Highlights
-
•
Hepatoblastoma is the most common primary liver tumor for children under 5 years of age, with an incidence of 5.2 per million.
-
•
Diagnosis depends on imaging studies, AFP levels and percutaneous biopsy.
-
•
Treatment modality is usually surgical with neoadjuvant chemotherapy.
-
•
Remission and long term survival can be achieved after complete anatomical resection and appropriate neoadjuvant chemotherapy.
-
•
Hepatoblastoma cases should be referred to specialized centers for management.
Abbreviations: AFP, alpha-feto protein; SGPT, serum glutamic-pyruvic transaminase; SGOT, serum glutamic-oxaloacetic transaminase; CRP, c-reactive protein; PO, per os; NVD, normal vaginal delivery; FAP, familial adenomatous polyposis; BWS, Beckwith–Wiedemann syndrome; CT scan, computed tomography scan; SIOPEL, Société Internationale d'Oncologie Pédiatrique—Epithelial Liver Tumor Study Group
Keywords: Hepatoblastoma case reports, Surgical resection, Chemotherapy, Total remission, Long term survival
Abstract
Introduction
Hepatoblastoma is the most common primary liver tumor for children under 5 years of age. It usually presents as an abdominal mass, symptomatic only when large enough to cause mass effect on nearby organs. Symptoms such as early satiety, anorexia, abdominal pain or weight loss are the most common. Diagnosis depends on imaging studies, AFP levels and percutaneous biopsy. Treatment modality is usually surgical with neoadjuvant chemotherapy.
Cases
In this article, we present 2 cases of hepatoblastoma treated 15 years ago by neoadjuvant chemotherapy and surgery, and are presenting for long term follow-up with complete disease remission.
Discussion
Complete resection and remission can be achieved as demonstrated below by our 2 cases of hepatoblastoma, especially when performing a true anatomical hepatectomy, along with a neoadjuvant chemotherapy regimen. Although one of the cases did not respond to chemotherapy very well a complete resection was achieved and therefore a disease free survival of 15 years.
Conclusion
Hepatoblastoma are rare tumors of the pediatric age group. Management depends highly on combined surgical and pediatric oncological knowledge. A complete disease remission can be achieved when both modalities are treatment are optimal. Therefore, hepatoblastoma cases should be referred to specialized centers for management.
1. Case 1
This is the case of a 3 ½ year old male patient, previously healthy, born on term, NVD, with no perinatal complications, presenting with abdominal pain and distention. History goes back to few days prior to presentation when the male complained of abdominal pain. Upon examination by his pediatrician, hepatomegaly was noticed. Investigations were initiated.
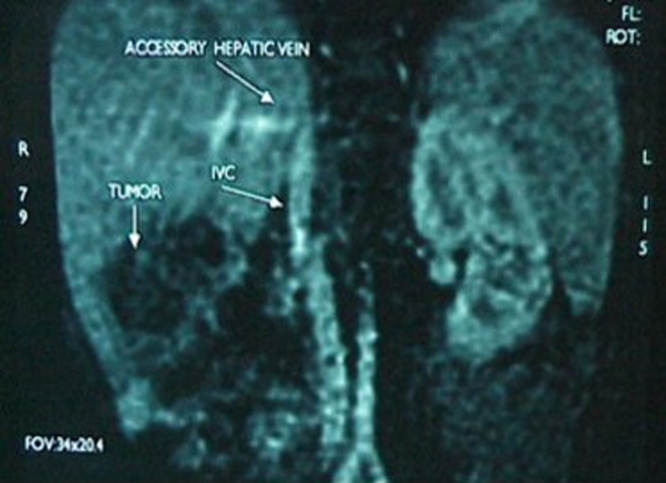
Ultrasound of the abdomen showed a solid hepatic mass, 9 × 6 cm. CT scan of the chest, abdomen and pelvis revealed an encapsulated, loculated hepatic mass, 9 × 9 cm in the posterior segment of the right lobe, involving mainly segments V & VI, with inferomedial expansion (Fig. 1). No other lesions were detected whatsoever, no enlarged lymph nodes. Laboratory studies showed: Hemoglobin 12.4 g/dl, Hematocrit 37%, White blood cells 8000/mm3, Neutrophils 53%, Platelets 534,000/mm3, SGPT 17 U/l, SGOT 42 U/l, Direct Bilirubin 0.62 mg/dl, Total Bilirubin 0.62 mg/dl, Alkaline Phosphatase 378 U/l, CRP 7.5 mg/dl, Alpha Feto-protein >35350 ng/ml.
