.6±3.8% (from 86.4±7.5 to 121.5±12.3 unit/min) in response to an increase in tubular NaCl concentration from 10 to 80 mM, a maneuver that initiates a TGF response. TGF-induced NO generation increased by 47.5±5.1% (from 81.2±8.7 to 119.8±13.9 unit/min) in NOS1αKO mice. There was no significant difference in the NO generation between WT and NOS1αKO mice (n=5, Fig 2A).
Figure 2. NO generation by the macula densa.
NO generation was measured with a NO-sensitive fluorescent dye in isolated perfused juxtaglomerular apparatus. A. NO generation during TGF when tubular NaCl was increase from 10 to 80 mM between NOS1αKO and WT mice. *p<0.05 vs 10 mM NaCl. B. TGF-induced NO generation in NOS1αKO and WT mice between with and without 7-NI treatment. *p<0.01 vs without 7-NI.
To determine whether 7-NI inhibits NO generation by the macula densa, we repeated the above experiments at the end of 7-NI treatment. The TGF-induced NO generation by the macula densa was reduced to 9.4±2.1% in WT mice (n=4) and 7.8±3.5% in NOS1αKO mice (n=4). In the WT mice without 7-NI treatment, TGF-induced NO generation was 54.2±6.5% (p<0.01 vs 7-NI treated animals, n=5, Fig 2B).
GFR measurement in conscious mice
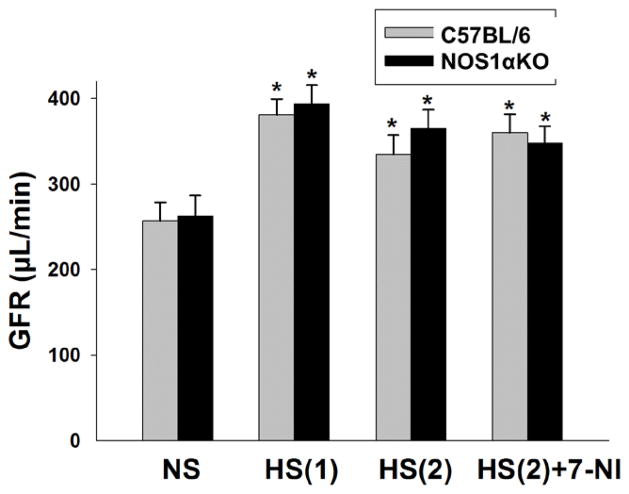
To determine whether high salt diet and 7-NI treatment had any effect on GFR, we measured GFR in conscious animals in WT and NOS1αKO mice after 7-NI or high salt diet treatment. GFR was increased by about 40% following a high salt diet in both NOS1αKO and WT mice (p<0.01 vs normal salt diet). There were no significant differences in GFR among all groups during the first period of high salt diet (HS1) or second period of high salt diet (HS2), with and without 7-NI treatment (Fig 3, n=8/group).
Figure 3. Measurement of GFR in conscious mice.
GFR in conscious mice was measured with a single injection of FITC-inulin. GFR was compared between NOS1αKO and WT mice fed a normal salt diet (NS), first period high salt diet (HS1), second period high salt diet without 7-NI (HS2) and with 7-NI (Hs(2)+7-NI). * p<0.01 vs NS.
Kidney clearance function in response to acute volume expansion in NOS1αKO and WT mice
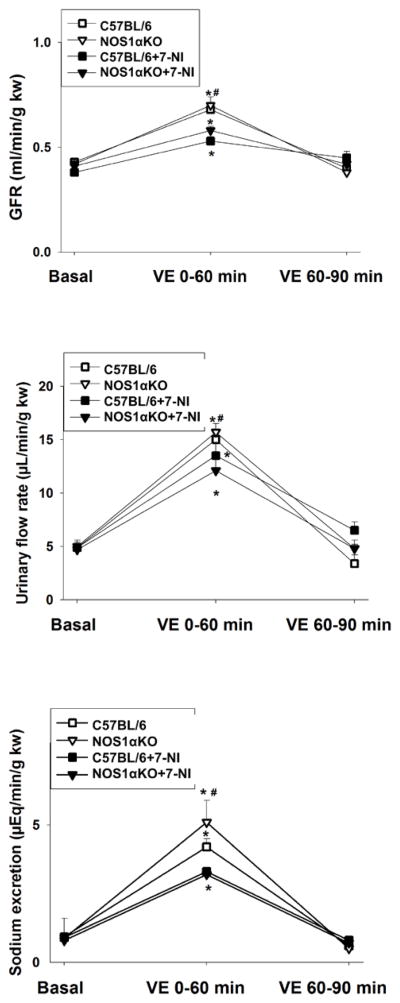
To evaluate the significance of NOS1α and further determine whether inhibition of NOS1 affects renal hemodynamics and sodium excretion in response to acute volume expansion, we measured kidney clearance function by intravenous infusion of saline in WT and NOS1αKO animals at the end of 7-NI or high salt diet treatment. The baseline GFR was similar in the WT and KO mice. GFR rose by about 60% (p<0.01 vs basal) in WT and KO mice without 7-NI treatment during 60 minutes following acute volume expansion. In contrast, GFR increased less than 40% in the animals treated with 7-NI (p<0.05 vs WT without 7-NI, Fig 4A). Urinary flow rate and sodium excretion were similar in WT and KO mice in basal and increased significantly in all groups of animals in the first hour following acute volume expansion. However, the diuretic and natriuretic response were significantly blunted in the WT and KO mice treated with 7-NI (Fig 4B and C, p<0.05 vs WT without 7-NI, n=5/group).
