Abstract
Turf algae increasingly dominate benthic communities on coral reefs. Given their abundance and high dissolved organic carbon (DOC) release rates, turf algae are considered important contributors to the DOC pool on modern reefs. The release of photosynthetically fixed carbon as DOC generally, but not always, increases with increased light availability. Nutrient availability was proposed as an additional factor to explain these conflicting observations. To address this proposed but untested hypothesis, we documented the interactive contributions of light and nutrient availability on the release of DOC by turf algae. DOC release rates and oxygen production were quantified in incubation experiments at two light levels (full and reduced light) and two nutrient treatments (natural seawater and enriched seawater). In natural seawater, DOC release at full light was four times higher than at reduced light. When nutrients were added, DOC release rates at both light levels were similar to the natural seawater treatment at full light. Our results therefore show that low light in combination with low nutrient availability reduces the release of DOC by turf algae and that light and nutrient availability interactively determine DOC release rates by this important component of Caribbean reef communities.
The concentration and composition of waterborne dissolved organic carbon (DOC) plays an important role in the functioning of coral reef ecosystems (e.g.1,2,3,4). The DOC pool on coral reefs is mainly fueled by benthic primary producers (i.e., benthic algae, benthic cyanobacterial mats and scleractinian corals)5,6,7,8, which release a substantial part of their photosynthetically fixed carbon as DOC into the water column7,9,10. Benthic algae generally release more DOC per surface area than corals (e.g.7,10,11) and algal DOC can promote the growth of opportunistic microbes in the interface of coral-algal interactions (reviewed in3). The combined effects of a shift in the microbial community towards opportunistic pathogens12,13, an increase in microbial abundance and respiration7,14 and as a result oxygen depletion15,16,17 can lead to mortality of the coral18,19,20 and therefore negatively influences the outcome of coral-algal competition3,21. The global increase of benthic algae will thus likely come with major implications for the DOC dynamics and the general functioning of coral reefs1,7,10,22.
Of all benthic algal groups, the abundance of turf algae has increased most dramatically in recent decades and turf algae presently represent the most dominant benthic component on many coral reefs around the world15,21,23,24,25. Turf algae are multi-species assemblages of Chlorophyta, Phaeophyta and Rhodophyta intermixed with filamentous cyanobacteria with a maximum height of approximately 1 cm and a distinct community of associated bacteria26,27,28. They exhibit fast growth rates29, rapidly take up nutrients30 and are capable of nitrogen fixation due to the presence of cyanobacteria within these communities31,32. Moreover, their high surface to volume ratio facilitate the exchange of metabolic products (e.g., DOC) between these algal communities and their environment33 and makes them one of the most productive benthic primary producers on coral reefs34,35. Given their abundance and high DOC release rates turf algae can be considered important contributors to the local DOC pool on modern coral reefs 7,8,10.
Light (e.g.36,37,38) and nutrient availability37,39 influence DOC release rates of aquatic primary producers. Potential interactive effects among these factors are less often considered and could underlie the sometimes contradicting observations of DOC release studies. For example, many authors have reported on the positive effect of light availability on DOC release37,39,40,41, whereby carbon fixation is hypothesized to outpace cell growth, particularly under nutrient limited conditions (e.g.37,38,39). Other authors were unable to confirm such a positive relationship between DOC release and light availability (e.g.42,43,44,45) and instead suggested that DOC passively diffuses through the cell membrane, independent of light availability. These contrasting observations are certainly not mutually exclusive46,47 and suggest that actual DOC release rates likely depend on more than one environmental condition (e.g., light and nutrient availability).
Light availability alone or in combination with increased nutrient availability due to eutrophication (e.g.48,49) could affect the rate of DOC release by Caribbean turf algae according to the light-dependent and/or light-independent mechanisms described by Carlson46. The purpose of this study was to determine if DOC release by turf algae depends on (1) light availability and/or (2) nutrient availability and if so (3) whether the contribution of light and nutrient availability is interactive or additive.
Materials and Methods
Experimental set up
The study was conducted in May 2012 on Curaçao, an island in the Southern Caribbean, 65 kilometers north of Venezuela. Fringing reefs run along its entire leeward coast50. Following the protocol of Den Haan, et al.32, turf algae were grown on the exterior of 0.5 L polyethylene (PET) bottles that were placed inside a 1 m3 chicken-wire cage (mesh size: 2.5 cm) to minimize grazing by larger herbivorous fish. The cage was deployed on the fore reef slope at 10 m depth at the site ‘Buoy 0’ (12°12′35″ N, 68°97′10″), which is located 500 m downstream of the outlet of the eutrophied Piscadera Bay. The benthic community at this site was dominated by macroalgae, turf algae and benthic cyanobacteria and hard coral cover is only 10%51. After 6 weeks, turf communities, representative of those growing on the reef bottom, had developed on the bottles27,51. Bottles covered with turf algae were collected 24 h prior to the experiment and the bottom and top part of the bottles were cut off. The resulting open cylinders (height = 8 cm, surface area = 282.4 ± 1.1 cm2 [mean ± SE]) were allowed to recover and acclimatize in flow-through seawater aquaria (27–29 °C) for at least 24 h. During this acclimatization period, light conditions were on average ~100 μmol photons m−2 s−1 during the daytime as measured with a Hydrolab DS5 (OTT Messtechnik GmbH & Co., Kempten, Germany; sampling interval 30 s), which is similar to the light conditions measured at 20 m depth at Buoy 0 at midday32. Remaining bottles were completely scraped to serve as controls (PET without turf algae) for all experiments.
Turf algae were incubated in transparent Plexiglas incubators (1.0 L) with an opaque bottom and lid. The lid had a removable (Ø 5 cm) PVC plug to allow water sampling and a magnetic stirrer to ensure mixing throughout the experiment. Prior to the experiments, incubators were acid-washed (0.4 M HCl) and rinsed twice with filtered treatment water (0.22 μm Whatman Cellulose acetate membrane filter) to remove phytoplankton and planktonic microbes that could release or consume DOC. Incubators were filled with 1.0 L of filtered treatment water and turf algae (n = 4 per treatment) or a control cylinder (n = 1 per treatment) were subsequently placed in the incubators. Two nutrient treatments were used: (1) natural seawater and (2) nutrient enriched seawater. Enriched seawater was prepared 1 h prior to the experiments by adding nutrients in the form of NH4Cl, NaNO3 and KH2PO4 (Sigma Aldrich) to filtered seawater (Table 1).
