Background
Despite a variety of pharmacologic and device therapies for persons with chronic heart failure (HF), prognosis and quality of life (QOL) remain poor. The need for new effective strategies to improve outcomes for patients with HF is underscored by persistently high mortality, morbidity, health care utilization and costs associated with HF, with over 1.million U.S. HF hospitalizations at an estimated direct and indirect cost in the U.S. of $ 40 billion in 2012. (1)
Exercise intolerance is a primary symptom in chronic HF patients, both those with preserved ejection fraction (HFpEF) and reduced ejection fraction (HFrEF), and is a strong determinant of prognosis and of reduced QOL. (2) Exercise training (ET) improves exercise intolerance and QOL in patients with chronic stable HFrEF, and has become an accepted adjunct therapy for these patients (Class B level of evidence) based on a fairly extensive evidence base of randomized trials, mostly small. (3)
The National Heart, Lung, and Blood Institute (NHLBI)-funded HF-ACTION trial compared an individualized supervised and home-based aerobic exercise program plus guideline-based pharmacologic and device therapy with guideline-based therapy alone in persons with HFrEF. The exercise arm showed a modest reduction in cardiovascular (CV) hospitalizations and mortality and improved QOL. (4,5) However, problems with adherence in the exercise arm likely dampened the potential benefit. This landmark study leaves unanswered a number of key questions, including the role of exercise dose; the relative benefit of different types of aerobic exercise including high intensity interval training, and resistance, training relative to aerobic training; combination of ET with other therapies; optimization of adherence; benefit for older HF patients, those with HFpEF or multiple comorbidities, and those with acute decompensated HF.
The NHLBI convened a working group of experts on June 11, 2012 in Bethesda, Maryland to identify knowledge gaps and to suggest general approaches to filling those gaps for exercise training as a treatment for HF. The NHLBI invited experts in a variety of areas, including basic and clinical exercise physiologists, HF and cardiac rehabilitation specialists, and clinical trial specialists to address these issues. Workshop participants were asked to identify knowledge gaps and to suggest general approaches in basic and clinical investigation to evaluate, optimize, and translate the potential role of exercise training in the treatment of HF.
They were asked to address the following specific questions:
What more needs to be learned about the pathophysiology of exercise intolerance in HFpEF and HFrEF in order to design better exercise treatments?
What do we need to learn regarding the mechanisms of exercise training, and of the training-related improvements (or lack thereof)?
What do we know about the need to tailor exercise regimens to specific HF populations, e.g., persons with multiple comorbidities, frail elderly, and women?
What evolving, innovative new exercise training modalities and combinations should be tested?
Can we begin rehabilitation earlier and in more severe, decompensated patients?
How can we improve long-term exercise adherence and maintenance?
How can we decrease the cost of exercise training interventions, while increasing their generalizability and dissemination (e.g., home therapy, community centers, avoidance of ECG monitoring)?
Is there a more efficient, yet clinically meaningful, outcome than mortality or exercise capacity in trials of HFpEF and HFrEF?
Given the focus of the current manuscript on these questions, the reader is referred to excellent recent reviews of exercise training in HF for additional general information on this topic. (6,7)
Pathophysiology of Exercise Intolerance in Heart Failure: Cardiac Limitations
Exercise intolerance, typically quantified by the reduction in peak oxygen consumed during maximal effort exercise (peak VO2), is a hallmark of HFpEF and HFrEF. (2) According to the Fick principle, VO2 is equal to the product of cardiac output (CO) and arteriovenous oxygen difference (a-vO2 diff).Thus, deficits in reserve capacity, i.e., the change from rest to peak effort, in either component or both may cause reduction in peak VO2 in HF. CO reserve limitation has been repeatedly though not invariably observed in HFpEF and HFrEF, and is related to impairments in both heart rate (HR) and stroke volume (SV) responses. (6–10) An early study identified limited ability to recruit preload (LV end diastolic volume, EDV) as the key mechanism limiting peak VO2 in HFpEF (9), but a more recent study observed that EDV reserve is similar in HFpEF and controls (10). Chronotropic reserve is typically blunted in both HFrEF and HFpEF (2,8–10), and it remains unknown whether EDV reserve would be similar if HR during exercise were higher in HFpEF, as with rate-adaptive pacing. Though EDV reserve is preserved in HFpEF, the increase in LV filling pressures (LVFP) required to achieve adequate EDV is much greater than what is observed in healthy controls. (11) This elevation in LVFP causes secondary elevation in pulmonary artery pressure which may affect right ventricular performance, and acute LVFP elevation during exercise is believed to play the dominant role in promoting symptoms of exertional dyspnea, though the underlying mechanisms remain poorly understood. Limitation in SV reserve in both HFrEF and HFpEF is related to decreased ability to reduce LV end systolic volume. (8–11) There is evidence that the latter finding is related to impairments in both contractile and vasodilatory reserve responses with exercise.
In HFrEF exercise training is generally associated with improved exercise cardiac output and stroke volume, lower heart rate at submaximal workloads, reductions in resting LV volumes and no changes in resting or exercise filling pressure or pulmonary artery pressures. (12,13) Central effects of training in HFpEF have been minimal in the few studies to date. (14,15)
The pathophysiology of HFpEF in many ways represents an exaggeration of “normal” cardiovascular aging. Even healthy aging leads to cardiac stiffening(16,17) that can be prevented by lifelong exercise training.(17) Aging also leads to slowing of relaxation, a seemingly inevitable consequence of senescence that is not modified even by prolonged and intensive training.(18) Patients with HFpEF appear to have hearts that are less distensible than those of sedentary, age-matched controls, with increased wall stress, slower relaxation), and impaired ventriculo-arterial coupling.(19) These changes leads to markedly increased filling pressures during exercise which likely contributes to dyspnea and exercise intolerance.(20,21) This slowed cardiac relaxation may be compounded by abnormalities in skeletal muscle oxygen utilization which augment the CO response to exercise, increasing flow into a small, stiff, slowly relaxing heart. (10,11)
Although short-term exercise training studies in the healthy elderly (22) or patients with HFpEF (23) typically show significant improvements in functional capacity as estimated by VO2max, the mechanism of this improvement is uncertain. Evidence is strongest for improvements in oxygen extraction by skeletal muscle (a-vO2 diff) (14), with little evidence for altered CV structure even in long-term studies. For example, one year of training of sedentary seniors failed to improve ventricular compliance or estimated aortic age although it did increase VO2max and facilitate ventriculo-arterial coupling. (24) Similarly, a full year of training in 12 invasively studied HFpEF patients failed to alter cardiac compliance or improve ventricular-arterial coupling. (15) One potential mechanism for the apparent limited plasticity of cardiac training responses in HFpEF patients may be the presence of advanced glycation end-products, which increase with “normal” aging but are present to a greater degree in patients with HF and diabetes. (25) Recent data in rats suggests that breaking these end-products, combined with exercise training, may reverse the consequences of sedentary aging (26), though this must be confirmed in human studies.
Key Knowledge Gaps:
Are there overarching, systemic processes in HFpEF or HFrEF that underlie the global impairments in cardiac and peripheral reserve that might be targeted therapeutically to improve overall exercise capacity and reduce morbidity/mortality?
Would approaches to “phenotype” the predominant mechanism(s) of exercise intolerance (central versus peripheral) in the individual patient improve understanding of pathophysiology and optimize treatment approaches in HFpEF or HFrEF?
What is the optimal “dose” (frequency, duration, intensity) and modality of exercise training that will be most effective in HFpEF?
Are there pharmacologic strategies that can be combined with exercise training in HFpEF to facilitate an improvement in cardiac and vascular compliance, blood flow delivery, or speed relaxation (cross-link breakers, nitrite donors, SERCA2a up-regulators, pericardial resection)?