Figure 4. Kidney clearance function measurement.
GFR (A), urine flow rate (B) and sodium excretion rate (C) were measured in mice following an acute volume expansion by a bolus infusion of saline of 3% body weight. Kidney clearance function was measured during 0–60 min, and 60–90 min after volume expansion. * p<0.05 vs basal, # p<0.05 vs with 7-NI.
Changes in blood pressure in response to a high salt intake plus 7-NI in NOS1αKO and WT mice
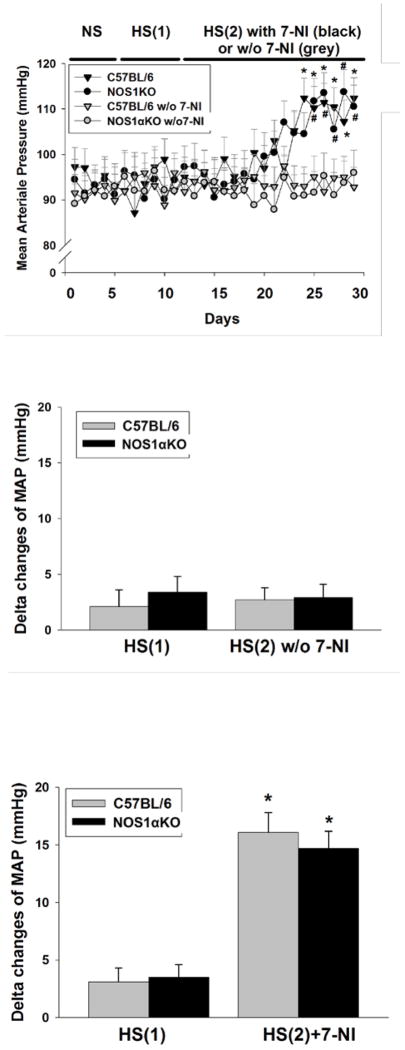
To determine if inhibition of NOS1 promotes the development of salt-sensitive hypertension, we compared changes in MAP measured by telemetry in WT and NOS1αKO mice. Baseline MAP measured on the normal salt diet averaged 91.7±4.5 mmHg in all groups of animals. After switching to a high salt diet (HS-1), the MAP of the mice did not change significantly. The MAP of mice maintained a high salt diet (HS-2) and treated with 7-NI increased to 16.1±3.5 mmHg in WT mice (n=5) and 14.7 ±3.1 mmHg in NOS1αKO mice (p<0.01 vs basal, n=6), while it was not significantly altered in mice without 7-NI (Fig 5, p<0.01 vs 7-NI treated groups, n=5).
Figure 5. Measurement of MAP in response to a high salt diet with and without 7-NI.
MAP was measured with telemetry between NOS1αKO and WT mice fed a normal salt diet (NS), first period high salt diet (HS1), second period high salt diet (HS2) without 7-NI and with 7-NI. A. Changes in MAP over time. * p<0.01 vs basal and w/o 7-NI in C57BL/6 mice, # p<0.01 vs basal and w/o 7-NI in NOS1αKO mice. B. Delta changes in MAP in mice without 7-NI treatment. C. Delta changes in MAP in mice with 7-NI treatment. * p<0.01 vs high salt.
