Summary
Atherosclerosis is initiated and sustained by hypercholesterolemia, which results in the generation of oxidized LDL (OxLDL) and other metabolic byproducts that trigger inflammation. Specific immune responses have been shown to modulate the inflammatory response during atherogenesis. The sialic acid-binding immunoglobulin-like lectin G (Siglec-G) is a negative regulator of the functions of several immune cells, including myeloid cells and B-1 cells. Here, we show that deficiency of Siglec-G in atherosclerosis-prone mice inhibits plaque formation and diet-induced hepatic inflammation. We further demonstrate that selective deficiency of Siglec-G in B cells alone is sufficient to mediate these effects. Levels of B-1 cell-derived natural IgM with specificity for OxLDL were significantly increased in the plasma and peritoneal cavity of Siglec-G-deficient mice. Consistent with the neutralizing functions of OxLDL-specific IgM, Siglec-G-deficient mice were protected from OxLDL-induced sterile inflammation. Thus, Siglec-G promotes atherosclerosis and hepatic inflammation by suppressing protective anti-inflammatory effector functions of B cells.
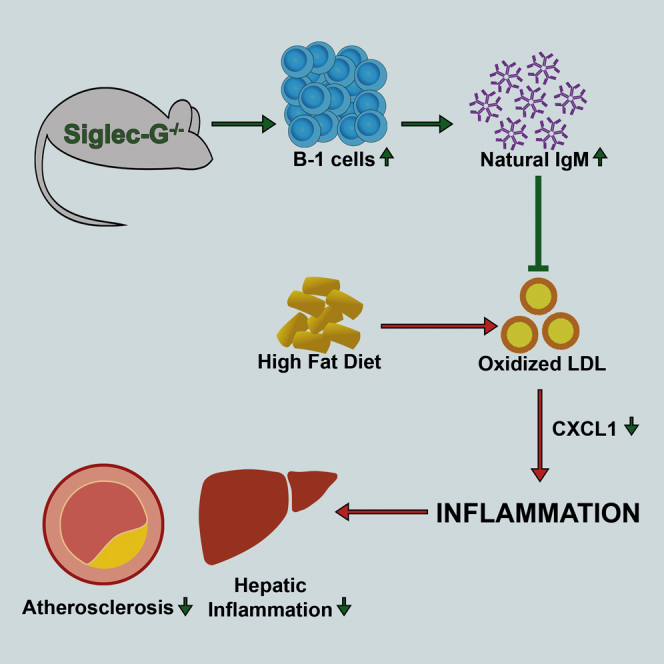
Graphical Abstract
Highlights
-
•
Siglec-G deficiency reduces atherosclerosis and hepatic inflammation in Ldlr KO mice
-
•
B cells secreting natural IgM mediate the protective effects of Siglec-G deficiency
-
•
Siglec-G deficiency protects from inflammation induced by oxidized LDL
-
•
CXCL1 is a common mediator of inflammation that is decreased by Siglec-G deficiency
Gruber et al. demonstrate that Siglec-G deficiency protects from oxidized LDL-induced inflammation through the expansion of B-1 cells secreting natural IgM antibodies, leading to reduced atherosclerosis and hepatic inflammation. CXCL1 represents a common pro-inflammatory factor that is reduced as a result of Siglec-G deficiency.
Introduction
Atherosclerosis is a lipid-driven chronic disease of the artery wall and the underlying cause of heart attacks and strokes, which accounts for the majority of mortalities and morbidities in the world (Libby et al., 2011). It is characterized by chronic inflammatory responses to endogenous sterile triggers, such as oxidized LDL (OxLDL), dying cells, and their metabolic byproducts that trigger tissue inflammation if not efficiently cleared (Tabas, 2010, Hotamisligil, 2006). Persistence of this inflammatory response or its impaired resolution paves the way for chronic inflammatory responses, which have been shown to propagate associated pathologies such as vascular and hepatic inflammation (Tall and Yvan-Charvet, 2015). Thus, there is growing interest in identifying mechanisms that enhance the immune system’s capacity to prevent endogenously triggered inflammation and/or promote its resolution.
B cells, which can be subdivided into B-1 and B2 cells, are emerging players in the chronic inflammation of metabolic diseases, such as obesity, diabetes, and atherosclerosis (Tsiantoulas et al., 2014, Winer et al., 2014, Zouggari et al., 2013, Perry et al., 2012). B2 cells, which include follicular (FO) B cells and marginal zone (MZ) B cells, have been shown to promote atherosclerotic lesion formation in murine models of atherosclerosis via mechanisms that are largely unclear (Kyaw et al., 2010, Ait-Oufella et al., 2010). On the other hand, selective transfer of B-1 cells, which can be further divided into B-1a and B-1b cells, protects mice from atherosclerosis (Kyaw et al., 2011, Rosenfeld et al., 2015). One of the main functions of B-1 cells is the production of natural IgM antibodies (NAb), which are pre-existing germline encoded antibodies that arise without any conventional T cell help and comprise approximately 80% of IgM antibodies in unchallenged mice (Baumgarth et al., 2005). B-1a cells seem to exhibit their atheroprotective effects via the secretion of NAb (Tsiantoulas et al., 2014). Indeed, atherosclerosis-prone soluble IgM-deficient mice develop accelerated atherosclerosis, though the exact mechanism by which NAb protect is not entirely clear (Lewis et al., 2009). We and others have suggested that NAb promote the neutralization and clearance of self-antigens, such as dying cells and oxidized lipids (Tsiantoulas et al., 2012). These studies indicate the importance of selective regulation of individual B cell subsets for appropriate responses to inflammatory triggers. Moreover, the role of B-1 cells in atherosclerosis has only been studied in immune-compromised animals, and their role in animals that do not lack major compartments of the immune system remains elusive. In this regard, the sialic acid-binding immunoglobulin-like lectin G (Siglec-G) is of particular interest as it acts as negative regulator of the B-1a cell population size, presumably via inhibiting B cell receptor dependent signaling (Hoffmann et al., 2007, Ding et al., 2007). We and others have previously shown that mice deficient in Siglec-G exhibit a nearly 10-fold expansion of B-1a cells along with a robust increase in total serum IgM (Hoffmann et al., 2007, Ding et al., 2007). Moreover, we also found that Siglec-G deficiency results in an expansion of IgM with specificity for oxidation-specific epitopes (OSE), which represent prototypic metabolic byproducts present on OxLDL, dying cells, and circulating microparticles (Chou et al., 2009, Tsiantoulas et al., 2015, Chang et al., 1999, Chang et al., 2004, Jellusova et al., 2010). As excessive accumulation of OSE has been suggested to be a key driver for inflammatory reactions in metabolic diseases, such as atherosclerosis, non-alcoholic steatohepatitis, and diabetes (Miller et al., 2011, Walenbergh et al., 2013, Horie et al., 1997), targeting Siglec-G may have beneficial therapeutic effects in chronic inflammation.
The expansion of B-1a cells has also been associated with increased autoimmunity (Chan et al., 1997, Pao et al., 2007, Ishida et al., 2006), which could accelerate atherosclerosis (Roman and Salmon, 2007, Ma et al., 2008). Siglec-G deficiency has been shown to result in an earlier onset of autoimmune disease in the Murphy Roths Large/lymphoproliferative (MRL/lpr) lupus mouse model and leads to mild autoimmunity in aging mice with an over-activation of adaptive T and B cells (Müller et al., 2015, Bökers et al., 2014). In addition, Siglec-G has also been found to be expressed in and influence responses of myeloid cells. For example, Siglec-G has been shown to be upregulated by RNA viruses and to inhibit retinoic acid-inducible gene 1 (RIG-I) mediated IFN-β secretion by macrophages and dendritic cells. In line with this, vesicular stomatitis virus (VSV)-infected Siglec-G-deficient mice were found to display increased IFN-β production and decreased viral load compared to control mice (Chen et al., 2013). Moreover, dendritic cells of Siglec-G-deficient mice have been found to exhibit increased pro-inflammatory cytokine secretion in response to multiple danger-associated molecular patterns (DAMPs) (e.g., HSP70, HSP90, and HMGB1). A detrimental role of Siglec-G deficiency is further supported by the findings that Siglec-G-deficient mice exhibit increased mortality in models of acetaminophen-induced liver necrosis (Chen et al., 2009) and cecal ligation and puncture-induced sepsis (Chen et al., 2011). Thus, all studies so far indicate that Siglec-G functions as negative regulator of inflammation, and Siglec-G deficiency may actually propagate inflammatory responses. Because pro-inflammatory cytokine production is a hallmark of metabolic inflammation, the role of Siglec-G and the consequences of Siglec-G deficiency in chronic inflammation and specifically in atherosclerosis are entirely unknown.
Here, we investigated the role of Siglec-G in sterile chronic inflammation in vivo. We demonstrate that total as well as B cell-specific Siglec-G deficiency reduces atherosclerotic lesion formation as well as hepatic inflammation in hypercholesterolemic Ldlr−/− mice. Moreover, we show that Siglec-G-deficient mice are protected form OxLDL-induced inflammation in vivo.
