Highlights
-
•
G-CSF producing gallbladder carcinoma is rare and has a poor prognosis.
-
•
We report a long term survival of G-CSF producing gallbladder carcinoma.
-
•
We suggest multidisciplinary therapy prolong the survival of patients with G-CSF producing gallbladder carcinoma.
Keywords: Granulocyte-colony stimulating factor, Gallbladder carcinoma, Long survival
Abstract
Introduction
It is extremely rare for gallbladder carcinoma to produce granulocyte-colony stimulating factor (G-CSF) and such tumors have a poor prognosis.
Presentation of case
A 67-year-old man was admitted with continuous fever. Laboratory tests showed a leukocyte count of 27,980/μL, serum C-reactive protein (CRP) of 9.2 mg/dL and serum G-CSF of 225 pg/mL. Imaging revealed an irregular gallbladder mass about 90 mm in diameter with peripheral enhancement that also involved the liver and transverse colon. G-CSF producing gallbladder carcinoma was diagnosed. We performed cholecystectomy, partial resection of segments 4 and 5 of the liver, partial resection of the transverse colon, and gastrostomy. Histopathological examination showed gallbladder carcinoma (pT3, pN0, M0, G2, and pStage IIIA by the UICC classification, version 7). On immunohistochemical staining, tumor cells were positive for G-CSF. The leukocyte count was normalized postoperatively and fever subsided immediately after surgery. Two months later, the leukocyte count rose to 56,820/μL and metastases to the liver and lymph nodes were detected by CT. Chemotherapy (gemcitabine plus cisplatin) was started and the leukocyte count was normalized after the first course. The patient has continued chemotherapy and has survived for 16 months postoperatively.
Discussion
G-CSF producing gallbladder carcinoma has a poor prognosis and most patients die within 12 months of starting therapy. It is rare for patients with recurrence to survive for 16 months after surgery, as in the present case.
Conclusion
Multidisciplinary therapy (surgery and chemotherapy) may prolong the survival of patients with G-CSF producing gallbladder carcinoma, especially those with recurrence.
1. Introduction
Production of granulocyte-colony stimulating factor (G-CSF) by tumor tissue was initially demonstrated by Robinson [1] in 1974 and G-CSF-producing lung cancer was first reported by Asano et al. in 1977 [2]. The diagnostic criteria for G-CSF producing tumor are as follows: (1) a very high leukocyte count, (2) an increased serum level of G-CSF, (3) both the leukocyte count and G-CSF decrease after treatment, and (4) G-CSF production by tumor cells is demonstrated.
Carcinoma of the gallbladder rarely produces G-CSF and such tumors generally have a poor prognosis. Here we report a patient with recurrence of G-CSF producing gallbladder carcinoma who has survived for 16 months after surgery.
2. Presentation of case
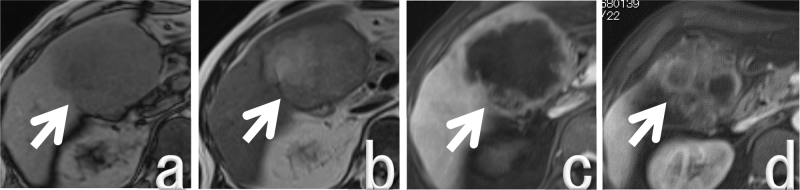
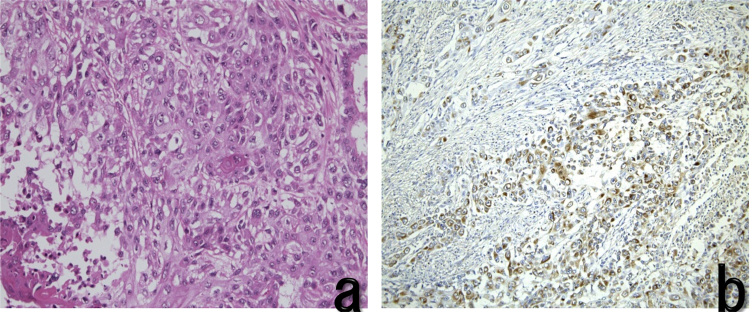
