Abstract
The post-translational modification of arginine residues represents a key mechanism for the epigenetic control of gene expression. Aberrant levels of histone arginine modifications have been linked to the development of several diseases including cancer. In recent years, great progress has been made in understanding the physiological role of individual arginine modifications and their effects on chromatin function. The present review aims to summarize the structural and functional aspects of histone arginine modifying enzymes and their impact on gene transcription. We will discuss the potential for targeting these proteins with small molecules in a variety of disease states.
Epigenetic regulation of gene expression is essential to eukaryotic life, and its dysregulation is involved in numerous human diseases. This regulatory mechanism is controlled, at least in part, by a diverse set of post-translational modifications (PTMs) of histone proteins.1 Histone proteins are small, basic proteins that constitute the building blocks of nucleosomal particles. These proteins form octamers around which the genomic DNA is spooled. Projecting out of this nucleosomal core are unstructured lysine/arginine-rich N-terminal tails.2 Notably, the N-terminal tails of each histone harbor the majority of known PTMs that are critical for the epigenetic control of gene expression. Since arginine residues are important for DNA binding and protein–protein interactions, it is not surprising that they are subject to extensive modification. Currently, there are four known types of enzymatic arginine modifications, i.e., methylation, citrullination, phosphorylation, and ADP-ribosylation,3,4 and all four have been shown to occur on histone arginine residues.4 The best characterized modifications, however, are arginine methylation and citrullination. In this review, we discuss the chemical biology of protein arginine modifications in the epigenetic control of gene transcription, focusing on the enzymes that catalyze protein citrullination and arginine methylation as well as their regulatory effects on the core histone tails and chromatin function. Additionally, we highlight the recent progress in targeting these proteins using small molecule inhibitors.
The Epigenetic Role of Arginine Modifications
The Biological Effects of Histone Arginine Methylation
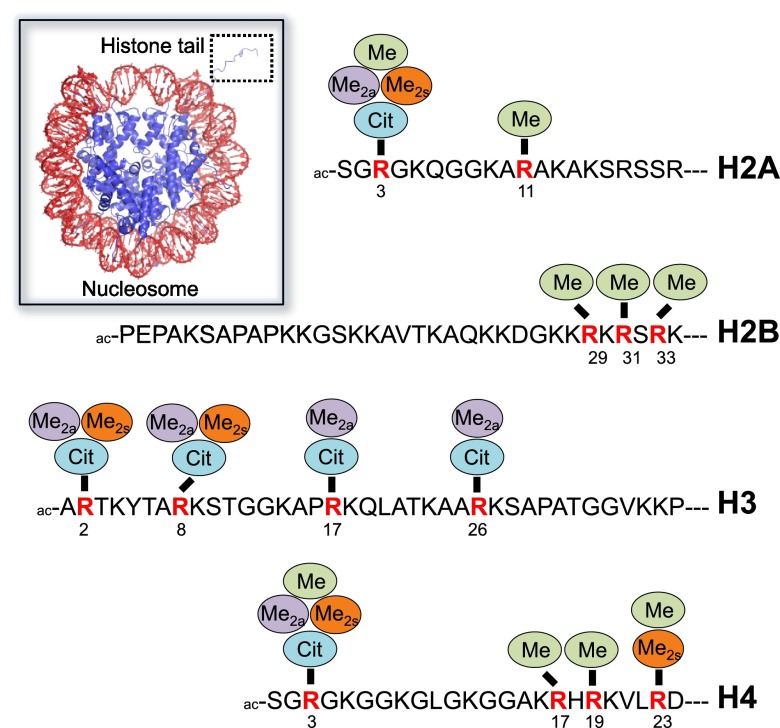
Protein arginine methylation is a common post-translational modification, with many cytoplasmic and nuclear proteins being methylated on arginines.5−7 In fact, arginine methylation impacts numerous cellular pathways, and, when dysregulated, human disease, particularly the development and progression of cancer.8 This modification is mediated by a family of nine protein arginine methyltransferases (PRMTs) that can be grouped into three types based on their arginine methylation products, i.e., monomethylarginine (MMA), asymmetric dimethylarginine (ADMA), and symmetric dimethylarginine (SDMA; for a detailed description, see below). Histone proteins are well-established PRMT substrates for all types of PRMTs.7 The main sites of histone arginine methylation include H2AR3 and R11, H2BR29, R31 and R33, H3R2, R8, R17 and R26, H4R3, R17, R19, and R23 (Figure 1). In addition, there is evidence that arginine methylation affects not only the histone tails but also the histone core, such as in H3R42me2a, where it is implicated in transcriptional activation by weakening the histone–DNA interactions.9 Typically, asymmetric dimethylation of histones has been associated with transcriptional activation while symmetric dimethylation is linked to transcriptional repression.10 Here, we provide a brief overview about individual PRMT members and their influence on histone methylation.
Figure 1.
Sites and types of histone arginine modifications. Arginine methylation and citrullination sites of individual histone N-terminal tails. Abbreviations: Me, monomethylation; Me2a, asymmetric dimethylation; Me2s, symmetric dimethylation; Cit, citrullination. The inset on the left depicts the nucleosome core particle (PDB code: 1AOI); DNA is colored in red, and the histone octamer is highlighted in blue, including a protruding H3-derived histone tail that is otherwise barely defined for the other histone proteins in the crystal structure.
PRMT1 is an essential gene product and is responsible for the majority of ADMA modifications in mammalian cells.11 The PRMT1 deposited methylation mark (H4R3me2a) is associated with transcriptional activation of nuclear receptor regulated genes.12 This coactivator activity is facilitated by the subsequent acetylation of the H4 tails by the histone lysine acetyltransferase p300.12 Notably, the previous acetylation of H4 by p300 prevents the methylation by PRMT1,12 most likely by reducing the positive charges in the remote sequences that are required for efficient PRMT binding (see below). In addition, PRMT1 functions synergistically with CARM1 and p300 as transcriptional coactivators of the tumor suppressor p53.13 Blythe and colleagues showed that during embryonic development, β-catenin recruits PRMT2 to distinct promoters, where it asymmetrically dimethylates H3R8, thereby priming a genetic program for dorsal development.14 The PRMT4/CARM1 enzyme was shown to be responsible for transcriptional activation by the asymmetric dimethylation of H3R17 and H3R26 and to be required for the maintenance of cellular pluripotency15 and also muscle cell differentiation.16 Moreover, upon growth stimulation, PRMT4 is recruited to the Cyclin E1 encoding gene promoter where it methylates histone H3 at R17 and R26 and thereby functions as a transcriptional coactivator and likely accelerates tumor progression.17 In contrast to the ADMA mark deposited by type I PRMTs, the symmetric dimethylation of H4R3 by PRMT5 represents a repressive mark that is required for the formation of DNMT3A-mediated transcriptionally repressive DNA methylation.7,18,19 PRMT5 methylates histones H2A and H4 at R3, respectively, as well as H3 at R2 and R8. Interestingly, the cooperator of PRMT5, COPR5, binds to the amino terminus of histone H4 and thereby recruits PRMT5 to preferentially methylate histone H4 at R3.20 A similar recruitment of PRMT5 to histones is also mediated by the protein MEP50, which interacts with the histone fold of the H3–H4 tetramer and thus promotes the proper positioning of the substrate arginine to the catalytic site.21 Notably, Alinari and colleagues reported that B cells transformed with Epstein–Barr virus (EBV) show high levels of nuclear PRMT5 and a concomitant increase in PRMT5-mediated H4R3me2s and H4R8me2s symmetric dimethylation marks and a decrease of the type I PRMT-dependent asymmetric dimethylation of H4R3me2a.22
Similar to PRMT1, PRMT6 was shown to deposit ADMA marks on H2AR3 and H4R3, and these modifications have been shown to be linked to transcriptional activation.23 However, PRMT6 can also asymmetrically methylate H3R2, and this modification is associated with transcriptional repression by blocking the recruitment of transcriptional activators to trimethylated H3K4.24 PRMT7 mediates the monomethylation of H2AR3 and H4R3 that are both associated with DNA damage repair.25 The presence of these monomethylation marks blocks the transcription of DNA polymerase encoding genes.25
Furthermore, epigenetic regulation of gene expression by arginine methylation goes beyond histone methylation and can also directly impact the activity of diverse transcription factors such as CBP,26 ERα,27 p53,28 and BRCA1,29 as well as RNA polymerase II.30 Although, there is only limited information available regarding the readers of the histone methylarginine marks, it is now well established that several members of the Tudor protein family specifically recognize methylarginine.31 For instance, the Tudor domain containing protein TDRD3 recognizes ADMA modified H3R317 and H4R3 and acts as a transcriptional coactivator.32 It remains to be shown whether other proteins specifically bind to methylarginines or whether competition with other histone modifications is the primary mode of action of these marks.
The Biological Effects of Histone Citrullination
Protein citrullination is mediated by a family of five enzymes called protein arginine deiminases (PADs), which hydrolyze the arginine guanidinium into a urea group. Based on electrostatic considerations, histone arginine citrullination best compares to histone lysine acetylation. In both cases, the positively charged functionalities (guanidinium group in arginine and amino group in lysine) are converted into neutral forms (urea in citrulline and acetamide in acetyllysine). Since histone lysine acetylation is usually associated with an open chromatin structure that can be accessed by RNA polymerases as well as transcription factors and thus typically correlates with gene activation,1 a similar trend was expected for histone arginine citrullination. Indeed, it was recently shown that PAD4 induced citrullination of the linker histone H1 at R54 leads to extensive chromatin decondensation in pluripotent stem cells.33 The loosened chromatin structure allows for the enhanced expression of genes involved in stem cell development and maintenance such as Klf2, Tcl1, Tcfap2c, Kit, and Nanog.33 It was proposed that the observed overexpression of PADs in several cancers might induce a similar chromatin decondensation and thus promote a stem-cell-like state.34
However, more detailed analyses regarding the functional effects of histone citrullination reveals that this mark is associated with both transcriptional repression and activation.35−37 It was suggested that its distinct roles on gene expression can be mediated either by preventing activating arginine-methylation events or by the recruitment of further histone modifying enzymes.36,38 PAD4 was shown to citrullinate histone H3 on arginines 2, 17, and 26, as well as histones H2A and H4 on arginine 3, respectively (Figure 1).35,36,39 Specifically, the citrullination at H3R17 represses the expression of estrogen receptor regulated genes.36 Moreover, PAD4 seems to act as a p53 corepressor by H3 citrullination at the p21 promoter site, thereby blocking downstream gene transcription.40 Histone H3 citrullination at the promoter region of the pro-apoptotic tumor suppressor gene OKL38 was shown to associate with transcriptional repression as well.41 Estrogen-induced stimulation of PAD4 induces citrullination of H3R8 that is linked to transcriptional activation at ERα-dependent promoters by interfering with the H3K9me3 directed binding of HP1α.42 There, it was shown that the citrullination of H3R8 in peripheral blood mononuclear cells is involved in the increased expression of cytokines TNFα and IL8; the overexpression of these cytokines is associated with an uncontrolled immune response and T-cell activation in multiple sclerosis.42 Besides the direct effect of histone citrullination on transcriptional regulation, the citrullination of the histone acetyltransferase p300 was shown to enhance its coactivator ability to stimulate gene transcription indicating a role for nonhistone mediated epigenetic functions of protein citrullination.43
Based on cellular localization studies employing overexpressed PAD enzymes, PAD4 is the only isozyme located in the nucleus and thus has been suggested to be solely responsible for histone H2A, H3, and H4 citrullination.44 Moreover, sequence analysis revealed that only PAD4 contains a canonical nuclear localization signal.45 However, several recent studies revealed that PAD2 can also reside in the nucleus, where it citrullinates histone H3 at arginines R2, R8, R17, and R26.37,46,47 The PAD2 catalyzed citrullination of histone H3 in EGF stimulated mammary epithelial cells has been suggested to modulate the expression of lactation related genes during the estrous cycle.46 In addition, stimulation of estrogen receptor α (ERα)-positive cells with 17 β-estradiol (E2) induced PAD2 dependent citrullination of H3R26 at ERα target genes.37 This modification leads to local chromatin decondensation, thereby increasing the accessibility for ERα to its target sites and consequently transcriptional activation of ERα regulated genes.37,47 Guertin and colleagues further proposed that PAD2 mediated citrullination at H3R26 might be a potential prognostic marker for estrogen receptor positive (ER+) tumor development.47
