Abstract
Background
Mirror neurons have been localized in several locations, including the inferior parietal lobule (IPL). Increase of EEG alpha3/alpha2 frequency power ratio has been detected in mild cognitive impairment (MCI) subjects who will convert in Alzheimer's disease (AD). We investigated the association of alpha3/alpha2 frequency power ratio with cortical thickness in IPL in MCI subjects.
Methods
74 adult subjects with MCI underwent EEG recording and high resolution MRI. Alpha3/alpha2 frequency power ratio as well as cortical thickness were computed for each subject. Three MCI groups were obtained according to increasing tertile values of alpha3/alpha2 ratio. Difference of cortical thickness among the groups was estimated.
Results
Higher alpha3/alpha2 frequency power ratio group had wider cortical thinning than other groups, mapped on the IPL, supramarginal gyrus and precuneus bilaterally.
Conclusions
High EEG alpha3/alpha2 frequency power ratio was associated with atrophy of IPL areas in MCI subjects.
General significance
The scientific hypothesis is divided into the following main points: 1) the theoretical background considering two recent theories, an evolutionary perspective theory and the theory of mind (ToM), which both track a possible relationship between prodromal AD and mirror system; 2) the relationship has been focused on the prodromal stage of Alzheimer's disease, that is a peculiar and very debated phase of the disease itself; and 3) not a generical relationship, but a focused anatomo-functional association has been proposed.
Highlights
-
•
Mirror neurons have been localized in several locations, including the inferior parietal lobule (IPL)
-
•
Subjects with MCI with higher alpha3/alpha2 EEG frequency power ratio are more prone to develop AD
-
•
Subjects with MCI with higher alpha3/alpha2 EEG ratio had wider cortical thinning mapped on the IPL bilaterally
-
•
a possible relationship between prodromal AD and mirror system could be hypothesized
1. Introduction
“Mirror neurons” were first reported in the premotor cortex of macaque monkeys [1]. These neurons fire, both not only when a monkey performs a specific action, but also when the monkey simply watches another monkey carrying out the same action. This was the first description of a neural mechanism that allowed a “direct matching between the visual description of an action and its execution” [2]. Remarkably, it is now recognized that mirror neurons in humans do not function in isolation, but are rather part of a more extensive network involving other structures, including inferior parietal lobule (IPL) [3], [4]. These areas in the human brain appear to play a role in the imitation of action providing somatosensory information associated with the observed and to-be-executed action, a peculiar activity of mirror neurons. The IPL, even more than the rest of the cortex, underwent an accelerated enlargement in the phylogenetic line, leading to the great apes and hominids, splitting into the supramarginal gyrus (SG) and the angular gyrus (AG) [5], [6].
The possible link between the mirror system and AD has been suggested by two recent theories based on, respectively, an evolutionary perspective and on theory of mind (ToM). The first theory has linked the dysfunction of the mirror system with neurodegenerative diseases. In particular, in AD, the disruption of the mirror system would lead to the loss of a proper functioning of the hippocampus as regards the sampling of explicit memory episodes. This loss of function occurs through the alteration of the hippocampal link with associative secondary cortical and paralimbic areas processing explicit cognition and memory and leads to explicit amnesic syndrome as well as alterations of explicit hand and eye-derived treatment of information like as ideomotor apraxia, visuospatial deficit, agnosia, transcortical aphasia [7]. As regards the second theory, a recent research has studied patients with amnestic mild cognitive impairment (aMCI), or prodromal AD, performing a task strictly correlated with the activation of the mirror neurons system like the Reading the Mind in the Eyes test (RME). During the execution of this task which attributes mental states by focusing on eye-gaze, prodromal AD patients were found to have worse performances in two second order false belief exercises, confirming the decay of ToM on the behavioral and cognitive level. Moreover, the fMRI scans show a relative preservation of the anterior part of the mirror system, located in precentral gyrus (BA 6) and Broca area (BA 44) [8]. These results could suggest a major involvement of the posterior part of the mirror neurons network, located in IPL, in the cognitive decline in subjects with prodromal AD. The role of the “uncoupling” of the mirror system in neurological diseases has been also suggested by a recent EEG study [9]. EEG activity and readiness potential (RP) were observed from individuals with selective lesions in the inferior parietal lobe (IPL) when exposed to a video showing a person grasping a colored object. Specifically, three groups were compared: parietal and ventral premotor cortex-lesioned patients and neurologically healthy subjects. The results demonstrated that neurologically healthy individuals and premotor patients exhibit a significant RP prior to the observed action, although no such RP is seen in patients with parietal lesions. The findings also showed that parietal cortex damage changes the ability to regulate the early planning phases and mirroring process.