Results
Siglec-G Deficiency in Cholesterol-Fed Ldlr−/− Mice Increases B-1a Cells and Natural IgM Antibodies
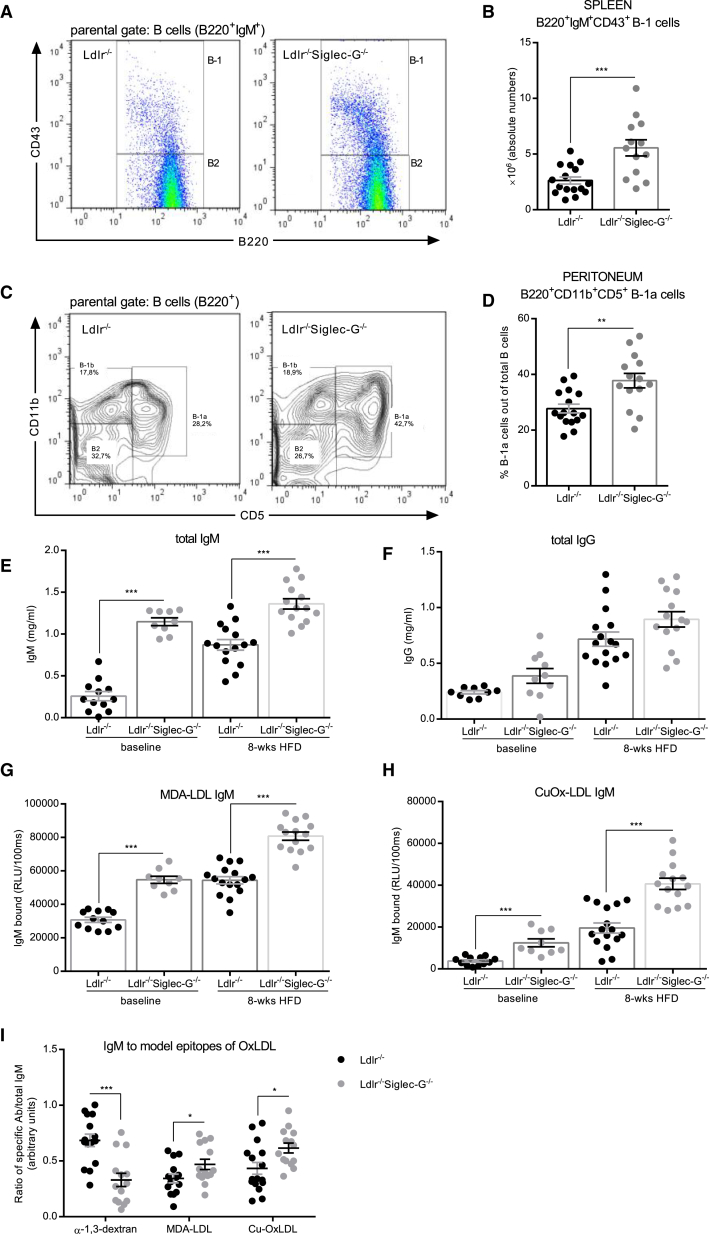
Siglec-G deficiency has been previously shown to result in an expansion of OSE-specific NAb, which are hypothesized to possess robust anti-inflammatory properties, particularly against products of increased oxidative stress (Jellusova et al., 2010). In agreement with previous data, non-atherosclerotic Siglec-G−/− mice have—compared to Siglec-G+/+ control mice—significantly increased numbers of B-1a cells in the spleen, increased numbers of CD138+ plasmablasts (B220+) and plasma cells (B220lo) in the spleen and bone marrow, and increased levels of OSE-specific IgM antibodies in the plasma (Figures S1A–S1G). In order to investigate the effect of Siglec-G deficiency in atherosclerosis, Ldlr−/−Siglec-G−/− mice as well as control Ldlr−/− mice were fed an atherogenic diet for 8 weeks. Siglec-G deficiency did not affect body weight, or plasma total cholesterol (TC), or triglycerides (TG) (Table 1). Moreover, there was no significant difference in frequencies of total or Ly6Chi and Ly6Clo monocytes in the peripheral blood of these mice (Figures S2E and S2F). Frequencies of peripheral blood B220+ B cells were also not different between the two groups (Figure S2D).
Table 1.
Parameters Overview of Experimental Groups: Atherosclerosis Study
|
Ldlr−/− |
Ldlr−/−Siglec-G−/− |
|
|---|---|---|
| (n = 16) | (n = 14) | |
|
Metabolic Parametersa | ||
| Weights (g) | 32.15 ± 0.53 | 31.2 ± 0.90 |
| TC (mg/dL) | 1,655.36 ± 70.24 | 1,633.65 ± 88.09 |
| TG (mg/dL) |
1,257.14 ± 55.37 |
1,284.38 ± 61.18 |
|
Atherosclerosis | ||
| en face (% of aorta) | 4.22 ± 0.25 | 2.22 ± 0.20∗∗∗ |
| Innominate (× 104 mm2) | 0.99 ± 0.17 | 0.43 ± 0.16∗ |
| Aortic origin (× 104 μm2/section) | 7.08 ± 0.88 | 8.69 ± 1.14 |
| Necrotic area (% of total lesion area) | 14.8 ± 4.0 | 4.65 ± 1.21∗ |
| mac-3+ (% of cellular area) |
65.83 ± 3.78 |
51.61 ± 2.53∗∗ |
|
Hepatic Inflammation | ||
| Liver cholesterol (μg TC/μg protein) | 0.19 ± 0.01 | 0.19 ± 0.02 |
| Liver TG (μg TG/μg protein) | 0.68 ± 0.04 | 0.62 ± 0.06 |
| Liver free fatty acids (μg FFA/μg protein) | 0.42 ± 0.02 | 0.37 ± 0.04 |
| ALT (U/l) | 72.9 ± 8.1 | 80.4 ± 6.6 |
| Mac-1 (# positive cells/mm2) | 150.04 ± 6.74 | 118.58 ± 6.14∗∗ |
| Ly6G (# positive cells/mm2) |
68.38 ± 2.61 |
55.91 ± 2.32∗∗ |
|
Serum Antibody Titers | ||
| Total IgM (mg/ml) | ||
| Baseline | 0.26 ± 0.05 | 1.15 ± 0.05∗∗∗ |
| 8 weeks HFD | 0.87 ± 0.06 | 1.36 ± 0.06∗∗∗ |
| MDA-LDL IgM (RLU/100 ms) | ||
| Baseline | 30,735 ± 1,534 | 54,680 ± 2,101∗∗∗ |
| 8 weeks HFD | 54,378 ± 2,196 | 80,773 ± 2,467∗∗∗ |
| CuOx-LDL IgM (RLU/100 ms) | ||
| Baseline | 3,767 ± 585 | 12,459 ± 1,894∗∗∗ |
| 8 weeks HFD | 19,549 ± 2,443 | 40,649 ± 2,698∗∗∗ |
| Total IgG (mg/ml) | ||
| Baseline | 0.24 ± 0.02 | 0.39 ± 0.07 |
| 8 weeks HFD | 0.72 ± 0.06 | 0.89 ± 0.07 |
| MDA-LDL IgG (RLU/100 ms) | ||
| Baseline | 14,879 ± 1,855 | 22,392 ± 2,383∗ |
| 8 weeks HFD | 20,646 ± 1,800 | 23,699 ±1,870 |
| CuOx-LDL IgG (RLU/100 ms) | ||
| Baseline | 1,473 ± 91 | 3,220 ± 525∗∗ |
| 8 weeks HFD |
3,267 ± 356 |
4,463 ± 449 |
|
Spleen (Absolute Numbers × 106) | ||
| T cells (CD3+) | 34.18 ± 2.30 | 37.97 ± 3.04 |
| B cells (B220+IgM+) | 33.14 ± 2.44 | 38.64 ± 4.25 |
| B-1 cells (B220+IgM+CD43+) | 2.63 ± 0.32 | 5.56 ± 0.73∗∗∗ |
| MZ B cells (B220+CD43−CD23−CD21hi) | 0.71 ± 0.11 | 0.54 ± 0.09 |
| T2/FO B cells (B220+CD43−CD23+CD21+) | 26.87 ± 2.26 | 34.0 ± 3.71 |
| T1 B cells (B220+CD43−CD23−CD21lo) | 1.96 ± 0.18 | 2.71 ± 0.36 |
| B220+CD43− CD23− CD21+ | 1.50 ± 0.15 | 1.84 ± 0.15 |
| IRA B cells (CD19+IgMhighCD43+CD5+CD138+CD93+MHCII+) |
0.09 ± 0.01 |
0.18 ± 0.03∗∗ |
|
Peritoneal Cavity (% Viable Cells) | ||
| B-1a cells (B220+CD11b+CD5+) out of B cells | 27.74 ± 1.59 | 37.78 ± 2.60∗∗ |
| B-1b cells (B220+CD11b+CD5−) out of B cells | 15.57 ± 1.46 | 12.39 ± 1.41 |
| B2 cells (B220+CD11b−CD5−) out of B cells | 35.29 ± 1.84 | 33.2 ± 1.84 |
| T cells (B220−CD5+) out of total | 8.99 ± 1.10 | 9.42 ± 0.86 |
See also Figure S5.
Data are shown as mean ± SEM (∗ p ≤ 0.05, ∗∗ p ≤ 0.01, and ∗∗∗ p ≤ 0.001).
Consistent with the previously described effect of Siglec-G deficiency on B cells, numbers of splenic CD43+ B-1 cells were increased in Ldlr−/−Siglec-G−/− mice (Figures 1A and 1B). Numbers of CD3+ T cells, CD23+ FO B cells, and MZB cells were not different between the groups (Table 1). Notably, the numbers of the recently identified pro-atherogenic subset of innate response activator (IRA) B cells in the spleen were significantly increased in Ldlr−/−Siglec-G−/− mice compared to Ldlr−/− mice (Hilgendorf et al., 2014) (Figure S2A). Moreover, compared to Ldlr−/− mice, Ldlr−/−Siglec-G−/− mice exhibited a significantly increased frequency of B-1a cells in the peritoneal cavity (Figures 1C and 1D), while the frequencies of B-1b or B2 cells were not significantly different between the groups (Figures S2B and S2C).
Figure 1.
Siglec-G Deficiency in Cholesterol-Fed Ldlr−/− Mice Increases B-1a Cell-Derived IgM Antibodies
(A) Representative flow cytometry plots for splenic B220+CD43− B2 cells and B220+CD43+ B-1 cell subpopulations. The parental gate was set on B220+IgM+ B cells.
(B) Absolute numbers of splenic B220+IgM+CD43+ B-1 cells.
(C) Representative flow cytometry plots for B cell subpopulations of the peritoneal cavity. The parental gate was set on B220+ B cells.
(D) Relative numbers of peritoneal B220+CD5+CD11b+ B-1a cells out of total B cells.
(E and F) Quantification of total IgM (E) and total IgG (F) antibodies in plasma. The samples were diluted between 1:30,000 and 1:70,000 and measured in triplicates.