Table 1. Mean light intensities (μmol photons m−2 s−1)(±SD) between 10:00 hrs and 16:00 hrs and mean (±SD) initial (t0) and final (tend) pH and nutrient concentrations (μmol L−1) for the four treatment combinations.
| Natural seawater& reduced light | Natural seawater &full light | Enriched seawater& reduced light | Enriched seawater& full light | ||
|---|---|---|---|---|---|
| Date | 30.5.2012 | 30.5.2012 | 23.5.2012 | 24.5.2012 | |
| light | 109 ± 44 | 622 ± 249 | 86 ± 48 | 585 ± 270 | |
| pH | t0 | 7.72 ± 0.15 | 7.53 ± 0.20 | 7.76 ± 0.18 | 7.76 ± 0.15 |
| tend | 8.50 ± 0.04 | 8.59 ± 0.13 | 8.31 ± 0.13 | 8.59 ± 0.21 | |
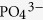 |
t0 | 0.019 ± 0.016 | 0.101 ± 0.010 | 11.341 ± 0.850 | 9.163 ± 1.517 |
| tend | 0.015 ± 0.018 | 0.090 ± 0.122 | 5.249 ± 1.304 | 6.071 ± 0.600 | |
 |
t0 | 2.831 ± 2.607 | 1.977 ± 1.309 | 69.029 ± 36.556 | 61.099 ± 32.825 |
| tend | 7.595 ± 4.954 | 13.415 ± 14.147 | 45.705 ± 14.025 | 57.633 ± 6.979 | |
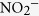 |
t0 | 0.129 ± 0.036 | 0.139 ± 0.004 | 0.240 ± 0.059 | 0.271 ± 0.170 |
| tend | 0.398 ± 0.317 | 0.491 ± 0.378 | 0.297 ± 0.102 | 0.196 ± 0.025 | |
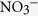 |
t0 | 0.434 ± 0.086 | 0.584 ± 0.197 | 3.620 ± 1.781 | 3.527 ± 1.767 |
| tend | 0.413 ± 0.343 | 1.454 ± 2.199 | 3.278 ± 0.547 | 3.751 ± 0.411 |
Resulting nutrient concentrations (23 times dissolved inorganic nitrogen and 171 times PO43− concentration compared to those of the natural seawater treatment) were not intended to mirror naturally occurring concentrations, but rather to create nutrient replete conditions during the course of the incubations. Furthermore, these concentrations are similar to those used in nutrient uptake experiments and were proven to be non-lethal for the used turf algal communities30. The incubators were placed in a flow-through seawater system to keep them at temperatures similar to those on the reef (27–29 °C). During the 6 h experiments (all conducted between 10:00 hrs and 16:00 hrs local time, to ensure sufficient light levels during the entire duration of the experiments), incubators were exposed to one of two light treatments: (1) “full light” and (2) “reduced light”. In the “full light” treatment, incubators were placed in full sunlight receiving an average light intensity of 631 ± 52 μmol photons m−2 s−1 (±SD). In the “reduced light” treatment incubators were wrapped in Neutral Density Filter shading foil (Modulor GmbH, Berlin, Germany) reducing the light intensity inside the incubators by 83% to an average of 105 ± 18 μmol photons m−2 s−1 (Table 1).
At the beginning and end of the 6 h incubation, a water sample (60 mL) was taken from each incubator for DOC analysis using a polypropylene syringe (100 mL) that was acid-washed and rinsed with filtered treatment water beforehand. Samples were immediately placed in the dark and processed within 60 minutes after sampling. Additional water samples were taken at the beginning of the experiment to determine the initial nutrient concentrations in each incubator using a 50 mL Terumo syringe. Water samples were immediately filtered using 0.22 μm Acrodisc filters and stored in 6 mL polyethylene vials (PerkinElmer, MA, USA) at −20 °C until further analysis. Oxygen concentrations in each incubator were measured at hourly intervals during the duration of the experiment using an oxygen optode (PreSens Fibox 3). Oxygen production was determined to serve as a proxy for carbon fixation and to express DOC release as percentage of primary production (e.g.7,8). PH was measured at the beginning and the end of the experiment with a pH meter (WTW pH 330). The variability in the amount of light entering the incubation chambers during the experiment was quantified using a Hydrolab DS5 (OTT Messtechnik GmbH & Co., Kempten, Germany; sampling interval 30 s) that was standing in the direct vicinity of the incubators. Obtained light values by the Hydrolab were transformed to light values that would have occurred inside the incubators using a pre-determined conversion factor. This conversion factor was obtained by measuring the light intensity inside and outside of the incubators, with and without shading foil, using a light meter (cosine LI-192SSA underwater quantum sensor connected to LI-1000 data logger; range: PAR 400–700). The two light and nutrient treatments were combined in a factorial sampling design and two light-nutrient combinations were simultaneously run per day, in which we ensured that abiotic variables were comparable amongst sampling days (see Table 1). After the experiment, turf algae were scraped off each open cylinder to determine their dry weight (DW). Samples were rinsed with distilled water, oven-dried to constant weight in pre-weighed aluminum cups (>72 h at 60 °C) and weighed (accuracy 0.001 g). The mean DW of turf algae per cylinder was 0.396 ± 0.149 g (±SD).
DOC and nutrient analyses
DOC samples were filtered (<20 kPa Hg suction pressure) over a 0.2 μm polycarbonate filter (Whatman, 25 mm). Prior to filtration, filters, glassware and pipette tips were rinsed three times with acid (10 mL 0.4 M HCl) and twice with sample water (10 mL). Afterwards, 20 mL of sample water was filtered and the filtrate that contained DOC was transferred to pre-combusted (4 h at 450 °C) Epa vials (40 mL). Samples were acidified with 6–7 drops of concentrated HCl (38%) to remove inorganic C and stored at 4 °C until analysis. DOC concentrations were measured using the high-temperature catalytic oxidation (HTCO) technique in a total organic C analyzer (TOC-VCPN; Shimadzu). The instrument was calibrated with a standard addition curve of Potassium Hydrogen Phthalate (0; 25; 50; 100; 200 μmol C L−1). Consensus Reference Materials (CRM) provided by DA Hansell and W Chen of the University of Miami (Batch 12; 2012; 41–44 μmol C L−1) were used as positive controls for our measurements. Concentrations measured of the batch gave average values (±SD) of 42 ± 6 μmol C L−1. Average analytical variation of the instrument was <3% (5–7 injections per sample). Concentrations of 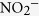 and
and 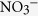 52,
52, 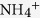 53 and
53 and 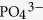 54 were analyzed using continuous flow analysis in a Quatro auto-analyzer (Seal Analytical, UK).
