Abstract
Background
Few studies have examined the efficacy of a Mindfulness-Based Stress Reduction (MBSR) intervention on psychological and physiological functioning in patients with fibromyalgia.
Purpose
We conducted a randomized prospective trial of MBSR among female fibromyalgia patients.
Methods
Effects on perceived stress, pain, sleep quality, fatigue, symptom severity, and salivary cortisol were tested in treatment (n=51) versus wait-list control participants (n=40) using data at baseline, post-program, and two-month follow-up.
Results
Analyses revealed MBSR significantly reduced perceived stress, sleep disturbance, and symptom severity, with gains maintained at follow-up. Greater home practice at follow-up was associated with reduced symptom severity. MBSR did not significantly alter pain, physical functioning, or cortisol profiles.
Conclusion
MBSR ameliorated some of the major symptoms of fibromyalgia and reduced subjective illness burden. Further exploration of MBSR effects on physiological stress responses is warranted. These results support use of MBSR as a complementary treatment for women with fibromyalgia. (ISRCTN: 34628811)
Keywords: Fibromyalgia, mindfulness meditation, perceived stress, sleep, cortisol
INTRODUCTION
Fibromyalgia is a chronic pain syndrome that may occur in up to 3.4% of US adult women, with the highest prevalence (7.4%) among women in their 7th decade (1). According to the 1990 American College of Rheumatology guidelines, fibromyalgia is defined by patient report of a history of widespread pain occurring in at least 11 of 18 tender points (2), accompanied by fatigue, cognitive symptoms, sleep, and somatic symptoms (2–4). Fatigue is one of the most prevalent symptoms reported by women with fibromyalgia, and fatigue may have the greatest impact of any symptom experienced (5). The recent expansion of the diagnostic criteria (2) recognizes the frequent co-morbidities and diverse symptoms of fibromyalgia.
Among a high proportion of fibromyalgia patients, illness onset immediately follows a major stressful event, such as trauma (6–8). Adding to the evidence for stress and trauma as etiological factors, abnormalities of neuroendocrine stress responses have often been noted. Most healthy individuals demonstrate clear circadian rhythms in hypothalamic-pituitary-adrenal axis activity characterized by a nocturnal cortisol nadir and peak secretion after awakening. Aberrant, or low morning cortisol and flattened diurnal rhythms have been noted among fibromyalgia patients who report childhood trauma, including physical or sexual abuse (9,10). Such circadian disruption may contribute to problems with sleep, fatigue, cognitive function, and pain (11). Indeed, more severe fibromyalgia pain has been associated with higher levels of cortisol recorded at first awakening, though not later in the day (10). Flattened diurnal cortisol patterns have been demonstrated among patients with various types of chronic pain, including fibromyalgia (12).
The 50–75% increase in cortisol secretion that occurs in most people 30–45 minutes after awakening is termed the cortisol awakening response (CAR; 13). The CAR may reflect a transition from sleep to waking (14) and is sensitive to numerous types of psychological stress (15,16) and other state factors (17). Despite the relationship of high waking cortisol with fibromyalgia pain (10), few studies have examined the CAR in fibromyalgia. Of those, a decreased CAR in patients with fibromyalgia has been demonstrated in comparison to healthy controls (18) and women with shoulder and neck pain (19). Research is needed to fully understand the relationships between stress and trauma, cortisol abnormalities, and fibromyalgia symptom severity.
The complex interactions between psychosocial factors, CAR, and symptomatology pose significant challenges to treatment. However, stress-reduction treatment interventions have shown promise in reducing or alleviating aberrant cortisol rhythms that can occur with chronic illnesses including fibromyalgia (20) and cancer (21,22). Fibromyalgia is sometimes managed with anti-inflammatory and/or anti-depressant medications, and agents targeting central pain mechanisms have recently shown promise (23,24). However, recent meta-analyses conclude that optimal treatment interventions should include components aimed at enhancing relaxation, cognitive and behavioral responses, exercise, health education, and pharmacotherapy (25,26). The Mindfulness-Based Stress Reduction (MBSR) program is a multi-component intervention designed to relieve suffering through the cultivation of mindfulness, or “awareness that emerges through paying attention on purpose, in the present-moment, and nonjudgmentally to the unfolding of experience” (27). During an MBSR program, participants' repeated exposure to mindfulness helps them learn to pause and choose their response to difficulty rather than reacting without thinking. The program supports the development of a nonjudgmental stance toward one's experiences, and helps participants find adaptive strategies to work with stressful circumstances (28).
There has been an explosion of interest and research into mindfulness, MBSR, and their impact on mental and physical symptoms across various populations including those with chronic illnesses, such as fibromyalgia. Research has demonstrated benefits with regard to symptoms of pain, depression, psychological distress, and anxiety (29,30). A review of studies assessing changes in cortisol after MBSR highlights a rapidly growing body of evidence that cortisol secretion may decrease after participation in an MBSR Program. However, the authors also note inconsistent results and methodological problems with current studies (31). Our laboratory has shown that MBSR produced significant reduction in depressive symptoms among women with fibromyalgia (32). Other studies that have examined the impact of MBSR on patients with fibromyalgia have also found significant improvements in depressive symptoms, as well as in anxiety, somatic symptoms, quality of life, sleep, and pain (33,34). In a small group of fibromyalgia patients screened for medications, MBSR participation was associated with a significant reduction in skin conductance level, indicating lowered sympathetic tone (35). Taken together, these results suggest that MBSR can produce positive psychosocial effects, while physiological effects need to be further studied.
Here we present results from a randomized controlled trial (RCT) regarding MBSR effects on the defining symptoms and neuroendocrine aberrations associated with fibromyalgia: perceived stress, pain, sleep problems, fatigue, symptom severity, and physiological stress responses measured in saliva using diurnal mean cortisol level, diurnal cortisol rhythm, and the cortisol awakening response (CAR).
METHODS
Participants and Procedure
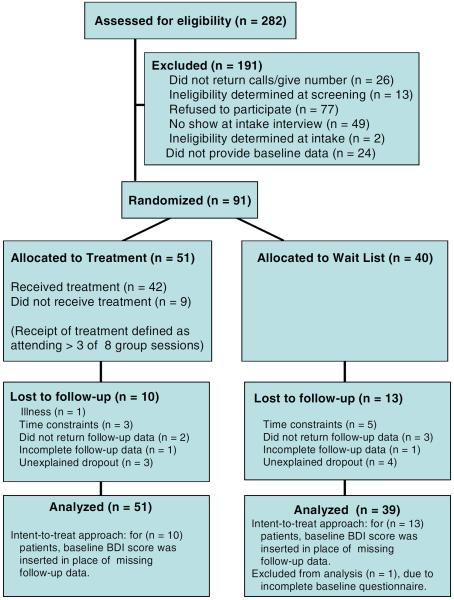
Subjects were 91 female fibromyalgia sufferers aged 18 years and older, able to attend a weekly group, with physician verified diagnosis. Two television news broadcasts and newspaper advertisements generated 282 respondents, of whom 166 were eligible. Participants attended a group training session where the investigators explained the procedures of salivary cortisol collection and provided packets of medical, control, and symptom questionnaires to complete at home. Seventy-three agreed to participate but did not complete baseline data collection, two were excluded due to severe mental illness, and 91 were randomized after baseline assessment (Figure 1).
Figure 1.
Flow diagram of participant progress through this randomized controlled trial (figure reprinted with permission (32).
