Abstract
Central chemoreception traditionally refers to a change in ventilation attributable to changes in CO2/H+ detected within the brain. Interest in central chemoreception has grown substantially since the previous Handbook of Physiology published in 1986. Initially, central chemoreception was localized to areas on the ventral medullary surface, a hypothesis complemented by the recent identification of neurons with specific phenotypes near one of these areas as putative chemoreceptor cells. However, there is substantial evidence that many sites participate in central chemoreception some located at a distance from the ventral medulla. Functionally, central chemoreception, via the sensing of brain interstitial fluid H+, serves to detect and integrate information on 1) alveolar ventilation (arterial PCO2), 2) brain blood flow and metabolism and 3) acid-base balance, and, in response, can affect breathing, airway resistance, blood pressure (sympathetic tone) and arousal. In addition, central chemoreception provides a tonic ‘drive’ (source of excitation) at the normal, baseline PCO2 level that maintains a degree of functional connectivity among brainstem respiratory neurons necessary to produce eupneic breathing. Central chemoreception responds to small variations in PCO2 to regulate normal gas exchange and to large changes in PCO2 to minimize acid-base changes. Central chemoreceptor sites vary in function with sex and with development. From an evolutionary perspective, central chemoreception grew out of the demands posed by air vs. water breathing, homeothermy, sleep, optimization of the work of breathing with the ‘ideal’ arterial PCO2, and the maintenance of the appropriate pH at 37°C for optimal protein structure and function.
Introduction
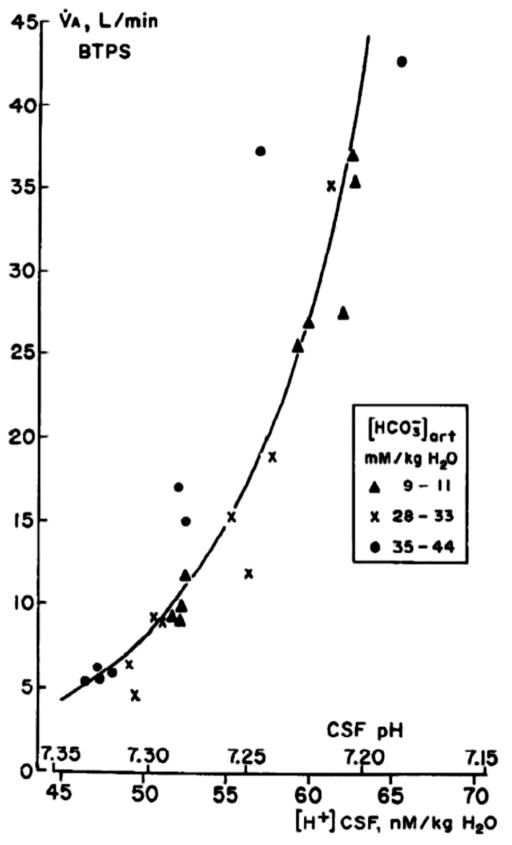
Central chemoreception refers to the detection of changes in CO2/H+ within the brain and the associated effects on breathing. In the conscious animal the response of ventilation to changes in brain interstitial fluid (ISF) pH is very sensitive (63, 199). Fig. 1 shows the relationship of alveolar ventilation to cerebrospinal fluid (CSF) pH in a single conscious goat subjected to both chronic acid-base disorders and acute CO2 inhalation. Note that a small change in CSF pH from 7.30 to 7.25 is associated with a doubling of alveolar ventilation; it is a very sensitive reflex response (63, 199). Note also that the relationship of alveolar ventilation to ISF pH is essentially the same for both types of stimulation, metabolic acid-base disorders and primary CO2 stimulation. The traditional concept of the function of central chemoreception is that it, along with peripheral chemoreception at the carotid body, 1) regulates arterial PCO2 within normal limits in response to primary changes in CO2, and 2) regulates blood and body pH in response to acid-base disturbances (166). The primary signal detected is thought by most to bepH either within or at the membranes of sensor cells although recent data suggests that CO2 itself can participate via glial activation (107) (see Cellular basis of CO2 sensitivity in neurons/glia). In the first case, the pH change arises from a primary alteration in the ratio of alveolar ventilation to CO2 production, which affects PCO2 and pH bringing about a ventilatory response that acts to correct the disturbance; in the second case the pH change arises from a metabolic or renal alteration in H+ ion balance, which affects ventilation and as a result PCO2 in a manner that acts to minimize the initial H+ disturbance. In the first case, chemoreceptor stimulation minimizes a primary disturbance in PCO2; in the second case, it changes PCO2 essentially using ventilation to minimize changes in pH of metabolic origin.
Figure 1. The ventilatory response to changes in brain interstitial fluid pH as studied in conscious goats.
The response of alveolar ventilation to CO2 inhalation in normal acid-base conditions (X) as well as in chronic metabolic acidosis (solid triangles) and alkalosis (solid circles) in a conscious goat. Fencl et al., Am. J. Physiol. 1966 (65), used with permission.
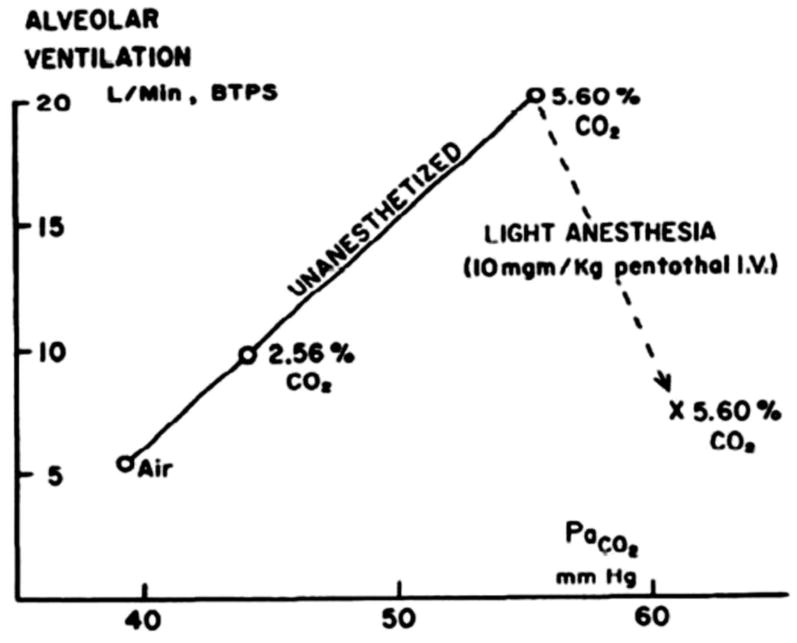
In this Chapter we ask the following questions about central chemoreception: 1) Where are central chemoreceptors? What kinds of neurons are involved? Are glia involved? 2) What are the functions of central chemoreceptors? Are they limited to arterial PCO2 and acid-base homeostasis? 3) How did central chemoreception evolve? Is there a unifying theory to explain how pH sensitivity, a ubiquitous property of proteins, is channeled by the whole organism to function in an integrative manner? We will not discuss specific mechanisms of pH detection but will discuss generic theories of such. Our emphasis in this Chapter, as in our own experimental approach, is on data obtained on the whole animal, especially in the unanesthetized animal studied in wakefulness and sleep. We are systems physiologists and our goal is to understand how central chemoreception operates within the awake (or sleeping) animal and the description of this function is the goal of this Chapter. One axiom for our work is the fact that anesthesia profoundly depresses chemoreception thus making it difficult to interpret the physiological significance of findings obtained under anesthesia and making it impossible to understand central chemoreceptor function in different arousal states. Fig. 2 shows the relationship of alveolar ventilation to arterial PCO2 in a conscious goat comparing the response in the unanesthetized state to that following light anesthesia (199). Note the marked decrease in response sensitivity. Effects of anesthesia on studies of the control of breathing have long been recognized. For example, lesions of the pontine regions involved in the control of breathing have large effects on the breathing pattern when studied under anesthesia. The animals breath more slowly and with larger breaths (60). But when such lesions are placed and then studied in unanesthetized animals there is little effect unless the animal is once again anesthetized (241).
Figure 2. The effect of anesthesia on the CO2 response.
Effect of light anesthesia on the ventilatory response to inhaled 2.56% and 5.6% CO2. While breathing 5.6% CO2, 10 mg/kg sodium pentothal was injected through a catheter in the jugular vein. Subsequent measurements were made during the last 5 min of a 15-min period of anesthesia. Pappenheimer et al., Am. J. Physiol. 1965 (200), used with permission.
Central chemoreception has been the focus of many recent reviews (31, 85, 86, 170, 177, 206), the 2010 Comroe Lecture (177), a Mini-series of nine papers in the Journal of Applied Physiology (40), and a Special Issue in Respiration Physiology and Neurobiology (188). The related topic of brain and cerebrospinal fluid acid-base regulation has also been recently reviewed (164, 165, 167).
The location of central chemoreceptors
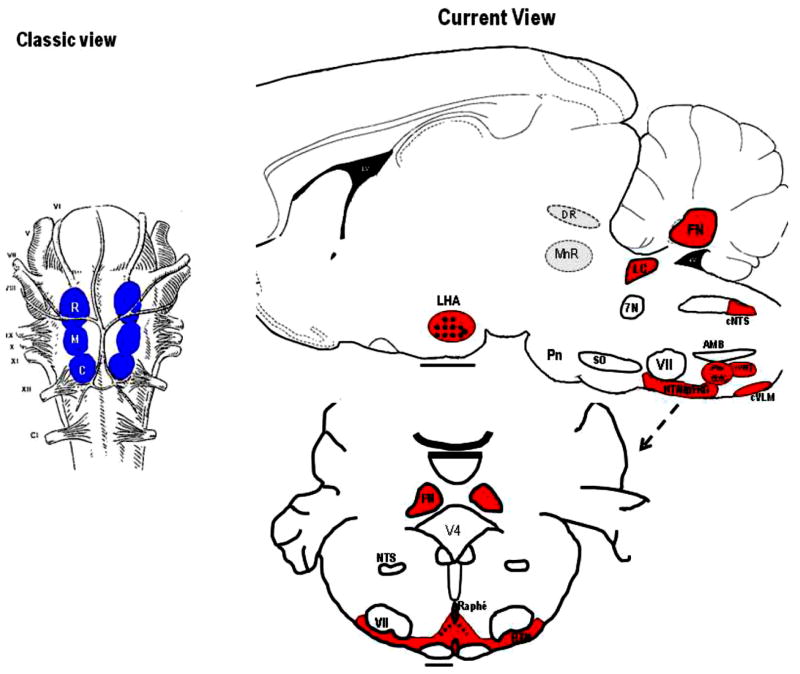
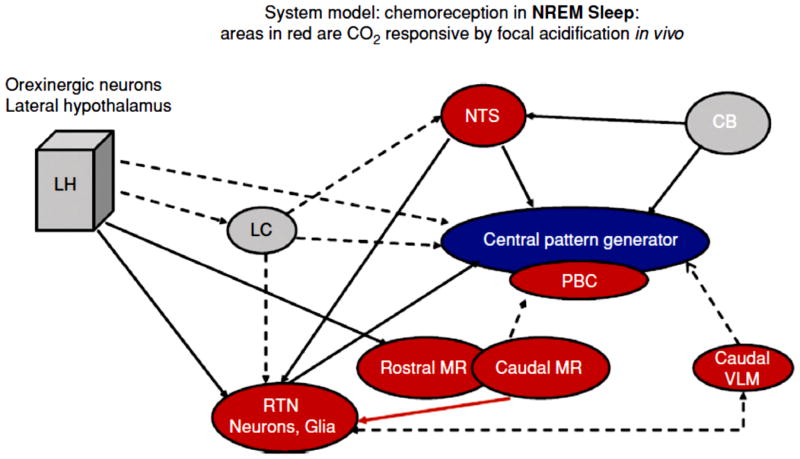
Central chemoreceptors, first localized to areas on the ventral surface of the medulla, now are thought to be present in many locations within the brainstem, cerebellum, hypothalamus and midbrain (133, 143, 144, 158, 166, 226, 257). Fig. 3 shows at left the Classic View of the location of central chemoreceptors on the ventral medulla with shaded areas outlining the three traditional areas (rostral, intermediate, caudal) while on the right the Current View of the location of central chemoreceptor areas in a variety of sites is summarized (27, 61, 128, 166, 168, 171). Leuson (133) showed in 1954 that application of acidic fluids within the cerebral ventricles produced an increase in breathing thus demonstrating the presence of central chemoreception. Subsequent application of such fluids to different locations led to the discovery of two aspects of the ventral medullary surface that were proposed as the central chemosensitive areas (143, 158, 257). The two areas included a rostral area located approximately at the rostral-caudal level of the more deeply located facial nucleus and a caudal area located approximately at the level of the hypoglossal nerve rootlets. This discovery and its schematic depiction has held an almost mystical sway on respiratory physiologists. However, these initial experiments were performed under anesthesia and required large pH changes in order to obtain effects on breathing. Questions were raised as to whether the cells involved in sensing these pH changes might be located more deeply within the medulla, an idea that is compelling given the anatomy of medullary blood vessel distribution, namely that the vessels originate on the ventral surface and penetrate into the medulla, and the presence of Virchow-Robins spaces, invaginations along blood vessels penetrating from the ventral surface that allow cerebrospinal fluid (and acidic pH fluids) easy access to deeper sites (see (164). Fig. 4 shows a view of the ventral medulla, the blood vessels filled with a visible dye, that demonstrates the profuse vascular network on the surface, which penetrates dorsally into the medulla. No cell is far from a source of blood supply.
Figure 3. Schematic location of central chemoreceptor sites.
Locations of central chemoreceptors; the classic view: chemoreception located at ventral medullary surface (left panel) and the current view: chemoreception is widely distributed in hindbrain (right panel). Abbreviations: R, rostral; M, middle; C, caudal; LHA, lateral hypothalamus; DR, dorsal raphe; FN, fastigial nucleus; 4v, fourth ventricle; LC, locus ceruleus; 7N, facial nerve; cNTS, caudal nucleus tractus solitarious; AMB, ambiguous; VII, facial nucleus; SO, superior olive; PBC, pre-BÖotzinger Complex; rVRG, rostral ventral respiratory group; cVLM, caudal ventrolateral medulla; RTH/pFRG, retrotrapezoid nucleus/parafacial respiratory group; and Pn, pons [modified from Figure 1 in Nattie (168) and used with permission].
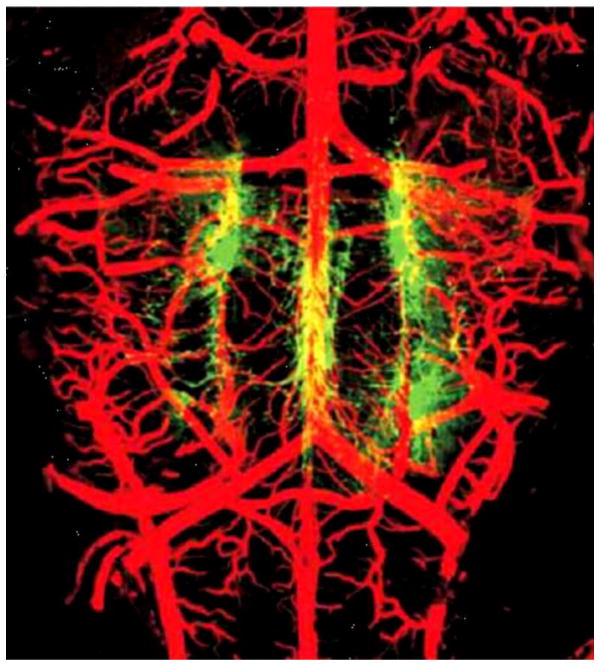
Figure 4. Blood vessels and serotonergic neurons on the ventral medullary surface.
A confocal image of arteries filled with fluorescein-tagged albumin (red) and serotonergic neurons stained with anti-TPH antibody (green). (A) TPH-IR neurons and arteries seen in on the ventral surface of the medulla en bloc; filled vessels include arteries and some veins. [Adapted with permission from Macmillan Publishers Ltd: Nature Neuroscience (20), 2002.]
Cells responsive to pH changes and located more deeply within the medulla were described in vivo (7, 109, 122) and in vitro in medullary slices with focal localization within the caudal aspect of the nucleus tractus solitarius (39) an interesting location in that this region is a primary relay site for afferents arising from the peripheral chemoreceptor, the carotid body. The issue of whether or not central chemoreceptors were solely located at one site just beneath the ventral medullary surface was fully opened to scrutiny by the findings of Lee Coates et al., (27, 28). Initially, Coates was asking whether acetazolamide, a known respiratory stimulant, produced its effects in the brainstem by simply applying it to the ventral medullary surface in anesthetized animals (28). Phrenic nerve activity, the measure of ventilatory output, increased and tissue pH decreased at the site of acetazolamide application; the mechanisms by which acetazolamide increases ventilation include stimulation of central chemoreceptors by a brain tissue acidosis. Acetazolamide was then applied by microinjection at a variety of regions to ask if specific sites would, when acidified by the focal injection of acetazolamide, increase ventilatory output (27). One nl injections of acetazolamide (5–10 μM) into the brainstem of anesthetized cats and rats in vivo decreased tissue pH in a focal, localized manner with a measured stimulus intensity like that associated with a 36 mm Hg increase in arterial PCO2 and a region of decreased pH limited to within 350 um from the center of the injection. Using focal acetazolamide injections as a probe for central chemoreceptor sites, ventilatory responses to injections were uncovered at the: 1) ventrolateral medullary surface (within 800 μm of the surface) at locations dorsal to the traditional rostral and caudal chemosensitive areas, 2) nucleus tractus solitarii, 3) locus coeruleus, 4) rostral aspect of the medullary raphé (raphé magnus), 5) pre-Bötzinger complex (237), and 6) fastigial nucleus of the cerebellum (124, 269). While the stimulus intensity, although focally applied, was large, and the use of a drug raised the issue of non-specific effects, these data nevertheless suggested the presence of a widespread distribution of central chemoreceptor sites that can affect breathing in the anesthetized animal in vivo. The presence of anesthesia likely explains the need for such a large focal stimulus intensity.
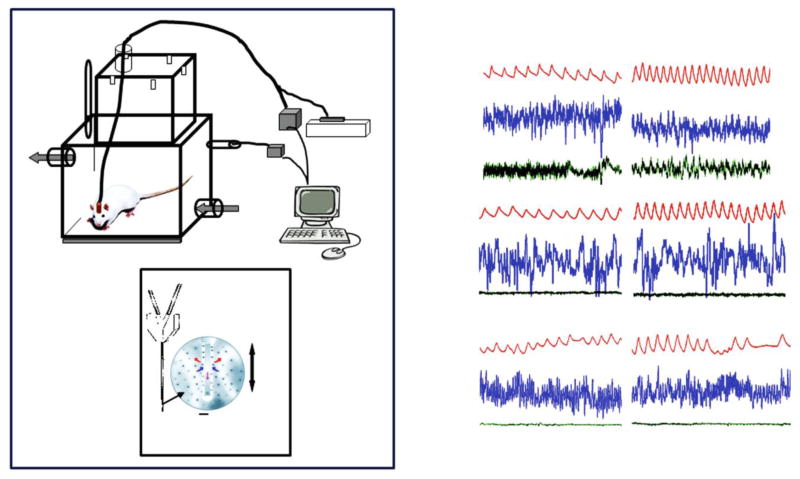
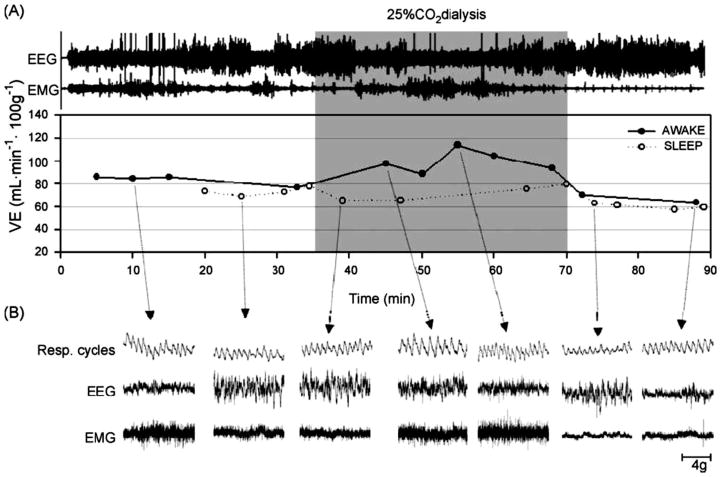
The challenge then became to find a method to produce a focal acidosis within specific brain regions in unanesthetized animals, a difficult task in that blood flow can quickly clear exogenously added CO2. Li et al., (4, 138, 140) discovered that by using reverse microdialysis with artificial cerebrospinal fluid (aCSF) equilibrated with 25% CO2 together with a high dialysis flow rate they could induce a steady-state situation of CO2 delivery and removal by brain blood flow that produced a focal tissue acidosis. Under anesthesia, this approach reduced tissue pH measured by microelectrode in situ by an amount like that found with an increase in arterial PCO2 of 35 mm Hg with the region of acidosis limited to within 550 μm of the probe tip. In conscious rats, this same dialysis in the retrotrapezoid nucleus (RTN; see below) decreased focal pH measured within 200 μm of the dialysis probe by much less, an amount equivalent to that induced by a 6.6 mm Hg increase in arterial PCO2 (138). Dialysis with aCSF equilibrated with 5% CO2 did not change brain pH. Fig. 5A shows a schematic drawing of this set-up, which allows measurement of ventilatiopn and metabolism during defined arousal states with focal dialysis in specific brain regions. Fig. 5B shows an example of recordings of breathing made in wakefulness, NREM and REM sleep. Using the dialysis technique, the Forster lab (97, 98) compared the pH change produced by dialysis of aCSF equilibrated with different concentrations of CO2 in vivo and in vitro. The pH change measured in vitro would reflect that throughout the unbuffered aCSF solution while that in vivo would reflect in addition the powerful ‘buffer’ effect of local cerebral blood flow. The measured pH change in vivo was on average about 20% of the in vitro change. This observation plus the observed difference in the degree of the brain tissue pH response to the same dialysis conditions in the anesthetized vs in the conscious rat described above underscores the importance of cerebral blood flow in the clearance of exogenous as well as of endogenous CO2. This technique was applied in the conscious, unanesthetized rat to examine putative central chemoreceptor sites using a physiologically relevant stimulus, one that is equivalent to a 6.6 mm Hg increase in arterial PCO2, focally applied at different regions. Note also that this approach does not directly affect the peripheral chemoreceptor and can be applied in different arousal states. The focal stimulus intensity is considerably milder than that associated with using 5 or 7 % inspired CO2 as a central chemoreceptor stimulus. When breathing 7% CO2 the arterial PCO2 in the conscious rat increased by 15 mm Hg and the degree of brain tissue acidosis was double that observed with focal acidification by dialysis (138).
Figure 5. Schematic view of set-up for study of conscious rats in wakefulness and sleep.
Drawing of experimental set-up including blow-up of dialysis probe tip (Panel A) and example of typical tracings (Breathing, EEG, neck EMG) in air and 7% CO2 during wakefulness (AW), non-rapid eye movement (NREM), and rapid eye movement (REM) sleep (Panel B).