(G and H) Titers of MDA-LDL IgM (G) and CuOx-LDL IgM (H) in plasma were determined by ELISA at baseline and after 8 weeks of atherogenic diet. The samples were diluted between 1:100 and 1:500 and antibody binding was measured in triplicates. The data are expressed as relative light units (RLU) per 100 ms.
(I) RLU of IgM antibodies to MDA-LDL and CuOx-LDL as well as α-1,3-dextran (1:100) were normalized to total IgM concentrations and expressed as ratio of specific IgM per total IgM (a.u.). Shown are data of Ldlr−/− and Ldlr−/−Siglec-G−/− mice after 8 weeks of atherogenic diet. The symbols represent individual mice. The horizontal bars represent the mean of each group and errors bars represent SEM (∗p ≤ 0.05, ∗∗p ≤ 0.01, and ∗∗∗p ≤ 0.001).
See also Figures S1 and S2.
We then assessed both total and OSE-specific IgM and IgG antibody levels in plasma at baseline and after 8 weeks of atherogenic diet. Levels of IgM antibodies were significantly increased in Ldlr−/−Siglec-G−/− mice at both time points (Figure 1E), while levels of total IgG antibodies were not different (Figure 1F). Moreover, IgM titers to MDA-LDL as well as to CuOx-LDL were also higher in Ldlr−/−Siglec-G−/− mice at baseline and even further increased in response to atherogenic diet feeding compared to controls (Figures 1G and 1H). Importantly, expression of the antigen-specific IgM as a ratio of total IgM, revealed a preferential expansion of IgM to MDA-LDL and CuOx-LDL, while the relative levels of IgM to the atherosclerosis-irrelevant antigen α-1,3-dextran were decreased (Figure 1I). On the other hand, IgG titers to these antigens were elevated in Ldlr−/−Siglec-G−/− mice only at baseline, but the differences disappeared after 8 weeks of atherogenic diet (Figures S2G and S2H). Thus, even in the presence of extreme hypercholesterolemia, Siglec-G deficiency results in an expansion of B-1a cells and a concomitant increase of total and OSE-specific IgM.
Total and B Cell-Selective Siglec-G Deficiency Protects from Atherosclerosis
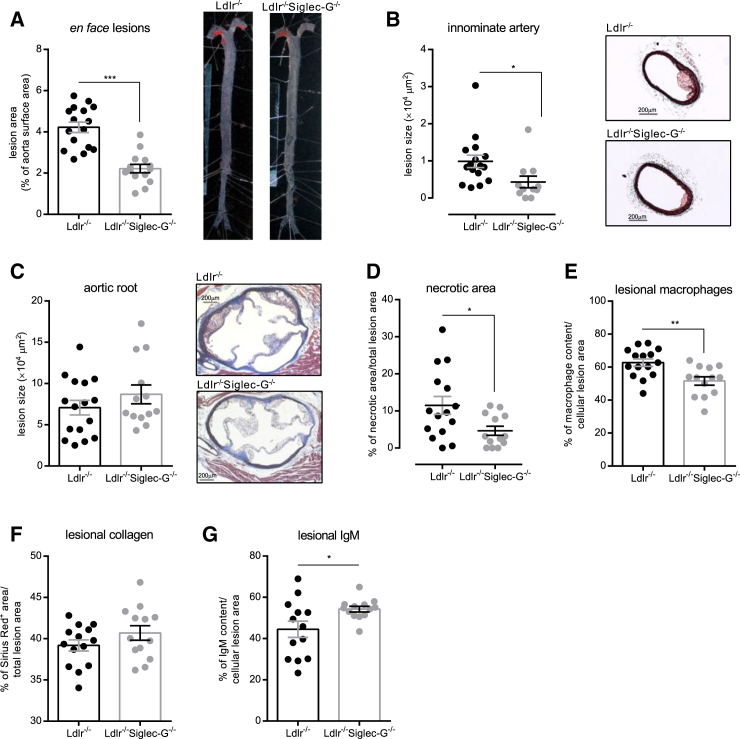
We then quantified the extent of atherosclerosis in the entire aorta of both groups of mice by en face analyses, which revealed a 50% reduced lesion size as a result of Siglec-G deficiency (Figure 2A). Decreased atherosclerosis was also found in the innominate arteries of Ldlr−/−Siglec-G−/− mice compared to Ldlr−/− mice (Figure 2B). Moreover, while cross-sectional analyses of the aortic origin did not reveal differences in lesion size between the two groups (Figure 2C), lesions of Ldlr−/−Siglec-G−/− mice were clearly less complex with smaller necrotic areas and reduced macrophage content (Figures 2D and 2E). Lesional collagen content was not significantly different between the two groups (Figure 2F), while deposition of IgM was significantly increased in lesions of Ldlr−/−Siglec-G−/− mice (Figure 2G). Thus, Siglec-G deficiency results in a profound reduction of lesion size and complexity.
Figure 2.
Siglec-G Deficiency Decreases Atherosclerosis in Cholesterol-Fed Ldlr−/−Siglec-G−/− Mice
(A) Quantitative analysis of atherosclerosis in the aorta. The data are expressed as percentage of Sudan IV stained area of the entire aorta. The representative pictures are shown on the right.
(B) Quantitative analysis of atherosclerotic lesions in cross-sections of innominate arteries. The values represent the average μm2 of four sections throughout the innominate artery (300 μm). The images show representative Masson’s trichrome stains. The original magnification is 50×, and the scale bar represents 200 μm.
(C) Quantification of atherosclerotic lesion size in cross-sections at the aortic origin. The values represent the average μm2 of nine sections throughout the entire aortic origin (400 μm). The images show representative Masson’s trichrome stains. The original magnification is 50Χ, and the scale bar represents 200 μm.
(D) Quantification of necrotic areas in cross-sections at the aortic origin. The values represent the percentages of necrotic areas of one section at 200 μm depth.
(E–G) Quantification of macrophage, collagen, and IgM content in cross-sections at the aortic origin.
(E) Values represent the percentage of mac-3+ area per cellular lesion area of one section per mouse at 200 μm depth.
(F) Values represent the percentage of Sirius Red+ area per total lesion area of one section per mouse at 200 μm depth.
(G) Values represent the percentage of IgM+ area per cellular lesion area of one section per mouse at 180 μm depth. Shown are the data of Ldlr−/− and Ldlr−/−Siglec-G−/− mice after 8 weeks of atherogenic diet. The symbols represent individual mice. The horizontal bars represent the mean of each group and errors bars represent SEM (∗p ≤ 0.05, ∗∗p ≤ 0.01, and ∗∗∗p ≤ 0.001).
See also Table 1.
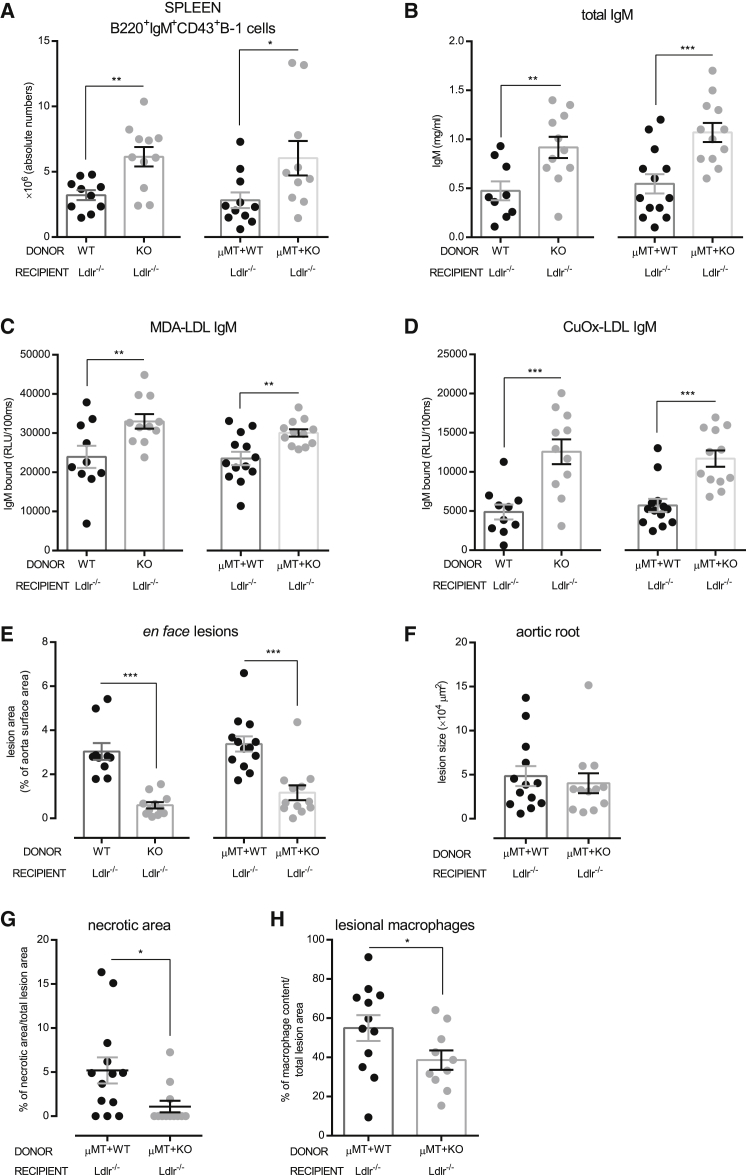
To identify whether the observed protective effects of Siglec-G deficiency are mainly conferred by B cells, we generated bone marrow chimeric Ldlr−/− mice with a selective Siglec-G deficiency on B cells using a previously established method (Sage et al., 2012, Fillatreau et al., 2002). Ldlr−/− mice were lethally irradiated and reconstituted with (1) bone marrow from Siglec-G−/− mice or Siglec-G+/+ littermate controls to assess the role of Siglec-G deficiency in hematopoietic cells in general, and (2) a mixture of 80% bone marrow from B cell-deficient μMT mice and 20% from either Siglec-G−/− mice or Siglec-G+/+ littermate controls to assess Siglec-G deficiency specifically in B cells. After a 4 week recovery period, mice were fed an atherogenic diet for 10 weeks. Successful engraftment of the respective bone marrow was confirmed by PCR of DNA isolated from bone marrow of recipients and of sorted splenic B and non-B cells to demonstrate selective Siglec-G deficiency (Figures S3A and S3B). In addition, the lack of Siglec-G expression on splenic B cells was confirmed by flow cytometry (Figure S3C).