Peripheral Mechanisms of Exercise Intolerance in Heart Failure
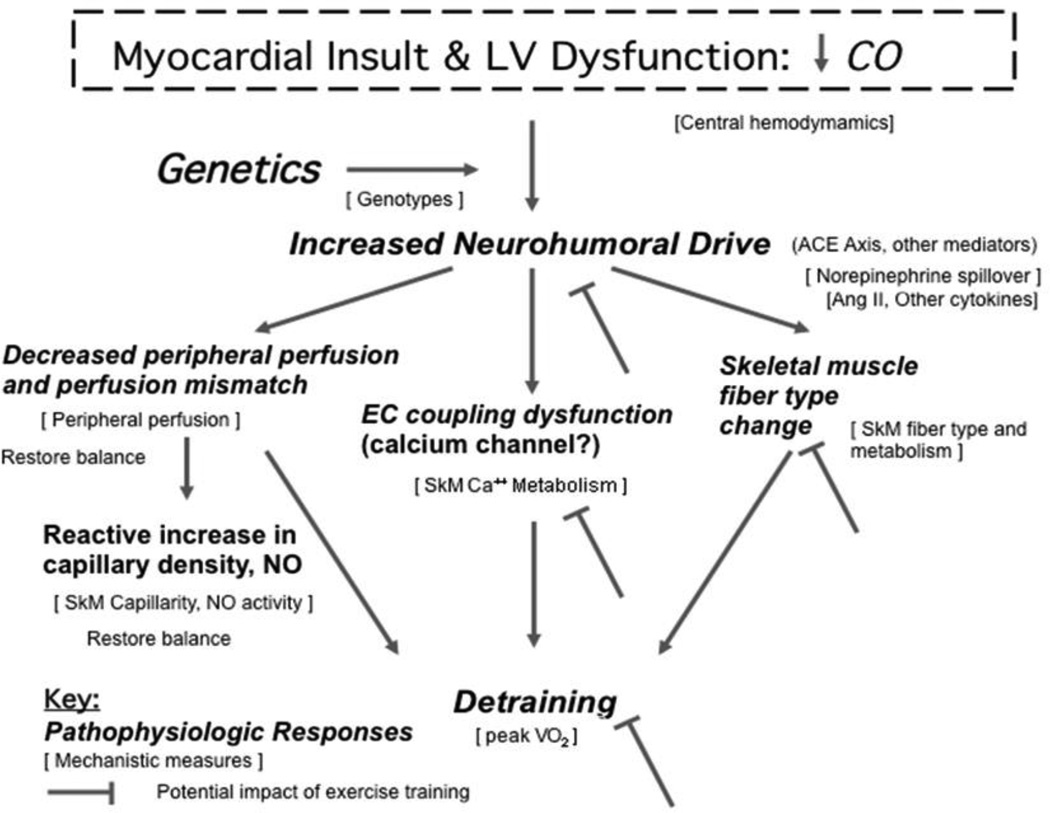
Substantial attention has focused on defining the central versus peripheral mechanisms underlying the reduced functional capacity and symptoms among patients with HF as recently reviewed. (27) To help redirect available blood flow and maintain arterial pressure during exercise in HF patients, locomotory muscles experience enhanced sympathetic vasoconstriction, down regulation of endothelial vasodilatory function, and elevated venous pressures that impair the muscle pumping action to facilitate blood flow. Compelling evidence supports the concept that there may be a peripheral ‘block’ in HF patients that limits the ability to translate changes in central hemodynamics into changes in functional capacity, potentially accounting for the failure of many therapies to improve exercise tolerance: Low LVEF, increased pulmonary wedge pressure, and other hemodynamic indices measured at rest do not predict exercise capacity in HF. (28,29) Furthermore, intrinsic abnormalities are present in skeletal muscles of HF patients compared to aerobically matched sedentary normal controls (30,31), resulting in anaerobic metabolism (measured using 31P–MRI) in leg skeletal muscle of HF patients, both under basal conditions and after occluding skeletal muscle blood flow. (32,33) In addition, acute use of inotropes and vasodilators does not translate into increases in exercise tolerance or reduction of early anaerobic metabolism despite improving leg blood flow and CO. (34,35) Conversely, exercise training improves lactate threshold and aerobic capacity, but without significantly improving CO in both HFrEF (12) and HFpEF . (14) What is less clear is the temporal sequence of central and peripheral changes in HF, which has important implications for informing new therapeutic strategies. Figure 1 presents a model of how left ventricular systolic dysfunction, induced by a myocardial insult with decreased CO, can lead to impaired exercise tolerance and how exercise training may reverse such changes.
Figure 1.
The figure presents a model of how left ventricular systolic dysfunction, induced by a myocardial insult with decreased CO, can lead to impaired exercise tolerance and how exercise training may reverse such changes. Pathophysiologic responses at each step are represented in large type and the corresponding mechanisms are represented in small type in brackets. Potential points at which exercise training has been shown to induce a physiologic response that might block progression to symptomatic exercise intolerance are shown with flat headed arrows. Adapted from reference 27.
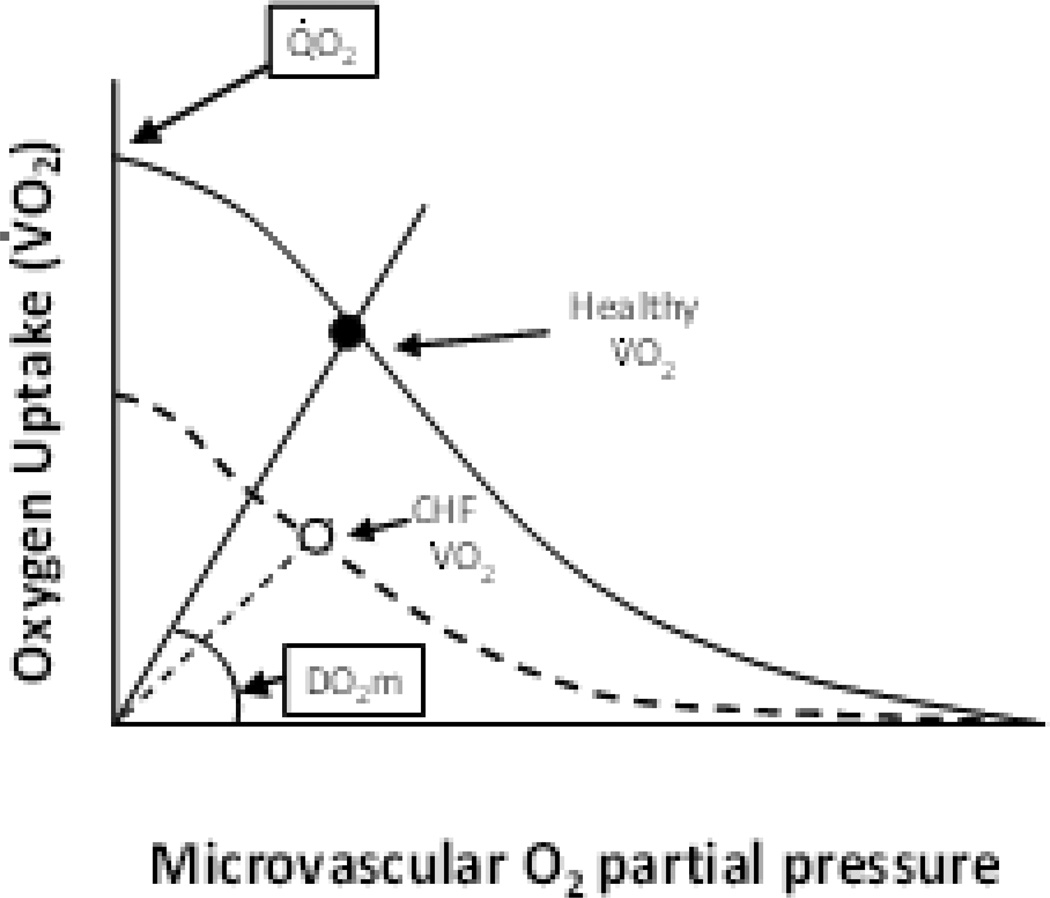
Esposito and colleagues (36) have demonstrated that HF severely reduces muscle oxygen diffusion conductance (DO2m), helping to explain why increasing O2 delivery to skeletal muscle via vasodilators in HF might not yield expected increases in muscle O2 consumption during aerobic exercise (Figure 2). The impaired DO2m may also help account for poor muscle function and exercise intolerance in both HFrEF and HFpEF. (37) Determining the mechanistic bases for this reduced DO2m and developing strategies to correct it are crucial for increasing blood-muscle O2 flux in the face of limited O2 delivery, which may be relatively refractory to exercise training in many HF patients.
Figure 2.
Schematic illustrating how the muscle perfusive (curved lines, Fick principle, VO2 = Qm x (arterial-venous O2 content) and diffusive O2 (straight lines from origin, Fick’s law, VO2 = DO2m x (PmicrovascularO2 – PintracellularO2) conductances conflate to yield VO2 during exercise (e.g., cycling). In chronic HF (dashed lines) VO2 is reduced by both impaired perfusive and diffusive O2 conductances and microvascular O2 partial pressures may either be the same or lower than found in health notwithstanding the presence of marked diffusional derangements (i.e., lower DO2m). Note that correction of DO2m deficits by improving capillary hemodynamics has the potential to increase VO2 even in the absence of improved muscle perfusion. From reference 37.