Apart from the epigenetic consequences of histone citrullination, PAD4-mediated hypercitrullination of histones is critical for the innate immune system and the development of inflammatory diseases such as rheumatoid arthritis (RA) and lupus. Specifically, it was shown that PAD4 is essential for neutrophil extracellular trap (NET) formation,48 also termed NETosis, a specialized pro-inflammatory form of cell death that is involved in the defense against bacterial infection.49 During NETosis, histone hypercitrullination promotes chromatin unraveling on such a massive scale that the chromatin complex is extruded from the cell to form a web-like structure that captures pathogens. These large extracellular structures of decondensed chromatin include hypercitrullinated histone H3, which is a key marker of this form of cell death.48,50 Notably, aberrantly increased NETosis has been recognized as a central player in the pathogenesis of several systemic autoimmune diseases, including lupus and RA, as well as Alzheimer’s disease.51−54 Interestingly, citrullination of H4R3 was also shown to be associated with apoptosis in osteosarcoma cells and suggested to promote apoptotic fragmentation by increasing the accessibility of genomic DNA for DNase attack.55
Structure and Function of Arginine Modifying Enzymes
The Structure and Function of PRMTs
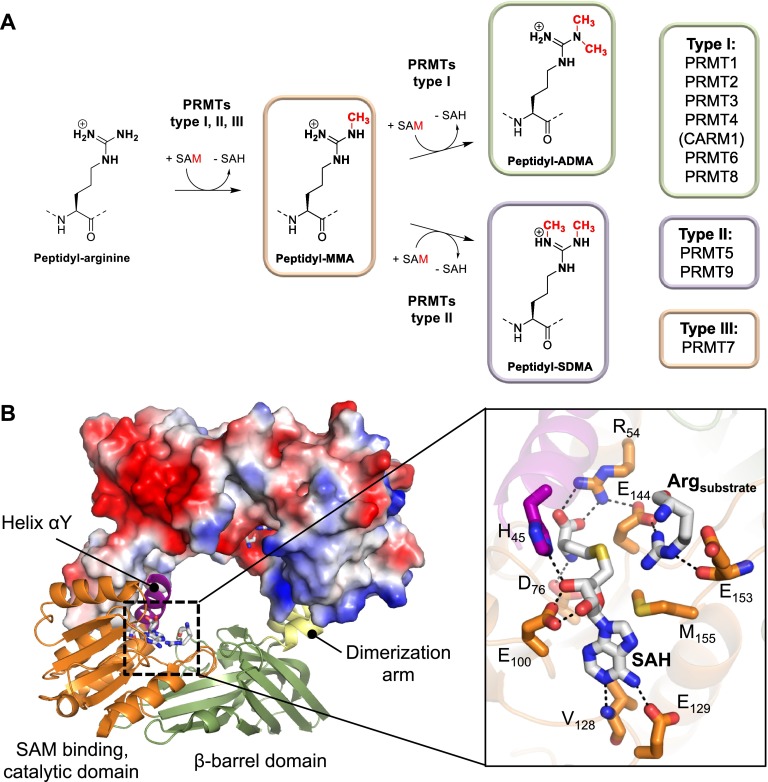
PRMTs catalyze the transfer of a methyl group from a donor molecule, S-adenosylmethionine (SAM), to the terminal guanidino nitrogens of arginine residues. As mentioned above, there are currently nine known PRMTs that can be further classified into three distinct types according to their regiospecificity, i.e., the generation of (i) ADMA, performed by type I enzymes, (ii) SDMA, mediated by type II PRMTs, and (iii) MMA, which is catalyzed by type III enzymes (Figure 2A).4,7 Notably, the mono- or dimethylation of arginine residues does not alter the overall positive charge on the arginine guanidinium group; however, it affects the hydrogen bonding capabilities of this residue.4 The availability of numerous crystal structures of type I and type II PRMTs reveals a conserved architecture wherein two monomers form a head-to-tail homodimer. The dimer interface is stabilized by interactions between the catalytic domain and the helix-turn-helix dimerization arm that protrudes from the C-terminal β-barrel domain (Figure 2B).56−60 Notably, the known structures of all the dimethylation specific PRMTs show a central hole and two opposing active sites that are separated by ∼3 nm. By contrast, the only type III enzyme, PRMT7, lacks the central cavity and consists of a monomer constituting two consecutive PRMT modules that fold into a homodimer-like structure.61,62 The PRMT active site contains a SAM binding pocket that consists of a series of highly conserved sequence motifs that are critical for SAM binding and the structural organization of the active site. In addition, the arginine binding pocket is characterized by two invariant glutamate residues (E144 and E153 in PRMT1), which are located on the double E-loop and are thought to properly align and orient the substrate guanidinium group for nucleophilic attack.57,63
Figure 2.
Structure and mechanism of PRMTs. (A) Schematic representation of the PRMT catalyzed arginine methylation reactions including the different types of PRMTs mediating these enzymatic reactions. The classification of individual PRMT members is shown on the right side. (B) The crystal structure of dimeric PRMT1 bound to SAH and arginine (PDB code: 1OR8). The protomer on top is shown as surface representation colored according to its electrostatic potential (negative electrostatic potentials are highlighted in red, whereas positive electrostatic potentials are illustrated in blue). The inset on the right depicts a close up view of the PRMT1 active site residues implicated in substrate and cofactor binding as well as catalysis.
The structural determinants that dictate product selectivity and thereby steer the formation of ADMA, SDMA, or MMA remain unclear. However, recent biochemical studies and the availability of high resolution crystal structures of several PRMT members reveals striking signature features specific for each PRMT type.56,57,59−61,64 For instance, a conserved methionine residue (M48 in PRMT1), present in all type I and type III enzymes, is replaced by a phenylalanine in the type II enzyme PRMT5 (F327). It was shown that swapping this residue from a methionine to a phenylalanine in PRMT1 led to the slight formation of SDMA.65 The complementary experiment, i.e., replacing the phenylalanine in PRMT5 to a methionine residue, results in the generation of both ADMA and SDMA.64 These experiments highlight the important role that this residue plays in specifying the regiospecificity of the PRMTs. However, the very recent demonstration that PRMT9 acts as a type II enzyme66,67 questions the general importance of this site for product specificity, as this enzyme possesses a methionine at this position.4 More recently, it was proposed that subtle steric constraints, among different PRMT types, may be important for conferring the observed product selectivity.4,61,68 In this respect, two major determinants have been suggested. The first one consists of differences in the THW loop-motif. This motif is only present in type I and type III enzymes, and the critical histidine residue is thought to narrow the substrate arginine binding pocket. In the case of type II enzymes, the histidine is replaced with a serine (in PRMT5) or cysteine (in PRMT9) residue that increases the available volume to fit a methylated nitrogen atom while placing the other nonmethylated guanidine nitrogen close to the SAM methyl transfer site.4 This orientation is compatible with the formation of symmetrically dimethylated arginine residues. The second critical determinant comprises the conserved YF/YXXY motif in the αY helix that is only found in type I PRMTs. There, the two invariant tyrosine hydroxyl groups hydrogen bond to one glutamate residue of the double E-loop, thereby forming a small pocket that allows the accommodation of a methyl group on the attacking nitrogen atom for asymmetric dimethylation.68
Apart from the generation of different methylation products, individual PRMTs also have distinct substrate specificities. Typically, PRMTs prefer to methylate glycine and arginine-rich (GAR) sequences as encountered in numerous RNA binding proteins and the histones H2A and H4.4,6,69,70 A plausible reason for the requirement of glycine residues is their enhanced conformational flexibility and the ability to form β-turn-like structures that are critical for the enzyme–substrate interaction as shown in the crystal structure of PRMT5 bound to a histone H4 peptide.60,71 However, the substrate specificity of PRMT5 is not only restricted to GAR sequences as it can also accommodate arginines within a wider spectrum of sequence contexts.72 Notably, PRMT4 (CARM1) mainly methylates arginine residues present within proline-, glycine-, and methionine-rich (PGM) sequence motifs as found in several splicing factors and histone H3.73 The type III enzyme, PRMT7, specifically monomethylates arginines within an RXR motif encountered in H2B and H4 (Figure 1).74 In addition, substrate recognition in most PRMTs is drastically enhanced by remote sequences that are typically more than 10 residues apart from the arginine methylation site.75−77 The recognition of distal substrate elements is mainly mediated by electrostatic interactions. In this respect, it is interesting to note that all PRMTs have acidic isoeletric points (pI), with typical pI values between 5.0 and 5.3. The only exceptions are PRMT5 (pI, 5.9), PRMT4 (pI, 6.3), and PRMT8 (pI, 6.5), which possess slightly higher pI’s. Notably, PRMTs contain large areas of negative electrostatic potentials located on the β-barrel domain and the catalytic domain (Figure 2B) that are proposed to bind the positively charged residues of the remote substrate regions.56,57,75,78 This effect is further enhanced by the presence of dimeric PRMTs that can act as a negatively charged “sponge” to tether the positively charged substrates close to the active site cavity. This in turn facilitates the processive dimethylation of arginine residues, where the remote sequence elements anchor the substrate, while the arginine methylation site can swing from one active site into the other thereby promoting efficient dimethylation reactions. Evidence for such a mechanism stems from kinetic studies that show a partially or semiprocessive mechanism of dimethylation for PRMT1 and PRMT6.75,79,80 Interestingly, the extent of processivity is influenced by the substrate employed, and thus different patterns of methylation can be obtained by the same PRMT enzyme in a substrate-dependent manner.80 Conversely, the type II enzyme PRMT5 uses a distributive mechanism for the symmetric dimethylation of histone H4.81 This might be a consequence of much slower reaction kinetics of symmetric methylation compared to asymmetric dimethylation reactions and a weaker interaction between the monomethylated substrate and PRMT5.21
The Structure and Function of PADs
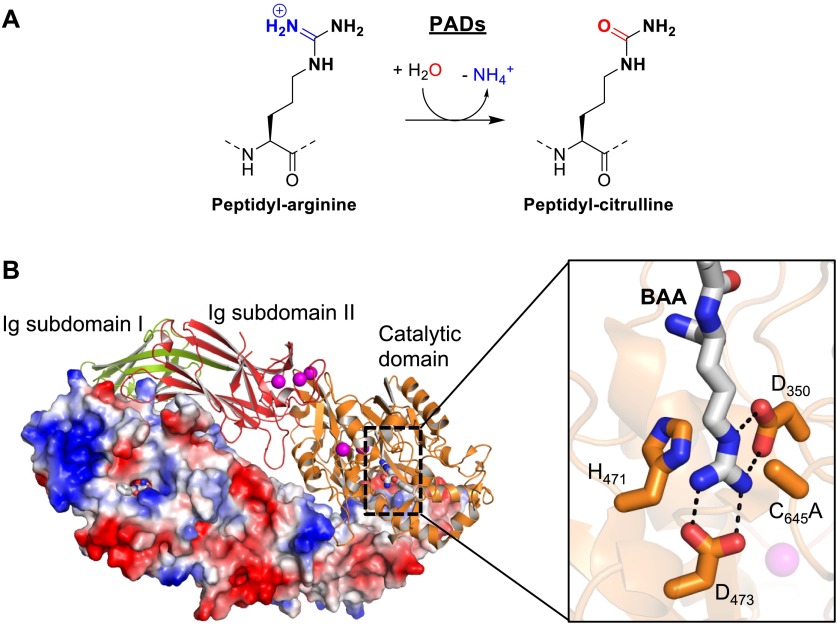
As mentioned above, citrullination is the conversion of arginine into citrulline via the hydrolysis of the guanidinium group to form the neutral urea. In essence, an imine is replaced by a carbonyl; therefore this is termed a deimination reaction. Protein citrullination is mediated by the PAD enzymes (Figure 3A). There are five human PAD isozymes that show distinct tissue distributions and cellular localizations.4,45,82 Four of these enzymes (PADs 1–4) are catalytically active, whereas PAD6 appears to be a pseudoenzyme with no detectable enzymatic activity.83 Interestingly, PAD activity is strictly dependent on the availability of high concentrations of calcium (K0.5,Ca = 130–710 μM) and the enzymes bind to five (PADs 1, 3, and 4) or six (PAD2) calcium ions at distinct sites.84−86 Although, calcium is not directly involved in catalysis, the recent crystal structures of apo and calcium-bound PAD2 show that calcium induces a series of structural rearrangements that are essential for the formation of a catalytically competent active site.86 In particular, the movement of the catalytically important cysteine, C645 in PAD4 and C647 in PAD2, into the active site is triggered by calcium. Interestingly, calcium binding itself occurs in an ordered fashion, and residues involved in calcium interactions are conserved across the PAD family.86
Figure 3.
Structure and mechanism of PADs. (A) Schematic representation of the PAD catalyzed citrullination reaction. (B) The crystal structure of the PAD4 C645A dimer bound to the arginine mimicking substrate BAA (PDB code: 1WDA). The structure on top is colored according to domain organization. The C-terminal catalytic domain (orange) contains the bound substrate (BAA, gray). The bound calcium ions are illustrated as purple spheres. The inset on the right shows the PAD4 active site residues implicated in catalysis. The protomer on the bottom is shown as a surface representation colored according to its electrostatic potential.