Body weights, TC and TG levels in plasma, as well as frequencies of peripheral blood monocytes and B cells were not different between the experimental groups (Table 2; Figures S4A–S4C). Splenic B-1 cells as well as peritoneal B-1a cells were significantly increased in recipients of Siglec-G−/− bone marrow (whole and mixed bone marrow) compared to recipients of control bone marrow (Figures 3A and S4D; Table 2). Moreover, total plasma IgM levels were significantly increased in mice reconstituted with Siglec-G−/− and μMT+Siglec-G−/− bone marrow compared to their controls (Figure 3B), while total plasma IgG levels were not different (Figure S4E). IgM—but not IgG—levels to MDA-LDL and CuOx-LDL were also robustly and preferentially increased in recipient mice of Siglec-G−/− bone marrow (Figures 3C, 3D, and S4F–S4H). Importantly, total and B cell-selective deficiency of Siglec-G in the hematopoetic compartment resulted in a similar reduction of atherosclerotic lesion formation (Figure 3E) and a decreased lesional complexity with smaller necrotic areas and fewer lesional macrophages in the aortic root (Figures 3F–3H). This indicates that Siglec-G deficiency on B cells is primarily responsible for the protective effects in atherogenesis.
Table 2.
Parameters Overview of Experimental Groups: Bone Marrow Transplantation Study
| Ldlr−/−← | C57BL/6 |
Siglec-G−/− |
μMT+C57BL/6 |
μMT+Siglec-G−/− |
|---|---|---|---|---|
| (n = 10) | (n = 11) | (n = 13) | (n = 13) | |
|
Metabolic Parametersa | ||||
| Weights (g) | 25.54 ± 0.63 | 25.15 ± 0.67 | 25.72 ± 0.49 | 25.29 ± 0.73 |
| TC (mg/dL) | 758.33 ± 79.47 | 821.59 ± 66.53 | 884.67 ± 92.20 | 925.96 ± 78.29 |
| TG (mg/dL) |
595.83 ± 49.43 |
567.05 ± 48.57 |
661.46 ± 73.94 |
585.58 ± 46.60 |
|
Atherosclerosis | ||||
| en face (% of aorta) | 3.04 ± 0.38 | 0.59 ± 0.15∗∗∗ | 3.11 ± 0.24 | 1.17 ± 0.34∗∗∗ |
| Aortic origin (× 104 μm2/section) | 4.84 ± 1.14 | 4.03 ± 1.13 | ||
| Necrotic area (% of total lesion area) | 5.20 ± 1.48 | 1.09 ± 0.66∗ | ||
| mac-3+ (% of total area) |
54.96 ± 6.57 |
38.60 ± 4.95∗ |
||
|
Hepatic Inflammation | ||||
| Liver cholesterol (μg TC/μg protein) | 0.22 ± 0.04 | 0.19 ± 0.03 | 0.23 ± 0.03 | 0.23 ± 0.03 |
| Liver TG (μg TG/μg protein) | 0.25 ± 0.04 | 0.21 ± 0.02 | 0.25 ± 0.03 | 0.22 ± 0.02 |
| Liver free fatty acids (μg FFA/μg protein) | 0.14 ± 0.01 | 0.12 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 |
| ALT (U/l) | 109.1 ± 27.2 | 58.8 ± 11.5 | 47.6 ± 4.8 | 38.6 ± 3.0 |
| Mac-1 (# positive cells/mm2) | 120.69 ± 13.08 | 86.72 ± 8.95∗ | 100.68 ± 6.33 | 79.41 ± 5.50∗ |
| Ly6G (# positive cells/mm2) |
79.67 ± 5.82 |
58.58 ± 7.41∗ |
74.40 ± 6.30 |
48.61 ± 4.04∗∗ |
|
Serum Antibody Titers—10 Weeks HFD | ||||
| Total IgM (mg/ml) | 0.47 ± 0.10 | 0.92 ± 0.11∗∗ | 0.55 ± 0.10 | 1.07 ± 0.10∗∗∗ |
| MDA-LDL IgM (RLU/100ms) | 23,939 ± 2,848 | 32,989 ± 1,848∗∗ | 23,514 ± 1,706 | 30,045 ± 943∗∗ |
| CuOx-LDL IgM (RLU/100ms) | 4,877 ± 951 | 12,566 ± 1,583∗∗∗ | 5,714 ± 829 | 11,704 ± 1,045∗∗∗ |
| Total IgG (mg/ml) | 0.47 ± 0.10 | 0.89 ± 0.10∗ | 0.61 ± 0.09 | 0.98 ± 0.27 |
| MDA-LDL IgG (RLU/100ms) | 3,220 ± 633 | 6,126 ± 1,248 | 4,405 ± 701 | 4,012 ± 752 |
| CuOx-LDL IgG (RLU/100ms) |
2,885 ± 754 |
3,476 ± 769 |
1,827 ± 274 |
1,990 ± 452 |
|
Spleen (Absolute Numbers × 106) | ||||
| T cells (CD3+) | 41.93 ± 4.76 | 37.51 ± 3.86 | 67.76 ± 17.05 | 42.90 ± 8.13 |
| B cells (B220+IgM+) | 66.80 ± 9.44 | 74.18 ± 9.22 | 43.85 ± 12.07 | 47.46 ± 13.18 |
| B1 cells (B220+IgM+CD43+) | 3.21 ± 0.38 | 6.15 ± 0.75∗∗ | 2.82 ± 0.60 | 6.04 ± 1.32∗∗ |
| MZ B cells (B220+CD43−CD23−CD21hi) | 1.98 ± 0.35 | 1.56 ± 0.26 | 4.58 ± 1.41 | 2.94 ± 1.28 |
| T2/FO B cells (B220+CD43−CD23+CD21+) | 46.04 ± 5.43 | 54.25 ± 7.49 | 23.47 ± 7.63 | 25.28 ± 8.42 |
| T1 B cells (B220+CD43−CD23−CD21lo) | 3.42 ± 0.77 | 3.46 ± 0.35 | 3.68 ± 2.40 | 2.24 ± 0.72 |
| B220+CD43− CD23− CD21+ |
5.99 ± 1.18 |
5.48 ± 0.67 |
4.16 ± 1.05 |
5.38 ± 2.08 |
|
Peritoneal Cavity (% Viable Cells) | ||||
| B-1a cells (B220+CD11b+CD5+) out of B cells | 17.13 ± 1.40 | 31.85 ± 1.14∗∗∗ | 25.18 ± 0.62 | 46.20 ± 1.07∗∗∗ |
| B-1b cells (B220+CD11b+CD5−) out of B cells | 14.17 ± 1.24 | 12.47 ± 0.97 | 19.45 ± 1.29 | 15.96 ± 0.83∗ |
| B2 cells (B220+CD11b−CD5−) out of B cells | 64.96 ± 2.32 | 48.47 ± 2.00∗∗∗ | 47.40 ± 1.94 | 29.88 ± 1.06∗∗∗ |
| T cells (B220−CD5+) out of total | 11.99 ± 1.19 | 15.07 ± 2.68 | 18.18 ± 5.04 | 13.97 ± 2.89 |
Data are shown as mean ± SEM (∗ p ≤ 0.05, ∗∗ p ≤ 0.01, and ∗∗∗ p ≤ 0.001).
Figure 3.
Selective Siglec-G Deficiency in B Cells Reduces the Development of Atherosclerosis
(A) Absolute numbers of splenic IgM+B220+CD43+ B-1 cells.
(B) Quantification of total IgM in plasma. The samples were diluted 1:60,000 and measured in triplicates.
(C and D) Binding of IgM antibodies to (C) MDA-LDL and (D) CuOx-LDL was determined by ELISA. The plasma samples were diluted between 1:100 and 1:500 and antibody binding was measured in triplicates. The data are expressed as RLU/100 ms.
(E) Quantitative analysis of atherosclerosis (en face) in the entire aorta. The data are expressed as percentage of Sudan IV stained area of the entire aorta.
(F) Quantification of atherosclerotic lesion size in cross-sections at the aortic origin. The values represent the average μm2 of nine sections throughout the entire aortic origin (400 μm).
(G) Quantification of necrotic areas in cross-sections at the aortic origin. The values represent percentages of necrotic areas of one section at 200 μm.
(H) Quantification of the macrophage content in cross-sections at the aortic origin. The values represent percentages of mac-3+ area per total lesion area of one section per mouse at 200 μm depth. Shown are data of Ldlr−/− mice reconstituted with Siglec-G+/+ [WT] versus Siglec-G−/− [KO] and μMT+Siglec-G+/+ [WT] versus μMT+Siglec-G−/− [KO] bone marrow after 10 weeks of atherogenic diet. The symbols represent individual mice. The horizontal bars represent the mean of each group and errors bars represent SEM (∗p ≤ 0.05, ∗∗p ≤ 0.01, and ∗∗∗p ≤ 0.00).
See also Figures S3 and S4; Table 2.