Using this approach, eight putative central chemoreceptor sites have been identified (Fig. 3): 1) the retrotrapzoid nucleus (RTN) (138, 140), 2) the rostral medullary raphe (MR) (raphe magnus) (97, 182), 3) the caudal MR (97, 98) (raphe obscurus) indirectly via the RTN (45), 4) the caudal NTS (181), 5) the region just dorsal to the caudal ventral medullary surface (35), 6) the pre-Bötzinger region (124), 7) the fastigial nucleus of the cerebellum (152), and 8) the orexin neuron containing regions of the hypothalamus (Li N, Li A, Nattie, E; personal communication). Thus a mild focal acidosis at many, but not all, brainstem sites can stimulate ventilation.
The ventral medulla
Here we focus on three specific anatomical regions within the ventral medulla, which are likely the sources of chemosensitivity for the initially described ventral medulla surface chemosensitive areas.
Retrotrapezoid nucleus
The retrotrapezoid nucleus (RTN) was originally identified and named by Smith et al. (236) as a cluster of neurons situated between the ventral border of the facial nucleus and the ventral medullary surface that was labeled by retrograde tracer after injection into the dorsal and ventral respiratory groups. At the same time, we had observed in anesthetized cats (Fig. 6) a dramatic decrease in phrenic nerve activity, often to apnea, and a severely reduced response to increased CO2 and to hypoxia after a 10 nl injection of kainic acid (4.7 mM) (186) at sites within 400 microns of the ventral medullary surface, which appeared to be within the RTN as described by Smith et al., (236). We subsequently explored this region in a series of studies initially in anesthetized then in unanesthetized rats. Using kainic acid to produce lesions we showed that the RTN contributes significantly to the CO2 response, more so in anesthesia than in wakefulness (4, 178, 183, 187). In conscious, unanesthetized rats with prior unilateral kainic acid induced lesions of the RTN there is no detectable effect on baseline resting breathing but the response to hypercapnia is reduced by 39% (4) an effect that differs markedly from the apnea and absent CO2 response observed in anesthetized animals with similar lesions (183). In fact, the effect of RTN inhibition or disruption on breathing under anesthesia was so powerful that it precluded the design of experiments to be performed in wakefulness as any perturbation performed while the rat was anesthetized often prohibited recovery. The application of different lesion producing techniques helped with this problem. First, taking advantage of the fact that the RTN is rich in its expression of neurokinin 1 receptors (NK1R), injections were made into the RTN of a conjugate of substance P (the NK1R ligand) to the mitochondrial toxin, saporin (184, 265). The conjugate is internalized into RTN cells and with release of saporin the cells are killed, a process that takes hours to days allowing the rats to recover from anesthesia before RTN cell death occurs. These rats had a 30% decrease in the CO2 response present both in wakefulness and in NREM sleep that was associated with a 44% loss of NK1R immunoreactivity in the RTN (184). These rats also had a decrease in ventilation under resting conditions along with an increase in arterial PCO2 indicating the loss of a tonic drive from RTN NK1R-expressing neurons. Second, taking advantage of the fact that RTN neurons express Phox2b (vida infra), injections of a lentivirus construct containing the PRSx8 promoter specific for Phox2b neurons along with allatostatin, the drosophila receptor, were made into the RTN region (149). Post mortem estimates via EGFP immunohistochemistry indicated 50–64% transduction of RTN neurons. In conscious rats, specific inhibition by injection of allatostatin reduced the CO2 response by ~ 60%, a substantial effect, without alteration of resting ventilation (149). Other experiments substantiated this chemoreceptor role for RTN neurons. The acute use of reverse microdialysis with a GABA receptor agonist, muscimol, into the RTN of conscious rats inhibited the CO2 response (172) while focal GABA receptor antagonism with bicuculline increased baseline ventilation (174) showing the presence of a tonic GABAergic inhibition in the RTN of unknown origin. And, focal acidification of the RTN in the rat increased ventilation in wakefulness but not sleep by about 24% due to increases in tidal volume (Fig. 7) (140).
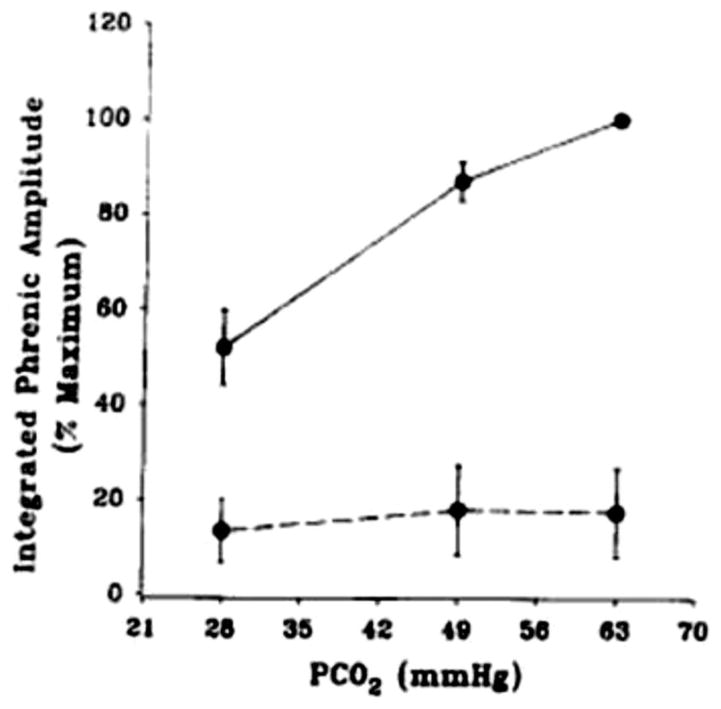
Figure 6. Lesions in the RTN region in anesthetized rats reduces the CO2 response dramatically.
The average response of integrated phrenic amplitude to increased end-tidal PCO2 before (solid line) and after (dotted line) injection of 100 nl kainic acid into the retrotrapezoid nucleus. These rats were anesthetized initially with halothane followed by chloralose-urethane. Mean +/− SEM values are shown (n = 4). (Reprinted from Respiration Physiology, 97, Nattie EE, and Li A. Retrotrapezoid nucleus lesions decrease phrenic activity and CO2 sensitivity in rats. 63–77, 1994, (183) with permission from Elsevier.)
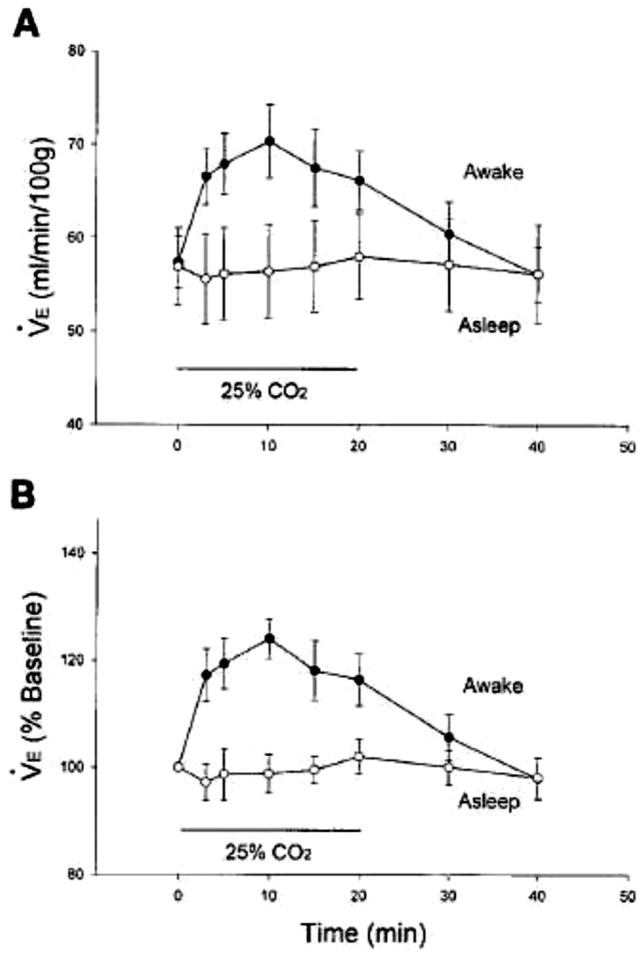
Figure 7. Focal acidification in the RTN increases ventilation in wakefulness.
Ventilation in absolute terms (A) and expressed as % baseline (B) in unanesthetized rats (n = 7) dialyzed with 25% CO2 in the retrotrapezoid nucleus during wakefulness (solid circles n = 13 trials) and behaviorally defined sleep (open circles, N = 10 trials). Mean +/− SE values are shown. Control room air values were obtained before and after 20-min period of dialysis. The 4 pre-exposure control values were combined into single value. Ventilation during focal RTN acidification was significantly greater during wakefulness when expressed in absolute terms or as % baseline. Note that ventilation increased to 24% of baseline. There was no response during sleep. Li et al., J. Appl. Physiol. 1999, (140), used with permission.
A series of parallel studies in conscious goats added important information on the functional significance of the rostral ventrolateral medulla (RVLM), a larger region that included the RTN as well as portions of the nucleus paragigantocellularis lateralis (PGCL) and the parapyramidal neurons of the caudal midline raphe. Cooling of RVLM neurons in goats by the placement of thermodes on the surface had much smaller effects on baseline and CO2 stimulated breathing when the goat was awake as compared to being anesthetized (69, 192). However, if the disruption of the RVLM is substantial in size, for example, by bilateral coagulation or bilateral and large neurotoxin injections (224), one can observe hypoventilation as well as reduced CO2 sensitivity, even in the unanesthetized state. It is not clear to what extent these experiments affected other, deeper regions. Overall, the effects of RVLM disruption on breathing are more dramatic when studied under anesthesia and in the absence of anesthesia, any remaining response to increased CO2 in the presence of RVLM disruption likely originates from other central chemoreceptor locations. Input to the brainstem from sources other than central chemoreceptors also likely contributes to the responses to RVLM disruption. For example, cooling of neurons in the RVLM has a slightly greater inhibitory effect on breathing in sleeping, unanesthetized goats than in awake goats. And peripheral chemoreceptor denervation together with surface cooling in sleep produces long lasting apnea (192). This suggests that carotid body and the RVLM (and perhaps other sources) together support respiration during sleep (192) (vida infra).
The Guyenet laboratory has studied the RTN region in anesthetized rats and has identified the specific types of neurons that account for the sensitivity to CO2/H+ along with the afferent and efferent connectivity of the RTN (80–82, 86, 160, 218, 242). With results similar to earlier work from our laboratory (179) they showed that the activity of RTN neurons is largely tonic but becomes more respiro-phasic if CO2 is increased (83) and they demonstrated afferent connections from a variety of brainstem sources (132) as did others (32). They have also shown that RTN neurons project to the main groups of brainstem respiratory neurons, respond to CO2/H+ in vivo and in slice preparations in vitro, receive functional afferent inputs from the peripheral chemoreceptor, the carotid body (250), and from the posterior hypothalamus (71), regions that are also chemoresponsive in vivo and in vitro. This lab has made a substantial contribution to the study of the RTN and central chemoreception via the identification, thru a series of beautiful studies, of the chemical phenotype of the RTN chemosensitive neurons. Fig. 8 summarizes some of their findings and demonstrates their approach. RTN neurons that respond to CO2/H+ in vitro and in vivo express the glutamate transporter VGLUT2 and the autonomic nervous system fate determining gene, Phox2b (118, 242), and some RTN neurons also express galanin (243). This phenotype characterization has led to the development of interesting ideas regarding chemoreception and the RTN and to experiments in vivo that alter the function of these specific neurons. For example, it has been shown via optical activation of channelrhodopsin 2 targeted to RTN neurons that breathing is stimulated, i.e., these neurons can when selectively activated increase breathing (1, 117, 149).
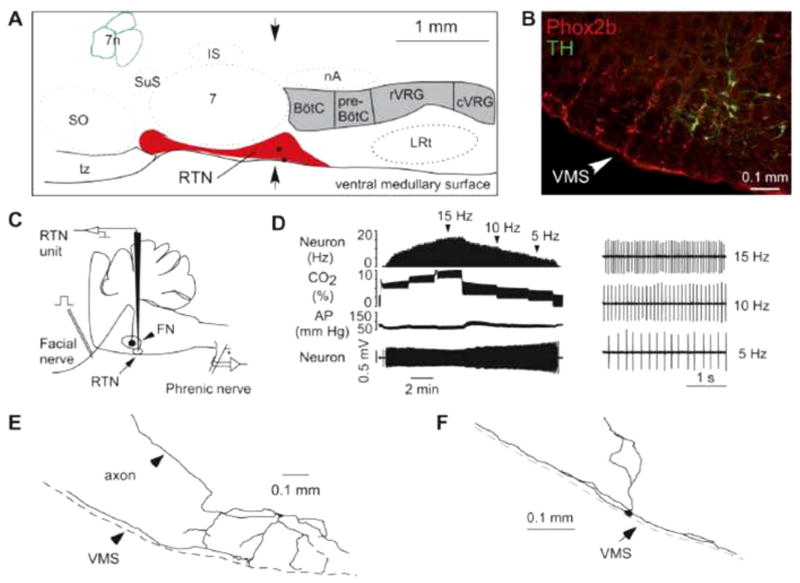
Figure 8. The location of putative chemoreceptor neurons in the RTN.
Location and general characteristics of retrotrapezoid nucleus (RTN) neurons. A: schematic but correctly scaled drawing of a parasagittal section through the pontomedullary region of the adult rat showing the location of the RTN. BötC, Bötzinger region of the ventral respiratory column; preBötC, pre-Bötzinger region; rVRG, rostral ventral respiratory group; cVRG, caudal ventral respiratory group; IS, inferior salivary nucleus; LRt, lateral reticular nucleus; nA, nucleus ambiguus pars compacta; SO, superior olive; tz, trapezoid body; 7, facial motor nucleus; 7n, seventh nerve. The two black dots are the cell bodies of the neurons shown in E and F. B: coronal section at the level indicated by the arrows in A showing the distribution of neurons that express Phox2b (left side of brain). The chemoreceptors are the Phox2b-positive neurons that do not express tyrosine-hydroxylase (TH). The cells that express both markers are the C1 neurons, which regulate blood pressure. C: method used to record from RTN neurons in vivo. D: effect of changing end-expiratory CO2 on the activity of an RTN neuron recorded in vivo after intracerebral injection of the glutamate blocker kynurenic acid. The neuron still encodes the level of arterial CO2 despite the fact that the drug has silenced the activity of the central pattern generator (CPG; evidence that it has is not shown in the figure). E and F: structure of two RTN neurons recorded in vivo illustrating the fact that a major portion of the dendritic domain of these cells resides within the marginal layer of the ventral medullary surface. Guyenet, PG, J. Appl. Physiol. 2008, (80) used with permission.
Recent work has begun to describe the genetic origins of Phox2b RTN neurons (22, 36, 201). One genetic advance that is directly pertinent to central chemoreception concerns the Congenital Central Hypoventilation Syndrome (CCHS) (262). Patients with CCHS have multiple autonomic nervous system defects and have a severely decreased ventilatory response to CO2. The syndrome does not cause death at birth or in early postnatal life but is usually detected in young children often as a result of the secondary sequellae of chronic hypoventilation caused by intermittent hypoxia and hypercapnia or as a result of other autonomic defects. The defect in the CO2 response is more severe in sleep and most of these patients require lifetime ventilatory support during sleep. Virtually all CCHS patients have been found to express a defect of some kind in the Phox2b gene most commonly a polyalanine expansion (5). This gene has been labeled the master gene for the development of the autonomic nervous system (22, 36). The fact that RTN chemosensitive neurons express Phox2b (242) together with this association of a Phox2b gene defect and a clinical syndrome identified in part by an abnormal CO2 response is of great interest. These associations along with the development of a transgenic mouse (49, 50) with a polyalanine expansion of the Phox2b gene that has very abnormal breathing and absent CO2 sensitivity in the few hours of life before it dies furthered this interest. That the Phox2b defect in this mouse is in the RTN region led to the hypothesis that the RTN Phox2b neurons are the sole or the most important central chemoreceptor neurons (49, 50, 81, 82). However, mice with severe apnea and unstable breathing that die at birth and have, as so far described, an isolated defect in only RTN Phox2b neurons, are not the same as CCHS patients who have multiple defects and, while expressing a severely reduced CO2 response, do not die at birth (49, 50). The mouse model does imply that the RTN is quite important in chemoreception in early postnatal life when rodents are developmentally quite immature.
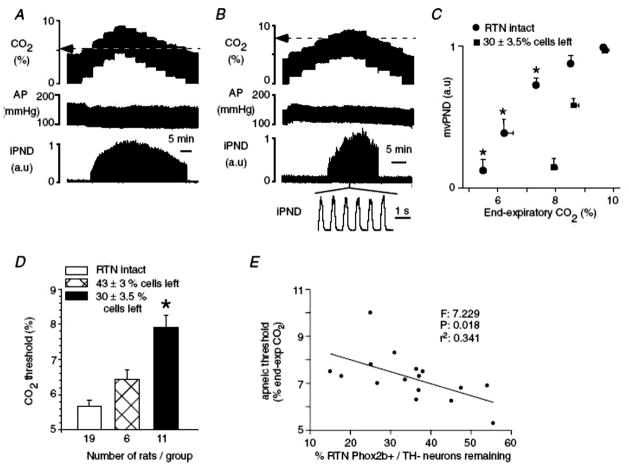
In a study by Takakura et al., (251), lesions of the RTN were made using SSP-saporin (SSP is a modified substance P moiety) and the lesion size measured using an anti-Phox2b antibody, which showed up to 70% loss of RTN Phox2b neurons. The rats with the greater cell loss, studied under anesthesia, had an increase in the apneic threshold, i.e., after hypocapnc apnea a higher baseline CO2 was required to initiate phrenic nerve activity. But once initiated the subsequent increase in phrenic activity with further increases in CO2 was not reduced. Fig. 9 summarizes these data. Thus, a substantial defect in RTN Phox2b neurons did not affect CO2 sensitivity but did remove a tonic drive to respiratory output, as studied under anesthesia. If we compare these data to the results obtained in earlier experiments with lesions of the RTN made using kainic acid and the rats studied under anesthesia there is a dramatic difference (183). The kainic acid lesions, only on one side, resulted in the complete absence of the CO2 response (Fig. 4) while these SSP-saporin induced lesions constrained solely to RTN Phox2b neurons did not alter the CO2 response slope but did shift the apneic threshold. These data suggest that there are non-Phox2b RTN neurons or non-RTN neurons participating in chemoreception at the higher CO2 stimulus levels but that Phox2b RTN neurons contribute to the resting drive to breathe at lower CO2 levels. The observations of Takakura et al., (251) allow a further deduction. They showed that a specific lesion of NK1R-expressing RTN cells and processes dramatically reduced the number of RTN Phox2b-immunoreactive neurons. In previous experiments we had injected SPsaporin into the cisterna magna in order to affect a wide range of NK1R-expressing neurons near the VLM surface (173). These rats exhibited a 79% reduction of RTN NK1R-ir, a very large lesion. In addition they had loss of NK1R-ir in other VLM locations, 65% in the A5 region, 38% in the medullary raphe, and 49% in the pre-Bötzinger complex. The CO2 response was dramatically reduced by 61% in wakefulness and up to 57% in NREM sleep and the level of baseline ventilation was reduced by 8–9% in wakefulness and NREM sleep. These results allow two conclusions: 1) NK1R-expressing neurons and processes in the VLM participate importantly in central chemoreception, and 2) even an ~ 80% lesion of RTN NK1R-ir neurons (presumably Phox2b-expressing based on the Takakura et al., data (251) along with moderate lesions in adjacent VLM cell groups could not abolish the CO2 response in the conscious rat. The remaining response (~40% of the original) must arise elsewhere, either at other central chemoreceptor sites or at the carotid body. The degree of participation of the carotid body is likely compromised based on the data of Takakura et al. (250) showing that carotid body afferent traffic travels in part through the RTN as well as the recent data supporting interdependence between central and peripheral chemoreceptors (19, 231).
Figure 9. Lesions of putative RTN chemosensitive neurons alters the apneic threshold.
Effect of bilateral injections of SSP-SAP into the RTN on the central chemoreflex. A, relationship between phrenic nerve discharge (PND) and end-expiratory CO2 in a control rat 2 weeks after bilateral injection of saline. The apnoeic threshold is 5.2%. B, same experiment in a different rat 2 weeks after bilateral treatment with 2 × 0.6 ng of SSP-SAP. The apnoeic threshold is 7.9%. PND above the apnoeic threshold appears normal. C, relationship between mv PND and end-expiratory CO2 in controls (n = 19) and in 11 rats treated bilaterally with 2 × 0.6 ng of SSPSAP causing the destruction of 70% of the Phox2b+TH− neurons of RTN. One arbitrary unit represents the highest value of mv PND registered at steady state with end-expiratory CO2 set at 9.5–10%. *Statistical significance by RM ANOVA (P < 0.05). D, effect of graded lesions of the Phox2b+TH− neurons of the RTN on the apnoeic threshold measured as shown in A and B. *Statistically significant difference from the other two groups by ANOVA (P < 0.05). E, correlation between apnoeic threshold and percentage Phox2b+TH− neurons remaining (10 rats with 2 injections of toxin on each side and 6 rats with one injection on each side). The F, r2 and probability values of the linear regression are indicated in the figure. Takakura et al., J. Physiol., John Wiley and Sons (251) used with permission.
These studies of RTN function have all been carried out using 6% inspired CO2 or greater as the stimulus, a choice that may not reflect what occurs if a lower stimulus intensity were applied (see below). A recent study examining the location and function of the Task2 channel with quite unexpected results is relevant to this issue (76). First, the expression of the Task2 channel was shown to be present only at a small number of focal sites within the brain, one being the RTN. The reasons for this are unclear. Transgenic mice with altered Task2 channels were then studied the expectation presumably being that the CO2 response would be diminished or absent. Surprisingly, when studied with 2–3% inspired CO2 the adult Task2 null mice had an exaggerated CO2 response while when studied with 5–6% inspired CO2 their CO2 response was reduced. Either the CO2/H+ response of RTN neurons requires more than just Task2 channels and their relative contribution varies with stimulus intensity or the RTN contribution to chemoreception varies with stimulus intensity.
The RTN contains Phox2b expressing neurons involved in chemoreception that are of crucial importance just after birth. Under anesthesia, specific loss of function of Phox2b RTN neurons affects the apneic threshold but not the sensitivity of the subsequent response to CO2. Under anesthesia, non-specific kainic acid induced lesions of the RTN region abolish the CO2 response while in the unanesthetized awake state, such lesions reduce but do not abolish the CO2 response. In the unanesthetized rat, SP-saporin induced lesions affecting 44% of RTN NK1R-ir neurons decreased the CO2 response by 30% while similarly induced lesions with measured effect on Phox2b-ir had no effect on the CO2 response sensitivity under anesthesia. In the unanesthetized rat, allatostatin induced focal inhibition of ~50–60% of RTN neurons decreased the CO2 response by ~60% without effect on resting breathing (149). The effects of specific lesions on baseline breathing and on the CO2 response in sleep await further experiments.
The RTN is also likely to be related to the parafacial respiratory group (pFRG) (196). The role of the RTN and the pFRG in rhythm generation is covered elsewhere (see Mechanisms of Respiratory Rhythm Generation).