Simple randomization was conducted using a 5:4 allocation strategy to protect the MBSR group from high attrition; a problem encountered in previous fibromyalgia intervention studies (36). After baseline data were collected from two cohorts of 32 and 59 women respectively, a blinded research associate computer-generated a random number between 1 and 9 for each subject ID: 1–4 were assigned to the control group, 5–9 to the treatment group. This allowed for illness-based absences of up to 25% in the treatment group while insuring that at least 10–12 participants would be present for each MBSR session. Sample size calculations were based on desired power of .80, alpha of .05, and the effect size and loss to follow-up reported in a previous study of a cognitive-behavioral stress reduction program for fibromyalgia (37), which suggested at least 82 participants. The treatment group (n=51) received MBSR, while wait-list control group participants (n=40) were offered MBSR after providing follow-up data. This trial of MBSR (ISRCTN: 34628811) was administered in 2 cycles of 25 women each. Assessments occurred in three waves: At baseline, immediately following the eight-week intervention (post-program), and 2 months after conclusion of the intervention (2-month follow-up).
The MBSR Intervention
The MBSR group followed the format of the traditional program, meeting for weekly 2.5-hour sessions over eight weeks with an experienced, trained MBSR instructor who had completed the Professional Internship training program at the University of Massachusetts Medical Center Stress Reduction Clinic (P.S.). Both formal and informal mindfulness practices were introduced (38,39), including instruction/discussion, an attention-focusing technique (body scan, directing attention throughout the body in a relaxed, supine state), sitting meditation (systematically directing attention to breath and immediate sensory and cognitive experiences), and a series of simple yoga positions taught as a means of encouraging relaxed and focused movement.
Participants were taught and encouraged to practice the three techniques 45 minutes per day, six days a week. Home practice assignments were guided by a workbook and audiotapes. Attendance data and logs of home practice were collected weekly. Absent participants received a reminder phone call to attend subsequent sessions. A half-day meditation retreat was offered to participants between the 6th and 7th week of the program.
Control Condition
A wait-list control group design was employed. Control participants were offered the MBSR program only after the conclusion of the study, subsequent to their provision of data at the same three assessment times as those randomized to MBSR: first at baseline, second at post-program (2 months), and third at follow-up (4 months after baseline, 2 months after the post-program assessment). Of the 40 wait-list control participants, 33 attended the first MBSR session. No further data were collected from control participants.
Medical and Control Variables
Medication use, smoking status, and medical comorbidities were assessed. Depressive symptoms (Beck Depression Inventory; 40) and childhood abuse or neglect (Childhood Trauma Questionnaire, Short Form; 41) were also assessed. At each follow-up assessment, all participants reported the number of times per week they practiced the mindfulness techniques at home.
Fibromyalgia Symptoms
The Perceived Stress Scale (42) was used to assess perceptions of stress over the past month. Pain was measured using an aggregate item assessing pain level over the preceding week using a Visual Analog Scale for pain, widely used among patients with chronic pain (43). Sleep quality was assessed using the summary score from the Stanford Sleep Questionnaire, which assesses problems getting to sleep, getting up at night, getting up in the morning and daytime sleepiness (44). Fatigue was measured using the summary score of the Fatigue Symptom Inventory (FSI), a measure of fatigue duration and impact with adequate reliability and validity (45). Physical functioning (rating of ability to do ten tasks requiring large muscle groups) and symptom severity (visual analog ratings of pain, fatigue, morning sleepiness, stiffness, anxiety, and depression) were measured using the Fibromyalgia Impact Questionnaire (46,47).
Neuroendocrine Function
Salivary cortisol was collected on two consecutive days at waking, 45 minutes post-waking (+45m), 1200, 1600, 2000 hours, and bedtime (48). There was no restriction imposed for day of the week of sample collection, or on dietary intake. Participants were instructed not to eat, drink, brush their teeth, or chew gum for 30 minutes prior to collecting each sample. Samples were centrifuged, aliquoted, and stored at −80° C until enzyme immunoassay (Salimetrics, Inc., State College, PA) in our laboratory. Assay sensitivity was 0.007 μg/dL, while inter- and intra-assay coefficients of variation for low and high controls were 8.9% and 6.0%, 6.4% and 4.3%, respectively. All raw cortisol values were log-transformed before calculation of the diurnal mean, diurnal slope (unstandardized beta of log-transformed cortisol values regressed on self-reported collection time, excluding +45m data), mean waking level, and CAR (mean percent increase from waking to +45m). The CAR slope was also calculated, consisting of the unstandardized beta of wake and +45m cortisol regressed on time. Analyses excluded two subjects for systemic steroid use and were adjusted for antidepressant and oral contraceptive use by entering these as covariates. Post-hoc analyses revealed no significant effects of menopausal status, smoking, caffeine intake, physical activity, menstrual phase, psychological distress, or weekday versus weekend sampling on cortisol.
Statistical Analyses
The success of randomization was tested using independent samples t-tests examining for baseline differences between treatment and control subjects on the major outcome variables. Following recommendations for RCTs in which attrition prevents collection of complete outcome data from all participants, all available data were utilized in primary analyses. Secondary analyses repeated hypothesis tests using only the data from patients with at least one follow-up point (49,50). This conservative method was intended to protect against inflated treatment effects that could result from examining only the patients with follow-up data (51).
Primary Analyses
Post-program effects were evaluated using separate analyses of covariance (ANCOVAs) for each outcome on data from wave one (baseline) and two (post-program), with treatment condition as the independent variable. Data from all three waves (i.e., including the 2-month follow-up) were incorporated in slopes analyses testing effects of MBSR. Outcome scores were regressed on time, yielding the intercept and slope of change over three assessments. According to recommendation (50,52) and consistent with other recent trials (53) an analytic strategy consistent with intent-to-treat principles was used: Baseline values were inserted in place of missing data for those lost to follow up, resulting in slopes of zero. Intervention effects were tested using ANCOVAs with slopes as the dependent variable and treatment condition as the independent variable. Baseline score was used as a covariate in tests of post-program effects, while intercepts were a covariate in slopes analyses.
Secondary Analyses
Hypotheses were re-analyzed using outcomes calculated without replacement of missing data (i.e., analyses included only data from participants who provided at least two assessments). Based upon a model of MBSR effects on health outcomes (54), potential mediators were considered, including stress appraisal (Perceived Stress Scale), psychological distress (Beck Depression Inventory), and mindfulness practice. According to previous recommendations (55), potential mediators that were significantly altered by treatment were included in linear models that simultaneously entered centered variables representing treatment group assignment, the mediator, and the treatment X mediator interaction term.
Weekly home practice logs were incomplete for a number of treatment participants; however, most participants (78%) reported the number of times per week they were meditating when completing follow-up assessments. Thus, within the treatment group, potential dose-response effects of MBSR were tested by entering attendance and self-reported home meditation practice in linear regressions predicting slopes of change. Potential systematic differences between the first and second treatment cycle were evaluated.
Finally, to examine whether effects of treatment could be attributed to worsening symptoms in the control group, outcome analyses that yielded significant results were repeated using only control group data in repeated measures ANOVAs. Secondary analyses were repeated with depressive symptoms and childhood trauma scores entered as covariates.
RESULTS
Sample Characteristics and Preliminary Analyses
The typical participant was a peri-menopausal woman of European descent, married, and college-educated with a household income over $40,000. Co-morbidities (including arthritis, headaches, chronic fatigue, and respiratory symptoms) were reported by 72.5%. A total of 63.7% used antidepressant and 23.1% used anxiolytic medication. Beck Depression Inventory scores indicative of clinical depression (>19; 59) were obtained by 29%, commensurate with proportions reported in other studies (56,57). The randomization procedure was successful; there were no baseline group differences in demographic, medical, or outcome variables between the treatment and the control group. Forty-three treatment group participants (84.3%) provided follow-up data. Forty-two treatment group participants (82.3%) completed the MBSR program (attending four or more session; 58), with mean attendance of 5.5 sessions (SD=2.1). Attendance rates fell during the first month of treatment from 90% at the first meeting to 57% by the fourth group meeting. Attendance rates were maintained between 57% and 64.7% during the second four weeks of the treatment, and are commensurate with or greater than those reported in other psychosocial interventions with fibromyalgia patients (30–45% dropout rates; 56,57,59). Independent t-tests showed significantly higher baseline sleep problems, fatigue, symptom severity, poorer physical functioning and lower log transformed mean cortisol (all p's<.05) among those subsequently lost to follow-up. However, a two-way factorial ANOVA demonstrated no differences in attrition between the treatment and the control group. Among the control group, 27 (67.5%) provided complete follow-up data.
