Highlights
-
•
Postprandial hypoglycemia may be a serious adverse effect following Roux-en-Y gastric bypass surgery.
-
•
Most patient can be treated with diet and pharmacological agents, but some patients need surgical reversals.
-
•
This Roux-en-Y gastric bypass reversal alleviates severe postprandial hyperinsulinaemic hypoglycaemia.
-
•
The technique retains some component of rapid transit of food into a shorter alimentary limb in an attempt to reduce weight regain.
-
•
This new surgical procedure also attenuates s-GLP-1 and s-insulin responses along with improved p-glucose.
Keywords: Roux-en-Y gastric bypass, Hypoglycaemia, Gastric bypass reversal, Obesity, Postprandial hyperinsulinemic hypoglycaemia, GLP-1, Case report
Abstract
Background
We describe an evaluation of the effects of partial Roux-en-Y gastric bypass (RYGB) reversal on postprandial hyperinsulinaemic hypoglycaemia, insulin and GLP-1 levels.
Case summary
A 37 year old man was admitted with neuroglycopenia (plasma–glucose 1.6 mmol/l) 18 months after RYGB, with normal 72 h fasting test and abdominal CT. Despite dietary modifications and medical treatment, the hypoglycaemic episodes escalated in frequency. Feeding by a gastrostomy tube positioned in the gastric remnant did not prevent severe episodes of hypoglycaemia. A modified reversal of the RYGB was performed. Mixed meal tests were done perorally (PO), through the gastrostomy tube 1 (GT1), 4 weeks (GT2) after placement and 4 weeks after reversal (POr), with assessment of glucose, insulin and GLP-1 levels.
Results
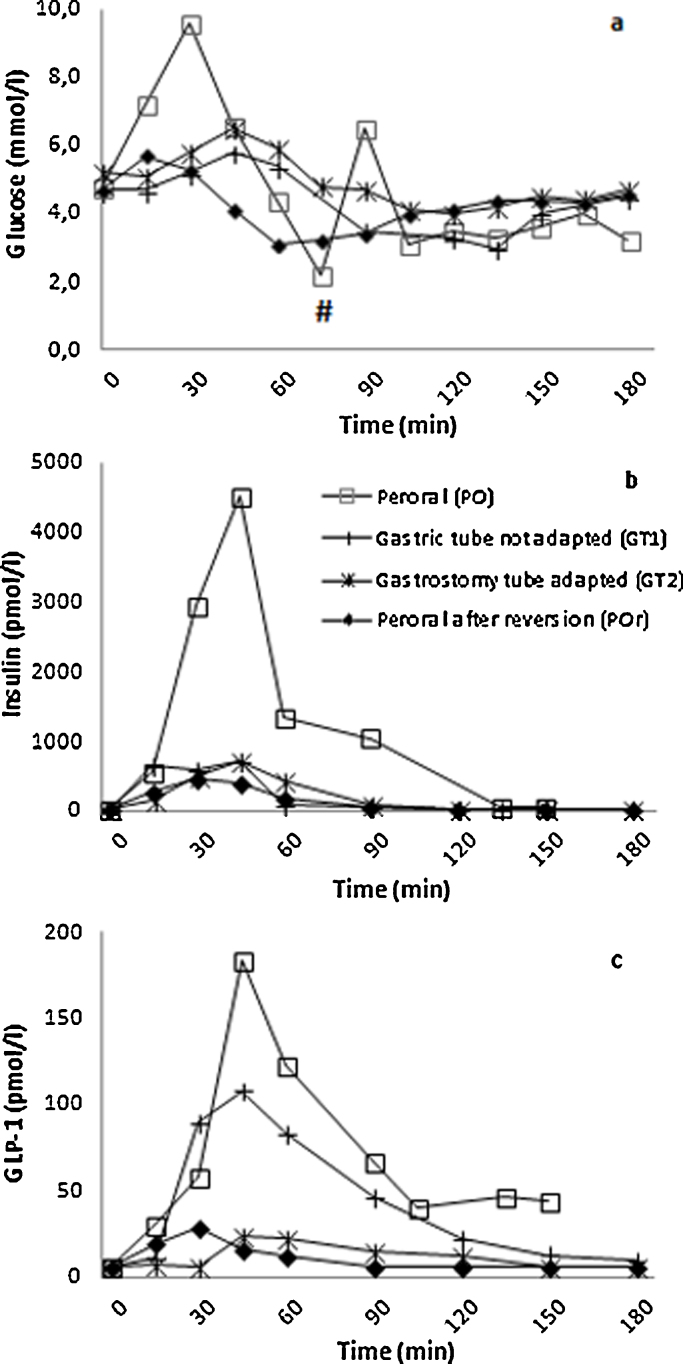
Plasma–glucose increased to a maximum of 9.6, 5.4, 6.5 and 5.8 mmol/l at the PO, GT1, GT2 and POr tests respectively. The corresponding insulin levels were 2939, 731, 725 and 463 pmol/l. A decrease of plasma–glucose followed: 2.2, 3.0, 3.9 and 2.9 mmol/l respectively and insulin levels were suppressed at 150 min: 45, 22, 21 and 14 pmol/l, respectively. GLP-1 levels increased in the PO test (60 min: 122 pmol/l, 21 fold of basal), but was attenuated in the two latter tests (12–23 pmol/l at 60 min).