Medullary raphe
The medullary raphe contains a prominent population (~25%) of serotonergic (5HT) neurons (31) and represents the caudal cluster of these monoaminergic cells. The rostral cluster in the pons projects mainly to more rostral brain structures and is concerned with the functions of these neurons while the caudal cluster projects to spinal cord as well as to other brainstem sites and is more concerned with the functions of these regions including the regulation of breathing and central chemoreception (20, 31, 99–101, 215–217). In 1995, George Richerson (214), in a study using medullary slices in vitro to look for neurons responsive to acidic stimulation in the rostral ventral medulla, described CO2/H+ responsive neurons at two locations, one possibly within the subsequently described RTN region, the other in the midline raphe. In anesthetized rats in vivo, focal acidification of the midline raphe by microinjection of acetazolamide increased respiratory output (15) indicating the presence of functionally significant chemoreception, a result substantiated shortly thereafter by focal acidification in conscious rats (182) and goats (97, 98) by reverse microdialysis of aCSF equilibrated with increased CO2. Non-specific inhibition of the medullary raphe by dialysis of muscimol decreased the CO2 response (253). Thus the medullary raphe became a putative central chemoreceptor site.
The neuron type within the medullary raphe responsible for chemoreception has been identified as serotonergic (Fig. 10). Recordings from slice preparations with subsequent anatomical verification, from 5HT cells in culture, and from neurons transgenically labeled as serotonergic all show a specific increase in activity of 5HT neurons to increased CO2/H+ and, in some neurons, a decrease in activity (31, 214–217). Medullary 5HT neurons exhibit an inherent sensitivity to CO2/H+. Data obtained in vivo support a role for medullary 5HT neurons in chemoreception. As mentioned above focal acidification increases ventilatory output and this effect, in rats, is via an increase in breathing frequency (182) while a similar effect in the nearby RTN is via an increase in tidal volume (140). Specific inhibition of 5HT neurons by dialysis of 8-OH-DPAT (252), a 5HT1A receptor agonist thought to inhibit 5HT neurons, decreases the CO2 response as do specific lesions of 5HT neurons by the conjugate of an anti-body to the serotonin transport protein (SERT) and the toxin saporin (anti-SERT-saporin) (185).
Figure 10. Serotonergic neurons respond to CO2.
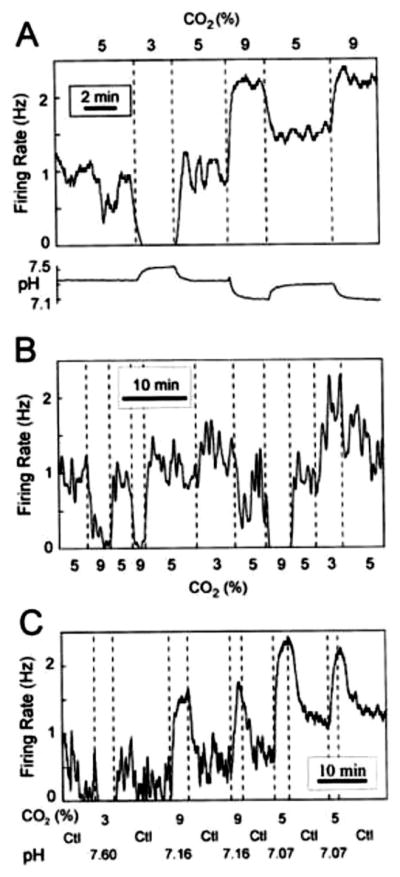
Neurons in cell cultures from the medullary raphe’ are chemosensitive to acidosis. (A) Example of the firing rate of an acidosis-stimulated neuron in response to respiratory acidosis and alkalosis. Lower trace is bath pH measured simultaneously at the inflow to the recording chamber. (B) Example of the firing rate of an acidosis-inhibited neuron in response to the same stimuli. (C) Acidosis-stimulated neurons respond to both respiratory acidosis and metabolic acidosis, indicating that a change in pHo (and/or intracellular pH), in the absence of changes in CO2, is sufficient for a response to occur. (Reprinted from Respiration Physiology, 129, Richerson et al., Chemosensitivity of serotonergic neurons of the rostral ventral medulla. 175–189, 2001, (217) with permission from Elsevier.)
Two sets of observations are of particular interest in respect to the physiological function of 5HT neurons in central chemoreception; those related to 1) age dependence, and 2) gender specificity. In newborn piglets, focal dialysis of the 5HT1A agonist, 8-OH-DPAT, increases the CO2 response at younger ages while by postnatal day 10 (P10) the CO2 response is decreased (156). In rats, the CO2 response is present in early postnatal life but is of smaller magnitude than in adults. At ~P12, the CO2 response begins to increase (37). In the rat at P12, the eyes open, the ears begin to function and the pup begins a transition that might be compared to birth in humans. It has been proposed that the role of 5HT neurons in chemoreception is minimal or absent until ~P12, when 5HT neuron participation would begin to increase (31). When studied in culture, 5HT neurons do not respond to CO2/H+ until 12 days of age or so. One hypothesis then is that the RTN is a dominant central chemoreceptor site in rodent early postnatal life, a time period that can be compared to premature human infants or to the last portion of gestation in humans, and then with postnatal development the 5HT neurons of the medullary raphe, and perhaps other neurons at other putative chemoreceptor sites, begin to play a greater role. This hypothesis would account for the lethal effects witnessed at birth in the polyalanine expansion and null Phox2b mice (49, 50, 52).
There are gender specific effects on chemoreception in some experiments in which 5HT function is disrupted. In newborn piglets with 5,7-dihydroxytryptamine induced lesions of medullary 5HT neurons the CO2 response is reduced only in males and only in sleep (202). In serotonin transporter null adult mice the CO2 response is reduced only in males (139). In a transgenic mouse with a c-fos promoter driven tau-lacZ reporter construct (FTL) that facilitates mapping of cell locations in the brainstem that respond to 5% CO2 exposure, sites with enhanced X-gal expression included the RTN, the medullary raphe, the nucleus of the solitary tract and the locus ceruleus with the male RTN region containing significantly more x-gal-labeled cells than the female (190).
Beautiful recent work has begun the task of describing the genetic origin of 5HT neurons (112). This work is at present beyond the scope of this review but is well worth reading.
Caudal medulla
As originally defined, the ventral medullary surface chemosensitive areas included a caudal area adjacent to the hypoglossal nerve rootlets (Figs. 3, 11). In the recent renaissance of interest in central chemoreception, this area has been largely ignored. There are data from anesthetized animals that complement the earlier findings implicating the caudal ventrolateral medulla as a chemosensitive site (143, 158, 257). The location of neurons that increase their firing rate in response to an acute infusion of CO2 enriched saline via the vertebral artery includes the caudal ventrolateral medulla (7). The distribution of c-fos expression after acidic stimulation includes neurons in the caudal ventrolateral medulla (193). In anaesthetized rats there are neurons in the caudal ventrolateral medulla that fired spontaneously, although not in a respirophasic manner, and are responsive to acidic stimulus (212). Focal acidic stimulation by microiontophoretic injection of H+ stimulated neurons in an area including the caudal region underlying Loeshcke’s area and this region was subsequently shown to contain projections to respiratory-related regions in the brain stem (213). This caudal ventrolateral medulla region contains 3rd order (propriobulbar) neurons identified by retrograde tracing of retrovirus injected into the diaphragm (48). Using the reverse microdialysis technique, focal acidification of the region just dorsal to the caudal ventral medullary surface chemosensitive area in the unanesthetized rat significantly increased ventilation by ~17% only in wakefulness (35) (see Fig. 11). Thus the caudal ventrolateral medulla chemosensitive area remains open for investigation as to cell type and physiological function in central chemoreception.
Figure 11. Focal acidification at the caudal chemosensitive zone increases ventilation in wakefulness.
Focal acidification just below the caudal ventral surface chemosensitive area increases ventilation. Illustrative example of a single experiment in a responsive animal. The period of focal acidification is depicted by the gray rectangle with control periods shown before and after. The EEG and EMG recordings shown in panel A indicate the presence of wakefulness and sleep periods before, during and after focal acidosis. The ventilation data in the bottom of panel A shows that the increase of ventilation during high CO2 dialysis is present only in wakefulness. Note that at the onset of high CO2 dialysis when the rat was asleep (open symbols) ventilation did not increase but quickly did so when the animal woke up (filled symbols). In panel B, we show actual recordings of the plethysmograph pressure signal (upper trace), the EEG (middle trace) and the EMG (lower trace) taken from the averaged data points depicted by the lines and arrows. (Reprinted from Respiration Physiology & Neurobiology, 171, da Silva et al., High CO2/H+ dialysis in the caudal ventrolateral medulla (Loeschcke’s area) increases ventilation in wakefulness. 46–53, 2010, (35) with permission from Elsevier.)
More widespread locations
Nucleus tractus solitarius (NTS)
The NTS as a CO2 detector
The NTS is part of one of the three main clusters of brainstem neurons involved in the control of breathing, the “dorsal respiratory group” (61), and it is a relay site for many cardiopulmonary reflexes with afferents from the periphery. NTS lesions in anesthetized cats decrease the CO2 response under anesthesia, an effect that largely disappears when the cat is allowed to awaken (13). This result along with the observations that the expression of the early gene c-fos is increased in the NTS region with increased CO2 (254), suggest that NTS neurons are important in chemoreception, at least under anesthesia, but this effect could be attributed to disruption of afferents from the peripheral chemoreceptor, the carotid body. A key experiment in the progression of thinking about locations of central chemoreceptors was the study of CO2/H+ responses of NTS neurons in slice preparations. NTS neurons studied in vitro exhibit CO2- dependent changes in membrane potential and firing rate (39) suggesting that NTS neurons can themselves be chemosensitive. In vivo studies corroborated this idea. Focal acidification of the NTS region by microinjection of acetazolamide in anesthetized cats and rats increased respiratory output (27). And, subsequently, focal acidification in unanesthetized rats significantly increased ventilation (181) with greater effects observed with caudal NTS stimulation, a 16% increase in sleep and a 28% increase in wakefulness, than with rostral NTS stimulation, an 11% increase in sleep and a 7% increase in wakefulness. Dialysis with control aCSF equilibrated with 5% CO2 had no effect at either location.
The NTS and the RTN
In anesthetized rats, lesions of the RTN by kainic acid injections abolished the CO2 response and severely reduced the ventilatory response to hypoxia (183) suggesting the possibility that the brainstem integration of carotid body afferents included a direct connection with the RTN. The presence of a direct connection from NTS neurons that receive carotid body afferents to the RTN was subsequently demonstrated by Takakura et al., (250). Anterograde tracing and double labeling revealed connections from the NTS to glutamatergic neurons in the RTN and retrograde tracing from injections into the RTN identified NTS neurons with hypoxia induced c-fos activation. In anesthetized rats, bilateral RTN inhibition by muscimol reduced phrenic activity both at baseline and with stimulation by either hypercapnia or carotid body excitation. Recorded RTN neurons were activated by both CO2 and carotid body stimulation. This study shows clearly that NTS activation by carotid body stimulation also activates RTN neurons that are chemoresponsive to CO2. These data support the concept of a functional interdependence between central and peripheral chemoreception (19, 231) (vida infra).
Hypothalamus-orexin neurons
There are two orexins (orexin-A and orexin-B) that are cleaved from a common precursor, prepro-orexin (221, 222), which is localized to neurons located in the lateral hypothalamus, perifornical area, and dorsomedial hypothalamus. There are two orexin receptors, the orexin-1 receptor (OX1R), more selective for orexin-A, and the orexin-2 receptor (OX2R), which binds to both orexins with equal affinity. Both nerve terminals containing orexin and the orexin receptors are widely distributed in the brain (56, 148, 163) and orexin participates in many physiological functions, e.g., energy homeostasis, feeding behavior, sleep–wake state control, the stress response, and cardiovascular and respiratory control (203, 221, 273).
While there is a broad spectrum of orexin effects, the clinical syndrome associated with an orexin deficit in man has a rather narrow phenotype, narcolepsy (26, 255). Orexin seems to stabilize wakefulness and promote arousal (24). The activity of orexin neurons does vary with sleep-wake state (249) and orexin neurons provide excitatory inputs to nuclei that regulate arousal (203, 221). But the degree of circadian variation in orexin levels in cerebrospinal fluid of rats is quite large; it is two-fold, being highest during the active period of the cycle, suggesting an important circadian role that acts above and beyond the wake-sleep state cycle (44, 271). Orexin neurons receive direct and indirect projections from the suprachiasmatic nucleus, a circadian rhythm oscillator, which may be a source for this large circadian variation in orexin levels (154, 223). Orexin levels also increase during exercise (154) and in heightened alertness (129) suggesting a role for orexins in activities related to increased arousal even within wakefulness.
Within either part of the circadian cycle, orexin levels still vary with sleep-wake state, but the change is much smaller, ~11%, than the circadian variation (121). And, orexin neuron firing rates vary by sleep-wake state even within a circadian period. Direct activation of orexin neurons via in vivo photostimulation of transfected channelrhodopsin-2 elicits rapid sleep state transitions regardless of circadian period (2, 24). Orexin seems to have dual roles; 1) circadian and 2) sleep-wake arousal state.
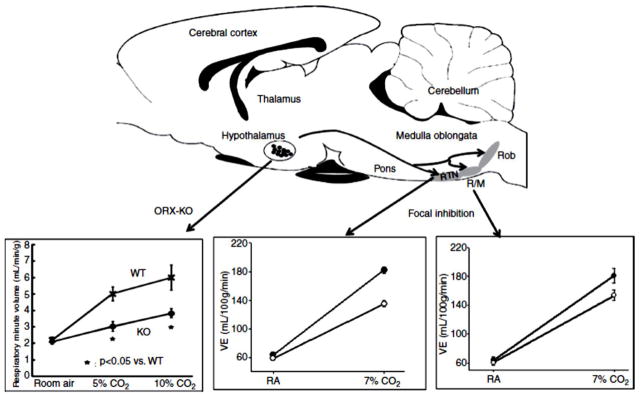
Orexin neurons are anatomically connected with neurons involved in the control of breathing (53, 127, 128, 219). In respect to central chemoreception, orexin neurons are activated by CO2/H+ in vitro as measured by direct neural recording (266) and in vivo as measured by c-fos activation (246). Prepro-orexin knockout mice have a 50% decrease in the ventilatory CO2 response measured during the light/inactive phase of the circadian cycle in quiet wakefulness but not during sleep, an effect that is reversible by administration of orexin (43). Administration via the cerebral ventricles of an OX1R-selective antagonist decreased the CO2 response by 24% in wildtype mice during wakefulness in the light/inactive phase of the circadian cycle (43). At the RTN region, where there is evidence for OX1Rs, OX2Rs, and for activation by hypothalamic stimulation (71, 148, 203), unilateral microdialysis of an OX1R antagonist in rats during the light/inactive phase of the circadian cycle resulted in a 30% reduction of the ventilatory response to breathing 7% CO2 during wakefulness, while during SWS the inhibitory effect was only 9% (47). These results are in accordance with the results in prepro-orexin knockout mice mentioned above and suggest that a portion of the decreased CO2 response in the knock-out mice can be explained by decreased activation of RTN neurons during wakefulness. This inhibitory effect of antagonism of OX1R in the RTN during wakefulness may well be greater if studied during the dark/active phase of the circadian cycle when orexin levels are up to 2-fold higher.
The rostral medullary raphe also receives projections from orexin-containing neurons (203) and expresses both OX1R and OX2R (148, 203). Focal inhibition of the OX1R by dialysis of antagonist in the medullary raphe decreased the CO2 response (7% CO2 inspired) by 16% during wakefulness in the dark period, but not in the light period (46). There was no significant effect in sleep. That focal antagonism of OX1R in the MR was only effective in wakefulness in the dark period of the circadian cycle when orexin levels are high while it was dramatically effective at the RTN during wakefulness in the light period of the diurnal cycle when orexin levels are lower (it was not studied in the RTN in the dark period) suggests a site specific sensitivity to orexin during the circadian cycle with the RTN being much more sensitive. This interpretation requires more evidence but it suggests the hypothesis that different central chemoreceptor sites may vary in function not only by vigilance state but by circadian period. Fig. 12 summarizes the CO2 response effects in orexin knock-out mice and in rats with focal inhibition of OX1R in RTN and MR.
Figure 12. Orexin participates in chemoreception.
The role of orexin in central chemoreception. The top panel is a schematic of a saggital section of rat brain showing the location of orexin containing neurons in the hypothalamus and their projection sites in the retrotrapezoid nucleus (RTN) and raphe magnus (RM) and raphe obscurus (Rob), which have been identified as participating in central chemoreception. The arrows point to plots demonstrating chemoreception effects at each site, At the left, the hypercapnic responses of ventilation in wild-type (WT) mice and prepro-orexin knockout mice (ORX-KO) during quiet Wakefulness are shown. Data are presented as means± SEM of five WT mice and five ORX-KO mice. *P < 0.05 compared with WT mice. At the right, the Figure shows the effects of dialysis of vehicle solution (solid circles; N= 6) and 5 mM SB-334867, an Ox1R antagonist, (open circles; N= 6) into the medullary raphe on ventilation while the rats were breathing air and 7% CO2 during wakefulness in the dark period. Mean ± SEM values are shown. The −16% effect is significant comparing vehicle to SB-334867 treatment during 7% CO2 breathing for ventilation (P < 0.001, repeated measures ANOVA interaction with gas type). In the middle, the Figure shows the effects of dialysis of vehicle solution (filled circles; n = 6) and 5 mM SB-334867 (open circles; n = 6) into the RTN on ventilation while the rats were breathing air and 7% CO2 during wakefulness. Mean ± SEM values are shown. The −30% effect is significant comparing vehicle to SB-334867 treatment during 7% CO2 breathing (P < 0.01 post hoc comparison). This composite is modified from Fig. 1 in Nattie (166) and used with permission (top panel), Fig 3 from Respiration Physiology& Neurobiology, 164, Kuwacki, Orexinergic modulation of breathing across vigilance states, 204–212, 2008, (127) and used with permission of Elsevier (bottom left panel), Fig. 2 from Respiration Physiology & Neurobiology, 170, Dias et al., The orexin receptor 1 (OX1R) in the rostral medullary raphe contributes to the hypercapnic chemoreflex in wakefulness, during the active period of the diurnal cycle, 96–102, 2010, (46) and used with permission of Elsevier (bottom right panel), and Fig. 2 in Dias et al., (47) J. Physiol. used with permission, John Wiley and Sons (bottom middle panel).
One can produce in conscious rats widespread pharmacological blockade of both orexin receptors by oral gavage of Almorexant, an antagonist of both OX1R and OX2R (Actelion Pharm., Ltd.), which promotes sleep in animals and man (21). The CO2 response was measured in wakefulness and sleep during both the light/inactive and dark/active periods of the rat diurnal cycle (136, 171). Almorexant decreased rat body temperature independent of diurnal cycle and, during only the active phase of the diurnal cycle, it decreased oxygen consumption, presumably by decreasing activity, and decreased the CO2 response normalized to metabolic rate by 16% in wakefulness and 15% in NREM sleep. The smaller effect in comparison to the orexin knock-out mice is likely due to less thorough antagonism of all orexin receptors by systemic drug administration. These data strongly support a role for orexin in determining the CO2 response during the active part of the diurnal cycle.
There are two other aspects of altered ventilatory control in orexin knock-out mice that could involve central chemoreception. First, these orexin deficient mice express an increase in sleep apnea occurrence (162). In that orexin provides an excitatory stimulus to the pre-Bötzinger complex and phrenic motoneurons (272) and to central chemoreceptor sites, its absence could be viewed as removal of a necessary excitatory input, e.g., from central chemoreceptors, during sleep states that promotes neural responses that prevent apnea. Second, orexin participates in the ‘defense response’ (129, 162, 273). Prepro-orexin knockout mice and orexin neuron-ablated mice (87) exhibit a reduced defense response (smaller increases in ventilation, heart rate and blood pressure) induced under anesthesia by disinhibition of the perifornical area via injection of the GABA-A receptor antagonist, bicuculline (120, 273). And, in unanesthetized mice, the defense response (heart rate and blood pressure) is reduced when tested by air jet to the nose or confrontation of an intruder mouse (120, 261). Orexin neurons may act to activate multiple efferent pathways of the defense response working via adjustment of central ventilatory and autonomic regulation. Animal arousal, or alertness, is minimal during sleep, increases during quiet wakefulness, and further increases during active wakefulness with activities such as exercise, stress, or panic. The level of this arousal activation by orexin in rodents will be greater in the dark, active period of the circadian cycle than in the light, inactive period.
Locus ceruleus and A5
There is strong evidence that the locus ceruleus (LC) participates in central chemoreception (18, 54, 75, 88, 114, 137, 198, 244). Located bilaterally in the dorsal pons at the floor of the fourth ventricle, the LC contains the largest concentration of catecholamine containing neurons in the central nervous system, exhibits activity that is arousal state-dependent, and modulates sensory information, arousal, feeding, pain processing, and cardiovascular control (8, 16, 67, 110). LC neurons play a role in the development of the respiratory network (94) and can modulate respiratory rhythm (95, 198). CO2 stimulation in vivo induces c-fos expression in the LC (93, 254). In vitro studies of LC neuron responses to CO2/H+ have shown that the large majority (~80%) are responsive with a relatively low sensitivity (88, 114). Reducing the CO2 from 5% to 0% decreased firing rate and increasing CO2 from 2.5% to 10% increased the firing rate by 53% (88, 114). The intrinsic sensitivity of LC neurons to CO2/H+ has been studied via primary cell culture and patch-clamp recordings of LC neurons identified by endogenous expression of green fluorescent protein (GFP) obtained from the Prp57 transgenic mouse (114). As in slice preparations the % of LC neurons that responded was quite large, here ~90%. The responses observed were larger, up to 250% of baseline with 9% CO2, than in slices. The degree of the neuronal response depended on the baseline firing rate, ranging from ~156 % when baseline firing rate was ~3 Hz to 381% when baseline firing rate was ~1Hz. The reasons why on average the response was greater in culture than in slices are unclear. One possibility is the presence of gap junctions. The response of LC neurons to CO2/H+ is in part related to the presence of gap junctions (38, 88), which can influence the percentage of LC neurons that express intrinsic chemosensitivity at different postnatal ages from P0 up to P18. LC neurons from younger ages are less dependent on the presence of gap junctions suggesting that the role of the LC in chemoreception may change with development and is dependent on gap junction coupling within the region.
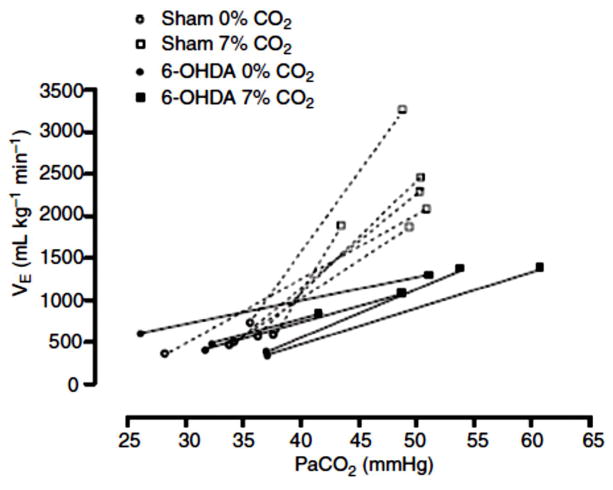
Studies in vivo provide support for the LC being involved in chemoreception. LC cells increase their firing rate with systemic CO2 stimulation before and after peripheral chemoreceptor denervation (54) and focal acidosis within the LC by injection of acetazolamide in anesthetized cats increases respiratory output (27). Lesions (137) of brainstem catecholamine neurons by injection of antidopamine β-hydroxylase–saporin via the fourth ventricle decreased the ventilatory response to 7% CO2 during sleep and wakefulness by 28% in rats, without significant effect on baseline ventilation. More focal deletion of only LC cells by 80% with 6-hydroxydopamine injection into the LC (18) decreased the CO2 response by 64% in unanesthetized rats without effect on baseline ventilation. The former study produced a 73–85% loss of catelcholamine neurons in A5, A6, and A7 and a 50–60% loss in the C1, and C3 regions while the latter study affected only the LC (A6) region. Still it is not clear why there is such a discrepancy in the degree of the effect between these two studies. One possibility is that some catecholamine containing regions may provide a net inhibitory effect. Fig. 13 shows the effects of specific LC NA neuron lesions on the CO2 response in conscious rats.