Siglec-G Deficiency in Mice Protects from Hepatic and Systemic Inflammation
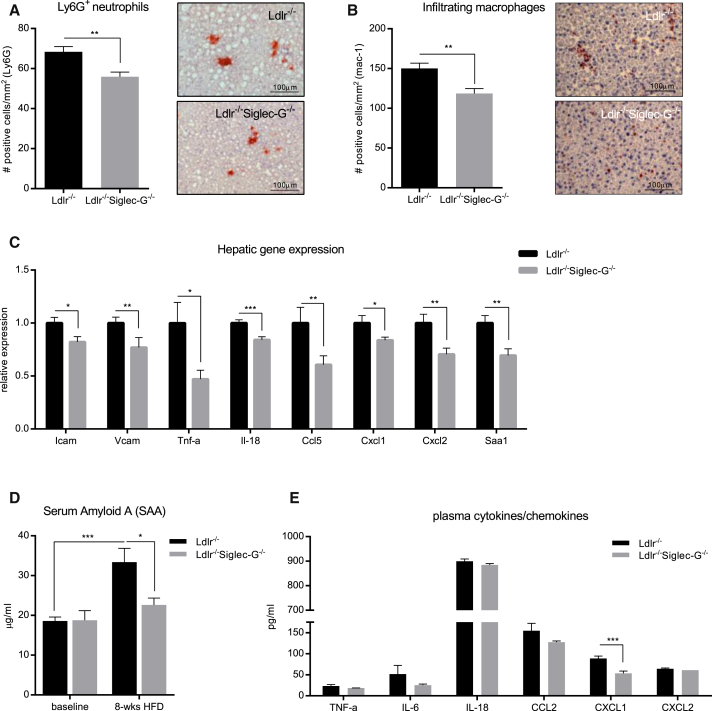
We have recently demonstrated that Ldlr−/− mice develop hepatic inflammation when fed an atherogenic diet (Bieghs et al., 2012b). Therefore, we investigated the effect of Siglec-G deficiency on hepatic inflammation in these mice. Atherogenic diet feeding resulted in a robust steatosis of the liver of all experimental mice. We found no significant differences in hepatic cholesterol, hepatic TG, and hepatic free fatty acids between the experimental groups. Consistent with only minimal liver damage in this model, alanine aminotransferase (ALT) levels were only moderately elevated, but also not different between the two groups (Tables 1 and 2). In contrast, immunohistochemical analyses of liver sections revealed a significantly reduced infiltration of Ly6G+ neutrophils and mac-1+ macrophages in the livers of Ldlr−/−Siglec-G−/− mice compared to Ldlr−/− mice (Figures 4A and 4B). A similar significant reduction in neutrophil and macrophage infiltration was found in the livers of Ldlr−/− mice that were reconstituted with either Siglec-G−/− or μMT+Siglec-G−/− bone marrow (Figures S5A and S5B). To further define the effects on hepatic inflammation between Ldlr−/− and Ldlr−/−Siglec-G−/− mice, we analyzed the expression of adhesion molecules, pro-inflammatory cytokines, and chemokines in the liver of these mice. In line with the reduced cell infiltration, expression of intercellular adhesion molecule (Icam) and vascular cell adhesion protein (Vcam), tumor necrosis factor-alpha (Tnf-α) and interleukin-18 (Il-18), C-C motif ligand 5 (Ccl5), C-X-C motif ligand 1 (Cxcl1), and C-X-C motif ligand 2 (Cxcl2) were significantly reduced in the livers of Ldlr−/−Siglec-G−/− mice compared to Ldlr−/− mice (Figure 4C). A similar though less pronounced decrease of inflammatory gene expression was also observed in the livers of mice lacking Siglec-G in B cells only (Figure S5C). Thus, Siglec-G deficiency and Siglec-G deficiency on B cells reduces hepatic inflammation, suggesting an important role in the development of steatohepatitis.
Figure 4.
Siglec-G Deficiency Protects from Atherogenic Diet-Induced Hepatic and Systemic Inflammation
(A and B) Quantification of infiltrating neutrophils (A) and macrophages (B) in liver sections of Ldlr−/− versus Ldlr−/−Siglec-G−/− mice after 8 weeks of atherogenic diet. The sections were stained with anti-Ly6G and anti-mac-1 antibody. The positively stained cells were counted and expressed as number of positive cells per mm2. The representative pictures (original magnification 200× and the scale bar represents 100 μm) are shown on the right.
(C) Relative gene expression of Icam, Vcam, Tnf-α, Il-18, Ccl5, Cxcl1, Cxcl2, and Saa1 mRNA in livers of Ldlr−/− versus Ldlr−/−Siglec-G−/− mice after 8 weeks of atherogenic diet. The expression data of individual genes was normalized to the house-keeping gene S12 and expressed relative to the expression in Ldlr−/− control mice.
(D) Quantification of SAA levels in plasma of Ldlr−/− versus Ldlr−/−Siglec-G−/− mice at baseline and after 8 weeks of atherogenic diet. The samples were diluted 1:300 and measured in triplicates.
(E) Quantification of TNFα, IL-6, IL-18, CCL2, CXCL1, and CXCL2 in plasma of Ldlr−/− versus Ldlr−/−Siglec-G−/− mice after 8 weeks of atherogenic diet by multiplex assay. The samples were diluted 1:2. The data represent mean ± SEM of 9–13 mice per group (∗p ≤ 0.05, ∗∗p ≤ 0.01, and ∗∗∗p ≤ 0.001).
See also Figure S5.
To assess systemic markers of inflammation in these mice, we quantified hepatic mRNA expression and the circulating levels of serum amyloid A (SAA), which in mice represents an acute phase protein that is induced during chronic inflammation (Uhlar and Whitehead, 1999). Expression of Saa1 was significantly reduced in the livers of Ldlr−/−Siglec-G−/− mice compared to Ldlr−/− mice (Figure 4C). Moreover, while plasma SAA levels increased after 8 weeks of atherogenic diet in Ldlr−/−, this was not the case for Ldlr−/−Siglec-G−/− mice (Figure 4D). SAA levels in Ldlr−/− mice that were reconstituted with μMT+Siglec-G−/− or μMT+wild-type (WT) bone marrow after 10 weeks of atherogenic diet were lower in both groups, consistent with a more moderate degree of inflammation, but still showed a tendency toward reduced levels as a result of Siglec-G deficiency (Figure S5D). To better characterize the inflammatory responses in these mice, we quantified the plasma levels of cytokines (TNF-α, IL-6, and IL-18) and chemokines (CCL2, CXCL1, and CXCL2) that have been previously identified to contribute to different pathogenic responses in atherosclerosis and hepatic inflammation (Ait-Oufella et al., 2011, Weber and Noels, 2011). Of all cytokines and chemokines tested, plasma levels of CXCL1 were significantly reduced both in Ldlr−/−Siglec-G−/− mice (Figure 4E) and Ldlr−/− mice lacking Siglec-G in B cells only compared to their respective controls (Figure S5E). Notably, Cxcl1 has been shown to be prominently induced in macrophages and endothelial cells stimulated with OxLDL and mediates the recruitment of neutrophils and monocytes (Stewart et al., 2010, Berliner et al., 1990).
Thus, atherogenic diet is associated with increased inflammation in Ldlr−/− mice, and this effect is suppressed in mice lacking Siglec-G. Collectively, these data point to a strong anti-inflammatory effect of Siglec-G deficiency in diet-induced hepatic and systemic inflammation.
Siglec-G Deficiency Protects from OxLDL-Induced Sterile Peritonitis
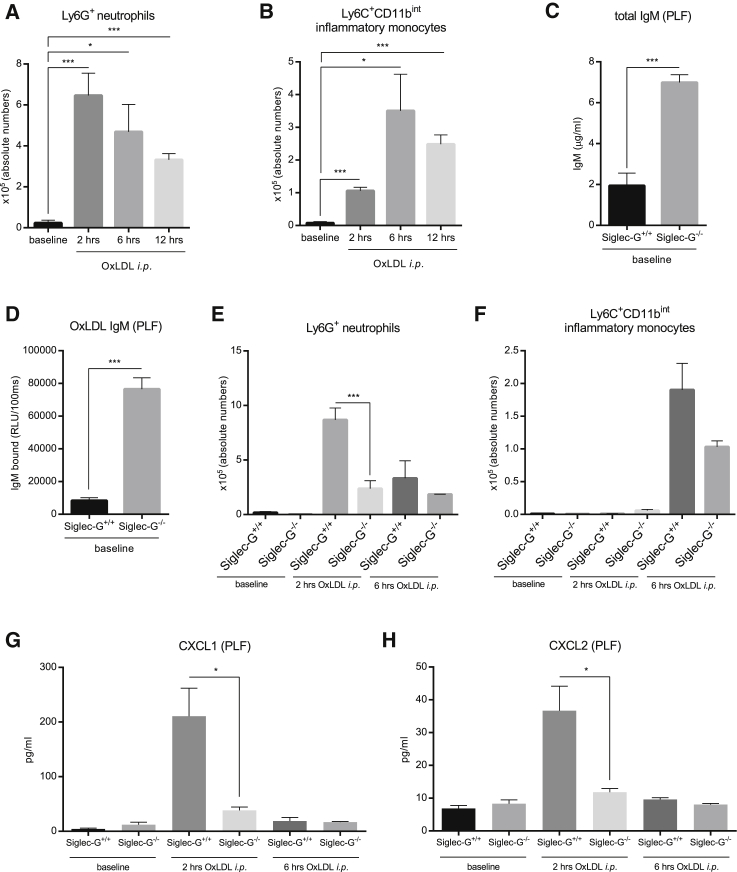
Because we have previously shown that OSE-specific NAb inhibit pro-inflammatory effects of epitopes of OxLDL (Tsiantoulas et al., 2015, Imai et al., 2008), which is considered a major driver of vascular and hepatic inflammation (Miller et al., 2011, Walenbergh et al., 2013), we evaluated the effect of Siglec-G deficiency in an OxLDL-induced sterile inflammation model in vivo. For this, we first established a sterile peritonitis model that allowed us to monitor the recruitment of neutrophils and monocytes in the peritoneal lavage fluid (PLF) of WT C57BL/6 mice 2, 6, and 12 hr after they had received an intraperitoneal (i.p.) injection of OxLDL. Compared to baseline, mice injected with OxLDL exhibited a marked recruitment of Ly-6G+ neutrophils into the peritoneal cavity already after 2 hr, which remained significantly different, but gradually declined after 6 and 12 hr (Figures 5A, S6, and S7A). Similarly, peritoneal recruitment of Ly6C+CD11bint inflammatory monocytes was also induced following i.p. injection of OxLDL, but only peaked 6 hr after the injection, consistent with the delayed recruitment of monocytes during inflammatory processes (Figures 5B and S7A).
Figure 5.