Conclusions
Reduction of plasma–glucose, insulin and GLP-1 excursions and symptoms were seen after gastric tube placement and partial RYGB reversal. This attenuation of GLP-1 response to feeding could reflect an adaptation to nutrients.
1. Background
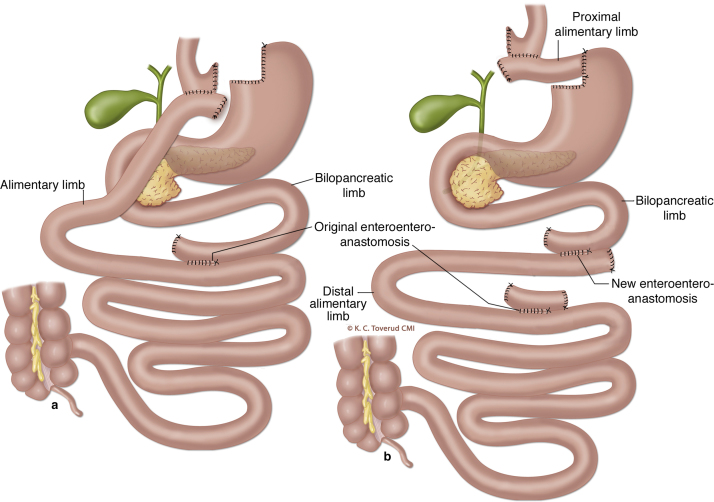
Roux-en-Y gastric bypass (RYGB) is widely used to treat morbid obesity (Fig. 1a). Postprandial hyperinsulinemic hypoglycaemia (PHH) can be a complication after RYGB and potentially difficult to treat [1], [2]. The mechanisms for PHH are not fully understood, but exaggerated incretin responses play a role [3], [4]. A small subgroup of patients does not respond to dietary changes and medical therapy. For these patients, surgical reversal or pancreatectomy have been proposed although the optimal surgical treatment has not been defined [5], [6].
Fig. 1.
Panel a depicts the entero–entero anastomosis (OEE) and the gastro–jejunostomy after Roux-en-Y gastric bypass. Panel b demonstrates the division of the alimentary limb 6 cm below the gastro–jejunostomy and the anastomosis of the proximal part of the alimentary limb to the gastric remnant. A new entero–entero anastomosis is made between the former alimentary and bilio–pancreatic limb following transection of the original entero–entero anastomosis at the bilio–pancreatic limb side. A is previously published in D. Hofsø et al.: Follow-up after bariatric surgery, in the Journal of the Norwegian Medical Association, 2011, by permission from K. Toverud, CMI. B was produced for our paper by K. Toverud, CMI. The images are not covered by the terms of the Creative Commons licence of this publication. For permission to reuse, please contact the rights holder (K. Toverud, CMI).
We describe a patient with severe PHH unresponsive to non-surgical treatment. A novel surgical approach of partial reversal of the RYGB was successfully used. Here we describe the pre- and postoperative physiological responses demonstrating the resolution of PHH.
2. Patient and methods
A 37-year-old man (weight 183 kg, height 185 cm, BMI 53.5 kg/m2) with known bradycardia, asthma and gout, but no diabetes, had a laparoscopic RYGB with a 150 cm (antecolic) alimentary and a 50 cm bilio–pancreatic limb (Fig. 1a). One year after surgery he had lost 88 kg (48% total weight loss) and his BMI was reduced to 27.7 kg/m2.
