Highlights
-
•
Mammary hamartoma is a rare benign lesion accounting for approximately 4.8% of all benign breast masses.
-
•
There are several variants depending on the composition of glandular and fibroadipose elements including adenolipoma, fibroadenolipoma, and myoid hamartoma.
-
•
Histologically the lesions show a haphazard admixture of benign mammary glandular tissue, fat, and fibrous strands present in varying proportions.
-
•
Breast hamartomas are rarely associated with malignancies.
-
•
Excision of hamartoma is considered curative; though recurrence is seen in 8% of cases.
Keywords: Breast, Hamartoma, Mass
Abstract
Objectives
Mammary hamartoma is a rare benign lesion accounting for approximately 4.8% of all benign breast masses. It is often underdiagnosed and therefore is underreported mostly due to lack of awareness of the characteristic clinical and histological features. Raising awareness of this poorly recognized benign entity is of utmost significance as it clinically mimics other breast tumors including both benign and malignant ones. This study is to report and present our experience of breast hamartomas from Johns Hopkins Aramco Healthcare in the Eastern province of Saudi Arabia from which there have not been previous studies in literature.
Method
A retrospective review of our pathology files was done from 1994 to 2014 for cases diagnosed as breast hamartoma during this 20 year period.
Results
A total of 14 cases with diagnosis of breast hamartoma were identified in our institute. Histologically the lesion is mostly sharply demarcated showing a mixture of varying proportions of fibrous, adipose, and glandular tissue. 13 cases were seen in females (93%) and only one rare occurrence in a male patient (7%). The age ranges quite vastly from 18 to 51 years (mean 33 years). Two-third of these lesions were seen involving the right breast (9 cases/64.3%) and only one-third in the left side (5 cases/35.7%). 13 out of 14 patients had a well circumscribed lesion (92.9%) while only 1 case showed irregular borders (7.1%). The size varied from 1.4 to 9.5 cm. Three cases (21.4%) showed evidence of myoid differentiation, a histopathologic variance which is important to identify however has no clinical significance. 3 cases had associated epithelial ductal hyperplasia of the usual type varying from mild (2 cases) to moderate (1 case); with two of these cases exhibiting additional features of fibrocystic mastopathy including adenosis, apocrine metaplasia, and cyst formation. None of our cases showed any malignancy or pseudoangiomatous stroma hyperplasia (PASH).
1. Introduction
Breast hamartoma is a well-demarcated mostly encapsulated nodule representing as a mass composed of haphazardly arranged breast tissue components. The lesion was initially described in 1968 by Hogeman and Osbterg, and the term “hamartoma” was introduced back in the year 1971 by Arrigoni et al. There are several variants described depending on the composition of glandular and fibroadipose elements that include adenolipoma, fibroadenolipoma, and myoid hamartoma, the latter shows prominent smooth muscle proliferation. The pathogenesis of the development of a breast hamartoma is still not fully understood. Pathologically, hamartomas lack a distinguishing appearance. And clinically, it is often discovered incidentally during screening mammography, and is considered to be underdiagnosed. However, with the increase in the usage of breast diagnostic procedures such as ultrasound, mammography, core needle and fine needle aspiration biopsy, the number of breast hamartomas discovered is on the rise. It is important to note that the lesion can be easily missed if not appreciated by clinical examination or radiologically. Herein, we report 14 cases of breast hamartomas including 3 myoid hamartomas.
2. Materials and methods
We performed a retrospective review of all breast cases seen in our institute from 1994 to 2014. A total of 14 cases were identified (Table 1).
Table 1.