DISCUSSION
The present study demonstrated that inhibiting NOS1 with 7-NI promoted salt sensitivity of blood pressure to an equal extant in both the NOS1αKO and WT mice, since the expression levels and activity of the NOS1β splice variant in the macula densa are intact in NOS1αKO mice. In response to an acute volume expansion, the diuretic and natriuretic response were blunted in both WT and NOS1αKO mice treated with 7-NI. NOS1α did not play a significant role in control of sodium excretion and renal hemodynamics, while NOS1β dominated the function of NOS1 in control of salt sensitivity of blood pressure.
Alternative 5′-end splicing of NOS1 mRNA results in at least three different N-terminal NOS1 protein variants, which are NOS1α at about 155 kDa, NOS1β at about 145 kDa, and NOS1λ at about 125 kDa 41,42. NOS1α exhibits full enzymatic activity, NOS1β has about 80%, while NOS1λ has only about 2% catalytic activity compared with that of NOS1α 41,43–46. The predominant splice variant in brain is NOS1α, which accounts for more than 95% of NOS1 activity 32,41,47. In the kidney, splice variants of NOS1 have been found in both cortex and medulla 48,49. NOS1 is a predominant isoform expressed in the macula densa cells 15,22. Recently, we found that macula densa expresses α, β, and γ splice variants of NOS1 30,31. Mice with deletion of NOS1 specifically from the macula densa developed salt-sensitive hypertension. This previous study using the macula densa specific knockout model clearly demonstrated the significance of NOS1 in the macula densa and TGF response in long-term control of volume homeostasis and blood pressure. However, we sought to further confirm the significance of NOS1β in the present study to determine whether pharmacological inhibition of NOS1β induces salt-sensitive hypertension in NOS1αKO mice to levels similar to those in WT mice. This pharmacological approach also avoided potential complications from any long-term adaptation that might occur in the tissue specific KO mice. In addition, we also investigated whether NOS1α plays an important role in control of sodium excretion and renal hemodynamics in response to an acute salt loading.
Similar to our previous findings31, we confirmed in the present study that NOS1β was the primary splice variant expressed in the macula densa and accounts for most of the NO generated by the macula densa. We found that a high salt diet enhanced NO generation by the macula densa to a similar level in both NOS1αKO and WT mice. These data provided additional evidence indicating NOS1β is a salt-sensitive splice variant. In addition, we observed that a high salt diet enhanced NOS1β activity as we previously reported 30,31.
Our findings about salt intake and NO generation were in agreement with previous studies. Rats on a high salt diet had higher plasma levels, increased renal excretion rates of nitrite/nitrates 50–53, and increased cGMP levels 50, suggesting that NO activity was higher during high NaCl intake. Inhibition of NOS1 in vitro augmented TGF responses to a greater extent in animals on a high salt diet 23,54, while inhibition of NOS1 in vivo had a greater effect on RBF, GFR and renal vascular resistance in animals fed a high-salt diet 50,52,55, also indicating a higher NO generation in response to a high salt diet. Similar findings have been reported in clinical trials in normal and hypertensive humans. A high salt diet was associated with an elevation in GFR, RBF, sodium and cGMP excretion compared with that in a low salt diet 56,57, and these effects were significantly enhanced following L-arginine administration, suggesting that they were possibly due to the increased NO production.
In present study, we found that the NO generation by the macula densa was inhibited in mice treated with 7-NI, confirming an effective NOS1 inhibition. In response to a salt loading, GFR increased significantly in both conscious NOS1αKO and WT mice. Similar findings have been reported in animals 10,13,58–60 and humans 61–65, which is considered as an important mechanism for rapid elimination of a salt load, possibly modulated by TGF responsiveness 31. No differences were found in GFR in mice fed a high salt diet with or without 7-NI. The reason that 7-NI inhibited NO generation but did not alter GFR may be due to the increased blood pressure in 7-NI treated mice. For GFR measurement in conscious mice, completely intravenous injection of FITC-inulin without leaking is critical for accurate measurement. We found that injection via penile vein is a very easy and reliable way for FITC-inulin injection.
Accurate measurement of salt-water balance in mice on a high salt diet is notoriously difficult. Therefore, to determine whether chronic inhibition of NOS1 with 7-NI impairs sodium excretion, we measured kidney clearance function in response to an acute volume expansion in anesthetized mice. We found that elevations of GFR in response to an acute volume expansion was significantly blunted in mice treated with 7-NI, for both NOS1αKO and WT mice. The inhibition of GFR increase may be mediated by inhibition of NO generation by the macula densa with 7-NI, which enhances TGF response. Similarly, sodium excretion rate in response to an acute volume expansion was also significantly lower in mice treated with 7-NI. These data indicated that one of the mechanisms underlying the effects of 7-NI was mediated by inhibition of NO in the macula densa, enhancement of TGF responsiveness and inhibition of GFR increases following salt loading. These data also indicated that NOS1α did not play a significant role in control of sodium excretion and renal hemodynamics.
