Abstract
Promising results in several clinical studies have emphasized the potential of gene therapy to address important medical needs and initiated a surge of investments in drug development and commercialization. This enthusiasm is driven by positive data in clinical trials including gene replacement for Hemophilia B, X-linked Severe Combined Immunodeficiency, Leber's Congenital Amaurosis Type 2 and in cancer immunotherapy trials for hematological malignancies using chimeric antigen receptor T cells. These results build on the recent licensure of the European gene therapy product Glybera for the treatment of lipoprotein lipase deficiency. The progress from clinical development towards product licensure of several programs presents challenges to gene therapy product manufacturing. These include challenges in viral vector-manufacturing capacity, where an estimated 1–2 orders of magnitude increase will likely be needed to support eventual commercial supply requirements for many of the promising disease indications. In addition, the expanding potential commercial product pipeline and the continuously advancing development of recombinant viral vectors for gene therapy require that products are well characterized and consistently manufactured to rigorous tolerances of purity, potency and safety. Finally, there is an increase in regulatory scrutiny that affects manufacturers of investigational drugs for early-phase clinical trials engaged in industry partnerships. Along with the recent increase in biopharmaceutical funding in gene therapy, industry partners are requiring their academic counterparts to meet higher levels of GMP compliance at earlier stages of clinical development. This chapter provides a brief overview of current progress in the field and discusses challenges in vector manufacturing.
Introduction
The field of clinical gene therapy has advanced rapidly and now accounts for over 2200 clinical trials initiated since 1989, of which ∼65% are being conducted in the USA (1). Progress in the field has contributed to a new generation of gene therapy commercial initiatives, including several startup companies. Since the beginning of 2013, at least $600 million of venture capital funding was raised to support gene therapy, a strong comeback after several years of slow progress (2,3). The estimated global gene therapy market value is estimated to exceed $10 billion by 2025 (4,5). In October of 2012, the European Medicines Agency approved the use of a gene therapy product (Glybera) for the treatment of adult patients diagnosed with familial lipoprotein lipase deficiency (6). The approval comes 9 years after the State Food and Drug Administration of China (SFDA; Beijing, China) approved the world's first gene therapy product (Gendicine) for the treatment of head and neck squamous cell carcinoma in 2003 (7). This was followed in 2005 by the approval of Oncorine by the SFDA, the first commercialized oncolytic adenovirus for patients with late-stage refractory nasopharyngeal cancer (8) and the approval of Rexin-G, a tumor-targeted gamma-retroviral vector expressing cytocidal cyclin G1 by the Philippine FDA in 2007 (9). Also, the Russian Ministry of Healthcare and Social Development issued a market authorization in 2013 for Neovasculgen, a plasmid vector that expresses Vascular Endothelial Growth Factor gene for the treatment of peripheral arterial disease through angiogenesis (10). In October 2015, positive results from a Phase III clinical trial for LCA2 (meeting its primary endpoints) were reported by Spark Therapeutics (11). These results may lead to the first commercial product to be approved in the USA (12). The acceleration in the total number of gene therapy clinical trials, as well as the increased number of later stage clinical trials, signals an exciting era that promises to lead to the emergence of this new therapeutic paradigm for previously unmet therapeutic needs. The increased interest also stimulated the emergence of several new gene therapy companies, many closely affiliated with the academic centers that fostered the technology (13). This trend has led to a greater demand for both pre-clinical and clinical grade viral vector manufacturing capacity to support the increasing number of gene therapy clinical development programs. As these programs advance towards licensure, more rigorous product characterization using improved analytical methods, and progressively higher regulatory compliance will be required. Herein, we highlight several significant clinical successes in the field of gene therapy and provide examples of challenges in vector manufacturing, vector characterization and compliance.
Promising Clinical Results
The increasing interest in gene therapy is driven by the successful outcomes in several clinical trials, including for Hemophilia B, X-linked Severe Combined Immunodeficiency (SCID-X1), Leber's Congenital Amaurosis Type 2 (LCA2), and for hematological malignancies using autologous Chimeric Antigen Receptor (CAR)-T-cell therapy.
Hemophilia B is a congenital X-linked recessive bleeding disorder caused by a deficiency of coagulation Factor 9 (FIX). The use of recombinant adeno-associated virus (AAV) expressing human FIX (hFIX) delivered to liver or muscle is a promising alternative to the expensive recurrent replacement therapy with recombinant clotting factor (14–17). As demonstrated in a Phase 1/2 dose escalation study, intramuscular injection of an AAV serotype 2 vector-encoding hFIX was safe (18), and sustained presence of vector genome and FIX could be detected long term up to 3.7 years after administration (19). However, the levels of circulating FIX achieved at the highest dose administered were <1% of physiological levels, considered the threshold for therapeutic benefit (19). The investigators next developed a version of the recombinant AAV2 vector with an optimized liver-specific promoter for liver-directed gene transfer. They reported therapeutic but transient levels of factor IX expression in their clinical trial, which revealed immunological barriers to sustained expression (20). These unanticipated immune responses preventing long-term transgene expression were not predicted by pre-clinical studies in animal models. Building on these pioneering clinical studies, Nathwani and colleagues developed an optimized vector with a self-complementary, codon-optimized genome packaged with an AAV8 capsid. With this vector they reported sustained levels of circulating hFIX at therapeutically relevant levels in a majority of subjects in a Phase I/II clinical trial (21–24).
A second example of a successful outcome are several studies conducted by independent labs focused on sub-retinal delivery of recombinant AAV expressing retinal pigment epithelial 65-KDa protein (RPE65) for Leber Congenital Amaurosis Type 2 (25–28). Teams in the USA and Europe concurrently developed and independently performed clinical trials using AAV2–RPE65 vectors. While safety was reported in all trials, the clinical efficacy reported ranged from modest in Phase I/II clinical trials (25,27), to highly significant, as recently reported in a Phase III clinical study (meeting the primary endpoint with significance at P = 0.001) by Spark Therapeutics (11). The recombinant AAV vectors developed by these groups, which are similar in general design but reveal significant differences upon a detailed comparative analysis, resulted in divergent clinical outcomes. This observation emphasizes the importance of details of vector design and manufacturing methods that likely contributed to the effectiveness of the respective investigational products. These differences include the degree of optimization of the expression cassette (e.g. promoter design and transgene codon optimization), the manufacturing process and resulting final product purity and the product formulation (29). As illustrated in this example, the cumulative effect in details of vector chemistry, manufacturing and controls may have been key determinants of clinical success.
As a third example, in two Phase I/II trials conducted in the USA and Europe, nine children with SCID-X1 were treated with autologous bone marrow CD34+ cells transduced with a self-inactivating (SIN) γ-retroviral vector expressing the IL-2 receptor γ-chain (30). Eight of nine children presented with restored immune function without adverse effects after a median follow up of 33 months (range 16–43). One patient in the trial died prior to reconstitution with genetically modified T cells as a result of an overwhelming adenoviral infection unrelated to the treatment. The viral vector used in this trial included several improvements over the first-generation Moloney murine leukemia virus vector that was used in two similar trials conducted several years earlier. Although in earlier trials, immunity was substantially restored in 19 of 20 infants, 25% of the children developed T-cell acute lymphoblastic leukemia as a result of enhancer-mediated mutagenesis (31–33) driven by the viral long terminal repeats (LTRs). In response to these events, an SIN γ-retroviral vector was developed in which the LTR U3 enhancer was deleted, rendering the LTR ineffective. Instead, the human elongation factor 1-α short promoter was used as an internal promoter to drive transgene expression (34). To be able to effectively manufacture the SIN γ-retroviral vector, the manufacturing process was modified to a transient transfection-based system and optimized for titer (35,36). The similar T-cell recovery rates in both the previous and recent trials, but distinctly different outcomes in terms of insertional mutagenesis, again illustrate the importance of viral vector design and vector-specific optimization of manufacturing. For SCID-X1, comparison of gene therapy with haploidentical hematopoietic stem cell transplantation, the gold standard since 1968 (37), showed gene therapy to be an equal if not superior alternative, with a faster T-cell recovery (38).