Demographics and clinicopathalogic features of 14 cases of breast hamartoma.
| # | Age | Site (R/L) | Lesion Size | Lesion Description | Sex | Diagnosis & Comment |
|---|---|---|---|---|---|---|
| 1 | 28 | Left | 2 × 1.6 × 1 cm | Discrete well circumscribed nodule | F | Mammary hamartoma |
| 2 | 51 | Left | 9.5 × 6.5 × 2.8 cm | Lobulated, well circumscribed nodule. Cut section; soft lobulated white tan tissue with foci of yellow fatty tissue and white fibrous tissue | F | Mammary hamartoma |
| 3 | 32 | Right | 3 × 2 × 1.5 cm | Well circumscribed whitish tan nodule with occasional clefts | F | Mammary hamartoma |
| 4 | 39 | Right | 3.5 × 1 × 1 cm | Fibrofatty nodular appearance | F | Mammary Hamartoma with focal moderate epithelial ductal hyperplasia of the usual type |
| 5 | 21 | Right | 4.3 × 4 × 2.8 cm | Well circumscribed firm tan fibrofatty nodule with smooth cut surface | F | Mammary hamartoma |
| 6 | 22 | Left | 1 × 0.8 × 0.5 cm | Well circumscribed nodule with homogenous rubbery tan cut surface | F | Mammary hamartoma |
| 7 | 35 | Right | 1.5 × 3.4 × 2.1 | Circumscribed tan white lobulated firm nodule with homogenous tan cut surface | F | Myoid Hamartoma |
| 8 | 42 | Left | 2 × 1 × 0.5 | Circumscribed nodular rubbery tissue with homogenous soft white cut surface showing area of yellow fatty tissue | M | Mammary hamartoma |
| 9 | 18 | Right | 2 × 1.4 × 0.7 cm | Oval nodule of rubbery white tan tissue with attached scanty fatty tissue and homogenous soft tan cut surface | F | Myoid hamartoma |
| 10 | 43 | Right | 5.3 × 3.8 × 2.7 cm | Lobulated fatty nodular tissue with irregular area of firm tan cut surface | F | Mammary hamartoma, fibroadenolipoma variant |
| 11 | 39 | Right | 5.5 × 4.5 × 3.5 cm | Nodule of fatty tissue with thin shiny capsule and homogenous yellow soft cut surface | F | Mammary hamartoma, adenolipoma variant. With fibrocystic changes including adenosis, apocrine metaplasia, and mild usual epithelial ductal hyperplasia without atypia |
| 12 | 42 | Right | 6 × 5.5 × 3 cm | Well circumscribed fibrofatty soft nodule | F | Mammary hamartoma |
| 13 | 35 | Left | 3 × 2.3 × 2.4 cm | Well circumscribed lobulated tan firm encapsulated nodule with white firm whorly cut surface | F | Myoid hamartoma. With Fibrocystic changes including mild epithelial ductal hyperplasia without atypia, adenosis, and cyst formation |
| 14 | 21 | Right | 5 × 4 × 2 cm | Nodular well circumscribed rubbery white tan nodule with tan yellow cut surface and foci of congestion and hemorrhage | F | Mammary hamartoma. With dominance of dense fibrous tissue |
3. Results
Upon retrospective review of all breast cases seen in our institute during the period of 1994–2014, a total of 14 cases of breast hamartomas were identified (Table 1). They all presented as solitary nodules, the majority being in female patients (93%) with only one case diagnosed in a male patient (7%). The age ranged from 18 to 51 years (mean, 33). Lesions were found in the right breast in a significantly higher amount in comparison to the left breast with a ratio of 9:5 (64.3% and 35.7% respectively). 13 of the 14 patients presented with well circumscribed nodules (92.9%), with only one case having a nodular lesion with irregular borders (7.1%). The size of the lesions ranged from 1.4 to 9.5 cm. Two of the lesions were fairly well encapsulated with the majority of others showing a rim of pseudocapsule.