Fig. 1.
CT scan showing the encapsulated, loculated hepatic mass, 9 × 9 cm in the posterior segment of the right lobe, involving mainly segments V & VI, with inferomedial expansion.
Transhepatic biopsy was done, under CT guidance: sections showing cores of tumorous tissue comprising cohesive aggregates of polygonal cells having pleomorphic nuclei and basophilic cytoplasm forming solid sheets and few papillary structures associated with other islands of cuboidal cells having ample eosinophilic cytoplasm and central nucleolated nuclei forming multilayered trabeculae separated by sinusoids reminiscent of fetal liver. These cellular areas are separated by scanty myxoid edematous loose stroma.
Neoadjuvant chemotherapy course was initiated: Cisplatin + Doxorubicin. 4 months later, after 3 cycles of chemotherapy repeat abdominal CT scan showed a marked shrinking of the mass: 9–4 cm.
Patient was admitted, partial hepatectomy was performed: chevron incision, no peritoneal carcinomatosis, tumor identified in segments V & VI, cholecystectomy done, then bisegmentectomy done with Ultracision, removal of the specimen en-bloc, portocaval lymph nodes dissection performed (Fig. 2). No perioperative complications.
Fig. 2.
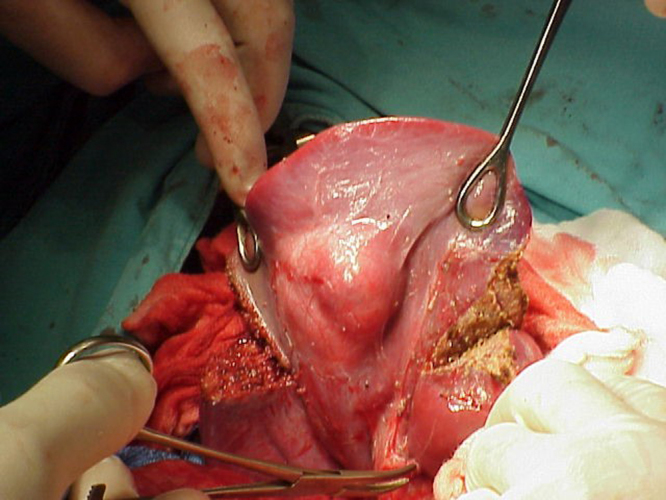
Intraoperative picture taken, showing anatomical resection of segments V and VI.
Final pathology showed (Fig. 3): mixed epithelial-mesenchymal hepatoblastoma with predominance of fetal morphology in the epithelial component. Low mitotic index, intact liver capsule, absence of vascular invasion with negative resection margins. Portocaval lymph nodes negative for tumor and no significant pathology in the gallbladder.
Fig. 3.