To determine whether 7-NI-induced impairment of sodium excretion promotes a development of salt-sensitive hypertension, we measured blood pressure in mice fed a high salt diet plus 7-NI. We found that a high salt diet did not significantly increase blood pressure in NOS1αKO and WT mice, indicating that neither of the strains are salt-sensitive. The findings are agreement with previous studies 29,66. However, a high salt diet plus 7-NI similarly elevated MAP about 15 mmHg in both NOS1αKO and WT mice. These data indicate that inhibition of NOS1 with 7-NI enhanced salt sensitivity, mediated by NOS1β. These data also indicated that NOS1α did not play a significant role in control of sodium excretion and renal hemodynamics.
7-NI shows little isoform selectivity in vitro. Its IC50 is ranging 0.5–0.8 μM similarly for both purified enzymes of NOS1 and NOS3 67–69. However, 7-NI shows high selectivity in tissue and in vivo for the NOS1. 7-NI at 100 μM failed to inhibit endothelium-dependent relaxation of the rabbit isolated aorta in response to acetylcholine70. In contrast, L-NAME at 1.5 and 15 μM produces about 20% and 70% inhibition of the response to the rabbit aorta to acetylcholine71. Several studies have demonstrated that acute intraperitoneal or intravenous administration of 7-NI did not affect MAP in either anesthetized or conscious mice or rats67,70,72–74. These studies demonstrated that 7-NI is a highly selective inhibitor for NOS1 in vivo. The mechanisms for the notable differences between in vitro and in vivo effect of 7-NI have not been fully clarified. The basis of selectivity of 7-NI appears to lie in the differential uptake of the inhibitor into cells express NOS1 vs NOS3 75,76.
PERSPECTIVES
In summary, we found that the NOS1β splice variant was intact both in expression and function in NOS1αKO mice. Increases of GFR and sodium excretion in response to acute salt loadings were blunted in mice treated with 7-NI in both NOS1αKO and WT mice. A high salt diet did not increase blood pressure, but adding 7-NI elevated blood pressure to a similar level both in NOS1αKO and WT mice. Taken together, the present study demonstrated that NOS1β, but not NOS1α, plays an important role in control of sodium excretion and salt sensitivity of blood pressure.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is New
NOS1α does not play an important role in control of sodium excretion and hemodynamics in response to acute salt loading. NOS1β is the primarily isoform that regulate sodium excretion and blood pressure.
What is Relevant
We determined the role of NOS1 in control of hypertension.
Summary
NOS1 β, but not NOS1α, plays an important role in control of sodium excretion and salt sensitivity of blood pressure.
Acknowledgments
SOURCES OF FUNDING
This work was supported by National Institutes of Health Grants DK099276 and DK098582 (RL); and by National Natural Science Foundation of China Grant 81371547 (XW) and 31471100 (EYL) and a Taishan Scholar Projection (XW).