On the basis of sequence analyses and structural comparisons, PADs belong to the pentein superfamily that is characterized by the pentameric arrangement of five subdomains around a central hollow, forming an α/β propeller.87,88 This central cavity accommodates the active site within the catalytic domain. Besides the C-terminal catalytic domain, PADs also contain an N-terminal domain that is further composed of two immunoglobulin-like (Ig) subdomains (Figure 3B). Like the PRMTs, PADs exist as homodimeric proteins, with the individual monomers arranged in a head-to-tail fashion such that both active site pockets are located on the same side of the dimer.88 The active site cavity of an individual monomer harbors all the critical residues for catalysis (Figure 3B). These residues include a strictly conserved cysteine (C645 in PAD4) that is important for nucleophilic attack onto the central guanidinium carbon atom of the arginine substrate, as well as two invariant aspartate residues (D350 and D473 in PAD4), which are thought to attract and properly position the arginine guanidinium group via electrostatic interactions. In addition, the active site contains a histidine (H471 in PAD4) that is important for the protonation of the ammonia leaving group and the subsequent activation of an incoming water molecule that ultimately cleaves the thiouronium reaction intermediate.89
The proposed reaction mechanism, including the covalent intermediate, is shared by other members of the guanidino-group modifying pentein superfamily, such as dimethylarginine dimethylaminohydrolase (DDAH) and arginine deiminases (ADI).90,91 However, in contrast to these proteins, PADs modify peptidyl-arginine residues.84 As a result, PADs have evolved a more accessible active site and contain several residues that specifically recognize the substrate peptide backbone.92 For example, residue R374 is critical for the formation of a bidendate hydrogen bond with two substrate peptide carbonyls. The structures of PAD4 bound to histone peptides reveal that substrate recognition is mainly mediated via interactions with the substrate peptide backbone.92 As such, there is limited sequence specificity regarding PAD4 substrate selection. In addition, and in contrast to PRMTs, substrate recognition by the PADs does not depend on long-range interactions originating from remote sequences in the substrate.84 Despite limited sequence specificity, it was shown that PAD4 bound peptide substrates adopt a β-turn-like conformation, similar to PRMT bound substrates.92 Therefore, the propensity of peptide substrates to adopt such kinked conformations might dictate substrate selection in PADs. Moreover, apart from structural constraints, PAD substrate specificity may be regulated by the accessibility of arginine residues in chromatin structures, through interaction of a PAD with other proteins and by cross-talk with distinct PTMs, such as arginine methylation. In this regard, it was claimed that PADs can also act on methylated arginine residues.35 However, several lines of evidence questioned the physiological relevance of this activity and even indicated that arginine methylation prevents arginines from being PAD substrates.36,84,93,94 Thus, citrullination and arginine methylation are now considered to be antagonistic modifications.4,95
Inhibitors of Arginine Modifying Enzymes
PRMT Inhibitors
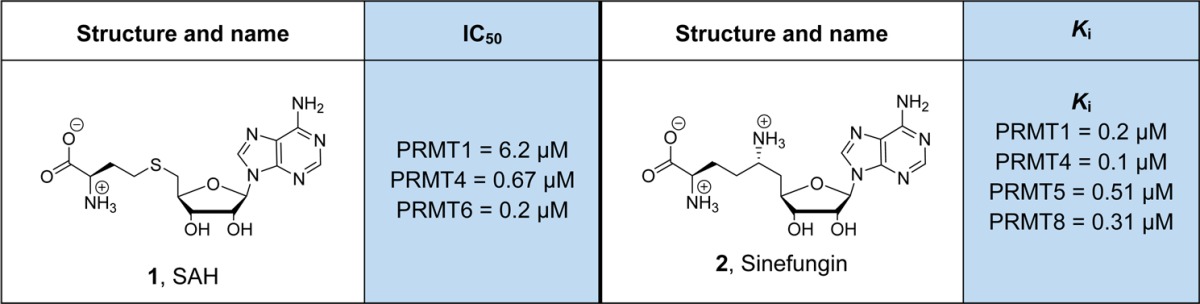
In recent years, a diverse set of PRMT inhibitors have been discovered. The most common and general PRMT inhibitors are S-adenosyl-L-homocysteine (SAH, 1) and sinefungin (2). These molecules are structurally related to SAM, and numerous biochemical and crystallographic data confirm that they are SAM-competitive inhibitors that block PRMT activity in the low micro- to nanomolar range (Table 1).96 SAH is the reaction product of SAM-dependent methyl transfer reactions. Normally, it is rapidly degraded by SAH hydrolase; however, its activity can be blocked by adenosine dialdehyde to artificially increase the endogenous level of SAH.97 This approach is frequently employed to study the global effects of inhibiting cellular methyltransferases including the PRMTs. The natural product sinefungin was originally isolated from Streptomyces species98 and was shown to act as a pan-methyltransferase inhibitor similar to SAH.99
Table 1. General Methyltransferase Inhibitors.
One of the first high-throughput screens to identify small molecule inhibitors of PRMT1 was performed by the Bedford group.100 These screening efforts led to the identification of several compounds termed arginine methyltransferase inhibitor (AMI). One of these small molecules, AMI-1, was shown to be cell-permeable and to inhibit cellular PRMT1 in a concentration dependent manner.100 However, most of these small molecules were nonspecific and also inhibited protein lysine methyltransferases. In addition, subsequent studies revealed that AMI-1 actually does not bind to PRMTs but rather interacts with the histone substrates via electrostatic interactions, thereby preventing substrate access.101
To obtain PRMT specific inhibitors, Spannhoff et al. employed a target-based virtual screening approach.102 The identified compounds include the diamidine stilbamidine (3; Table 2) and the dapsone derivative allantodapsone (4) that both act as competitive inhibitors of the protein substrate. Moreover, these compounds were shown to block PRMT1 methylation of H4R3 in cellular assays while having minimal inhibitory effects on lysine methylation of H3K4.102 However, they show limited potency and PRMT isozyme specificity. Recently, an improved diamidine compound, furamidine (5), was described.103 This small molecule is selective for PRMT1 with a ∼18-fold lower IC50 for PRMT1 compared to PRMT5. Based on molecular modeling studies, the positively charged amidinium in 5 was proposed to bind to the substrate binding site, occupying the position of the substrate guanidinium group. In addition, furamidine was shown to be cell permeable, resulting in inhibition of cellular PRMT1 and a decrease in cell proliferation in leukemia cell lines.103 However, care has to be taken regarding the utilization of diamidine derivatives as PRMT-specific inhibitors because they are also reported to bind DNA with high affinity;104 as such we do not recommend their use as PRMT inhibitors.
Table 2. Inhibitors of PRMTs.
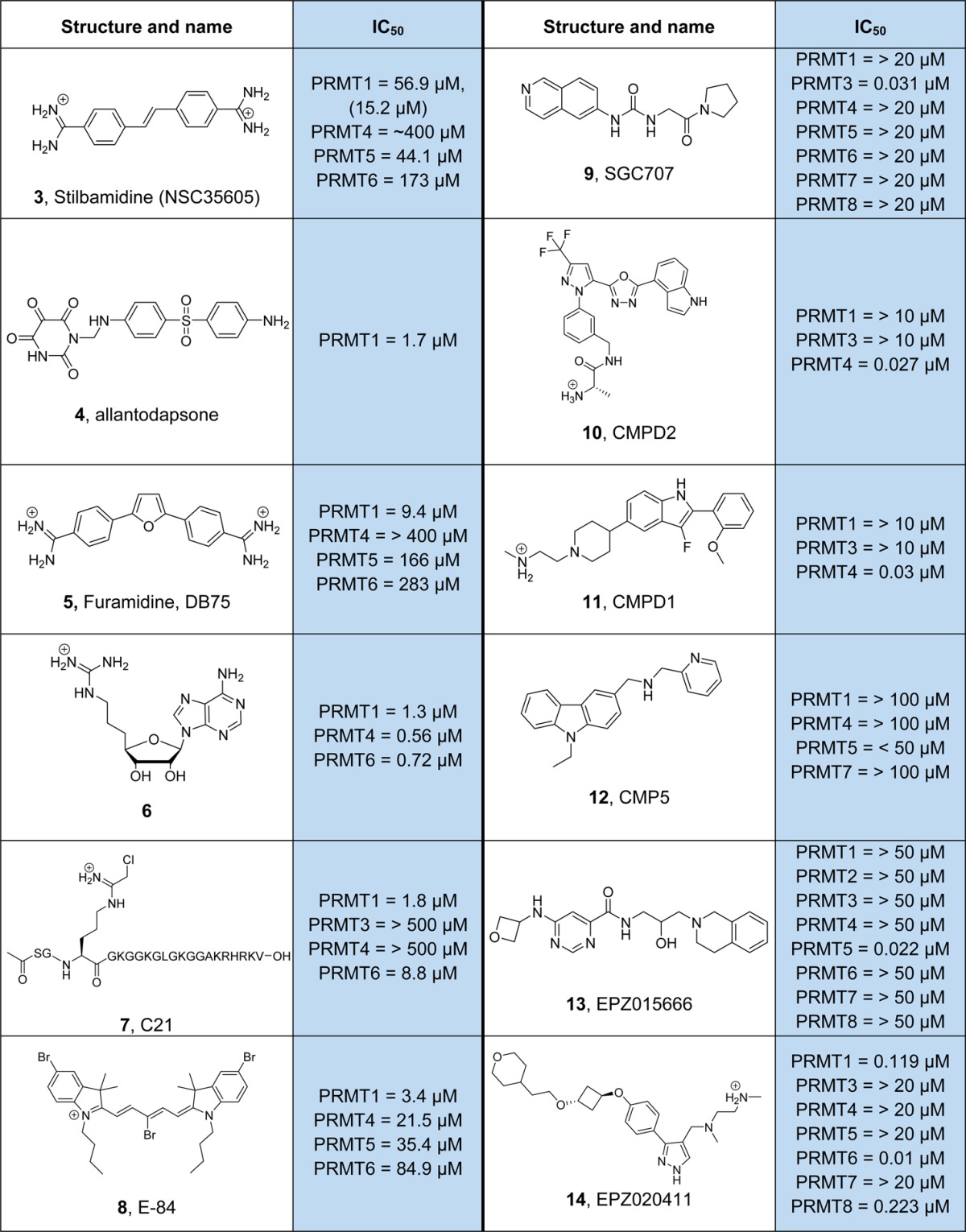
There are several studies on PRMT inhibitor development strategies aimed at substrate, cofactor, and in particular partial bisubstrate analogs.105−110 Although these compounds showed decent inhibition, they are usually nonspecific with regard to different PRMT isozymes. For example, one of the most potent compounds (6), consisting of the SAM adenosine moiety linked to a guanidinium group, shows an IC50 of 560 nM for PRMT4 but also effectively blocks the activity of PRMT1 and PRMT5.110 An exception is the peptide-based inhibitor C21 (7) that is composed of the first 21 residues of histone H4 and contains a chloroacetamidine warhead instead of the substrate arginine guanidinium group.106 This compound acts as an irreversible inhibitor wherein the chloroacetamidine group reacts with a hyperreactive cysteine, C101 present in the PRMT1 active site, to form a stable thioether bond.111 Notably, the peptide inhibitor C21 is >100-fold more potent than the chloroacetamidine warhead containing compound Cl-amidine (19, Table 3, discussed below).106 These data further highlight the requirement of remote sequences, distal from the arginine substrate site for efficient inhibitor/substrate peptide binding. C21 shows high preference for PRMT1 over PRMT3 and PRMT4 with an IC50 of 1.8 μM for PRMT1; however, it can also block the activity of PRMT6 (IC50, 8.8 μM). In addition, C21 was adapted as a chemical probe by incorporating either fluorescein or biotin reporter tags to monitor and isolate active PRMT1.112 On the basis of a previously identified cyanine scaffold,113 Hu and colleagues developed a PRMT1 inhibitor (8), denoted as E-84.114 This small molecule inhibitor blocks PRMT1 with an IC50 of 3.4 μM and shows 6-fold selectivity over PRMT4 and over 10- and 25-fold selectivity over PRMT5 and PRMT8. On the basis of molecular docking studies, it was proposed that E-84 binds to the SAM binding pocket as well as the arginine substrate binding site. In addition, this compound slightly reduced the level of asymmetrically dimethylated arginine in leukemia cells and displays cytotoxic activity toward these cells.114 However, experimental evidence of target engagement is still lacking, and it thus remains to be shown if this compound directly interacts with PRMTs.