54 were analyzed using continuous flow analysis in a Quatro auto-analyzer (Seal Analytical, UK).
Data analysis
DOC release and oxygen production rate were calculated as the change in their concentration through time. This change was solely based on the difference between initial and final concentration in case of DOC release (ESM Fig. S1), whereas for oxygen production it was based on a linear regression including all intermediate time points (ESM Fig. S2). The change of the respective control was subtracted and each rate was normalized to turf algal biomass (DW)7,55,56. Assumptions of heterogeneity and normality were met57. Differences in initial O2 and DOC concentrations between the treatment combinations were tested using one-way ANOVA followed by Tukey’s HSD post-hoc tests. A two-way ANOVA was used to assess whether the DOC release rates of turf algae differed among experimental treatments. All analyses were performed using software package SPSS 20 (IBM Corp., Armonk, NY, USA). As turf algae are multi-species assemblages including micro- and macroalgae, filamentous cyanobacteria and a distinct community of associated bacteria, all fluxes determined here must be considered to be net community fluxes of this turf algal holobiont (sensu Barott, et al.28).
Results
In natural seawater, DOC release rates were four times higher at full light than at reduced light (Tukey HSD, p = 0.012; Fig. 1A). In enriched seawater, DOC release rates at both light intensities were similar irrespective of light availability (Tukey HSD, p = 0.419), and similar to DOC release rates at full light in the natural seawater treatment (Tukey HSD, p = 0.665 and p = 0.970, respectively). A positive relation between DOC release and light availability was only observed in the natural seawater treatment, i.e. indicating that the occurrence of light-dependent DOC release depended on nutrient availability (significant interaction: light x nutrients, p = 0.002; ESM Table S3A).
Figure 1.
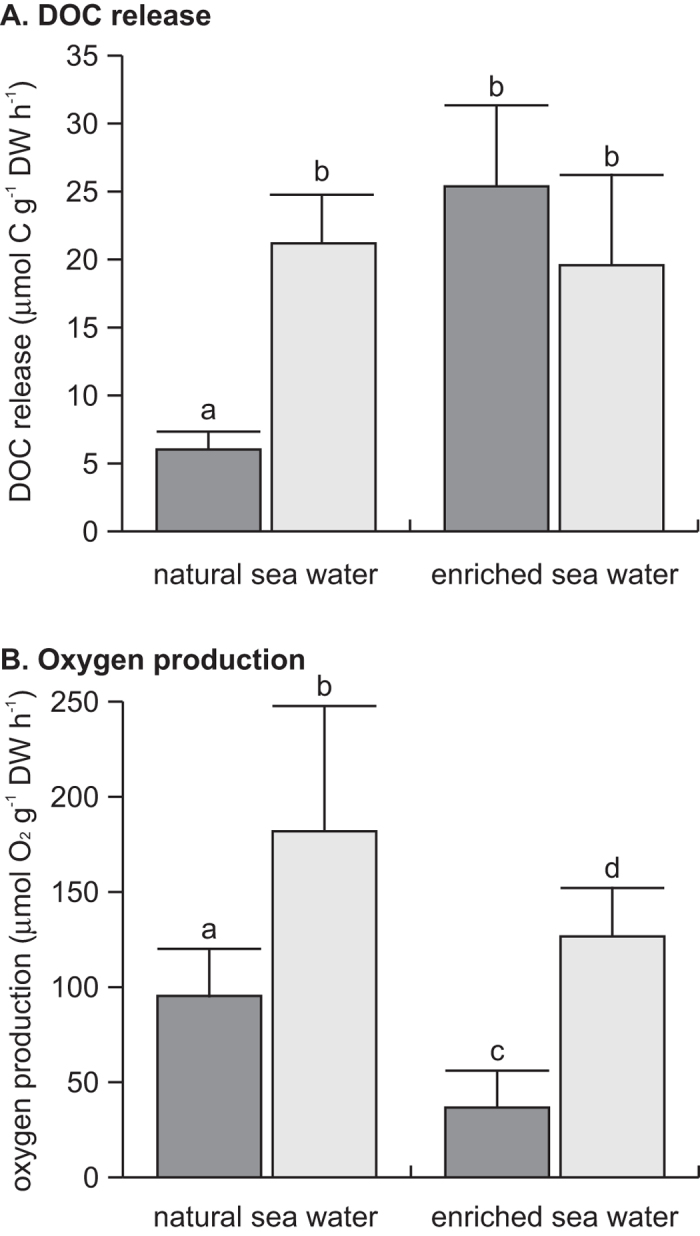
(A) DOC release (mean + SD) and (B) oxygen production (mean + SD) of turf algae for the natural and the enriched seawater treatment under reduced (dark grey) and full light conditions (light grey). In both panels n = 4 per treatment combination. Treatment combinations with the same letter are not significantly different at α = 0.05.
Initial DOC concentrations in the incubators with turf algae and controls were 98 ± 6 μmol C L−1 (mean ± SD) except for the enriched seawater treatment at full light, where the DOC concentration of the control was elevated by 28 μmol C L−1 (Tukey HSD, p < 0.05). During the course of the incubations DOC concentrations in the controls increased by 10–12% in the natural seawater, whereas a slight decrease of −2 and −5% occurred in the enriched seawater treatments (ESM Fig. S1). In contrast, changes in DOC concentrations in the incubators with turf algae ranged between 19–57 and 22–65% in the natural and enriched seawater treatment, respectively.
Light and nutrient availability had a strong effect on oxygen production by turf algae (ESM Table S3B). Net oxygen production at full light was twice as high compared to reduced light in the natural seawater treatment (Tukey HSD p < 0.039; Fig. 1B). In contrast to DOC release, oxygen production also differed between light treatments when nutrients were added (Tukey HSD, p < 0.031). In nutrient replete treatments net oxygen production at full light was 3.5 times higher than at reduced light. Despite comparable light availability, net oxygen production under enriched conditions was 59 and 55 μmol O2 g−1 h−1 DW lower in the full and reduced light treatment, respectively, compared to the natural seawater treatments (Fig. 1B). The initial oxygen concentration in the incubators with turf algae and in controls was 242 ± 16 μmol O2 L−1 (mean ± SD), except for the enriched seawater treatment at reduced light, which was 23 μmol O2 L−1 lower (Tukey HSD, p < 0.05). Changes in oxygen concentrations over time ranged between −5 and 11% in the controls, whereas the change in the incubators with turf algae ranged between 118 and 205% (ESM Fig. S2). The only exception to this general pattern occurred in the enriched seawater treatment at reduced light, where the oxygen concentration in the control changed by 45%, whereas the incubators with turf algae showed changes in oxygen concentrations between 66–74%. The net oxygen production rate of turf algae in the enriched seawater treatment at reduced light should therefore be considered with some caution.