Primary Analyses
Table 1 presents mean scores on all outcomes for treatment and control participants at each assessment, and Figures 2A and 2B depict the raw cortisol values obtained at each assessment. The MBSR program significantly reduced perceived stress, sleep problems, fatigue, and symptom severity at the post-program assessment (Table 2). Post-program effects on pain, physical functioning, and cortisol outcomes did not reach significance. Slopes analyses demonstrated that significant reductions of perceived stress, sleep problems, and symptom severity were maintained, while effects on fatigue did not reach significance.
Table 1.
Mean and SD outcome scores for treatment and control participants at Baseline, immediately post-intervention (Post) and two-month follow-up (2-Month). Slope of change and Intercept scores were calculated over all three assessments.
| Treatment (N = 51) | Control (N = 40) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post | 2-Month | Slope | Intercept | Baseline | Post | 2-Month | Slope | Intercept | |
| Primary Analyses | ||||||||||
| Symptom Variables | ||||||||||
| PSS | 22.0 (6.2) | 19.2 (7.2) | 20.2 (6.6) | −.88 (1.8) | 21.7 (6.2) | 21.4 (7.4) | 22.5 (6.5) | 20.8 (6.5) | −.04 (1.3) | 21.6 (7.3) |
| VAS | 68.1 (25.4) | 60.4 (26.4) | 65.2 (25.0) | −1.9 (6.7) | 67.1 (24.8) | 69.2 (19.6) | 68.5 (23.5) | 65.1 (22.1) | −1.1 (6.6) | 69.5 (20.0) |
| SSQ | 9.0 (3.2) | 8.5 (3.3) | 8.4 (4.0) | −.34 (.73) | 9.4 (3.3) | 9.3 (3.1) | 9.3 (3.1) | 9.5 (2.7) | −.05 (.64) | 9.5 (3.2) |
| FSI | 6.1 (1.4) | 5.6 (1.8) | 5.5 (1.8) | −.16 (.42) | 6.0 (1.5) | 6.1 (1.7) | 6 .4 (1.6) | 6.0 (1.9) | −.05 (.27) | 6.2 (1.6) |
| FIQ Symptom Severity | 67.5 (15.8) | 58.4 (21.0) | 62.0 (18.6) | −3.3 (6.0) | 66.5 (16.2) | 62.5 (18.1) | 67.2 (16.7) | 66.7 (16.8) | −.21 (3.6) | 67.8 (14.6) |
| FIQ Physical Functioning | 1.3 (.72) | 1.2 (.73) | 1.2 (.74) | −.05 (.15) | 1.3 (.70) | 1.2 (.75) | 1.2 (.84) | 1.2 (.76) | .01 (.15) | 1.2 (.78) |
| Salivary Cortisol | ||||||||||
| Diurnal Mean | −2.0 (.33) | −1.9 (.34) | −2.0 (.34) | .01 (.09) | −2.0 (.32) | −1.9 (.51) | −1.8 (.53) | −1.8 (.56) | .02 (.09) | −1.9 (.51) |
| Diurnal Slope | −.11 (.07) | −.12 (.05) | −.12 (.05) | −.00 (.02) | −.11 (.06) | −.10 (.05) | −.10 (.06) | −.11 (.04) | −.00 (.01) | −1.0 (.05) |
| Mean Waking Level | −1.2 (.63) | −1.1 (.54) | −1.2 (.61) | .03 (.30) | −1.2 (.59) | −1.2 (.70) | −1.2 (.72) | −1.1 (.71) | .07 (.21) | −1.2 (.68) |
| CAR | .45 (.71) | .33 (.56) | .43 (.69) | −.08 (.49) | .45 (.73) | .59 (.73) | .54 (.95) | .46 (.74) | −.06 (.55) | .60 (.71) |
| CAR Slope | .85 (1.58) | .56 (.69) | .76 (1.3) | −.05 (.37) | .76 (.93) | 1.19 (1.90) | 1.2 (2.1) | .83 (1.4) | −.04 (.64) | 1.2 (1.9) |
| Secondary Analyses | ||||||||||
| Symptom Variables | ||||||||||
| PSS | 22.0 (6.2) | 17.6 (7.0) | 18.2 (6.2) | −1.10 (2.0) | 21.1 (6.0) | 21.4 (7.4) | 22.4 (7.0) | 21.7 (6.3) | −.05 (1.61) | 21.7 (8.0) |
| VAS | 68.1 (25.4) | 52.8 (22.8) | 58.9 (22.8) | −2.38 (7.4) | 63.3 (22.8) | 69.2 (19.6) | 65.0 (27.6) | 62.0 (24.8) | −1.6 (8.2) | 68.4 (21.7) |
| SSQ | 9.0 (3.2) | 7.9 (3.2) | 6.8 (3.0) | −.41 (.79) | 8.9 (3.2) | 9.3 (3.1) | 8.5 (2.9) | 8.8 (2.4) | −.07 (.80) | 8.8 (3.0) |
| FSI | 6.1 (1.4) | 5.1 (1.7) | 5.1 (1.8) | −.21 (.47) | 5.9 (1.4) | 6.1 (1.7) | 6.3 (1.6) | 5.5 (1.9) | −.07 (.33) | 5.9 (1.5) |
| FIQ Symptom Severity | 67.5 (15.8) | 51.6 (19.6) | 54.3 (19.4) | −4.16 (6.4) | 64.5 (16.5) | 62.5 (18.1) | 62.5 (18.1) | 26.2 (16.4) | −.32 (4.5) | 63.4 (14.5) |
| FIQ Physical Functioning | 1.3 (.72) | .97 (.69) | 1.1 (.81) | −.06 (.17) | 1.2 (.71) | 1.2 (.75) | 1.0 (.89) | 1.0 (.76) | .01 (.19) | 1.1 (.76) |
| Salivary Cortisol | ||||||||||
| Diurnal Mean | −2.0 (.33) | −1.90 (.34) | −2.0 (.36) | .01 (.10) | −2.0 (.32) | −1.9 (.51) | −1.9 (.50) | −1.9 (.53) | .03 (.11) | −2.0 (.47) |
| Diurnal Slope | −.11 (.07) | −.11 (.05) | −.10 (.06) | .02 (.02) | −.11 (.07) | −.10 (.05) | −.10 (.06) | −.11 (.04) | −.01 (.02) | −.10 (.05) |
| Mean Waking Level | −1.18 (.63) | −1.17 (.52) | −1.28 (.60) | .04 (.33) | −1.24 (.58) | −1.22 (.70) | −1.18 (.68) | −1.06 (.72) | .09 (.24) | −1.28 (.24) |
| CAR | .85 (1.58) | .55 (.67) | .85 (1.58) | −.07 (.43) | .80 (.94) | 1.19 (1.90) | .84 (1.6) | .45 (.82) | −0.5 (.80) | 1.1 (1.8) |
| CAR Slope | .45 (.71) | .35 (.53) | .50 (.73) | −.10 (.55) | .50 (.73) | .59 (.73) | .41 (.90) | .30 (.70) | −.08 (.65) | .55 (.68) |
Note: PSS - Perceived Stress Scale, VAS -Visual Analog Scale, SSQ - Stanford Sleep Questionnaire, FSI - Fatigue Symptom Inventory, FIQ - Fibromyalgia Impact Questionnaire, CAR - cortisol awakening response
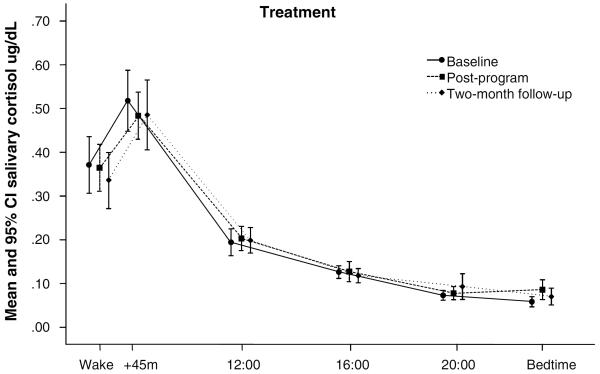
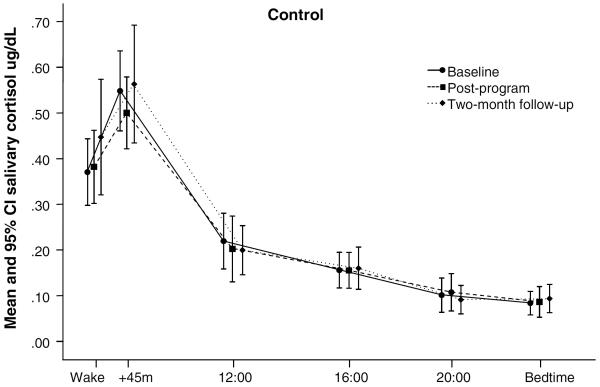
Figure 2.