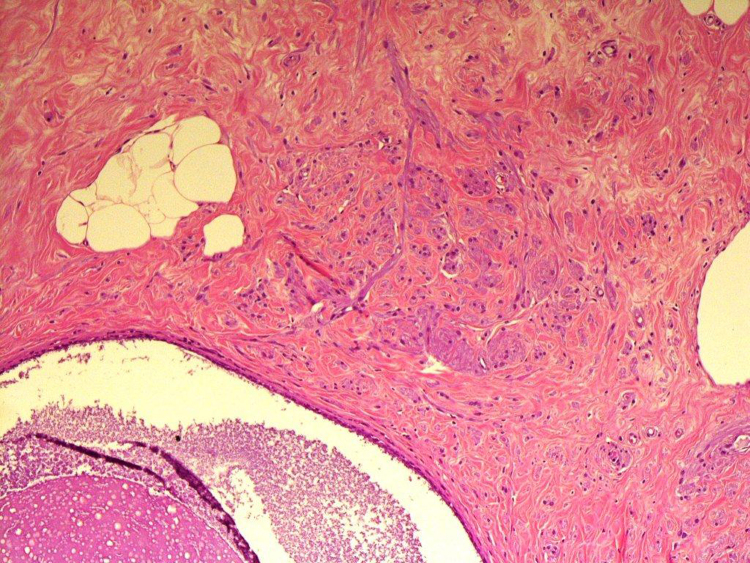
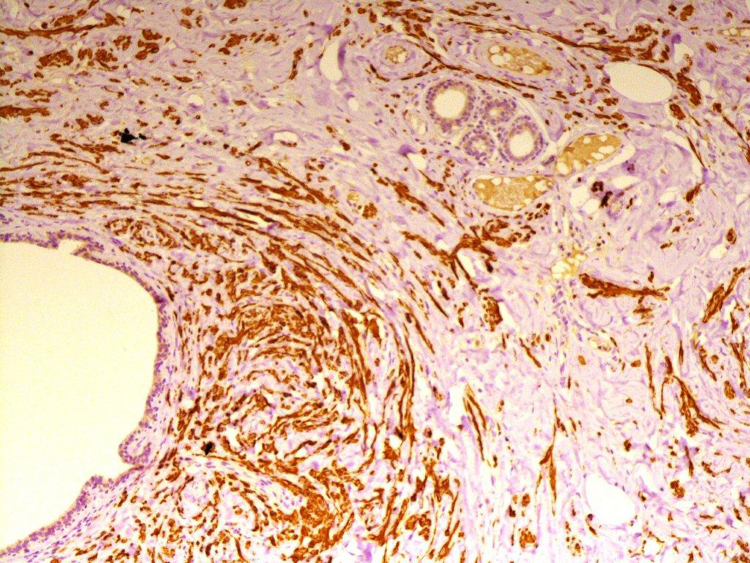
Histologically the lesions showed a haphazard admixture of benign mammary glandular tissue, fat, and fibrous strands present in varying proportions. Three cases, in addition, showed bundles of benign appearing spindled smooth muscle cells (Fig. 1, Fig. 2). Three cases showed additional fibrocystic changes with adenosis, apocrine metaplasia, cyst formation, and simple ductal epithelial hyperplasia of the usual type ranging from mild (2 cases) to moderate (1 case) hyperplasia without atypia. None of our cases had any evidence of atypical hyperplasia or malignancy whether in-situ or invasive.
Fig. 1.
Mammary glandular tissue admixed with adipose tissue and smooth muscle fibers in fibrotic background.
Fig. 2.
Desmin highlighting smooth muscle fibers in a myoid hamartoma.
Immunohistochemical studies were performed on 4 cases; CD34 stain was done in one case that showed focal positivity. S-100 protein was done on 2 cases with a negative result. P63 and CD10 were performed on 2 cases that highlighted myoepithelial cells. Desmin and Vimentin were done on one case and Actin was performed on two cases of Myoid hamartomas that all resulted in strong positivity.