Peripheral Mechanisms to Improve Exercise Tolerance with Training
In part because of limitations in O2 delivery, HF patients have an extremely slow increase of VO2 following the onset of acute exercise and also prolonged recovery. (38) These slow kinetics create a greater perturbation of intramuscular high-energy phosphates (i.e., Δ [creatine phosphate], [ADPfree]) and pH, which exacerbate glycogenolysis and premature fatigue. (37,38) Moreover, because these patients have a low lactate threshold, even at very modest activity levels they incur the increased energetic costs associated with slow VO2 kinetics , which decreases muscle efficiency and raises the VO2 demands, thereby increasing the O2 deficit. (37) Effectively improving blood-muscle O2 flux via exercise training has the potential to speed VO2 kinetics and reduce the VO2 requirement of exercise, i.e., improved muscle efficiency. In addition, emerging evidence suggests that enhancing nitric oxide bioavailability by beetroot juice or inorganic nitrate supplementation can effectively lower the mitochondrial O2 cost of ATP production, thereby lowering the exercising VO2 requirement. (39) Using these strategies, a therapeutic program that improves skeletal muscle O2 delivery while simultaneously improving mitochondrial and contractile efficiency might substantially improve metabolic function and exercise tolerance in HF patients.
Key Knowledge Gaps:
Do pre-existing skeletal muscle characteristics determine responses to HF or is the converse true - skeletal muscle alterations are a consequence of the disease process?
Does exercise training ameliorate skeletal muscle alterations induced in HF? If so, do such salutary changes in skeletal muscle morphology predict improved clinical outcomes?
How quantitatively do events in the capillary decrease DO2m in HF, are they similar in HFrEF and HFPpEF, and what are the most effective exercise training (duration, intensity, frequency: whole-body, small muscle mass) and/or alternative (↑nitric oxide , ↓cytokines) strategies to reverse this pathophysiology?
Do exercise therapy-induced improvements in capillary hemodynamics (if they occur) effectively speed O2 uptake kinetics and lower the O2 cost of exercise?
Impact of Aging, Frailty, and Comorbidities
Aging per se is associated with a progressive decline in exercise capacity and decreased physiological reserve in cardiovascular function as well as in most other organ systems, altered pharmacological responses, increased adverse effects of medical therapy, and prolonged and often incomplete recovery. The prevalence and incidence of HF increase sharply after middle-age. (1) In the subset over age 80 years, up to 20% have prevalent HF, and the incidence of HF is rising fastest in this group. Approximately 88% of HF deaths and over 75% of HF hospitalizations occur in person’s age ≥ 65 years. (40) Despite these demographics, older persons are significantly under-represented in HF studies, especially those involving exercise training. (41,42) In an analysis of 59 general HF trials conducted from 1985–1999 in > 45,000 patients, the average age of participants was 61.4 years, whereas it is >77 years in the community. (42) In the HF-ACTION trial, the largest trial of exercise training in HF, the mean age of participants was 59.5 years. (4)
Outcomes of HF in the elderly have not changed substantially in the past 2 decades despite advances in HF therapies. (43) This may be due to the combined impact of multiple comorbidities and frailty. The majority of older patients have multiple comorbidities, and a high proportion are frail. The adverse impacts of aging, frailty, and comorbidities on functional capacity and clinical outcomes are cumulative and synergistic. (43) This synergy may be mediated in large part by the reduction in physical activity that accompanies each condition.
Perhaps the most prominent difference between in older versus younger HF patients is the greater prevalence and severity of comorbidities in the former group. Common comorbidities in the elderly that further reduce exercise capacity and complicate therapy include diabetes, cerebrovascular and peripheral artery disease, musculoskeletal disorders, and renal, pulmonary, and cognitive dysfunction. It is noteworthy that patients with major or multiple comorbidities have often been actively excluded from clinical HF studies, thereby producing results that may not be applicable to typical older HF patients, who typically have 5 or more comorbidities, many of which are non-cardiac. (44) Mounting evidence indicates that non-cardiac comorbidities strongly contribute to adverse outcomes in HF patients. Over 50% of subsequent events in recently hospitalized HF patients are related to non-cardiac comorbidities. (45)
Frailty is highly prevalent in older HF patients. (43) Although there is incomplete consensus on its specific definition, frailty is marked by excess vulnerability to stressors, with reduced ability to maintain or regain homeostasis after a destabilizing event. It is manifested by slowness, weakness, perception of exhaustion, lower activity levels, and involuntary weight loss. (46) Frailty contributes to worse clinical outcomes, which may be ameliorated by disease management programs. The effects of aging, multiple comorbidities, and frailty on the use of exercise training in older HF patients are profound. The marked impairment of aerobic capacity, ambulatory function, strength, and balance often seen in this population presents major challenges to effectively and safely implement exercise training.
Key Knowledge Gaps:
What are the mechanisms whereby aging, non-cardiac comorbidities, and frailty impact physical function outcomes in HF.
How can we develop and test novel exercise and physical function interventions that directly address the adverse impact of multiple co-morbidities and frailty in older patients with HF.
Gender Differences in Heart Failure and Their Implications for Therapy
Several important gender differences in the clinical profile of HF patients have been consistently observed. First, women with HF are generally about a decade older than men, and are therefore more likely to have multiple comorbidities and greater frailty. (47) A non-coronary heart disease etiology of HF is more common in women than men, which may explain in part their higher ejection fraction and thus a greater proportion with preserved LVEF. (48) Conversely, diastolic dysfunction is a more commonly observed etiology for HF in women. Regardless of etiology or the contribution of diastolic versus systolic dysfunction, women with HF generally have a lower functional capacity than men with comparable levels of clinical HF severity. (49,50) The lower peak VO2 by NYHA Class for women versus men reported by others was also observed in the HF-ACTION trial, the largest database of cardiopulmonary testing in women with HF although the gender gap narrowed as NYHA Class worsened. (50) These values of peak VO2 must be analyzed in the context of typical values for sedentary women of similar age. From a nomogram developed by Gulati et al (51) for women without known heart disease, a typical 80-year old woman has a predicted aerobic capacity of 4.3 METS (15.1 ml/kg/min), which approximates that in younger, predominately male HF populations such as in HF-ACTION. (4,5) The contribution of deconditioning and adaptation of skeletal muscle to the HF milieu may vary by gender, with men but not women developing abnormalities not attributable to deconditioning alone. (30) Whether the mechanisms of exercise intolerance in women with HF differ from those in men is unresolved.
Although peak VO2 is strongly predictive of survival in both sexes, women show better survival for any given value. (49) However, women with an ischemic versus non-ischemic etiology of HF appear to have a worse outcome for a given peak VO2 that is especially prominent at lower values. (49) Thus, HF etiology may contribute strongly to prognosis in both sexes. The HF-ACTION trial demonstrated a greater benefit of training in women than men for the combined endpoints of all-cause mortality or all cause hospitalization, primarily due to lower hospitalization rates, with no reduction in mortality. (52) The mechanism for this differential benefit is unclear, but a significant interaction with etiology was not observed, and both sexes had similar adherence rates and achieved similar modest improvements in peak VO2 with training. (52)
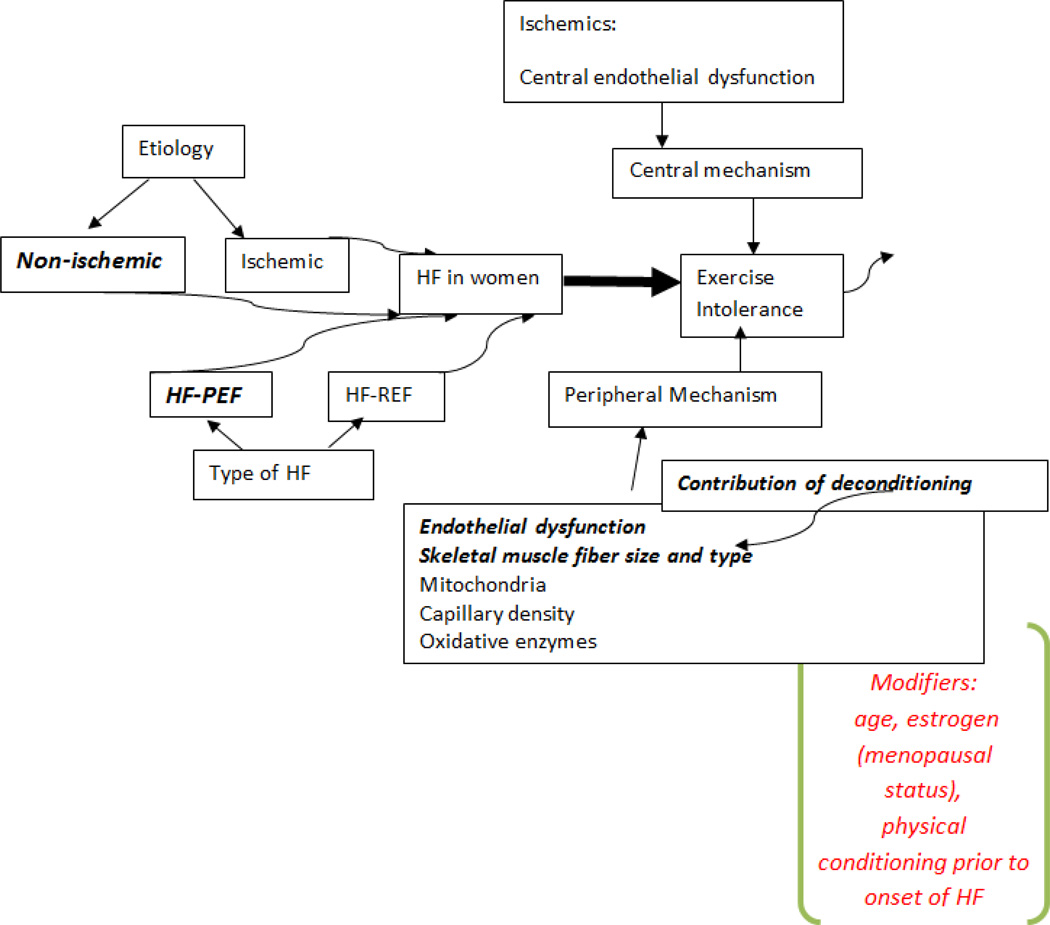
The contribution of hormonal status to exercise intolerance has not been systematically examined in women with HF. Estrogen levels decline with age and may be associated with endothelial dysfunction. Thus, the hormonal status of women with HF may contribute to their exercise intolerance. Although the overall concerns about estrogen replacement in older women have probably contributed to the paucity of work in this area, further studies of hormonal intervention in older women and men with HF are clearly needed. For example, testosterone supplementation has been shown in small studies to significantly improve exercise capacity and QOL in older women as well older men with HF. (53) Figure 3 is a model of the contributors to exercise intolerance in women versus men.