Raw cortisol values by collection time at baseline, immediate post-program, and two month follow-up assessments for treatment (n=42) and control (n=27) group participants. Baseline cortisol values did not differ significantly between treatment and control groups.
Table 2.
Primary results testing effects of the intervention on immediate post-program scores and on slopes of change over all three assessments. Results presented include the F score, the degrees of freedom (df), significance level of the analysis (P), measures of effect size (eta2 and Cohen's d), and observed power (1-β).
| Post-Program | Slopes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | df | P | Eta2* | d | 1-β | F | df | P | Eta2* | d | 1-β | |
| Symptom Variables | ||||||||||||
| PSS | 14.58 | 88 | .000 | .14 | .48 | .97 | 6.29 | 88 | .014 | .07 | .54 | .70 |
| VAS | 3.41 | 87 | .068 | .04 | .32 | .45 | .82 | 87 | .368 | .01 | .12 | .15 |
| SSQ | 4.46 | 88 | .038 | .05 | .25 | .55 | 4.06 | 88 | .047 | .04 | .42 | .51 |
| FSI | 9.87 | 84 | .002 | .11 | .47 | .87 | 3.59 | 87 | .062 | .04 | .31 | .47 |
| FIQ Symptom Severity | 6.54 | 87 | .012 | .07 | .46 | .72 | 9.03 | 87 | .003 | .09 | .62 | .84 |
| FIQ Physical Functioning | 1.90 | 88 | .172 | .02 | .00 | .28 | 2.32 | 88 | .132 | .03 | .40 | .33 |
| Salivary Cortisol | ||||||||||||
| Diurnal Mean | .727 | 80 | .396 | .01 | .22 | .13 | .86 | 80 | .357 | .01 | .11 | .15 |
| Diurnal Slope | .000 | 80 | .988 | .00 | .36 | .05 | .26 | 80 | .610 | .00 | .00 | .08 |
| Mean Waking Value | .093 | 77 | .761 | .00 | .16 | .06 | .113 | 77 | .738 | .00 | .15 | .06 |
| CAR | 3.73 | 73 | .057 | .05 | .27 | .48 | .58 | 73 | .450 | .01 | .04 | .12 |
| CAR Slope | 2.96 | 74 | .090 | .04 | .41 | .40 | .583 | 74 | .448 | .01 | .02 | .12 |
Note.
Eta2 is the effect size indicating the proportion of the total variance attributed to the effect of treatment (calculated by dividing the sum-of-squares for an effect by the sum-of-squares total; small=.01, medium=.06, large=.14). PSS - Perceived Stress Scale, VAS - Visual Analog Scale, SSQ - Stanford Sleep Questionnaire, FSI - Fatigue Symptom Inventory, FIQ - Fibromyalgia Impact Questionnaire, CAR - cortisol awakening response
Secondary Analyses
Secondary analyses replicated the primary post-program findings for perceived stress, fatigue, and symptom severity. Effects of MBSR on pain, sleep, and cortisol were not significant (Table 3). Slopes analyses replicated primary findings for stress and symptom severity; however, MBSR effects on sleep did not reach significance in this smaller sample of participants with follow-up data.
Table 3.
Secondary results testing effects of the intervention on immediate post-program scores and on slopes of change in outcomes over all three assessments. Analyses include 68 participants who provided baseline and at least one follow-up score. Results presented include the F score, the degrees of freedom (df), significance level of the analysis (P), measures of effect size (eta2 and Cohen's d), and observed power (1-β).
| Post-Program | Slopes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | df | P | Eta2* | d | 1-β | F | df | P | Eta2* | d | 1-β | |
| Symptom Variables | ||||||||||||
| PSS | 14.60 | 52 | .000 | .22 | .69 | 1.00 | 8.58 | 63 | .005 | .12 | .58 | .82 |
| VAS | 3.43 | 57 | .057 | .06 | .48 | .44 | .83 | 64 | .37 | .01 | .13 | .15 |
| SSQ | 2.89 | 60 | .094 | .05 | .20 | .39 | 3.39 | 65 | .07 | .05 | .43 | .44 |
| FSI | 10.16 | 50 | .002 | .17 | .73 | .89 | 2.00 | 64 | .16 | .03 | .34 | .29 |
| FIQ Symptom Severity | 5.92 | 56 | .018 | .10 | .58 | .67 | 7.44 | 63 | .008 | .11 | .69 | .77 |
| FIQ Physical Functioning | 1.40 | 60 | .241 | .02 | .04 | .21 | 1.63 | 65 | .21 | .02 | .39 | 24 |
| Salivary Cortisol | ||||||||||||
| Diurnal Mean | .39 | 61 | .53 | .01 | .00 | .10 | .35 | 61 | .56 | .01 | .19 | .14 |
| Diurnal Slope | .018 | 62 | .90 | .00 | .18 | .05 | .76 | 61 | .39 | .01 | 1.5 | .14 |
| Mean Waking Value | .006 | 55 | .938 | .00 | .02 | .05 | .288 | 65 | .594 | .01 | .17 | .08 |
| CAR | 1.60 | 51 | .21 | .03 | .24 | .24 | .39 | 57 | .54 | .01 | .67 | .09 |
| CAR Slope | 1.03 | 52 | .314 | .02 | .08 | .17 | .267 | 58 | .607 | .01 | .03 | .08 |
Note.
Eta2 is the effect size indicating the proportion of the total variance attributed to the effect of treatment (calculated by dividing the sum-of-squares for an effect by the sum-of-squares total; small=.01, medium=.06, large=.14). PSS - Perceived Stress Scale, VAS - Visual Analog Scale, SSQ - Stanford Sleep Questionnaire, FSI - Fatigue Symptom Inventory, FIQ - Fibromyalgia Impact Questionnaire, CAR - cortisol awakening response
Potential mediation of MBSR effects on sleep and symptom severity were examined using six linear regressions examining effects of change in Perceived Stress Scale, Beck Depression Inventory, and mindfulness practice on these outcomes. No mediation effects were observed.