Reference List
- 1.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 2.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men. The Framingham Heart Study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 4.Luft FC. Salt and hypertension: recent advances and perspectives. J Lab Clin Med. 1989;114:215–221. [PubMed] [Google Scholar]
- 5.Guyton AC, Young DB, DeClue JW, Ferguson JD, McCaa RE, Cevese A, Trippodo NC, Hall JE. The role of the kidney in hypertension. In: Berglund G, Hansson L, Werkö L, editors. Pathophysiology and Management of Arterial Hypertension; Proceedings of a Conference; Copenhagen, Denmark. April 10–11, 1975; Molndal, Sweden: A. Lindgren & Soner AB; 1975. pp. 78–89. [Google Scholar]
- 6.Roman RJ, Cowley AW, Jr, Garcia-Estan J, Lombard JH. Pressure-diuresis in volume-expanded rats: cortical and medullary hemodynamics. Hypertension. 1988;12:168–176. doi: 10.1161/01.hyp.12.2.168. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson H, Moritz K, Wintour EM, Walker DW, Kett MM. A comparative study of renal function in the desert-adapted spiny mouse and the laboratory-adapted C57BL/6 mouse: response to dietary salt load. Am J Physiol Renal Physiol. 2007;293:F1093–F1098. doi: 10.1152/ajprenal.00202.2007. [DOI] [PubMed] [Google Scholar]
- 8.Luft FC, Weinberger MH, Fineberg NS, Miller JZ, Grim CE. Effects of age on renal sodium homeostasis and its relevance to sodium sensitivity. Am J Med. 1987;82:9–15. doi: 10.1016/0002-9343(87)90266-x. [DOI] [PubMed] [Google Scholar]
- 9.Luft FC, Grim CE, Fineberg N, Weinberger MC. Effects of volume expansion and contraction in normotensive whites, blacks, and subjects of different ages. Circulation. 1979;59:643–650. doi: 10.1161/01.cir.59.4.643. [DOI] [PubMed] [Google Scholar]
- 10.Simchon S, Manger WM, Carlin RD, Peeters LL, Rodriguez J, Batista D, Brown T, Merchant NB, Jan KM, Chien S. Salt-induced hypertension in Dahl salt-sensitive rats. Hemodynamics and renal responses. Hypertension. 1989;13:612–621. doi: 10.1161/01.hyp.13.6.612. [DOI] [PubMed] [Google Scholar]
- 11.Campese VM, Parise M, Karubian F, Bigazzi R. Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension. 1991;18:805–812. doi: 10.1161/01.hyp.18.6.805. [DOI] [PubMed] [Google Scholar]
- 12.Stadler P, Pusterla C, Beretta-Piccoli C. Renal tubular handling of sodium and familial predisposition to essential hypertension. J Hypertens. 1987;5:727–732. doi: 10.1097/00004872-198712000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Hua JL, Kaskel FJ, Juno CJ, Moore LC, McCaughran JA., Jr Salt intake and renal hemodynamics in immature and mature Dahl salt-sensitive (DS/JR) and salt-resistant (DR/JR) rats. Am J Hypertens. 1990;3:268–273. doi: 10.1093/ajh/3.4.268. [DOI] [PubMed] [Google Scholar]
- 14.Schnermann J, Persson AE, Agerup B. Tubuloglomerular feedback. Nonlinear relation between glomerular hydrostatic pressure and loop of Henle perfusion rate. J Clin Invest. 1973;52:862–869. doi: 10.1172/JCI107250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HHW. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci USA. 1992;89:11993–11997. doi: 10.1073/pnas.89.24.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navar LG, Bell PD, Thomas CE, Ploth DW. Influence of perfusate osmolality on stop-flow pressure feedback responses in the dog. Am J Physiol. 1978;235:F352–F358. doi: 10.1152/ajprenal.1978.235.4.F352. [DOI] [PubMed] [Google Scholar]
- 17.Ito S, Ren Y. Evidence for the role of nitric oxide in macula densa control of glomerular hemodynamics. J Clin Invest. 1993;92:1093–1098. doi: 10.1172/JCI116615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu R, Ren Y, Garvin JL, Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int. 2004;66:268–274. doi: 10.1111/j.1523-1755.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 19.Thurau K, Schnermann J. The Na concentration of the macula densa cells as a factor regulating glomerular filtration rate (micropuncture studies). 1965. J Am Soc Nephrol. 1998;9:925–934. doi: 10.1681/ASN.V95925. [DOI] [PubMed] [Google Scholar]
- 20.Schnermann J, Briggs JP. Restoration of tubuloglomerular feedback in volume-expanded rats by angiotensin II. Am J Physiol. 1990;259:F565–F572. doi: 10.1152/ajprenal.1990.259.4.F565. [DOI] [PubMed] [Google Scholar]