Eighteen months after surgery the patient was admitted to hospital with neuroglycopenic symptoms relieved by consumption of carbohydrates and plasma–glucose of 1.6 mmol/l. He used standard nutritional supplements, but no drugs and rarely alcohol. Serum–cortisol, thyroid function tests, cerebral- and abdominal CT scans, cardiac evaluation and plasma–glucose after a 72 h fast were normal. Despite dietary modifications and self-monitoring of blood–glucose the hypoglycaemic episodes and hospital admissions increased in frequency. Acarbose was attempted but not tolerated due to gastrointestinal side effects. Diazoxide up to 600 mg daily had little effect and was discontinued due to dyspnea. Verapamil was not considered as the patient had bradycardia. Subcutaneous injection of octreotide (50 mcg 3 times daily) resulted in less severe PHH for some weeks, but the effect gradually attenuated and his alanine transaminase and aspartate transaminase increased.
Severe symptomatic hypoglycaemia occurred on a daily basis resulting in hospital admission for glucose infusions. In an attempt to alleviate the symptoms, feeding through a gastrostomy tube into the remnant stomach was started. The laparascopic positioning of the gastrostomy tube was complicated by subileus and the patient was reoperated. To evaluate if feeding through the gastrostomy tube would decrease the PHH a standard mixed meal test was performed orally before the gastrostomy tube placement (PO), and via the tube at 1 week (GT1) and 4 weeks (GT2) after the tube placement. The test meal; 200 ml of Fresubin 2 kcal™ (400 kcal, 45 g carbohydrates, 20 g protein and 15.6 g fat) was ingested or infused through the gastric tube over 12 min. During the tests, the Edinburgh symptom scale for hypoglycaemia [8] was used to evaluate hypoglycaemic symptoms every 15 min, in parallel with pulse and BP measurements. Plasma–glucose, serum–insulin and plasma–GLP-1 were measured every 15 min during the first hour, and then every 30 min until the end of the test.
Four weeks after the last test the patient developed recurrent abdominal pain, especially after ingestion of oral fiber-rich foods, and became dependent on continuous parenteral glucose infusions to prevent hypoglycemia. He developed signs of hypoglycaemia unawareness. Reduced oral feeding did not prevent the hypoglycaemia, in spite of gradually increased nutrition (250 ml × 3 Diaben™) through the gastric tube.
RYGB reversal: Due to prolonged hospitalization, the need for continuous glucose infusions and the development of hypoglycaemic unawareness surgery was considered. The patient was finally offered a modified reversal of the RYGB (Fig. 1b).
During laparoscopy the gastrostomy tube was removed and the alimentary limb was transected 6 cm below the gastro–jejunostomy (Fig. 1b). A side-to-side anastomosis was created between the highest part of the remnant stomach on the minor side and the small bowel 6 cm below the original RYGB pouch. The bilio–pancreatic limb was divided at the entero–entero anastomosis and a new side-to-side entero–entero anastomosis was created between the previously divided alimentary limb and the bilio–pancreatic limb.
The same mixed meal was given orally 4 weeks after the RYGB reversal (POr).
Biochemical analysis: Plasma–glucose was analyzed every 5 min by a glucose oxidase method (YSI Inc., Yellow Springs, Ohio, USA.). Serum–insulin was analyzed at the Hormone Laboratory (ECLIA, Roche diagnostic Inc.). Plasma–GLP-1 samples were centrifuged and frozen at −80° and analyzed later in one run by an EMD Millipore ELISA kit (CV 8%).
The present case report is compliant with the CARE Guidelines [7].
3. Results
During the mixed meal testing pre-intervention, Serum–insulin levels increased rapidly during the first 30 min in parallel with glucose levels, and were later suppressed. Plasma–glucose decreased from 30 to 90 min. Plasma–GLP-1 levels demonstrated exaggerated responses during the first two tests (PO, GT1), but was attenuated at both the GT2 and the POr tests (Fig. 2a–c).
Fig. 2.
Panel a–c respectively: Plasma–glucose, serum–insulin and plasma–GLP-1 levels during tests (peroral □), gastric tube unadapted (GT1 +), gastric tube adapted (GT2 ×), peroral after reversion (POr ♦). # indicates the point where the PO test was stopped due to a serious episode of hypoglycaemia (2,0 mmol/l), necessitating iv. glucose injection.
Symptoms and signs during both the PO and GT1 tests included palpitations, abdominal pain, nausea, sleepiness and redness. These symptoms were attenuated after gastric tube adaptation (GT2). Postoperatively (POr), the patient experienced neither symptoms of hypoglycaemia, nor episodes of abdominal pain. The patient had one episode of constipation successfully corrected by a barium enema.
The patient was reviewed two months after the RYGB reversal and reported no hypoglycaemic symptoms. His body weight was 87.3 kg. Three months after the partial reversal of RYGB, continuous glucose monitoring demonstrated plasma–glucose levels around 5 mmol/l, and no report of hypoglycaemic symptoms in spite of provocations with high glycaemic meals. At 10 months post operatively he weighed 94 kg, reported some dyspepsia, but had a normal upper endoscopy and infrequent low blood glucose levels (lowest at 2.7 mmol/l), but no hypoglycaemic symptoms.