Assuming a balanced molar ratio of carbon fixation to net oxygen production (1 mole C fixed equals 1 mole O2 released), 6 and 12% of the photosynthetically fixed carbon was released as DOC at reduced and full light in the natural seawater treatments. In contrast, in the enriched seawater treatments 82 and 15% of the photosynthetically fixed carbon was released as DOC at reduced and full light, respectively.
In the natural seawater treatments PO43− concentrations decreased by 23 and 11% during the course of the incubations at reduced and full light, respectively (Table 1). In contrast, dissolved inorganic nitrogen concentrations (DIN: 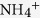 +
+ 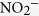 +
+ 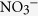 ) increased by 168 and 579%, respectively, indicating the release of fixed nitrogen by cyanobacteria within the turf algal communities, mainly in the form of
) increased by 168 and 579%, respectively, indicating the release of fixed nitrogen by cyanobacteria within the turf algal communities, mainly in the form of  . In the enriched seawater treatment at reduced light
. In the enriched seawater treatment at reduced light 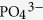 and DIN concentrations decreased by 54 and 45%, respectively, during the incubations. At full light
and DIN concentrations decreased by 54 and 45%, respectively, during the incubations. At full light 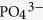 and DIN concentrations only decreased by 34 and 23%, respectively. Changes in DIN concentrations are mainly driven by the uptake of NH4+ by the turf algal community. At the end of the enriched seawater incubations PO43− and DIN concentrations were on average still 18 and 94 times higher than the initial concentrations in the natural seawater treatment, which suggests that both
and DIN concentrations only decreased by 34 and 23%, respectively. Changes in DIN concentrations are mainly driven by the uptake of NH4+ by the turf algal community. At the end of the enriched seawater incubations PO43− and DIN concentrations were on average still 18 and 94 times higher than the initial concentrations in the natural seawater treatment, which suggests that both 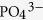 and DIN remained replete throughout the experiment. As a result of photosynthesis, the pH increased in the incubators containing turf algae by 0.55–1.07 units during the course of the experiments (Table 1). These changes are comparable to those experienced by benthic reef organisms on coral reefs over a diurnal cycle (e.g.58,59).
and DIN remained replete throughout the experiment. As a result of photosynthesis, the pH increased in the incubators containing turf algae by 0.55–1.07 units during the course of the experiments (Table 1). These changes are comparable to those experienced by benthic reef organisms on coral reefs over a diurnal cycle (e.g.58,59).
Discussion
In this study we demonstrate that DOC release by turf algae increases with increasing light availability under naturally occurring nutrient concentrations. Addition of nutrients resulted in the disappearance of the positive relationship with light availability and under nutrient replete conditions DOC release became similar as in the full light and natural seawater treatment (Fig. 1A). Our results therefore indicate that the release of DOC by turf algae is affected by light and nutrient availability simultaneously.
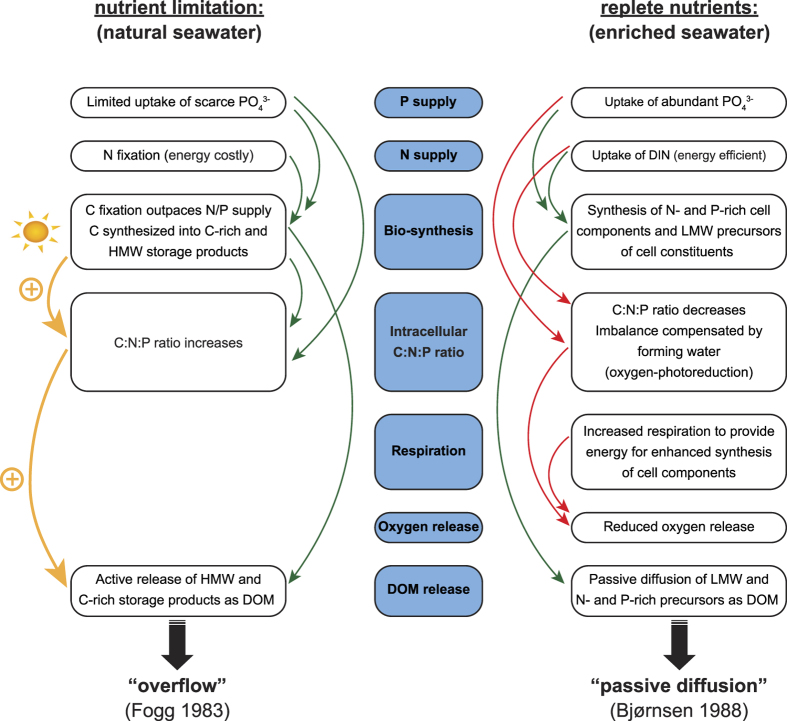
Two different pathways have been proposed to explain the DOC release of aquatic primary producers: (1) A light-dependent pathway where DOC is actively released in an overflow mechanism (e.g.37,38,39) and (2) a light-independent pathway where DOC diffuses through the cell membrane along a concentration gradient (e.g.42,43,44). Light-dependent and light-independent release of DOC by turf algae were both observed in our study, but under different nutrient conditions. Thus, our results support the hypothesis that the availability of nutrients determines which pathway dominates. Under natural nutrient conditions the DOC release of turf algae was four times higher at full light compared to reduced light (Fig. 1A), confirming the reports of light-dependent DOC release by benthic reef algae and sea grasses41,55,60 (and references therein). Under nutrient limited conditions fixed carbon is proposed to be predominantly synthesized into carbon-rich storage products (i.e., carbohydrates, polysaccharides), as the lack of nutrients confines the synthesis of other cell components61,62 (Fig. 2). Eventually, the carbon-rich storage products are actively released as DOC in an overflow mechanism39,61,63. Since the carbon fixation in photosynthates is directly related to light, the resulting DOC release is also suggested to follow a positive relationship with light37,40,64,65.
Figure 2. Proposed mechanisms involved in the dissolved organic matter (DOM) release of turf algae under nutrient limited and nutrient replete conditions.