Figure 13. Lesions of noradrenergic neurons of the locus ceruleus decrease the CO2 response.
The relationship between pulmonary ventilation (VE) and the arterial partial pressure of CO2 (PaCO2) of sham and rats treated with 6-OHDA induced lesions (>50%) of noradrenergic neurons of the locus ceruleus exposed to normocapnia (0% CO2) and hypercapnia (7% CO2). The overall effect was a 64% reduction in the CO2 response. With kind permission from Springer Science and Business Media: Pflügers Archiv, Locus coeruleus noradrenergic neurons and CO2 drive to breathing, 455, 2008, pp. 1119–28, Biancardi, V., Bicego, K. C., Almeida, M. C., and Gargaglioni, L. H Fig. 3. (18).
Fastigial nucleus
The rostral portion of the fastigial nucleus, one of the deep cerebellar nuclei, is implicated in central chemoreception from evidence obtained in vivo. Direct stimulation of this region increased ventilatory output (269) while lesions did not alter baseline breathing but did reduce the CO2 response when tested at moderate to high stimulus levels (151–153). In the anesthetized rat, focal acidosis within the rostral fastigial nucleus by injection of acetazolamide increases ventilatory output by ~38% suggesting the presence of chemoreception. In unanesthetized goats, focal dialysis with aCSF equilibrated with high levels of CO2 in the rostral fastigial nucleus increased ventilation by 12–16% but with dialysis in the caudal fastigial nucleus this effect was absent. Lesions of the rostral fastigial nucleus by injections of an excitatory amino acid toxin reduced the CO2 response by 27% in unanesthetized goats but the effect was present only at inspired CO2 levels of 7% but not at 3%. There have not been any studies of neurons within the rostral fastigial nucleus.
Rostral ventral respiratory group / pre-Bötzinger complex
The pre-Bötzinger complex (PBC) is importantly involved in rhythm generation although its precise role in early development and in the adult is still under study (see Mechanisms of Respiratory Rhythm Generation). There are data that indicate that the PBC is itself also involved in central chemoreception. In anesthetized cats (237), focal acidosis in the PBC region was produced by injection of acetazolamide (50 uM; 10–20 nl) into sites at which prior injection of d-l homocysteic acid (DLH), a glutamate analog, produced a tonic excitation of ventilatory output. Focal acidosis in the PBC increased ventilatory activity alone and produced an enhanced effect of DLH injections. In the awake goat (124), focal reverse microdialysis of aCSF equilibrated with high CO2 increased ventilatory output by 10% via an effect on breathing frequency. Strangely this effect was present with unilateral but not bilateral microdialysis. In anesthetized rats (170, 180), microinjections of acetazolamide (50 uM, 1 nl) into the region of the ventral respiratory group (VRG) increased ventilatory output (the amplitude of the integrated phrenic nerve recording) in 14 of 22 sites. In five rats with prior injection of glutamate (100 mM; 10 nl) to identify a region with a ventilatory effect, all demonstrated a response to acetazolamide injection.
Relationship of chemoreceptor sites to blood vessels and cerebrospinal fluid
The blood supply to the medulla arises from vessels that lie on the ventral medullary surface (see Figs. 3,4) and send penetrating branches deep into the tissue in a dorsal direction. CO2 is highly diffusible in brain tissue and a sudden surge of increased PCO2 in arteries supplying the medulla quickly is reflected in the tissue (7, 109). Given the diffuse network of brainstem capillaries and the high diffusibility of CO2 it is difficult to understand how the proximity of putative central chemosensitive cells to blood vessels serves any purpose in regard to the detection of CO2. The proximity of LC catecholaminergic cells (62), medullary raphe serotonergic cells (20) and RTN glutamatergic and Phox2b cells (132) to vessels has been noted. Processes from these cells that connect to vessels have been described. The purpose of this intimate relationship remains, to our minds, unclear. In contrast to CO2, H+ does not cross brain capillary walls quickly (167) so it is possible that the appositions of putative chemosensitive cells, of different types, to vessels may serve to allow easier and perhaps quicker detection of primary pH disturbances that are metabolic in nature. It is also possible that these appositions serve to detect other substances carried in the blood stream that by their nature diffuse slowly or with some difficulty through vessel and capillary walls.
Pertinent here is the observation made by many that some putative chemoreceptor neurons also contain processes that seem to reach out towards the ventral surface and provide access perhaps to large cavity cerebrospinal fluid (119, 198). CSF pH is carefully and independently regulated by a combination of choroid plexus and blood –brain barrier capillary endothelial cell transport mechanisms as well as by changes in PCO2 determined by ventilation and cerebral blood flow (165). It is possible that some central chemosensitive cells serve to measure or detect pH changes within CSF and that this information is integrated along with information on blood pH changes. Glia also participate in this brain tissue interstitial fluid and CSF pH regulation (see below and see Cellular basis of CO2 sensitivity in neurons/glia).
The role of glia
The Chapter by Putnam, “Cellular Basis of CO2 Sensitivity in Neurons/Glia” covers how glia might detect changes in CO2/H+. Here we discuss from a systems perspective data related to glial roles in central chemoreception including ATP.
Fukuda et al., (73) first described pH-sensitive glial cells located in the region now recognized as the RTN. Glia are well known to be important in the regulation of extracellular fluid K+ concentrations when neuronal activity is increased (126, 195). Brain tissue and extracellular fluid pH as well as neuronal cell pH are regulated by a variety of processes (165, 167). Glia are likely to participate in extracellular pH regulation thereby affecting neuronal excitability (57, 58, 210). Whether glia play a role in central chemoreception was directly tested in the RTN region in vivo by Erlichman et al. (59). In anesthetized and conscious rats, a glial specific toxin (fluorocitrate; 1 mM) was administered continually over 60 min into the RTN. The fluorocitrate depolarized glia, likely by depletion of ATP and K+ loss, resulted in decreased pH in the extracellular fluid and increased ventilatory output and CO2 sensitivity (59, 102). Anatomical analysis using a marker for dying cells showed only a small number of stained cells of glial size suggesting that the dose was non-toxic. These results indicated that glia in the RTN region contribute to the maintenance of normal extracellular pH and, possibly, in the modulation of central chemoreceptor function (68). It is also possible that the fluorocitrate caused release of substances from glia that increased the activity of pH-sensitive neurons.
Purogenic signaling (ATP) has been proposed as important within the autonomic nervous system (79) and, more specifically, within chemosensory pathways including central chemoreception (239). For central chemoreception, one source of ATP would be glial cells (77, 159, 161). There are interesting data to support this idea. ATP, as measured by a unique electrode in vivo, is released within or adjacent to the RTN region when an anesthetized rat is exposed to high CO2 (78). In such anesthetized rats, direct application of ATP stimulates ventilatory output and the application of a purogenic receptor antagonist inhibits the CO2 response. One fly in this ointment is the absence of any abnormality in chemoreception in transgenic mice lacking the P2X2 receptor proposed as the mediator of the ATP effect in the RTN region (78). Studies in vitro of RTN chemosensitive neurons have shown that activation of P2X receptors inhibits them while activation of P2Y receptors excites them. These results suggest that glial release of ATP during CO2/H+ stimulation may act by modulation of RTN neuronal CO2/H+ sensitivity. Specific stimulated of astrocytres by activation of channelrhodopsin 2 localized within them (77) show increased astrocyte Ca++ and ATP release that accompany increased ventilation.
An intriguing extension of this glial-ATP-RTN neuron hypothesis is embodied in the suggestion that glia modulate blood flow within the RTN, and perhaps other chemosensitive regions (161). ATP can effect arteriole dilation directly via P2Y-receptors on smooth muscle or endothelial cells and/or indirectly via the metabolite adenosine, which can affect vasodilation by activation of P1 receptors (see (58, 161)).
Summary: Location of Central Chemoreceptors
Central chemoreception was initially localized to two areas on the ventral medullary surface by experiments using direct application of acidic fluids in anesthetized animals. Subsequent work has substantially added to our knowledge of one of these, the rostral chemosensitive area-now identified as the retrotrapezoid nucleus (RTN), and has identified an additional wide array of putative chemoreceptor sites within the hindbrain and hypothalamus. For the RTN we now have considerable information on chemosensitive cell phenotype but we remain less certain of its physiological role. For other putative sites our information on cell phenotype is less complete. Changes in CO2/H+ are powerful determinants of alveolar ventilation. Most, but not all, investigators support the view that central chemoreception involves a complex system of detector sites that vary in effectiveness depending on stimulus intensity, arousal state, and gender. The ventilatory response to central chemoreceptor activation is very dependent on the simultaneous level of peripheral chemoreceptor activation.
The function of central chemoreceptors
Central chemoreception has traditionally been associated with two physiological functions: 1) the maintenance of a constant, normal arterial PCO2 as a negative feed-back control loop, and 2) the maintenance of a constant pH by using ventilatory exchange of CO2 to minimize metabolically induced acid-base disturbances. We also suggest that central chemoreception is involved in a broader set of physiological processes.
Relationship of alveolar ventilation to the rate of CO2 production
CO2/H+ sensitive central (and peripheral) chemoreceptors provide ongoing and rapid feedback to the brainstem respiratory control system concerning the levels of CO2 in arterial blood and in alveolar gas. This PCO2 value is determined by the ratio of metabolic CO2 production by body tissues and the amount of alveolar ventilation such that a decrease in alveolar ventilation with a constant rate of CO2 production results in an increase in arterial and alveolar PCO2, and vice versa. An increase in PCO2 would stimulate chemoreceptors, which would increase alveolar ventilation and decrease PCO2 correcting the initial increase. CO2/H+ sensitive chemoreceptors provide information concerning the adequacy of alveolar ventilation relative to metabolism and provide a source of excitatory or inhibitory afferent information to the respiratory control system, a classic feed-back control loop.
While this chemical feed-back system is well accepted, there are a series of fascinating experiments that have linked CO2 production, both above and below the normal rate, directly with the level of ventilation. In these experiments, which have never been thoroughly explained (123, 204, 205), an extracorporeal gas exchange circuit isapplied to add or remove CO2. Mixed venous blood enters the extracorporeal circuit and, after gas exchange, the blood is returned to the animal via the vena cava. If CO2 is removed via the external circuit, the level of the animal’s ventilation decreases to match the remaining amount of CO2 production that is excreted by the lungs. If the extracorporeal circuit adds CO2, ventilation increases to excrete both the CO2 produced metabolically and that added by the circuit. If such an experiment is performed in an unanesthetized, awake sheep (204) or lamb (205), when the rate of CO2 removal by the extracorporeal circuit equals the rate of CO2 production by the animal and there is no CO2 exchange in the animal’s lungs, arterial PCO2 remains normal but ventilation actually ceases. The animal is awake and alert but not breathing! How does the animal sense the pulmonary excretion of CO2? In the absence of any detectable change in arterial PCO2 it is difficult to explain these data by the known peripheral and central chemoreceptors.
Experimental evaluation of central chemoreceptor function
The experimental study of central chemoreception has for the most part relied on the use of increased inspired CO2 as a mean to increase arterial and brain PCO2/H+. Most commonly, 5% or 7% CO2 is added to the inspired gas and the resultant increase in ventilation is measured. This approach yields insight into the sensitivity of the central (and peripheral) chemoreception system and is extremely useful in this regard. There are concerns associated with this approach, which are commonly ignored.
Stimulus intensity
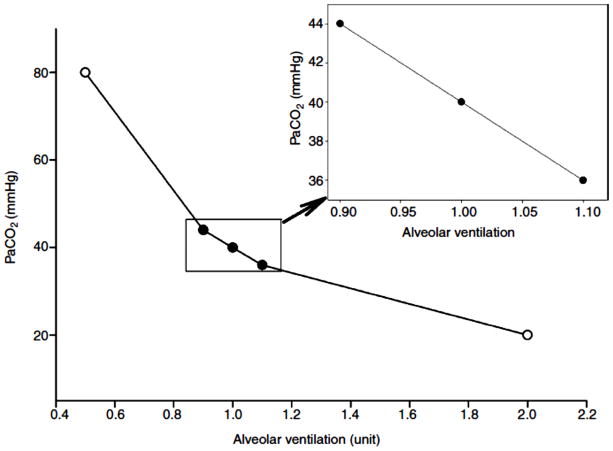
Breathing 7% CO2 in the conscious rat results in an approximately 15 mm Hg increase in arterial PCO2 (138) which when added to a baseline value of 40 mm Hg would result in an arterial value of 55 mm Hg. This demonstrates a powerful response as the inspired PCO2 is ~49 mm Hg. If there were no ventilatory stimulation, the arterial value would be the baseline value, 40 mm Hg, plus the inspired value or 89 mm Hg. Thus the chemoreceptor response has limited the increase in arterial PCO2 from 89 to 55 mm Hg. This is brought about by an increase in ventilation that is >3 x baseline, which is impressive. However, under normal physiological conditions this degree of CO2 rise almost never occurs. We presume that the function of central (and peripheral) chemoreception to maintain normal arterial PCO2 values most commonly takes place with perturbations of arterial PCO2 of say ~5 mm Hg or less. This is difficult to examine experimentally because of the following physiological relationship. Fig. 14 shows how changes in alveolar ventilation affect arterial PCO2 (assuming no change in the rate of CO2 production). The normal arterial PCO2 is 40 mm Hg and this value is determined by the ratio of metabolic CO2 production and alveolar ventilation. Fig. 14 shows that if alveolar ventilation increases x2, arterial PCO2 decreases by ½ from 40 mm Hg to 20 mm Hg and if alveolar ventilation decreases by ½ arterial PCO2 increases x2 to 80 mm Hg. Fig. 14 (insert) shows that if alveolar ventilation changes by 10%, then arterial PCO2 would change by 4 mm Hg. Thus, the control system can adjust to 4 mm Hg changes in arterial PCO2 with 10% changes in alveolar ventilation. It is difficult to measure reliably a 10% change in alveolar ventilation in small conscious animals. And unless arterial PCO2 or dead space data are obtained, the commonly measured variable is expired ventilation, which includes both alveolar and dead space ventilations. So, experimentally, greater stimulus intensities are applied with the implicit assumption that the observed response sensitivity is applicable to these more physiologically relevant perturbations. This may not be so.
Figure 14. The relationship of alveolar ventilation and PCO2.
The arterial partial pressure of CO2 (PaCO2) in mm Hg is shown as a function of alveolar ventilation relative to a ‘normal’ value (that associated with PaCO2 = 40 mm Hg) of 1.0 and multiples thereof. A constant and normal metabolic rate is assumed. The insert magnifies the central region of the plot. As alveolar ventilation increases, PaCO2 decreases, and vice versa. A 10% change in alveolar ventilation is associated with a 4 mm Hg change in PaCO2.
Assumed linearity
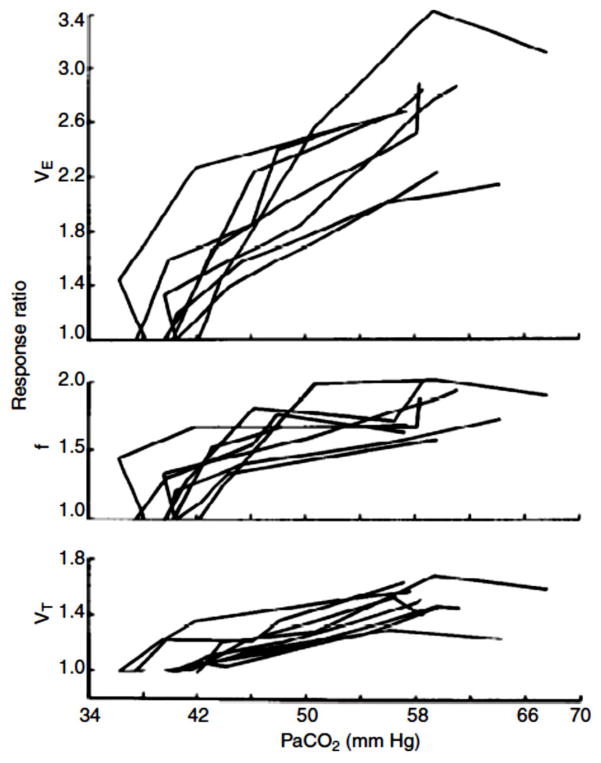
Is the ventilatory response to CO2 proportionally the same with low and high stimulus intensities? The answer is no. Application of 1–2% CO2 in the inspired gas seems to result in greater increases in ventilation than expected, which are associated with very small, almost undetectable, increases in arterial PCO2 (130). Fig. 15 (130) illustrates this phenomenon. Note that with small increases in inspired CO2 the response ratio abruptly increases and that this abrupt increase is largely due to an increase in breathing frequency. The control system has almost perfect arterial PCO2 regulation when the perturbation is small. The mechanism by which the breathing frequency response to small increases in CO2 occurs is unknown. Of relevance, the response to focal low level acidic stimulation by reverse microdialysis is via tidal volume at the RTN (140) and via frequency and tidal volume at the caudal NTS (181) and via frequency at the caudal ventral medullary region (182). The system can easily regulate small changes in arterial PCO2 by small changes in alveolar ventilation but, for unknown reasons, chooses to increase total ventilation more than predicted. Which central chemoreceptor sites participate in this level of regulation at low stimulus intensities is unknown.
Figure 15. Breathing responses to low levels of inspired CO2.
Responses of VE, VT, and f to 0 – 7.6% CO2 in 8 awake, unrestrained rats. Note that the response at low inspired CO2 levels is substantial such that PaCO2 is unchanged, i.e. PaCO2 regulation is “perfect”. Lai et al., J. Appl. Physiol. 1978, (130) used with permission.
Airway vs blood and brain CO2/H+
Does the manner in which we apply the stimulus, e.g., by increasing inspired PCO2, affect the response? The data of Pappenheimer and colleagues (63, 199) (Fig. 1) show that the steady-state ventilatory responses to chronic acid-base disturbances, ventriculo-cisternal perfusion of aCSF with differing bicarbonate concentrations, and CO2 inhalation all have the same relationship to brain interstitial fluid pH suggesting that the responses are the same. Studies with comparison of inhaled vs intravenous infusion with increased CO2 show inconsistent results. Some show a greater CO2 response with intravenous infusion (142, 260, 270) others find no difference (131, 134). The findings of an increased response to intravenous CO2 loading have been interpreted to be due to increased oscillations of arterial PCO2 that are detected by chemoreceptors. Direct application of increased CO2 to specific focal brain regions by reverse microdialysis decreases tissue pH by an amount like that observed with a ~6.6 mm Hg increase in arterial PCO2 (138). This results in an ~10–20% increase in ventilation when performed at the RTN, rostral medullary raphe, caudal NTS, caudal ventrolateral medulla, and within the lateral hypothalamus (170). These observations indicate that, overall, the use of increased inspired CO2 results in response characteristics similar to when the stimulus is applied in blood or brain. But the crucial issue of what determines the responses at low systemic stimulus intensities remains unresolved.
Each central chemoreceptor site alone appears to be capable of maintaining arterial PCO2 and when more than one site is simultaneously stimulated the response can be synergistic
The fact that the direct application of CO2 to specific focal brain regions by reverse microdialysis decreases tissue pH by an amount like that observed with a ~6.6 mm Hg increase in arterial PCO2 (138) by chance yields insight into the potential role of individual chemoreceptor sites in the maintenance of normal arterial PCO2 values if the perturbation is small. The observed effects of such stimulation on ventilation was ~24% at the RTN (140), 20% at the rostral medullary raphe (182), 20–30% at the caudal NTS (181), and 17% at the caudal ventrolateral medulla (35). Each of these sites acting alone has the potential to significantly affect the regulation of arterial PCO2 if the perturbation is small.
Central chemoreception and the chemical control of breathing
Brain interstitial fluid pH reflects the integration of: a) arterial PCO2, b) cerebral blood flow, and c) cerebral metabolic rate
We favor the hypothesis that central chemoreceptors monitor brain interstitial fluid (ISF) pH as originally suggested by the studies in conscious goats by Fencl, Miller and Pappenheimer (63, 199). This ISF pH value is in turn determined by tissue PCO2 and tissue bicarbonate. The tissue bicarbonate is regulated by ionic exchange mechanisms that are themselves pH sensitive (63, 165, 167, 199). We shall not discuss these further in this context. Tissue PCO2 then is determined by three factors; the arterial PCO2, the rate of CO2 production by medullary tissue and the medullary blood flow. Tissue (central chemoreceptor) PCO2 therefore varies directly with arterial PCO2 and inversely with medullary blood flow for any constant brain metabolic state. Thus, ISF pH reflects blood PCO2, cerebral blood flow (CBF) and neuronal metabolism and reflects an integrated estimate of these variables (3, 189). In this view, central chemoreceptors may detect arterial PCO2 and serve as a chemical feedback loop in the control of breathing as well as changes in tissue pH that result from acid-base disorders that arise either in the periphery or centrally. And changes in CBF may modulate the intensity of the ISF pH signal or, in some cases, be the direct cause of a change in ISF pH.
CBF is very sensitive to changes in arterial PCO2. Increased PCO2 vasodilates cerebral vessels and CBF increases; decreased PCO2 vasoconstricts cerebral vessels and CBF decreases. An increase in arterial PCO2 increases ISF H+ and stimulates central chemoreceptors but also vasodilates cerebral vessels and the resultant increase in CBF decreases tissue PCO2 widening the arterial-tissue PCO2 difference and minimizes the initial stimulus intensity at the chemoreceptors. Conversely, a decrease in arterial PCO2 decreases ISF H+ and inhibits central chemoreceptors but also vasoconstricts cerebral vessels and the resultant decrease in CBF increases tissue PCO2 diminishing the arterial-tissue PCO2 difference and minimizing the degree of central chemoreceptor inhibition. Thus, the responses of CBF to changes in PCO2 serve both to maintain ISF pH relatively constant and to modulate the central chemoreceptor response to a level appropriate for the ISF pH stimulus intensity. A recent paper by Ainslie and Duffin (3) discusses and models this interaction.
Experimental support for an effect of changes in CBF on chemoreception is provided by studies in which the CBF was altered primarily and the result change in ventilation observed. In unanesthetized goats (25) CBF was altered by partial occlusion of an exteriorized shunt. A 30% reduction in CBF increased baseline ventilation and the response to increased CO2. In conscious human subjects (268), inhibition of the CBF response to CO2 was produced by administration of indomethacin, which inhibits cycloxygenase and both reduces CBF and its response sensitivity to CO2. With a ~25% reduction in CBF the subjects increased alveolar ventilation (arterial PCO2 decreased by 2–3 mm Hg) and increased the ventilatory response to CO2 by 40%. These twotechnically challenging and important studies demonstrate that primary changes in CBF can affect ventilation and the sensitivity of the ventilatory CO2 response providing indirect support for the hypothesis that central chemoreception involves detection of ISF pH.