4. Discussion
In our patient the symptoms of PHH resolved after partial reversal of the RYGB and he returned to work 6 weeks after surgery. We found a reduction in hypoglycaemia and symptoms three weeks after gastric tube feeding was started, with further improvement after RYGB reversal. The attenuation of plasma–GLP-1 and insulin responses were associated with improved plasma–glucose and symptoms during to the two latter meal tests (GT2 and POr).
The exaggerated plasma–GLP-1 response at the GT1 visit, which involved feeding through the gastrostomy tube, suggests that the duodenum and jejunum in the bilio–pancreatic limb had an exacerbated response to the mixed meal. The subsequent attenuated GLP-1 response during GT2 and POr tests, suggests small bowel adaptation, resulting in a diminutive response triggered by the mixed meals.
The optimal surgical approach to patients with post-RYGB hypoglycaemia not responding to diet and/or pharmacotherapy has not been defined [6]. A gastrostomy tube has been used by some authors to test the potential effects of RYGB reversal. Our findings suggest time to pass for an enteral adaptation to nutrition by the gastric tube, prior to testing the effect of tube feeding on plasma–glucose levels and symptoms of hypoglycaemia.
Pancreatic resection and reversal have both been used in an attempt to relieve PHH [6]. To prevent weight regain a sleeve gastrectomy has been performed together with the RYGB reversal [5]. We describe a technically easier reversal while potentially retaining some component of nutritional restriction as the original gastric pouch is preserved. This may reduce the risk of weight regain. This approach has to our knowledge, not previously been described in the context of surgical treatment to PHH after RYGB. Our short-term findings indicate that the procedure alleviates the symptoms of hypoglycaemia. Further study of long-term outcome is necessary for evaluation of the clinical applicability of this technique of RYGB reversal.
Conflict of interest
The authors declare that they have no conflicts of interests.
Funding
The authors declare that they have no sources of funding for the research.
Ethical approval
This is an observational case report. No ethical approval is required.
Consent
Written informed consent was obtained from the patient for publication of this case report.
Authors’ contributions
EQ, TM, HR and JAK performed the clinical work-up, EQ and HLG planned and performed the tests, TM and JAK planned and performed the surgical procedures. CLR analyzed the GLP-1 samples. EQ, HLG, HR, CLR, TJB, TM and JAK interpreted the test results and wrote the manuscript.
Guarantor
EQ, JAK.
References
- 1.Marsk R., Jonas E., Rasmussen F., Naslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia. 2010;53:2307–2311. doi: 10.1007/s00125-010-1798-5. [DOI] [PubMed] [Google Scholar]
- 2.Foster-Schubert K.E. Hypoglycemia complicating bariatric surgery: incidence and mechanisms. Curr. Opin. Endocrinol. Diabetes Obes. 2011;18:129–133. doi: 10.1097/MED.0b013e32834449b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dirksen C., Hansen D.L., Madsbad S., Hvolris L.E., Naver L.S., Holst J.J., Worm D. Postprandial diabetic glucose tolerance is normalized by gastric bypass feeding as opposed to gastric feeding and is associated with exaggerated GLP-1 secretion: a case report. Diabetes Care. 2010;33:375–377. doi: 10.2337/dc09-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patti M.E., Li P., Goldfine A.B. Insulin response to oral stimuli and glucose effectiveness increased in neuroglycopenia following gastric bypass. Obesity. 2015;23:798–807. doi: 10.1002/oby.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilallonga R., van de Vrande S., Himpens J. Laparoscopic reversal of Roux-en-Y gastric bypass into normal anatomy with or without sleeve gastrectomy. Surg. Endosc. 2013;27:4640–4648. doi: 10.1007/s00464-013-3087-0. [DOI] [PubMed] [Google Scholar]
- 6.Mala T. Postprandial hyperinsulinemic hypoglycemia after gastric bypass surgical treatment. Surg. Obes. Relat. Dis. 2014;10:1220–1225. doi: 10.1016/j.soard.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 7.www.care-statement.org.
- 8.Geddes J., Wright R.J., Zammitt N.N., Deary I.J., Frier B.M. An evaluation of methods of assessing impaired awareness of hypoglycemia in type 1 diabetes. Diabetes Care. 2007;30:1868–1870. doi: 10.2337/dc06-2556. [DOI] [PubMed] [Google Scholar]