A 67-year-old man was admitted to our hospital with continuous fever. He had received antibiotic therapy at his local hospital for 3 months, but symptoms had not improved so he was referred to our hospital. He had hypertension, but was a nonsmoker and did not drink alcohol. He had not worked in the printing industry. On examination, his temperature was 37.2 °C. Laboratory tests showed a severe inflammatory response, with a leukocyte count of 27,980/μL and a serum C-reactive protein (CRP) level of 9.2 mg/dL Serum levels of alkaline phosphatase (ALP) and γ-glutamyl transpeptidase (γ-GTP) were elevated to 488 U/L and 118 U/L, respectively. In addition, serum G-CSF was elevated to 225 pg/mL and IL-6 was 52.9 pg/dL. Computed tomography (CT) showed an irregular gallbladder mass about 90 mm in diameter with peripheral enhancement (Fig. 1). Magnetic resonance imaging (MRI) also revealed an irregular mass about 90 mm in diameter with peripheral enhancement by gadolinium in the gallbladder. The mass was low intensity on T1-weighted images and high intensity on T2-weighted images (Fig. 2), and it extended to involve the liver (segments 4 and 5) and the transverse colon. Magnetic resonance cholangiopancreatography revealed that there was no pancreaticobiliary maljunction. G-CSF producing gallbladder carcinoma was diagnosed and the patient underwent resection of the tumor (cholecystectomy, partial resection of segments 4 and 5 of the liver, and partial resection of the transverse colon) and gastrostomy. Histopathological examination of the resected specimen showed gallbladder carcinoma, biliary type, adenocarcinoma (pT3, pN0, M0, G2, and pStage IIIA by the UICC classification, version 7). The margins were healthy. Tumor cells were positive for G-CSF by immunohistochemical staining (Fig. 3). After surgery, the leukocyte count decreased rapidly to the normal range and fever subsided immediately. Two months later, the leukocyte count increased to 56820/μL and serum level of total bilirubin was elevated to 6.2 mg/dL. CT scans revealed liver and lymph node metastases with slight dilatation of the intrahepatic bile ducts. He underwent percutaneous transhepatic cholangio drainage, followed by chemotherapy with gemcitabine (800 mg per body) plus cisplatin (30 mg per body) each administered on days 1 and 8, every 3 weeks. After the first course of chemotherapy, the leukocyte count decreased dramatically to the normal range. The patient has continued chemotherapy and is still alive at 16 months after surgery with stable disease (revised RECIST guideline, version 1.1) [3]. The patient provided written permission for publication of his case.
Fig. 1.
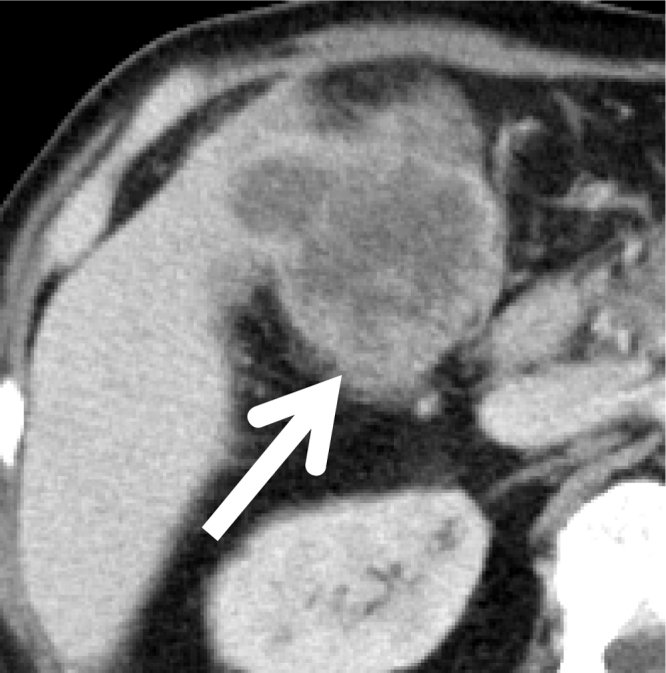
CT.
There is an irregular gallbladder mass about 90 mm in diameter with peripheral enhancement (arrows). The mass appears to invade the liver (segments 4 and 5) and the transverse colon.