Dose-response effects of MBSR were significant for home mindfulness practice but not session attendance. Specifically, slopes analyses revealed that mindfulness practice (times per week at two-month follow-up; M=4.8) predicted persistent reductions in pain (Visual Analog Scale; R2=.42; p<.01, partial r=−.45) and symptom severity (Fibromyalgia Impact Questionnaire; R2=.24, p<.05, partial r=−.40). In contrast, significant dose-response effects were not observed on perceived stress, sleep, fatigue, physical functioning, or cortisol (all p's>.223).
The 8-week MBSR treatment was completed in two cycles of 25 participants. A significant effect of treatment cycle emerged in post program analyses: Participants in the second cycle reported a greater decline in the level of perceived stress at follow-up after adjusting for baseline perceived stress level (F1,30=4.41, p<.05).
Analyses adjusted for childhood trauma history produced no change in results. Analyses adjusted for changes in depressive symptoms over the course of the study revealed that, while MBSR effects on sleep were unchanged, effects on perceived stress, fatigue, and impairment no longer reached significance. Repeated measures ANOVAs revealed no significant change in any outcome measure within the wait-list control group, as expected.
DISCUSSION
Results of this randomized prospective trial show that the 8-week MBSR Program reduced perceived stress and lessened the severity of fibromyalgia symptoms. These gains were maintained at a follow-up two months after the conclusion of the intervention. These findings persisted in secondary analyses. MBSR may be particularly well suited as a complementary intervention for fibromyalgia patients, given that they frequently report that major life stressors preceded the onset of their illness, and psychological distress worsens their symptoms.
Improvements in self-reported sleep were demonstrated in primary, but not secondary, analyses. Transient (post-program) improvements in self-reported fatigue were also noted. One might expect improvements in sleep and fatigue to confer a physical benefit; however, physical function did not improve. Study participants had to function physically well enough to participate, and this study's physical function scores were relatively high compared to others (47), possibly making it difficult to detect improvement. Compelling and persistent improvements on measures of pain and fatigue were not observed. Perhaps MBSR ameliorated the threat of illness more than its actual symptoms as measured. Our data are consistent with evidence of benefits related to mindfulness practice among diverse medical groups (30,33,60–63). With regard to the effect sizes observed here, findings were consistent across the two parameters used: Both eta2 and Cohen's d suggest at least medium effects for reduction of distress and impact of illness, with 61% of treatment participants (vs. 48% of controls) reporting a reduction in perceived stress, and 75% (vs. 69% of controls) experiencing a reduction in symptom severity. This suggests a meaningful impact of MBSR in reducing patient's burden related to fibromyalgia.
After adjusting for changes in depressive symptoms over the course of the study, MBSR effects on depression could not be distinguished from effects on perceived stress and symptom severity, but could be distinguished from effects on sleep. This is not surprising given known interrelationships between depression, stress, and impairment. However, mediation analysis showed that none of the effects of MBSR on symptom outcomes were mediated by changes in depressive symptoms.
Additionally, we noted that frequency of home mindfulness practice (times per week) was significantly associated with greater symptom relief. Analyses of process variables related to mindfulness practice are beginning to shed light on how such benefits may be achieved. Recent work has underscored individual meditation practice, as well as neural underpinnings and other aspects of MBSR (64). Our results highlight these potential variables. Similarly, a recent study suggests that greater amount of home practice is associated with improved psychological distress, somatization symptoms, and self-rated health in patients with chronic pain conditions (60). Home practice has been shown to increase self-reported mindfulness, which in turn mediates improved well-being and depressive symptom reduction (65). Practice time effects may also extend to physiological responses, including inflammatory response to laboratory stress (66). While dose-response findings differ (67,68), available data suggest that regular formal mindfulness practice is tied to beneficial outcomes, at least in some participants.
Other mechanisms by which mindfulness can improve clinical health outcomes have been recently conceptualized among models focusing chiefly on attention and emotion regulation (64,69,70), metacognitive factors (60,71,72), improved stress coping (73), and bridging of Eastern and Western psychology (74,75). Perhaps most intriguing is a conceptualization that explores mindfulness within the context of the Transactional Model of Stress and Coping (54,73,76). Such a model suggests that, among fibromyalgia patients, mindfulness may enhance the perception of symptom control, while present-moment focus may reduce distress stemming from preoccupations with past or future concerns related to the illness.
In addition to measuring effects on appraisal, it may be informative to test effects on other components of Lazarus's model, as well as on physiological mediators of effects of stress on health. It is possible that there are both direct and indirect effects of mindfulness on appraisal, coping, positive and negative mood, and physiologic stress responses (52,54). For example, simply focusing on the breath may result in a slower breathing rate and concomitant reduction of sympathetic arousal (77). In support, we have observed a reduction of sympathetic activation during meditation (body scan) sessions, as well as over the course of MBSR training among fibromyalgia patients (35). Activation-reducing qualities of both yoga and sitting meditation have also been documented (78,79), suggesting formal mindfulness practices may reduce physiological activation patterns associated with sympathetic nervous and/or hypothalamic-pituitary-adrenal axis arousal.
MBSR effects on neuroendocrine function did not reach significance in this study. In comparison to normative data from healthy subjects (Salimetrics, Inc.), this chronically ill sample appeared to have a diminished CAR, with relatively normal evening cortisol levels. Because treatment effects on CAR just nearly reached significance, we explored the possibility that the high number and diversity of medications used by our study participants introduced physiological variance that may have masked MBSR effects on cortisol. Therefore, we conducted an additional non-randomized trial that produced complete cortisol data from 24 new fibromyalgia patients. Participants were screened for smoking and medications likely to affect cortisol or circadian rhythms (barbiturate, benzodiazepine, hypnotic, muscle relaxant, opiate, anticonvulsant, antipsychotic, and systemic glucocorticoids). This small MBSR trial produced symptom reductions similar to those obtained in the current RCT. As expected, ANCOVAs adjusted for antidepressant use revealed the CAR was significantly reduced after MBSR, as measured both by percent increase and by CAR slope. Taken together with other data showing MBSR can reduce sympathetic activation (35,65), these pilot results suggest future studies should continue to explore effects of mindfulness and MBSR on adrenocortical and autonomic stress responses, and employ careful assessment and control when examining these complex and sensitive biological parameters.
Despite the advantages of a randomized controlled design, employment of CONSORT guidelines (80), multiple assessments, and analyses that addressed loss to follow-up (51), limitations of attrition and racial homogeneity are present in this sample. Further, symptom severity measured at baseline was significantly related to attrition, limiting generalizability to healthier fibromyalgia patients. The high percentage of those with a co-morbid medical disorder (72.5%) may have reduced the effectiveness of MBSR able to be observed amongst this sample. Having such a large percentage of non-smokers in this sample (83%) may have skewed some of the results, since smokers often experience more severe symptoms than non-smokers with fibromyalgia, including increased pain and functional disability (81). Future studies that assess a more equal distribution in smoking status may reveal differential MBSR outcomes between the two groups, since smokers may experience increased benefits from the MBSR intervention. In addition, patient reported increases in mindfulness were not assessed in this study due to the absence of validated mindfulness measures at the time, though these are now available (82–85).
Future studies are needed to investigate the possible interactions between changes in psychosocial variables, symptoms, and endocrine function. As a recent study suggests, changes in psychological distress may be at least partially mediated by mechanisms that include reperceiving, values clarification, flexibility, and exposure (86). Future studies could also better account for effects of meditation practice and clinician attention. The group nature of MBSR introduces social factors as potential intervention elements, such as mutual support and encouragement. The potential multiple effects of mindfulness and specific pathways of change in symptoms could be more closely examined in studies which make use of multiple groups, dismantling designs, and assessment of social support and group interaction (38). Future studies could also examine the impact of the MBSR intervention at a later point in time, such as 6 or 12 months post-intervention. Studies designed in such a way could achieve a more complete picture of the benefits achieved from home practice, formal practice, group interaction, as well as generalized improved outcomes.