The most compelling achievement in gene therapy for common life-threatening diseases are the dramatic clinical results obtained by several independent teams using the CAR-T-cell technology. This therapeutic strategy involves ex vivo gene transfer using recombinant retroviral or lentiviral vectors of chimeric antigen receptors composed of antibody-binding domains fused to T-cell-signaling domains into patient T lymphocytes. The transduced T lymphocytes are engineered to recognize and destroy autologous tumor cells. The most convincing results to date have been obtained using recombinant viral vectors to reprogram T cells to recognize CD19, a cell surface antigen expressed on B lymphocytic leukemic cells in acute lymphocytic leukemia and chronic lymphocytic leukemia (39–44). Treatment of patients with ex vivo expanded transduced autologous T cells caused dramatic and highly significant cancer remission in several independent trials [reviewed in (45)]. This promising therapeutic strategy will require the manufacture and quality control (QC) testing of potentially large quantities of clinical grade viral vectors because of the large number of patients that may be amenable to treatment by this approach.
Culture Systems for Viral Vector Production
Manufacturing processes for viral vectors include a variety of approaches, predominantly based on the use of mammalian cells in either adherent or suspension-cell-based systems, as reviewed by Merten et al. (46,47). Traditional laboratory-scale systems with adherent cells are generally difficult to scale up due to the large number of flasks, roller bottles or cell factories that need to be manipulated during a clinical production run. Not only does this pose a challenge in terms of available incubator space, the manipulation of a large number of culture vessels increases processing time and increases risk due to the number of steps that include open manipulation during aseptic processing. To address this challenge, and to reduce risk during clinical manufacturing, the field is moving to larger single-use disposable culture systems and bioreactors. A variety of different systems have been used, mostly defined by the type of vector to be manufactured and the type of cells used, with examples of each provided in Table 1 and as briefly discussed here. The choice of system is also affected by whether or not virus is harvested from the cell culture media, from the cells or from both.
Table 1.
Manufacturing systems for the production of gene therapy viral vectors organized by system, vector and method
| System | Vector | Method and cells | References |
|---|---|---|---|
| HYPERFlask (Corning) | LV vector | Transfection, HEK 293T adherent cells (CaPhos) | (48) |
| Roller Bottles | AAV | Transfection HEK293 adherent cells (CaPhos) | (49,50) |
| γ-Retroviral vector | PG13 and GPE-Am12-based stable producer cell lines | (51) | |
| Cell Factories (Nunc) or CellSTACKS (Corning) | LV vector | Transfection, HEK293 or 293T adherent cells (CaPhos, Lipofectamine 2000) | (52–55) |
| γ-Retroviral vector | PG13-based stable producer cell line; Transfection, HEK 293T adherent cells (CaPhos) | (35,56) | |
| AAV | Transfection, HEK293 (CaPhos) | (57) | |
| Foamy virus | Transfection, 293T, (PEI) | (58,59) | |
| iCELLis™ (Pall) | Adenoviral vector | Infection | (60) |
| AAV | Transfection, HEK 293T (PEI) | (61) | |
| γ-Retroviral vector | PG13 and 293Vec stable producer cell lines | (62) | |
| LV vector | Transfection, 293T adherent cells (CaPhos) | (63) | |
| Spinner Flask, Stirred Bioreactor | γ-Retroviral vector | Suspension-based stable producer cell lines, PA317-based stable producer cell line on Cytodex 1, 2 and Cytopore 2 microcarriers | (64,65) |
| Adenovirus | PA317-based stable producer cell line on Cytodex 1, 2 and Cytopore 2 microcarriers | (64) | |
| Rabies virus | Vero cells on Cytodex 1 microcarriers | (66) | |
| Baculovirus/AAV | SF9 cells in suspension infected using baculovirus | (67–72) | |
| LV vector | Transfection, HEK293, HEK293SF-3F6, suspension (PEI) | (73,74) | |
| CellCube (Costar) | γ-Retroviral vector | 203T Phoenix amphotropic packaging cell lines and TE Fly GA18 stable producer cell line | (75,76) |
| HSV-1 | Vero cell-derived 7b complementing cell line | (77) | |
| Wave Bioreactor (GE Healthcare) | γ-Retroviral vector | Transfection, HEK 293T adherent cells on FibraCel (CaPhos) | (35,36) |
| LV vector | GPRG stable producer cell line on FibraCel; producer cell lines suspension | (78,79) | |
| AAV | Transfection, HEK293, suspension (PEI); HeLa-based producer cell line/Ad5 infection; BHK-21 cells/HSV-1 infection | (80–83) | |
| Chemap CF-2000 bioreactor (Mannedorf) | LV vector | Transfection, 293E suspension cells (PEI) | (84) |
| AAV | Transfection, HEK293, 293E, 293F suspension cells (PEI) | (85) | |
| Spinferm Bioreactor (Braun Biotechnology) | AAV | Transfection, HEK293 suspension cells (CaPhos) | (86) |
| Celligen Plus (NewBrunswick Scientific) | Adenovirus | Infection, HEK293 | (7) |
An alternative to the standard tissue culture flask and cell factory are the Corning HYPERFlask and HYPERStack. In these systems, cells grow on a gas permeable surface, which omits the need for a headspace above the media thereby allowing more cells to be cultured within the same incubator foot print (87). The HYPERStack 36 provides 18 000 cm2 of cell growth area, which is almost three times the capacity of a 10-layer CellSTACK. In a side-by-side comparison, the productivity of a lentivirus (LV) vector produced by transient transfection in HYPERflask was 10-fold higher, up to 0.75 × 108 TU per cm2 as compared with 150-cm2 dishes (48), and one- to two-orders of magnitude higher as reported by others using cell factories (52,53).
Alternatively, cells can be cultured at even higher cell densities in fixed-bed culture systems such as the CellCube (Costar), iCELLIS™ (Pall) bioreactor or Celligen (NewBrunswick Scientific) bioreactor (48,60–62,75). The Celligen bioreactor, with a FibraCel cell carrier that serves as an HEK293 cell substrate, was the system used to manufacture the worlds' first approved gene therapy product in China (7). These fixed-bed culture systems are designed to increase cell culture density while maintaining adequate gas exchange and supply of nutrients and prevent accumulation of cell culture byproducts such as ammonia and lactic acid. This is generally accomplished by active control of the conditions in the bioreactor including dissolved oxygen and pH through control of the perfusion rate or by supplementation. A comparison of the CellCube and Celligen bioreactors versus stirred tank reactors (using Cytodex 1 microcarriers or suspension-adapted clump culture) showed superior growth of γ-retroviral vector producer cells in the fixed-bed reactors, with TE Fly GA18 cell densities 3- to 7-fold higher up to 5 × 1010 cells in a 21 250 cm2 CellCube (2.4 × 106 cells/cm2) resulting in higher titer (75). For transfection however, the fixed-bed reactor design may be less optimal as the high density of the biomass may not allow effective transfection of all cells. During process development of a γ-retroviral vector produced by calcium phosphate (CaPhos) transfection in 293T cells on FibraCel, viral titers were low when cells were transfected after being established within the FibraCel matrix and high when cells were seeded onto the carriers in the presence of transfection reagent and plasmid (35). In contrast, others have shown that 293T cells could be effectively transfected in the iCELLis™ Nano bioreactor (63) suggesting that the iCELLis™ scalable high-density cell culture production platform may be a viable option for transfection-based technologies. The iCELLis™ was used to develop a manufacturing process for the production of γ-retroviral vector suitable for Phase I/II trials (62). The investigators reported that vector production from 293Vec or PG13 packaging cells was 10 to 19-times more efficient in the bioreactor as compared with cell factories, with 293Vec cell densities of up to 106 cells per cm2, providing enough material in a single 30-l lot for the treatment of potentially up to 500 patients with ex vivo transduced autologous T cells (62). These data demonstrate that the iCELLis fixed-bed bioreactor may be used as a platform for scalable clinical grade γ-retroviral vector production for both stable producer cell-based and transfection-based production methodology.