Noteworthy, the inferior parietal cortex (IPC), including the intraparietal sulcus (IPS), AG, and SG, plays an important role in episodic memory, and is considered to be one of the specific neuroimaging markers in predicting the conversion of mild cognitive impairment (MCI) to Alzheimer's disease (AD). With functional magnetic resonance imaging (fMRI) at the resting state, SG displayed decreased connectivity with different regions including the frontal and parietal cortical areas [10]. Increasing thinning of temporo-parietal brain areas is peculiar of AD and it could predict conversion from MCI state to AD dementia [10]. Moreover, a positron emission tomography (PET) study has demonstrated that hypoperfusion in SG and AG could differentiate AD from fronto-temporal dementia (FTD) even in the prodromal state [10]. It is widely accepted that the cerebral EEG rhythms reflect the underlying brain network activity [11]. In turn, modifications in EEG rhythms could be an early sign of disease in preclinical stage associated with AD-related structural and functional networks alterations. Furthermore, a specific EEG marker, namely the increase in alpha3/alpha2 frequency power ratio, has been demonstrated predictive of conversion of patients with mild cognitive impairment (MCI) in AD, but not in non-AD dementia [11]. As a consequence, the working hypothesis of the present study is that an increase in alpha3/alpha2 EEG frequency power ratio would like to be associated with brain atrophy in temporo-parietal brain networks, in particular with the IPL. If the case, a link between disrupture of mirror system network and AD could be hypothesized.
Results show that subjects with higher a3/a2 when compared to subjects with lower and middle a3/a2 frequency power ratio showed significant and wide thinning of both global cortical volume and IPL specific brain areas, like the supramarginal gyrus and precuneus bilaterally. Results were discussed in the view of the possible disruption of the mirror neuron system in prodromal AD.
In our knowledge, this is the first study linking the resting EEG to the mirror system in prodromal AD and proposing the uncoupling of the mirror system as a possible explanation of the relationship between structural alterations and symptoms in patients with prodromal AD. The novelty of the study is to propose a different interpretation of the cognitive deficits observed in Alzheimer's disease, shedding new light on a disease of great complexity. Many studies have previously addressed the correlation between the EEG findings with atrophies or metabolic and functional alterations in other brain regions in patients with mild cognitive impairment, but it was beyond the scope of this study which is only focused on the possible relationship between uncoupling of mirror system and prodromal AD.
2. Materials and methods
2.1. Subjects
74 patients were selected from a prospective study on the natural history of cognitive impairment (the translational outpatient memory clinic — TOMC study) carried out in the outpatient facility and memory clinic of the National Institute for the Research and Care of Alzheimer's Disease (IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy), aimed to study the natural history of persons without dementia with apparently primary cognitive deficits, i.e. not due to psychic or physical conditions, in the absence of functional impairment. A group of 40 elderly subjects, cognitively non-impaired, has been chosen among spouses as a control group. All experimental protocols had been approved by the Local Ethics Committee. Informed consent was obtained from all participants or their caregivers, according to the Code of Ethics of the World Medical Association (Declaration of Helsinki).
2.2. Diagnostic criteria
The selection criteria have the aim to include as much as possible primary prodromal AD. Demographic and cognitive features of the subjects in study are summarized in Table 1.
Table 1.
Demographic and cognitive characteristics in the MCI and normal control groups. Numbers denote mean ± standard deviation, p denotes significance on ANOVA (MMSE, mini mental state examination; WMHs, white matter hyperintensities).