In a subsequent test of the functional implications of a primary reduction in CBF via indomethcin administration, it was found that breathing stability was reduced in sleep in subjects with reduced CBF (267). This is explained as a loss, in the hypocapnic range, of the stabilizing influence of the normal CBF response on ventilation. With a reduced CBF response to hypocapnia, a transient drop in arterial PCO2 will produce a greater decrease in ISF H+ detected by central chemoreceptors and a resultant greater propensity for apnea. Insofar as the CBF response to CO2 is reduced in NREM sleep in normal humans (155), this may contribute to sleep apnea and unstable breathing.
Importance of central versus peripheral chemoreception –interdependence
How the two chemoreception systems, the peripheral and the central, interact has been a topic of avid and heated interest since their discovery. Peripheral chemoreception is covered in a separate chapter (Peripheral Chemoreceptors in Comprehensive Physiology). The function and relative importance of the peripheral and central chemoreceptors has been beautifully studied in conscious goats and dogs in an approach that feature surgical isolation and perfusion of one carotid body with denervation of the other by Bisgard, Dempsey, Forster, Smith and their colleagues. This approach along with the use of various inspired gas mixtures and applied artificial ventilation has allowed separation of the two systems without the problems of adaptation associated with denervation or lesions. This work has been recently review (70, 231). Two main concepts have emerged: (i) interdependence, and (ii) the importance of hypocapnia.
Interdependence means that
“central and peripheral chemoreceptors are not functionally separate but rather that they are dependent upon one another such that the sensitivity of the medullary chemoreceptors is critically determined by input from the carotid body chemoreceptors and vice versa i.e., they are interdependent” (231).
This idea is the end result of a series of incremental steps in the understanding of how these two chemoreceptor systems function together. Initial denervation studies, which suffered from time-dependent adaptations following total loss of one sensory input, were replaced by the application of isolated perfusion of one carotid body (the other denervated), which allowed separate manipulation of each chemoreceptor site and, importantly, allowed there to be some type of activation at each site.
One anatomical pathway for interdependence emerged from the observations of Takakura et al., (250) showing, in anesthetized rats, that carotid afferents synapse at NTS neurons that, in turn, communicate directly with the RTN, one central chemoreceptor site. Other central sites likely also contribute to this newly defined “two-way street” but the story at present is most compelling for the RTN. Of interest is the fact that the transcription factor, Phox2b, labeled the master gene for the autonomic nervous system (22, 36, 201) is expressed in carotid body, its afferent pathways and the RTN. The data to date raise the possibility that carotid body afferent inputs that are funneled to the RTN (and perhaps other central chemoreceptor sites) alter the response of the RTN neurons to chemostimulation or inhibition.
The review by Smith et al., (231) nicely summarizes studies in a variety of preparations that have examined the interaction of peripheral and central chemoreceptors. Most relevant is the study of awake animals (231) using the isolated and perfused carotid body approach that allows separate control of the stimulus at each site. Stimulation of the carotid bodies increased the ventilatory response to central CO2 by 223% of normal while with inhibition of the carotid body the central response was 19% of normal (19, 231). These data support a potent interaction between central and peripheral chemoreceptors. In the framework of the interdependence hypothesis, in eupnea in conscious dogs with an isolated, perfused carotid body, inhibition of the carotid body alone produced hypoventilation and CO2 retention that was only partially corrected by central chemoreceptors (19). Thus the carotid bodies contribute a significant tonic drive to breath under normal conditions but part of this effect may be mediated via connections to central chemoreceptors, i.e., interdependence (19, 231).
The importance of central chemoreception in hypocapnia
We mentioned above two main new concepts emerging from the study of peripheral and central chemoreceptor interactions, the second being hypocapnia. We suggest here that a major function of chemoreception is to maintain an organized and effective ventilatory output by providing a tonic drive that keeps brainstem respiratory neurons coordinated in an optimal manner. In adults, the generation and maintenance of normal respiratory rhythm and ventilation requires what is often called a tonic ‘drive’, which maintains the excitability of the respiratory neurons. Drive can arise from many sources, e.g., activity of the reticular formation, excitatory input from afferents involved in respiratory control (245), and inputs related to CO2 sensed by central (and peripheral) chemoreceptors (29, 42, 66, 197). Here we focus on the chemoreceptor aspect of drive.
In anesthesia, hyperventilation with the associated decrease in arterial PCO2 below the normal level, hypocapnia, can result in apnea. With the gradual build-up of CO2 during the apnea the threshold for CO2 stimulation is reached and breathing resumes. In NREM sleep, this same phenomenon, hypocapnic apnea, can be observed. It is not present in wakefulness, when additional drives to breathe over-ride the hypocapnic inhibition at chemoreceptors. This drive has been called ‘wakefulness drive’ for breathing (66, 197).
In NREM sleep, apneas can occur due to the hypocapnia associated with a brief ventilatory overshoot. Studies in healthy human subjects using mechanical ventilation to induce hypocapnia have demonstrated that the apneic threshold is <5 mmHg below the normal, eupneic PaCO2 (157, 227). And the addition of very small amounts of inspired CO2 to sleeping subjects who exhibit periodic breathing stabilizes breathing (17, 191). Thus, central and peripheral CO2 sensitivity are very important in determining apnea and periodic breathing (10, 108, 264).
Are the peripheral or the central chemoreceptors primarily responsible for sensing the hypocapnia and triggering apneas in sleep and anesthesia? There is good evidence that the carotid bodies are the primary sensors, i.e., that they more quickly detect the hypocapnia. For example, in experiments with quick changes in PCO2, the apneas occur within 20 secs (234). It seems that the carotid bodies are also a required sensor for hypocapnia. Following bilateral carotid body denervation (229), large decreases in PCO2 failed to induce apnea in sleeping dogs, and in dogs with an isolated, perfused carotid body, perfusion of by normocapnic blood prevented apnea when systemic PCO2 was made hypocapnic (229).
However, hypocapnia that is isolated to the central chemoreceptors in dog and cat does not cause apnea (14, 231, 233) nor does hypocapnia isolated to the carotid body (232) not even perfusion with hyperoxic hypocapnic blood (19). Thus hypocapnia at the carotid bodies is required for apnea. But there must be additional inhibition (231). In interdependence, the decreased input from the carotid body would affect the response of central chemoreceptors and, conversely, the hypocapnic inhibition sensed by central chemoreceptors would affect the carotid body response (227).
Hypocapnic apnea is associated with a marked change in the firing pattern of brainstem respiratory neurons. Inspiratory neurons become quiescent and expiratory neurons are tonically active and with greater activity (29, 225). It seems that some level of CO2 is needed to maintain the normal interactions among the different types of respiratory neurons and to provide a ‘drive’ to the system, at least in NREM sleep and anesthesia. This has been demonstrated dramatically by Bruce Lindsey and his colleagues (191) using their unique approach in decerebrate, ventilated cats with simultaneous multi-array recording of neurons each with individual electrode depth adjustment in the regions of the medullary raphe nuclei, the ventral respiratory column, and the pontine respiratory group (Nuding et al., 2005 abst). Hyperventilation decreased end-tidal PCO2 from 29 to 10 mm Hg and abolished rhythmic phrenic nerve activity. This was associated with elimination of respiratory modulated neuronal activity and neurons in all three regions examined became tonically, not phasically, active during the apnea with evidence of continued connections among them. In recovery, as CO2 increased respiro-phasic activity gradually emerged from this tonic activity. In one animal, during recovery multiple respiratory-related rhythms were observed that were absent during the apnea. One interpretation of these data is that the normal PCO2 value sets the environment for the normal interaction among large numbers of respiratory neurons, which determines the normal phasic respiratory output. Central chemoreception is essential for this task.
This outlook is supported by the three phase model of respiratory control of Rybak et al (220, 235). Using three levels of transaction in the isolated perfused brainstem preparation, which is decerebrate, they define1) an intact-ponto-medullary preparation, 2) a medullary preparation, 3) a BötC-VRG preparation, and 4) a pre-BötC-VRG preparation. They measure whole nerve activities that represent a postulated three phase respiratory output model, inspiratory, postinspiratory, and late expiratory, which together define eupnea. Their data and model support the idea that respiratory rhythm is due to multiple nonlinear state-dependent interactions and, further, they postulate that potentially different rhythmogenic mechanisms may emerge in a state dependent manner. Relevant to chemoreception, this system requires a drive, part of which must arise from chemoreception. In their preparation, and model, hypocapnia converts the eupneic three-phase rhythm to a two-phase rhythm that appears identical to that obtained by pontine transaction. This is reminiscent of the multi-array data from Lindsey and colleagues in that the interactions among respiratory neurons at different sites varies with hypocapnia and the normal, eupneic configuration requires the drive provided by a normal, eucapnic CO2 level.
There are data in anesthetized preparations with hypocapnia that indicate expiratory neurons convert from rhythmic to tonic activity (11, 12, 30, 103, 104) and that both inspiratory and expiratory motor nerves and muscles convert from rhythmic to tonic activity (11, 225). The level of this tonic motor activity increases as CO2 increases even below the apneic threshold (11, 225). In conscious dogs with hypocapnia produced by mechanical hyperventilation, when apnea began expiratory muscle activity converted from rhythmic to tonic discharge and changes in arterial PCO2 altered expiratory muscle activity (104). In NREM sleep, the same changes occurred but the level of tonic expiratory activity in hypocapnia was less (105).
Interaction among central chemoreceptor sites
The medullary raphe (MR) and the retrotrapezoid nucleus (RTN) in the ventral medulla are two putative central chemoreceptor sites. To study how these two sites might interact in conscious rats, the RTN was inhibited by microdialysis with muscimol, which produced hypoventilation and a 24% decrease in the CO2 response (141). The 5HT neurons of the caudal medullary raphe were inhibited by dialysis with the 5-HT1A receptor agonist 8-OH-DPAT, which produced hypoventilation but had no significant effect on the CO2 response (141). This lack of inhibition of the CO2 response differed from the significant 22% inhibition previously observed with 8-OH-DPAT dialysis in the rostral medullary raphe (252) suggesting quite different functional roles within the medullary raphe. When both the RTN and the caudal MR were simultaneously inhibited, the result was enhanced hypoventilation and an enhanced 51% decrease in the CO2 response (141). These effects were similar in wakefulness and sleep. Serotonergic neurons within the caudal medullary raphe provide a non-CO2-dependent tonic drive to breathe and potentiate the effects of RTN neurons that contribute to a resting chemical ‘drive to breathe’ as well as to the CO2 response. These effects of caudal medullary 5HT neurons could be at a chemoreceptor site, e.g. the RTN, or at ‘downstream’ sites involved in rhythm and pattern generation.
In a separate study designed to examine interactions between the medullary raphe and the RTN (Fig. 16), focal acidification was produced by reverse microdialysis with aCSF equilibrated with increased CO2 in the caudal medullary raphe of the conscious rat, which had little effect on ventilation. This lack of effect in the caudal medullary raphe stands in contrast to the ~20% increase in ventilation when the rostral medullary raphe is focally acidified (182). Again, these data indicate different functional roles for rostral and caudal medullary raphe. If the caudal medullary raphe is acidified simultaneously with focal acidification of the RTN, there was a 51% increase in ventilation, a much greater response than the 24% increase observed with focal RTN acidification alone (140). Thus the caudal medullary can detect CO2/H+ but the ventilatory response requires an interaction with the RTN.
Figure 16. Interdependent response to simultaneous focal acidification of medullary raphe and RTN.
Ventilatory response, expressed as % baseline, to focal acidification of: (A) the retrotrapezoid nucleus (RTN) alone (with simultaneous acidification of sites lying outside the raphe obscurus (ROb)), (B) the ROb alone (with simultaneous acidification of the sites lying outside the RTN), and (A + B) both the RTN and the ROb simultaneously. Asterisk at far right of A and of A + B indicates a significant (P < 0.01) increase as determined by repeated-measures ANOVA on the absolute values for ventilation. Asterisks at individual time periods indicate a significant (P < 0.05) difference from baseline as determined by post hoc comparison with Dunnett’s test. Modified from Figs. 3, 4, and 5 in Dias et al., J. Appl. Physiol. 2008, (45) and used with permission.
The role of separate central chemoreceptor sites; sleep, wakefulness, and chemoreception
Based on the data discussed above we have made a simple preliminary organization scheme to illustrate the possible interactions among putative chemoreceptor sites and the respiratory Central Pattern Generator (CPG) in wakefulness (Fig. 17) and NREM sleep (Fig. 18).
Figure 17. Chemoreception in wakefulness.
A schematic model for central chemoreception in wakefulness that represents our current working hypothesis. The areas in red represent sites at which focal acidification by dialysis with aCSF equilibrated with high CO2 produced an increase in ventilation in wakefulness. The areas in gray represent sites at which we anticipate a response to focal acidification in wakefulness. Solid lines show established functional connections related to chemoreception, e.g., dialysis of an OX1R antagonist at the RTN decreased the CO2 response in wakefulness. Dotted lines show likely connections that remain to be established. That linking the caudal MR to the PBC reflects observations obtained in a slice preparation. The red line between the caudal MR and the RTN reflects a CO2 linked connection, i.e., focal acidification of the caudal MR enhances the response to focal acidification of the RTN. Abbreviations are: LH, a designation that includes orexin neurons in lateral hypothalamus, dorsomedial hypothalamus and perifornical area; LC, locus ceruleus; CB, carotid body; NTS, nucleus tractus solitarius; PBC, pre-Bötzinger complex; RTN, retrotrapezoid nucleus; MR, medullary raphe; VLM, ventrolateral medulla. Nattie & Li, (169) J. Appl. Physiol. 2010, used with permission.
Figure 18. Chemoreception in NREM sleep.
A schematic model for central chemoreception in NREM (non-rapid eye movement) sleep that represents our current working hypothesis. The symbols and lines are as in Figure 17. Nattie & Li, (169) J. Appl. Physiol. 2010, used with permission.
In wakefulness, orexin inputs to the RTN and rostral medullary raphe enhance the overall sensitivity of the system, which includes responses that originate at the RTN, caudal NTS, the carotid body, the caudal ventral medulla, and likely the locus ceruleus. The caudal medullary raphe can affect the CO2 response indirectly via an amplification of the response at the RTN (141) and perhaps elsewhere. The newly described responses to focal acidification of the caudal ventrolateral medulla (35) may affect the system response via the RTN and/or the CPG. While many inputs exist to the RTN from other sites, so too are there many inputs directly to the CPG. It is unclear at present how the various central chemoreceptor sites proportion their efferent activity between the RTN and the CPG. Further it is possible that orexin inputs may affect central chemoreceptor responses at sites other than the RTN and the rostral medullary raphe.
In NREM sleep, orexin excitation of CCR is much less or absent. The main sources of currently known chemoreception at the stimulus levels obtained by our in vivo dialysis approach are the caudal NTS (181), rostral medullary raphe (182), and carotid body. The role of the LC in sleep is unknown. We do not know if there is any synergy between central chemoreceptor sites during sleep.
Acid-base regulation
Central (and peripheral) chemoreceptors also serve to help regulate body pH if changes in pH result from metabolic acid-base disorders. The acidic pH that accompanies a metabolic acidosis stimulates ventilation via peripheral and central chemoreceptors causing an increase in alveolar ventilation, which decreases arterial PCO2 and tends to correct the acidic pH. The ventilatory control system excretes acid in the form of CO2 to help correct the acidic pH caused by a metabolic derangement. The alkaline pH that accompanies a metabolic alkalosis inhibits alveolar ventilation and the resultant rise in arterial PCO2 alleviates the alkalosis (41, 63, 164, 165, 167, 199). In general the central chemoreceptor sites within the brainstem are protected to some degree from rapid changes in blood pH that arise from a metabolic disturbance. The blood brain barrier and associated ion transport processes provide this protection. Perhaps proximity of neuronal and glial processes to blood vessels that have been described for many putative central chemoreceptor cells act not to more quickly sense the easily diffusible CO2 but to detect changes in blood pH in a manner that minimizes the influence of the blood-brain barrier. Arita and colleagues have suggested in a more general way that central chemoreceptor cells are located in medullary regions in which tissue pH is less well protected (7, 109).
Modulation of airway caliber
Lower airways
Musa Haxhiu and colleagues have proposed that airway vagal preganglionic neurons (AVPN), which promote bronchoconstriction by governing airway smooth muscle tone and thus airway caliber, are regulated by inputs from neurons and sites that are also putative central chemosensors (92). AVPNs are inhibited by 5HT from the medullary raphe, and NA from the locus ceruleus, sites that in turn are regulated by excitatory orexin inputs from the lateral hypothalamus. Further, this system is arousal state dependent. Current thinking on the neural origins of sleep emphasize the role of inhibitory (gabaergic, glycinergic) neurons in the ventrolateral preoptic (VLPO) area (223, 248). These neurons become active at the onset of sleep and inhibit the orexin neurons in the lateral hypothalamus removing their excitation of the histaminergic neurons of the tuberomammlillary nucleus, the NA neurons of the locus ceruleus, and the 5HT neurons of the raphe nuclei. The activation in sleep of the VLPO neurons also directly inhibits these nuclei. In wakefulness, the VLPO neurons are inactive and the lateral hypothalamic orexin neurons are active as are the histaminergic, 5HT and NA neurons. The net result is inhibition of AVPNs and diminished bronchoconstriction. In sleep the system switches with a net loss of inhibition of the AVPNs and enhanced bronchoconstriction.
Retrograde tracing indicates that NA inputs to AVPNs arise at multiple brainstem NA neuron groups; A6 (locus ceruleus), A5, A1, A2, and stimulation of the locus ceruleus in anesthetized ferrets causes release of NA at the AVPNs and bronchodilation, an effect that is partially blocked by local administration of the α2A-adrenergic receptor antagonist yohimbine at the AVPNs,(91, 92). Retrograde tracing also indicates that 5HT inputs to AVPNs arise over a wide expanse of the medulla including the raphe obscurus, pallidus, and magnus, the nucleus gigantocellularis and the parapyramidal region (90, 92). Stimulation of the medullary raphe in anesthetized cats causes release of 5HT at the AVPNs and bronchodilation an effect that is partially blocked by local administration on the ventral medullary surface of the non-specific 5HT receptor antagonist methysergide (89, 92). The major 5HT receptor in AVPNs seems to be the 5HT1A receptor.
Insofar as 5HT and NA neurons function in central chemoreception and in this AVPN modulatory system, we hypothesize that changes in CO2 affect lower airway tone as a vital part of this chemoreflex. Increased CO2 activates locus ceruleus NA neurons and medullary 5HT neurons, which inhibit AVPNs and promote bronchodilation, which facilitates the increase in ventilation by lowering airway resistance. Hypocapnia would facilitate bronchoconstriction and increase airway resistance as long as brainstem neuronal network interactions allowed an organized output (see discussion of hypocapnia). There are as yet no experiments that examine the role of RTN neurons, or other putative chemoreceptor neurons, on airway caliber.
Upper airways
The hypoglossal motor nucleus contributes importantly to upper airway muscle tone, caliber, and resistance. The activity of hypoglossal motor neurons is modulated by 5HT. Perfusion of the rat hypoglossal nucleus with 5HT in sleep increases genioglossus muscle activity to levels observed in wakefulness (111, 116). 5HT stimulates hypoglossal motoneuron and hypoglossal nerve activity via 5-HT2 receptors (64, 125). Inhibition of serotonin reuptake, which should increase 5HT levels, increases genioglossal activity in normal subjects (247). Thus, activation of 5HT2 receptors by 5HT in the hypoglossal nucleus produces changes that decrease upper airway resistance. Increased CO2 decreases upper airway resistance in dogs and humans (194, 259) and increases genioglossal activity in goats (200) and rats (113). Is this due to CO2 induced release of 5HT? Kanamaru and Homma (116) examined this issue by using dialysis within the dorsal medial medulla of anesthetized mice. The dorsal medial medulla included the regions of the hypoglossal nucleus as well as the NTS. They measured airway resistance and ventilation and dialyzed a serotonin re-uptake inhibitor to enhance 5HT availability either with or without a 5HT2 receptor antagonist. With increased inspired CO2, in both cases, measured 5HT release in the dorsomedial medulla increased ~ 2.5 fold. The highest CO2 (9%) decreased airway resistance to the same minimum value in both groups. As inspired CO2 was decreased, airway resistance increased in both groups but the increase in airway resistance as CO2 decreased was much greater in the group with 5HT2 receptor antagonism. This greater airway airway resistance with dorsomedial medulla 5HT2 receptor antagonism resulted in a lower ventilation at each CO2 level, an effect that was mediated by a lower tidal volume at each CO2 level. These data suggest that 5HT release with CO2 stimulation can importantly modulate upper airway resistance via effects on hypoglossal activity.
There are NA inputs to hypoglossal neurons that produce an excitatory drive during wakefulness that arises in part from the A7 group as injection of an alpha 2-adrenergic receptor agonist into A7 reduced hypoglossal nerve activity (65). But whether the effects of NA cell groups on upper airway function are linked to CO2 stimulation is not known. Whether other putative central chemoreceptor sites, most notably the RTN, contribute to the regulation of upper airway resistance is at present unknown.
Modulation of sympathetic tone and blood pressure
Central chemoreception, sympathetic tone and blood pressure
The regulation of sympathetic activity to the heart and to the arterioles responsible for most of the systemic vascular resistance is a key component of systemic blood pressure regulation (see Guyenet et al., (84) for recent review). This sympathetic output is governed by a host of central and peripheral mechanisms perhaps the most well studied being the baroreflex. It is, however, well known that increased ventilatory output caused by CO2 stimulation also increases sympathetic nerve activity (SNA) (84). Each breath is associated with an increase in SNA and, with CO2 stimulation, both are greater. The source of this respiro-phasic increase in SNA involves the rostral ventro-lateral medulla (RVLM) presympathetic neurons as they fire in phase with respiro-phasic SNA in deafferented anesthetized animals. The exact source of input to these RVLM neurons is not fully known but could be neurons within the caudal ventrolateral medulla (CVLM), which are GABAergic and fire with a strong respiratory modulation (see (84)) and are known to provide a major source of inhibitory input to the RVLM.
In addition to this respiro-phasic change in SNA that is CO2 sensitive, there appears to be a tonic CO2 sensitive activity that is independent of respiratory events. This is uncovered by studies in anesthetized, deafferented and ventilated cats (258) in which single unit activity in the cervical sympathetic nerve and spontaneous mass activity in the cervical, splanchnic, renal sympathetic and phrenic nerves were recorded. With increased CO2, phrenic nerve activity increased as did blood pressure and sympathetic discharge. Hypocapnia such as to produce apnea was associated with decreased blood pressure by 17 mm Hg on average. As CO2 was increased, a pressor and excitatory sympathetic response preceded, in all experiments, the onset of the phrenic nerve rhythmic activity. The arterial PCO2 threshold for the pressor and sympathetic response was ~ 36 mm Hg while that for the phrenic nerve was ~44 mm Hg. Similar data have been obtained in conscious humans (240). Mechanical hyperventilation in sleep silenced ventilatory output while SNA and blood pressure were maintained. These data indicate the presence of a tonic CO2 dependent drive for SNA and blood pressure that is present during phrenic apnea and not dependent on links to respiration.
Where does this chemoreceptor drive for SNA arise? Given the existence of multiple sites for central chemoreception related to ventilation one might reasonably presume that one or more of these sites is involved. There is not much evidence to draw upon here. Lesions of catecholamine neurons limited to the brainstem in A5, A6, A2 and A3 result in decreased blood pressure (135). Orexin neurons innervate the presympathetic neurons of the RVLM and can increase SNA (6, 84) and orexin null mice have decreased blood pressure in wakefulness (120). There are little data on the role of 5-HT neurons in blood pressure control but recent data in developing rodents indicate a vital role for 5-HT in the control of heart rate (33, 34).