Under nutrient limitation carbon fixation is proposed to outpace the N and P supply, leading to the formation of C-rich and high molecular weight (HMW) storage products which are actively released as DOM in an overflow mechanism. An increase in light availability further increases the intracellular C:N:P ratio and thus stimulates DOM release. When nutrients are replete N- and P-rich cell components and low molecular weight (LMW) precursors of cell components are synthesized. These LMW molecules passively diffuse through the cell membrane as long as a concentration gradient prevails. This mechanism is not affected by an increase in light availability. A combination of increased respiration due to an enhanced synthesis and the formation of oxygen radicals and thereafter water to compensate for the imbalance of the intracellular C:N ratio are further suggested to reduce the oxygen release under such conditions. Green and red arrows indicate positive effects (stimulation) and negative effects (reduction), respectively.
Furthermore, cyanobacteria, that account for approximately 20% of the total turf algal biomass27,32,66,67, are capable of nitrogen (N2) fixation32,68. In fact, an increase in DIN concentrations by 5 and 13 μmol L−1 in natural seawater at reduced and full light, respectively, suggests nitrogen fixation and a subsequent release of excess N mainly in the form of NH4+ during the course of the experiment. As nitrogen fixation is an energy-costly process, which requires 16 ATP and 8 reduction equivalents to reduce one N2 molecule69, less energy can be allocated to carbon fixation, particularly at reduced light, and less carbon will be released as DOC. While nitrogen fixation provided turf algae with a continuous supply of N, rapid uptake of 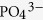 led to P depletion at the end of the natural seawater incubations (Table 1). Light-dependent DOC release, as observed in the natural seawater treatments, may therefore foremost be a result of P-limitation (e.g.70).
led to P depletion at the end of the natural seawater incubations (Table 1). Light-dependent DOC release, as observed in the natural seawater treatments, may therefore foremost be a result of P-limitation (e.g.70).
Under nutrient replete conditions DOC release rates were similar irrespective of light availability (reduced vs full light) and were comparable to those at full light of the natural seawater treatment (Fig. 1A). These findings can possibly be explained by two mechanisms (Fig. 2): Firstly, DOC release rates that do not differ under different light intensities could be explained by the ‘passive diffusion theory’ that was used as explanation for a similar phenomenon in mixed phytoplankton communities in the open ocean43,44. According to this theory, low molecular weight molecules are believed to constantly diffuse through the cell membrane as long as a concentration gradient exists42,43. And secondly, the higher DOC release rate at reduced light in nutrient enriched conditions compared to natural seawater could be explained by the ceasing of nitrogen fixation in enriched seawater. Less energy is invested in nitrogen fixation, and thus more photosynthates can be produced and eventually released as DOC than in the natural seawater treatments at similar light. In the enriched seawater treatments NH4+ concentrations decreased at both reduced and full light (Table 1). Caribbean turf algae are known to be capable of rapidly taking up 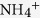 30, which suggests that the uptake of NH4+ was the predominant source of N for turf algae under nutrient replete conditions, in contrast to nitrogen fixation in the natural seawater treatments. The higher net uptake of N at reduced light in the enriched seawater treatment may further indicate a lower contribution of nitrogen fixation to the N budget compared to full light. This could compensate for the lower carbon fixation due to a lower light availability and thus, at least partly, explain similar DOC release rates at reduced and full light.
30, which suggests that the uptake of NH4+ was the predominant source of N for turf algae under nutrient replete conditions, in contrast to nitrogen fixation in the natural seawater treatments. The higher net uptake of N at reduced light in the enriched seawater treatment may further indicate a lower contribution of nitrogen fixation to the N budget compared to full light. This could compensate for the lower carbon fixation due to a lower light availability and thus, at least partly, explain similar DOC release rates at reduced and full light.
High DOC release rates by turf algae at both light treatments under nutrient replete conditions that were similar to release rates under full light in natural seawater were unexpected. This observation might however be of interest in the face of climate change, where coral reefs are increasingly subjected to run-off from land that increases nutrient availability and reduces the availability of light via suspended matter transport (e.g.49,71,72). Den Haan30 reported DIN and 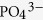 concentrations in run-off plumes coming out of the Piscadera Bay after heavy rainfall approaching those used in our enriched nutrient treatments. During such an event, DOC release rates of turf algae are therefore expected to remain high and constant, despite a reduction in light availability.
concentrations in run-off plumes coming out of the Piscadera Bay after heavy rainfall approaching those used in our enriched nutrient treatments. During such an event, DOC release rates of turf algae are therefore expected to remain high and constant, despite a reduction in light availability.
Reported DOC release rates of benthic algae vary widely between 0.14 and 5.53 mmol C m−2 h−1 and those of turf algae cover most of this spectrum (0.52 and 5.53 mmol C m−2 h−1) (Brocke, et al.8 and references therein). When expressing our release rates per surface area, they range between 0.07 (natural seawater at reduced light) and 0.32 mmol C m−2 h−1 (enriched seawater at reduced light) and are therefore lower than those reported in aforementioned studies. However, when our DOC release rates are normalized to DW (6.0 and 21.2 μmol C g−1 h−1 of the natural seawater treatment with reduced and full light, respectively), they are comparable to the rates reported by Mueller, et al.41 from Curaçao (8.5 μmol C g−1 h−1). This may indicate that the turf algal biomasses per m2 in our experiments were lower than in previous studies. The release of photosynthetically fixed carbon as DOC is related to the level of primary productivity38,40,73. Thus, DOC release is commonly expressed independent of biomass or surface area, but as a percentage of primary production. It can be assumed that 6 and 12% of the photosynthetically fixed carbon was released as DOC at reduced and full light, respectively, based on a balanced molar ratio of carbon fixation to net oxygen production in the natural seawater treatments. These percentages are within the range of reported values of coral reef benthic primary producers in previous studies: between 436 and 51%74.
Net oxygen production increased with increasing light availability independent of nutrient conditions (Fig. 1B). However, in the enriched seawater treatments the net oxygen production of turf algae was lower than in the natural seawater treatments at comparable light intensities. Based on the dynamic energy budget75, this difference in net oxygen release could be explained by increased respiration under nutrient replete conditions to provide energy for the enhanced synthesis of cell compounds (e.g.76) (Fig. 2). Moreover, the high addition of DIN in the enriched seawater treatment is likely to have caused an imbalance in the relative abundance of C and N within the turf algae (lowered the C:N ratio). To compensate, electrons could have been donated to oxygen forming oxygen radicals , and thereafter water in the Mehler reaction77. This so-called oxygen-photoreduction would thus result in a lower net oxygen production. Both mechanisms imply that the DOC release in the enriched seawater treatments expressed as a percentage of net oxygen production (reduced light: 82%; full light: 15%) might be an overestimation and should therefore be considered with caution.