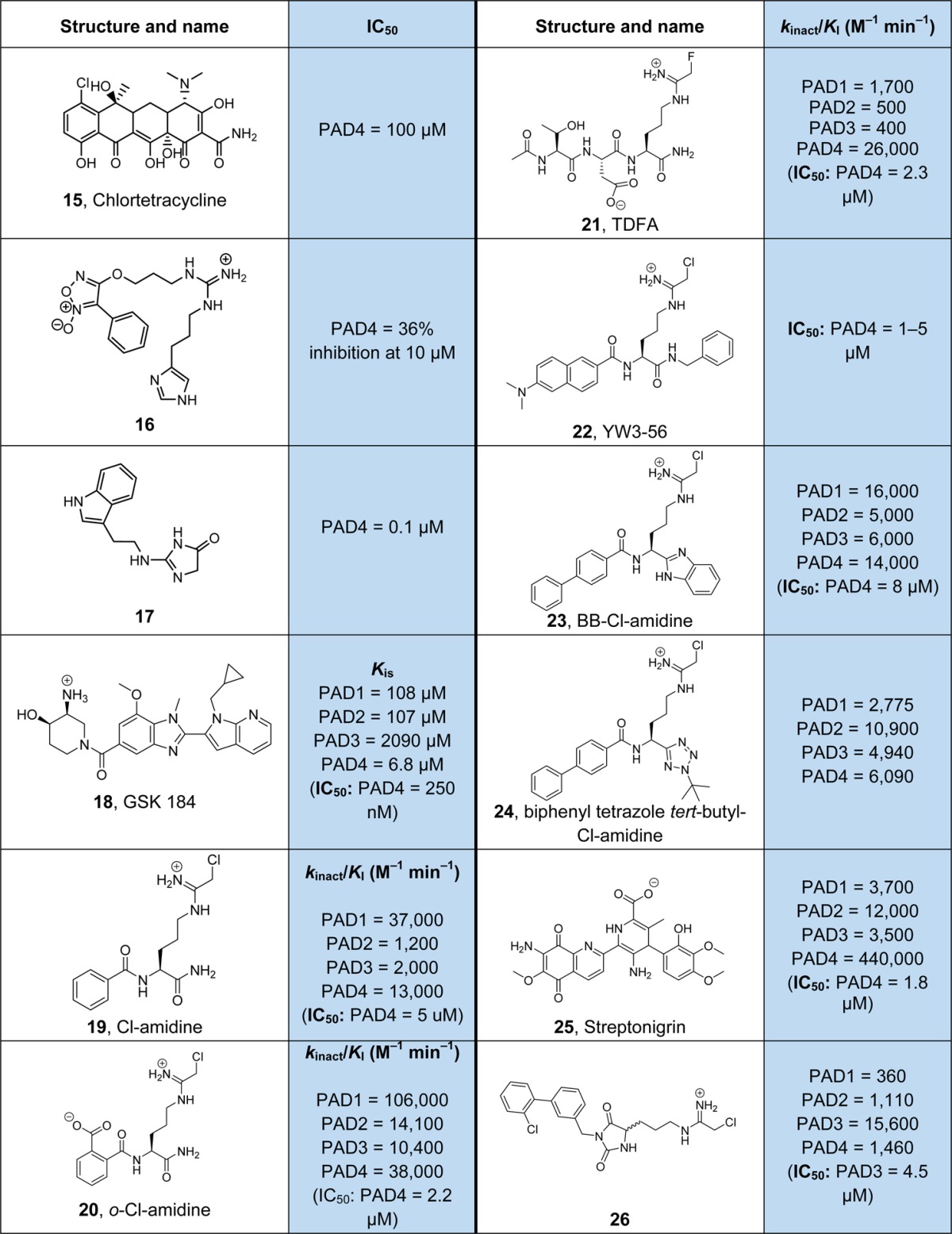
Table 3. Inhibitors of PADs.
In recent years, several highly potent isozyme-specific and more drug-like PRMT inhibitors have been developed. One of these compounds, 9, represents an optimized hit derived from a HTS approach that selectively targets PRMT3.115,116 This compound is an allosteric inhibitor that does not bind the active site pocket. Structural analysis revealed that 9 binds at the PRMT3 homodimerization interface and prevents the proper orientation of helix αY for catalysis.116 In addition, it was shown that 9 is active in cellular assays and efficiently blocks the PRMT3-dependent dimethylation of H4R3 with nanomolar efficacy.116 Sack and colleagues described the identification of two PRMT4 (CARM1) selective inhibitors, the pyrazole derivative 10 and the indole derivative 11.117 Both of these compounds were derived after optimization of compounds identified from initial HTS screens.118 Detailed structural investigations showed that these small molecule inhibitors bind to the arginine-substrate binding cavity of PRMT4 and require bound SAH.117 In addition to PRMT3 and PRMT4, specific inhibitors of PRMT5 have also been identified. The carbazole ring containing CMP5 (12) was shown to block PRMT5 activity but did not inhibit PRMT1, PRMT4, or PRMT7.22 This compound was predicted to occupy the SAM binding pocket and form π–π stacking interactions with the signature phenylalanine (F327) residue implicated in product selectivity of PRMT5. Cellular studies indicate that treatment of transformed B-cells, expressing high levels of PRMT5, with CMP5 blocks Epstein–Barr virus driven B-lymphocyte transformation while leaving normal B cells unaffected.22 Another highly selective and even more potent PRMT5 inhibitor was recently developed by Chan-Penebre et al.(119) This small molecule, EPZ015666 (13), is an optimized version of a compound derived from a library of 370 000 compounds. Inhibition studies with PRMT5 revealed that EPZ015666 is competitive with the peptide substrate, and this was further confirmed by structural analyses.119 Similar to PRMT4 inhibitors, it was shown that the binding affinity of EPZ015666 for PRMT5 was greatly increased by SAM binding. Interestingly, EPZ015666 also engages in π–π stacking interaction, via its critical tetrahydroisoquinoline moiety, with F327 of PRMT5 as predicted for compound CMP5. These data highlight the potential for developing selective PRMT inhibitors by harnessing isozyme specific differences in the active site such as the characteristic phenylalanine residue, F327, of PRMT5. On the basis of functional studies, EPZ015666 reduces the level of global symmetric dimethylation in the mantle cell lymphoma (MCL) cell line Z-138. Moreover, EPZ015666 exerts antiproliferative effects in numerous MCL cell lines at nanomolar concentrations, and oral administration of this compound induces antitumor activity in different MCL xenograft mouse models.119 Very recently, a PRMT6 specific inhibitor, EPZ020411 (14), has been reported.120 This compound shows high potency, IC50 = 10 nM, for PRMT6, and good selectivity over PRMT1 (12-fold) and PRMT8 (22-fold) and excellent selectivity (>100-fold) compared to PRMT3, PRMT4, PRMT5, and PRMT7. The crystal structure of EPZ020411 bound to a ternary PRMT6-SAH complex revealed that the inhibitor binds into the arginine substrate site via its diamine side chain and the pyrazole core structure.120
PAD Inhibitors
Since dysregulated citrullination levels have been implicated in numerous diseases including rheumatoid arthritis, several autoimmune diseases, as well as cancer, PADs represent a promising target for pharmaceutical intervention.121 Thus, several small molecules have been developed that block the activity of this enzyme class. Because of the involvement of PADs in the development of RA, a small panel of disease modifying antirheumatic drugs were tested for the presence of potential PAD inhibitors.122 Interestingly, some of these compounds showed modest PAD inhibition in the low milli- to micromolar range. One of the most potent inhibitors was the tetracycline derivative minocycline. Further investigations, using different tetracycline derivatives, revealed chlortetracycline (15) as a modest PAD4 inhibitor with an IC50 of ∼100 μM (Table 3).122 However, due to the weak inhibitory activity, it is unlikely that these compounds exert inhibition of cellular PAD4. Since the arginine guanidinium moiety is the major contributor for efficient PAD–substrate interactions, several guanidinium group containing compounds have also been evaluated. In this respect, the derivatized guanidine compound 16 displays 36% inhibition of PAD4 activity at 10 μM.123 Moreover, the acylguanidine derivative 17 was shown to block PAD3 with an IC50 of 100 nM.124 However, given that acylguanidines have a low pKa value, typically several orders of magnitude lower than that of guanidines, and are expected to be poorly suited as arginine substrate mimicking inhibitors, the strong inhibitory activity of 17 needs further validation. Although it was hypothesized that these compounds act as competitive inhibitors, their detailed mode of inhibition has not been studied. The limited potency for these reversible PAD inhibitors likely relates to the small active site pocket that only accommodates the side chain of an arginine residue. Therefore, the recent discovery of a distinct binding site occupied by compounds GSK 199 and its more potent derivative GSK 484 (18) opens a promising approach to develop high affinity reversible PAD inhibitors.125 Specifically, structural studies revealed that these compounds bind to the solvent exchange channel in PADs and induce large conformational changes around the active site.125 In addition, compound 18 is a PAD4 specific inhibitor that displays at least 35-fold selectivity for PAD4 over the other PAD isozymes. Interestingly, 18 preferentially binds a calcium-deficient form of PAD4 that lacks calcium in the Ca2 binding site and has an IC50 value in the nanomolar range in the absence of calcium, whereas calcium binding decreases its potency by at least 5-fold.125 These data further highlight the dynamic nature of the PAD4 active site that fluctuates between different conformations in a calcium dependent manner. Moreover, it further indicates that these conformational states (calcium-deficient, inactive and calcium-bound, active) can be targeted by distinct inhibitors and pave the way for developing conformer-specific PAD inhibitors. Inhibitors targeting the resting apoenzyme are of particular interest as they stabilize the inactive conformation that likely represents the major form inside the cell. By shifting the equilibrium toward the inactive conformation, these inhibitors do not directly compete with endogenous substrates and might be better suited to prevent burst activation of PADs such as during NETosis, triggered by massive calcium influx. In this respect, pretreatment of stimulated mouse neutrophils with 10 μM GSK484 markedly diminished hypercitrullination of histone H3 and NET formation, thus highlighting the biological activity of this compound.125 Apart from these reversible inhibitors, substantial progress has been made in developing irreversible PAD inhibitors. One of the most widely used and best characterized irreversible PAD inhibitors is Cl-amidine (19), which blocks PAD4 activity with an IC50 of 5 μM.126 This compound contains a reactive haloacetamidine warhead, as in the PRMT inhibitor C21 (7). The positively charged electrophilic group acts as a mimic of the substrate guanidinium group and covalently attaches to the active site cysteine residue (C645 in PAD4) forming a stable thioether bond.126,127 By varying the side chain length in a Cl-amidine derivative, it was confirmed that a three-carbon linker between the chloroacetamidine moiety and the amino acid backbone is most effective.128 Notably, and in contrast to GSK484, haloacetamidine inhibitors require the high-calcium bound form of PAD4, thus confirming their substrate mimicking mode of inhibition and preference for the calcium primed conformation of PAD4.126 Cl-amidine has been used successfully in several preclinical models of RA,129 lupus,130 colitis,131 and even breast cancer,132 by effectively reducing aberrant hypercitrullination levels. To improve the potency and specificity of Cl-amidine, several derivatives were developed. For instance, the ortho-carboxylate containing Cl-amidine derivative 20 has a more than 2-fold higher inhibitory activity compared to 19 but a similar PAD isozyme selectivity profile.133 On the basis of a peptide library approach, the fluoroacetamidine containing compound TDFA (21) has been identified.134 The tripeptide TDFA shows high selectivity for PAD4 compared to the other PADs with more than 15-fold preference for PAD4 inhibition over PAD1. Interestingly, both, 20 and 21, contain a negatively charged carboxylate group that occupies a similar position in PAD4-inhibitor crystal structures and is involved in direct or water mediated interactions with the side chain amide of Q346 that might explain the increased potency of these compounds over Cl-amidine.4,133,134 Since Cl-amidine is a polar and highly water-soluble compound and thus exhibits poor bioavailability, several attempts to increase its hydrophobicity have been undertaken. For example, Wang et al. attached a diverse set of hydrophobic groups to the amide backbone of Cl-amidine.135 One of the most potent compounds of this series, YW3–56 (22), contains an N-terminal dimethyl-naphthylamine and C-terminal methylbenzene moiety. Yw3–56 shows similar rates of inhibition compared to Cl-amidine, but its antiproliferative activity toward mouse sarcoma cells was increased by a factor of 50.135 The same trend of increased cellular activity, bioavailability, and in vivo half-life was observed with BB-Cl-amidine (23) that possesses an N-terminal biphenyl and a C-terminal benzimidazole group.136 Notably, using the PAD4 expressing U2OS osteosarcoma cell line, the EC50 value of BB-Cl-amidine has been demonstrated to be 8.8 μM compared to >200 μM for Cl-amidine.136 Biphenyl-tetrazole-tert-butyl-Cl-amidine (24), a further apolar derivative of Cl-amidine, preferentially inhibits PAD2.137 This compound harbors a C-terminal tert-butyl-tetrazole group that was shown to increase the specificity toward PAD2 over other PAD isozymes. Employing a fluorophore labeled Cl-amidine derivative in a fluorescence polarization HTS assay, Knuckley et al. described the identification of streptonigrin (25) as a potent and very selective PAD4 inhibitor.138 Streptonigrin acts as an irreversible inactivator of PAD4 and was shown to block PAD4 activity in cellular studies; however, it also binds to several off targets thereby limiting its physiological utility.138,139 Recently, Jamali and colleagues employed a substrate-fragment discovery approach to identify a PAD3 isozyme selective inhibitor (26) that shows >10 selectivity for PAD3 over other PAD enzymes.140 This compound contains a chloroacetamidine warhead for reactivity as well as a biphenyl-hydantoin group for selectivity.