Arousal
The presence among putative central chemoreceptor regions of neurons whose firing rate varies with the level of arousal (orexin, 5HT and NA neurons all fire more actively in wakefulness and less so in sleep) raises the question as to whether central chemoreceptor regions participate in arousal from sleep if they are activated. There are few direct studies of central chemoreceptor activation and arousal from sleep. Increased CO2 can produce arousal from sleep (9). But the degree of increased CO2 and the site of action are not well understood.
Adding low levels of CO2 to the inspired gas has been reported to improve breathing in some patients with sleep apnea (256). To examine whether low vs high levels of CO2 affected sleep, Fraigne et al., (72) exposed cats for 3 hrs to 0%, 2%, 4%, and 6% CO2 in room air. An inspired CO2 of 2% improved sleep by increasing sleep duration and decreasing time awake. In contrast, an inspired CO2 of 6% CO2 made sleep worse. This study and others suggest that at low inspired CO2 levels there is little effect on arousal (23, 105) even though ventilation may be stimulated. This suggests that some central chemoreceptor sites that primarily affect breathing will have a different threshold than those that bring about arousal. This is an intriguing hypothesis. Of interest is that hypocapnia per se also disrupts sleep in cats the effect being largely on REM sleep (145).
One specific central chemoreceptor site has been examined in respect to arousal threshold, the caudal NTS. Focal acidification of the caudal NTS by reverse microdialysis of aCSF equilibrated with high CO2 stimulates ventilatory output by about the same amount whether 25% or 50% CO2 is added (181). But, when using 25% CO2 in the dialysate no arousal was detected while when 50% CO2 was used, the rats awoke if they had been sleeping. This is the only single chemoreceptor site study that examined arousal with different stimulus intensities during focal acidification. We can estimate that the focal tissue pH change with 25% CO2 in the dialysate would be like that associated in the rat with a 6.6 mm Hg increase in arterial PCO2 (138), which is associated with an ~3.5% inspired CO2. We can guess that the use of 50% CO2 in the dialysate would be like that with an inspired of 5% CO2, a rough agreement with the whole animal data above that the threshold for arousal is around a value associated with 5% CO2 inspired. When single central chemoreceptor sites are focally acidified using the lower stimulus, 25% CO2 in aCSF in the dialysate, rats routinely cycle between wakefulness and NREM sleep (see Fig. 20).
A central chemoreceptor site involved with CO2 induced arousal is the 5HT neurons in the medulla, which are likely central respiratory chemoreceptors, and in the midbrain, which project to thalamocortical circuitry involved with regulation of sleep and wakefulness. Buchanan and Richerson (23) have examined the effect of CO2 on arousal in mice with conditional genetic deletion of Lmx1b in Pet-1 expressing cells (Lmx1bf/f/p) rendering them selectively and nearly completely deficient in central 5-HT neurons. In 24 hr EEG and EMG recordings the 5HT deficient mice compared to controls are awake more and in NREM sleep less. When exposed to 3% CO2 in the inspired gas, the time for arousal in 5HT deficient mice is ~ 75% longer than in control, with exposure to 5% CO2 it is ~ 200% longer and with exposure to 7% or 10% CO2 it is ~ 350% longer. These effects reflect decreasing arousal times in controls as CO2 increased, while in 5HT deficient mice the arousal time was independent of inspired gas. There was no difference in arousal time in response to other stimuli, i.e., hypoxia, auditory, air puff. These results indicate that 5HT neurons are important in CO2 dependent arousal.
Summary: Functions of Central Chemoreception
Central and peripheral chemoreceptors are interdependent and can respond quickly and sensitively to changes in arterial PCO2. This allows rapid and sensitive regulation of alveolar ventilation relative to metabolism. Central chemoreceptors are responsive to brain interstitial fluid pH, which allows an integrative sensing process that detects and responds to changes in a) alveolar ventilation, b) cerebral blood flow, and c) cerebral metabolism. While ventilation has been almost the exclusive focus of studies of central chemoreception, their output can also affect airway resistance and blood pressure and their sensitivity varies with arousal state.
The evolution of central chemoreception
Our considerations of central chemoreception so far have been constrained to homeothermic mammals. Here we discuss how the evolution of homeothermy and the transition from a water-breathing to an air-breathing environment determined the functions of central chemoreception as studied in the homeotherm.
Transition from water-to-air breathing and the development of central chemoreception work of breathing, appropriate pH for chosen temperature, and gas exchange
Using data from experiments in comparative physiology, one can gain insight into the evolutionary steps that likely resulted in the present normal value for the arterial PCO2 in homeotherm mammals of 40 mm Hg, which represents the balance of two processes, the rate of CO2 production and the rate of alveolar ventilation. An initial major event was the transition from water to air breathing (207). Water contains less oxygen than air because the solubility of oxygen in water is low. This means that animals living entirely in water must have high rates of water flow through their gas exchange apparatus in order to supply their metabolic needs. In contrast, CO2, a metabolic by-product of oxygen utilization, has greater solubility than oxygen in water so the high fluid flows required for oxygen uptake easily eliminate large amounts of CO2. The result is a PCO2 in aquatic animals that is just a few millimeters Hg, a very low value compared to the normal value in air breathers.
When breathing air with relatively large amounts of oxygen available for diffusion in the gas exchange spaces, the convective requirements are less than for water. The lower values of ventilation required for oxygen uptake in air breathing are associated with a lower rate of CO2 elimination as compared to water breathing. Air breathers, in comparison to water breathers retain CO2. The evolutionary transition from water to air breathing resulted in higher PCO2 values.
An interesting experimental model for this transition from water to air breathing is amphibian metamorphosis (115). Water breathing tadpoles with gill ventilation have PCO2 values of a few millimeters Hg; with metamorphosis into air breathing frogs with lungs, PCO2 increases to values in the 30 mm Hg range. Central chemoreception for CO2 may also have appeared during this period in which relative CO2 retention became the mode (228). Most air breathing aquatic animals show little or no ‘ventilatory’ response to increased CO2. Again, using metamorphosis as a model, frogs in comparison to tadpoles exhibit greater chemosensitivity. Another example is the neotenus amphibian, the axolotol, ambystoma mexicanum. The axolotol is, in nature, fixed in the gill breathing stage but metamorphosis can be induced exogenously by means of treatment with thyroid hormone. With such treatment, the gills regress, lungs grow and a response to increased CO2 appears (74) (Kamath, Nattie and Li, unpublished observations).
Given that the PCO2 increases in the transition from water to air breathing and the development of central chemoreception accompanied this event, we can then ask why the normal value settled at 40 mm Hg (208)? Why not 20 mm Hg? Or 60 mm Hg? The factors that determine this normal value appear to include the energetic costs of ventilation-increasing ventilation uses more energy, the achievement of optimal gas exchange-the level of ventilation must supply oxygen needs, and the maintenance of acid-base balance-the level of CO2 is an important determinant of pH. The evolutionary solution presumably minimizes the metabolic cost of breathing while allowing adequate gas exchange and appropriate acid-base balance.
In blood and tissue, CO2 is hydrolyzed into a proton and a bicarbonate ion so the level of PCO2, which is determined by the balance of CO2 production and alveolar ventilation, is a key determinant of cell and extracellular pH. The level of ventilation, which determines arterial PCO2, also determines the level of oxygen in the alveolar gas exchange space. In normal mammals, the level of ventilation which maintains a PCO2 in the alveolar gas exchange space of 40 mm Hg also results in an oxygen partial pressure, PO2, in the alveolar space of 100 mm Hg. A higher level of ventilation would increase the PO2 and decrease the PCO2 but would be more costly energetically and would increase the pH. A lower level of ventilation might save energy but would be associated with a higher PCO2, a more acidic pH, and a lower PO2.
The arterial PO2 does not play an important role in the determination of the optimal level of resting ventilation. The response of the peripheral chemoreceptor, the carotid body, to decreased values of arterial PO2 does not really have a large effect on ventilation until the values fall from the normal of 90 mm Hg to 70 mm Hg or so (263). Further, because of the sigmoid shape of the oxy-hemoglobin dissociation curve the arterial PO2 value can decrease to values of 70 mm Hg or so before the oxygen content decreases very much. This phenomenon provides some latitude in the amount of ventilation required to maintain nearly full hemoglobin saturation with O2. Thus, control of the level of alveolar ventilation just above and below the normal value is not likely to be determined by chemoreceptors responding to PO2. That the ventilatory response to decreased arterial PO2 begins in ernest at values of 70 mm Hg suggests that these O2 sensors provide an emergency system to detect and respond to levels of PO2 at or below 70 mm Hg. At normal levels of ventilation, the baseline firing of the peripheral chemoreceptors does contribute to the tonic drive to the respiratory control system (229, 230).
Temperature and pH-the alphastat hypothesis
The signal responsible for the maintenance of normal levels of alveolar ventilation must be CO2 acting via both peripheral and central chemoreceptors, which are quite sensitive in that they produce ventilatory responses to small increases of PCO2 above the normal value as well as to small decreases in PCO2 in anesthesia and in sleep. The choice, by nature, of 40 mm Hg as the normal mammalian value seems linked to acid-base balance and the maintenance of a normal extracellular pH of 7.4 (106, 150, 175, 209, 211). The initial evolutionary appearance of chemoreceptors is suggested to have occurred in ectotherm air breathing vertebrates. In ectotherms, blood and cell pH vary with temperature. This temperature effect is not trivial; the coefficient is ~0.015 pH units/°C (209, 211). Thus these early chemoreceptors did not likely sense pH per se. It is possible that they sensed CO2 directly.
The hypothesis that some central chemoreceptors sense CO2 directly is an attractive one given the fact that CO2 is the variable being controlled. It is also possible that these early chemoreceptors sensed pH changes that occurred independently from the temperature induced effects on pH. A mechanism by which such a temperature independent pH change - as separate from temperature induced pH changes - might be detected has been proposed. This mechanism, which focuses on imidazole-histidine and is called the alpha-stat hypothesis, evolved from consideration of the effects of temperature on pH. Reeves (211) noted that, in solution, the pK of imidazole-histidine had a similar temperature relationship as that of ectotherm extra- and intracellular pH (96, 147, 150), that is, they varied by ~0.015 units/°C. He deduced that with changes in temperature, the fractional dissociation of imidazole-histidine (alpha-imidazole) would remain constant even as pH changed. In contrast, if pH was changed in isothermal conditions, alpha-imidazole would change. The alphastat hypothesis predicts that proteins with tertiary structure and function determined by histidine groups would be functionally unaffected by pH changes caused by temperature. Isothermal pH changes could affect them in a pH dependent manner. In a more general form, the hypothesis predicts that proteins would be unaffected by temperature if their structure (and function) were determined by moieties with pK –temperature relationships like that of pH in that system [see also (175, 238). This hypothesis explains the relative alkalinity of extracellular fluid, 7.4, and the neutral pH of intracellular fluid, 7.0, at normal mammalian body temperature, as the values, which maintain appropriate protein charge state. In the mammal, these pH values are obtained with a PCO2 of 40 mm Hg.
This hypothesis is difficult to prove because the precise pK of imidazole can be affected by neighbor amino acids [see (175) for discussion] and most physiologically relevant proteins contain many histidines (175, 238). Direct measurements, using nuclear magnetic resonance, of alpha-imidazole in skeletal muscle of the newt show that it is constant when temperature varies even though intracellular pH changes dramatically (96). Treatment with diethylpyrocarbonate (DEPC), a substance that is relatively specific for binding to imidazole-histidine, interferes with the pH sensitivity of many proteins [see (176)] and application of DEPC to the ventral medullary surface diminishes baseline ventilatory output and the response to increased CO2 (176). DEPC treatment also blocks the CO2 response in a snail model for central chemosensitivity (146). Any protein that is pH sensitive may detect pH changes via an alphastat-like mechanism. The final evolution choice for the normal PCO2 40mmHg in all likelihood represents a balance of minimal energy costs for breathing, optimal gas exchange, and the maintenance of a pH value that allows pH sensitive proteins to function at the normal body temperature of homeotherms, 37°C.
Is PCO2 sensed independently from interstitial fluid pH?
The experimental study of whether CO2 itself or pH is the signal for chemoreception in modern homeotherms is technically challenging due to the high solubility and easy diffusibility of CO2. Most presume that pH is the stimulus for chemoreception. One study in vivo in anesthetized, ventilated, peripherally chemodenervated animals used either systemic hypercapnia or isocapnic metabolic acidosis to evaluate CO2 versus pH (57). The measured ventilatory response expressed per unit change in measured medullary surface pH, used as a index of the stimulus at the central chemoreceptor site, was much greater for systemic hypercapnia than isocapnic metabolic acidosis. In this study, either medullary surface pH does not represent the pH at the central chemoreceptor site or CO2 and pH act independently. Recent data suggest that CO2 per se can be a chemoreceptor stimulus acting via stimulation of glial ATP release at connexin hemichannels within the ventrolateral medulla (109). This mechanism at these sites was estimated to account for ~20% of the total CO2 response in anesthetized rats (109). The relative contribution and importance of this glial mechanism in the unanesthetized state is unknown.
There are numerous studies of the CO2 versus pH issue using a variety of in vitro preparations (see Cellular Basis of CO2 Sensitivity in Neurons/Glia in Comprehensive Physiology).
Summary: Evolution of central chemoreception
The evolution from water to air breathing along with the development of homeothermy created a new chemical environment for sensor neurons/glia that participate in chemoreception. The movement from water, which due to the low O2 solubility requires high flow rates thru gills to achieve sufficient O2, to air, which has higher O2 levels and requires lower air flow rates, resulted in evolutionary choices that increased blood PCO2 from a few mmHg to~40mmHg. Further, in ectotherms, blood and cell pH vary with temperature making it unlikely that pH per se is a sensed or regulated variable. Thus, mammals are a special case in respect to acid-base balance. The theory that best explains what is actually “sensed” by chemosensors is the alphastat hypothesis. Here, it is the fractional dissociation of histidine that determines protein charge state and physiological function. This explains why pH can vary considerably in ectotherms without effect on physiological function. In ectotherms as temperature changes, the alphastat is constant, while when pH changes in a homeotherm, alphastat also changes.
Conclusions
The study of central chemoreception has moved beyond the initial descriptions of participating areas on the ventral medullary surface to a realization that multiple locations can participate.
Application of transgenic, viral transfection and opticogenetic strategies has allowed identification of specific neurons involved in the process of chemoreception. Identification and evaluation in vivo of a cell-specific CO2/H+ detection molecule remains as an experimental goal.
The concept of interdependence in which functions of the peripheral and central chemoreceptors depend importantly on each other has taken new life. When and how this interdependence functions remains worthy of study.
The RTN is poised as an important chemoreceptor site that can participate in interdependence.
Glial cells in and around chemosensitive areas, e.g., the RTN, may contribute to chemoreception.
Studies of central chemoreception should be undertaken in conditions that allow evaluation in both sleep and wakefulness.
Why there are so many putative central chemoreceptor sites and what their individual contributions might be is at present uncertain.
Acknowledgments
The authors would like to thank the Heart, Lung and Blood Institute (HL 28066) and the Institute of Child Health and Development (P01 36379) of the National Institutes of Health, the Parker B. Francis Foundation, and the CJ Foundation for SIDS.
References
- 1.Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: Mechanisms of regulation, measurement, and interpretation. Am J Physiol. 2009;296:R1473–R1495. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- 4.Akilesh MR, Kamper M, Li A, Nattie EE. Effects of unilateral lesions of retrotrapezoid nucleus on breathing in awake rats. J Appl Physiol. 1997;82:469–479. doi: 10.1152/jappl.1997.82.2.469. [DOI] [PubMed] [Google Scholar]
- 5.Amiel J, Dubreuil V, Ramanantsoa N, Fortin G, Gallego J, Brunet JF, Goridis C. PHOX2B in respiratory control: Lessons from congenital central hypoventilation syndrome and its mousemodels. Respir Physiol Neurobiol. 2009;168:125–132. doi: 10.1016/j.resp.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ. Orexins/ hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol. 2001;281:R1801–R1807. doi: 10.1152/ajpregu.2001.281.6.R1801. [DOI] [PubMed] [Google Scholar]
- 7.Arita H, Ichikawa K, Kuwana S, Kogo N. Possible locations of pHdependent central chemoreceptors: Intramedullary regions with acidic shift of extracellular fluid pH during hypercapnia. Brain Res. 1989;485:285–293. doi: 10.1016/0006-8993(89)90572-6. [DOI] [PubMed] [Google Scholar]
- 8.Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- 9.Ayas NT, Brown R, Shea SA. Hypercapnia can induce arousal from sleep in the absence of altered respiratory mechanoreception. AmJ Respir Crit Care Med. 2000;162:1004–1008. doi: 10.1164/ajrccm.162.3.9908040. [DOI] [PubMed] [Google Scholar]
- 10.Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/ occlusion during central sleep apnea. J Appl Physiol. 1995;78:1806–1815. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 11.Bainton CR, Kirkwood PA. The effect of carbon dioxide on the tonic and the rhythmic discharges of expiratory bulbospinal neurones. J Physiol. 1979;296:291–314. doi: 10.1113/jphysiol.1979.sp013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batsel HL. Activity of bulbar respiratory neurons during passive hyperventilation. Exp Neurol. 1967;19:357–374. doi: 10.1016/0014-4886(67)90032-5. [DOI] [PubMed] [Google Scholar]
- 13.Berger AJ, Cooney KA. Ventilatory effects of kainic acid injection of the ventrolateral solitary nucleus. J Appl Physiol. 1982;52:131–140. doi: 10.1152/jappl.1982.52.1.131. [DOI] [PubMed] [Google Scholar]
- 14.Berkenbosch A, van Beek JH, Olievier CN, De Goede J, Quanjer PH. Central respiratory CO2 sensitivity at extreme hypocapnia. Respir Physiol. 1984;55:95–102. doi: 10.1016/0034-5687(84)90119-1. [DOI] [PubMed] [Google Scholar]
- 15.Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphe. J Appl Physiol. 1996;80:108–115. doi: 10.1152/jappl.1996.80.1.108. [DOI] [PubMed] [Google Scholar]
- 16.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 17.Berssenbrugge A, Dempsey J, Iber C, Skatrud J, Wilson P. Mechanisms of hypoxia-induced periodic breathing during sleep in humans. J Physiol. 1983;343:507–524. doi: 10.1113/jphysiol.1983.sp014906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biancardi V, Bicego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflugers Arch. 2008;455:1119–1128. doi: 10.1007/s00424-007-0338-8. [DOI] [PubMed] [Google Scholar]
- 19.Blain GM, Smith CA, Henderson KS, Dempsey JA. Contribution of the carotid body chemoreceptors to eupneic ventilation in the intact, unanesthetized dog. J Appl Physiol. 2009;106:1564–1573. doi: 10.1152/japplphysiol.91590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- 21.Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, Mueller C, Nayler O, van Gerven J, de Haas SL, Hess P, Qiu C, Buchmann S, Scherz M, Weller T, Fischli W, Clozel M, Jenck F. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- 22.Brunet JF, Pattyn A. Phox2 genes - from patterning to connectivity. Curr Opin Genet Dev. 2002;12:435–440. doi: 10.1016/s0959-437x(02)00322-2. [DOI] [PubMed] [Google Scholar]
- 23.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter ME, Borg JS, de Lecea L. The brain hypocretins and their receptors: Mediators of allostatic arousal. Curr Opin Pharmacol. 2009;9:39–45. doi: 10.1016/j.coph.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapman RW, Santiago TV, Edelman NH. Effects of graded reduction of brain blood flow on chemical control of breathing. J Appl Physiol. 1979;47:1289–1294. doi: 10.1152/jappl.1979.47.6.1289. [DOI] [PubMed] [Google Scholar]
- 27.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 28.Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- 29.Coates EL, Li AH, Nattie EE. Acetazolamide on the ventral medulla of the cat increases phrenic output and delays the ventilatory response to CO2. J Physiol. 1991;441:433–451. doi: 10.1113/jphysiol.1991.sp018760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen MI. Tonic chemoreceptor input as the background for respiratory rhythm. In: Trouth CO, Millis RM, Kiwull-Schone HF, Schlafke ME, editors. Ventral Brainstem Mechanisms and Control of Respiration and Blood Pressure. New York: Marcel Dekker; 1995. pp. 797–799. [Google Scholar]
- 31.Cohen MI, Piercey MF, Gootman PM, Wolotsky P. Respiratory rhythmicity in the cat. Fed Proc. 1976;35:1967–1974. [PubMed] [Google Scholar]
- 32.Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol. 2009;168:49–58. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cream C, Li A, Nattie E. The retrotrapezoid nucleus (RTN): Local cytoarchitecture and afferent connections. Respir Physiol Neurobiol. 2002;130:121–137. doi: 10.1016/s0034-5687(01)00338-3. [DOI] [PubMed] [Google Scholar]