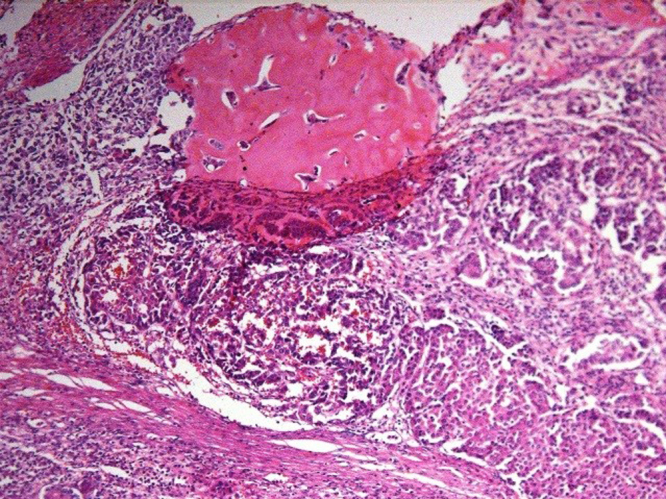
Upper half shows mesenchymal proliferation with large osteoid component and sheets of well-defined fetal type hepatocytes are seen in the right lower aspect of the field.
Post-operative abdominal ultrasound was performed 6 days later and was normal. Patient underwent 2 more cycles of Cisplatin + Doxorubicin and stopped. Follow-up CT scan 10 months later showed no lesions in the liver or abdomen, no masses detected, no adenopathies.
15 years later, patient is still alive with no recurrence of his disease on annual follow-up, laboratory and imaging studies.
2. Case 2
This is the case of an 8 months old female patient, previously healthy, born on term, NVD, with no perinatal complications, presenting with anorexia and epigastric mass. History goes back to 2 months prior to presentation when the female started to refuse PO intake, and increased her crying episodes during the day. An epigastric bulging mass was noted by her mother. Few days prior to presentation, patient started experiencing several episodes of vomiting, not profuse, non-bloody, and non-bilious. Upon examination by her pediatrician, hepatomegaly was noticed. Investigations were initiated.
CT-scan was done showing a hepatic mass about 11 × 9 cm replacing almost all the right lobe of the liver, showing heterogeneous contrast enhancement with punctate central calcification. Hepatic parenchyma intact only in segments 1 and 2 which are both displaced to the left upper quadrant, showing mild intrahepatic biliary dilatation. Inferior vena cava being displaced 2 cm paramedian to the left, the right kidney displaced down to the pelvis.
Laboratory studies showed: Hemoglobin 9.5 g/dl, Hematocrit 30.9%, White blood cells 13,500/mm3, Neutrophils 20%, Platelets 536,000/mm3, SGPT 34 U/l, SGOT 65 U/l, Direct Bilirubin 2 mg/dl, Total Bilirubin 7 mg/dl, Alkaline Phosphatase 167 U/l, CRP 1 mg/dl, Alpha Feto-protein >35350 ng/ml.
Transhepatic biopsy was done, under CT guidance: sections showing a cellular tumor composed of sheets of hepatocytes having granular eosinophilic cytoplasm and central round nuclei forming thick trabeculae with absence of bile ducts. These are associated with other smaller areas which are composed of embryonal type basophilic cells separated by areas of immature chondroid and osteoid tissue (Fig. 4).
Fig. 4.
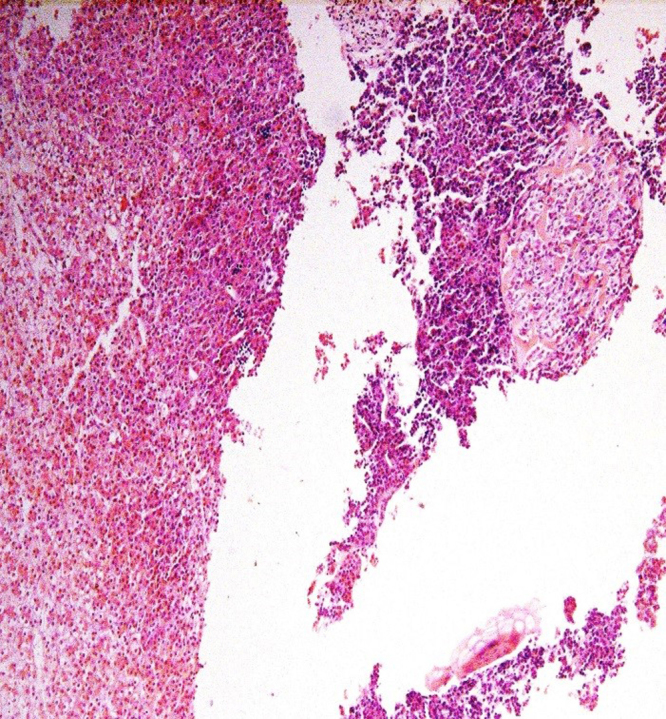
Fetal hepatoblastoma featuring alternating pale and dark areas on the left side. Right side shows immature embryonal elements forming osteoid. Extramedullary hematopoiesis is also evident.