Fig. 2.
MRI findings.
a: Low intensity mass on a T1-weighted image (arrow).
b: High intensity mass on a T2-weighted image (arrow).
c, d: Irregular gallbladder mass about 90 mm in diameter with peripheral gadolinium enhancement (arrows).
Fig. 3.
Histopathological findings.
a. Moderately differentiated tubular adenocarcinom
b. Immunostaining of tumor cells was positive for G-CSF.
(a: HE ×400, b: An immunohistochemistry with anti-G-CSF monoclonal antibodies ×400)
3. Discussion
In our patient, gallbladder carcinoma was suspected from imaging findings. Despite persistent fever and a very high leukocyte count, no microorganism was detected by blood culture and administration of antibiotics did not lead to improvement. These points suggested G-CSF-producing gallbladder carcinoma and G-CSF was found to be elevated to 225 pg/ml.
(18)F-fluorodeoxy-glucose positron emission tomography (FDG-PET) is a useful method for diagnosis of G-CSF producing gallbladder carcinoma. But it is difficult to diagnosis of bone metastasis. It is described that the diffuse FDG uptake present in the spine and in fact, no bone metastases were detected [4]. Increased FDG uptake by bone marrow was induced by incerased in bone marrow metabolism and celularity in response to G-CSF. [5]. When the patients underwent FDG-PET, it is necessary for paying attention to the diagnosis of bone metastasis.
Because his general condition was poor, it was difficult to perform radical resection or chemotherapy, so we performed limited surgery to control inflammation. While G-CSF causes leukocytosis, it is thought that signs or symptoms of inflammation, such as fever or elevation of CRP, are due to concomitant production of interleukin (IL)-6 by the tumor [6]. In our patient, IL-6 was also increased to52.9 pg/dL, which could explain the patient’s inflammatory symptoms.
We searched PubMed using “Gallbladder carcinoma”, “G-CSF”, and “CSF” as key words and excluded duplicate reports; revealing only 11 patients including our case [4], [7], [8], [9], [10], [11], [12], [13], [14], [15] (Table 1). They comprised 5 men and 6 women with a mean age of 66.2 years (range: 48–79 years). The mean tumor diameter was 100 (45–130) mm; mean leukocyte count was 32,057 (9400–57,900)/μL; and mean G-CSF level was 342.0 (46–1311). Tumor histology was moderately differentiated tubular adenocarcinoma in 3 patients; poorly differentiated tubular adenocarcinoma in 1 patient; adenosquamous carcinoma in 3 patients; squamous cell carcinoma in 1 patient; and undifferentiated carcinoma in 3 patients. Mean survival time is 11.8 months. Five patients died within 6 months after surgery or presentation and only 4 patients survived for ≥1 year. Unlike our patient; the other patients who survived for ≥1 year underwent surgery alone and did not experience recurrence. G-CSF-producing gallbladder carcinoma generally has a poor prognosis because the tumor is often poorly differentiated; G-CSF promotes the growth of non-hematologic tumor cells [16]; and G-CSF-producing cells may express G-CSF receptors allowing it to act as an autocrine growth factor promoting rapid tumor growth and/or metastasis [17]. In addition; persistent inflammation can compromise the general condition; making it difficult to perform chemotherapy.
Table 1.
Reported cases of G-CSF producing gallbladder carcinoma.