Figure 3.
Possible mechanisms of exercise intolerance in HF as related to gender. Areas that have been associated with women are in bold. The modifiers of the responses are in red in italics. Thus, age may have a more profound effect on the exercise intolerance of women with HF as well as the level of fitness prior to disease onset. Estrogen as a modifier has been poorly studied in HF.
Key Knowledge Gaps:
How do the mechanisms of exercise intolerance in HF differ in women from men, and what role do sex hormone deficiencies play in these differences?
What, if any, fundamental differences in response to exercise training are seen in women with HF compared to men?
Beginning Rehabilitation Earlier and in More Severely Decompensated Patients
Physical impairments associated with chronic HF often worsen markedly with decompensation and can be further compounded by prolonged immobility associated with the hospital environment, creating profound impairments in physical function. (54) For instance, six minute walk distance in patients hospitalized with HF is approximately half of that seen in chronic stable HF patients. (2,4,55,56) However, physical impairments are not limited to endurance. Most patients hospitalized with acute HF syndrome (AHFS) are frail elders with multiple comorbidities, including deficits in mobility, strength and balance. (43,54) Resulting functional impairments may persist or even progress after hospital discharge (57) and are associated with an increased risk of adverse clinical outcomes, including re-hospitalization and death. (55–57) These findings suggest the potential for extending physical function interventions to patients hospitalized with AHFS.
Studies of exercise training in HF have focused almost exclusively on chronic, stable HF patients. (58) HF-ACTION, the largest study of exercise training in a HF population, specifically excluded patients with any clinical instability, including hospitalization, within 6 weeks of enrollment. (4) The current literature regarding the safety and efficacy of exercise interventions that specifically target patients with AHFS is limited to observational data (59) and a small randomized trial (60), both of which showed benefit. A state-of-the-art review (7) and the recent Center for Medicare and Medicaid Services (CMS) coverage memo for cardiac rehabilitation (CR) in patients with chronic stable HF specifically exclude patients with AHFS or recent (4–6 weeks) hospitalization, and call for a period of stability prior to enrollment in CR. Furthermore, traditional aerobic exercise training alone, the primary focus of prior HF exercise research, does not address the multi-domain deficits present in this frail population, including muscle wasting, and impaired balance and flexibility. Initiating traditional exercise training without addressing these deficits and other special needs could cause injuries and worsen outcomes. Indeed, there are reports of increased adverse events, including injuries and falls, associated with standard rehabilitation interventions in frail older populations (61,62).
Given the severity and multi-domain nature of their physical impairment, multiple comorbidities, and limited applicability of prior exercise training trials in chronic HF, additional research is needed to guide the development and implementation of rehabilitation programs specifically designed for older patients hospitalized with AHFS. Longitudinal studies are needed to fully define the physical function impairments in these patients and their trajectory of functional recovery novel rehabilitation interventions that address the specific needs of frail, older patients hospitalized with AHFS should be carefully developed and formally tested in clinical trials.
Key Knowledge Gaps:
What are the contributions of frailty, multiple comorbidities, cognitive deficits, and other factors to the functional impairments after hospitalization for AHFS?
Can exercise interventions during or immediately after hospitalization in frail older patients with AHFS, be implemented safely and improve key outcomes such as physical function, QOL, and readmissions? If so, what is the optimal design for such interventions?
Traditional and Innovative Exercise Training Modalities in Heart Failure
Exercise intervention trials for clinically stable patients with HFpEF and HFrEF have primarily focused on continuous moderate-intensity aerobic training. (4,58,63) Despite favorable anti-remodeling and QOL benefits, moderate-intensity training is associated with only modest improvements in peak VO2. (63,64), which averaged 3.0 ml/kg/min in meta-analyses of both HFrEF (63) and HFpEF (64) patients. Accordingly, there has been recent interest in the role of high intensity interval training (HIIT) on improving peak VO2 in individuals with clinically stable HF. Wisloff’s laboratory (65) reported that HIIT, characterized by acute bouts of brief (4 minutes per bout) repeated vigorous near-maximal exercise (≥85% peak VO2) alternated with lower-intensity recovery exercise, is superior to continuous moderate-intensity aerobic training for improving peak VO2, LVEF, and brachial artery flow-mediated dilation in clinically stable older HFrEF patients. Fu et al. (66) extended these findings by demonstrating that 3 months of HIIT significantly increased peak VO2 secondary to enhanced peak exercise SV and CO in older HFrEF patients, with no significant change in A-VO2 diff. In contrast, Dimopoulos et al(67) and Iellamo et al (68) found that the increase in peak VO2 was similar after 3 months of continuous moderate-intensity versus HIIT exercise in HFrEF patients. A meta-analysis of 7 small trials in patients with HFrEF showed that HIIT was more effective than traditional continuous moderate-intensity exercise in augmenting peak VO2 (difference of 2.1 ml/kg/min) whereas increases in LVEF did not differ significantly.(69) To date, the safety and efficacy of HIIT in HFpEF patients has not been studied.
Traditionally, exercise training guidelines for HF patients recommend large muscle mass (walking, cycling) aerobic exercise. However, in the setting of reduced convective O2 delivery as occurs in HF (Figure 2), whole body exercise may not be the most effective mode of training to increase peak VO2. Esposito et al.(36) demonstrated that 2 months of one leg knee extensor exercise resulted in a significant increase in leg and total body VO2 during cycle or knee extensor exercise, secondary to increased convective and diffusive O2 transport. Vastus lateralis fiber cross-sectional area, percent type I fibers, capillary to fiber ratio, number of capillaries surrounding a muscle fiber and mitochondrial volume density were also significantly higher after training. Accordingly, localized muscle training may be an important type of training to improve convective and diffusive O2 transport in HF and could be particularly useful in severely disabled patients with minimal reserve capacity.
Key Knowledge Gaps:
1. What is the optimal training intensity (high-intensity aerobic interval versus moderate-intensity continuous exercise), mode (whole body versus small muscle mass training +/− resistance training) and duration of training (short-term: 2–3 months versus one year or longer) to improve cardiovascular and skeletal muscle function, health status, physical functional performance and survival in HFrEF and HFpEF patients?
Exercise Training Combined with Other Treatment Strategies for Heart Failure Patients
Because chronic HF patients receive multiple cardiac medications and often device therapy or surgical interventions, it is important to assess the utility of exercise training in combination with such background therapy. For example, a small crossover trial demonstrated additive effects of exercise training and lisinopril on exercise capacity in patients with moderate to severe systolic HF. (70) Prior studies have shown that HF patients receiving guideline-based beta blocker therapy exhibit training-induced increases in peak VO2 similar to those not receiving beta blockers. (3,71)
The ability of cardiac resynchronization therapy to improve peak VO2 has been shown in randomized controlled trials (RCTs). At least two studies have demonstrated additive effects of cardiac resynchronization therapy and exercise training on peak VO2 as well as hemodynamic indices and QOL. (72,73) More recently, an 8 week program of aerobic and strength training improved peak VO2 by an average of 3.0 ml/kg/min in patients with a left ventricular assist device as a bridge to transplantation. (74) These encouraging findings require confirmation in larger trials, including the growing number of HF patients receiving these devices as destination therapy. Finally, exercise training has been increasingly employed in HF patients after cardiac transplantation, in who muscle wasting and exercise intolerance are common. A meta-analysis of 6 studies reported a significant 2.3 ml/kg/min mean increase of peak VO2 and significant improvements in chest and leg press strength after exercise training in transplant recipients. (75)
Most studies that focus on exercise training in HF patients provide limited information regarding other interventions that patients may have received during the training. Since many of these studies take place in CR or other healthcare settings, it is likely that patients experienced opportunities to obtain further lifestyle education or to engage healthcare providers regarding a change in their symptoms. This additional access to healthcare providers in many ways mirrors disease-management interventions. (76) In prior studies of older HF patients, a CR program that included exercise training and additional patient education improved NYHA status, QOL, six minute walk distance and reduced all-cause and CV hospitalizations and days in the hospital. (77,78) The benefit of participating in a multifaceted training program on exercise capacity, QOL, and HF hospitalizations has been shown to extend up to 10 years. (79) However, addition of an exercise training program to a nurse-directed HF clinic and home visits that were received by both the exercise training and the usual care cohorts did not improve QOL or reduce clinical events, including mortality or hospitalization. (80)
Knowledge Gaps:
What are the optimal strategies to complement, extend, and magnify the beneficial effects of exercise training in HF patients?