Conclusions and Perspective
In the past decade, there has been tremendous progress in our understanding of the epigenetic influences of histone arginine methylation and citrullination; however, much remains to be learned about the chemistry and biology of these fascinating modifications. In future studies, it will be interesting to test whether protein arginine methylation and citrullination are reversible modifications. Although it was proposed that the Jmjd6 protein acts as an arginine demethylase,141 subsequent studies showed that it does not remove the methyl mark from methylated arginine residues and actually acts as a lysine hydroxylase.142,143 Nonetheless, the dynamic appearance and disappearance of citrullination and arginine methylation marks on histones hints at the existence of enzymes that might reverse these modifications.36,144 In this respect, it will also be of great importance to identify proteins that act as “readers” to recognize the modified arginine residues such as the tudor domain-containing proteins that were shown to bind methylated arginine residues.31 Moreover, it will be interesting to evaluate the scope and impact of other enzymatic and nonenzymatic arginine modifications such as phosphorylation,145−147 ADPribosylation,148,149 carbonylation,150 and the formation of arginine derived advanced glycation end products151 on epigenetic regulation. The goal is to combine this information with other histone PTMs to generate a map of individual histone modifications and to delineate the underlying crosstalk to ultimately decipher the “language” of histone PTMs.
Acknowledgments
We apologize to those researchers whose original work could not be cited due to space limitations. This work was supported in part by National Institutes of Health grants GM079357, GM110394, and CA151304 (to P.R.T.).
Glossary
Keywords
- Epigenetics
describes the heritable alterations in states of gene expression apart and beyond alterations in the genomic DNA sequence. It comprises DNA and/or histone modifications
- Histones
small, highly basic proteins that combine into octamers. These histone octamers tightly bind to genomic DNA forming nucleosome particles that represent the building blocks of higher chromatin structures
- Posttranslational modification (PTM)
protein alterations that affect the mature protein after translation. Typically, these modifications include but are not limited to the covalent addition of chemical groups to the amino acid side chains as well as cleavage of existing bonds
- Protein arginine methylation
a PTM in which one or two methyl groups are added to an arginine residue on the nitrogen(s) of its guanidine side chain. There are three types of arginine methylation: monomethylation, symmetric dimethylation, and asymmetric dimethylation
- Protein arginine methyltransferases (PRMTs)
enzymes that methylate arginine residues. They can be subdivided into three types: type I, yielding asymmetrically dimethylated arginine (ADMA); type II, forming symmetrically dimethylated arginine (SDMA); and type III enzymes, i.e., monomethyltransferases, generating monomethyl arginine (MMA)
- S-adenosyl-l-methioline (SAM)
a cofactor that serves as a methyl donor for PRMT catalyzed methylation reactions
- Protein citrullination
also known as arginine deimination; defines the post-translational conversion of arginine to citrulline. Specifically, it is characterized by the replacement of an imine by a carbonyl group, thereby forming a neutral urea
- Protein arginine deiminases (PADs)
an enzyme family composed of five members in humans that specifically deiminates peptidyl arginine residues yielding citrulline
Author Contributions
The manuscript was written through contributions of both authors. Both authors have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): The authors declare competing financial interests. P.R.T. is a cofounder and consultant to Padlock Therapeutics.
References
- Jenuwein T.; Allis C. D. (2001) Translating the histone code. Science 293, 1074–1080. 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Luger K.; Mader A. W.; Richmond R. K.; Sargent D. F.; Richmond T. J. (1997) Crystal structure of the nucleosome core particle at 2.8 angstrom resolution. Nature 389, 251–260. 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Slade D. J.; Subramanian V.; Fuhrmann J.; Thompson P. R. (2014) Chemical and Biological Methods to Detect Post-Translational Modifications of Arginine. Biopolymers 101, 133–143. 10.1002/bip.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann J.; Clancy K. W.; Thompson P. R. (2015) Chemical biology of protein arginine modifications in epigenetic regulation. Chem. Rev. 115, 5413–5461. 10.1021/acs.chemrev.5b00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert F. M.; Cote J.; Boulanger M. C.; Richard S. (2003) A proteomic analysis of arginine-methylated protein complexes. Mol. Cell. Proteomics 2, 1319–1330. 10.1074/mcp.M300088-MCP200. [DOI] [PubMed] [Google Scholar]
- Bedford M. T.; Richard S. (2005) Arginine methylation: An emerging regulator of protein function. Mol. Cell 18, 263–272. 10.1016/j.molcel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Bedford M. T.; Clarke S. G. (2009) Protein Arginine Methylation in Mammals: Who, What, and Why. Mol. Cell 33, 1–13. 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Z.; Bedford M. T. (2013) Protein arginine methyltransferases and cancer. Nat. Rev. Cancer 13, 37–50. 10.1038/nrc3409. [DOI] [PubMed] [Google Scholar]
- Casadio F.; Lu X. D.; Pollock S. B.; LeRoy G.; Garcia B. A.; Muir T. W.; Roeder R. G.; Allis C. D. (2013) H3R42me2a is a histone modification with positive transcriptional effects. Proc. Natl. Acad. Sci. U. S. A. 110, 14894–14899. 10.1073/pnas.1312925110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo A.; Bedford M. T. (2011) Histone arginine methylation. FEBS Lett. 585, 2024–2031. 10.1016/j.febslet.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.; Frankel A.; Cook R. J.; Kim S.; Paik W. K.; Williams K. R.; Clarke S.; Herschman H. R. (2000) PRMT1 is the predominant type I protein arginine methyltransferase in mammalian cells. J. Biol. Chem. 275, 7723–7730. 10.1074/jbc.275.11.7723. [DOI] [PubMed] [Google Scholar]
- Wang H. B.; Huang Z. Q.; Xia L.; Feng Q.; Erdjument-Bromage H.; Strahl B. D.; Briggs S. D.; Allis C. D.; Wong J. M.; Tempst P.; Zhang Y. (2001) Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. Science 293, 853–857. 10.1126/science.1060781. [DOI] [PubMed] [Google Scholar]
- An W.; Kim J.; Roeder R. G. (2004) Ordered cooperative functions of PRMT1, p300, and CARM1 in transcriptional activation by p53. Cell 117, 735–748. 10.1016/j.cell.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Blythe S. A.; Cha S. W.; Tadjuidje E.; Heasman J.; Klein P. S. (2010) beta-Catenin Primes Organizer Gene Expression by Recruiting a Histone H3 Arginine 8 Methyltransferase, Prmt2. Dev. Cell 19, 220–231. 10.1016/j.devcel.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q.; Bruce A. W.; Jedrusik A.; Ellis P. D.; Andrews R. M.; Langford C. F.; Glover D. M.; Zernicka-Goetz M. (2009) CARM1 is Required in Embryonic Stem Cells to Maintain Pluripotency and Resist Differentiation. Stem Cells 27, 2637–2645. 10.1002/stem.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. L.; Loffler K. A.; Chen D. G.; Stallcup M. R.; Muscat G. E. O. (2002) The coactivator-associated arginine methyltransferase is necessary for muscle differentiation - CARM1 coactivates myocyte enhancer factor-2. J. Biol. Chem. 277, 4324–4333. 10.1074/jbc.M109835200. [DOI] [PubMed] [Google Scholar]
- El Messaoudi S.; Fabbrizio E.; Rodriguez C.; Chuchana P.; Fauquier L.; Cheng D. H.; Theillet C.; Vandel L.; Bedford M. T.; Sardet C. (2006) Coactivator-associated arginine methyltransferase 1 (CARM1) is a positive regulator of the Cyclin E1 gene. Proc. Natl. Acad. Sci. U. S. A. 103, 13351–13356. 10.1073/pnas.0605692103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S.; Yun R.; Datta A.; Lacomis L.; Erdjument-Bromage H.; Kumar J.; Tempst P.; Sif S. (2003) mSin3A/histone deacetylase 2- and PRMT5-containing Brg1 complex is involved in transcriptional repression of the Myc target gene cad. Mol. Cell. Biol. 23, 7475–7487. 10.1128/MCB.23.21.7475-7487.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.; Hoang S.; Mayo M. W.; Bekiranov S. (2010) Application of machine learning methods to histone methylation ChIP-Seq data reveals H4R3me2 globally represses gene expression. BMC Bioinf 11, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix M.; El Messaoudi S.; Rodier G.; Le Cam A.; Sardet C.; Fabbrizio E. (2008) The histone-binding protein COPR5 is required for nuclear functions of the protein arginine methyltransferase PRMT5. EMBO Rep. 9, 452–458. 10.1038/embor.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos E. S.; Wilczek C.; Onikubo T.; Bonanno J. B.; Jansong J.; Reimer U.; Shechter D. (2015) Histone H2A and H4 N-Terminal Tails are Positioned by the MEP50 WD-Repeat Protein for Efficient Methylation by the PRMT5 Arginine Methyltransferase. J. Biol. Chem. 290, 9674–9689. 10.1074/jbc.M115.636894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alinari L.; Mahasenan K. V.; Yan F.; Karkhanis V.; Chung J. H.; Smith E. M.; Quinion C.; Smith P. L.; Kim L.; Patton J. T.; Lapalombella R.; Yu B.; Wu Y.; Roy S.; De Leo A.; Pileri S.; Agostinelli C.; Ayers L.; Bradner J. E.; Chen-Kiang S.; Elemento O.; Motiwala T.; Majumder S.; Byrd J. C.; Jacob S.; Sif S.; Li C.; Baiocchi R. A. (2015) Selective inhibition of protein arginine methyltransferase 5 blocks initiation and maintenance of B-cell transformation. Blood 125, 2530–2543. 10.1182/blood-2014-12-619783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M. J.; Tang Y. H.; Dowhan D. H. (2010) Protein arginine methyltransferase 6 regulates multiple aspects of gene expression. Nucleic Acids Res. 38, 2201–2216. 10.1093/nar/gkp1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyllus D.; Stein C.; Schnabel K.; Schiltz E.; Imhof A.; Dou Y. L.; Hsieh J.; Bauer U. M. (2007) PRMT6-mediated methylation of R2 in histone H3 antagonizes H3K4 trimethylation. Genes Dev. 21, 3369–3380. 10.1101/gad.447007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis V.; Wang L.; Tae S.; Hu Y. J.; Imbalzano A. N.; Sif S. (2012) Protein Arginine Methyltransferase 7 Regulates Cellular Response to DNA Damage by Methylating Promoter Histones H2A and H4 of the Polymerase delta Catalytic Subunit Gene, POLD1. J. Biol. Chem. 287, 29801–29814. 10.1074/jbc.M112.378281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevillard-Briet M.; Trouche D.; Vandel L. (2002) Control of CBP co-activating activity by arginine methylation. EMBO J. 21, 5457–5466. 10.1093/emboj/cdf548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Romancer M.; Treilleux I.; Leconte N.; Robin-Lespinasse Y.; Sentis S.; Bouchekioua-Bouzaghou K.; Goddard S.; Gobert-Gosse S.; Corbo L. (2008) Regulation of estrogen rapid signaling through arginine methylation by PRMT1. Mol. Cell 31, 212–221. 10.1016/j.molcel.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Jansson M.; Durant S. T.; Cho E. C.; Sheahan S.; Edelmann M.; Kessler B.; La Thangue N. B. (2008) Arginine methylation regulates the p53 response. Nat. Cell Biol. 10, 1431–U1122. 10.1038/ncb1802. [DOI] [PubMed] [Google Scholar]
- Guendel I.; Carpio L.; Pedati C.; Schwartz A.; Teal C.; Kashanchi F.; Kehn-Hall K. (2010) Methylation of the Tumor Suppressor Protein, BRCA1, Influences Its Transcriptional Cofactor Function. PLoS One 5, e11379. 10.1371/journal.pone.0011379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims R. J.; Rojas L. A.; Beck D.; Bonasio R.; Schuller R.; Drury W. J.; Eick D.; Reinberg D. (2011) The C-Terminal Domain of RNA Polymerase II Is Modified by Site-Specific Methylation. Science 332, 99–103. 10.1126/science.1202663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Nott T. J.; Jin J.; Pawson T. (2011) Deciphering arginine methylation: Tudor tells the tale. Nat. Rev. Mol. Cell Biol. 12, 629–642. 10.1038/nrm3185. [DOI] [PubMed] [Google Scholar]