Diagnosis: hepatoblastoma, mixed mesenchymal and epithelial type.
Neoadjuvant chemotherapy course was initiated: Cisplatin + Doxorubicin. 8 months later, after 4 cycles of chemotherapy repeat abdominal CT scan showed 10 × 10 × 8.9 cm right liver tumor with central calcifications, in close proximity to the hepatic pedicle, with marked hypertrophy of the left hepatic lobe. No significant change in the mass dimensions was noted after 4 cycles of chemotherapy but AFP decreased: >35350 to 272 ng/ml. MRI with gadolinium was scheduled showing a 10 × 10 × 8 cm heterogeneous right liver tumor, with hyperintensity signaling on T2 and hypointensity signaling on T1. No vascular invasion noted and no intrahepatic biliary ducts dilatation. A compensatory hypertrophy of the left lobe was noted.
Patient was admitted, right hepatectomy was performed: chevron incision, no peritoneal carcinomatosis, tumor identified in segments V–VIII, cholecystectomy done, then right hepatectomy done with Ultracision, removal of the specimen en-bloc, portocaval lymph nodes dissection performed. No perioperative complications.
Final pathology showed: well differentiated mixed fetal epithelial type hepatoblastoma with abundant osteoid throughout. Intact liver capsule, absence of vascular invasion with negative resection margins. Portocaval lymph nodes negative for tumor and no significant pathology in the gallbladder.
Post-operative abdominal ultrasound was performed 4 days later and was normal. 1 month later, we repeated AFP levels and turned to be 12 ng/ml, abdominal CT-scan was also normal, revealing no lesions or lymphadenopathies. Patient underwent 2 more cycles of Cisplatin + Doxorubicin and stopped. Follow-up ultrasound 6 months later was normal, AFP levels 5.9 ng/ml.
15 years later, patient is still alive with no recurrence of her disease on annual follow-up, laboratory and imaging studies.
3. Discussion
In a time hepatoblastoma incidence is around 10.5 per million in children <1 year, and 5.2 per million in those between 1 and 4 years, according to the SEER program [1], it is thought to have risen 4% between 1992 and 2004 in the United States [2], versus only 1% in Europe between 1978 and 1997 as per the ACCIS.
A review article published by Spector & Birch in 2012 [3], assessed the incidence of hepatoblastoma with various factors such as inherited syndromes, congenital anomalies, gestational risk factors and parental tobacco use.
In the inherited dyndromes section, the highest associations were found with Familial Adenomatous Polyposis syndrome, Beckwith–Wiedemann syndrome and Edwards syndrome (Table I).
Table I.
The incidence of hepatoblastoma in inherited syndromes.
| Syndrome | Source | Incidence |
|---|---|---|
| FAP | John Hopkins polyposis registry [4] | 847 times the SEER population incidence (95% CI: 230–2168) |
| BWS | BWS registry [5] | 2280 times the US population incidence (95% CI: 928–11,656) |
| Edwards syndrome | J. Med. Case reports [6] | 7 case reports in very rare disease with poor survival rate beyond the first year of life |
(a) FAP: familial adenomatous polyposis, (b) BWS: Beckwith–Wiedemann syndrome.