Are there structured approaches to uniform reporting that could facilitate translation of interventions into patient care?
Adherence Issues in Exercise Training
There are substantive potential health benefits of exercise for individuals with HF. However, the major clinical obstacle confronting the use of exercise training as a therapeutic option in the HF population is how to get individuals to initiate and maintain an exercise training program. This is illustrated by the experience of the HF-ACTION trial. Despite a well-organized and resourced effort to optimize adherence, only ~40% of patients in the intervention arm achieved the target of 90 minutes of exercise/week at 3 months.(4,5) Perhaps as a result, the mean increase in peak VO2 was only 0.6 ml/kg/min., potentially limiting the ability to fully evaluate the benefits of training on clinical outcomes. This experience is similar to that of some other exercise training trials in HF though better adherence was found in some smaller, single center trials, as summarized in the Table. (13,81–84) Adherence issues also played a major role in the conduct and evaluation of other major lifestyle intervention trials, including the Multiple Risk Factor Intervention Trial (85) and the Diabetes Prevention Program. (86) Conventional wisdom suggests that adherence issues are primarily related to neurobehavioral and social issues, and barriers can be identified and addressed using behavioral approaches. Although most attention regarding adherence to lifestyle interventions has traditionally focused around psychosocial/behavioral factors, it is conceivable that biological factors may also help determine whether individuals maintain an exercise program once initiated. There are studies of biological predictors of physical activity behavior, including genetic markers. (87) If we are to maximize the salutary effects of regular exercise in individuals with heart failure, more information about the predictors of adherence to regular physical activity must be acquired, so as to identify individuals for whom more effective strategies to increase adherence can be applied. In addition, novel interventions to improve adherence are critically needed.
Key Knowledge Gaps:
What are the predictors of adherence to exercise in chronic HF patients, beyond what can currently be identified?
What interventions optimize adherence to exercise in chronic HF patients?
How Can We Reduce Costs and Increase Use of Exercise Training in Heart Failure Patients?
Despite the demonstrated benefits of exercise training in HF patients, widespread implementation of formal CR and home-based training in this population presents special challenges. HF patients are often older; more deconditioned, and have more comorbidities than the typical coronary patient. Clinicians may be concerned about asking HF patients to increase their activity, due to fears of worsening ventricular function and symptoms. Many physicians lack awareness of the physiologic benefits of exercise training, its safety and its potential to improve health status and QOL in patients with HF. Lack of financial coverage has been another major deterrent to CR referral. In addition, primary care providers are typically unfamiliar with exercise prescriptions or how to derive them. Since the vast majority of HF patients are in primary care practices, educating these physicians about the benefits and basics of exercise training in HF is a high priority.
In February 2014, the CMS approved coverage for CR for selected patients with chronic HF. The criteria match the HF-ACTION inclusion criteria, with stable medications for at least 6 weeks and LVEF ≤35%. This generally excludes patients with recent hospitalization as well as those with HFpEF. The dearth of supportive data in this latter group should be priorities for future studies. This important extension of CR coverage should facilitate CR utilization in chronic HFrEF patients. Although private insurers and Medicaid traditionally follow CMS policy, CR may remain inaccessible for uninsured patients. Since cardiac monitoring was not required in the chronic HF patients enrolled in HF-ACTION, costs could potentially be lower than for conventional CR. Future studies should address the feasibility and safety of offering community-based CR programs, potentially at YMCA’s, community centers, and churches.
Equipment for CR in HF patients can be simple and relatively inexpensive. Walking programs require only appropriate footwear and a safe place to walk. Although home-based training programs are financially attractive, they introduce additional non-adherence issues. The greatest challenge facing the widespread use of CR is clinician education to overcome the current gap in evidence-based care, as illustrated by the low referral rates of coronary patients to traditional CR programs despite over 2 decades of favorable published outcome data. (88) Convincing clinicians about the benefits of CR in HF patients will likely be more difficult due to concerns of worsening LV function with exercise. Since patients respect the advice of their physicians, the latter should emphasize the importance of physical activity and CR for their HF patients.
Key Knowledge Gaps
How can we optimize physician adherence to CR and exercise therapy guidelines for HF patients?
Can we develop cost-effective models of CR in HF patients, including CR initiated early after hospitalization for AHFS?
Is There a More Efficient, Yet Clinically Meaningful Outcome Than Mortality or Exercise Capacity in Heart Failure Trials?
The RCT is the “gold standard” for evaluating the efficacy and safety of a therapeutic intervention. In designing and conducting a RCT, the balance between resources available and obtaining a reliable answer to the primary hypothesis generally requires making many compromises. Undoubtedly, the most critical decisions concerning design of a RCT are the selection of the patient population and the determination of the primary endpoint. Both the estimated effectiveness of the intervention and the numbers of endpoint events are key factors in the sample size calculations.
In early RCTs of patients with HFrEF, all-cause mortality was frequently the primary outcome. The beneficial results on this, the most definitive of clinical endpoints, in several well done RCTs evaluating ACE inhibitors, beta blockers and aldosterone receptor antagonists were impressive, convincing, and practice changing. In these RCTs the benefits of the tested therapies on deaths attributed to CV causes had to be so pronounced that robust statistical significance could be demonstrated despite the presumption that the therapy would not have a positive impact on rates of non-CV deaths such as cancer, trauma, infectious and other etiologies.
As mortality rates for CV disease, including HF, have declined over time, all-cause mortality, the undisputed heavyweight champion of RCT endpoints, is not a viable option for most RCTs of typical HF populations. In addition, the lower absolute mortality and the higher proportion of non-CV modes of death in patients with HFpEF versus HFrEF render all-cause mortality an impractical and nonspecific primary outcome in RCTs of HFpEF patients. (89)
Composite outcomes combining nonfatal and fatal outcomes have been frequently adopted. In a cohort selected for symptomatic HF, combining CV death with nonfatal hospitalization for HF is a frequent and reasonable primary target for therapeutic interventions. (90) Using a cause-specific clinical outcome as the primary objective of RCTs would be anticipated to be more sensitive to the effects of a targeted intervention, resulting in a lower sample size. (91) Of course, data on all-cause mortality and other serious nonfatal events must still be collected and presented as supporting efficacy and safety information. The interpretation of composite outcome results can be straightforward when there is congruence of the effect of the intervention on all components of the composite outcome, but is more complicated when there is discordance between the effects of the therapy on fatal and nonfatal events. (92)
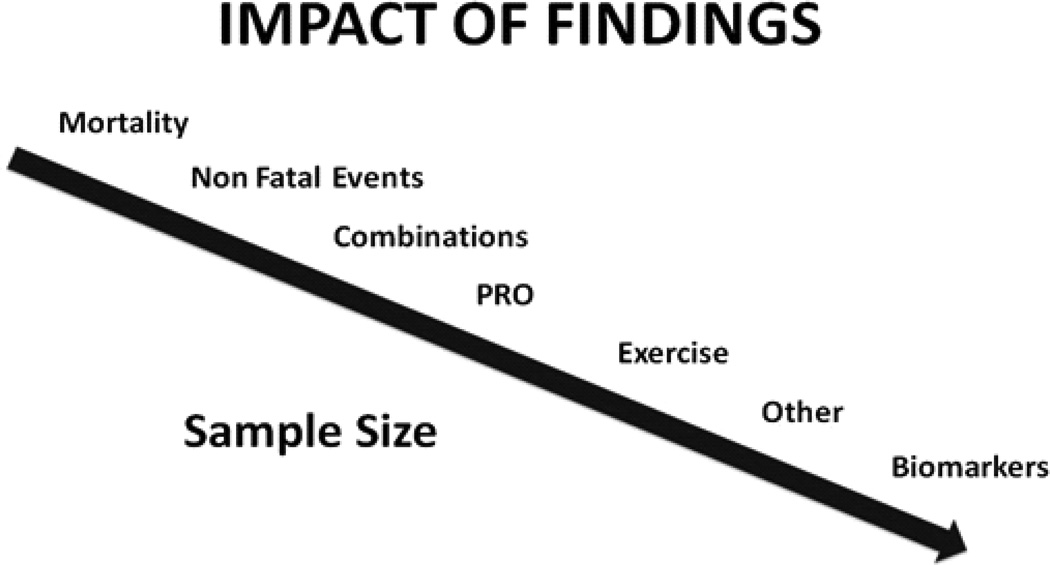
Other important goals of therapy such as improving symptomatology, QOL as perceived by the patient using validated instruments, and exercise capacity are all acknowledged as clinically meaningful. The sample size required for a RCT powered for these outcomes, ascertained at multiple times in all enrolled patients, is generally much lower than in RCTs with a primary endpoint of major morbidity and mortality. Similarly, pilot interventions probing whether biomarkers associated with adverse outcomes, such as brain natriuretic peptides, are altered by therapy can also be conducted with a smaller patient sample. However, it must be acknowledged that these smaller trials using surrogate endpoints cannot provide a reliable estimate of either the effect on clinical outcomes or the safety of the therapeutic intervention being evaluated. Figure 4 represents a theoretical hierarchy of possible RCT outcomes and their relationship to the sample size required.