The present study contributes to the body of research supporting the notion that MBSR may be effective in reducing the impact and some symptoms of illness among women with fibromyalgia (33,34,59,87). In addition, rigorous physiological outcome measurement that included salivary cortisol collection across multiple days, combined with analyses that controlled for known confounds (i.e., sleep, diet, activity level, etc.), offers a substantial contribution to the MBSR literature (56). Overall, these results support the use of MBSR as a complementary intervention for fibromyalgia.
Abbreviations
- MBSR
Mindfulness-Based Stress Reduction
- CAR
cortisol awakening response
- RCT
randomized controlled trial
Footnotes
Authors' Statement of Conflict of Interest and Adherence to Ethical Standards Author Elizabeth Cash, Author Paul Salmon, Author Inka Weissbecker, Author Whitney N. Rebholz, Author Rene Bayley-Veloso, Author Lauren Zimmaro, Author Andrea Floyd, Author Eric Dedert, and Author Sandra E. Sephton declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
REFERENCES
- 1.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38(1):19–28. doi: 10.1002/art.1780380104. doi:10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Clauw DJ, Fitzcharles M-A, et al. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010;62(5):600–610. doi: 10.1002/acr.20140. doi:10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 3.Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet. Disord. 2007;8(1):27. doi: 10.1186/1471-2474-8-27. doi:10.1186/1471-2474-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutledge DN, Jones K, Jones CJ. Predicting High Physical Function in People With Fibromyalgia. J. Nurs. Scholarsh. 2007;39(4):319–324. doi: 10.1111/j.1547-5069.2007.00187.x. doi:10.1111/j.1547-5069.2007.00187.x. [DOI] [PubMed] [Google Scholar]
- 5.Shillam CR, Dupree Jones K, Miller L. Fibromyalgia Symptoms, Physical Function, and Comorbidity in Middle-Aged and Older Adults. Nurs. Res. 2011;1 doi: 10.1097/NNR.0b013e31822bbdfa. doi:10.1097/NNR.0b013e31822bbdfa. [DOI] [PubMed] [Google Scholar]
- 6.Crofford LJ. Violence, Stress, and Somatic Syndromes. Trauma Violence Abuse. 2007;8(3):299–313. doi: 10.1177/1524838007303196. doi:10.1177/1524838007303196. [DOI] [PubMed] [Google Scholar]
- 7.Van Houdenhove BV, Egle U, Luyten P. The role of life stress in fibromyalgia. Curr. Rheumatol. Rep. 2005;7(5):365–370.. doi: 10.1007/s11926-005-0021-z. doi:10.1007/s11926-005-0021-z. [DOI] [PubMed] [Google Scholar]
- 8.Mease P. Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatment. J. Rheumatol. 2005;75:6–21. [PubMed] [Google Scholar]
- 9.Weissbecker I, Salmon P, Studts JL, Floyd AR, Dedert EA, Sephton SE. Mindfulness-Based Stress Reduction and Sense of Coherence Among Women with Fibromyalgia. J. Clin. Psychol. Med. Settings. 2002;9(4):297–307. doi:10.1023/A:1020786917988. [Google Scholar]
- 10.McLean SA, Williams DA, Harris RE, et al. Momentary relationship between cortisol secretion and symptoms in patients with fibromyalgia. Arthritis Rheum. 2005;52(11):3660–3669. doi: 10.1002/art.21372. doi:10.1002/art.21372. [DOI] [PubMed] [Google Scholar]
- 11.Korszun A. Sleep and circadian rhythm disorders in fibromyalgia. Curr. Rheumatol. Rep. 2000;2(2):124–130. doi: 10.1007/s11926-000-0052-4. doi:10.1007/s11926-000-0052-4. [DOI] [PubMed] [Google Scholar]
- 12.McCain GA, Tilbe KS. Diurnal hormone variation in fibromyalgia syndrome: a comparison with rheumatoid arthritis. J. Rheumatol. Suppl. 1989;19:154–157. [PubMed] [Google Scholar]
- 13.Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163–171. doi: 10.1016/j.psyneuen.2008.10.026. doi:10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32(4):358–366. doi: 10.1016/j.psyneuen.2007.01.008. doi:10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom. Med. 1999;61(2):197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29(4):516–528. doi: 10.1016/s0306-4530(03)00072-6. doi:10.1016/S0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 17.Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology. 2007;32(1):80–86. doi: 10.1016/j.psyneuen.2006.10.005. doi:10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Riva R, Mork PJ, Westgaard RH, Rø M, Lundberg U. Fibromyalgia Syndrome is Associated with Hypocortisolism. Int. J. Behav. Med. 2010;17(3):223–233. doi: 10.1007/s12529-010-9097-6. doi:10.1007/s12529-010-9097-6. [DOI] [PubMed] [Google Scholar]
- 19.Riva R, Mork PJ, Westgaard RH, Lundberg U. Comparison of the cortisol awakening response in women with shoulder and neck pain and women with fibromyalgia. Psychoneuroendocrinology. 2012;37(2):299–306. doi: 10.1016/j.psyneuen.2011.06.014. doi:10.1016/j.psyneuen.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Curtis K, Osadchuk A, Katz J. An eight-week yoga intervention is associated with improvements in pain, psychological functioning and mindfulness, and changes in cortisol levels in women with fibromyalgia. J. Pain Res. 2011;4:189–201. doi: 10.2147/JPR.S22761. doi:10.2147/JPR.S22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain. Behav. Immun. 2007;21(8):1038–1049. doi: 10.1016/j.bbi.2007.04.002. doi:10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Carlson LE, Garland SN. Impact of mindfulness-based stress reduction (MBSR) on sleep, mood, stress and fatigue symptoms in cancer outpatients. Int. J. Behav. Med. 2005;12(4):278–285. doi: 10.1207/s15327558ijbm1204_9. doi:10.1207/s15327558ijbm1204_9. [DOI] [PubMed] [Google Scholar]
- 23.Crofford LJ, Appleton BE. The treatment of fibromyalgia: A review of clinical trials. Curr. Rheumatol. Rep. 2000;2(2):101–103. doi: 10.1007/s11926-000-0048-0. doi:10.1007/s11926-000-0048-0. [DOI] [PubMed] [Google Scholar]
- 24.Crofford LJ. Pain management in fibromyalgia. Curr. Opin. Rheumatol. 2008;20(3):246–250. doi: 10.1097/BOR.0b013e3282fb0268. doi:10.1097/BOR.0b013e3282fb0268. [DOI] [PubMed] [Google Scholar]
- 25.Glombiewski JA, Sawyer AT, Gutermann J, Koenig K, Rief W, Hofmann SG. Psychological treatments for fibromyalgia: A meta-analysis. PAIN. 2010;151(2):280–295. doi: 10.1016/j.pain.2010.06.011. doi:10.1016/j.pain.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA. 2004;292(19):2388–2395. doi: 10.1001/jama.292.19.2388. doi:10.1001/jama.292.19.2388. [DOI] [PubMed] [Google Scholar]
- 27.Kabat-Zinn J. Mindfulness-Based Interventions in Context: Past, Present, and Future. Clin. Psychol. Sci. Pract. 2003;10(2):144–156. [Google Scholar]
- 28.Keng S-L, Smoski MJ, Robins CJ. Effects of mindfulness on psychological health: A review of empirical studies. Clin. Psychol. Rev. 2011;31(6):1041–1056. doi: 10.1016/j.cpr.2011.04.006. doi:10.1016/j.cpr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro SL. The integration of mindfulness and psychology. J. Clin. Psychol. 2009;65(6):555–560. doi: 10.1002/jclp.20602. doi:10.1002/jclp.20602. [DOI] [PubMed] [Google Scholar]