OxLDL-Induced Sterile Peritonitis Is Suppressed by Siglec-G Deficiency
(A and B) Quantification of absolute numbers of Ly6G+ neutrophils (A) and Ly6Chi inflammatory monocytes (B) in the PLF of C57BL/6 mice at baseline, as well as 2, 6, and 12 hr after OxLDL injections.
(C) Quantification of total IgM in the PLF of Siglec-G+/+ and Siglec-G−/− mice. The samples were diluted 1:20 and measured in triplicates.
(D) Binding of IgM to CuOx-LDL in the PLF of Siglec-G+/+ and Siglec-G−/− mice was determined by ELISA. The samples were undiluted and antibody binding was measured in triplicates. The data are expressed as relative RLU/100 ms.
(E and F) Quantification of absolute numbers of Ly6G+ neutrophils (E) and Ly6Chi monocytes (F) in the PLF of Siglec-G+/+ and Siglec-G−/− mice at baseline, 2, and 6 hr after i.p. injection of OxLDL.
(G and H) Quantification of CXCL1 (G) and CXCL2 (H) in the PLF of Siglec-G+/+ and Siglec-G−/− mice at indicated time points by ELISA. The samples were undiluted and measured in duplicates. The data represent mean ± SEM of 3–7 mice per group and are a representative example of three individual experiments (∗p ≤ 0.05, ∗∗p ≤ 0.01, and ∗∗∗p ≤ 0.001). See also Figures S6 and S7.
To investigate the anti-inflammatory effect of natural IgM associated with Siglec-G deficiency, we compared the dynamics of inflammatory cell recruitment during OxLDL-induced peritonitis in Siglec-G+/+ and Siglec-G−/− mice, respectively. Consistent with the dramatic increase of IgM antibodies in the plasma of Siglec-G−/− mice, we observed >3-fold higher levels of total and >9-fold higher levels of OxLDL-specific IgM in the PLF of Siglec-G−/− mice at baseline (Figures 5C and 5D). At baseline, the numbers of peritoneal macrophages, which represent the cellular population that is primarily involved in responding to OxLDL, were not significantly different between Siglec-G+/+ and Siglec-G−/− mice (Figure S7C). Siglec-G−/− mice showed a significantly reduced recruitment of neutrophils 2 hr after i.p. injection of OxLDL compared to Siglec-G+/+ mice, while after 6 hr, no differences between the two groups were observed (Figure 5E). Consistent with this, OxLDL-induced secretion of CXCL1 and CXCL2 was nearly absent in the peritoneal cavity of Siglec-G−/− mice at this time point (Figures 5G and 5H). At 6 hours after i.p. injection of OxLDL, the recruitment of Ly-6C+CD11bint inflammatory monocytes appeared lower in Siglec-G−/− mice (Figure 5F). In contrast, Siglec-G deficiency had no effect on neutrophil recruitment in response to injection of thioglycollate (another sterile trigger), indicating that Siglec-G-deficient mice do not have defective inflammatory responses in general and demonstrating specificity for inflammatory responses triggered by OSE (Figure S7D). In summary, Siglec-G deficiency results in a significant inhibition of the inflammatory response to OxLDL in vivo. The markedly reduced infiltration of neutrophils in Siglec-G−/− mice in response to i.p. injection of OxLDL indicates direct neutralization of its pro-inflammatory moieties and more efficient clearance mediated by OSE-specific IgM.
Discussion
In this study, we demonstrate a pathogenic role for Siglec-G in atherosclerosis and hepatic inflammation. We could show that Siglec-G deficiency in atherosclerosis-prone Ldlr−/− mice leads to a marked reduction of atherosclerotic lesion burden as well as decreased hepatic inflammation. This effect is specific for Siglec-G deficiency in B cells that results in higher B-1 cell numbers and a robust and preferential increase in OSE-specific IgM antibodies, which neutralize OxLDL-induced inflammation in vivo.
Siglec-G is primarily expressed on B cells, with slightly higher expression levels on B-1a cells than on conventional B2 cells (Hoffmann et al., 2007), but it has also been shown to be expressed on dendritic cells and eosinophils (Hutzler et al., 2014, Pfrengle et al., 2013). A suppressive role of Siglec-G in non-B cells has been suggested in drug-induced liver damage, as Siglec-G deficiency results in increased production of pro-inflammatory cytokines, including IL-6, TNF-α, and MCP-1, following stimulation with DAMPs that are released in this setting (Chen et al., 2009). Nevertheless, our data clearly identify an anti-inflammatory effect of Siglec-G deficiency in cholesterol-fed Ldlr−/− mice as documented by reduced hepatic expression of adhesion molecules, pro-inflammatory cytokines, and chemokines as well as lower CXCL1 levels in plasma. Moreover, our data demonstrate Siglec-G deficiency to be protective in a model of OxLDL-induced peritonitis, while it has been shown to aggravate inflammation in cecal ligation and puncture-induced polybacterial peritonitis. These detrimental effects of Siglec-G deficiency have been shown to be dependent on its interaction with the glycosylphosphatidylinositol (GPI)-anchored co-stimulatory protein CD24 (Chen et al., 2011). CD24 has been shown to recognize DAMPs and to allow Siglec-G to suppress pro-inflammatory signaling via pattern-recognition receptors, which can be disrupted by bacterial sialidases, but not by LPS. In our study, Siglec-G deficiency conferred a protective effect against another type of sterile trigger, OxLDL, as Siglec-G-deficient mice exhibited decreased inflammation in an OxLDL-induced peritonitis model. Presumably, the sterile inflammation induced by OxLDL also does not disrupt the interaction of CD24 and Siglec-G, suggesting that Siglec-G deficiency protects via other mechanisms than CD24.
It has also been shown that RNA viruses evade anti-viral defenses by upregulating Siglec-G in macrophages and dendritic cells, resulting in diminished production of type I interferons that are essential in the host defense against viruses (Chen et al., 2013). As a consequence of this, Siglec-G deficiency results in increased production of type I interferons, which notably have been shown to promote atherosclerosis in several studies (Ait-Oufella et al., 2011). Because we have shown that Siglec-G deficiency protects from atherosclerosis, this pathway seems to be not relevant in this setting. In line with this, stimulation of peritoneal and bone-marrow derived macrophages with OxLDL does not induce Siglec-G expression (data not shown). Taken together, our data argue against a role of Siglec-G in myeloid cells with respect to atherosclerosis and hepatic inflammation. Moreover, we provide clear evidence that the protective effect of Siglec-G deficiency is mediated by B cells, indicating that the function of Siglec-G on B cells is dominant. In B cells, Siglec-G is expressed in all stages throughout B cell development (Pfrengle et al., 2013, Hutzler et al., 2014). Therefore, its functional role in autoimmunity has been studied, particularly in aging mice (Müller et al., 2015). Old Siglec-G−/− mice have been shown to exhibit an increased expression of activation markers on T cells and conventional B2 cells, suggesting a more general over-activation of the adaptive immune system in these settings. However, the effects described in our study are more consistent with the increased production of protective B-1 cell-derived NAb by Siglec-G-deficient mice. In line with previous data, we demonstrate that Siglec-G deficiency also resulted in a dramatic expansion of peritoneal B-1a as well as splenic B-1 cells in atherosclerotic mice. FO B cells and MZB cells remained unchanged in Ldlr−/−Siglec-G−/− mice as well as in the bone marrow chimeric mice. Thus, our data support a protective role of B-1 cells in atherosclerosis (Tsiantoulas et al., 2014). Notably, we did observe a robust increase of the recently described IRA B cells in the spleens of atherosclerotic Ldlr−/−Siglec-G−/− mice. These cells have been suggested to originate from B-1a cells and have been shown to promote atherosclerotic lesion formation (Hilgendorf et al., 2014). Thus, although our data are in line with a B-1 cell origin of IRA B cells, the protective effects of B-1 cells dominate in atherosclerotic mice lacking Siglec-G. Previous studies demonstrated an atheroprotective effect of adoptive transfer of B-1a cells into splenectomized Apoe−/− mice and of B-1b cells into Rag-1−/−Apoe−/− mice (Kyaw et al., 2011, Rosenfeld et al., 2015). These studies address the role of B-1 cells only in immune-compromised mice on top of existing differences in various immune cell populations, such as lack of splenocytes or total lymphocytes as a result of splenectomy or Rag-1 deficiency, respectively. However, they do not provide a conclusive answer on the role of B-1 cells in atherosclerosis in an intact host. Our data provide clear and direct genetic evidence that expansion of B-1a cells in intact mice mediates atheroprotection.
One of the major functions of B-1 cells is the secretion of natural IgM antibodies, which have been suggested to mediate the atheroprotective effect of B-1 cells. Kyaw et al. (2011) showed that accelerated lesion formation in splenectomized Ldlr−/− mice, which exhibit diminished peritoneal B-1a cell numbers and reduced serum IgM, is reversed by the adoptive transfer of B-1a cells. This effect was dependent on the capacity of B-1a cells to secrete IgM, as adoptive transfer of B-1 cells from secreted IgM−/− donors did not have any effect. The atheroprotective role of NAbs is further supported by the fact that Ldlr−/− mice deficient in secreted IgM develop increased atherosclerosis compared to Ldlr−/− control mice (Lewis et al., 2009). These data also suggested that increasing IgM titers above and beyond normal plasma levels could confer atheroprotection. Interestingly, hypercholesterolemia raises plasma IgM levels, presumably as part of an endogenous defense mechanism against increased accumulation of metabolic waste products (Khoo et al., 2015). However, this protective response is not sufficient to prevent the formation of atherosclerotic plaques and hepatic inflammation. Deficiency of Siglec-G results in an even greater increase of IgM antibodies despite excessive hypercholesterolemia, and thus Siglec-G may be an attractive target for therapeutic intervention, for example, by blocking antibodies.