While systems such as the fixed-bed bioreactors allow efficient collection of virus from cell supernatant, collecting virus such as AAV from the biomass is more challenging. Cells could potentially be chemically lysed inside of the bioreactor to liberate intracellular virus. However, it remains to be evaluated whether chemical lysis and microfluidization, a process where harvested cells are mechanically disrupted with a very high efficiency, provide comparable yields. The latter has been used successfully in the large-scale manufacturing of AAV (49,67–71,88).
Suspension-based cell cultures, on the other hand, provide true scalability from laboratory size systems to very large industry-scale stirred tank bioreactors. As compared with fixed-bed bioreactors, these systems allow for easy collection of both cells and culture media. However, not all producer or packaging cell lines allow adaptation to a serum-free suspension culture while maintaining high productivity. In addition, cell densities on a per volume basis are generally lower as compared with fixed-bed reactors. The Wave Bioreactor and Sartorius stirred tank reactor have been successfully used for the manufacture of AAV in SF9 suspension cells using the baculovirus system up to a 200-l scale (67–71). Grieger et al. developed a simple but effective transfection system of suspension-adapted human embryonic kidney (HEK293) cells to generate AAV serotypes 1 through 6, 8 and 9, as well as various chimeric capsids using the Wave Bioreactor, generating >105 vg/cell, or >1014 vg/l at ∼106cells/ml (80). Others have used transient transfection of suspension HEK293 or 293 EBNA-1 cells or established stable producer cell lines for the production of LV vectors (73,74,84,89). Similarly, an investigational LV vector for a Phase I/II Parkinson's disease clinical trial was manufactured in the Wave Bioreactor using inducible producer cell lines adapted to suspension growth (78,90). In addition, these systems can be adapted for adherent cells using microcarriers, as shown in the manufacture of adenovirus and rabies virus (64,66).
Ultimately, the choice of system for the large-scale manufacture of clinical grade viral vector requires a substantial investment in time and capital as each system requires deliberate and careful optimization and validation, in compliance with Federal Drug Administration (FDA) current good manufacturing practices (cGMP).
Transfection versus Stable Producer Cell Lines
Transient transfection of HEK293 or HEK293-derived cells with vector and helper or packaging plasmids is the most widely used method to generate γ-retroviral, LV and AAV-based viral vectors. Transfection-based manufacturing methods have been developed and optimized by a number of centers in terms of cell culture, transfection technique, harvesting method, purification and fill and finish (53,91–94). However, the efficient transfection of large amounts of cells is considered a bottleneck in clinical manufacturing that is susceptible to variation.
Available transfection reagents include calcium phosphate (CaPhos), polyethylenimine (PEI) and cationic lipids such as Lipofectamine 2000. Although CaPhos is the most affordable and most commonly used, CaPhos transfection is difficult to scale up and requires rigorous control for consistent results. The procedure is pH dependent and even small variations in pH can affect the quality of the precipitate, leading to lower vector titer. Batch-to-batch variation in the 2× HEPES-buffer saline (HBS) used to control pH has been reported to negatively affect transfection efficiencies (95). N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES) is an alternative to HBS which, when compared, was found to be more efficient (96–98). Transfection efficiency critically depends on the appropriate mixing of plasmid DNA/CaCl2 with HBS or BES. While at small scale bubbling of air into the HBS or BES solution while adding the DNA/CaCl2 provides effective mixing, at larger volumes alternate methods are required to achieve the same result. Methods that involve timely mixing at different scales are difficult to standardize and strongly depend on operator skill and training. Also, the amount of DNA added affects the quality of the transfection mixture. For example, the addition of a large amount of DNA (40 μg per 10-cm plate equivalent) produces a course precipitate associated with lower titer, and a lower amount of DNA (20 μg per 10-cm plate equivalent) produces a fine-to-medium precipitate associated with a higher titer (53). In clinical manufacturing, it is critical that procedures produce consistent results with minimal lot-to-lot variability. The variability in transfection efficiency and the lack of scalability and consistency is therefore problematic and requires rigorous control.
Cationic lipids, as reviewed by Ma et al. (99), are not subjected to the same variability and are effective in a large range of human cells. However, cationic lipids cause cellular toxicity (100) and therefore are less suitable for large-scale vector manufacturing. In a side-by-side comparison, CaPhos produced higher LV vector titers as compared with Lipofectamine 2000 (53). Polyethylenimine (PEI), on the other hand, is nontoxic, is also less sensitive to operator variability as compared to CaPhos and can effectively transfect cells in suspension (101–103). In addition, transfection with PEI only requires the addition of one reagent whereas CaPhos transfection requires the mixing of two components. Interestingly, in the production of foamy virus vectors, the use of PEI as compared with CaPhos significantly increased viral titer (58,59). Finally, the development of the MaxCyte, a scalable transfection system that uses flow electroporation, allows for the rapid transfection of large numbers of cells (104). However, for early-phase clinical trial vector manufacturing, the low cost, low toxicity and high transfection rates of CaPhos, without the need to invest in additional equipment, frequently make it the reagent of choice.
While transfected cells typically generate viral particles within the first 72 to 96 h post-transfection, stable cell lines produce vector over extended periods of time (47). In addition, processes using stable producer lines are easier to scale to levels required for commercial manufacture. Last but not least, vector derived from stable producer cells is free of contaminating plasmid DNA, which simplifies downstream vector purification. However, a challenge in creating producer cell lines for recombinant viral vector production is that some of the gene products required for vector generation are toxic to cells, and strategies are required to overcome these toxicities. Also, the use of stable producer lines for clinical manufacturing requires certification of each vector-specific producer cell line as a master cell bank. Finally, cell lines do not necessarily continue to produce high titer vector upon expansion (105,106).
Nonetheless, a series of packaging lines for the manufacturing of LV vector was developed by Throm et al. using a novel concatemeric array transfection technique for the effectively introduction of an SIN vector into the genome of the packaging cell line, combined with the Tetoff inducible expression of the cytotoxic proteins Rev and VSV-G (89). This system provided high titer LV vector at >107 transducing (TU)/ml at a large scale. Briefly, vector was generated in a Wave Bioreactor 20/50 system in a Cellbag supplemented with FibraCel disks, and vector was collected daily starting at day-2 post-induction for up to 6 to 8 days. Vector was clarified using a 1.2-/0.45-μm filter, purified on a Pall Mustang Q anion exchange capsule, diafiltered using PBS, formulated, 0.2-μm filtered and stored frozen (79). This vector is currently being used for a Phase I trial for the treatment of SCID-X1. Other groups have similarly developed inducible cell lines for LV vector production that may be used for clinical application (107–109). Since stable producer cell lines provide scalability where transfection does not, their use may ultimately be more cost effective when larger volumes of product are needed, especially in late phase and commercial manufacture.
Infection-Based Systems
For AAV, manufacturing methods based on transient transfection currently allow manufacturing with yields in the range of 1015 to 1016 viral genomes (110). This level, representing the currently established and validated manufacturing capacity, is sufficient for a number of promising programs involving relative low doses and for orphan disease indications such as LCA2. However, with the general maturation of the more successful clinical programs towards later Phase clinical trials, and for disease applications with a greater number of patients and/or where higher per patient vector doses are needed, an estimated one to two order of magnitude increase in manufacturing capacity is predicted to be needed to meet the increasing number of disease indications that may be targeted by gene therapy. An approach to meet these higher yields is the baculovirus system, which was employed by uniQure in the manufacture of Glybera. Originally developed by Kotin and colleagues (70), this system utilizes SF9 suspension insect cells for the manufacture of AAV. Rather than the introduction of genes required for vector production by transfection, SF9 cells are infected using recombinant baculoviruses that carry the vector genome, capsid and helper genes (72). Originally developed using three baculoviruses, recent improvements resulted in the consolidation of the AAV rep and cap genes into one baculovirus (111). The system has been shown to generate a yield of approaching 1014 viral genomes per liter with over 1016 vg produced from 100–200-l bioreactors. Moreover, the system has been shown to be scalable from 20 ml to 200 l, with 500-l size productions planned (69). These findings support the notion that further scale-up can result in a proportional increase in manufacturing capacity, where a 100-fold increase in volume to a 20 000-l scale may provide yields in the range of 1018 vg (93,112) sufficient to meet the expected needs in the field.