- 21.Thomson SC, Blantz RC, Vallon V. Increased tubular flow induces resetting of tubuloglomerular feedback in euvolemic rats. Am J Physiol. 1996;270:F461–F468. doi: 10.1152/ajprenal.1996.270.3.F461. [DOI] [PubMed] [Google Scholar]
- 22.Mundel P, Bachmann S, Bader M, Fischer A, Kummer W, Mayer B, Kriz W. Expression of nitric oxide synthase in kidney macula densa cells. Kidney Int. 1992;42:1017–1019. doi: 10.1038/ki.1992.382. [DOI] [PubMed] [Google Scholar]
- 23.Welch WJ, Wilcox CS. Role of nitric oxide in tubuloglomerular feedback: effects of dietary salt. Clin Exp Pharmacol Physiol. 1997;24:582–586. doi: 10.1111/j.1440-1681.1997.tb02095.x. [DOI] [PubMed] [Google Scholar]
- 24.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol. 2003;65:501–529. doi: 10.1146/annurev.physiol.65.050102.085738. [DOI] [PubMed] [Google Scholar]
- 25.Ollerstam A, Pittner J, Persson AEG, Thorup C. Increased blood pressure in rats after long-term inhibition of the neuronal isoform of nitric oxide synthase. J Clin Invest. 1997;99:2212–2218. doi: 10.1172/JCI119394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan DY, Meng S, Manning RD., Jr Role of neuronal nitric oxide synthase in Dahl salt-sensitive hypertension. Hypertension. 1999;33:456–461. doi: 10.1161/01.hyp.33.1.456. [DOI] [PubMed] [Google Scholar]
- 27.Hannan RL, John MC, Kouretas PC, Hack BD, Matherne GP, Laubach VE. Deletion of endothelial nitric oxide synthase exacerbates myocardial stunning in an isolated mouse heart model. J Surg Res. 2000;93:127–132. doi: 10.1006/jsre.2000.5953. [DOI] [PubMed] [Google Scholar]
- 28.Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O’Rourke B, Rodriguez ER, Huang PL, Lima JAC, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–340. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 29.Sallstrom J, Carlstrom M, Jensen BL, Skott O, Brown RD, Persson AE. Neuronal nitric oxide synthase-deficient mice have impaired renin release but normal blood pressure. Am J Hypertens. 2008;21:111–116. doi: 10.1038/ajh.2007.16. [DOI] [PubMed] [Google Scholar]
- 30.Lu D, Fu Y, Lopez-Ruiz A, Zhang R, Juncos R, Liu H, Manning RD, Jr, Juncos LA, Liu R. Salt-sensitive splice variant of nNOS expressed in the macula densa cells. Am J Physiol Renal Physiol. 2010;298:F1465–F1471. doi: 10.1152/ajprenal.00650.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Y, Wei J, Stec DE, Roman RJ, Ge Y, Cheng L, Liu E, Zhang J, Hansen PB, Fan F, Juncos LA, Wang L, Pollock JS, Huang P, Fu Y, Wang S, Liu R. Macula Densa Nitric Oxide Synthase 1β Protects against Salt-Sensitive Hypertension. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015050515. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 33.Song J, Lu Y, Lai EY, Wei J, Wang L, Chandrashekar K, Wang S, Shen C, Juncos LA, Liu R. Oxidative status in the macula densa modulates tubuloglomerular feedback responsiveness in Angiotensin II-induced hypertension. Acta Physiol (Oxf) 2015;213:249–258. doi: 10.1111/apha.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Chandrashekar K, Lu Y, Duan Y, Qu P, Wei J, Juncos LA, Liu R. Enhanced expression and activity of Nox2 and Nox4 in the macula densa in ANG II-induced hypertensive mice. Am J Physiol Renal Physiol. 2014;306:F344–F350. doi: 10.1152/ajprenal.00515.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 2004;286:F590–F596. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- 36.Liu R, Pittner J, Persson AE. Changes of cell volume and nitric oxide concentration in macula densa cells caused by changes in luminal NaCl concentration. J Am Soc Nephrol. 2002;13:2688–2696. doi: 10.1097/01.asn.0000033275.17169.67. [DOI] [PubMed] [Google Scholar]