| Demographic and clinical futures |
|||
|---|---|---|---|
| Healthy Subjects | MCI | P | |
| Number of subjects | 74 | 40 | – |
| Age | 68.5 ± 6.3 | 69.7 ± 5.4 | 0.53 |
| Sex, female | 17% | 19% | 0.52 |
| Education | 7.9 ± 4.3 | 7.4 ± 3.6 | 0.32 |
| MMSE | 27.4 ± 5.9 | 27.1 ± 4.7 | 0.56 |
| WMHs (mm3) | 3.34 ± 4.72 | 3.64 ± 5.91 | 0.12 |
| Alpha3/alpha2 | 0.3 ± 5.4 | 1.09 ± 7.9 | 0.005 |
The Mini-Mental State Examination (MMSE), the Clinical Dementia Rating Scale (CDRS), the Hachinski Ischemic Scale (HIS) and the Instrumental and Basic Activities of Daily Living (IADL, BADL) have been used to evaluate the patients [12], [13], [14], [15]. Furthermore, magnetic resonance imaging, (MRI) and laboratory testing were performed to rule out cognitive impairment due to non-degenerative origins. Inclusion criteria, based on previous studies [16], [17], [18], were all of the following: (i) memory or other cognitive disturbances reported by the patient, by a relative or by the general practitioner; (ii) MMSE score of 24–27/30, or MMSE of 28/30 and higher associated to low performance (score of 2–6 or higher) on the clock drawing test; and (iii) no evidence of functional impairment in instrumental and basic activities of daily living. Exclusion criteria were: (i) patients aged 90 years and older (no minimum age to participate in the study); (ii) psychiatric diseases history (from mild to moderate or major depression; juvenile-onset psychosis); (iii) present or anamnestic neurological signs of major stroke; (iv) epilepsy, drug addiction, alcohol dependence; (v) use of any psychotropic drugs, that could enhance the brain cognitive functions or bias the EEG activity (including acetylcholinesterase inhibitors); and (vi) uncontrolled systemic diseases (including diabetes mellitus); and (vii) traumatic brain injuries. All subjects were right-handed. A geriatrician or neurologist performed: (i) semi-structured interview with the relatives (usually, the patient's spouse or a child of the patient) or the patients themselves; (ii) physical and neurological examinations; and (iii) tests of, gait, balance and physical function. A skilled neuropsychologist performed full neuropsychological evaluation assessing: verbal and non-verbal memory, attention and executive functions, abstract reasoning thinking, frontal functions, language, apraxia and visuo-constructional abilities [19]. The presence and severity of depressive syndrome were also assessed [20]. All the neuropsychological tests were standardized on Italian population, thus scores were corrected according age, education and gender normative values of an Italian population of healthy people.
2.3. EEG recordings
Electrodes set in an elastic cap (Electro-Cap International, Inc.), positioned according to the 10–20 international systems, was used to record the EEG activity from 19 sites. Fz was the site for the ground electrode whereas the left and right mastoids were used as reference for all electrodes. All recordings were obtained in the morning with subjects resting comfortably. The level of vigilance was maintained constant by an operator who checked on-line both the subject and the EEG traces. The subject was alerted if there were signs of behavioral and/or EEG drowsiness. Off-line re-reference of the scalp recordings to the common average was done prior to perform the detection and analysis of the EEG artifacts. A band-pass filter of 0.3–70 Hz was used to record EEG data, and a sampling rate of 250 Hz was used for digitizing (BrainAmp, BrainProducts, Germany). Impedance of the electrodes was set below 5 kHz. The electrooculogram (EOG) was recorded to detect horizontal and vertical eye movements. The EEG recording lasted 5 min, with subjects resting with closed eyes. The variability of the data would be reduced with longer recordings. Anyway, the lengthening of the recordings would also have increased the possibility to cause the slowing of EEG oscillations due to reduced vigilance and arousal. EEG data were fragmented off-line in consecutive epochs of 2 s, with a frequency resolution of 0.5 Hz and then analyzed. The epochs analyzed were, on average, 140 ranging from 130 to 150. At first, a computerized automatic procedure preliminary identified the EEG epochs with ocular, muscular and other types of artifact [21], [22], [23], [24], [25], [26], [27], [28], [29]. Afterwards, the automatic selection was manually confirmed by two expert electroencephalographists. The epochs with ocular, muscular and other types of artifacts were no longer considered.