In the Congenital Anomalies section, and according to the British National Registry of Childhood Tumors, 6.4% of the 165 hepatoblastoma cases reviewed, showed congenital anomalies which is way higher than the reported proportion in reference populations [7].
In the gestational risk factors section, a strong association was found between hepatoblastoma and very low birth weight (<1500 g). The advances in medical resuscitation and thus the improved survival of VLBW children, is thought to have participated in the increase in incidence of hepatoblastoma. Various studies handled the possible reasons behind this increase in incidence, attributing it to the exposures in NICU: chemicals used for resuscitation and support (TPN [8], [9], furosemide [10], oxygen [11], antibiotics etc.), and diagnostic irradiations [12]. Studies are being conducted now on a larger scale to find a stronger association between these exposures and hepatoblastoma [13].
In the parenteral tobacco use section, enough evidence was gathered to label parental smoking as carcinogenic to the fetal liver preconception [14].
Hepatoblastoma is considered to be a heterogeneous tumor arising in an otherwise healthy liver. A cirrhotic or diseased liver is not usually associated with hepatoblastoma, rather one can find an association with hepatocellular carcinoma in 75% of cases.
Hepatoblastoma exists as many histological types, notably epithelial, mesenchymal, mixed, or undifferentiated [15]. Within the epithelial type one can find many subtypes: embryonal, fetal, pleomorphic, anaplastic, small cell undifferentiated and cholangioblastic macrotrabecular. Mixed hepatoblastoma comprises both epithelial and mesenchymal components.
Before any surgical resection attempt, one must classify hepatoblastoma in categories where risk stratification, staging and extent of resectability can be assessed. SIOPEL group introduced a classification theme, revised and updated in 2005 for assessment of liver involvement, distant organs involvement and possible resectability/transplant. The PRETEXT and POSTEXT classification is based on: involvement of liver sections, vascular invasion, nearby organs affected and distant metastasis.
PRETEXT: pre-neoadjuvant chemotherapy extent of disease.
POSTEXT: post-neoadjuvant chemotherapy extent of disease.
PRETEXT, POSTEXT I represent 1 section involved with 3 adjoining sections tumor free. PRETEXT, POSTEXT II represent 1 or 2 sections involved with 2 adjoining sections tumor free. PRETEXT, POSTEXT III represent 2 or 3 sections involved with 1 adjoining section tumor free. PRETEXT, POSTEXT IV represent 4 sections involved.
Annotations were also added to the classification for better disease assessment: V refers to the involvement of retrohepatic vena cava or all 3 hepatic veins. P refers to right and left portal veins involved. E refers to contiguous organ involved such as the diaphragm, the abdominal wall, colon, stomach… M represents the distant metastasis. C refers to the caudate lobe involvement. F refers to multifocal tumor nodules. And finally, N represents lymph nodes involvement.
Regarding the surgical approach in hepatoblastoma, all studies have converged towards the same end principles [16], [17], [18]. In cases of PRETEXT I, II or III, segmentectomy, bisegmentectomy, or trisegmentectomy/mesohepatectomy are warranted. In cases of bilobar macrovascular invasion or PRETEXT IV, surgical management tends towards liver transplant. Achieving negative surgical margins is of crucial importance. Non-anatomic resections are contraindicated due to their possible association with tumor cells dissemination, unidentified microscopic positive margins, and tumor cells regeneration under the influence of hepatocyte growth factor which levels increase post-hepatectomy. Atypical resections can be justified in cases of multifocal liver involvement and distant metastasis when transplant is not to be performed. Residual disease was found to be associated with worst outcomes and relapses. Low CVP, Trendelenburg position and short intervals of warm ischemia (10–15 min) with short intervals of reperfusion in between (5–10 min), were found to minimize blood loss and damage to the remaining disease-free liver. Various instruments such as Argon beam, LigaSure, vascular clips, bipolar coagulation etc. can be used according their availability and the surgeon’s preferences. Hepatoduodenal and portocaval lymph nodes sampling are also performed.