- 34.Cummings KJ, Commons KG, Fan KC, Li A, Nattie EE. Severe spontaneous bradycardia associated with respiratory disruptions in rat pups with fewer brain stem 5-HT neurons. Am J Physiol. 2009;296:R1783–R1796. doi: 10.1152/ajpregu.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cummings KJ, Li A, Deneris ES, Nattie EE. Bradycardia in serotonindeficient Pet-1−/− mice: Influence of respiratory dysfunction and hyperthermia over the first 2 postnatal weeks. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1333–R1342. doi: 10.1152/ajpregu.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.da Silva GS, Li A, Nattie E. High CO2/H+dialysis in the caudal ventrolateral medulla (Loeschcke’s area) increases ventilation in wakefulness. Respir Physiol Neurobiol. 2010;171:46–53. doi: 10.1016/j.resp.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, Brunet JF. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- 38.Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol. 2006;101:1097–1103. doi: 10.1152/japplphysiol.00378.2006. [DOI] [PubMed] [Google Scholar]
- 39.Dean JB. Theory of gastric CO2 ventilation and its control during respiratory acidosis: Implications for central chemosensitivity, pH regulation, and diseases causing chronic CO2 retention. Respir Physiol Neurobiol. 2011;175:189–209. doi: 10.1016/j.resp.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Dean JB, Kinkade EA, Putnam RW. Cell-cell coupling in CO(2)/H(+)- excited neurons in brainstem slices. Respir Physiol. 2001;129:83–100. doi: 10.1016/s0034-5687(01)00284-5. [DOI] [PubMed] [Google Scholar]
- 41.Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res. 1989;76:656–661. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- 42.Dean JB, Nattie EE. Central CO2 chemoreception in cardiorespiratory control. J Appl Physiol. 2010;108:976–978. doi: 10.1152/japplphysiol.00133.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dempsey JA, Forster HV. Mediation of ventilatory daptations. PhysiolmRev. 1982;62:262–346. doi: 10.1152/physrev.1982.62.1.262. [DOI] [PubMed] [Google Scholar]
- 44.Dempsey JA, Smith CA, Przybylowski T, Chenuel B, Xie A, Nakayamam H, Skatrud JB. The ventilatory responsiveness to CO2 below eupnoea as a determinant of ventilatory stability in sleep. J Physiol. 2004;560:1–11. doi: 10.1113/jphysiol.2004.072371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: Evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103:1772–1779. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- 46.Desarnaud F, Murillo-Rodriguez E, Lin L, Xu M, Gerashchenko D, Shiromani SN, Nishino S, Mignot E, Shiromani PJ. The diurnal rhythm of hypocretin in young and old F344 rats. Sleep. 2004;27:851–856. doi: 10.1093/sleep/27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dias MB, Li A, Nattie E. Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus. J Appl Physiol. 2008;105:83–90. doi: 10.1152/japplphysiol.00120.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dias MB, Li A, Nattie E. The orexin receptor 1 (OX1R) in the rostral medullary raphe contributes to the hypercapnic chemoreflex in wakefulness, during the active period of the diurnal cycle. Respir Physiol Neurobiol. 2010;170:96–102. doi: 10.1016/j.resp.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dias MB, Li A, Nattie EE. Antagonism of orexin receptor-1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol. 2009;587:2059–2067. doi: 10.1113/jphysiol.2008.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. JComp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- 51.Dubreuil V, Barhanin J, Goridis C, Brunet JF. Breathing with phox2b. Philos Trans R Soc Lond B Biol Sci. 2009;364:2477–2483. doi: 10.1098/rstb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C. Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci. 2009;29:14836–14846. doi: 10.1523/JNEUROSCI.2623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durand E, Dauger S, Pattyn A, Gaultier C, Goridis C, Gallego J. Sleepdisordered breathing in newborn mice heterozygous for the transcription factor Phox2b. Am J Respir Crit Care Med. 2005;172:238–243. doi: 10.1164/rccm.200411-1528OC. [DOI] [PubMed] [Google Scholar]
- 55.Dutschmann M, Kron M, Morschel M, Gestreau C. Activation of Orexin B receptors in the pontine Kolliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respir Physiol Neurobiol. 2007;159:232–235. doi: 10.1016/j.resp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Elam M, Yao T, Thoren P, Svensson TH. Hypercapnia and hypoxia: Chemoreceptor-mediated control of locus coeruleus neurons and splanchnic, sympathetic nerves. Brain Res. 1981;222:373–381. doi: 10.1016/0006-8993(81)91040-4. [DOI] [PubMed] [Google Scholar]
- 57.Eldridge FL, Kiley JP, Millhorn DE. Respiratory responses to medullary hydrogen ion changes in cats: Different effects of respiratory and metabolic acidoses. J Physiol. 1985;358:285–297. doi: 10.1113/jphysiol.1985.sp015551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 59.Erlichman JS, Leiter JC. Glia modulation of the extracellular milieu as a factor in central CO2 chemosensitivity and respiratory control. J Appl Physiol. 2010;108:1803–1811. doi: 10.1152/japplphysiol.01321.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erlichman JS, Leiter JC, Gourine AV. ATP, glia and central respiratory control. Respir Physiol Neurobiol. 2010;173:305–311. doi: 10.1016/j.resp.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erlichman JS, Li A, Nattie EE. Ventilatory effects of glial dysfunction in a rat brain stem chemoreceptor region. J Appl Physiol. 1998;85:1599–1604. doi: 10.1152/jappl.1998.85.5.1599. [DOI] [PubMed] [Google Scholar]
- 62.Feldman JL, Gautier H. Interaction of pulmonary afferents and pneumotaxic center in control of respiratory pattern in cats. J Neurophysiol. 1976;39:31–44. doi: 10.1152/jn.1976.39.1.31. [DOI] [PubMed] [Google Scholar]
- 63.Feldman JL, Mitchell GS, Nattie EE. Breathing: Rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Felten DL, Crutcher KA. Neuronal-vascular relationships in the raphe nuclei, locus coeruleus, and substantia nigra in primates. Am J Anat. 1979;155:467–481. doi: 10.1002/aja.1001550405. [DOI] [PubMed] [Google Scholar]
- 65.Fencl V, Miller TB, Pappenheimer JR. Studies on the respiratory response to disturbances of acid-base balance, with deductions concerning the ionic composition of cerebral interstitial fluid. Am J Physiol. 1966;210:459–472. doi: 10.1152/ajplegacy.1966.210.3.459. [DOI] [PubMed] [Google Scholar]
- 66.Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. AmJ Respir Crit CareMed. 2003;167:563–569. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- 67.Fenik VB, Rukhadze I, Kubin L. Inhibition of pontine noradrenergic A7 cells reduces hypoglossal nerve activity in rats. Neuroscience. 2008;157:473–482. doi: 10.1016/j.neuroscience.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fink BR. The stimulant effect of wakefulness on respiration: Clinical aspects. Br J Anaesth. 1961;33:97–101. doi: 10.1093/bja/33.2.97. [DOI] [PubMed] [Google Scholar]
- 69.Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Forster HV. Ventilatory effects of glial dysfunction in a rat brain stem chemoreceptor region. J Appl Physiol. 1998;85:1597–1598. doi: 10.1152/jappl.1998.85.5.1597. [DOI] [PubMed] [Google Scholar]
- 71.Forster HV, Ohtake PJ, Pan LG, Lowry TF. Effect on breathing of surface ventrolateral medullary cooling in awake, anesthetized and asleep goats. Respir Physiol. 1997;110:187–197. doi: 10.1016/s0034-5687(97)00083-2. [DOI] [PubMed] [Google Scholar]
- 72.Forster HV, Smith CA. Contributions of central and peripheral chemoreceptors to the ventilatory response to CO2/H+ J Appl Physiol. 2010;108:989–994. doi: 10.1152/japplphysiol.01059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fortuna MG, Stornetta RL, West GH, Guyenet PG. Activation of the retrotrapezoid nucleus by posterior hypothalamic stimulation. J Physiol. 2009;587:5121–5138. doi: 10.1113/jphysiol.2009.176875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fraigne JJ, Dunin-Barkowski WL, Orem JM. Effect of hypercapnia on sleep and breathing in unanesthetized cats. Sleep. 2008;31:1025–1033. [PMC free article] [PubMed] [Google Scholar]
- 75.Fukuda Y, Honda Y, Schlafke ME, Loeschcke HH. Effect of H+ on the membrane potential of silent cells in the ventral and dorsal surface layers of the rat medulla in vitro. Pflugers Arch. 1978;376:229–235. doi: 10.1007/BF00584955. [DOI] [PubMed] [Google Scholar]
- 76.Gahlenbeck H, Bartels H. Blood gas transport properties in gill and lung forms of the axolotl (ambystoma mexicanum) Respir Physiol. 1970;9:175–182. doi: 10.1016/0034-5687(70)90069-1. [DOI] [PubMed] [Google Scholar]
- 77.Gargaglioni LH, Hartzler LK, Putnam RW. The locus coeruleus and central chemosensitivity. Respir Physiol Neurobiol. 2010;173:264–273. doi: 10.1016/j.resp.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J, Benfriha C, Tegtmeier I, Ehnes H, Georgieff M, Lesage F, Brunet JF, Goridis C, Warth R, Barhanin J. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci U S A. 107:2325–2330. doi: 10.1073/pnas.0910059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- 81.Gourine AV, Wood JD, Burnstock G. Purinergic signalling in autonomic control. Trends Neurosci. 2009;32:241–248. doi: 10.1016/j.tins.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 82.Guyenet PG. The 2008 Carl Ludwig Lecture: Retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol. 2008;105:404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guyenet PG, Bayliss DA, Mulkey DK, Stornetta RL, Moreira TS, Takakura AT. The retrotrapezoid nucleus and central chemoreception. Adv Exp Med Biol. 2008;605:327–332. doi: 10.1007/978-0-387-73693-8_57. [DOI] [PubMed] [Google Scholar]
- 84.Guyenet PG, Bayliss DA, Stornetta RL, Fortuna MG, Abbott SB, DePuy SD. Retrotrapezoid nucleus, respiratory chemosensitivity and breathing automaticity. Respir Physiol Neurobiol. 2009;168:59–68. doi: 10.1016/j.resp.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Fortuna MG, Kanbar R. Central CO2 chemoreception and integrated neural mechanisms of cardiovascular and respiratory control. J Appl Physiol. 2010;108:995–1002. doi: 10.1152/japplphysiol.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol. 2010;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol. 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 90.Hartzler LK, Dean JB, Putnam RW. The chemosensitive response of neurons from the locus coeruleus (LC) to hypercapnic acidosis with clamped intracellular pH. Adv Exp Med Biol. 2008;605:333–337. doi: 10.1007/978-0-387-73693-8_58. [DOI] [PubMed] [Google Scholar]
- 91.Haxhiu MA, Erokwu B, Bhardwaj V, Dreshaj IA. The role of the medullary raphe nuclei in regulation of cholinergic outflow to the airways. J Auton Nerv Syst. 1998;69:64–71. doi: 10.1016/s0165-1838(98)00009-5. [DOI] [PubMed] [Google Scholar]
- 92.Haxhiu MA, Jansen AS, Cherniack NS, Loewy AD. CNS innervation of airway-related parasympathetic preganglionic neurons: A transneuronal labeling study using pseudorabies virus. Brain Res. 1993;618:115–134. doi: 10.1016/0006-8993(93)90435-p. [DOI] [PubMed] [Google Scholar]
- 93.Haxhiu MA, Kc P, Neziri B, Yamamoto BK, Ferguson DG, Massari VJ. Catecholaminergic microcircuitry controlling the output of airwayrelated vagal preganglionic neurons. J Appl Physiol. 2003;94:1999–2009. doi: 10.1152/japplphysiol.01066.2002. [DOI] [PubMed] [Google Scholar]
- 94.Haxhiu MA, Rust CF, Brooks C, Kc P. CNS determinants of sleeprelated worsening of airway functions: Implications for nocturnal asthma. Respir Physiol Neurobiol. 2006;151:1–30. doi: 10.1016/j.resp.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 95.Haxhiu MA, Yung K, Erokwu B, Cherniack NS. CO2-induced c-fos expression in the CNS catecholaminergic neurons. Respir Physiol. 1996;105:35–45. doi: 10.1016/0034-5687(96)00034-5. [DOI] [PubMed] [Google Scholar]
- 96.Hilaire G. Endogenous noradrenaline affects the maturation and function of the respiratory network: Possible implication for SIDS. Auton Neurosci. 2006;126–127:320–331. doi: 10.1016/j.autneu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 97.Hilaire G, Viemari JC, Coulon P, Simonneau M, Bevengut M. Modulation of the respiratory rhythm generator by the pontine noradrenergicA5 and A6 groups in rodents. Respir Physiol Neurobiol. 2004;143:187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 98.Hitzig BM, Perng WC, Burt T, Okunieff P, Johnson DC. 1H-NMR measurement of fractional dissociation of imidazole in intact animals. Am J Physiol. 1994;266:R1008–R1015. doi: 10.1152/ajpregu.1994.266.3.R1008. [DOI] [PubMed] [Google Scholar]
- 99.Hodges MR, Klum L, Leekley T, Brozoski DT, Bastasic J, Davis S, Wenninger JM, Feroah TR, Pan LG, Forster HV. Effects on breathing in awake and sleeping goats of focal acidosis in the medullary raphe. J Appl Physiol. 2004;96:1815–1824. doi: 10.1152/japplphysiol.00992.2003. [DOI] [PubMed] [Google Scholar]
- 100.Hodges MR, Martino P, Davis S, Opansky C, Pan LG, Forster HV. Effects on breathing of focal acidosis at multiple medullary raphe sites in awake goats. J Appl Physiol. 2004;97:2303–2309. doi: 10.1152/japplphysiol.00645.2004. [DOI] [PubMed] [Google Scholar]
- 101.Hodges MR, Richerson GB. Interaction between defects in ventilatory and thermoregulatory control in mice lacking 5-HT neurons. Respir Physiol Neurobiol. 2008;164:350–357. doi: 10.1016/j.resp.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci. 2009;29:10341–10349. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holleran J, Babbie M, Erlichman JS. Ventilatory effects of impaired glial function in a brain stem chemoreceptor region in the conscious rat. J Appl Physiol. 2001;90:1539–1547. doi: 10.1152/jappl.2001.90.4.1539. [DOI] [PubMed] [Google Scholar]
- 105.Horner RL, Kozar LF, Kimoff RJ, Phillipson EA. Effects of sleep on the tonic drive to respiratory muscle and the threshold for rhythm generation in the dog. J Physiol. 1994;474:525–537. doi: 10.1113/jphysiol.1994.sp020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Horner RL, Kozar LF, Phillipson EA. Tonic respiratory drive in the absence of rhythm generation in the conscious dog. J Appl Physiol. 1994;76:671–680. doi: 10.1152/jappl.1994.76.2.671. [DOI] [PubMed] [Google Scholar]
- 107.Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleepwake state on the genioglossus vs. diaphragmmuscle response to CO(2) in rats. J Appl Physiol. 2002;92:878–887. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- 108.Howell BJ, Baumgardner FW, Bondi K, Rahn H. Acid-base balance in cold-blooded vertebrates as a function of body temperature. Am J Physiol. 1970;218:600–606. doi: 10.1152/ajplegacy.1970.218.2.600. [DOI] [PubMed] [Google Scholar]
- 109.Huckstepp RT, Id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes, to central respiratory chemosensitivity. J Physiol. 2010;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hudgel DW, Hendricks C, Dadley A. Alteration in obstructive apnea pattern induced by changes in oxygen- and carbon-dioxide-inspired concentrations. Am Rev Respir Dis. 1988;138:16–19. doi: 10.1164/ajrccm/138.1.16. [DOI] [PubMed] [Google Scholar]
- 111.Ichikawa K, Kuwana S, Arita H. ECF pH dynamics within the ventrolateral medulla: A microelectrode study. J Appl Physiol. 1989;67:193–198. doi: 10.1152/jappl.1989.67.1.193. [DOI] [PubMed] [Google Scholar]
- 112.Jacobs BL. Single unit activity of locus coeruleus neurons in behaving animals. Prog Neurobiol. 1986;27:183–194. doi: 10.1016/0301-0082(86)90008-0. [DOI] [PubMed] [Google Scholar]
- 113.Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol. 2001;532:467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.John J, Bailey EF, Fregosi RF. Respiratory-related discharge of genioglossus muscle motor units. Am J Respir Crit Care Med. 2005;172:1331–1337. doi: 10.1164/rccm.200505-790OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Johnson SM, Haxhiu MA, Richerson GB. GFP-expressing locus ceruleus neurons from Prp57 transgenic mice exhibit CO2/H+ responses in primary cell culture. J Appl Physiol. 2008;105:1301–1311. doi: 10.1152/japplphysiol.90414.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Just JJ, Gatz RN, Crawford EC., Jr Changes in respiratory functions during metamorphosis of the bullfrog, Rana catesbeiana. Respir Physiol. 1973;17:276–282. doi: 10.1016/0034-5687(73)90002-9. [DOI] [PubMed] [Google Scholar]
- 118.Kanamaru M, Homma I. Compensatory airway dilation and additive ventilatory augmentation mediated by dorsomedial medullary 5-hydroxytryptamine 2 receptor activity and hypercapnia. Am J Physiol. 2007;293:R854–R860. doi: 10.1152/ajpregu.00829.2006. [DOI] [PubMed] [Google Scholar]
- 119.Kanbar R, Stornetta RL, Cash DR, Lewis SJ, Guyenet PG. Photostimulation of Phox2b medullary neurons activates cardiorespiratory function in conscious rats. Am J Respir Crit Care Med. 2010;182:1184–1194. doi: 10.1164/rccm.201001-0047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kang BJ, Chang DA, Mackay DD, West GH, Moreira TS, Takakura AC, Gwilt JM, Guyenet PG, Stornetta RL. Central nervous system distribution of the transcription factor Phox2b in the adult rat. J Comp Neurol. 2007;503:627–641. doi: 10.1002/cne.21409. [DOI] [PubMed] [Google Scholar]
- 121.Kawai A, Ballantyne D, Muckenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem–spinal cord preparation of the neonatal rat. J Physiol. 1996;492(Pt 1):277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basalblood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–R593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- 123.Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kogo N, Arita H. In vivo study on medullary H(+)-sensitive neurons. J Appl Physiol. 1990;69:1408–1412. doi: 10.1152/jappl.1990.69.4.1408. [DOI] [PubMed] [Google Scholar]
- 125.Kolobow T, Gattinoni L, Tomlinson T, Pierce JE. An alternative to breathing. J Thor Cardiovasc Sur. 1978;75:261–266. [PubMed] [Google Scholar]
- 126.Krause KL, Forster HV, Davis SE, Kiner T, Bonis JM, Pan LG, Qian B. Focal acidosis in the pre-Botzinger complex area of awake goats induces a mild tachypnea. J Appl Physiol. 2009;106:241–250. doi: 10.1152/japplphysiol.90547.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- 128.Kuffler SW. Neuroglial cells: Physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond B Biol B. 1967;168:1–21. doi: 10.1098/rspb.1967.0047. [DOI] [PubMed] [Google Scholar]
- 129.Kuwaki T. Orexinergic modulation of breathing across vigilance states. Respir Physiol Neurobiol. 2008;164:204–212. doi: 10.1016/j.resp.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 130.Kuwaki T, Li A, Nattie E. State-dependent central chemoreception: A role of orexin. Respir Physiol Neurobiol. 2010;173:223–229. doi: 10.1016/j.resp.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kuwaki T, Zhang W, Nakamura A, Deng BS. Emotional and statedependent modification of cardiorespiratory function: Role of orexinergic neurons. Auton Neurosci. 2008;142:11–16. doi: 10.1016/j.autneu.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 132.Lai YL, Tsuya Y, Hildebrandt J. Ventilatory responses to acute CO2 exposure in the rat. J Appl Physiol. 1978;45:611–618. doi: 10.1152/jappl.1978.45.4.611. [DOI] [PubMed] [Google Scholar]
- 133.Lamb TW. Ventilatory responses to intravenous and inspired carbon dioxide in anesthetized cats. Respir Physiol. 1966;2:99–104. doi: 10.1016/0034-5687(66)90041-7. [DOI] [PubMed] [Google Scholar]
- 134.Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol. 2009;517:69–86. doi: 10.1002/cne.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leusen I. Chemosensitivity of the respiratory center: Influence of CO2 in the cerebral ventricles on respiration. Am J Physiol. 1954;176:390–444. doi: 10.1152/ajplegacy.1953.176.1.39. [DOI] [PubMed] [Google Scholar]
- 136.Lewis SM. Awake baboon’s ventilatory response to venous and inhaled CO2 loading. J Appl Physiol. 1975;39:417–422. doi: 10.1152/jappl.1975.39.3.417. [DOI] [PubMed] [Google Scholar]
- 137.Li A, Emond L, Nattie E. Brainstem catecholaminergic neurons modulate both respiratory and cardiovascular function. Adv Exp Med Biol. 2008;605:371–376. doi: 10.1007/978-0-387-73693-8_65. [DOI] [PubMed] [Google Scholar]
- 138.Li A, Nattie E. CO2 dialysis in one chemoreceptor site, the RTN: Stimulus intensity and sensitivity in the awake rat. Respir Physiol Neurobiol. 2002;133:11–22. doi: 10.1016/s1569-9048(02)00134-9. [DOI] [PubMed] [Google Scholar]
- 139.Li A, Nattie E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J Physiol. 2006;570:385–396. doi: 10.1113/jphysiol.2005.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li A, Nattie E. Serotonin transporter knockout mice have a reduced ventilatory response to hypercapnia (predominantly in males) but not to hypoxia. J Physiol. 2008;586:2321–2329. doi: 10.1113/jphysiol.2008.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li A, Nattie E. Antagonism of rat orexin receptors by almorexant attenuates central chemoreception in wakefulness in the active period of the diurnal cycle. J Physiol. 2010;588:2935–2944. doi: 10.1113/jphysiol.2010.191288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li A, Randall M, Nattie EE. CO(2) microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol. 1999;87:910–919. doi: 10.1152/jappl.1999.87.3.910. [DOI] [PubMed] [Google Scholar]
- 143.Li A, Zhou S, Nattie E. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol. 2006;577:307–318. doi: 10.1113/jphysiol.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Linton RA, Miller R, Cameron IR. Ventilatory response to CO2 inhalation and intravenous infusion of hypercapnic blood. Respir Physiol. 1976;26:383–394. doi: 10.1016/0034-5687(76)90008-6. [DOI] [PubMed] [Google Scholar]
- 145.Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Loeschcke HH, Mitchell RA, Katsaros B, Perkins JF, Konig A. Interaction of intracranial chemosensitivity with peripheral afferents to the respiratory centers. Ann N Y Acad Sci. 1963;109:651–660. doi: 10.1111/j.1749-6632.1963.tb13495.x. [DOI] [PubMed] [Google Scholar]
- 147.Lovering AT, Fraigne JJ, Dunin-Barkowski WL, Vidruk EH, Orem JM. Hypocapnia decreases the amount of rapid eye movement sleep in cats. Sleep. 2003;26:961–967. doi: 10.1093/sleep/26.8.961. [DOI] [PubMed] [Google Scholar]
- 148.Lu DC, Erlichman JS, Leiter JC. Diethyl pyrocarbonate (DEPC) inhibits CO2 chemosensitivity in Helix aspersa. Respir Physiol. 1998;111:65–78. doi: 10.1016/s0034-5687(97)00111-4. [DOI] [PubMed] [Google Scholar]
- 149.Malan A, Wilson TL, Reeves RB. Intracellular pH in cold-blooded vertebrates as a function of body temperature. Respir Physiol. 1976;28:29–47. doi: 10.1016/0034-5687(76)90083-9. [DOI] [PubMed] [Google Scholar]
- 150.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 151.Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci. 2010;30:12466–12473. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Marjanovic M, Elliott AC, Dawson MJ. The temperature dependence of intracellular pH in isolated frog skeletal muscle: Lessons concerning the Na(+)-H+ exchanger. J Membr Biol. 1998;161:215–225. doi: 10.1007/s002329900328. [DOI] [PubMed] [Google Scholar]