| Age | Sex | WBC (/μL) | G-CSF (pg/mL) | Size (mm) | Treatment | Histology | Immunostaining | Outcome | Author |
|---|---|---|---|---|---|---|---|---|---|
| 67 | Male | 27980 | 225 | 115 | Surgery + chemotherapy |
Moderately differentiated tubular adenocarcinoma | Positive | Alive with a recurrence (16 months) | Our case |
| 78 | Male | 26050 | 120 | 120 | Surgery | Adenosquamous carcinoma | Positive | Alive (27 months) | Suzumura et al. [4] |
| 50 | Female | 19800 | 800 | 45 | Surgery | Moderately differentiated tubular adenocarcinoma | Positive | Recurrence-free survival (27 months) | Ikeda et al. [7] |
| 62 | Male | 21400 | 50.8 | 120 | Surgery | Squamous carcinoma | Positive | Dead (8 months) | Murata et al. [8] |
| 73 | Female | 36500 | 1311 | 100 | Chemotherapy | Moderately differentiated tubular adenocarcinoma | Positive | Dead (3 months) | Kuroki et al. [9] |
| 48 | Female | 15700 | 54 | 80 | Surgery + chemotherapy | Poorly differentiated adenocarcinoma | Positive | Alive (11 months) | Furihata et al. [10] |
| 73 | Male | 75200 | 129 | ND | Surgery | Undifferentiated carcinoma | Positive | Alive (18 months) | Omura et al. [11] |
| 71 | Male | 18600 | 46 | 90 | Surgery + TAE | Adenosquamous carcinoma | Negative | Dead (6 months) | Nakajima et al. [12] |
| 79 | Female | 9400 | Activity(+) | ND | Surgery + chemotherapy | Undifferentiated carcinoma | Positive | Dead (6 months) | Takeda et al. [13] |
| 55 | Female | 57900 | Activity(+) | 130 | Surgery + chemotherapy + radiation | Undifferentiated carcinoma | ND | Dead (6 months) | Sakamoto et al. [14] |
| 72 | Female | 44100 | Activity(+) | ND | Immunotherapy | Adenosquamous carcinoma | ND | Dead (54 days) | Takahashi et al. [15] |
TAE: transcatheter arterial embolization. ND: Not described.
As chemotherapy for unresectable biliary carcinoma, studies performed in the UK and Japan [18], [19] have led to a Level 1 recommendation for treatment with gemcitabine plus cisplatin (GC therapy) [20]. Accordingly, our patient received multidisciplinary therapy with surgical resection and GC therapy. One of the reasons for his relatively long survival may be the tumor histology, since he had moderately differentiated adenocarcinoma while many G-CSF-producing tumors are poorly differentiated. In patients with G-CSF-producing gallbladder carcinoma, surgeons may hesitate to operate because of inflammatory features, but this may lead the patient's general condition to deteriorate further. In our patient, a good outcome may have been achieved because G-CSF-producing gallbladder carcinoma was promptly diagnosed and resected, after which recurrence was suspected when the leukocyte count increased again and chemotherapy was promptly started following treatment of mild obstructive jaundice
4. Conclusion
While G-CSF producing gallbladder carcinoma generally has a poor prognosis, surgery to control inflammation should be performed as soon as possible and the leukocyte count should be monitored to detect recurrence. If recurrence is found, the patient should immediately be offered chemotherapy. Multidisciplinary therapy (surgery combined with chemotherapy) may achieve longer survival of patients with G-CSF producing gallbladder carcinoma, especially after recurrence.
Conflicts of interest
None.
Funding
None.
Ethical approval
Not applicable.
References
- 1.Robinson W.A. Granulocytosis in neoplasia. Ann. N. Y. Acad. Sci. 1974;203:212–218. doi: 10.1111/j.1749-6632.1974.tb14451.x. [DOI] [PubMed] [Google Scholar]
- 2.Asano S., Urabe A., Okabe T., Sato N., Kondo Y. Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood. 1977;49:845–852. [PubMed] [Google Scholar]