Other systems developed for the large-scale manufacture of AAV, and an alternative to the use of baculovirus, include recombinant replication-defective Herpes simplex virus 1 (HSV-1) and wild-type Adenovirus type 5 (Ad5), as recently reviewed (46). A HeLa-based producer cell line-based manufacturing process with Ad5 was established at a 250-l production scale (81,82). On the other hand, the recombinant HSV-1 expression system has been used in the Wave Bioreactor with suspension baby hamster kidney (BHK-21) cells for the production of AAV 1, 2, 5 and 8 showing high productivity with up to 1014 vg/l at a 10-l scale (83), with evidence of improved virus viability resulting in higher expression per virion as compared with vector created by plasmid transfection (113).
Vector Characterization and QC
Extensive characterization and QC testing is required for recombinant viral vectors for human clinical trials. This assures that each batch of investigational product meets pre-determined specifications for purity, potency, safety and identity. Furthermore, these QC tests should be sufficiently precise and accurate to provide the clinical investigation team and regulatory authorities with confidence regarding batch-to-batch consistency and comparability. The evolution from pre-clinical animal studies, to early- and then late-phase clinical studies, and ultimately to commercialization, correlates with the development, qualification and validation of a broad range of QC tests to ensure sufficient characterization of each investigational product. Similarly, over the course of clinical development, manufacturing process improvements and multi-lot experience enable progressive tightening of specifications. One of the challenges of characterization and QC testing of viral vectors is the high degree of complexity of this class of biologics. Even recombinant AAV, the smallest and least complex type of recombinant viral vectors, has a structure more complex than the most complex recombinant proteins. Each AAV particle consists of 60-VP protein subunits assembled into a capsid of defined architecture and stoichiometry (114) containing a single strand of DNA. Retroviral vectors are even more complex containing a double-stranded genome and lipid bilayer envelope surrounding the viral capsid. Thus, unique analytical methods must be developed and validated for each different viral vector-based gene therapy product and for each serotype. A second challenge for QC testing of gene therapy products is that most products are still at early stages of product development, with relatively little advanced stage experience in the field.
Methods for QC testing of viral vector-based gene therapy products can be divided into those that are substantially similar to existing tests that were developed and validated for licensed recombinant protein or vaccine products, and those that are more vector-specific and unique. The former include assays for process-related impurities such as residual production (host) cell proteins and nuclease-sensitive nucleic acids. For example, AAV vector-specific analytical methods and QC tests have had to be developed to address the complexity and unique features of vector product-related impurities in recombinant AAV investigational products (115). These include the development of product-specific potency assays required to demonstrate biological activity in each lot of manufactured product, a critical quality attribute of the vector (116). Potency assays are designed to measure the functional activity of the product resulting from expressing the vector transgene (e.g. a therapeutic protein). Measurement of potency is also critical to demonstrate lot-to-lot consistency and stability of the drug after periods of storage. Another vector-specific challenge for QC testing of viral vector gene therapy products relates to residual DNA impurities that are packaged within vector particles (117–119). Unlike residual nucleic impurities in recombinant proteins, which are typically reduced by in-process nuclease digestion to meet established specifications for recombinant therapeutic proteins (120), packaged nucleic impurities in AAV cannot be removed by nuclease treatment (e.g. Benzonase). Viral vector-encapsidated nucleic acid impurities may include fragments from any available source of DNA in the production milieu, including DNA from helper components (plasmid or bacmid DNA) and from producer cells [reviewed in (121)]. For AAV vectors, the predominant species of packaged DNA impurities are the plasmid or bacmid sequences adjacent to the ITR flanking the expression cassette (117,122), likely generated by reverse packaging from the ITR. The mechanism of packaging of fragments of host cell DNA is not understood, perhaps involving interaction of packaging components with motifs in the host cell genome that share some degree of homology with the viral vector-packaging signals. Regardless of the mechanism, nucleic acids impurities packaged and thereby protected by AAV capsid are especially difficult to remove because of their close resemblance to the vector itself. Approaches to mitigate the potential risks associated with packaged nucleic acid impurities include optimization of design of vector production plasmids used during the cell culture and vector production processes, as well as purification steps that exploit any minor differences in physico-chemical properties between true vectors and vector related impurities (88,117). Many of the methods relating to the unique challenges of gene therapy vectors are not yet validated to the standards required for licensed products, and additional manufacturing experience and innovations in analytical methods development will be required.
Regulatory Expectations
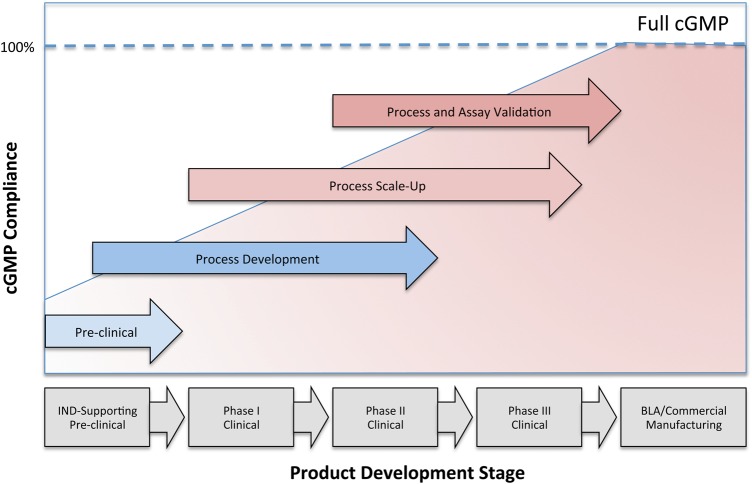
The manufacture of investigational new drugs, including viral vectors used in gene therapy, needs to comply with the CGMP as required under section 501(a)(2)(B) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) and as described in 21 Code of Federal Regulations (CFR) parts 210 and 211. Given the large investments needed to establish a fully compliant GMP-manufacturing operation, it is generally recognized that it is difficult to establish all aspects of GMP at early phases of clinical development. For example, while product QC testing for safety attributes of an investigational product (sterility, absence of mycoplasma and adventitious viral contaminants) must be established and validated from the beginning of clinical development, certain product characterization tests may be performed using non-validated assays and broad specifications, with the regulatory expectation of full assay validation and progressive tightening of specification to occur in a timely manner during clinical development. Similarly it is recognized that process changes, including process optimization and scale-up, are likely to occur during clinical development of most investigational products. In 2008, the FDA published a guidance for industry-entitled CGMP for Phase 1 Investigational Drugs that distinguishes Phase 1 drugs from those manufactured commercially and for later Phase clinical trials (123). This document formally recognized that certain requirements in part 211, such as the validation of manufacturing processes or requirements for warehousing, written for manufacturing processes that are typically characterized by large, repetitive, commercial batch production, may not be appropriate to the manufacture of most investigational drugs used for Phase 1 clinical trials. The guidance not only reflects the fact that some manufacturing controls differ between investigational and commercial manufacturing but also among various phases of the clinical trial spectrum. The relationship between the development stage of a product and the level of cGMP compliance is illustrated in Figure 1. As such, the regulations exempt the manufacture of Phase 1 investigational drugs from full cGMP compliance provided that adequate controls are in place to ensure that the drug meets appropriate standards of safety, identity, strength, quality and purity. This exception, however, is at odds with the European guidelines that mandate that products are fully cGMP compliant irrespective of the clinical trial stage (124,125). Such discrepancies often surface when US-based academic manufacturers of Phase 1 investigational drugs are audited by an international pharmaceutical company. An example is the requirement for identity testing of raw materials. Where ICH Q7A–7.30 requires all pharmaceutical ingredients intended for human use to be tested regardless of clinical phase, the FDA recognizes that for some materials, all relevant attributes or acceptance criteria may not be known at the Phase 1 stage of product development (123). Another example is compliance with 21 CFR 11 Electronic records; Electronic Signatures (126) that proves to be challenging for academic manufacturing facilities. While in industry robust validated enterprise-based systems support manufacturing, raw material and document control as well as quality assurance and a variety of laboratory and warehousing functions, such systems are generally less established or simply too large and costly for smaller academic-based manufacturing units. Software systems in place are frequently the same ones that were used when the academic-based manufacturing facility was initiated, sometimes on a minimal budget. With the field expanding, this leaves many institutions with a variety of software solutions that may no longer fit the size of the operation, are nonintegrated and sometimes difficult or impossible to validate. Ultimately, capital investments will be needed to assure compliance with 21 CFR 11 and to position academic institutions for growth as the field matures. The trend for the pharmaceutical industry and biotech to require full compliance of their academic-based partners, even at an early phase of clinical development, is partly driven by the complexity of the biological license application and the need, from a business perspective, to lower risk when products move towards commercialization. Although the FDA does not require full cGMP compliance for Phase 1 product manufacturing, academic manufacturers are increasingly being asked to conform to the needs of their industry partners.