In cases of pulmonary metastasis, wedge resection or lobectomy can be performed if primary tumor is resectable and good prognosis is anticipated. Whether pulmonary metastasis are to be treated before or after primary tumor resection is still debatable. In liver recurrences situations, an additional resection or rescue transplant can be performed, but long term survival is not high, and correlates mainly with the initial disease presentation severity, and whether good initial measures were undertaken.
After major hepatic resections, complications such as bile leak, biliary strictures, bleeding, coagulopathy, blood flow to and though the liver obstruction, liver failure and infection may be encountered [19], [20]. Therefore patients must be closely monitored post-operatively in a paediatrics critical care unit for the first few days to anticipate, prevent and treat possible clinical deterioration.
Our two patients presented with PRETEXT II/III. Neoadjuvant chemotherapy was of marked benefit on the epithelial type but of minimal benefit on the mixed type. It is unclear for us whether the presence of osteoid and chondroid components in the mixed type contributed to its resistance to chemotherapy or some genes at the cellular level were responsible of that. Further specimens analysis might be of benefit in this context. The surgical resections we carried were anatomical, with negative margins and of minimal damage to the adjacent structures. In combination with the chemotherapy course given post-operatively we managed to leave a disease-free liver with no evidence of recurrence 15 years later. And finally, the importance for the treatment regimens to be precise and optimized, makes hepatoblastoma a disease to be referred to specialized centers for ideal management.
Conflict of interest
None.
Funding
None.
Ethical approval
This is not a research study.
Consent
Written informed consent was obtained from the patients and patients’ families for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Authors’ contributions
ZER was the physician in charge of this patient, made the treatment decisions and performed the surgery. AEAreviewed the cases, did the literature review, contacted the patients’ families and wrote the article. Both authors read and approved the final manuscript.
Guarantor
This is not a research study. All authors read and approved the final manuscript. Therefore, all authors are responsible for this report.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijscr.2016.02.019.
Contributor Information
Antoine El Asmar, Email: antoine.el.asmar@gmail.com.
Ziad El Rassi, Email: ziadelrassi@gmail.com.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Howlader N., Noone A.M., Krapcho M., Neyman N., Aminou R., Waldron W., Altekruse S.F., Kosary C.L., Ruhl J., Tatalovich Z., Cho H., Mariotto A., Eisner M.P., Lewis D.R., Chen H.S., Feuer E.J., Cronin K.A., Edwards B.K. National Cancer Institute; Bethesda, MD: 2011. SEER Cancer Statistics Review 1975–2008. [Google Scholar]
- 2.Linabery A.M., Ross J.A. Trends in childhood cancer incidence in the U.S. (1992–2004) Cancer. 2008;112:416–432. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 3.Spector L.G., Birch J. The epidemiology of hepatoblastoma. Pediatr. Blood Cancer. 2012 doi: 10.1002/pbc.24215. [DOI] [PubMed] [Google Scholar]
- 4.Giardiello F.M., Offerhaus G.J., Krush A.J., Booker S.V., Tersmette A.C., Mulder J.R., Kelley C.N., Hamilton S.R. Risk of hepatoblastoma in familial adenomatous polyposis. J. Pediatr. 1991;119:766–768. doi: 10.1016/s0022-3476(05)80297-5. [DOI] [PubMed] [Google Scholar]
- 5.DeBaun M.R., Tucker M.A. Risk of cancer during the first four years of life in children from the Beckwith–Wiedemann syndrome registry. J. Pediatr. 1998;132:398–400. doi: 10.1016/s0022-3476(98)70008-3. [DOI] [PubMed] [Google Scholar]