2.4. Analysis of individual frequency bands
The power density of EEG rhythms with a 0.5 Hz frequency resolution – ranging from 2 to 45 Hz – was computed by a digital power spectrum analysis based on the Fourier Fast Transform (FFT; Welch technique, Hanning windowing function, no phase shift). Power spectra were averaged across all recording electrodes and the theta/alpha transition frequency (TF) and the individual alpha frequency (IAF) peak were selected as anchor frequencies, according to the literature guidelines [30], [31]. Since our EEG recordings were performed at rest, the TF, representing an estimate of the frequency at which the theta and alpha spectra intersect, was computed as the minimum power value in the alpha frequency range. On turn, the IAF frequency power represents the power value of higher peak within the extended alpha range (6–14 Hz). Based on TF and IAF, the frequency band range for each subject was estimated as follows: delta from TF-4 to TF- 2, theta from TF-2 to TF, low alpha band (alpha1 and alpha2) from TF to IAF, and high alpha band (or alpha3) from IAF to IAF + 2. The alpha1 and alpha2 bands were computed for each subject as follows: alpha1 from TF to the middle point of the TF-IAF range, and alpha2 from such middle point to the IAF peak [21], [22], [23], [24], [25], [26], [27], [28], [29]. The mean frequency range computed in the whole group of MCI subjects is: delta 2.9–4.9 Hz; theta 4.9–6.9 Hz; alpha1 6.9–8.9 Hz; alpha2 8.9–10.9 Hz; alpha3 10.9–12.9 Hz. Finally, the relative power spectra were computed for each subject in the individual-determined frequency bands. The ratio between the absolute power and the mean power spectra from 2 to 45 Hz engenders the relative power density for each frequency band. In particular, the relative band power at each band was defined as the mean of the relative band power for each frequency bin within that band. Increasing tertile values of alpha3/alpha2 frequency power ratio were estimated in all subjects and 3 groups were thus formed: low tertile (a3/a2 < 1) middle tertile (1 < a3/a2 < 1.17) and high tertile (a3/a2 > 1.17). The three groups of MCI has been demonstrated in previous studies to be different in nature. In particular, the higher alpha3/alpha 2 EEG frequency power ratio MCI group is at major risk to convert to Alzheimer's disease, as well as to have different patterns of hippocampal atrophy as compared to the other alpha3/alpha2 frequency power ratio MCI groups [23], [32]. Although these previous results have been obtained at a group level, given the extensive initial assessment and the prolonged follow-up, we are quite confident that the MCI patients individuated with higher alpha3/alpha 2 EEG frequency power ratio belong to prodoromal AD group. Moreover, this group subdivision has been chosen for reason of homogeneity and comparability with the previous studies.
2.5. MRI scans
For each subject, a high-resolution sagittal T1 weighted volumetric MR scan was acquired at the Neuroradiology Unit of the ‘Citta` di Brescia’ Hospital, Brescia, by using a 1.0 T Philips Gyroscan scanner, with a gradient echo 3D technique: TR = 20 ms, TE = 5 ms, flip angle = 30, field of view = 220 mm, acquisition matrix 256 · 256, and slice thickness 1.3 mm.
2.6. Cortical thickness estimation steps
Cortical thickness measurements for 74 MCI patients were made using a fully automated magnetic resonance imaging-based analysis technique: FreeSurfer, a set of software tools for the study of cortical and subcortical anatomy. Briefly, in the cortical surface stream, the models of the boundary between white matter and cortical gray matter as well as the pial surface were constructed. Once these surfaces are known, an array of anatomical measures becomes possible, including: cortical thickness, surface area, curvature, and surface normal at each point on the cortex. In addition, a cortical surface-based atlas has been defined based on average folding patterns mapped to a sphere and surfaces from individuals can be aligned with this atlas with a high-dimensional nonlinear registration algorithm. The surface-based pipeline consists of several stages previous described in [33], [34].
2.6.1. Single subject analysis
For each subject the T1-weighted, anatomical 3-D MRI dataset was converted from Dicom format into .mgz format, then intensity variations are corrected and a normalized intensity image is created. The volume is registered with the Talairach atlas through an affine registration. Next, the skull is stripped using a deformable template model [35] and extracerebral voxels are removed. The intensity normalized, skull-stripped image is then operated on by a segmentation procedure based on the geometric structure of the gray–white interface. Voxels are classified as white or gray matter, cutting planes are chosen to separate the hemispheres from each other. A white matter surface is then generated for each hemisphere by tiling the outside of the white matter mass for that hemisphere. This initial surface is then refined to follow the intensity gradients between the white and gray matter. The white surface is then nudged to follow the intensity gradients between the gray matter and CSF, obtaining the pial surface. Cortical thickness measurements were obtained by calculating the distance between those surfaces (white and pial surface) at each of approximately 160,000 points per hemisphere across the cortical mantle [36], [37], [38], [39].