Figure 1.
Progression of cGMP compliance at different stages of product development: illustration of the increase in the level of compliance with cGMP as products progress from a pre-clinical stage to the Biological License Application and Commercial Manufacturing.
Conclusion
There is great momentum and excitement in the field of gene therapy with an increasing number of clinical programs moving to later-phase clinical trials and towards market approval. This trend, however, does come with challenges, including a need for additional vector-manufacturing capacity to meet the increased demand for different products and higher vector quantities per product, and the requirement for more highly purified and better-characterized drugs. It is expected that the biotech sector will respond with additional engineering solutions for single-use closed manufacturing systems to meet this need. In addition, the various newly formed partnerships between academia and industry are expected to be complementary and instrumental in addressing these challenges with academia providing the pioneering experience gathered over the past decades in manufacturing protocol development for early-phase clinical programs, and industry providing experience and long-standing expertise in large-scale cGMP-manufacturing and regulatory compliance.
Conflict of Interest statement. J.F.W. is a co-founder, employee and stockholder of Spark Therapeutics, Inc. and is an inventor on a number of patents in the field of AAV vector technology.
Funding
J.V.D.L. is supported by the NHLBI Gene Therapy Resource Program (Contract HHSN268201200004I).
References
- 1.Edelstein M. The Journal of Gene Medicine Clinical Trial site. Gene Therapy Clinical Trials Worldwide http://www.wiley.com//legacy/wileychi/genmed/clinical/ (October 2015, date last accessed).
- 2.Herper M. (2014) Gene Therapy's Big Comeback. In: Forbes, April 14 http://www.forbes.com/sites/matthewherper/2014/03/26/once-seen-as-too-scary-editing-peoples-genes-with-viruses-makes-a-618-million-comeback/ (October 2015, date last accessed). [Google Scholar]
- 3.Philippidis A. (2014) Gene therapy briefs. Hum. Gene. Ther., 25, 262–264. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary G. (2015) Gene therapy market to be worth over USD 10 Billion by 2025, Predicts Roots Analysis. Business Wire, London. March 3, 2015.
- 5.Roots Analysis. (2015) Gene therapy market, 2015–2025.
- 6.Yla-Herttuala S. (2012) Endgame: glybera finally recommended for approval as the first gene therapy drug in the European Union. Mol. Ther., 20, 1831–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng Z. (2005) Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum. Gene. Ther., 16, 1016–1027. [DOI] [PubMed] [Google Scholar]
- 8.Liang M. (2012) Clinical development of oncolytic viruses in China. Curr. Pharm. Biotechnol., 13, 1852–1857. [DOI] [PubMed] [Google Scholar]
- 9.Gordon E.M., Hall F.L. (2010) Rexin-G, a targeted genetic medicine for cancer. Expert. Opin. Biol. Ther., 10, 819–832. [DOI] [PubMed] [Google Scholar]
- 10.Human Stem Cells Institute (2011) HSCI receives approval to market neovasculgen – the first Russian gene-therapy drug for treatment of peripheral arterial disease. Human Stem Cells Institute, Moscow, Russia. December 7, 2011.
- 11.Spark Therapeutics (2015) Spark therapeutics announces positive top-line results from pivotal Phase 3 Trial of SPK-RPE65 for genetic blinding conditions. Globe Newswire, Philadelphia, PA. October 5, 2015.
- 12.Schimmer J., Breazzano S. (2015) Investor outlook: focus on upcoming LCA2 gene therapy Phase III results. Hum. Gene. Ther. Clin. Dev., 26, 144–149. [DOI] [PubMed] [Google Scholar]
- 13.Huggett B. (2014) Innovative startups 2013. Nat. Biotechnol., 32, 127. [DOI] [PubMed] [Google Scholar]
- 14.Rogers G.L., Herzog R.W. (2015) Gene therapy for hemophilia. Front. Biosci. (Landmark Ed)., 20, 556–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herzog R.W., Yang E.Y., Couto L.B., Hagstrom J.N., Elwell D., Fields P.A., Burton M., Bellinger D.A., Read M.S., Brinkhous K.M. et al. (1999) Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat. Med., 5, 56–63. [DOI] [PubMed] [Google Scholar]
- 16.Herzog R.W., Hagstrom J.N., Kung S.H., Tai S.J., Wilson J.M., Fisher K.J., High K.A. (1997) Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc. Natl Acad. Sci. USA., 94, 5804–5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder R.O., Miao C.H., Patijn G.A., Spratt S.K., Danos O., Nagy D., Gown A.M., Winther B., Meuse L., Cohen L.K. et al. (1997) Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat. Genet., 16, 270–276. [DOI] [PubMed] [Google Scholar]
- 18.Manno C.S., Chew A.J., Hutchison S., Larson P.J., Herzog R.W., Arruda V.R., Tai S.J., Ragni M.V., Thompson A., Ozelo M. et al. (2003) AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood., 101, 2963–2972. [DOI] [PubMed] [Google Scholar]
- 19.Jiang H., Pierce G.F., Ozelo M.C., de Paula E.V., Vargas J.A., Smith P., Sommer J., Luk A., Manno C.S., High K.A. et al. (2006) Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol. Ther., 14, 452–455. [DOI] [PubMed] [Google Scholar]
- 20.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B. et al. (2006) Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med., 12, 342–347. [DOI] [PubMed] [Google Scholar]
- 21.Patel N., Reiss U., Davidoff A.M., Nathwani A.C. (2014) Progress towards gene therapy for haemophilia B. Int. J. Hematol., 99, 372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathwani A.C., Nienhuis A.W., Davidoff A.M. (2014) Our journey to successful gene therapy for hemophilia B. Hum. Gene. Ther., 25, 923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathwani A.C., Tuddenham E.G., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. et al. (2011) Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med., 365, 2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathwani A.C., Reiss U.M., Tuddenham E.G., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D. et al. (2014) Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med., 371, 1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cideciyan A.V., Jacobson S.G., Beltran W.A., Sumaroka A., Swider M., Iwabe S., Roman A.J., Olivares M.B., Schwartz S.B., Komaromy A.M. et al. (2013) Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc. Natl Acad. Sci. USA, 110, E517–E525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Testa F., Maguire A.M., Rossi S., Pierce E.A., Melillo P., Marshall K., Banfi S., Surace E.M., Sun J., Acerra C. et al. (2013) Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology, 120, 1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bainbridge J.W., Mehat M.S., Sundaram V., Robbie S.J., Barker S.E., Ripamonti C., Georgiadis A., Mowat F.M., Beattie S.G., Gardner P.J. et al. (2015) Long-term effect of gene therapy on Leber's congenital amaurosis. N. Engl. J. Med., 372, 1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N. Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K.A., Testa F., Surace E.M. et al. (2008) Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med., 358, 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennicelli J., Wright J.F., Komaromy A., Jacobs J.B., Hauck B., Zelenaia O., Mingozzi F., Hui D., Chung D., Rex T.S. et al. (2008) Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol. Ther., 16, 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hacein-Bey-Abina S., Pai S.Y., Gaspar H.B., Armant M., Berry C.C., Blanche S., Bleesing J., Blondeau J., de Boer H., Buckland K.F. et al. (2014) A modified gamma-retrovirus vector for X-linked severe combined immunodeficiency. N. Engl. J. Med., 371, 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E. et al. (2003) LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science, 302, 415–419. [DOI] [PubMed] [Google Scholar]