With this study we provide evidence that light and nutrient availability simultaneously and interactively affected the release of DOC in turf algae. The nature of the light-nutrient interaction and its influence on underlying DOC release mechanisms, which were not addressed in this study, remain to be investigated.
Additional Information
How to cite this article: Mueller, B. et al. Effect of light and nutrient availability on the release of dissolved organic carbon (DOC) by Caribbean turf algae. Sci. Rep. 6, 23248; doi: 10.1038/srep23248 (2016).
Supplementary Material
Acknowledgments
We thank the staff of Carmabi for their hospitality and logistic support during the field work. We are grateful to S. Gonzalez for his contribution to the DOC analysis. We further thank J. van Ooijen, K. Bakker and S. Crawford for the nutrient analysis. The research leading to these results has received funding from the European Union Seventh Framework Programme (P7/2007–2013) under grant agreement no. 244161 (Future of Reefs in a Changing Environment).
Footnotes
Author Contributions All authors conceived the experiment, analyzed the results and reviewed the manuscript. B.M. and J.d.H. conducted the experiment. B.M. wrote the manuscript.
References
- Wild C., Haas A., Naumann M., Mayr C. & el-Zidbah M. Phase shifts in coral reefs - comparative investigation of corals and benthic algae as ecosystem engineers. Proc 11th Int Coral Reef Symp. Ft. Lauderdale pp. 1319–1323 (2008). [Google Scholar]
- Rohwer F. & Youle M. Coral reefs in the microbial seas (Plaid Press, 2010). [Google Scholar]
- Barott K. L. & Rohwer F. L. Unseen players shape benthic competition on coral reefs. Trends Microbiol. 20, 621–628, doi: 10.1016/j.tim.2012.08.004 (2012). [DOI] [PubMed] [Google Scholar]
- Mueller B. et al. Natural diet of coral-excavating sponges consists mainly of dissolved organic carbon (DOC). PloS one 9, e90152, doi: 10.1371/journal.pone.0090152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torréton J., Pagès J., Dufour P. & Cauwet G. Bacterioplankton carbon growth yield and DOC turnover in some coral reef lagoons. In Proc 8th Int Coral Reef Symp. 947–952 (1997). [Google Scholar]
- Van Duyl F. C. & Gast G. J. Linkage of small-scale spatial variations in DOC, inorganic nutrients and bacterioplankton growth with different coral reef water types. Aquat. Microb. Ecol. 24, 17–26, doi: 10.3354/ame024017 (2001). [DOI] [Google Scholar]
- Haas A. F. et al. Effects of coral reef benthic primary producers on dissolved organic carbon and microbial activity. PloS one 6, e27973, doi: 10.1371/journal.pone.0027973 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocke H. J. et al. High dissolved organic carbon release by benthic cyanobacterial mats in a Caribbean reef ecosystem. Scientific reports 5, 8852, doi: 10.1038/srep08852 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson M. M. & Ward D. M. Photoexcretion and fate of glycolate in a hot spring cyanobacterial mat. Appl. Environ. Microbiol. 54, 1738–1743 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A. F. et al. Influence of coral and algal exudates on microbially mediated reef metabolism. PeerJ 1, e108, doi: 10.7717/peerj.108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A., Jantzen C., Naumann M., Iglesias-Prieto R. & Wild C. Organic matter release by the dominant primary producers in a Caribbean reef lagoon: implication for in situ O2 availability. Mar. Ecol. Prog. Ser. 409, 27–39, doi: 10.3354/meps08631 (2010). [DOI] [Google Scholar]
- Dinsdale E. A. et al. Microbial ecology of four coral atolls in the northern Line Islands. PloS one 3, e1584, doi: 10.1371/journal.pone.0001584 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. E. et al. Coral and macroalgal exudates vary in neutral sugar composition and differentially enrich reef bacterioplankton lineages. Isme j 7, 962–979, doi: 10.1038/ismej.2012.161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild C., Niggl W., Naumann M. & Haas A. Organic matter release by Red Sea coral reef organisms - potential effects on microbial activity and in situ O2 availability. Mar. Ecol. Prog. Ser. 411, 61–71, doi: 10.3354/meps08653 (2010). [DOI] [Google Scholar]
- Wangpraseurt D. et al. In situ oxygen dynamics in coral-algal interactions. PloS one 7, e31192, doi: 10.1371/journal.pone.0031192 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg A. K. et al. Biological oxygen demand optode analysis of coral reef-associated microbial communities exposed to algal exudates. PeerJ 1, e107, doi: 10.7717/peerj.107 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A. F. et al. Visualization of oxygen distribution patterns caused by coral and algae. PeerJ 1, e106, doi: 10.7717/peerj.106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. E. et al. Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecol. Lett. 9, 835–845, doi: 10.1111/j.1461-0248.2006.00937.x (2006). [DOI] [PubMed] [Google Scholar]
- Kuntz N. M., Kline D. I., Sandin S. A. & Rohwer F. Pathologies and mortality rates caused by organic carbon and nutrient stressors in three Caribbean coral species. Mar. Ecol. Prog. Ser. 294, 173–180, doi: 10.3354/meps294173 (2005). [DOI] [Google Scholar]
- Kline D. I., Kuntz N. M., Breitbart M., Knowlton N. & Rohwer F. Role of elevated organic carbon levels and microbial activity in coral mortality. Mar. Ecol. Prog. Ser. 314, 119–125, doi: 10.3354/meps314119 (2006). [DOI] [Google Scholar]
- Barott K. L. et al. Natural history of coral-algae competition across a gradient of human activity in the Line Islands. Mar. Ecol. Prog. Ser. 460, 1–12, doi: 10.3354/meps09874 (2012). [DOI] [Google Scholar]