4. Discussion
The word Hamartoma, Greek in origin meaning “Bodily defect”, refers to the presence of a disorganized mixture of components which are endogenous to a particular site. Hamartomas are considered benign non-neoplastic lesions that may recur. They can be found in various locations including but not limited to the lungs, kidneys, and breasts. Breast hamartomas are composed of a random mix of glandular epithelial, fibrous, and fatty tissue. Hogeman and Osbterg were the first to describe the lesion back in 1968 [1], while the actual term “hamartoma” was created in 1971 by Arrigoni et al. In 1994, a case series done by Charpin et al. concluded that breast hamartomas account for approximately 4.8% of all benign breast lesions [2]. The exact pathogenesis for breast hamartoma development remains a mystery. However, it is believed to be a result of a developmental abnormality rather than a true neoplastic process. An additional factor that has been noted to affect breast hamartoma development is female sex hormones; estrogen (ER) and progesterone (PR). Both the epithelial and stromal components of breast hamartomas may express hormone receptors [3]. In one study, Chiacchio et al. reported 9 out of the 10 breast hamartoma cases to be positive for both estrogen and progesterone receptors, while the remaining case showed estrogen receptor positivity and progesterone receptor negativity [4]. This study was later supported by Herbert et al. in 2002 who confirmed the presence of both estrogen and progesterone receptors in all of his 24 breast hamartoma cases [5].
The lesion occurs predominantly in premenopausal women, mostly in their 40s with a wide age range of teen age to women in their 80s. They are currently underdiagnosed, but with the surge of breast lump diagnostic tools usage, the number of diagnosed cases is on the rise.
Clinically, breast hamartomas are painless, usually mobile, soft to firm lumps typically found in the outer breast quadrants [6]. Such a clinical presentation often misleads physicians to think of other entities such as fibroadenoma amongst other tumors. Nearly 60% of breast hamartomas are non-palpable and are diagnosed radiologically. Ultrasound, mammography, magnetic resonance imaging, core needle and fine needle aspiration biopsy are more beneficial diagnostic utilities for breast hamartomas. Ultrasound shows these lesions to be well circumscribed with a smooth border and internally being hyperechoic or of heterogenous echogenicity. Breast hamartomas lack a retrotumor acoustic phenomenon which is one sonographic finding used to differentiate between benign and malignant lesions [7]. There might also be a halo separating most lesions from the surrounding tissue. This halo is mostly echogenic, composed of condensed breast tissue, but may appear to be anechoic, in which it is most likely formed of fat compressed between the hamartoma and the surrounding breast tissue. In one case series by Georgian et al., 94% of the cases were reported to show a thin echogenic halo [8]. On conventional T1 and T2 weighted MRI, breast hamartomas showed heterogenous intensities consistent with the results of other imaging modalities in addition to a thin capsule [9]. The appearance in mammography differs depending on the varying degree of each of its constituents. Classically, they appear nonhomogenous with dense nodules consisting of fibrous tissue surrounded by a thin radiopaque pseudocapsule formed from the displaced parenchyma of the breast. Sometimes, a hamartoma may appear to be homogeneously dense when it is rich in fibrous tissue making it hard to distinguish from a fibroadenoma, which is both uniformly dense and often has a thin radiolucent capsule of compressed fat. Despite the fact that they are used to accurately diagnose most breast lesions, needle core biopsy and fine needle aspiration cytology are not very helpful in diagnosing hamartomas and differentiating them from other entities which mimic them, mainly fibroadenomas. However, fibroadenomas can be distinguished from breast hamartomas by histological examination which typically shows the proliferation of ducts and/or stroma resulting in an intercanalicular or pericanalicular growth. On the other hand, breast hamartomas that consist mainly of fat may easily be misdiagnosed as lipoma, fat necrosis, or a cyst [10].
Grossly, the lesion is round to oval and may measure up to 20 cm in size. The cut surface mostly resembles normal breast parenchyma or fibroadenoma, again, depending on the consistency of the lesion. Histologically, hamartomas are mostly encapsulated whether by a true capsule or a surrounding rim of pseudocapsule. The lesion is lobulated and exhibits mammary ducts, lobules, fibrous tissue, and adipose tissue in varying proportions. Some cases may show additional changes including pseudoangiomatous stromal hyperplasia (PASH), and prominent smooth muscle proliferation [3].