- 32.Howe S.J., Mansour M.R., Schwarzwaelder K., Bartholomae C., Hubank M., Kempski H., Brugman M.H., Pike-Overzet K., Chatters S.J., de Ridder D. et al. (2008) Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest., 118, 3143–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hacein-Bey-Abina S., Garrigue A., Wang G.P., Soulier J., Lim A., Morillon E., Clappier E., Caccavelli L., Delabesse E., Beldjord K. et al. (2008) Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest., 118, 3132–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thornhill S.I., Schambach A., Howe S.J., Ulaganathan M., Grassman E., Williams D., Schiedlmeier B., Sebire N.J., Gaspar H.B., Kinnon C. et al. (2008) Self-inactivating gammaretroviral vectors for gene therapy of X-linked severe combined immunodeficiency. Mol. Ther., 16, 590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Loo J.C., Swaney W.P., Grassman E., Terwilliger A., Higashimoto T., Schambach A., Baum C., Thrasher A.J., Williams D.A., Nordling D.L. et al. (2012) Scale-up and manufacturing of clinical-grade self-inactivating gamma-retroviral vectors by transient transfection. Gene. Ther., 19, 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Loo J.C., Swaney W.P., Grassman E., Terwilliger A., Higashimoto T., Schambach A., Hacein-Bey-Abina S., Nordling D.L., Cavazzana-Calvo M., Thrasher A.J. et al. (2012) Critical variables affecting clinical-grade production of the self-inactivating gamma-retroviral vector for the treatment of X-linked severe combined immunodeficiency. Gene. Ther., 19, 872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pai S.Y., Logan B.R., Griffith L.M., Buckley R.H., Parrott R.E., Dvorak C.C., Kapoor N., Hanson I.C., Filipovich A.H., Jyonouchi S. et al. (2014) Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N. Engl. J. Med., 371, 434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Touzot F., Moshous D., Creidy R., Neven B., Frange P., Cros G., Caccavelli L., Blondeau J., Magnani A., Luby J.M. et al. (2015) Faster T-cell development following gene therapy compared with haploidentical HSCT in the treatment of SCID-X1. Blood, 125, 3563–3569. [DOI] [PubMed] [Google Scholar]
- 39.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med., 365, 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalos M., Levine B.L., Porter D.L., Katz S., Grupp S.A., Bagg A., June C.H. (2011) T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med., 3, 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kochenderfer J.N., Dudley M.E., Feldman S.A., Wilson W.H., Spaner D.E., Maric I., Stetler-Stevenson M., Phan G.Q., Hughes M.S., Sherry R.M. et al. (2012) B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood, 119, 2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brentjens R.J., Riviere I., Park J.H., Davila M.L., Wang X., Stefanski J., Taylor C., Yeh R., Bartido S., Borquez-Ojeda O. et al. (2011) Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood, 118, 4817–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F. et al. (2013) Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med., 368, 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., Bartido S., Stefanski J., Taylor C., Olszewska M. et al. (2013) CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med., 5, 177ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gill S., June C.H. (2015) Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol. Rev., 263, 68–89. [DOI] [PubMed] [Google Scholar]
- 46.Merten O.-W., Schweizer M., Chahal P., Kamen A.A. (2014) Manufacturing of viral vectors for gene therapy: part I. Upstream processing. Pharm. Bioprocess, 2, 183–203. [Google Scholar]
- 47.Merten O.W. (2004) State-of-the-art of the production of retroviral vectors. J. Gene. Med., 6(Suppl 1), S105–S124. [DOI] [PubMed] [Google Scholar]
- 48.Kutner R.H., Puthli S., Marino M.P., Reiser J. (2009) Simplified production and concentration of HIV-1-based lentiviral vectors using HYPERFlask vessels and anion exchange membrane chromatography. BMC. Biotechnol., 9, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright J.F., Wellman J., High K.A. (2010) Manufacturing and regulatory strategies for clinical AAV2-hRPE65. Curr. Gene. Ther., 10, 341–349. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y.L., Wagner K., Robinson N., Sabatino D., Margaritis P., Xiao W., Herzog R.W. (2003) Optimized production of high-titer recombinant adeno-associated virus in roller bottles. Biotechniques, 34, 184–189. [DOI] [PubMed] [Google Scholar]
- 51.Cornetta K., Matheson L., Ballas C. (2005) Retroviral vector production in the National Gene Vector Laboratory at Indiana University. Gene. Ther., 12(Suppl 1), S28–S35. [DOI] [PubMed] [Google Scholar]
- 52.Geraerts M., Michiels M., Baekelandt V., Debyser Z., Gijsbers R. (2005) Upscaling of lentiviral vector production by tangential flow filtration. J. Gene. Med., 7, 1299–1310. [DOI] [PubMed] [Google Scholar]
- 53.Karolewski B.A., Watson D.J., Parente M.K., Wolfe J.H. (2003) Comparison of transfection conditions for a lentivirus vector produced in large volumes. Hum. Gene. Ther., 14, 1287–1296. [DOI] [PubMed] [Google Scholar]
- 54.Merten O.W., Charrier S., Laroudie N., Fauchille S., Dugue C., Jenny C., Audit M., Zanta-Boussif M.A., Chautard H., Radrizzani M. et al. (2011) Large-scale manufacture and characterization of a lentiviral vector produced for clinical ex vivo gene therapy application. Hum. Gene. Ther., 22, 343–356. [DOI] [PubMed] [Google Scholar]
- 55.Slepushkin V., Chang N., Cohen R., Gan Y., Jiang B., Deausen E., VBerlinger D., Binder G., Andre K., Hummeau L. et al. (2003) Large-scale purification of a lentiviral vector by size exclusion chromatography or mustang Q ion exchange capsule. Bioprocessing. J., 2, 89–95. [Google Scholar]
- 56.Przybylowski M., Hakakha A., Stefanski J., Hodges J., Sadelain M., Riviere I. (2006) Production scale-up and validation of packaging cell clearance of clinical-grade retroviral vector stocks produced in cell factories. Gene. Ther., 13, 95–100. [DOI] [PubMed] [Google Scholar]
- 57.Lock M., Alvira M., Vandenberghe L.H., Samanta A., Toelen J., Debyser Z., Wilson J.M. (2010) Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum. Gene. Ther., 21, 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nasimuzzaman M., Lynn D., Beuerlein M., Cross S., Link K., Lutzko C.M., Nordling D.L., Russell D.W., Malik P., van der Loo J.C.M. (2015) Scale-up and manufacturing of high-titer foamy virus vector containing human CD18 for the treatment of leukocyte adhesion deficiency. Poster 461, In American Society of Gene & Cell Therapy, New Orleans, LA, May 13–16, 2015. [Google Scholar]
- 59.Nasimuzzaman M., Kim Y.S., Wang Y.D., Persons D.A. (2014) High-titer foamy virus vector transduction and integration sites of human CD34(+) cell-derived SCID-repopulating cells. Mol. Ther. Methods. Clin. Dev., 1, 14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lesch H.P., Heikkila K.M., Lipponen E.M., Valonen P., Muller A., Rasanen E., Tuunanen T., Hassinen M.M., Parker N., Karhinen M. et al. (2015) Process development of adenoviral vector production in fixed bed bioreactor: from bench to commercial scale. Hum. Gene. Ther., 26, 560–571. [DOI] [PubMed] [Google Scholar]
- 61.Emmerling V.V., Pegel A., Milian E.G., Venereo-Sanchez A., Kunz M., Wegele J., Kamen A.A., Kochanek S., Hoerer M. (2015) Rational plasmid design and bioprocess optimization to enhance recombinant adeno-associated virus (AAV) productivity in mammalian cells. Biotechnol. J., 10.1002/biot.201500176. [DOI] [PubMed] [Google Scholar]