Figure 4.
The figure presents a theoretical hierarchy of possible RCT outcomes and their relationship to the sample size required.
Key Knowledge Gaps:
What outcomes, including novel patient-centered composite outcomes, best capture the relative effectiveness of exercise interventions in HF patients, and when are surrogate outcomes appropriate?
Working Group Recommendations
The Working Group participants voted the following recommendations as the highest priority in advancing exercise training as a therapy for HF patients:
Better elucidate the basic mechanisms of impaired cardiac, vascular, and peripheral muscle function and the impact of exercise training on them. Examples might include determining the mechanistic basis for the decreased muscle oxygen diffusing capacity in HF and the effect of exercise training in reversing it.
Better phenotype the predominant mechanism of exercise intolerance in individual patients to optimize exercise training approaches. Clarify differences between patients with HFrEF vs. HFpEF; effects of obesity, sarcopenia, chronotropic incompetence, impaired peripheral vascular responses, etc.
Determine the best measures to assess and quantify exercise intolerance in HF patients and their responses to exercise training. Potential candidates include peak VO2, ventilatory threshold, other ventilatory variables, critical power, treadmill time/estimated METS, 6-minute walk distance.
Develop interventions to improve adherence to exercise training programs/regimens. Examples include better defining causes of non-adherence and developing educational and motivational tools, user-engaging and personalized training programs.
Optimize exercise training regimens through better tailoring to different types of patients. Variables to consider include exercise mode, program duration, frequency, and intensity, and use of novel training techniques (high intensity intervals, prolonged sessions, optimal mix of aerobic, resistance, balance, flexibility). Patient variables to consider include age, gender, comorbidities, frailty, and socioeconomic factors. Other variables include the potential safety and efficacy of beginning training early after acute decompensated heart failure or even during hospitalization.
Test combinations of exercise training with other lifestyle interventions, drugs, and devices. Examples might include formal CR, caloric and sodium restriction, new drugs, cardiac resynchronization therapy, and conventional cardiac pacing.
Table.
Adherence of Heart Failure Patients to Exercise Training in Some Prior Trials
| Trial | Adherence Methodology | Adherence Findings |
|---|---|---|
| HF-ACTION (4,5) | Follow-up phone calls and Physical Activity Questionnaire | 40% performing exercise training as prescribed at month 3 |
| Coats AJS et al.(13) | Percentage of expected bicycle wheel revolutions | Mean adherence 77.3% (range 26–116%) |
| Evangelista et al.(81) | Pedometers (10% improvement in scores) | 20/38 (53%) patients found to be adherent |
| Corvera-Tindel et al.(82) | Pedometers (actual walking time/prescribed time) | Mean adherence 74.3±37% (N=42 in exercise training arm) |
| McKelvie RS et al.(83) | Pre-randomization screening | 43% attended >80% of sessions, 16% attended <50% of sessions. Patients exercised 2.3±0.4 sessions/wk. during the firstt month and 1.7±0.4 sessions/wk. by Month 12. |
| Oka et al.(84) | Activity logs and telephone contact | Average adherence 110% for aerobic, 87% for upper body, and 75% for lower body |
Acknowledgments
The authors appreciate the assistance of Nina Hall in preparing this document.
Footnotes
Disclaimer
The views expressed in this document are the authors’ and do not necessarily represent those of the National Institutes of Health or the Department of Health and Human Services.
Disclosures
Drs. Fleg, Cooper, Haykowsky, Kraus, Levine, Piña, Poole, Reeves, Whallen, and Kitzman have no conflicts to declare. Dr. Borlaug reports research grant support from Atcor Medical and consultancy for Amgen, Merck, Glaxo- SmithKline, and Cardiokinetix. Dr. Pfeffer reports research grant support from Amgen, Celladon, Hamilton Health Sciences, Novartis, and Sanofi Aventis and consultancy for Aastrom, Abbott Vascular, Amgen, Bristol-Myers Squibb, Cerenis, Concert, Keryx, Medtronic, Merck, Novartis, Roche, Servier, Teva, and University of Oxford.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 3.Executive Summary: Heart Failure Society of America 2010 Comprehensive Heart Failure Practice Guidelines. J Cardiac Fail. 2010;16:475–539. [Google Scholar]
- 4.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL, Investigators H-A. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O’Connor CM, Weinfurt KP. HF-ACTION Investigators. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Downing J, Balady GD. The role of exercise training in heart failure. J Am Coll Cardiol. 2011;58:561–569. doi: 10.1016/j.jacc.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Ades PA, Keteyian SJ, Balady GJ, Houston-Miller N, Kitzman DW, Mancini DM, Rich MW. Cardiac rehabilitation and self-care for chronic heart failure. J Am Coll Cardiol HF. 2013;1:540–547. doi: 10.1016/j.jchf.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80:769–781. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- 9.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: Failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 10.Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BD, Redfield MM. Global Cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2012;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction: Hemodynamic and metabolic effects. Circulation. 1988;78:506–515. doi: 10.1161/01.cir.78.3.506. [DOI] [PubMed] [Google Scholar]
- 13.Coats AJ, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, Solda PL, Davey P, Ormerod O, Forfar C. Controlled trial of physical training in chronic heart failure Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992;85:2119–2131. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- 14.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimoto N, Prasad A, Hastings J, Bhella PS, Shibata S, Palmer D, Levine B. Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. A pilot study. Amer Heart J. 2012;164:869–877. doi: 10.1016/j.ahj.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimoto N, Hastings JL, Bhella PS, Shibata S, Gandhi NK, Carrick-Ranson G, Palmer D, Levine BD. Effect of ageing on left ventricular compliance and distensibility in healthy sedentary humans. J Physiol. 2012;590:1871–1880. doi: 10.1113/jphysiol.2011.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 18.Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol. 2007;99:1629–1636. doi: 10.1016/j.amjcard.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad A, Hastings J, Shibata S, Popovic ZB, Arbab-Zadeh A, Bhella PS, Okazaki K, Fu Q, Berk M, Palmer D, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. Characterization of static and dynamic left ventricular diastolic function in patients with heart failure and a preserved ejection fraction. Circulation: Heart Fail. 2010;3:617–626. doi: 10.1161/CIRCHEARTFAILURE.109.867044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circulation:Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibata S, Hastings JL, Prasad A, Fu Q, Bhella PS, Pacini E, Krainski F, Palmer MD, Zhang R, Levine BD. Congestive heart failure with preserved ejection fraction is associated with severely impaired dynamic Starling mechanism. J Appl Physiol. 2011;110:964–971. doi: 10.1152/japplphysiol.00826.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagberg JM, Graves JF, Limacher M, Woods DR, Leggett SH, Cononie C, Gruber JJ, Pollock ML. Cardiovascular response of 70–79-year old men to exercise training. J Appl Physiol. 1989;66:2589–2594. doi: 10.1152/jappl.1989.66.6.2589. [DOI] [PubMed] [Google Scholar]
- 23.Kitzman DW, Brubaker, Morgan TM, Stewart KP, Little WC. Exercise treaining in older patients with heart failure and preserved ejection fraction. Circulation Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2011;122:1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbely A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 26.Steppan J, Tran H, Benjo AM, Pellakuru L, Barodka V, Ryoo S, Nyhan SM, Lussman C, Gupta G, White AR, Daher JP, Shoukas AA, Levine BD, Berkowitz DE. Alagebrium in combination with exercise ameliorates age-associated ventricular and vascular stiffness. Exp Gerontol. 2012;47:565–572. doi: 10.1016/j.exger.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraus WE. In: Exercise in Heart Failure: Roles of Testing and Training, in Heart Failure: A Companion to Braunwald’s Heart Disease. Mann DL, editor. Philadelphia, PA: Elsevier; 2010. pp. 834–844. [Google Scholar]