- 6.Kitanovski L., Ovcak Z., Jazbec J. Multifocal hepatoblastoma in a 6-month-old girl with trisomy 18: a case report. J. Med. Case Reports. 2009;3:8319. doi: 10.4076/1752-1947-3-8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narod S.A., Hawkins M.M., Robertson C.M., Stiller C.A. Congenital anomalies and childhood cancer in Great Britain. Am. J. Hum. Genet. 1997;60:474–485. [PMC free article] [PubMed] [Google Scholar]
- 8.Chessex P., Harrison A., Khashu M., Lavoie J.C. In preterm neonates is the risk of developing bronchopulmonary dysplasia influenced by the failure to protect total parenteral nutrition from exposure to ambient light. J. Pediatr. 2007;151:213–214. doi: 10.1016/j.jpeds.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Latini G., Gallo F., De Felice C. Birth characteristics and hepatoblastoma risk in young children. Cancer. 2004;101:210. doi: 10.1002/cncr.20357. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda H., Hirato J., Suzuki N., Kuroiwa M., Maruyama K., Tsuchida Y. Detection of hepatic oxidative DNA damage in patients with hepatoblastoma and children with non-neoplastic disease. Med. Pediatr. Oncol. 2001;37:505–510. doi: 10.1002/mpo.1243. [DOI] [PubMed] [Google Scholar]
- 11.Saugstad O.D. Oxidative stress in the newborn—a 30-year perspective. Biol. Neonate. 2005;88:228–236. doi: 10.1159/000087586. [DOI] [PubMed] [Google Scholar]
- 12.Donadieu J., Zeghnoun A., Roudier C., Maccia C., Pirard P., Andre C., Adamsbaum C., Kalifa G., Legmann P., Jarreau P.H. Cumulative effective doses delivered by radiographs to preterm infants in a neonatal intensive care unit. Pediatrics. 2006;117:882–888. doi: 10.1542/peds.2005-0817. [DOI] [PubMed] [Google Scholar]
- 13.Ross J.A., Olshan A.F. Pediatric cancer in the United States: the Children's Oncology Group Epidemiology Research Program. Cancer Epidemiol. Biomarkers Prev. 2004;13:1552–1554. [PubMed] [Google Scholar]
- 14.Secretan B., Straif K., Baan R., Grosse Y., El Ghissassi F., Bouvard V., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., Cogliano V. A review of human carcinogens-part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 15.Kasai M., Watanabe I. Histologic classification of liver-cell carcinoma in infancy and childhood and its clinical evaluation. A study of 70 cases collected in Japan. Cancer. 1970;25:551–563. doi: 10.1002/1097-0142(197003)25:3<551::aid-cncr2820250309>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Lautz T.B., Ben-Ami T., Tantemsapya N., Gosiengfiao Y., Superina R.A. Successful nontransplant resection of POST-TEXT III and IV hepatoblastoma. Cancer. 2011;117:1976–1983. doi: 10.1002/cncr.25722. [DOI] [PubMed] [Google Scholar]
- 17.Czauderna P., Otte J.B., Aronson D.C., Gauthier F., Mackinlay G., Roebuck D., Plaschkes J., Perilongo G. Guidelines for surgical treatment of hepatoblastoma in the modern era: recommendations from the childhood liver tumour strategy group of the international society of paediatric oncology (SIOPEL) Eur. J. Cancer. 2005;41:1031–1036. doi: 10.1016/j.ejca.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Otte J.B., deVille de Goyet J., Reding R. Liver transplantation for hepatoblastoma: indications and contraindications in the modern era. Pediatr. Transpl. 2005;9:557–656. doi: 10.1111/j.1399-3046.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 19.Howat J.M. Major hepatic resections in infancy and childhood. Gut. 1971;12:212–217. doi: 10.1136/gut.12.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price J.B., Schullinger J.N., Santali T.V. Major hepatic resections for neoplasia in children. Arch. Surg. 1982;117:1139–1141. doi: 10.1001/archsurg.1982.01380330007003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.