Natural IgM exhibit house-keeping functions by promoting the clearance of dying cells and cellular debris (Avrameas, 1991, Notkins, 2004). These properties of natural IgM are in part mediated by their ability to recognize OSE, which are also present in OxLDL (Miller et al., 2011). We have previously shown that immunization of high-fat diet-fed Ldlr−/− mice with heat-killed pneumococci led to the expansion of a natural IgM clone recognizing OxLDL (T15/E06) and significantly decreased atherosclerotic lesion formation and hepatic inflammation (Binder et al., 2003, Bieghs et al., 2012b). These data suggested that anti-OxLDL IgM protect from atherosclerosis and liver inflammation. Indeed, epidemiological studies in humans indicate that high levels of OxLDL-specific IgM are associated with a lower incidence of cardiovascular disease (Tsiantoulas et al., 2014). Of note, Siglec-G deficiency, which results in atheroprotection, leads to a preferential increase of IgM with specificity for different epitopes of OxLDL in plasma.
Several mechanisms exist by which OxLDL-specific IgM exhibit atheroprotective properties, including their ability to inhibit foam cell formation by blocking scavenger receptor-mediated uptake of OxLDL by macrophages (Tsiantoulas et al., 2014). In addition, anti-OxLDL IgM prevent the accumulation of apoptotic cells by promoting their uptake by macrophages (Ogden et al., 2005, Chou et al., 2009). In line with this, we show smaller necrotic areas in lesion of Ldlr−/−Siglec-G−/− mice. Moreover, anti-OxLDL IgM inhibit inflammation by components of OxLDL in vitro (Imai et al., 2008). In agreement with a neutralizing effect of anti-OxLDL IgM, we now show reduced CXCL1 and CXCL2 secretion as well as neutrophil infiltration into the peritoneum in vivo following injection of OxLDL into Siglec-G−/− mice, which have >9-fold higher levels of anti-OxLDL IgM in the peritoneal cavity compared to WT controls. Moreover, plasma levels of CXCL1 were significantly reduced in Siglec-G-deficient atherosclerotic mice. OxLDL has been shown to induce Cxcl1 expression in macrophages via a co-operation of CD36/TLR4/TLR6, and CXCL1 represents a key mediator of leukocyte recruitment in atherosclerosis (Stewart et al., 2010). Moreover, both macrophage CD36 and TLR-4 expression have been shown to promote atherogenesis and hepatic inflammation. Thus, increased natural IgM with specificity for OxLDL have the capacity to inhibit atherosclerotic lesion formation and hepatic inflammation in Siglec-G-deficient mice by neutralizing the pro-inflammatory properties of OxLDL.
In conclusion, we provide evidence that expansion of natural IgM antibodies beyond physiological plasma levels protect from atherosclerosis and hepatic inflammation. Exploiting these mechanisms, for example, by blocking the inhibitory effect of Siglec-G, may represent a therapeutic approach to enhance and strengthen the endogenous defense mechanisms to protect from cardiovascular disease and associated metabolic disorders, such as non-alcoholic steatohepatitis.
Experimental Procedures
An expanded version of experimental procedures can be found in Supplemental Information.
Animals and Intervention Studies
All experimental protocols were approved by the institutional animal experimentation committee and the Austrian Ministry of Science. All mice were on a C57BL/6 background. Ldlr−/− and μMT mice were purchased originally from The Jackson Laboratories (Bar Harbor, Maine, USA). The generation of C57BL/6 Siglec-G−/− mice has been described elsewhere (Müller et al., 2015). Siglec-G−/− mice were further crossed with Ldlr−/− mice to obtain Ldlr−/−Siglec-G−/− and Ldlr−/−Siglec-G+/+ mice. For intervention studies, three cohorts of 12-week-old male Ldlr−/−Siglec-G−/− mice (n = 14) and Ldlr−/−Siglec-G+/+ (n = 16) littermate controls were fed an atherogenic diet containing 21% milk fat and 0.2% cholesterol (TD88137, Ssniff Spezialdiäten) for 8 weeks. Bone marrow transplantation studies were performed as previously described (Binder et al., 2004, Sage et al., 2012, Fillatreau et al., 2002) and as described in Supplemental Information.
Analysis of Atherosclerotic Lesions
The extent of atherosclerosis was assessed in a blinded fashion in en face preparations of the entire aorta and in cross-sections through the aortic origin by computer-assisted image analysis as previously described (Binder et al., 2003, Binder et al., 2004, Cardilo-Reis et al., 2012).
Liver Histology
The left lobes of livers were harvested and four equal pieces were snap frozen in liquid nitrogen for further analyses. Frozen liver sections (7 μm) were stained for the presence of infiltrating macrophages and neutrophils as described previously (Bieghs et al., 2012a). For the presence of neutrophils, sections were stained with mouse anti-Ly6G (1:50; BD PharMingen). Cell nuclei were counterstained with hematoxylin (Klinipath). Pictures were taken with a Nikon digital camera DMX1200 and ACT-1 v2.63 software (Nikon). The number of positive-stained cells was counted in six pictures (200 ×) per liver per mouse to determine the level of liver inflammation.
Flow Cytometry
Peritoneal exudate cells (PEC), splenocytes, bone marrow cells, and blood leukocytes were harvested as previously described (Cardilo-Reis et al., 2012) and stained as indicated in Supplemental Information. Stained cell populations were analyzed by multiparameter flow cytometry using a BD FACSCalibur (BD Bioscience) or BD FACS Fortessa, respectively. Dead cells and doublets were excluded by forward- and side-scatter and data were analyzed using the FlowJo v10 data analysis software (Tree Star). Criteria used to identify the various cell populations are provided in Table 1.
Plasma Antibody and Protein Analyses
Total IgM levels were measured by a chemiluminescent-based sandwich ELISA as described previously (Chou et al., 2009). Total IgG levels were measured in plasma using a commercially available ELISA (Mouse IgG total ELISA Ready-SET-Go!, eBioscience). Cu2+-OxLDL (CuOx-LDL) and MDA-LDL were prepared as described previously (Chou et al., 2009). α-1,3-dextran was a gift of John F. Kearney (University of Alabama at Birmingham, Alabama, USA). Antigen-specific antibody titers were measured by chemiluminescent ELISA as previously described (Binder et al., 2003). OxLDL-specific IgM per total IgM ratios were calculated based on the measurements of specific IgM at non-saturating dilutions (expressed as RLU/100 ms) and total IgM quantities of each individual sample. Data are expressed as a.u. of these ratios. SAA levels were measured in plasma diluted 1:300 using a commercially available ELISA for mouse SAA (Tridelta Development).
Measurement of Chemokines/Cytokines in Plasma and PLF
A panel of chemokines and cytokines (IL-6, TNF-α, CCXL1, CXCL2, CCL2, and IL-18) was measured in mouse plasma (1:2 dilution) with ProcartaPlex Multiplex Immunoassays (eBioscience) according to the manufacturer’s protocol. Analysis was performed with the xMAP Technology by Luminex. Levels of CXCL1 and CXCL2 in the PLF were determined by using mouse CXCL1/KC DuoSet ELISA and Mouse CXCL2/MIP-2 DuoSet ELISA, respectively according to the manufacturer’s protocol (R&D Systems).
Murine Sterile Peritonitis
Siglec-G−/− mice and WT littermate controls (8–12 weeks of age) were injected i.p. with sterile CuOx-LDL (tested for endotoxin levels by chromogenic Limulus amoebocyte assay; <0.05 ng LPS/mg protein) suspended in 600 μl of sterile PBS-BSA (25 μg/gram body weight) or a comparable volume of sterile thioglycollate (Lipid MAPS) (25 μl/g body weight). At selected time points, mice were sacrificed, and PEC were collected by lavaging the peritoneum with 6 ml of sterile PBS+1% BSA. PEC were pelleted for 10 min at 300 g, and processed for flow cytometry, as described above. Cell-free lavage fluid was stored in aliquots and further processed for ELISA measurements.
Statistical Analysis
Data were analyzed using GraphPad Prism version 6.0 for Windows (GraphPad Software). Normal distribution of data was assessed, and statistical analyses were performed by unpaired Student’s t-test to determine statistical significance between the groups. Data points, which were more than 2 × SD of the mean, were excluded as statistical outliers. Exclusion of these values did not change the significance of the results. Data are presented as the mean ± SEM and considered significant at p ≤ 0.05 (∗p ≤ 0.05, ∗∗p ≤ 0.01, and ∗∗∗p ≤ 0.001, respectively).
Author Contributions
S.G. designed and performed experiments, analyzed and interpreted data, and wrote the manuscript. T.H. and D.T. performed experiments, analyzed and interpreted data, and critically revised the manuscript. M.O.-K. and L.G. performed experiments. J.L.W. provided intellectual input and critically revised the manuscript. Z.M., R.S.-S., and L.N. provided technical and material support and critically revised the manuscript. C.J.B. conceived, designed, and supervised the study and drafted and critically revised the manuscript.
Acknowledgments
The authors thank Robert Kralovics, Sylvia Knapp, Riem Gawish, and Anastasiya Hladik for technical and material support. We are grateful to Georg Obermayer for help with the illustrations. This work was supported by grants of the Austrian Academy of Sciences (C.J.B.), the Austrian Science Fund (SFB-54; C.J.B.), the European Union (FP7 programme VIA; C.J.B.), the German Research Foundation (TRR130, L.N.), the Fondation Leducq (C.J.B., Z.M., and J.L.W.), and The Netherlands Organisation for Scientific Research (NWO) (Vidi 016.126.327, R.S.-S.).
Published: March 3, 2016
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Supplemental Information includes Supplemental Experimental Procedures and seven figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2016.02.027.