- 30.Bohlmeijer E, Prenger R, Taal E, Cuijpers P. The effects of mindfulness-based stress reduction therapy on mental health of adults with a chronic medical disease: A meta-analysis. J. Psychosom. Res. 2010;68(6):539–544. doi: 10.1016/j.jpsychores.2009.10.005. doi:10.1016/j.jpsychores.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Matousek RH, Dobkin PL, Pruessner J. Cortisol as a marker for improvement in mindfulness-based stress reduction. Complement. Ther. Clin. Pract. 2010;16(1):13–19. doi: 10.1016/j.ctcp.2009.06.004. doi:10.1016/j.ctcp.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Sephton SE, Salmon P, Weissbecker I, et al. Mindfulness meditation alleviates depressive symptoms in women with fibromyalgia: Results of a randomized clinical trial. Arthritis Care Res. 2007;57(1):77–85. doi: 10.1002/art.22478. doi:10.1002/art.22478. [DOI] [PubMed] [Google Scholar]
- 33.Grossman P, Tiefenthaler-Gilmer U, Raysz A, Kesper U. Mindfulness training as an intervention for fibromyalgia: evidence of postintervention and 3-year follow-up benefits in well-being. Psychother. Psychosom. 2007;76(4):226–233. doi: 10.1159/000101501. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt S, Grossman P, Schwarzer B, Jena S, Naumann J, Walach H. Treating fibromyalgia with mindfulness-based stress reduction: Results from a 3-armed randomized controlled trial. PAIN. 2011;152(2):361–369. doi: 10.1016/j.pain.2010.10.043. doi:10.1016/j.pain.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 35.Lush E, Salmon P, Floyd A, Studts JL, Weissbecker I, Sephton SE. Mindfulness Meditation for Symptom Reduction in Fibromyalgia: Psychophysiological Correlates. J. Clin. Psychol. Med. Settings. 2009;16(2):200–207. doi: 10.1007/s10880-009-9153-z. doi:10.1007/s10880-009-9153-z. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan KH, Goldenberg DL, Galvin-Nadeau M. The impact of a meditation-based stress reduction program on fibromyalgia. Gen. Hosp. Psychiatry. 1993;15(5):284–289. doi: 10.1016/0163-8343(93)90020-o. doi:10.1016/0163-8343(93)90020-O. [DOI] [PubMed] [Google Scholar]
- 37.Goldenberg DL, Kaplan KH, Nadeau MG, Brodeur C, Smith S, Schmid CH. A Controlled Study of a Stress-Reduction, Cognitive-Behavioral Treatment Program in Fibromyalgia. J. Musculoskelet. Pain. 1994;2(2):53–66. doi:10.1300/J094v02n02_05. [Google Scholar]
- 38.Shapiro SL, Carlson LE, Astin JA, Freedman B. Mechanisms of mindfulness. J. Clin. Psychol. 2006;62(3):373–386. doi: 10.1002/jclp.20237. doi:10.1002/jclp.20237. [DOI] [PubMed] [Google Scholar]
- 39.Salmon P, Sephton S, Weissbecker I, Hoover K, Ulmer C, Studts JL. Mindfulness meditation in clinical practice. Cogn. Behav. Pract. 2004;11(4):434–446. doi:10.1016/S1077-7229(04)80060-9. [Google Scholar]
- 40.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 1988;8(1):77–100. doi:10.1016/0272-7358(88)90050-5. [Google Scholar]
- 41.Bernstein DP, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report: Manual. Harcourt Brace & Company; 1998. [Google Scholar]
- 42.Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983;24(4):385. doi:10.2307/2136404. [PubMed] [Google Scholar]
- 43.Callahan LF, Brooks RH, Summey JA, Pincus T. Quantitative pain assessment for routine care of rheumatoid arthritis patients, using a pain scale based on activities of daily living and a visual analog pain scale. Arthritis Rheum. 1987;30(6):630–636. doi: 10.1002/art.1780300605. doi:10.1002/art.1780300605. [DOI] [PubMed] [Google Scholar]
- 44.Douglass A, Bornstein R, Nino-Murcia G, et al. Sleep Disorders Questionnaire. Sleep. 1994;17(2):160–167. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 45.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of Fatigue in Cancer Patients: Development and Validation of the Fatigue Symptom Inventory. Qual. Life Res. 1998;7(4):301–310. doi: 10.1023/a:1024929829627. doi:10.1023/A:1024929829627. [DOI] [PubMed] [Google Scholar]
- 46.Burckhardt CS, Clark SR, Bennett RM, et al. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18(5):728–33. [PubMed] [Google Scholar]
- 47.Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin. Exp. Rheumatol. 2005;23(5):S154. [PubMed] [Google Scholar]
- 48.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19(4):313–333. doi: 10.1016/0306-4530(94)90013-2. doi:10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 49.Pledger GW. Basic statistics: importance of compliance. J. Clin. Res. Pharmacoepidemiol. 1992;6(2):77–81. [Google Scholar]
- 50.Friedman LM, Furberg C, DeMets DL. Fundamentals of Clinical Trials. Springer; New York, NY: [Accessed September 5, 2014]. 2010. Available at: http://link.springer.com/content/pdf/10.1007/978-1-4419-1586-3.pdf. [Google Scholar]
- 51.Lachin JM. Statistical Considerations in the Intent-to-Treat Principle. Control. Clin. Trials. 2000;21(3):167–189. doi: 10.1016/s0197-2456(00)00046-5. doi:10.1016/S0197-2456(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 52.Lambert MJ, editor. Bergin and Garfield's Handbook of Psychotherapy and Behavior Change. 5th ed John Wiley & Sons Inc; New York, NY: 2004. [Google Scholar]
- 53.Hughes JW, Fresco DM, Myerscough R, H. M. van Dulmen M, Carlson LE, Josephson R. Randomized Controlled Trial of Mindfulness-Based Stress Reduction for Prehypertension. Psychosom. Med. 2013;75(8):721–728. doi: 10.1097/PSY.0b013e3182a3e4e5. doi:10.1097/PSY.0b013e3182a3e4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herbert JD, Forman EM, editors. Acceptance and Mindfulness in Cognitive Behavior Therapy: Understanding and Applying the New Therapies. John Wiley & Sons; 2011. [Google Scholar]
- 55.Kraemer H, Wilson G, Fairburn CG, Agras W. Mediators and moderators of treatment effects in randomized clinical trials. Arch. Gen. Psychiatry. 2002;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. doi:10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 56.Offenbaecher M, Glatzeder K, Ackenheil M. Self-reported depression, familial history of depression and fibromyalgia (FM), and psychological distress in patients with FM. Z. Für Rheumatol. 1998;57(2):S94–S96. doi: 10.1007/s003930050245. doi:10.1007/s003930050245. [DOI] [PubMed] [Google Scholar]
- 57.Thieme K, Turk DC, Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: relationship to somatic and psychosocial variables. Psychosom. Med. 2004;66(6):837–844. doi: 10.1097/01.psy.0000146329.63158.40. [DOI] [PubMed] [Google Scholar]
- 58.Teasdale JD, Segal ZV, Mark J, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J. Consult. Clin. Psychol. 2000;68(4):615–623. doi: 10.1037//0022-006x.68.4.615. doi:10.1037/0022-006X.68.4.615. [DOI] [PubMed] [Google Scholar]
- 59.Astin JA, Berman BM, Bausell B, Lee W-L, Hochberg M, Forys KL. The efficacy of mindfulness meditation plus Qigong movement therapy in the treatment of fibromyalgia: a randomized controlled trial. J. Rheumatol. 2003;30(10):2257–2262. [PubMed] [Google Scholar]