- 37.Liu R, Carretero OA, Ren Y, Wang H, Garvin JL. Intracellular pH regulates superoxide production by the macula densa. Am J Physiol Renal Physiol. 2008;295:F851–F856. doi: 10.1152/ajprenal.90204.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu Y, Zhang R, Lu D, Liu H, Chandrashekar K, Juncos LA, Liu R. NOX2 is the primary source of angiotensin II-induced superoxide in the macula densa. Am J Physiol Regul Integr Comp Physiol. 2010;298:R707–R712. doi: 10.1152/ajpregu.00762.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang R, Harding P, Garvin JL, Juncos R, Peterson E, Juncos LA, Liu R. Isoforms and Functions of NADPH Oxidase at the Macula Densa. Hypertension. 2009;53:556–563. doi: 10.1161/HYPERTENSIONAHA.108.124594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu Y, Hall JE, Lu D, Lin L, Manning RD, Jr, Cheng L, Gomez-Sanchez C, Juncos LA, Liu R. Aldosterone blunts tubuloglomerular feedback by activating macula densa mineralocorticoid receptors. Hypertension. 2012;59:599–606. doi: 10.1161/HYPERTENSIONAHA.111.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and a1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 42.Eliasson MJ, Blackshaw S, Schell MJ, Snyder SH. Neuronal nitric oxide synthase alternatively spliced forms: prominent functional localizations in the brain. Proc Natl Acad Sci U S A. 1997;94:3396–3401. doi: 10.1073/pnas.94.7.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee MA, Cai L, Hubner N, Lee YA, Lindpaintner K. Tissue- and development-specific expression of multiple alternatively spliced transcripts of rat neuronal nitric oxide synthase. J Clin Invest. 1997;100:1507–1512. doi: 10.1172/JCI119673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bredt DS. Targeting nitric oxide to its targets. Proc Soc Exp Biol Med. 1996;211:41–48. doi: 10.3181/00379727-211-43950f. [DOI] [PubMed] [Google Scholar]
- 45.Hecker M, Mulsch A, Busse R. Subcellular localization and characterization of neuronal nitric oxide synthase. J Neurochem. 1994;62:1524–1529. doi: 10.1046/j.1471-4159.1994.62041524.x. [DOI] [PubMed] [Google Scholar]
- 46.Jaffrey SR, Snyder SH. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science. 1996;274:774–777. doi: 10.1126/science.274.5288.774. [DOI] [PubMed] [Google Scholar]
- 47.Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- 48.Hyndman KA, Boesen EI, Elmarakby AA, Brands MW, Huang P, Kohan DE, Pollock DM, Pollock JS. Renal collecting duct NOS1 maintains fluid-electrolyte homeostasis and blood pressure. Hypertension. 2013;62:91–98. doi: 10.1161/HYPERTENSIONAHA.113.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith C, Merchant M, Fekete A, Nyugen HL, Oh P, Tain YL, Klein JB, Baylis C. Splice variants of neuronal nitric oxide synthase are present in the rat kidney. Nephrol Dial Transplant. 2009;24:1422–1428. doi: 10.1093/ndt/gfn676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shultz PJ, Tolins JP. Adaptation to increased dietary salt intake in the rat. Role of endogenous nitric oxide. J Clin Invest. 1993;91:642–650. doi: 10.1172/JCI116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritthaler T, Scholz H, Ackermann M, Riegger G, Kurtz A, Kramer BK. Effects of endothelins on renin secretion from isolated mouse renal juxtaglomerular cells. Am J Physiol. 1995;268:F39–F45. doi: 10.1152/ajprenal.1995.268.1.F39. [DOI] [PubMed] [Google Scholar]
- 52.Tolins JP, Shultz PJ. Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int. 1994;46:230–236. doi: 10.1038/ki.1994.264. [DOI] [PubMed] [Google Scholar]
- 53.Griffin KA, Picken M, Bidani AK. Radiotelemetric BP monitoring, antihypertensives and glomeruloprotection in remnant kidney model. Kidney Int. 1994;46:1010–1018. doi: 10.1038/ki.1994.361. [DOI] [PubMed] [Google Scholar]
- 54.Wilcox CS, Welch WJ. TGF and nitric oxide: effects of salt intake and salt-sensitive hypertension. Kidney Int. 1996;49(Suppl 55):S-9–S-13. [PubMed] [Google Scholar]
- 55.Deng X, Welch WJ, Wilcox CS. Renal vasodilation with L-arginine. Effects of dietary salt. Hypertension. 1995;26:256–262. doi: 10.1161/01.hyp.26.2.256. [DOI] [PubMed] [Google Scholar]
- 56.Barri YM, Wilcox CS. Salt intake determines the renal response to L-arginine infusion in normal human subjects. Kidney Int. 1998;53:1299–1304. doi: 10.1046/j.1523-1755.1998.00857.x. [DOI] [PubMed] [Google Scholar]
- 57.Parmer RJ, Stone RA, Cervenka JH. Renal hemodynamics in essential hypertension. Racial differences in responses to changes in dietary sodium. Hypertension. 1994;24:752–757. doi: 10.1161/01.hyp.24.6.752. [DOI] [PubMed] [Google Scholar]
- 58.Kassab S, Novak J, Miller T, Kirchner K, Granger J. Role of endothelin in mediating the attenuated renal hemodynamics in Dahl salt-sensitive hypertension. Hypertension. 1997;30:682–686. doi: 10.1161/01.hyp.30.3.682. [DOI] [PubMed] [Google Scholar]