- 153.Martino PF, Davis S, Opansky C, Krause K, Bonis JM, Czerniak SG, Pan LG, Qian B, Forster HV. Lesions in the cerebellar fastigial nucleus have a small effect on the hyperpnea needed to meet the gas exchange requirements of submaximal exercise. J Appl Physiol. 2006;101:1199–1206. doi: 10.1152/japplphysiol.00330.2006. [DOI] [PubMed] [Google Scholar]
- 154.Martino PF, Davis S, Opansky C, Krause K, Bonis JM, Pan LG, Qian B, Forster HV. The cerebellar fastigial nucleus contributes to CO2-H+ ventilatory sensitivity in awake goats. Respir Physiol Neurobiol. 2007;157:242–251. doi: 10.1016/j.resp.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Martino PF, Hodges MR, Davis S, Opansky C, Pan LG, Krause K, Qian B, Forster HV. CO2/H+chemoreceptors in the cerebellar fastigial nucleus do not uniformly affect breathing of awake goats. J Appl Physiol. 2006;101:241–248. doi: 10.1152/japplphysiol.00968.2005. [DOI] [PubMed] [Google Scholar]
- 156.Martins PJ, D’Almeida V, Pedrazzoli M, Lin L, Mignot E, Tufik S. Increased hypocretin-1 (orexin-a) levels in cerebrospinal fluid of rats after short-term forced activity. Regul Pept. 2004;117:155–158. doi: 10.1016/j.regpep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 157.Meadows GE, Dunroy HM, Morrell MJ, Corfield DR. Hypercapnic cerebral vascular reactivity is decreased, in humans, during sleep compared with wakefulness. J Appl Physiol. 2003;94:2197–2202. doi: 10.1152/japplphysiol.00606.2002. [DOI] [PubMed] [Google Scholar]
- 158.Messier ML, Li A, Nattie EE. Inhibition of medullary raphe serotonergic neurons has age-dependent effects on the CO2 response in newborn piglets. J Appl Physiol. 2004;96:1909–1919. doi: 10.1152/japplphysiol.00805.2003. [DOI] [PubMed] [Google Scholar]
- 159.Meza S, Giannouli E, Younes M. Control of breathing during sleep assessed by proportional assist ventilation. J Appl Physiol. 1998;84:3–12. doi: 10.1152/jappl.1998.84.1.3. [DOI] [PubMed] [Google Scholar]
- 160.Mitchell RA, Loeschcke HH, Massion WH, Severinghaus JW. Respiratory responses mediated through superficial chemosensitive areas on the medulla. J Appl Physiol. 1963;96:523–533. doi: 10.1152/jappl.1963.18.3.523. [DOI] [PubMed] [Google Scholar]
- 161.Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci. 2006;26:7230–7233. doi: 10.1523/JNEUROSCI.1696-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 163.Mulkey DK, Wenker IC, Kreneisz O. Current ideas on central chemoreception by neurons and glial cells in the retrotrapezoid nucleus. J Appl Physiol. 2010;108:1433–1439. doi: 10.1152/japplphysiol.01240.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol. 2007;102:241–248. doi: 10.1152/japplphysiol.00679.2006. [DOI] [PubMed] [Google Scholar]
- 165.Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- 166.Nattie E. Control and disturbances of cerebrospinal fluid pH. In: Kaila K, Silver RB, editors. pH and Brain Function. New York: Wiley-Liss; 1998a. pp. 629–650. [Google Scholar]
- 167.Nattie E. Central chemoreceptors, pH and respiratory control. In: Kaila K, Silver RB, editors. pH and Brain Function. New York: Wiley-Liss; 1998b. pp. 535–560. [Google Scholar]
- 168.Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 169.Nattie E. Chemoreceptors, pH, and respiratory control. In: Giebisch GSD, editor. The Kidney: Physiology and Pathophysiology. Philadelphia: Lippincott-Raven; 2001. pp. 1983–1993. [Google Scholar]
- 170.Nattie E, Li A. Muscimol dialysis in the retrotrapezoid nucleus region inhibits breathing in the awake rat. J Appl Physiol. 2000;89:153–162. doi: 10.1152/jappl.2000.89.1.153. [DOI] [PubMed] [Google Scholar]
- 171.Nattie E, Li A. Central chemoreception 2005: A brief review. Auton Neurosci. 2006a;126–127:332–338. doi: 10.1016/j.autneu.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 172.Nattie E, Li A. Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J Appl Physiol. 2006b;101:1596–1606. doi: 10.1152/japplphysiol.00347.2006. [DOI] [PubMed] [Google Scholar]
- 173.Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol. 2009;106:1464–1466. doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nattie E, Li A. Central chemoreception in wakefulness and sleep: Evidence for a distributed network and a role for orexin. J Appl Physiol. 2010;108:1417–1424. doi: 10.1152/japplphysiol.01261.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nattie E, Shi J, Li A. Bicuculline dialysis in the retrotrapezoid nucleus (RTN) region stimulates breathing in the awake rat. Respiration physiology. 2001;124:179–193. doi: 10.1016/s0034-5687(00)00212-7. [DOI] [PubMed] [Google Scholar]
- 176.Nattie EE. Diethyl pyrocarbonate (an imidazole binding substance) inhibits rostral VLM CO2 sensitivity. J Appl Physiol. 1986;61:843–850. doi: 10.1152/jappl.1986.61.3.843. [DOI] [PubMed] [Google Scholar]
- 177.Nattie EE. The alphastat hypothesis in respiratory control and acid-base balance. J Appl Physiol. 1990;69:1201–1207. doi: 10.1152/jappl.1990.69.4.1201. [DOI] [PubMed] [Google Scholar]
- 178.Nattie EE, Blanchford C, Li A. Retrofacial lesions: Effects on CO2 sensitive phrenic and sympathetic nerve activity. J Appl Physiol. 1992;73:1317–1325. doi: 10.1152/jappl.1992.73.4.1317. [DOI] [PubMed] [Google Scholar]
- 179.Nattie EE, Comroe JH., Jr Distinguished lecture of the American Physiological Society Respiration Section: Experimental biology 2010. J Appl Physiol. 2011;110:1–8. doi: 10.1152/japplphysiol.01061.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nattie EE, Fung ML, Li A, St John WM. Responses of respiratory modulated and tonic units in the retrotrapezoid nucleus to CO2. Respir Physiol. 1993;94:35–50. doi: 10.1016/0034-5687(93)90055-f. [DOI] [PubMed] [Google Scholar]
- 181.Nattie EE, Li A. Retrotrapezoid nucleus lesions decrease phrenic activity and CO2 sensitivity in rats. Respir Physiol. 1994;97:63–77. doi: 10.1016/0034-5687(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 182.Nattie EE, Li A. Central chemoreception in the region of the ventral respiratory group in the rat. J Appl Physiol. 1996;81:1987–1995. doi: 10.1152/jappl.1996.81.5.1987. [DOI] [PubMed] [Google Scholar]
- 183.Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- 184.Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol. 2002a;92:2119–2130. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- 185.Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol. 2002b;544:603–616. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nattie EE, Li A, Richerson G, Lappi DA. Medullary serotonergic neurons and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol. 2004;556:235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nattie EE, Li AH. Fluorescence location of RVLM kainate microinjections that alter the control of breathing. J Appl Physiol. 1990;68:1157–1166. doi: 10.1152/jappl.1990.68.3.1157. [DOI] [PubMed] [Google Scholar]
- 188.Nattie EE, Li AH, St John WM. Lesions in retrotrapezoid nucleus decrease ventilatory output in anesthetized or decerebrate cats. J Appl Physiol. 1991;71:1364–1375. doi: 10.1152/jappl.1991.71.4.1364. [DOI] [PubMed] [Google Scholar]
- 189.Nattie EE, Forster HV. Special issue: Central chemoreception. Respir Physiol & Neurobiol. 2010;173:193–336. doi: 10.1016/j.resp.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 190.Nattie G, Li A. Multiple central chemoreceptor sites: Cell types and function in vivo. Adv Exp Med Biol. 2008;605:343–347. doi: 10.1007/978-0-387-73693-8_60. [DOI] [PubMed] [Google Scholar]
- 191.Niblock MM, Gao H, Li A, Jeffress EC, Murphy M, Nattie EE. Fos-Tau-LacZ mice reveal sex differences in brainstem c-fos activation in response to mild carbon dioxide exposure. Brain Res. 1311:51–63. doi: 10.1016/j.brainres.2009.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nuding SC, Segers LS, Shannon R, O’Connor R, Morris KF, Lindsey BG. Central and peripheral chemoreceptors evoke distinct responses in simultaneously recorded neurons of the raphe-pontomedullary respiratory network. Philos Trans R Soc Lond Ser B. 2009;364:2501–2516. doi: 10.1098/rstb.2009.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ohtake PJ, Forster HV, Pan LG, Lowry TF, Korducki MJ, Whaley AA. Effects of cooling the ventrolateral medulla on diaphragm activity during NREM sleep. Respir Physiol. 1996;104:127–135. doi: 10.1016/0034-5687(96)00025-4. [DOI] [PubMed] [Google Scholar]
- 194.Okada Y, Chen Z, Jiang W, Kuwana S, Eldridge FL. Anatomical arrangement of hypercapnia-activated cells in the superficial ventral medulla of rats. J Appl Physiol. 2002;93:427–439. doi: 10.1152/japplphysiol.00620.2000. [DOI] [PubMed] [Google Scholar]
- 195.Oliven A, Odeh M, Gavriely N. Effect of hypercapnia on upper airway resistance and collapsibility in anesthetized dogs. Respir Physiol. 1989;75:29–38. doi: 10.1016/0034-5687(89)90084-4. [DOI] [PubMed] [Google Scholar]
- 196.Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: FromK+buffering to cell differentiation. J Neurochem. 2008;107:589–601. doi: 10.1111/j.1471-4159.2008.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Onimaru H, Ikeda K, Kawakami K. Phox2b, RTN/pFRG neurons and respiratory rhythmogenesis. Respir Physiol Neurobiol. 2009;168:13–18. doi: 10.1016/j.resp.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 198.Orem J. The nature of the wakefulness stimulus for breathing. Prog Clin Biol Res. 1990;345:23–30. discussion 31. [PubMed] [Google Scholar]
- 199.Oyamada Y, Ballantyne D, Muckenhoff K, Scheid P. Respirationmodulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem-spinal cord of the neonatal rat. J Physiol. 1998;513(Pt 2):381–398. doi: 10.1111/j.1469-7793.1998.381bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pappenheimer JR, Fencl V, Heisey SR, Held D. Role of cerebral fluids in control of respiration as studied in unanesthetized goats. AmJ Physiol. 1965;208:436–450. doi: 10.1152/ajplegacy.1965.208.3.436. [DOI] [PubMed] [Google Scholar]
- 201.Parisi RA, Neubauer JA, Frank MM, Edelman NH, Santiago TV. Correlation between genioglossal and diaphragmatic responses to hypercapnia during sleep. Am Rev Respir Dis. 1987;135:378–382. doi: 10.1164/arrd.1987.135.2.378. [DOI] [PubMed] [Google Scholar]
- 202.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–370. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- 203.Penatti EM, Berniker AV, Kereshi B, Cafaro C, Kelly ML, Niblock MM, Gao HG, Kinney HC, Li A, Nattie EE. Ventilatory response to hypercapnia and hypoxia after extensive lesion of medullary serotonergic neurons in newborn conscious piglets. J Appl Physiol. 2006;101:1177–1188. doi: 10.1152/japplphysiol.00376.2006. [DOI] [PubMed] [Google Scholar]
- 204.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Phillipson EA, Duffin J, Cooper JD. Critical dependence of respiratory rhythmicity on metabolic CO2 load. J Appl Physiol. 1981;50:45–54. doi: 10.1152/jappl.1981.50.1.45. [DOI] [PubMed] [Google Scholar]
- 206.Praud JP, Diaz V, Kianicka I, Chevalier JY, Canet E, Thisdale Y. Abolition of breathing rhythmicity in lambs by CO2 unloading in the first hours of life. Respir Physiol. 1997;110:1–8. doi: 10.1016/s0034-5687(97)00064-9. [DOI] [PubMed] [Google Scholar]
- 207.Putnam RW. CO2 chemoreception in cardiorespiratory control. J Appl Physiol. 2010;108:1796–1802. doi: 10.1152/japplphysiol.01169.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rahn H. Evolution of the gas transport system in vertebrates. Proc R Soc Med. 1966;59:493–494. [PMC free article] [PubMed] [Google Scholar]
- 210.Rahn H. Why are pH of 7.4 and PCO2 of 40 normal values for man? Bull Eur Physiopathol Respir. 1976;12:5–13. [PubMed] [Google Scholar]
- 211.Rahn H, Reeves RB, Howell BJ. Hydrogen ion regulation, temperature, and evolution. Am Rev Respir Dis. 1975;112:165–172. doi: 10.1164/arrd.1975.112.2.165. [DOI] [PubMed] [Google Scholar]
- 212.Ransom BR, Sontheimer H. The neurophysiology of glial cells. J Clin Neurophysiol. 1992;9:224–251. doi: 10.1097/00004691-199204010-00005. [DOI] [PubMed] [Google Scholar]
- 213.Reeves RB. The interaction of body temperature and acid-base balance in ectothermic vertebrates. Annu Rev Physiol. 1977;39:559–586. doi: 10.1146/annurev.ph.39.030177.003015. [DOI] [PubMed] [Google Scholar]
- 214.Ribas-Salgueiro JL, Gaytan SP, Crego R, Pasaro R, Ribas J. Highly H+-sensitive neurons in the caudal ventrolateral medulla of the rat. J Physiol. 2003;549:181–194. doi: 10.1113/jphysiol.2002.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ribas-Salgueiro JL, Gaytan SP, Ribas J, Pasaro R. Characterization of efferent projections of chemosensitive neurons in the caudal parapyramidal area of the rat brain. Brain Res Bull. 2005;66:235–248. doi: 10.1016/j.brainresbull.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 216.Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol. 1995;73:933–944. doi: 10.1152/jn.1995.73.3.933. [DOI] [PubMed] [Google Scholar]
- 217.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 218.Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol. 2005;90:259–266. doi: 10.1113/expphysiol.2005.029843. discussion 266–259. [DOI] [PubMed] [Google Scholar]
- 219.Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol. 2001;129:175–189. doi: 10.1016/s0034-5687(01)00289-4. [DOI] [PubMed] [Google Scholar]
- 220.Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- 221.Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465:593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- 222.Rybak IA, Abdala AP, Markin SN, Paton JF, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res. 2007;165:201–220. doi: 10.1016/S0079-6123(06)65013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sakurai T. Roles of orexins and orexin receptors in central regulation of feeding behavior and energy homeostasis. CNS Neurol Disord Drug Targets. 2006;5:313–325. doi: 10.2174/187152706777452218. [DOI] [PubMed] [Google Scholar]
- 224.Sakurai T. The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 225.Saper CB, Cano G, Scammell TE. Homeostatic, circadian, and emotional regulation of sleep. J Comp Neurol. 2005;493:92–98. doi: 10.1002/cne.20770. [DOI] [PubMed] [Google Scholar]
- 226.Schlaefke ME, Kille JF, Loeschcke HH. Elimination of central chemosensitivity by coagulation of a bilateral area on the ventral medullary surface in awake cats. Pflugers Arch. 1979;378:231–241. doi: 10.1007/BF00592741. [DOI] [PubMed] [Google Scholar]
- 227.Sears TA, Berger AJ, Phillipson EA. Reciprocal tonic activation of inspiratory and expiratory motoneurones by chemical drives. Nature. 1982;299:728–730. doi: 10.1038/299728a0. [DOI] [PubMed] [Google Scholar]
- 228.Severinghaus JW. Hans Loeschcke, Robert Mitchell and the medullary CO2 chemoreceptors: A brief historical review. Respir Physiol. 1998;114:17–24. doi: 10.1016/s0034-5687(98)00075-9. [DOI] [PubMed] [Google Scholar]
- 229.Skatrud JB, Dempsey JA. Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation. J Appl Physiol. 1983;55:813–822. doi: 10.1152/jappl.1983.55.3.813. [DOI] [PubMed] [Google Scholar]
- 230.Smatresk NJ. Chemoreceptor modulation of endogenous respiratory rhythms in vertebrates. Am J Physiol. 1990;259:R887–R897. doi: 10.1152/ajpregu.1990.259.5.R887. [DOI] [PubMed] [Google Scholar]
- 231.Smith CA, Chenuel BJ, Henderson KS, Dempsey JA. The apneic threshold during non-REM sleep in dogs: Sensitivity of carotid body vs. central chemoreceptors. J Appl Physiol. 2007;103:578–586. doi: 10.1152/japplphysiol.00017.2007. [DOI] [PubMed] [Google Scholar]
- 232.Smith CA, Chenuel BJ, Nakayama H, Dempsey JA. Ventilatory responsiveness to CO2 above & below eupnea: Relative importance of peripheral chemoreception. Adv Exp Med Biol. 2004;551:65–70. doi: 10.1007/0-387-27023-x_11. [DOI] [PubMed] [Google Scholar]
- 233.Smith CA, Forster HV, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: Evidence and implications for ventilatory control. Respir Physiol Neurobiol. 2010;173:288–297. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Smith CA, Henderson KS, Dempsey JA. Interactive ventilatory effects of carotid body hypoxia and hypocapnia in the unanesthetized dog. Adv Exp Med Biol. 1995;393:313–316. doi: 10.1007/978-1-4615-1933-1_59. [DOI] [PubMed] [Google Scholar]
- 235.Smith CA, Nakayama H, Dempsey JA. The essential role of carotid body chemoreceptors in sleep apnea. Can J Physiol Pharmacol. 2003;81:774–779. doi: 10.1139/y03-056. [DOI] [PubMed] [Google Scholar]
- 236.Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: Central vs. peripheral chemoreceptors. J Appl Physiol. 2006;100:13–19. doi: 10.1152/japplphysiol.00926.2005. [DOI] [PubMed] [Google Scholar]
- 237.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory networknetwork: A hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- 239.Solomon IC. Focal CO2/H+ alters phrenic motor output response to chemical stimulation of cat pre-Botzinger complex in vivo. J Appl Physiol. 2003;94:2151–2157. doi: 10.1152/japplphysiol.01192.2002. [DOI] [PubMed] [Google Scholar]
- 240.Somero GN. Proteins and temperature. Annu Rev Physiol. 1995;57:43–68. doi: 10.1146/annurev.ph.57.030195.000355. [DOI] [PubMed] [Google Scholar]
- 241.Spyer KM, Gourine AV. Chemosensory pathways in the brainstem controlling cardiorespiratory activity. Philos Trans R Soc Lond Ser B. 2009;364:2603–2610. doi: 10.1098/rstb.2009.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.St Croix CM, Satoh M, Morgan BJ, Skatrud JB, Dempsey JA. Role of respiratory motor output in within-breath modulation of muscle sympathetic nerve activity in humans. Circ Res. 1999;85:457–469. doi: 10.1161/01.res.85.5.457. [DOI] [PubMed] [Google Scholar]
- 243.St John WM, Glasser RL, King RA. Apneustic breathing after vagotomy in cats with chronic pneumotaxic center lesions. Respir Physiol. 1971;12:239–250. doi: 10.1016/0034-5687(71)90056-9. [DOI] [PubMed] [Google Scholar]
- 244.Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Stornetta RL, Spirovski D, Moreira TS, Takakura AC, West GH, Gwilt JM, Pilowsky PM, Guyenet PG. Galanin is a selective marker of the retrotrapezoid nucleus in rats. J Comp Neurol. 2009;512:373–383. doi: 10.1002/cne.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respir Physiol. 2001;127:135–155. doi: 10.1016/s0034-5687(01)00242-0. [DOI] [PubMed] [Google Scholar]
- 247.Sullivan CE, Murphy E, Kozar LF, Phillipson EA. Waking and ventilatory responses to laryngeal stimulation in sleeping dogs. J Appl Physiol. 1978;45:681–689. doi: 10.1152/jappl.1978.45.5.681. [DOI] [PubMed] [Google Scholar]
- 248.Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T. CO2 activates orexin-containing neurons in mice. Respir Physiol Neurobiol. 2009;166:184–186. doi: 10.1016/j.resp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 249.Sunderram J, Parisi RA, Strobel RJ. Serotonergic stimulation of the genioglossus and the response to nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:925–929. doi: 10.1164/ajrccm.162.3.9907077. [DOI] [PubMed] [Google Scholar]
- 250.Szymusiak R, McGinty D. Hypothalamic regulation of sleep and arousal. Ann N Y Acad Sci. 2008;1129:275–286. doi: 10.1196/annals.1417.027. [DOI] [PubMed] [Google Scholar]
- 251.Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and nonorexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–870. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 252.Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol. 2008;586:2975–2991. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Taylor NC, Li A, Nattie EE. Ventilatory effects of muscimol microdialysis into the rostral medullary raphe region of conscious rats. Respir Physiol Neurobiol. 2006;153:203–216. doi: 10.1016/j.resp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 256.Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 257.Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Thomas RJ, Daly RW, Weiss JW. Low-concentration carbon dioxide is an effective adjunct to positive airway pressure in the treatment of refractory mixed central and obstructive sleep-disordered breathing. Sleep. 2005;28:69–77. doi: 10.1093/sleep/28.1.69. [DOI] [PubMed] [Google Scholar]
- 259.Trouth CO, Loeschcke HH, Berndt J. Topography of the respiratory responses to electrical stimulation in the medulla oblongata. Pflugers Arch. 1973;339:153–170. doi: 10.1007/BF00587181. [DOI] [PubMed] [Google Scholar]
- 260.Trzebski A, Kubin L. Is the central inspiratory activity responsible for pCO2-dependent drive of the sympathetic discharge? J Auton Nerv Syst. 1981;3:401–420. doi: 10.1016/0165-1838(81)90078-3. [DOI] [PubMed] [Google Scholar]
- 261.Verin E, Tardif C, Marie JP, Buffet X, Lacoume Y, Delapille P, Pasquis P. Upper airway resistance during progressive hypercapnia and progressive hypoxia in normal awake subjects. Respir Physiol. 2001;124:35–42. doi: 10.1016/s0034-5687(00)00189-4. [DOI] [PubMed] [Google Scholar]
- 262.Wasserman K, Whipp BJ, Casaburi R, Huntsman DJ, Castagna J, Lugliani R. Regulation of arterial PCO2 during intravenous CO2 loading. J Appl Physiol. 1975;38:651–656. doi: 10.1152/jappl.1975.38.4.651. [DOI] [PubMed] [Google Scholar]
- 263.Watanabe S, Kuwaki T, Yanagisawa M, Fukuda Y, Shimoyama M. Persistent pain and stress activate pain-inhibitory orexin pathways. Neuroreport. 2005;16:5–8. doi: 10.1097/00001756-200501190-00002. [DOI] [PubMed] [Google Scholar]
- 264.Weese-Mayer DE, Berry-Kravis EM, Zhou L, Maher BS, Silvestri JM, Curran ME, Marazita ML. Idiopathic congenital central hypoventilation syndrome: Analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2b. Am J Med Genet A. 2003;123:267–278. doi: 10.1002/ajmg.a.20527. [DOI] [PubMed] [Google Scholar]
- 265.Weil JV, Byrne-Quinn E, Sodal IE, Friesen WO, Underhill B, Filley GF, Grover RF. Hypoxic ventilatory drive in normal man. J Clin Invest. 1970;49:1061–1072. doi: 10.1172/JCI106322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz ES, Schory K, Edwards JK, White DP. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wiley RG, Lappi DA. Targeting neurokinin-1 receptor-expressing neurons with [Sar9, Met(O2)11 substance P-saporin. Neurosci Lett. 1999;277:1–4. doi: 10.1016/s0304-3940(99)00846-0. [DOI] [PubMed] [Google Scholar]
- 268.Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Xie A, Skatrud JB, Barczi SR, Reichmuth K, Morgan BJ, Mont S, Dempsey JA. Influence of cerebral blood flow on breathing stability. J Appl Physiol. 2009;106:850–856. doi: 10.1152/japplphysiol.90914.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Xie A, Skatrud JB, Morgan B, Chenuel B, Khayat R, Reichmuth K, Lin J, Dempsey JA. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J Physiol. 2006;577:319–329. doi: 10.1113/jphysiol.2006.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Xu F, Zhang Z, Frazier DT. Microinjection of acetazolamide into the fastigial nucleus augments respiratory output in the rat. J Appl Physiol. 2001;91:2342–2350. doi: 10.1152/jappl.2001.91.5.2342. [DOI] [PubMed] [Google Scholar]
- 272.Yamamoto WS, Edwards MW., Jr Homeostasis of carbon dioxide during intravenous infusion of carbon dioxide. J Appl Physiol. 1960;15:807–818. doi: 10.1152/jappl.1960.15.5.807. [DOI] [PubMed] [Google Scholar]
- 273.Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, Mignot E, Nishino S. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14:1075–1081. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- 274.Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol. 2005;98:1387–1395. doi: 10.1152/japplphysiol.00914.2004. [DOI] [PubMed] [Google Scholar]
- 275.Zhang W, Shimoyama M, Fukuda Y, Kuwaki T. Multiple components of the defense response depend on orexin: Evidence from orexin knockout mice and orexin neuron-ablated mice. Auton Neurosci. 2006;126–127:139–145. doi: 10.1016/j.autneu.2006.02.021. [DOI] [PubMed] [Google Scholar]