- Yang Y. Z.; Lu Y.; Espejo A.; Wu J. C.; Xu W.; Liang S. D.; Bedford M. T. (2010) TDRD3 Is an Effector Molecule for Arginine-Methylated Histone Marks. Mol. Cell 40, 1016–1023. 10.1016/j.molcel.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophorou M. A.; Castelo-Branco G.; Halley-Stott R. P.; Oliveira C. S.; Loos R.; Radzisheuskaya A.; Mowen K. A.; Bertone P.; Silva J. C. R.; Zernicka-Goetz M.; Nielsen M. L.; Gurdon J. B.; Kouzarides T. (2014) Citrullination regulates pluripotency and histone H1 binding to chromatin. Nature 507, 104–108. 10.1038/nature12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade D. J.; Subramanian V.; Thompson P. R. (2014) Pluripotency: citrullination unravels stem cells. Nat. Chem. Biol. 10, 327–328. 10.1038/nchembio.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Wysocka J.; Sayegh J.; Lee Y. H.; Perlin J. R.; Leonelli L.; Sonbuchner L. S.; McDonald C. H.; Cook R. G.; Dou Y.; Roeder R. G.; Clarke S.; Stallcup M. R.; Allis C. D.; Coonrod S. A. (2004) Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 306, 279–283. 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- Cuthbert G. L.; Daujat S.; Snowden A. W.; Erdjument-Bromage H.; Hagiwara T.; Yamada M.; Schneider R.; Gregory P. D.; Tempst P.; Bannister A. J.; Kouzarides T. (2004) Histone deimination antagonizes arginine methylation. Cell 118, 545–553. 10.1016/j.cell.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Zhang X. S.; Bolt M.; Guertin M. J.; Chen W.; Zhang S.; Cherrington B. D.; Slade D. J.; Dreyton C. J.; Subramanian V.; Bicker K. L.; Thompson P. R.; Mancini M. A.; Lis J. T.; Coonrod S. A. (2012) Peptidylarginine deiminase 2-catalyzed histone H3 arginine 26 citrullination facilitates estrogen receptor alpha target gene activation. Proc. Natl. Acad. Sci. U. S. A. 109, 13331–13336. 10.1073/pnas.1203280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis H.; Deplus R.; Putmans P.; Yamada M.; Metivier R.; Fuks F. (2009) Functional Connection between Deimination and Deacetylation of Histones. Mol. Cell. Biol. 29, 4982–4993. 10.1128/MCB.00285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara T.; Hidaka Y.; Yamada M. (2005) Deimination of histone H2A and H4 at arginine 3 in HL-60 granulocytes. Biochemistry 44, 5827–5834. 10.1021/bi047505c. [DOI] [PubMed] [Google Scholar]
- Li P. X.; Yao H. J.; Zhang Z. Q.; Li M.; Luo Y.; Thompson P. R.; Gilmour D. S.; Wang Y. M. (2008) Regulation of p53 target gene expression by peptidylarginine deiminase 4. Mol. Cell. Biol. 28, 4745–4758. 10.1128/MCB.01747-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H.; Li P.; Venters B. J.; Zheng S.; Thompson P. R.; Pugh B. F.; Wang Y. (2008) Histone Arg modifications and p53 regulate the expression of OKL38, a mediator of apoptosis. J. Biol. Chem. 283, 20060–20068. 10.1074/jbc.M802940200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P.; Azebi S.; England P.; Christensen T.; Moller-Larsen A.; Petersen T.; Batsche E.; Muchardt C. (2012) Citrullination of Histone H3 Interferes with HP1-Mediated Transcriptional Repression. PLoS Genet. 8, e1002934. 10.1371/journal.pgen.1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. H.; Coonrod S. A.; Kraus W. L.; Jelinek M. A.; Stallcup M. R. (2005) Regulation of coactivator complex assembly and function by protein arginine methylation and demethylimination. Proc. Natl. Acad. Sci. U. S. A. 102, 3611–3616. 10.1073/pnas.0407159102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K.; Hagiwara T.; Yamada M. (2002) Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J. Biol. Chem. 277, 49562–49568. 10.1074/jbc.M208795200. [DOI] [PubMed] [Google Scholar]
- Vossenaar E. R.; Zendman A. J.; van Venrooij W. J.; Pruijn G. J. (2003) PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. BioEssays 25, 1106–1118. 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- Cherrington B. D.; Morency E.; Struble A. M.; Coonrod S. A.; Wakshlag J. J. (2010) Potential Role for Peptidylarginine Deiminase 2 (PAD2) in Citrullination of Canine Mammary Epithelial Cell Histones. PLoS One 5, e11768. 10.1371/journal.pone.0011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin M. J.; Zhang X.; Anguish L.; Kim S.; Varticovski L.; Lis J. T.; Hager G. L.; Coonrod S. A. (2014) Targeted H3R26 deimination specifically facilitates estrogen receptor binding by modifying nucleosome structure. PLoS Genet. 10, e1004613. 10.1371/journal.pgen.1004613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P. X.; Li M.; Lindberg M. R.; Kennett M. J.; Xiong N.; Wang Y. M. (2010) PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207, 1853–1862. 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V.; Reichard U.; Goosmann C.; Fauler B.; Uhlemann Y.; Weiss D. S.; Weinrauch Y.; Zychlinsky A. (2004) Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535. 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Wang Y. M.; Li M.; Stadler S.; Correll S.; Li P. X.; Wang D. C.; Hayama R.; Leonelli L.; Han H.; Grigoryev S. A.; Allis C. D.; Coonrod S. A. (2009) Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 184, 205–213. 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah E.; Andrade F. (2013) NETs: the missing link between cell death and systemic autoimmune diseases?. Front. Immunol. 3, 428. 10.3389/fimmu.2012.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandpur R.; Carmona-Rivera C.; Vivekanandan-Giri A.; Gizinski A.; Yalavarthi S.; Knight J. S.; Friday S.; Li S.; Patel R. M.; Subramanian V.; Thompson P.; Chen P. J.; Fox D. A.; Pennathur S.; Kaplan M. J. (2013) NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 5, 178ra140. 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner F. L.; Ransohoff R. M.; Becher B. (2015) Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 16, 358–372. 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- Zenaro E.; Pietronigro E.; Della Bianca V.; Piacentino G.; Marongiu L.; Budui S.; Turano E.; Rossi B.; Angiari S.; Dusi S.; Montresor A.; Carlucci T.; Nani S.; Tosadori G.; Calciano L.; Catalucci D.; Berton G.; Bonetti B.; Constantin G. (2015) Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 21, 880–886. 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- Tanikawa C.; Espinosa M.; Suzuki A.; Masuda K.; Yamamoto K.; Tsuchiya E.; Ueda K.; Daigo Y.; Nakamura Y.; Matsuda K. (2012) Regulation of histone modification and chromatin structure by the p53-PADI4 pathway. Nat. Commun. 3, 676. 10.1038/ncomms1676. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Zhou L.; Cheng X. D. (2000) Crystal structure of the conserved core of protein arginine methyltransferase PRMT3. EMBO J. 19, 3509–3519. 10.1093/emboj/19.14.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Cheng X. D. (2003) Structure of the predominant protein arginine methyltransferase PRMT1 and analysis of its binding to substrate peptides. Structure 11, 509–520. 10.1016/S0969-2126(03)00071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W. W.; Hassler M.; Roe S. M.; Thompson-Vale V.; Pearl L. H. (2007) Insights into histone code syntax from structural and biochemical studies of CARM1 methyltransferase. EMBO J. 26, 4402–4412. 10.1038/sj.emboj.7601856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troffer-Charlier N.; Cura V.; Hassenboehler P.; Moras D.; Cavarelli J. (2007) Functional insights from structures of coactivator-associated arginine methyltransferase 1 domains. EMBO J. 26, 4391–4401. 10.1038/sj.emboj.7601855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonysamy S.; Bonday Z.; Campbell R. M.; Doyle B.; Druzina Z.; Gheyi T.; Han B.; Jungheim L. N.; Qian Y. W.; Rauch C.; Russell M.; Sauder J. M.; Wasserman S. R.; Weichert K.; Willard F. S.; Zhang A. P.; Emtage S. (2012) Crystal structure of the human PRMT5:MEP50 complex. Proc. Natl. Acad. Sci. U. S. A. 109, 17960–17965. 10.1073/pnas.1209814109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cura V.; Troffer-Charlier N.; Wurtz J. M.; Bonnefond L.; Cavarelli J. (2014) Structural insight into arginine methylation by the mouse protein arginine methyltransferase 7: a zinc finger freezes the mimic of the dimeric state into a single active site. Acta Crystallogr., Sect. D: Biol. Crystallogr. 70, 2401–2412. 10.1107/S1399004714014278. [DOI] [PubMed] [Google Scholar]
- Hasegawa M.; Toma-Fukai S.; Kim J. D.; Fukamizu A.; Shimizu T. (2014) Protein arginine methyltransferase 7 has a novel homodimer-like structure formed by tandem repeats. FEBS Lett. 588, 1942–1948. 10.1016/j.febslet.2014.03.053. [DOI] [PubMed] [Google Scholar]
- Rust H. L.; Zurita-Lopez C. I.; Clarke S.; Thompson P. R. (2011) Mechanistic Studies on Transcriptional Coactivator Protein Arginine Methyltransferase 1. Biochemistry 50, 3332–3345. 10.1021/bi102022e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L. T.; Wang M. Z.; Lv Z. Y.; Yang N.; Liu Y. F.; Bao S. L.; Gong W. M.; Xu R. M. (2011) Structural insights into protein arginine symmetric dimethylation by PRMT5. Proc. Natl. Acad. Sci. U. S. A. 108, 20538–20543. 10.1073/pnas.1106946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui S.; Gathiaka S.; Li J.; Qu J.; Acevedo O.; Hevel J. M. (2014) A remodeled protein arginine methyltransferase 1 (PRMT1) generates symmetric dimethylarginine. J. Biol. Chem. 289, 9320–9327. 10.1074/jbc.M113.535278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Hadjikyriacou A.; Xia Z.; Gayatri S.; Kim D.; Zurita-Lopez C.; Kelly R.; Guo A.; Li W.; Clarke S. G.; Bedford M. T. (2015) PRMT9 is a Type II methyltransferase that methylates the splicing factor SAP145. Nat. Commun. 6, 6428. 10.1038/ncomms7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikyriacou A.; Yang Y.; Espejo A.; Bedford M. T.; Clarke S. G. (2015) Unique Features of Human Protein Arginine Methyltransferase 9 (PRMT9) and Its Substrate RNA Splicing Factor SF3B2. J. Biol. Chem. 290, 16723–16743. 10.1074/jbc.M115.659433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira M.; de Freitas R. F. (2014) Structural biology and chemistry of protein arginine methyltransferases. MedChemComm 5, 1779–1788. 10.1039/C4MD00269E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najbauer J.; Johnson B. A.; Young A. L.; Aswad D. W. (1993) Peptides with sequences similar to glycine, arginine-rich motifs in proteins interacting with RNA are efficiently recognized by methyltransferase(s) modifying arginine in numerous proteins. J. Biol. Chem. 268, 10501–10509. [PubMed] [Google Scholar]
- Thandapani P.; O’Connor T. R.; Bailey T. L.; Richard S. (2013) Defining the RGG/RG Motif. Mol. Cell 50, 613–623. 10.1016/j.molcel.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Kolbel K.; Ihling C.; Kuhn U.; Neundorf I.; Otto S.; Stichel J.; Robaa D.; Beck-Sickinger A. G.; Sinz A.; Wahle E. (2012) Peptide Backbone Conformation Affects the Substrate Preference of Protein Arginine Methyltransferase I. Biochemistry 51, 5463–5475. 10.1021/bi300373b. [DOI] [PubMed] [Google Scholar]
- Branscombe T. L.; Frankel A.; Lee J. H.; Cook J. R.; Yang Z. H.; Pestka S.; Clarke S. (2001) PRMT5 (Janus kinase-binding protein 1) catalyzes the formation of symmetric dimethylarginine residues in proteins. J. Biol. Chem. 276, 32971–32976. 10.1074/jbc.M105412200. [DOI] [PubMed] [Google Scholar]