- 59.Persson AEG, Morsing P, Boberg U. The tubuloglomerular feedback mechanism in Milan hypertensive rats. Kidney Int. 1990;38(Suppl 30):S-102–S-106. [PubMed] [Google Scholar]
- 60.Vallon V, Schroth J, Satriano J, Blantz RC, Thomson SC, Rieg T. Adenosine A(1) receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus. Nephron Physiol. 2009;111:30–38. doi: 10.1159/000208211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pechere-Bertschi A, Maillard M, Stalder H, Bischof P, Fathi M, Brunner HR, Burnier M. Renal hemodynamic and tubular responses to salt in women using oral contraceptives. Kidney Int. 2003;64:1374–1380. doi: 10.1046/j.1523-1755.2003.00239.x. [DOI] [PubMed] [Google Scholar]
- 62.Rasmussen MS, Simonsen JA, Sandgaard NC, Hoilund-Carlsen PF, Bie P. Mechanisms of acute natriuresis in normal humans on low sodium diet. J Physiol. 2003;546:591–603. doi: 10.1113/jphysiol.2002.027425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cannon PJ, Svahn DS, Demartini FE. The influence of hypertonic saline infusions upon the fractional reabsorption of urate and other ions in normal and hypertensive man. Circulation. 1970;41:97–108. doi: 10.1161/01.cir.41.1.97. [DOI] [PubMed] [Google Scholar]
- 64.Kawabe H, Furukawa T, Takenaka T, Saito I, Saruta T. Importance of the renin-angiotensin system in sodium regulation in essential hypertension. Am J Hypertens. 1991;4:119–125. doi: 10.1093/ajh/4.2.119. [DOI] [PubMed] [Google Scholar]
- 65.Chiolero A, Maillard M, Nussberger J, Brunner HR, Burnier M. Proximal sodium reabsorption: An independent determinant of blood pressure response to salt. Hypertension. 2000;36:631–637. doi: 10.1161/01.hyp.36.4.631. [DOI] [PubMed] [Google Scholar]
- 66.Brown RD, Thoren P, Steege A, Mrowka R, Sallstrom J, Skott O, Fredholm BB, Persson AE. Influence of the adenosine A1 receptor on blood pressure regulation and renin release. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1324–R1329. doi: 10.1152/ajpregu.00313.2005. [DOI] [PubMed] [Google Scholar]
- 67.Moore PK, Babbedge RC, Wallace P, Gaffen ZA, Hart SL. 7-nitro indazole, an inhibitor of nitric oxide synthase, exhibits anti-nociceptive activity in the mouse without increasing blood pressure. J Pharmacol. 1993;108:296–297. doi: 10.1111/j.1476-5381.1993.tb12798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Babbedge RC, Bland-Ward PA, Hart SL, Moore PK. Inhibition of rat cerebellar nitric oxide synthase by 7-nitro indazole and related substituted indazoles. Br J Pharmacol. 1993;110:225–228. doi: 10.1111/j.1476-5381.1993.tb13796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mayer B, Klatt P, Werner ER, Schmidt K. Molecular mechanisms of inhibition of porcine brain nitric oxide synthase by the antinociceptive drug 7-nitro-indazole. Neuropharmacology. 1994;33:1253–1259. doi: 10.1016/0028-3908(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 70.Moore PK, Wallace P, Gaffen Z, Hart SL, Babbedge RC. Characterization of the novel nitric oxide synthase inhibitor 7-nitro indazole and related indazoles: antinociceptive and cardiovascular effects. Br J Pharmacol. 1993;110:219–224. doi: 10.1111/j.1476-5381.1993.tb13795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moore PK, al-Swayeh OA, Chong NWS, Evans RA, Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990;99:408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bland-Ward PA, Moore PK. 7-nitro indazole derivatives are potent inhibitors of brain, endothelium and inducible isoforms of nitric oxide synthase. Life Sci. 1995;57:131–135. doi: 10.1016/0024-3205(95)02046-l. [DOI] [PubMed] [Google Scholar]
- 73.Beierwaltes WH. Macula densa stimulation of renin is reversed by selective inhibition of neuronal nitric oxide synthase. Am J Physiol. 1997;272:R1359–R1364. doi: 10.1152/ajpregu.1997.272.5.R1359. [DOI] [PubMed] [Google Scholar]
- 74.Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 1993;52:127–136. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- 75.Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- 76.Mukherjee P, Cinelli MA, Kang S, Silverman RB. Development of nitric oxide synthase inhibitors for neurodegeneration and neuropathic pain. Chem Soc Rev. 2014;43:6814–6838. doi: 10.1039/c3cs60467e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.