- De Goeij J. M. et al. Surviving in a marine desert: The sponge loop retains resources within coral reefs. Science 342, 108–110, doi: 10.1126/science.1241981 (2013). [DOI] [PubMed] [Google Scholar]
- Kramer P. A. Synthesis of coral reef health indicators for the western Atlantic: results of the AGRRA program (1997–2000). Atoll. Res. Bull. 496, 1–55 (2003). [Google Scholar]
- Barott K. et al. Hyperspectral and physiological analyses of coral-algal interactions. PloS one 4, e8043, doi: 10.1371/journal.pone.0008043 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeij M. J. et al. The effects of nutrient enrichment and herbivore abundance on the ability of turf algae to overgrow coral in the Caribbean. PloS one 5, e14312, doi: 10.1371/journal.pone.0014312 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steneck R. & Dethier M. A functional group approach to the structure of algaldominated communities. Oikos 69, 476–498 (1994). [Google Scholar]
- Fricke A., Teichberg M., Beilfuss S. & Bischof K. Succession patterns in algal turf vegetation on a Caribbean coral reef Bot. Mar. 54, 111–126 (2011). [Google Scholar]
- Barott K. L. et al. Microbial diversity associated with four functional groups of benthic reef algae and the reef-building coral Montastraea annularis. Environ. Microbiol. 13, 1192–1204, doi: 10.1111/j.1462-2920.2010.02419.x (2011). [DOI] [PubMed] [Google Scholar]
- Littler M. M., Littler D. S. & Brooks B. L. Harmful algae on tropical coral reefs: Bottom-up eutrophication and top-down herbivory. Harmful Algae 5, 565–585, doi: 10.1016/j.hal.2005.11.003 (2006). [DOI] [Google Scholar]
- Den Haan J. Effects of nutrient enrichment on the primary producers of a degraded coral reef, University of Amsterdam, p 129 (2015). [Google Scholar]
- Charpy L. et al. Dinitrogen-fixing cyanobacteria in microbial mats of two shallow coral reef ecosystems. Microb. Ecol. 59, 174–186, doi: 10.1007/s00248-009-9576-y (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Haan J. et al. Nitrogen fixation rates in algal turf communities of a degraded versus less degraded coral reef. Coral Reefs 33, 1003–1015, doi: 10.1007/s00338-014-1207-5 (2014). [DOI] [Google Scholar]
- Littler M. & Littler D. In Progress in phycological research (eds Round F. E. & Chapman D. J.) 815–825 (Biopress, 1984). [Google Scholar]
- Adey W. H. & Goertemiller T. Coral reef algal turfs: master producers in nutrient poor seas. Phycologia 26, 374–386, doi: 10.2216/i0031-8884-26-3-374.1 (1987). [DOI] [Google Scholar]
- Hatcher B. G. Coral reef primary productivity: A beggar’s banquet. Trends Ecol. Evol. 3, 106–111, doi: 10.1016/0169-5347(88)90117-6 (1988). [DOI] [PubMed] [Google Scholar]
- Brylinsky M. Release of dissolved organic matter by some marine macrophytes. Mar. Biol. 39, 213–220, doi: 10.1007/BF00390995 (1977). [DOI] [Google Scholar]
- Fogg G. E. The ecological significance of extracellular products of phytoplankton photosynthesis Bot. Mar. 26, 3–14 (1983). [Google Scholar]
- Cherrier J., Valentine S., Hamill B., Jeffrey W. H. & Marra J. F. Light-mediated release of dissolved organic carbon by phytoplankton. J. Mar. Syst. 147, 45–51, doi: http://dx.doi.org/10.1016/j.jmarsys.2014.02.008 (2014). [Google Scholar]
- Wood A. & Van Valen L. Paradox lost? On the release of energy rich compounds by phytoplankton. Mar Microb Food Webs 4, 103–116 (1990). [Google Scholar]
- Zlotnik I. & Dubinsky Z. The effect of light and temperature on DOC excretion by phytoplankton. Limnol. Oceanogr. 34, 831–839, doi: 10.4319/lo.1989.34.5.0831 (1989). [DOI] [Google Scholar]
- Mueller B. et al. Effect of light availability on dissolved organic carbon release by Caribbean reef algae and corals. Bull. Mar. Sci. 90, 875–893, doi: 10.5343/bms.2013.1062 (2014). [DOI] [Google Scholar]
- Bjørnsen P. K. Phytoplankton exudation of organic matter: Why do healthy cells do it? Limnol Oceanogr. 33, 151–154 (1988). [Google Scholar]
- Marañón E., Cermeño P., Fernández E., Rodríguez J. & Zabala L. Significance and mechanisms of photosynthetic production of dissolved organic carbon in a coastal eutrophic ecosystem. Limnol. Oceanogr. 49, 1652–1666, doi: 10.4319/lo.2004.49.5.1652 (2004). [DOI] [Google Scholar]
- Marañón E., Cermeño P. & Pér V. Continuity in the photosynthetic production of dissolved organic carbon from eutrophic to oligotrophic waters. Mar. Ecol. Prog. Ser. 299, 7–17, doi: 10.3354/meps299007 (2005). [DOI] [Google Scholar]
- Sorrell B. K., Hawes I., Schwarz A.-M. & Sutherland D. Inter-specific differences in photosynthetic carbon uptake, photosynthate partitioning and extracellular organic carbon release by deep-water characean algae. Freshwat. Biol. 46, 453–464, doi: 10.1046/j.1365-2427.2001.00686.x (2001). [DOI] [Google Scholar]
- Carlson C. Production and removal processes. In Biogeochemistry of marine dissolved organic matter (eds Hansell D. A. & Carlson C. A.) 91–151 (Elsevier Science, 2002). [Google Scholar]
- Borchard C. & Engel A. Size-fractionated dissolved primary production and carbohydrate composition of the coccolithophore Emiliania huxley. Biogeosciences 12, 1271–1284, doi: 10.5194/bg-12-1271-2015 (2015). [DOI] [Google Scholar]
- Nyström M., Folke C. & Moberg F. Coral reef disturbance and resilience in a human-dominated environment. Trends Ecol. Evol. 15, 413–417, doi: http://dx.doi.org/10.1016/S0169-5347(00)01948-0 (2000). [DOI] [PubMed] [Google Scholar]
- Fabricius K. E. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar. Pollut. Bull. 50, 125–146, doi: http://dx.doi.org/10.1016/j.marpolbul.2004.11.028 (2005). [DOI] [PubMed] [Google Scholar]
- Van Duyl F. C. Atlas of the living reefs of Curaçao and Bonaire (Netherlands Antilles). Vol. 117 (1985).