Supplemental Information
References
- Ait-Oufella H., Herbin O., Bouaziz J.D., Binder C.J., Uyttenhove C., Laurans L., Taleb S., Van Vré E., Esposito B., Vilar J. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 2010;207:1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Oufella H., Taleb S., Mallat Z., Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- Avrameas S. Natural autoantibodies: from “horror autotoxicus” to “gnothi seauton”. Immunol. Today. 1991;12:154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- Baumgarth N., Tung J.W., Herzenberg L.A. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin. Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- Berliner J.A., Territo M.C., Sevanian A., Ramin S., Kim J.A., Bamshad B., Esterson M., Fogelman A.M. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J. Clin. Invest. 1990;85:1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieghs V., Rensen P.C., Hofker M.H., Shiri-Sverdlov R. NASH and atherosclerosis are two aspects of a shared disease: central role for macrophages. Atherosclerosis. 2012;220:287–293. doi: 10.1016/j.atherosclerosis.2011.08.041. [DOI] [PubMed] [Google Scholar]
- Bieghs V., van Gorp P.J., Walenbergh S.M., Gijbels M.J., Verheyen F., Buurman W.A., Briles D.E., Hofker M.H., Binder C.J., Shiri-Sverdlov R. Specific immunization strategies against oxidized low-density lipoprotein: a novel way to reduce nonalcoholic steatohepatitis in mice. Hepatology. 2012;56:894–903. doi: 10.1002/hep.25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder C.J., Hörkkö S., Dewan A., Chang M.K., Kieu E.P., Goodyear C.S., Shaw P.X., Palinski W., Witztum J.L., Silverman G.J. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- Binder C.J., Hartvigsen K., Chang M.K., Miller M., Broide D., Palinski W., Curtiss L.K., Corr M., Witztum J.L. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bökers S., Urbat A., Daniel C., Amann K., Smith K.G., Espéli M., Nitschke L. Siglec-G deficiency leads to more severe collagen-induced arthritis and earlier onset of lupus-like symptoms in MRL/lpr mice. J. Immunol. 2014;192:2994–3002. doi: 10.4049/jimmunol.1303367. [DOI] [PubMed] [Google Scholar]
- Cardilo-Reis L., Gruber S., Schreier S.M., Drechsler M., Papac-Milicevic N., Weber C., Wagner O., Stangl H., Soehnlein O., Binder C.J. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med. 2012;4:1072–1086. doi: 10.1002/emmm.201201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan V.W., Meng F., Soriano P., DeFranco A.L., Lowell C.A. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- Chang M.K., Bergmark C., Laurila A., Hörkkö S., Han K.H., Friedman P., Dennis E.A., Witztum J.L. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. USA. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M.K., Binder C.J., Miller Y.I., Subbanagounder G., Silverman G.J., Berliner J.A., Witztum J.L. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J. Exp. Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.Y., Tang J., Zheng P., Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.Y., Chen X., King S., Cavassani K.A., Cheng J., Zheng X., Cao H., Yu H., Qu J., Fang D. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat. Biotechnol. 2011;29:428–435. doi: 10.1038/nbt.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Han C., Xie B., Hu X., Yu Q., Shi L., Wang Q., Li D., Wang J., Zheng P. Induction of Siglec-G by RNA viruses inhibits the innate immune response by promoting RIG-I degradation. Cell. 2013;152:467–478. doi: 10.1016/j.cell.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Chou M.Y., Fogelstrand L., Hartvigsen K., Hansen L.F., Woelkers D., Shaw P.X., Choi J., Perkmann T., Bäckhed F., Miller Y.I. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C., Liu Y., Wang Y., Park B.K., Wang C.Y., Zheng P., Liu Y. Siglecg limits the size of B1a B cell lineage by down-regulating NFkappaB activation. PLoS ONE. 2007;2:e997. doi: 10.1371/journal.pone.0000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillatreau S., Sweenie C.H., McGeachy M.J., Gray D., Anderton S.M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Hilgendorf I., Theurl I., Gerhardt L.M., Robbins C.S., Weber G.F., Gonen A., Iwamoto Y., Degousee N., Holderried T.A., Winter C. Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity. Circulation. 2014;129:1677–1687. doi: 10.1161/CIRCULATIONAHA.113.006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A., Kerr S., Jellusova J., Zhang J., Weisel F., Wellmann U., Winkler T.H., Kneitz B., Crocker P.R., Nitschke L. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat. Immunol. 2007;8:695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- Horie K., Miyata T., Maeda K., Miyata S., Sugiyama S., Sakai H., van Ypersole de Strihou C., Monnier V.M., Witztum J.L., Kurokawa K. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J. Clin. Invest. 1997;100:2995–3004. doi: 10.1172/JCI119853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hutzler S., Özgör L., Naito-Matsui Y., Kläsener K., Winkler T.H., Reth M., Nitschke L. The ligand-binding domain of Siglec-G is crucial for its selective inhibitory function on B1 cells. J. Immunol. 2014;192:5406–5414. doi: 10.4049/jimmunol.1302875. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y.H., Wang H. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida D., Su L., Tamura A., Katayama Y., Kawai Y., Wang S.F., Taniwaki M., Hamazaki Y., Hattori M., Minato N. Rap1 signal controls B cell receptor repertoire and generation of self-reactive B1a cells. Immunity. 2006;24:417–427. doi: 10.1016/j.immuni.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Jellusova J., Düber S., Gückel E., Binder C.J., Weiss S., Voll R., Nitschke L. Siglec-G regulates B1 cell survival and selection. J. Immunol. 2010;185:3277–3284. doi: 10.4049/jimmunol.1001792. [DOI] [PubMed] [Google Scholar]
- Khoo L.H., Thiam C.H., Soh S.Y., Angeli V. Splenic extrafollicular reactions and BM plasma cells sustain IgM response associated with hypercholesterolemia. Eur. J. Immunol. 2015;45:1300–1312. doi: 10.1002/eji.201344347. [DOI] [PubMed] [Google Scholar]
- Kyaw T., Tay C., Khan A., Dumouchel V., Cao A., To K., Kehry M., Dunn R., Agrotis A., Tipping P. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J. Immunol. 2010;185:4410–4419. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- Kyaw T., Tay C., Krishnamurthi S., Kanellakis P., Agrotis A., Tipping P., Bobik A., Toh B.H. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ. Res. 2011;109:830–840. doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- Lewis M.J., Malik T.H., Ehrenstein M.R., Boyle J.J., Botto M., Haskard D.O. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- Ma Z., Choudhury A., Kang S.A., Monestier M., Cohen P.L., Eisenberg R.A. Accelerated atherosclerosis in ApoE deficient lupus mouse models. Clin. Immunol. 2008;127:168–175. doi: 10.1016/j.clim.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Y.I., Choi S.H., Wiesner P., Fang L., Harkewicz R., Hartvigsen K., Boullier A., Gonen A., Diehl C.J., Que X. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J., Lunz B., Schwab I., Acs A., Nimmerjahn F., Daniel C., Nitschke L. Siglec-G deficiency leads to autoimmunity in aging C57BL/6 mice. J. Immunol. 2015;195:51–60. doi: 10.4049/jimmunol.1403139. [DOI] [PubMed] [Google Scholar]
- Notkins A.L. Polyreactivity of antibody molecules. Trends Immunol. 2004;25:174–179. doi: 10.1016/j.it.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Ogden C.A., Kowalewski R., Peng Y., Montenegro V., Elkon K.B. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38:259–264. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- Pao L.I., Lam K.P., Henderson J.M., Kutok J.L., Alimzhanov M., Nitschke L., Thomas M.L., Neel B.G., Rajewsky K. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Perry H.M., Bender T.P., McNamara C.A. B cell subsets in atherosclerosis. Front. Immunol. 2012;3:373. doi: 10.3389/fimmu.2012.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrengle F., Macauley M.S., Kawasaki N., Paulson J.C. Copresentation of antigen and ligands of Siglec-G induces B cell tolerance independent of CD22. J. Immunol. 2013;191:1724–1731. doi: 10.4049/jimmunol.1300921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman M.J., Salmon J.E. Cardiovascular manifestations of rheumatologic diseases. Circulation. 2007;116:2346–2355. doi: 10.1161/CIRCULATIONAHA.106.678334. [DOI] [PubMed] [Google Scholar]
- Rosenfeld S.M., Perry H.M., Gonen A., Prohaska T.A., Srikakulapu P., Grewal S., Das D., Mcskimming C., Taylor A.M., Tsimikas S. B-1b cells secrete atheroprotective IgM and attenuate atherosclerosis. Circ. Res. 2015;117:e28–39. doi: 10.1161/CIRCRESAHA.117.306044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage A.P., Tsiantoulas D., Baker L., Harrison J., Masters L., Murphy D., Loinard C., Binder C.J., Mallat Z. BAFF receptor deficiency reduces the development of atherosclerosis in mice--brief report. Arterioscler. Thromb. Vasc. Biol. 2012;32:1573–1576. doi: 10.1161/ATVBAHA.111.244731. [DOI] [PubMed] [Google Scholar]
- Stewart C.R., Stuart L.M., Wilkinson K., van Gils J.M., Deng J., Halle A., Rayner K.J., Boyer L., Zhong R., Frazier W.A. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall A.R., Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiantoulas D., Gruber S., Binder C.J. B-1 cell immunoglobulin directed against oxidation-specific epitopes. Front. Immunol. 2012;3:415. doi: 10.3389/fimmu.2012.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiantoulas D., Diehl C.J., Witztum J.L., Binder C.J. B cells and humoral immunity in atherosclerosis. Circ. Res. 2014;114:1743–1756. doi: 10.1161/CIRCRESAHA.113.301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiantoulas D., Perkmann T., Afonyushkin T., Mangold A., Prohaska T.A., Papac-Milicevic N., Millischer V., Bartel C., Hörkkö S., Boulanger C.M. Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J. Lipid Res. 2015;56:440–448. doi: 10.1194/jlr.P054569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlar C.M., Whitehead A.S. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- Walenbergh S.M., Koek G.H., Bieghs V., Shiri-Sverdlov R. Non-alcoholic steatohepatitis: the role of oxidized low-density lipoproteins. J. Hepatol. 2013;58:801–810. doi: 10.1016/j.jhep.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Weber C., Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- Winer D.A., Winer S., Chng M.H., Shen L., Engleman E.G. B Lymphocytes in obesity-related adipose tissue inflammation and insulin resistance. Cell. Mol. Life Sci. 2014;71:1033–1043. doi: 10.1007/s00018-013-1486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouggari Y., Ait-Oufella H., Bonnin P., Simon T., Sage A.P., Guérin C., Vilar J., Caligiuri G., Tsiantoulas D., Laurans L. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 2013;19:1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.