A rare subtype of breast hamartomas is myoid hamartoma, which is known to contain varying amounts of smooth muscle cells within its stroma. They are typically well-circumscribed lesions that clinically appear to be firm, mobile, well demarcated, and of high density in mammography. Myoid hamartomas are considered to be an exclusively female tumor; however, one single case in a male patient has been reported by Ravakhah et al. [11]. The origin of the smooth muscle seen in these hamartomas has not been fully supported in detail by any study in literature; yet potential sources include blood vessel walls, areolar muscularis tissue, undifferentiated breast stroma, and myoepithelial cells. Another hypothesis concerning the origin of smooth muscle is by metaplasia of the breast stromal cells. Presence of CD34 is believed to be a crucial indicator of metaplasia of breast stromal cells into smooth muscle [12]. Other immunohistochemical markers commonly sought out in breast hamartomas, specifically myoid hamartomas, include desmin, vimentin, and SMA. The general belief however is that myoid hamartomas reflect nodular sclerosing adenosis with hyperplasia of myoid myoepithelial cells by some and an adenomyoepithelioma by others [13].
There were attempts in the past to try to classify breast hamartomas into specific categories based on their histological appearance. Fibrous, fatty, and fibro-fatty were the categories created by McGuire et al., while adenolipoma, fibroadenoma-like, fibroadenoma with a fibrous stroma, and encapsulated fibrocystic changes were categories created by Jones et al. [2]. These classifications are not generally accepted by most authorities, mostly since they are not really easily reproducible and have no clinical impact.
Very seldom, breast hamartomas have been associated with malignancies. Only a few cases are reported in literature of breast hamartomas that are associated with in-situ or even invasive carcinomas.
Hamartomas are mostly benign lesions with recurrence seen in 8% of cases. It is speculated that these cases most likely represent multifocal disease rather than true recurrence.
Multiple breast hamartomas have been associated with certain genetic abnormalities, specifically Cowden syndrome which is also known as Multiple Hamartoma syndrome. Genetic data is limited; however, alterations involving chromosomal regions 12q12-15 and 6p21 have been described in literature [3].
Today, surgical excision of breast hamartomas is considered to be curative. Generally, they have an excellent prognosis with or without surgical excision. Some suggest merely observing the hamartoma without excision if diagnosis can be confirmed by mammography [14]; however, since there have been reports of malignancies associated with breast hamartomas, the general recommendation is surgical excision of the lesion. Seeing as most of these breast hamartomas are circumscribed, often encapsulated with well-delineated borders, they can be easily enucleated [13]. Post-operatively, patients are suggested to follow up with mammography and ultrasound every 6 months for 1–2 years to ensure stability of their current condition and monitor for any recurrence [15].
5. Conclusion
Breast hamartoma is a rare benign lesion composed of varying degrees of normal breast components including glandular, fatty, and fibrous tissue. It is underdiagnosed and thus underreported. However, there are increasing rates of breast hamartoma diagnoses due to the huge surge of usage of breast diagnostic procedures such as ultrasound and mammography. Breast hamartomas are rarely associated with malignancies, and excision is considered curative. Yet recurrence is seen in about 8% of reported cases. Clinicians in particular surgeons need to be aware of this entity as a differential diagnosis of a breast mass in particular if it is well demarcated.
Consent
Not applicable.
Author contribution
Rawan Amir: Writing the manuscript.
Salwa Sheikh: Reviewer.
Acknowledgments
There is no conflict of interest or financial acknowledgments for this case report.