- Den Haan J. et al. Fast detection of nutrient limitation in macroalgae and seagrass with nutrient-induced fluorescence. PloS one 8, e68834, doi: 10.1371/journal.pone.0068834 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasshoff K., Kremling K. & Manfred E. Methods of Seawater Analysis 1–600 (Wiley-VCH Verlag GmbH, 1983). [Google Scholar]
- Helder W. & De Vries R. T. P. An automatic phenol-hypochlorite method for the determination of ammonia in sea- and brackish waters. Netherlands Journal of Sea Research 13, 154–160, doi: http://dx.doi.org/10.1016/0077-7579(79)90038-3 (1979). [Google Scholar]
- Murphy J. & Riley J. P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 27, 31–36, doi: http://dx.doi.org/10.1016/S0003-2670(00)88444-5 (1962). [Google Scholar]
- Haas A. F. et al. Organic matter release by coral reef associated benthic algae in the Northern Red Sea. J. Exp. Mar. Biol. Ecol. 389, 53–60, doi: http://dx.doi.org/10.1016/j.jembe.2010.03.018 (2010). [Google Scholar]
- Naumann M. S. et al. Organic matter release by dominant hermatypic corals of the Northern Red Sea. Coral Reefs 29, 649–659, doi: 10.1007/s00338-010-0612-7 (2010). [DOI] [Google Scholar]
- Zuur A. F., Ieno E. N. & Elphick C. S. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1, 3–14, doi: 10.1111/j.2041-210X.2009.00001.x (2010). [DOI] [Google Scholar]
- Anthony K. R. N., A. Kleypas J. & Gattuso J.-P. Coral reefs modify their seawater carbon chemistry – implications for impacts of ocean acidification. Global Change Biol. 17, 3655–3666, doi: 10.1111/j.1365-2486.2011.02510.x (2011). [DOI] [Google Scholar]
- Kleypas J. A., Anthony K. R. N. & Gattuso J.-P. Coral reefs modify their seawater carbon chemistry – case study from a barrier reef (Moorea, French Polynesia). Global Change Biol. 17, 3667–3678, doi: 10.1111/j.1365-2486.2011.02530.x (2011). [DOI] [Google Scholar]
- Barron C., Apostolaki E. T. & Duarte C. M. Dissolved organic carbon fluxes by seagrass meadows and macroalgal beds. Frontiers in Marine Science 1, doi: 10.3389/fmars.2014.00042 (2014). [DOI] [Google Scholar]
- Konopka A. & Schnur M. Biochemicl composition and photosynthetic carbon metabolism of nutrient limited cultures of Merimopedia tenuissima (Cyanophyceae). J. Phycol. 17, 118–122, doi: 10.1111/j.1529-8817.1981.tb00829.x (1981). [DOI] [Google Scholar]
- Hama T. & Yanagi K. Production and neutral aldose composition of dissolved carbohydrates excreted by natural marine phytoplankton populations. Limnol. Oceanogr. 46, 1945–1955, doi: 10.4319/lo.2001.46.8.1945 (2001). [DOI] [Google Scholar]
- Fogg G. E. Photosynthesis and formation of fats in a diatom. Ann. Bot. 20, 265–285 (1956). [Google Scholar]
- Mague T. H., Friberg E., Hughes D. J. & Morris I. Extracellular release of carbon by marine phytoplankton; a physiological approach1. Limnol. Oceanogr. 25, 262–279, doi: 10.4319/lo.1980.25.2.0262 (1980). [DOI] [Google Scholar]
- Verity P. Effects of temperature, irradiance, and day length on the marine diatom Leptocylindrus danicus Qeve II. Photosynthesis and cellular composition. J. Exp. Mar. Biol. Ecol. 55, 79–91 (1981). [Google Scholar]
- Williams S. L. & Carpenter R. C. Grazing effects on nitrogen fixation in coral reef algal turfs. Mar. Biol. 130, 223–231, doi: 10.1007/s002270050242 (1997). [DOI] [Google Scholar]
- Williams S. L. & Carpenter R. C. Effects of unidirectional and oscillatory water flow on nitrogen fixation (acetylene reduction) in coral reef algal turfs, Kaneohe Bay, Hawaii. J. Exp. Mar. Biol. Ecol. 226, 293–316, doi: http://dx.doi.org/10.1016/S0022-0981(97)00252-9 (1998). [Google Scholar]
- Charpy L., Palinska K. A., Abed R. M. M., Langlade M. J. & Golubic S. Factors influencing microbial mat composition, distribution and dinitrogen fixation in three western Indian Ocean coral reefs. Eur. J. Phycol. 47, 51–66, doi: 10.1080/09670262.2011.653652 (2012). [DOI] [Google Scholar]
- Zehr J. P. Nitrogen fixation by marine cyanobacteria. Trends Microbiol. 19, 162–173, doi: 10.1016/j.tim.2010.12.004 (2011). [DOI] [PubMed] [Google Scholar]
- Wyatt K. H., Tellez E., Woodke R. L., Bidner R. J. & Davison I. R. Effects of nutrient limitation on the release and use of dissolved organic carbon from benthic algae in Lake Michigan. Freshwater Science 33, 557–567, doi: 10.1086/675453 (2014). [DOI] [Google Scholar]
- Bryant D. G., Burke L., McManus J. & Spalding M. Reefs at Risk: a Map-based Indicator of Threats to the World’s Coral Reefs World Resources Institute (1998). [Google Scholar]
- Selman M., Greenhalgh S., Diaz R. & Sugg Z. Eutrophication and hypoxia in coastal areas: a global assessment of the state of knowledge (World Resources Institute, 2008). [Google Scholar]
- Baines S. B. & Pace M. L. The production of dissolved organic matter by phytoplankton and its importance to bacteria: Patterns across marine and freshwater systems. Limnol. Oceanogr. 36, 1078–1090, doi: 10.4319/lo.1991.36.6.1078 (1991). [DOI] [Google Scholar]
- Davies P. S. The role of zooxanthellae in the nutritional energy requirements of Pocillopora eydouxi. Coral Reefs 2, 181–186, doi: 10.1007/BF00263571 (1984). [DOI] [Google Scholar]
- Kooijman S. A. L. M. The dynamic energy budget theory for metabolic organization P 66 (Cambridge University Press, 2000). [Google Scholar]
- Baretta-Bekker J. G., Baretta J. W. & Ebenhöh W. Microbial dynamics in the marine ecosystem model ERSEM II with decoupled carbon assimilation and nutrient uptake. J. Sea Res. 38, 195–211, doi: http://dx.doi.org/10.1016/S1385-1101(97)00052-X (1997). [Google Scholar]
- Helman Y., Barkan E., Eisenstadt D., Luz B. & Kaplan A. Fractionation of the three stable oxygen isotopes by oxygen-producing and oxygen-consuming reactions in photosynthetic organisms. Plant Physiol. 138, 2292–2298, doi: 10.1104/pp.105.063768 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.