- 28.Higginbotham MB, Morris KG, Conn EH, Coleman RE, Cobb FR. Determinants of variable exercise performance among patients with severe left ventricular dysfunction. Am J Cardiol. 1983;51:52–60. doi: 10.1016/s0002-9149(83)80010-1. [DOI] [PubMed] [Google Scholar]
- 29.Franciosa JA, Park M, Levine TB. Lack of correlation between exercise capacity and indexes of resting left ventricular performance in heart failure. Am J Cardio. 1981;47:33–39. doi: 10.1016/0002-9149(81)90286-1. [DOI] [PubMed] [Google Scholar]
- 30.Duscha BD, Annex BH, Green HJ, Pippen AM, Kraus WE. Deconditioning fails to explain peripheral skeletal muscle alterations in men with chronic heart failure. J Am Coll Cardiol. 2002;39:170–174. doi: 10.1016/s0735-1097(02)01740-0. [DOI] [PubMed] [Google Scholar]
- 31.Vescovo G, Serafini F, Facchin L, Tenderini P, Carraro U, Dalla Libera L, Catani C, Ambrosio GB. Specific changes in skeletal muscle myosin heavy chain composition in cardiac failure: differences compared with disuse atrophy as assessed on microbiopsies by high resolution electrophoresis. Heart. 1996;76:337–343. doi: 10.1136/hrt.76.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiener DH, Fink LI, Maris J, Jones RA, Chance B, Wilson JR. Abnormal skeletal muscle bioenergetics during exercise in patients with heart failure: role of reduced muscle blood flow. Circulation. 1986;73:1127–1136. doi: 10.1161/01.cir.73.6.1127. [DOI] [PubMed] [Google Scholar]
- 33.Massie BM, Conway M, Rajagopalan B, Yonge R, Frostick S, Ledingham J, Sleight P, Radda G. Skeletal muscle metabolism during exercise under ischemic conditions in congestive heart failure. Evidence for abnormalities unrelated to blood flow. Circulation. 1988;78:320–326. doi: 10.1161/01.cir.78.2.320. [DOI] [PubMed] [Google Scholar]
- 34.Maskin CS, Forman R, Sonnenblick EH, Frishman WH, LeJemtel TH. Failure of dobutamine to increase exercise capacity despite hemodynamic improvement in severe chronic heart failure. Am J Cardiol. 1983;51:177–182. doi: 10.1016/s0002-9149(83)80032-0. [DOI] [PubMed] [Google Scholar]
- 35.Wilson JR, Martin JL, Ferraro N, Weber KT. Effect of hydralazine on perfusion and metabolism in the leg during upright bicycle exercise in patients with heart failure. Circulation. 1983;68:425–432. doi: 10.1161/01.cir.68.2.425. [DOI] [PubMed] [Google Scholar]
- 36.Esposito F, Reese V, Shabetai R, Wagner PD, Richardson RS. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol. 2011;58:1353–1362. doi: 10.1016/j.jacc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poole DC, Hirai DM, Copp SW, Musch TM. Muscle oxygen transort and utilization in heart failure.: implications for exercise (in)tolerance. Am J PhysiolHeart Circ Pohysiol. 2012:302. doi: 10.1152/ajpheart.00943.2011. H1050-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossiter HB. In: Comprehensive Physiology:Respiration. Wiley Blackwell; 2011. Exercise kinetic considerations for gas exchange; pp. 203–244. [DOI] [PubMed] [Google Scholar]
- 39.Vanhatalo A, Fulford J, Bailey SJ, Blackwell JR, Winyard PG, Jones AM. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J Physiol. 2011;589:5517–5528. doi: 10.1113/jphysiol.2011.216341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 41.Kitzman DW, Rich MW. Age disparities in heart failure research. JAMA. 2010;304:1950–1951. doi: 10.1001/jama.2010.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heist A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Int Med. 2002;162:1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 43.Murad K, Kitzman D. Frailty and multiple comorbidities in the elderly patient with heart failure: implications for management. Heart Failure Reviews. 2012;17:581–588. doi: 10.1007/s10741-011-9258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 45.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Kan GA, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas Botbot GAP. The I.A.N.A. Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 47.Hsich EM, Pina IL. Heart failure in women: a need for prospective data. J Am Coll Cardiol. 2009;54:491–498. doi: 10.1016/j.jacc.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 48.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 49.Hsich E, Chadalavada S, Krishnaswamy G, Starling RC, Pothier CE, Blackstone EH, Lauer MS. Long-term prognostic value of peak oxygen consumption in women versus men with heart failure and severely impaired left ventricular systolic function. Am J Cardiol. 2007;100:291–295. doi: 10.1016/j.amjcard.2007.02.096. [DOI] [PubMed] [Google Scholar]
- 50.Piña IL, Kokkinos P, Kao A, Bittner V, Saval M, Clare B, Goldberg L, Johnson M, Swank A, Ventura H, Moe G, Fitz-Gerald M, Ellis SJ, Vest M, Cooper L, Whellan D. HF-ACTION Investigators. Baseline differences in the HF-ACTION trial by sex. Am Heart J. 2009;158:S16–S23. doi: 10.1016/j.ahj.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gulati M, Black HR, Shaw LJ, Arnsdorf MF, Merz CN, Lauer MS, Marwick TH, Pandey DK, Wicklund RH, Thisted RA. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353:468–475. doi: 10.1056/NEJMoa044154. [DOI] [PubMed] [Google Scholar]
- 52.Pina IL, Bittner V, Clare B, Swank AM, Kao AC, Safford RE, Nigam A, Barnard D, Walsh MM, Ellis SJ, Keteyian SJ for the HF-ACTION Investigators. Effects of exercise training on outcomes in women with heart failure: Analysis of HF- ACTION by sex. JACC Heart Fail. 2014;2:180–186. doi: 10.1016/j.jchf.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Iellamo F, Rosano G, Volterrani M. Testosterone deficiency and exercise intolerance in heart failure: treatment implications. Curr Heart Fail Rep. 2010;7:59–65. doi: 10.1007/s11897-010-0008-6. [DOI] [PubMed] [Google Scholar]
- 54.Krumholz HM. Post-hospital syndrome. An acquired transient condition of generalized risk. N Engl J Med. 2013;368:100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alahdab MT, Mansour IN, Napan S, Stamos TD. Six minute walk test predicts long-term all-cause mortality and heart failure rehospitalization in African-American patients hospitalized with acute decompensated heart failure. J Card Fail. 2009;15:130–135. doi: 10.1016/j.cardfail.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Kommuri NV, Johnson ML, Koelling TM. Six-minute walk distance predicts 30-day readmission in hospitalized heart failure patients. Arch Med Res. 2010;41:363–368. doi: 10.1016/j.arcmed.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Delgado Parada E, Suárez García FM, López Gaona V, Gutiérrez Vara S, Solano Jaurrieta JJ. Mortality and functional evolution at one year after hospital admission due to heart failure in elderly patients. Arch Gerontol Geriatr. 2012;54:261–265. doi: 10.1016/j.archger.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Davies EJ, Moxham T, Rees K, Singh S, Coats AJ, Ebrahim S, Lough F, Taylor RS. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur J Heart Fail. 2010;12:706–715. doi: 10.1093/eurjhf/hfq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scrutinio D, Passantino A, Catanzaro R, Farinola G, Lagioia R, Mastropasqua F, Ricci A, Santoro D. Inpatient cardiac rehabilitation soon after hospitalization for acute decompensated heart failure: a propensity score study. J Cardiopulm Rehabil Prev. 2012;32:71–77. doi: 10.1097/HCR.0b013e31823be124. [DOI] [PubMed] [Google Scholar]
- 60.Babu AS, Maiya AG, George MM, Padmakumar R, Guddattu V. Effects of combined early In-patient cardiac rehabilitation and structured home-based program on function among patients with congestive heart failure: A randomized controlled trial. Heart Views. 2011;12:99–103. doi: 10.4103/1995-705X.95064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giallauria F, Vigorito C, Tramarin R, Fattirolli F, Ambrosetti M, De Feo S, Griffo R, Riccio C, Piepoli M. ISYDE-2008 Investigators of the Italian Association for Cardiovascular Prevention, Rehabilitation Prevention Cardiac rehabilitation in very old patients: data from the Italian Survey on Cardiac Rehabilitation-2008 (ISYDE-2008)--official report of the Italian Association for Cardiovascular Prevention, Rehabilitation, and Epidemiology. J Gerontol A Biol Sci Med Sci. 2010;65:1353–1361. doi: 10.1093/gerona/glq138. [DOI] [PubMed] [Google Scholar]