- 62.Wang X., Olszewska M., Qu J., Wasielewska T., Bartido S., Hermetet G., Sadelain M., Riviere I. (2015) Large-scale clinical-grade retroviral vector production in a fixed-bed bioreactor. J. Immunother., 38, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valkama A.J., Lesch H.P., Martikainen A., Malinen J., Salonen T., Mahonen M., Heikura T., Yla-Herttuala S. (2015) Optimization of lentiviral vector production in a fixed-bed bioreactor. In European Society of Gene and Cell Therapy, Vol. P155. [DOI] [PMC free article] [PubMed]
- 64.Wu S.C., Huang G.Y., Liu J.H. (2002) Production of retrovirus and adenovirus vectors for gene therapy: a comparative study using microcarrier and stationary cell culture. Biotechnol. Prog., 18, 617–622. [DOI] [PubMed] [Google Scholar]
- 65.Pizzato M., Merten O.W., Blair E.D., Takeuchi Y. (2001) Development of a suspension packaging cell line for production of high titre, serum-resistant murine leukemia virus vectors. Gene. Ther., 8, 737–745. [DOI] [PubMed] [Google Scholar]
- 66.Rourou S., van der Ark A., Majoul S., Trabelsi K., van der Velden T., Kallel H. (2009) A novel animal-component-free medium for rabies virus production in Vero cells grown on Cytodex 1 microcarriers in a stirred bioreactor. Appl. Microbiol. Biotechnol., 85, 53–63. [DOI] [PubMed] [Google Scholar]
- 67.Virag T., Cecchini S., Kotin R.M. (2009) Producing recombinant adeno-associated virus in foster cells: overcoming production limitations using a baculovirus-insect cell expression strategy. Hum. Gene. Ther., 20, 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cecchini S., Negrete A., Kotin R.M. (2008) Toward exascale production of recombinant adeno-associated virus for gene transfer applications. Gene. Ther., 15, 823–830. [DOI] [PubMed] [Google Scholar]
- 69.Cecchini S., Virag T., Kotin R.M. (2011) Reproducible high yields of recombinant adeno-associated virus produced using invertebrate cells in 0.02- to 200-liter cultures. Hum. Gene. Ther., 22, 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Urabe M., Ding C., Kotin R.M. (2002) Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum. Gene. Ther., 13, 1935–1943. [DOI] [PubMed] [Google Scholar]
- 71.Negrete A., Kotin R.M. (2007) Production of recombinant adeno-associated vectors using two bioreactor configurations at different scales. J. Virol. Methods, 145, 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kotin R.M. (2011) Large-scale recombinant adeno-associated virus production. Hum. Mol. Genet., 20, R2–R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Segura M.M., Garnier A., Durocher Y., Ansorge S., Kamen A. (2010) New protocol for lentiviral vector mass production. Methods. Mol. Biol., 614, 39–52. [DOI] [PubMed] [Google Scholar]
- 74.Ansorge S., Lanthier S., Transfiguracion J., Durocher Y., Henry O., Kamen A. (2009) Development of a scalable process for high-yield lentiviral vector production by transient transfection of HEK293 suspension cultures. J. Gene. Med., 11, 868–876. [DOI] [PubMed] [Google Scholar]
- 75.Merten O.W., Cruz P.E., Rochette C., Geny-Fiamma C., Bouquet C., Goncalves D., Danos O., Carrondo M.J. (2001) Comparison of different bioreactor systems for the production of high titer retroviral vectors. Biotechnol. Prog., 17, 326–335. [DOI] [PubMed] [Google Scholar]
- 76.Wikstrom K., Blomberg P., Islam K.B. (2004) Clinical grade vector production: analysis of yield, stability, and storage of gmp-produced retroviral vectors for gene therapy. Biotechnol. Prog., 20, 1198–1203. [DOI] [PubMed] [Google Scholar]
- 77.Ozuer A., Wechuck J.B., Goins W.F., Wolfe D., Glorioso J.C., Ataai M.M. (2002) Effect of genetic background and culture conditions on the production of herpesvirus-based gene therapy vectors. Biotechnol. Bioeng., 77, 685–692. [DOI] [PubMed] [Google Scholar]
- 78.Palfi S., Gurruchaga J.M., Ralph G.S., Lepetit H., Lavisse S., Buttery P.C., Watts C., Miskin J., Kelleher M., Deeley S. et al. (2014) Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson's disease: a dose escalation, open-label, phase 1/2 trial. Lancet, 383, 1138–1146. [DOI] [PubMed] [Google Scholar]
- 79.Greene M.R., Lockey T., Mehta P.K., Kim Y.S., Eldridge P.W., Gray J.T., Sorrentino B.P. (2012) Transduction of human CD34+ repopulating cells with a self-inactivating lentiviral vector for SCID-X1 produced at clinical scale by a stable cell line. Hum. Gene. Ther. Methods., 23, 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grieger J.C., Soltys S.M., Samulski R.J. (2015) Production of recombinant adeno-associated virus vectors using suspension HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Mol. Ther., 10.1038/mt.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thorne B.A., Takeya R.K., Peluso R.W. (2009) Manufacturing recombinant adeno-associated viral vectors from producer cell clones. Hum. Gene. Ther., 20, 707–714. [DOI] [PubMed] [Google Scholar]
- 82.Farson D., Harding T.C., Tao L., Liu J., Powell S., Vimal V., Yendluri S., Koprivnikar K., Ho K., Twitty C. et al. (2004) Development and characterization of a cell line for large-scale, serum-free production of recombinant adeno-associated viral vectors. J. Gene. Med., 6, 1369–1381. [DOI] [PubMed] [Google Scholar]
- 83.Thomas D.L., Wang L., Niamke J., Liu J., Kang W., Scotti M.M., Ye G.J., Veres G., Knop D.R. (2009) Scalable recombinant adeno-associated virus production using recombinant herpes simplex virus type 1 coinfection of suspension-adapted mammalian cells. Hum. Gene. Ther., 20, 861–870. [DOI] [PubMed] [Google Scholar]
- 84.Segura M.M., Garnier A., Durocher Y., Coelho H., Kamen A. (2007) Production of lentiviral vectors by large-scale transient transfection of suspension cultures and affinity chromatography purification. Biotechnol. Bioeng., 98, 789–799. [DOI] [PubMed] [Google Scholar]
- 85.Durocher Y., Pham P.L., St-Laurent G., Jacob D., Cass B., Chahal P., Lau C.J., Nalbantoglu J., Kamen A. (2007) Scalable serum-free production of recombinant adeno-associated virus type 2 by transfection of 293 suspension cells. J. Virol. Methods., 144, 32–40. [DOI] [PubMed] [Google Scholar]
- 86.Park J.Y., Lim B.P., Lee K., Kim Y.G., Jo E.C. (2006) Scalable production of adeno-associated virus type 2 vectors via suspension transfection. Biotechnol. Bioeng., 94, 416–430. [DOI] [PubMed] [Google Scholar]
- 87.Titus K., Klimovich V., Rothenberg M., Pardo P., Tanner A., Martin G. (2010) Closed system cell culture protocol using HYPERStack vessels with gas permeable material technology. J. Vis. Exp., 10.3791/2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qu G., Bahr-Davidson J., Prado J., Tai A., Cataniag F., McDonnell J., Zhou J., Hauck B., Luna J., Sommer J.M. et al. (2007) Separation of adeno-associated virus type 2 empty particles from genome containing vectors by anion-exchange column chromatography. J. Virol. Methods., 140, 183–192. [DOI] [PubMed] [Google Scholar]
- 89.Throm R.E., Ouma A.A., Zhou S., Chandrasekaran A., Lockey T., Greene M., De Ravin S.S., Moayeri M., Malech H.L., Sorrentino B.P. et al. (2009) Efficient construction of producer cell lines for a SIN lentiviral vector for SCID-X1 gene therapy by concatemeric array transfection. Blood, 113, 5104–5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guy H.M., McCloskey L., Lye G.J., Mitrophanous K.A., Mukhopadhyay T.K. (2013) Characterization of lentiviral vector production using microwell suspension cultures of HEK293T-derived producer cells. Hum. Gene. Ther. Methods, 24, 125–139. [DOI] [PubMed] [Google Scholar]
- 91.Graham F.L., van der Eb A.J. (1973) A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology, 52, 456–467. [DOI] [PubMed] [Google Scholar]