- 60.Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, Beasley D. Mindfulness-based stress reduction for chronic pain conditions: Variation in treatment outcomes and role of home meditation practice. J. Psychosom. Res. 2010;68(1):29–36. doi: 10.1016/j.jpsychores.2009.03.010. doi:10.1016/j.jpsychores.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 61.Singh BB, Berman BM, Hadhazy VA, Creamer P. A pilot study of cognitive behavioral therapy in fibromyalgia. Altern. Ther. Health Med. 1998;4(2):67–70. [PubMed] [Google Scholar]
- 62.Chiesa A, Serretti A. Mindfulness-Based Stress Reduction for Stress Management in Healthy People: A Review and Meta-Analysis. J. Altern. Complement. Med. 2009;15(5):593–600. doi: 10.1089/acm.2008.0495. doi:10.1089/acm.2008.0495. [DOI] [PubMed] [Google Scholar]
- 63.Musial F, Büssing A, Heusser P, Choi K-E, Ostermann T. Mindfulness-Based Stress Reduction for Integrative Cancer Care – a Summary of Evidence. Forsch. Komplementärmedizin Res. Complement. Med. 2011;18(4):192–202. doi: 10.1159/000330714. doi:10.1159/000330714. [DOI] [PubMed] [Google Scholar]
- 64.Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How Does Mindfulness Meditation Work? Proposing Mechanisms of Action From a Conceptual and Neural Perspective. Perspect. Psychol. Sci. 2011;6(6):537–559. doi: 10.1177/1745691611419671. doi:10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- 65.Ditto B, Eclache M, Goldman N. Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Ann. Behav. Med. 2006;32(3):227–234. doi: 10.1207/s15324796abm3203_9. doi:10.1207/s15324796abm3203_9. [DOI] [PubMed] [Google Scholar]
- 66.Pace TWW, Negi LT, Adame DD, et al. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34(1):87–98. doi: 10.1016/j.psyneuen.2008.08.011. doi:10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-Based Stress Reduction in Relation to Quality of Life, Mood, Symptoms of Stress, and Immune Parameters in Breast and Prostate Cancer Outpatients. Psychosom. Med. 2003;65(4):571–581. doi: 10.1097/01.psy.0000074003.35911.41. doi:10.1097/01.PSY.0000074003.35911.41. [DOI] [PubMed] [Google Scholar]
- 68.Davidson RJ, Kabat-Zinn J, Schumacher J, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosom. Med. 2003;65(4):564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- 69.Baer RA. Self-Focused Attention and Mechanisms of Change in Mindfulness-Based Treatment. Cogn. Behav. Ther. 2009;38(sup1):15–20. doi: 10.1080/16506070902980703. doi:10.1080/16506070902980703. [DOI] [PubMed] [Google Scholar]
- 70.Shapiro SL, Astin JA, Bishop SR, Cordova M. Mindfulness-Based Stress Reduction for Health Care Professionals: Results From a Randomized Trial. Int. J. Stress Manag. 2005;12(2):164–176. doi:10.1037/1072-5245.12.2.164. [Google Scholar]
- 71.Jain S, Shapiro SL, Swanick S, et al. A randomized controlled trial of mindfulness meditation versus relaxation training: Effects on distress, positive states of mind, rumination, and distraction. Ann. Behav. Med. 2007;33(1):11–21. doi: 10.1207/s15324796abm3301_2. doi:10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- 72.Teasdale JD. Emotional processing, three modes of mind and the prevention of relapse in depression. Behav. Res. Ther. 1999;37(Supplement 1):S53–S77. doi: 10.1016/s0005-7967(99)00050-9. doi:10.1016/S0005-7967(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 73.Garland E, Gaylord S, Park J. The Role of Mindfulness in Positive Reappraisal. EXPLORE J. Sci. Heal. 2009;5(1):37–44. doi: 10.1016/j.explore.2008.10.001. doi:10.1016/j.explore.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alan B, Shapiro SL. Mental balance and well-being: Building bridges between Buddhism and Western psychology. Am. Psychol. 2006;61(7):690–701. doi: 10.1037/0003-066X.61.7.690. doi:10.1037/0003-066X.61.7.690. [DOI] [PubMed] [Google Scholar]
- 75.Hofmann SG, Asmundson GJG. Acceptance and mindfulness-based therapy: New wave or old hat? Clin. Psychol. Rev. 2008;28(1):1–16. doi: 10.1016/j.cpr.2007.09.003. doi:10.1016/j.cpr.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 76.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. Springer Publishing Company; New York, NY: 1984. [Google Scholar]
- 77.Narkiewicz K, Borne P van de, Montano N, Hering D, Kara T, Somers VK. Sympathetic Neural Outflow and Chemoreflex Sensitivity Are Related to Spontaneous Breathing Rate in Normal Men. Hypertension. 2006;47(1):51–55. doi: 10.1161/01.HYP.0000197613.47649.02. doi:10.1161/01.HYP.0000197613.47649.02. [DOI] [PubMed] [Google Scholar]
- 78.Lehrer PM, Woolfolk RL, Sime WE. Principles and Practice of Stress Management. Third Edition Guilford Press; 2007. [Google Scholar]
- 79.Khalsa SB. Yoga as a therapeutic intervention: a bibliometric analysis of published research studies. Indian J. Physiol. Pharmacol. 2004;48(3):269–85. [PubMed] [Google Scholar]
- 80.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Med. Res. Methodol. 2001;1(1):2. doi: 10.1186/1471-2288-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yunus MB, Arslan S, Aldag JC. Relationship between fibromyalgia features and smoking. Scand. J. Rheumatol. 2002;31(5):301–305. doi: 10.1080/030097402760375214. doi:10.1080/030097402760375214. [DOI] [PubMed] [Google Scholar]
- 82.Shapiro SL, Oman D, Thoresen CE, Plante TG, Flinders T. Cultivating mindfulness: effects on well-being. J. Clin. Psychol. 2008;64(7):840–862. doi: 10.1002/jclp.20491. doi:10.1002/jclp.20491. [DOI] [PubMed] [Google Scholar]
- 83.Baer RA, Smith GT, Allen KB. Assessment of Mindfulness by Self-Report The Kentucky Inventory of Mindfulness Skills. Assessment. 2004;11(3):191–206. doi: 10.1177/1073191104268029. doi:10.1177/1073191104268029. [DOI] [PubMed] [Google Scholar]
- 84.Brown KW, Ryan RM. The benefits of being present: Mindfulness and its role in psychological well-being. J. Pers. Soc. Psychol. 2003;84(4):822–848. doi: 10.1037/0022-3514.84.4.822. doi:10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- 85.Chadwick P, Hember M, Symes J, Peters E, Kuipers E, Dagnan D. Responding mindfully to unpleasant thoughts and images: Reliability and validity of the Southampton mindfulness questionnaire (SMQ) Br. J. Clin. Psychol. 2008;47(4):451–455. doi: 10.1348/014466508X314891. doi:10.1348/014466508X314891. [DOI] [PubMed] [Google Scholar]
- 86.Carmody J, Baer RA, L. B. Lykins E, Olendzki N. An empirical study of the mechanisms of mindfulness in a mindfulness-based stress reduction program. J. Clin. Psychol. 2009;65(6):613–626. doi: 10.1002/jclp.20579. doi:10.1002/jclp.20579. [DOI] [PubMed] [Google Scholar]
- 87.Sampalli T, Berlasso E, Fox R, Petter M. A controlled study of the effect of a mindfulness-based stress reduction technique in women with multiple chemical sensitivity, chronic fatigue syndrome, and fibromyalgia. J. Multidiscip. Healthc. 2009;2:53–59. doi: 10.2147/jmdh.s5220. [DOI] [PMC free article] [PubMed] [Google Scholar]