References
- 1.Hogamen K., Ostberg G. Three cases of pastlactational breast tumour of peculiar type. Acta Pathol. Microbiol. Scand. 1968;73:169–176. doi: 10.1111/j.1699-0463.1968.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 2.Sevim Y., Kocaay A., Eker T., Celasin H., Karabork A., Erden E., Genc V. Breast hamartoma: a clinicopathologic analysis of 27 cases and a literature review. Clinics (Sao Paulo) 2014;69(8):515–523. doi: 10.6061/clinics/2014(08)03. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4129555/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.S. Lakhani, I. Ellis, S. Schnitt, P. Hoon Tan, M. Van de Vivjer (Eds.). WHO Classification of Tumours of the Breast. WHO Classification of Tumours, WHO Press, Geneva, Switzerland, 4, (2012), 147.
- 4.Chiacchio R., Panico L., D'antonio A., Delrio P., Bifano D., Avallone M., Pettinato G. Mammary hamartomas: an immunohistochemical study of ten cases. Pathology—Res. Pract. 1999;195(4):231–236. doi: 10.1016/S0344-0338(99)80039-9. http://www.ncbi.nlm.nih.gov/pubmed/10337660. [DOI] [PubMed] [Google Scholar]
- 5.Herbert M., Sandbank J., Liokumovich P., Yanai O., Pappo I., Karni T., Segal M. Breast hamartomas: clinicopathological and immunohistochemical studies of 24 cases. Histopathology. 2002;41(1):30–34. doi: 10.1046/j.1365-2559.2002.01429.x. http://www.ncbi.nlm.nih.gov/pubmed/12121234 [DOI] [PubMed] [Google Scholar]
- 6.Tatar C., Erozgen F., Tuzun S., Karsidag T., Yilmaz E., Aydin H., Ozer B. Surgical approach to breast hamartoma and diagnostic accuracy in preoperative biopsies. J. Breast Health. 2013;9:186–190. http://thejournalofbreasthealth.com/sayilar/33/buyuk/186-90.pdf [Google Scholar]
- 7.Chao T., Chao H., Chen M. Sonographic features of breast hamartomas. J. Ultrasound Med. 2007;26(4):447–452. doi: 10.7863/jum.2007.26.4.447. http://www.jultrasoundmed.org/content/26/4/447.abstract Retrieved from. [DOI] [PubMed] [Google Scholar]
- 8.Cazorla S., Arentz C. Breast hamartomas—differntial consideration in slow developing breast asymmetry. JPRAS Open. 2015;3:17–21. http://www.sciencedirect.com/science/article/pii/S2352587814000096 Retrieved from. [Google Scholar]
- 9.Erdem G., Karakas H., Isik B., Firat A. Advanced MRI findings in patients with breast hamartomas. Diagn. Interv. Radiol. 2011;17:33–37. doi: 10.4261/1305-3825.DIR.1892-08.2. http://www.dirjournal.org/sayilar/34/buyuk/pdf_DIR_326.pdf Retrieved from. [DOI] [PubMed] [Google Scholar]
- 10.Farrokh D., Hashemi J., Ansaripour E. Breast hamartoma: mammographic findings. Iran. J. Radiol. 2011;8(4):258–260. doi: 10.5812/iranjradiol.4492. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3522368/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravakhah K., Javadi N., Simms R. Hamartoma of the breast in a man: first case report. Breast J. 2001;7(4):266–268. doi: 10.1046/j.1524-4741.2001.20079.x. http://www.ncbi.nlm.nih.gov/pubmed/11678806. [DOI] [PubMed] [Google Scholar]
- 12.Kajo K., Zubor P., Danko J. Myoid (Muscular) hamartoma of the breast: case report and review of the literature. Breast Care. 2010;5(5):331–334. doi: 10.1159/000321341. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3132958/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavassoli F., Eusebi V. Tumours of the mammary gland. AFIP Atlas Tumor Pathol. 2009;4:21–35. [Google Scholar]
- 14.Bope E., Kellerman R. Elsevier Health Sciences; 2016. Conn's Current Therapy. [Google Scholar]
- 15.McGarry K. 2nd ed. Lippincott Williams & Wilkins; 2013. The 5 Minute Consult Clinical Companion to Women's Health. [Google Scholar]