- 3.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Suzumura K., Iimura Y., Asano Y., Kuroda N., Hirano T., Yamanaka J. Granulocyte-colony stimulating factor-producing gallbladder carcinoma. Int. Surg. 2014;99:577–583. doi: 10.9738/INTSURG-D-13-00129.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugawara Y., Fisher S.J., Zasodny K.R., Kison P.V., Baker L.H., Wahl R.L. Preclinical and clinical studies of bone marrow uptake of fluorine-18-fluorodeoxyglucose with or without granulocyte colony-stimulating factor during chemotherapy. J. Clin. Oncol. 1998;16:173–180. doi: 10.1200/JCO.1998.16.1.173. [DOI] [PubMed] [Google Scholar]
- 6.Kitagawa S. CSF-producing tumors. In: Takaku F., editor. Hematologic Syndromes I―Other Hematologic Diseases; Syndromes Classified by Organ Systems. Nippon Rinsho; Osaka, Japan: 1998. pp. 25–27. [Google Scholar]
- 7.Ikeda T., Ohgaki K., Miura M., Aishima S., Shimizu T., Maehara Y. Granulocyte-colony stimulating factor-producing gallbladder cancer without recurrence more than 2 years after resection. Surg. Today. 2005;35:90–593. doi: 10.1007/s00595-004-2981-4. [DOI] [PubMed] [Google Scholar]
- 8.Murata M., Tateishi H., Nishiyama H., Ito M., Zushi S., Imai Y. A case of granulocyte-colony stimulating factor producing squamous cell carcinoma of the gallbladder. Clin. J. Gastroenterol. 2001;98:53–57. [PubMed] [Google Scholar]
- 9.Kuroki M., Uto H., Ido A., Kuwata G., Nakama T., Ochiai T. A case of gallbladder cancer producing granulocyte-colony stimulating factor and possible parathyroid hormone related protein. Clin. J. Gastroenterol. 2000;97:478–483. [PubMed] [Google Scholar]
- 10.Furihata M., Sonobe O., Ohtsuki Y., Enzan H., Tokuoka H., Nakanuma Y. An immunohistochemical study on a case of granulocyte-colony stimulating factor-producing gallbladder carcinoma. Pathol. Int. 1999;49:1010–1013. doi: 10.1046/j.1440-1827.1999.00970.x. [DOI] [PubMed] [Google Scholar]
- 11.Omura N., Abe S., Hirai K., Aoki T. A case of granulocyte-colony stimulating factor producing gallbladder cancer. Am. J. Gastroenterol. 1999;94:273–275. doi: 10.1111/j.1572-0241.1999.00817.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima Y., Takashima T., Naitou E., Yoshida J., Senmaru H., Oka M. Colony-stimulating factor producing carcinoma of the gallbladder. J. Jpn. Soc. Intern. Med. 1996;85:1931–1933. [PubMed] [Google Scholar]
- 13.Takeda T., Ichiyanagi A., Sano K., Yoshida J., Tsutsumi Y., Miyaji T. Granulocyte-colony stimulating factor- producing gallbladder cancer. Gastroenterol. Jpn. 1990;25:762–767. doi: 10.1007/BF02779193. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto K., Egami H., Yoshimura R., Nakamura S., Ikei S., Mori K. Colony-stimulating factor producing carcinoma of the gallbladder. Jpn. J. Clin. Oncol. 1986;16:87–96. [PubMed] [Google Scholar]
- 15.Takahashi M., Fujiwara M., Kishi K., Sakai C., Sanada M., Moriyama Y. CSF-producing gallbladder cancer: case report and characteristics of the CSF produced by tumor cells. Int. J. Cell Cloning. 1985;3:294–303. doi: 10.1002/stem.5530030502. [DOI] [PubMed] [Google Scholar]
- 16.Berdel W.E., Danhauser-Riedl S., Steinhauser G., Winoton E.F. Various human hematopoietic factors (interleukin-3, GM-CSF, G-CSF) stimulate clonal growth of nonhematopoietic tumor cells. Blood. 1989;73:80–83. [PubMed] [Google Scholar]
- 17.Tachibana M., Miyazaki A., Tazaki H., Nakamura K., Kudo A., Hata J. Autocrine growth of transitional cell carcinoma of the bladder induced by granulocyte-colony stimulating factor. Cancer. 1995;55:3438–3443. [PubMed] [Google Scholar]
- 18.Valle J., Wasan H., Palmer D.H., Cunningham D., Anthoney A., Maraveyas A. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 19.Okusaka T., Nakachi K., Fukutomi A., Mizuno N., Ohkawa S., Funakoshi A. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br. J. Cancer. 2010;103:469–474. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Committee for clinical practice guidelines for the management of biliary tract and ampullary carcinoma of the Japanese Society of Hepato-biliary & Pancreatic Surgery (Ed.), Evidence-based Clinical Practice Guidelines for the Management of Biliary Tract and Ampullary Carcinoma, second ed., Tokyo, Igaku Tosho Shuppan, Japan, 2014.