- Cheng D. H.; Cote J.; Shaaban S.; Bedford M. T. (2007) The arginine methyltransferase CARM1 regulates the coupling of transcription and mRNA processing. Mol. Cell 25, 71–83. 10.1016/j.molcel.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Feng Y.; Maity R.; Whitelegge J. P.; Hadjikyriacou A.; Li Z. W.; Zurita-Lopez C.; Al-Hadid Q.; Clark A. T.; Bedford M. T.; Masson J. Y.; Clarke S. G. (2013) Mammalian Protein Arginine Methyltransferase 7 (PRMT7) Specifically Targets RXR Sites in Lysine- and Arginine-rich Regions. J. Biol. Chem. 288, 37010–37025. 10.1074/jbc.M113.525345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne T. C.; Obianyo O.; Zhang X.; Cheng X.; Thompson P. R. (2007) Protein arginine methyltransferase 1: Positively charged residues in substrate peptides distal to the site of methylation are important for substrate binding and catalysis. Biochemistry 46, 13370–13381. 10.1021/bi701558t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooderchak W. L.; Zang T. Z.; Zhou Z. S.; Acuna M.; Tahara S. M.; Hevel J. M. (2008) Substrate profiling of PRMT1 reveals amino acid sequences that extend beyond the ″RGG″ paradigm. Biochemistry 47, 9456–9466. 10.1021/bi800984s. [DOI] [PubMed] [Google Scholar]
- Wang M.; Xu R. M.; Thompson P. R. (2013) Substrate Specificity, Processivity, and Kinetic Mechanism of Protein Arginine Methyltransferase 5. Biochemistry 52, 5430–5440. 10.1021/bi4005123. [DOI] [PubMed] [Google Scholar]
- Lee D. Y.; Ianculescu I.; Purcell D.; Zhang X.; Cheng X. D.; Stallcup M. R. (2007) Surface-scanning mutational analysis of protein arginine methyltransferase 1: Roles of specific amino acids in methyltransferase substrate specificity, oligomerization, and coactivator function. Mol. Endocrinol. 21, 1381–1393. 10.1210/me.2006-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A.; Yadav N.; Lee J. H.; Branscombe T. L.; Clarke S.; Bedford M. T. (2002) The novel human protein arginine N-methyltransferase PRMT6 is a nuclear enzyme displaying unique substrate specificity. J. Biol. Chem. 277, 3537–3543. 10.1074/jbc.M108786200. [DOI] [PubMed] [Google Scholar]
- Gui S. Y.; Wooderchak-Donahue W. L.; Zang T. Z.; Chen D.; Daly M. P.; Zhou Z. S.; Hevel J. M. (2013) Substrate-Induced Control of Product Formation by Protein Arginine Methyltransferase 1. Biochemistry 52, 199–209. 10.1021/bi301283t. [DOI] [PubMed] [Google Scholar]
- Wang M.; Fuhrmann J.; Thompson P. R. (2014) Protein Arginine Methyltransferase 5 Catalyzes Substrate Dimethylation in a Distributive Fashion. Biochemistry 53, 7884–7892. 10.1021/bi501279g. [DOI] [PubMed] [Google Scholar]
- van Beers J. J. B. C.; Zendman A. J. W.; Raijmakers R.; Stammen-Vogelzangs J.; Pruijn G. J. M. (2013) Peptidylarginine deiminase expression and activity in PAD2 knock-out and PAD4-low mice. Biochimie 95, 299–308. 10.1016/j.biochi.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Hirofumi T.; Tomoharu G.; Bryan K.; Paul R T.; Oliver V.; Kazuya H.; Tatsurou M.; Kouichiro S.; Hiroyuki H.; Eiji S. (2011) Purification of enzymatically inactive peptidylarginine deiminase type 6 from mouse ovary that reveals hexameric structure different from other dimeric isoforms. Adv. Biosci. Biotechnol. 2, 304–310. 10.4236/abb.2011.24044. [DOI] [Google Scholar]
- Kearney P. L.; Bhatia M.; Jones N. G.; Yuan L.; Glascock M. C.; Catchings K. L.; Yamada M.; Thompson P. R. (2005) Kinetic characterization of protein arginine deiminase 4: A transcriptional corepressor implicated in the onset and progression of rheumatoid arthritis. Biochemistry 44, 10570–10582. 10.1021/bi050292m. [DOI] [PubMed] [Google Scholar]
- Liu Y. L.; Chiang Y. H.; Liu G. Y.; Hung H. C. (2011) Functional Role of Dimerization of Human Peptidylarginine Deiminase 4 (PAD4). PLoS One 6, e21314. 10.1371/journal.pone.0021314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade D. J.; Fang P.; Dreyton C. J.; Zhang Y.; Fuhrmann J.; Rempel D.; Bax B. D.; Coonrod S. A.; Lewis H. D.; Guo M.; Gross M. L.; Thompson P. R. (2015) Protein arginine deiminase 2 binds calcium in an ordered fashion: implications for inhibitor design. ACS Chem. Biol. 10, 1043–1053. 10.1021/cb500933j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai H.; Blundell T. L.; Mizuguchi K. (2001) A novel superfamily of enzymes that catalyze the modification of guanidino groups. Trends Biochem. Sci. 26, 465–468. 10.1016/S0968-0004(01)01906-5. [DOI] [PubMed] [Google Scholar]
- Arita K.; Hashimoto H.; Shimizu T.; Nakashima K.; Yamada M.; Sato M. (2004) Structural basis for Ca2+-induced activation of human PAD4. Nat. Struct. Mol. Biol. 11, 777–783. 10.1038/nsmb799. [DOI] [PubMed] [Google Scholar]
- Knuckley B.; Causey C. P.; Jones J. E.; Bhatia M.; Dreyton C. J.; Osborne T. C.; Takahara H.; Thompson P. R. (2010) Substrate specificity and kinetic studies of PADs 1, 3, and 4 identify potent and selective inhibitors of protein arginine deiminase 3. Biochemistry 49, 4852–4863. 10.1021/bi100363t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai H.; Mokrab Y.; Mizuguchi K. (2006) The guanidino-group modifying enzymes: Structural basis for their diversity and commonality. Proteins: Struct., Funct., Genet. 64, 1010–1023. 10.1002/prot.20863. [DOI] [PubMed] [Google Scholar]
- Linsky T.; Fast W. (2010) Mechanistic similarity and diversity among the guanidine-modifying members of the pentein superfamily. Biochim. Biophys. Acta, Proteins Proteomics 1804, 1943–1953. 10.1016/j.bbapap.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita K.; Shimizu T.; Hashimoto H.; Hidaka Y.; Yamada M.; Sato M. (2006) Structural basis for histone N-terminal recognition by human peptidylarginine deiminase 4. Proc. Natl. Acad. Sci. U. S. A. 103, 5291–5296. 10.1073/pnas.0509639103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka Y.; Hagiwara T.; Yamada M. (2005) Methylation of the guanidino group of arginine residues prevents citrullination by peptidylarginine deiminase IV. FEBS Lett. 579, 4088–4092. 10.1016/j.febslet.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Raijmakers R.; Zendman A. J. W.; Egberts W. V.; Vossenaar E. R.; Raats J.; Soede-Huijbregts C.; Rutjes F. P. J. T.; van Veelen P. A.; Drijfhout J. W.; Pruijn G. J. M. (2007) Methylation of arginine residues interferes with citrullination by peptidylarginine deiminases in vitro. J. Mol. Biol. 367, 1118–1129. 10.1016/j.jmb.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Thompson P. R.; Fast W. (2006) Histone citrullination by protein arginine deiminase: Is arginine methylation a green light or a roadblock?. ACS Chem. Biol. 1, 433–441. 10.1021/cb6002306. [DOI] [PubMed] [Google Scholar]
- Richon V. M.; Johnston D.; Sneeringer C. J.; Jin L.; Majer C. R.; Elliston K.; Jerva L. F.; Scott M. P.; Copeland R. A. (2011) Chemogenetic Analysis of Human Protein Methyltransferases. Chem. Biol. Drug Des. 78, 199–210. 10.1111/j.1747-0285.2011.01135.x. [DOI] [PubMed] [Google Scholar]
- Chen D. H.; Wu K. T.; Hung C. J.; Hsieh M.; Li C. (2004) Effects of adenosine dialdehyde treatment on in vitro and in vivo stable protein methylation in HeLa cells. J. Biochem. 136, 371–376. 10.1093/jb/mvh131. [DOI] [PubMed] [Google Scholar]
- Hamill R. L.; Hoehn M. M. (1973) A9145, a new adenine-containing antifungal antibiotic. I. Discovery and isolation. J. Antibiot. 26, 463–465. 10.7164/antibiotics.26.463. [DOI] [PubMed] [Google Scholar]
- Luo M. K. (2012) Current Chemical Biology Approaches to Interrogate Protein Methyltransferases. ACS Chem. Biol. 7, 443–463. 10.1021/cb200519y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D.; Yadav N.; King R. W.; Swanson M. S.; Weinstein E. J.; Bedford M. T. (2004) Small molecule regulators of protein arginine methyltransferases. J. Biol. Chem. 279, 23892–23899. 10.1074/jbc.M401853200. [DOI] [PubMed] [Google Scholar]
- Feng Y.; Li M. Y.; Wang B. H.; Zheng Y. G. (2010) Discovery and Mechanistic Study of a Class of Protein Arginine Methylation Inhibitors. J. Med. Chem. 53, 6028–6039. 10.1021/jm100416n. [DOI] [PubMed] [Google Scholar]
- Spannhoff A.; Heinke R.; Bauer I.; Trojer P.; Metzger E.; Gust R.; Schule R.; Brosch G.; Sippl W.; Jung M. (2007) Target-based approach to inhibitors of histone arginine methyltransferases. J. Med. Chem. 50, 2319–2325. 10.1021/jm061250e. [DOI] [PubMed] [Google Scholar]
- Yan L. L.; Yan C. L.; Qian K.; Su H. R.; Kofsky-Wofford S. A.; Lee W. C.; Zhao X. Y.; Ho M. C.; Ivanov I.; Zheng Y. G. (2014) Diamidine Compounds for Selective Inhibition of Protein Arginine Methyltransferase 1. J. Med. Chem. 57, 2611–2622. 10.1021/jm401884z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munde M.; Ismail M. A.; Arafa R.; Peixoto P.; Collar C. J.; Liu Y.; Hu L. X.; David-Cordonnier M. H.; Lansiaux A.; Bailly C.; Boykin D. W.; Wilson W. D. (2007) Design of DNA minor groove binding diamidines that recognize GC base pair sequences: A dimeric-hinge interaction motif. J. Am. Chem. Soc. 129, 13732–13743. 10.1021/ja074560a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne T.; Roska R. L.; Rajski S. R.; Thompson P. R. (2008) In situ generation of a bisubstrate analogue for protein arginine methyltransferase 1. J. Am. Chem. Soc. 130, 4574–4575. 10.1021/ja077104v. [DOI] [PubMed] [Google Scholar]
- Obianyo O.; Causey C. P.; Osborne T. C.; Jones J. E.; Lee Y. H.; Stallcup M. R.; Thompson P. R. (2010) A Chloroacetamidine-Based Inactivator of Protein Arginine Methyltransferase 1: Design, Synthesis, and In Vitro and In Vivo Evaluation. ChemBioChem 11, 1219–1223. 10.1002/cbic.201000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowden J.; Hong W.; Parry R. V.; Pike R. A.; Ward S. G. (2010) Toward the development of potent and selective bisubstrate inhibitors of protein arginine methyltransferases. Bioorg. Med. Chem. Lett. 20, 2103–2105. 10.1016/j.bmcl.2010.02.069. [DOI] [PubMed] [Google Scholar]
- ’T Hart P.; Lakowski T. M.; Thomas D.; Frankel A.; Martin N. I. (2011) Peptidic Partial Bisubstrates as Inhibitors of the Protein Arginine N-Methyltransferases. ChemBioChem 12, 1427–1432. 10.1002/cbic.201100074. [DOI] [PubMed] [Google Scholar]
- Thomas D.; Koopmans T.; Lakowski T. M.; Kreinin H.; Vhuiyan M. I.; Sedlock S. A.; Bui J. M.; Martin N. I.; Frankel A. (2014) Protein Arginine N-Methyltransferase Substrate Preferences for Different N eta-Substituted Arginyl Peptides. ChemBioChem 15, 1606–1612. 10.1002/cbic.201402045. [DOI] [PubMed] [Google Scholar]
- van Haren M.; van Ufford L. Q.; Moret E. E.; Martin N. I. (2015) Synthesis and evaluation of protein arginine N-methyltransferase inhibitors designed to simultaneously occupy both substrate binding sites. Org. Biomol. Chem. 13, 549–560. 10.1039/C4OB01734J. [DOI] [PubMed] [Google Scholar]
- Rust H. L.Decoding PRMT1: Studies On the Catalytic Mechanism, Regulation, Inhibition, and Crosstalk of PRMT1-Dependent Methylation. Ph.D., University of South Carolina, 2013. [Google Scholar]