- 62.Faber MJ, Bosscher RJ, Chin Paw AMJ, van Wieringen PC. Effects of exercise programs on falls and mobility in frail and pre-frail older adults: A multicenter randomized controlled trial. Arch Phys Med Rehabil. 2006;87:885–896. doi: 10.1016/j.apmr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Taylor RS, Davies EJ, Dalal HM, Davis R, Doherty P, Cooper C, Holland DJ, Jolly K, Smart NA. Effects of exercise training for heart failure with preserved ejection fraction: A systematic review and meta-analysis of comparative studies. Int J Cardiol. 2012;162:6–13. doi: 10.1016/j.ijcard.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 64.Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: The benefit depends on the type of training performed. J Am Coll Cardiol. 2007;49:2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 65.Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 66.Fu TC, Wang CH, Lin PS, Hsu CC, Cherng WJ, Huang SC, Liu MH, Chiang CL, Wang JS. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int J Cardiol. 2013;167:41–50. doi: 10.1016/j.ijcard.2011.11.086. [DOI] [PubMed] [Google Scholar]
- 67.Dimopoulos S, Anastasiou-Nana M, Sakellariou D, Drakos S, Kapsimalakou S, Maroulidis G, Roditis P, Papazachou O, Vogiatzis I, Roussos C, Nanas S. Effects of exercise rehabilitation program on heart rate recovery in patients with chronic heart failure. Eur J Cardiovasc Prev Rehab. 2006;13:67–73. doi: 10.1097/01.hjr.0000198449.20775.7c. [DOI] [PubMed] [Google Scholar]
- 68.Iellamo F, Manzi V, Caminiti G, Vitale C, Castagna C, Massaro M, Franchini A, Rosano G, Volterrani M. Matched dose interval and continuous exercise training induce similar cardiorespiratory and metabolic adaptations in patients with heart failure. Int J Cardiol. 2012;167:2561–2565. doi: 10.1016/j.ijcard.2012.06.057. [DOI] [PubMed] [Google Scholar]
- 69.Haykowsky MJ, Timmons MP, Kruger C, McNelly M, Taylor DA, Clark AM. Meta-analysis of aerobic interval training in patients with heart failure and reduced ejection fraction. Am J Cardiol. 2013;111:1466–1469. doi: 10.1016/j.amjcard.2013.01.303. [DOI] [PubMed] [Google Scholar]
- 70.Meyer TE, Casadei B, Coats AJ, Davey PP, Adamopoulos S, Radaelli A, Conway J. Angiotensin-converting enzyme inhibition and physical training in heart failure. J Intern Med. 1991;230:407–413. doi: 10.1111/j.1365-2796.1991.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 71.Forissier JF, Vernochet P, Bertrand P, Charbonnier B, Monpere C. Influence of carvedilol on the benefits of physical training in patients with moderate chronic heart failure. Eur J Heart Fail. 2001;3:335–342. doi: 10.1016/s1388-9842(00)00152-5. [DOI] [PubMed] [Google Scholar]
- 72.Conraads VM, Vanderheyden M, Paelinck B, Verstreken S, Blankoff I, Miljoen H, De Sutter J, Beckers P. The effect of endurance training on exercise capacity following cardiac resynchronization therapy in chronic heart failure patients: a pilot trial. Eur J Cardiovasc Prev Rehabil. 2007;14:99–106. doi: 10.1097/HJR.0b013e32801164b3. [DOI] [PubMed] [Google Scholar]
- 73.Patwala AY, Woods PR, Sharp L, Goldspink DF, Tan LB, Wright DJ. Maximizing patient benefit from cardiac resynchronization therapy with the addition of structured exercise training: a randomized controlled study. J Am Coll Cardiol. 2009;53:2332–2339. doi: 10.1016/j.jacc.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 74.Hayes K, Leet AS, Bradley SJ, Holland AE. Effects of exercise training on exercise capacity and quality of life in patients with a left ventricular assist device: a preliminary randomized controlled trial. J Heart Lung Transplant. 2012;31:729–734. doi: 10.1016/j.healun.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 75.Hsieh PL, Wu YT, Chao WJ. Effects of exercise training in heart transplant recipients: a meta-analysis. Cardiology. 2011;120:27–35. doi: 10.1159/000332998. [DOI] [PubMed] [Google Scholar]
- 76.Whellan DJ, Hasselblad V, Peterson E, O’Connor CM, Schulman KA. Meta-analysis and review of heart failure disease management randomized controlled clinical trials. Am Heart J. 2005;149:722–729. doi: 10.1016/j.ahj.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 77.Austin J, Williams R, Ross L, Moseley L, Hutchinson S. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail. 2005;7:411–417. doi: 10.1016/j.ejheart.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 78.Davidson PM, Cockburn J, Newton PJ, Webster JK, Betihavas V, Howes L, Owensby DO. Can a heart failure-specific cardiac rehabilitation program decrease hospitalizations and improve outcomes in high-risk patients. Eur J Cardiovasc Prev Rehabil. 2010;17:393–402. doi: 10.1097/HJR.0b013e328334ea56. [DOI] [PubMed] [Google Scholar]
- 79.Belardinelli R, Georgiou D, Cianci G, Purcaro A. 10-year exercise training in chronic heart failure. J Am Coll Cardiol. 2012;60:1521–1528. doi: 10.1016/j.jacc.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 80.Jolly K, Taylor RS, Lip GY, Davies M, Davis R, Mant J, Singh S, Greenfield S, Ingram J, Stubley J, Bryan S, Stevens A. A randomized trial of the addition of home-based exercise to specialist heart failure nurse care: the Birmingham Rehabilitation Uptake Maximisation study for patients with Congestive Heart Failure. Eur J Heart Fail. 2009;11:205–213. doi: 10.1093/eurjhf/hfn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evangelista LS, Dracup K, Erickson V, McCarthy WJ, Hamilton MA, Fonarow GC. Validity of pedometers for measuring exercise adherence in heart failure patients. J Card Fail. 2005;11:366–371. doi: 10.1016/j.cardfail.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 82.Corvera-Tindel T, Doering LV, Woo MA, Khan S, Dracup K. Effects of a home walking program on functional status and symptoms in heart failure. Am Heart J. 2004;147:339–346. doi: 10.1016/j.ahj.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 83.McKelvie RS, Teo KK, Roberts R, McCartney N, Humen D, Montague T, Hendrican K, Yusuf S. Effects of exercise training in patients with heart failure: the Exercise Rehabilitation Trial (EXERT) Am Heart J. 2002;144:23–30. doi: 10.1067/mhj.2002.123310. [DOI] [PubMed] [Google Scholar]
- 84.Oka RK, De Marco T, Haskell WL, Botvinick E, Dae MW, Bolen K, Chatterjee K. Impact of a home-based walking and resistance training program on quality of life in patients with heart failure. Am J Cardiol. 2000;85:365–369. doi: 10.1016/s0002-9149(99)00748-1. [DOI] [PubMed] [Google Scholar]
- 85.Mark SD, Robins JM. A method for the analysis of randomized trials with compliance information: an application to the Multiple Risk Factor Intervention Trial. Control Clin Trials. 1993;14:79–97. doi: 10.1016/0197-2456(93)90012-3. [DOI] [PubMed] [Google Scholar]
- 86.Diabetes Prevention Program Research. Reduction in the incidence of type 2 diabetes with lifestyle interventionor metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJF, Martin BW. Correlates of physical activity: why are some people physically active and others not. Lancet. 2012;380:258–271. doi: 10.1016/S0140-6736(12)60735-1. [DOI] [PubMed] [Google Scholar]
- 88.Wenger NK. Current status of cardiac rehabilitation. J Am Coll Cardiol. 2008;51:1619–1631. doi: 10.1016/j.jacc.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 89.Solomon SD, Wang D, Finn P, Skali H, Zornoff L, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Pocock S, Pfeffer MA. Effect of candesartan on cause-specific mortality in heart failure patients: the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2004;110:2180–2183. doi: 10.1161/01.CIR.0000144474.65922.AA. [DOI] [PubMed] [Google Scholar]
- 90.Skali H, Pfeffer MA, Lubsen J, Solomon SD. Variable impact of combining fatal and nonfatal endpoints in heart failure trials. Circulation. 2006;114:2298–2303. doi: 10.1161/CIRCULATIONAHA.106.620039. [DOI] [PubMed] [Google Scholar]
- 91.Yusuf S, Negassa A. Choice of clinical outcomes in randomized trials of heart failure therapies: Disease-specific or overall outcomes. Am Heart J. 2002;143:22–28. doi: 10.1067/mhj.2002.119770. [DOI] [PubMed] [Google Scholar]
- 92.Freemantle N, Clavert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials: Greater precision but with greater uncertainty. JAMA. 2003;289:2554–2559. doi: 10.1001/jama.289.19.2554. [DOI] [PubMed] [Google Scholar]