- 92.Pear W.S., Nolan G.P., Scott M.L., Baltimore D. (1993) Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl Acad. Sci. USA, 90, 8392–8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galibert L., Merten O.W. (2011) Latest developments in the large-scale production of adeno-associated virus vectors in insect cells toward the treatment of neuromuscular diseases. J. Invertebr. Pathol., 107(Suppl), S80–S93. [DOI] [PubMed] [Google Scholar]
- 94.Segura M.M., Mangion M., Gaillet B., Garnier A. (2013) New developments in lentiviral vector design, production and purification. Expert. Opin. Biol. Ther., 13, 987–1011. [DOI] [PubMed] [Google Scholar]
- 95.Lee J.H., Welsh M.J. (1999) Enhancement of calcium phosphate-mediated transfection by inclusion of adenovirus in coprecipitates. Gene. Ther., 6, 676–682. [DOI] [PubMed] [Google Scholar]
- 96.Chen C., Okayama H. (1987) High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol., 7, 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen C.A., Okayama H. (1988) Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques, 6, 632–638. [PubMed] [Google Scholar]
- 98.Tognon M., Cattozzo E.M., Bianchi S., Romanelli M.G. (1996) Enhancement of HSV-DNA infectivity, in Vero and RS cells, by a modified calcium-phosphate transfection technique. Virus. Genes., 12, 193–197. [DOI] [PubMed] [Google Scholar]
- 99.Ma B., Zhang S., Jiang H., Zhao B., Lv H. (2007) Lipoplex morphologies and their influences on transfection efficiency in gene delivery. J. Control. Release., 123, 184–194. [DOI] [PubMed] [Google Scholar]
- 100.Nguyen L.T., Atobe K., Barichello J.M., Ishida T., Kiwada H. (2007) Complex formation with plasmid DNA increases the cytotoxicity of cationic liposomes. Biol. Pharm. Bull., 30, 751–757. [DOI] [PubMed] [Google Scholar]
- 101.Kuroda H., Kutner R.H., Bazan N.G., Reiser J. (2009) Simplified lentivirus vector production in protein-free media using polyethylenimine-mediated transfection. J. Virol. Methods., 157, 113–121. [DOI] [PubMed] [Google Scholar]
- 102.Hildinger M., Baldi L., Stettler M., Wurm F.M. (2007) High-titer, serum-free production of adeno-associated virus vectors by polyethyleneimine-mediated plasmid transfection in mammalian suspension cells. Biotechnol. Lett., 29, 1713–1721. [DOI] [PubMed] [Google Scholar]
- 103.Feng L., Guo M., Zhang S., Chu J., Zhuang Y., Zhang S. (2008) Improvement in the suspension-culture production of recombinant adeno-associated virus-LacZ in HEK-293 cells using polyethyleneimine-DNA complexes in combination with hypothermic treatment. Biotechnol. Appl. Biochem., 50, 121–132. [DOI] [PubMed] [Google Scholar]
- 104.Li L.H., Shivakumar R., Feller S., Allen C., Weiss J.M., Dzekunov S., Singh V., Holaday J., Fratantoni J., Liu L.N. (2002) Highly efficient, large volume flow electroporation. Technol. Cancer. Res. Treat., 1, 341–350. [DOI] [PubMed] [Google Scholar]
- 105.Broussau S., Jabbour N., Lachapelle G., Durocher Y., Tom R., Transfiguracion J., Gilbert R., Massie B. (2008) Inducible packaging cells for large-scale production of lentiviral vectors in serum-free suspension culture. Mol. Ther., 16, 500–507. [DOI] [PubMed] [Google Scholar]
- 106.Ikeda Y., Takeuchi Y., Martin F., Cosset F.L., Mitrophanous K., Collins M. (2003) Continuous high-titer HIV-1 vector production. Nat. Biotechnol., 21, 569–572. [DOI] [PubMed] [Google Scholar]
- 107.Sanber K.S., Knight S.B., Stephen S.L., Bailey R., Escors D., Minshull J., Santilli G., Thrasher A.J., Collins M.K., Takeuchi Y. (2015) Construction of stable packaging cell lines for clinical lentiviral vector production. Sci. Rep., 5, 9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hu P., Li Y., Sands M.S., McCown T., Kafri T. (2015) Generation of a stable packaging cell line producing high-titer PPT-deleted integration-deficient lentiviral vectors. Mol. Ther. Methods. Clin. Dev., 2, 15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stornaiuolo A., Piovani B.M., Bossi S., Zucchelli E., Corna S., Salvatori F., Mavilio F., Bordignon C., Rizzardi G.P., Bovolenta C. (2013) RD2-MolPack-Chim3, a packaging cell line for stable production of lentiviral vectors for anti-HIV gene therapy. Hum. Gene. Ther. Methods., 24, 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wright J.F. (2009) Transient transfection methods for clinical adeno-associated viral vector production. Hum. Gene. Ther., 20, 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith R.H., Levy J.R., Kotin R.M. (2009) A simplified baculovirus-AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol. Ther., 17, 1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wright J.F. (2011) Adeno-associated viral vector manufacturing: keeping pace with accelerating clinical development. Hum. Gene. Ther., 22, 913–914. [DOI] [PubMed] [Google Scholar]
- 113.Chulay J.D., Ye G.J., Thomas D.L., Knop D.R., Benson J.M., Hutt J.A., Wang G., Humphries M., Flotte T.R. (2011) Preclinical evaluation of a recombinant adeno-associated virus vector expressing human alpha-1 antitrypsin made using a recombinant herpes simplex virus production method. Hum. Gene. Ther., 22, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xie Q., Bu W., Bhatia S., Hare J., Somasundaram T., Azzi A., Chapman M.S. (2002) The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc. Natl Acad. Sci USA, 99, 10405–10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wright J.F., Zelenaia O. (2011) Vector characterization methods for quality control testing of recombinant adeno-associated viruses. Methods. Mol. Biol., 737, 247–278. [DOI] [PubMed] [Google Scholar]
- 116.FDA. (2008) Guidance for industry. Potency tests for cellular and gene therapy products. Draft guidance. Center Biologics Eval. Res. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm072571.htm. [Google Scholar]
- 117.Hauck B., Murphy S.L., Smith P.H., Qu G., Liu X., Zelenaia O., Mingozzi F., Sommer J.M., High K.A., Wright J.F. (2009) Undetectable transcription of cap in a clinical AAV vector: implications for preformed capsid in immune responses. Mol. Ther., 17, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chadeuf G., Ciron C., Moullier P., Salvetti A. (2005) Evidence for encapsidation of prokaryotic sequences during recombinant adeno-associated virus production and their in vivo persistence after vector delivery. Mol. Ther., 12, 744–753. [DOI] [PubMed] [Google Scholar]
- 119.Dolgin E. (2012) Gene therapies advance, but some see manufacturing challenges. Nat. Med., 18, 1718–1719. [DOI] [PubMed] [Google Scholar]
- 120.World Health Organization. (1998) Requirements for the use of animal cells as in vitro substrates for the production of biologicals (Requirement for Biological substance No. 50). WHO Expert Committee on Biological Standardization, Annex 1 (WHO Technical Report Series, No. 878), Geneva. [Google Scholar]
- 121.Wright J.F. (2014) Product-related impurities in clinical-grade recombinant AAV vectors: Characterization and risk assessment. Biomedicines, 2, 80–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.European Medicines Agency: Committee for Medicinal Products for Human Use (CHMP). (2012) Assessment report: Glybera (Procedure No. EMEA/H/C/002145), European Medicines Agency, Geneva.
- 123.U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) and Office of Regulatory Affairs (ORA). (2008) Guidance for industry: CGMP for Phase 1 Investigational Drugs. FDA, USA.
- 124.International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use. (2000) ICH harmonised tripartite guideline: Good manufacturing practice guide for active pharmaceutical ingredients. Q7 European Medicines Agency. [DOI] [PMC free article] [PubMed]
- 125.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER). (2001) Guidance for industry: Q7A good manufacturing practice guidance for active pharmaceutical ingredients. FDA, USA.
- 126.FDA. (2015) 21 CFR 11 Electronic records; Electronic Signatures. Code of Federal Regulations Title 21 – Food and Drugs. FDA, USA.