- Obianyo O.; Causey C. P.; Jones J. E.; Thompson P. R. (2011) Activity-Based Protein Profiling of Protein Arginine Methyltransferase 1. ACS Chem. Biol. 6, 1127–1135. 10.1021/cb2001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S. H.; Owens E. A.; Feng Y.; Yang Y. T.; Xie Y.; Tu Y. P.; Henary M.; Zheng Y. G. (2012) Synthesis and evaluation of carbocyanine dyes as PRMT inhibitors and imaging agents. Eur. J. Med. Chem. 54, 647–659. 10.1016/j.ejmech.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H.; Owens E. A.; Su H. R.; Yan L. L.; Levitz A.; Zhao X. Y.; Henary M.; Zheng Y. G. (2015) Exploration of Cyanine Compounds as Selective Inhibitors of Protein Arginine Methyltransferases: Synthesis and Biological Evaluation. J. Med. Chem. 58, 1228–1243. 10.1021/jm501452j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siarheyeva A.; Senisterra G.; Allali-Hassani A.; Dong A.; Dobrovetsky E.; Wasney G. A.; Chau I.; Marcellus R.; Hajian T.; Liu F.; Korboukh I.; Smil D.; Bolshan Y.; Min J.; Wu H.; Zeng H.; Loppnau P.; Poda G.; Griffin C.; Aman A.; Brown P. J.; Jin J.; Al-Awar R.; Arrowsmith C. H.; Schapira M.; Vedadi M. (2012) An allosteric inhibitor of protein arginine methyltransferase 3. Structure 20, 1425–1435. 10.1016/j.str.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Kaniskan H. U.; Szewczyk M. M.; Yu Z.; Eram M. S.; Yang X.; Schmidt K.; Luo X.; Dai M.; He F.; Zang I.; Lin Y.; Kennedy S.; Li F.; Dobrovetsky E.; Dong A.; Smil D.; Min S. J.; Landon M.; Lin-Jones J.; Huang X. P.; Roth B. L.; Schapira M.; Atadja P.; Barsyte-Lovejoy D.; Arrowsmith C. H.; Brown P. J.; Zhao K.; Jin J.; Vedadi M. (2015) A Potent, Selective and Cell-Active Allosteric Inhibitor of Protein Arginine Methyltransferase 3 (PRMT3). Angew. Chem., Int. Ed. 54, 5166–5170. 10.1002/anie.201412154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack J. S.; Thieffine S.; Bandiera T.; Fasolini M.; Duke G. J.; Jayaraman L.; Kish K. F.; Klei H. E.; Purandare A. V.; Rosettani P.; Troiani S.; Xie D. L.; Bertrand J. A. (2011) Structural basis for CARM1 inhibition by indole and pyrazole inhibitors. Biochem. J. 436, 331–339. 10.1042/BJ20102161. [DOI] [PubMed] [Google Scholar]
- Purandare A. V.; Chen Z.; Huynh T.; Pang S.; Geng J.; Vaccaro W.; Poss M. A.; Oconnell J.; Nowak K.; Jayaraman L. (2008) Pyrazole inhibitors of coactivator associated arginine methyltransferase 1 (CARM1). Bioorg. Med. Chem. Lett. 18, 4438–4441. 10.1016/j.bmcl.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Chan-Penebre E.; Kuplast K. G.; Majer C. R.; Boriack-Sjodin P. A.; Wigle T. J.; Johnston L. D.; Rioux N.; Munchhof M. J.; Jin L.; Jacques S. L.; West K. A.; Lingaraj T.; Stickland K.; Ribich S. A.; Raimondi A.; Scott M. P.; Waters N. J.; Pollock R. M.; Smith J. J.; Barbash O.; Pappalardi M.; Ho T. F.; Nurse K.; Oza K. P.; Gallagher K. T.; Kruger R.; Moyer M. P.; Copeland R. A.; Chesworth R.; Duncan K. W. (2015) A selective inhibitor of PRMT5 with in vivo and in vitro potency in MCL models. Nat. Chem. Biol. 11, 432–437. 10.1038/nchembio.1810. [DOI] [PubMed] [Google Scholar]
- Mitchell L. H.; Drew A. E.; Ribich S. A.; Rioux N.; Swinger K. K.; Jacques S. L.; Lingaraj T.; Boriack-Sjodin P. A.; Waters N. J.; Wigle T. J.; Moradei O.; Jin L.; Riera T.; Porter-Scott M.; Moyer M. P.; Smith J. J.; Chesworth R.; Copeland R. A. (2015) Aryl Pyrazoles as Potent Inhibitors of Arginine Methyltransferases: Identification of the First PRMT6 Tool Compound. ACS Med. Chem. Lett. 6, 655–659. 10.1021/acsmedchemlett.5b00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. E.; Causey C. P.; Knuckley B.; Slack-Noyes J. L.; Thompson P. R. (2009) Protein arginine deiminase 4 (PAD4): Current understanding and future therapeutic potential. Curr. Opin. Drug Discovery 12, 616–627. [PMC free article] [PubMed] [Google Scholar]
- Knuckley B.; Luo Y.; Thompson P. R. (2008) Profiling Protein Arginine Deiminase 4 (PAD4): A novel screen to identify PAD4 inhibitors. Bioorg. Med. Chem. 16, 739–745. 10.1016/j.bmc.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdag M.; Dreker T.; Henry C.; Tosco P.; Vallaro M.; Fruttero R.; Scozzafava A.; Carta F.; Supuran C. T. (2013) Novel small molecule protein arginine deiminase 4 (PAD4) inhibitors. Bioorg. Med. Chem. Lett. 23, 715–719. 10.1016/j.bmcl.2012.11.102. [DOI] [PubMed] [Google Scholar]
- Ferretti P.; Pong U. K.; Vagaska B.; Merchant R.; Matthews C. J.; Marson C. M. (2013) Discovery of a structurally novel, drug-like and potent inhibitor of peptidylarginine deiminase. MedChemComm 4, 1109–1113. 10.1039/c3md00091e. [DOI] [Google Scholar]
- Lewis H. D.; Liddle J.; Coote J. E.; Atkinson S. J.; Barker M. D.; Bax B. D.; Bicker K. L.; Bingham R. P.; Campbell M.; Chen Y. H.; Chung C. W.; Craggs P. D.; Davis R. P.; Eberhard D.; Joberty G.; Lind K. E.; Locke K.; Maller C.; Martinod K.; Patten C.; Polyakova O.; Rise C. E.; Rudiger M.; Sheppard R. J.; Slade D. J.; Thomas P.; Thorpe J.; Yao G.; Drewes G.; Wagner D. D.; Thompson P. R.; Prinjha R. K.; Wilson D. M. (2015) Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 11, 189–191. 10.1038/nchembio.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y.; Arita K.; Bhatia M.; Knuckley B.; Lee Y. H.; Stallcup M. R.; Sato M.; Thompson P. R. (2006) Inhibitors and inactivators of protein arginine deiminase 4: functional and structural characterization. Biochemistry 45, 11727–11736. 10.1021/bi061180d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y.; Knuckley B.; Lee Y. H.; Stallcup M. R.; Thompson P. R. (2006) A fluoroacetamidine-based inactivator of protein arginine deiminase 4: Design, synthesis, and in vitro and in vivo evaluation. J. Am. Chem. Soc. 128, 1092–1093. 10.1021/ja0576233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello A. M.; Wasiewski E.; Wei L. H.; Moscarello M. A.; Kotra L. P. (2013) Interrogation of the Active Sites of Protein Arginine Deiminases (PAD1,-2, and-4) Using Designer Probes. ACS Med. Chem. Lett. 4, 249–253. 10.1021/ml300377d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis V.; Gizinski A. M.; Banda N. K.; Causey C. P.; Knuckley B.; Cordova K. N.; Luo Y. A.; Levitt B.; Glogowska M.; Chandra P.; Kulik L.; Robinson W. H.; Arend W. P.; Thompson P. R.; Holers V. M. (2011) N-alpha-Benzoyl-N5-(2-Chloro-1-Iminoethyl)-L-Ornithine Amide, a Protein Arginine Deiminase Inhibitor, Reduces the Severity of Murine Collagen-Induced Arthritis. J. Immunol. 186, 4396–4404. 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J. S.; Zhao W. P.; Luo W.; Subramanian V.; O’Dell A. A.; Yalavarthi S.; Hodgin J. B.; Eitzman D. T.; Thompson P. R.; Kaplan M. J. (2013) Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J. Clin. Invest. 123, 2981–2993. 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumanevich A. A.; Causey C. P.; Knuckley B. A.; Jones J. E.; Poudyal D.; Chumanevich A. P.; Davis T.; Matesic L. E.; Thompson P. R.; Hofseth L. J. (2011) Suppression of colitis in mice by Cl-amidine: a novel peptidylarginine deiminase inhibitor. Am. J. Physiol Gastrointest Liver Physiol 300, G929–G938. 10.1152/ajpgi.00435.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee J. L.; Mohanan S.; Griffith O. L.; Breuer H. C.; Anguish L. J.; Cherrington B. D.; Palmer A. M.; Howe L. R.; Subramanian V.; Causey C. P.; Thompson P. R.; Gray J. W.; Coonrod S. A. (2012) Identification of PADI2 as a potential breast cancer biomarker and therapeutic target. BMC Cancer 12, 500. 10.1186/1471-2407-12-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causey C. P.; Jones J. E.; Slack J. L.; Kamei D.; Jones L. E.; Subramanian V.; Knuckley B.; Ebrahimi P.; Chumanevich A. A.; Luo Y.; Hashimoto H.; Sato M.; Hofseth L. J.; Thompson P. R. (2011) The Development of N-alpha-(2-Carboxyl)benzoyl-N-5-(2-fluoro-1-iminoethyl)-L-ornithine Amide (o-F-amidine) and N-alpha-(2-Carboxyl)benzoyl-N-5-(2-chloro-1-iminoethyl)-L-ornithine Amide (o-Cl-amidine) As Second Generation Protein Arginine Deiminase (PAD) Inhibitors. J. Med. Chem. 54, 6919–6935. 10.1021/jm2008985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. E.; Slack J. L.; Fang P. F.; Zhang X. S.; Subramanian V.; Causey C. P.; Coonrod S. A.; Guo M.; Thompson P. R. (2012) Synthesis and Screening of a Haloacetamidine Containing Library To Identify PAD4 Selective Inhibitors. ACS Chem. Biol. 7, 160–165. 10.1021/cb200258q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. J.; Li P. X.; Wang S.; Hu J.; Chen X. A.; Wu J. H.; Fisher M.; Oshaben K.; Zhao N.; Gu Y.; Wang D.; Chen G.; Wang Y. M. (2012) Anticancer Peptidylarginine Deiminase (PAD) Inhibitors Regulate the Autophagy Flux and the Mammalian Target of Rapamycin Complex 1 Activity. J. Biol. Chem. 287, 25941–25953. 10.1074/jbc.M112.375725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J. S.; Subramanian V.; O’Dell A. A.; Yalavarthi S.; Zhao W.; Smith C. K.; Hodgin J. B.; Thompson P. R.; Kaplan M. J. (2015) Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann. Rheum. Dis. 74, 2199–2206. 10.1136/annrheumdis-2014-205365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian V.; Knight J. S.; Parelkar S.; Anguish L.; Coonrod S. A.; Kaplan M. J.; Thompson P. R. (2015) Design, synthesis, and biological evaluation of tetrazole analogs of Cl-amidine as protein arginine deiminase inhibitors. J. Med. Chem. 58, 1337–1344. 10.1021/jm501636x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuckley B.; Jones J. E.; Bachovchin D. A.; Slack J.; Causey C. P.; Brown S. J.; Rosen H.; Cravatt B. F.; Thompson P. R. (2010) A fluopol-ABPP HTS assay to identify PAD inhibitors. Chem. Commun. 46, 7175–7177. 10.1039/c0cc02634d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyton C. J.; Anderson E. D.; Subramanian V.; Boger D. L.; Thompson P. R. (2014) Insights into the mechanism of streptonigrin-induced protein arginine deiminase inactivation. Bioorg. Med. Chem. 22, 1362–1369. 10.1016/j.bmc.2013.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali H.; Khan H. A.; Stringer J. R.; Chowdhury S.; Ellman J. A. (2015) Identification of Multiple Structurally Distinct, Nonpeptidic Small Molecule Inhibitors of Protein Arginine Deiminase 3 Using a Substrate-Based Fragment Method. J. Am. Chem. Soc. 137, 3616–3621. 10.1021/jacs.5b00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B. S.; Chen Y.; Zhao Y. M.; Bruick R. K. (2007) JMJD6 is a histone arginine demethylase. Science 318, 444–447. 10.1126/science.1145801. [DOI] [PubMed] [Google Scholar]
- Webby C. J.; Wolf A.; Gromak N.; Dreger M.; Kramer H.; Kessler B.; Nielsen M. L.; Schmitz C.; Butler D. S.; Yates J. R.; Delahunty C. M.; Hahn P.; Lengeling A.; Mann M.; Proudfoot N. J.; Schofield C. J.; Bottger A. (2009) Jmjd6 Catalyses Lysyl-Hydroxylation of U2AF65, a Protein Associated with RNA Splicing. Science 325, 90–93. 10.1126/science.1175865. [DOI] [PubMed] [Google Scholar]
- Hahn P.; Wegener I.; Burrells A.; Bose J.; Wolf A.; Erck C.; Butler D.; Schofield C. J.; Bottger A.; Lengeling A. (2010) Analysis of Jmjd6 Cellular Localization and Testing for Its Involvement in Histone Demethylation. PLoS One 5, e13769. 10.1371/journal.pone.0013769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metivier R.; Penot G.; Hubner M. R.; Reid G.; Brand H.; Kos M.; Gannon F. (2003) Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115, 751–763. 10.1016/S0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- Wakim B. T.; Aswad G. D. (1994) Ca(2+)-calmodulin-dependent phosphorylation of arginine in histone 3 by a nuclear kinase from mouse leukemia cells. J. Biol. Chem. 269, 2722–2727. [PubMed] [Google Scholar]
- Fuhrmann J.; Schmidt A.; Spiess S.; Lehner A.; Turgay K.; Mechtler K.; Charpentier E.; Clausen T. (2009) McsB Is a Protein Arginine Kinase That Phosphorylates and Inhibits the Heat-Shock Regulator CtsR. Science 324, 1323–1327. 10.1126/science.1170088. [DOI] [PubMed] [Google Scholar]
- Fuhrmann J.; Subramanian V.; Thompson P. R. (2015) Synthesis and Use of a Phosphonate Amidine to Generate an Anti-Phosphoarginine-Specific Antibody. Angew. Chem., Int. Ed. 54, 14715–14718. 10.1002/anie.201506737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzio L. O.; Riquelme P. T.; Koide S. S. (1979) ADP Ribosylation of Rat-Liver Nucleosomal Core Histones. J. Biol. Chem. 254, 3029–3037. [PubMed] [Google Scholar]
- Golderer G.; Grobner P. (1991) ADP-Ribosylation of Core Histones and Their Acetylated Subspecies. Biochem. J. 277, 607–610. 10.1042/bj2770607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom T. (2005) Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 24, 1311–1317. 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M.; Cerami A.; Vlassara H. (1988) Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N. Engl. J. Med. 318, 1315–1321. 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]