2.6.2. Group analysis
In order to relate and compare anatomical features across subjects, it is necessary to establish a mapping that specifies a unique correspondence between each location in one brain and the corresponding location in another. Thus, the pial surface of an individual subject is inflated to determine the large-scale folding patterns of the cortex and subsequently transformed into a sphere to minimize metric distortion. The folding patterns of the individual are then aligned with an average folding pattern using a high-resolution surface-based averaging. Thickness measures were mapped to the inflated surface of each participant's brain reconstruction allowing visualization of data across the entire cortical surface. Finally, cortical thickness was smoothed with a 20-mm full width at half height Gaussian kernel to reduce local variations in the measurements for further analysis.
2.7. Statistical analysis
Differences between groups in sociodemographic and neuropsychological features were analyzed using SPSS version 13.0 (SPSS, Chicago, IL) performing an analysis of variance (ANOVA) for continuous variables and paired χ2 test for dichotomous variables. For continuous variables, post-hoc pairwise comparisons among groups were performed with the Games–Howell or Bonferroni tests depending on homogeneity of variance tested with Levene's test.
Concerning the neuroimaging analysis, the Qdec interface in Freesurfer software was used: a vertex-by-vertex analysis was carried out performing a general linear model to analyze whether any difference in mean cortical thickness existed between groups (low: a3/a2 < 1 μV2; middle: 1 < a3/a2 < 1.17 μV2; high: a3/a2 > 1.17 μV2). The following comparisons were carried out: High versus Low, High vs Middle and Middle vs. Low. Age, sex, education, global cognitive level (MMSE score) and white matter hyperintensity (WMH) computation were introduced as covariates in the analysis to avoid confounding factors. Our results did not survive at p < 0.05 corrected, so we choose to apply an uncorrected but more restrictive significance threshold than 0.05 (p < .001) and we considered as significant only the clusters which also were wide equal or major to 30 mm2. Finally, a surface map has been generated to display the results on an average brain. For illustrative purpose significance was set to a p-value of < 0.01 uncorrected for multiple comparisons.
3. Results
Table 1 shows the sociodemographic and neuropsychological characteristics of MCI group as a whole compared with the matched healthy controls. The ANOVA analysis showed that there were not statistically significant differences between groups which resulted well paired for age, sex, WMH burden, education and global cognitive level. The only significant difference in ANOVA analysis was detected in the alpha3/alpha2 frequency power ratio (p = 0005). Table 2 shows the sociodemographic and neuropsychological characteristics of MCI subgroups defined by the tertile values of alpha3/alpha2 frequency power ratio. The ANOVA analysis showed that there were no statistically significant differences between groups which resulted well paired for age, sex, WMH burden, education and global cognitive level. Anyway, age, sex, education, global cognitive level (MMSE score) and WMHs were introduced as covariates in the subsequent analysis to avoid confounding factors. Alpha3/alpha2 frequency power ratio levels were significantly different at Games–Howell post-hoc comparisons (p = 0.000).
Table 2.
Demographic and cognitive characteristics in the whole sample, disaggregated for increased levels of alpha3/alpha2 numbers denote mean ± standard deviation, number and [range]. p denotes significance on ANOVA (MMSE, mini mental state examination; WMHs, white matter hyperintensities).
| Alpha3/alpha2 |
||||
|---|---|---|---|---|
| High |
Middle |
Low |
p |
|
| Demographic and clinical futures | ||||
| Number of subjects | 18 | 38 | 18 | – |
| Age, years | 70.4 ± 6.7 [60–85] |
68.4 ± 8.2 [52–83] |
70.4 ± 7.4 [57–80] |
.55 |
| Sex, female | 13 (%) | 24 (%) | 14 (%) | .51 |
| Education, years | 6.6 ± 3.6 [4–18] |
7.6 ± 3.7 [3–17] |
8.3 ± 4.7 [3–18] |
.42 |
| MMSE | 27 ± 1.7 [23–29] |
27.4 ± 1.3 [24–30] |
26.9 ± 1.2 [23–30] |
.46 |
| WMHs (mm3) | 2.78 ± 2.58 | 5.59 ± 6.60 | 2.57 ± 2.76 | .09 |
| Alpha3/alpha2 | 1.29 ± 0.1 [1.17–1.52] |
1.08 ± 0.0 [1–1.16] |
0.9 ± 0.1 [0.77–0.98] |
.000 |
3.1. Pattern of cortical thickness between groups
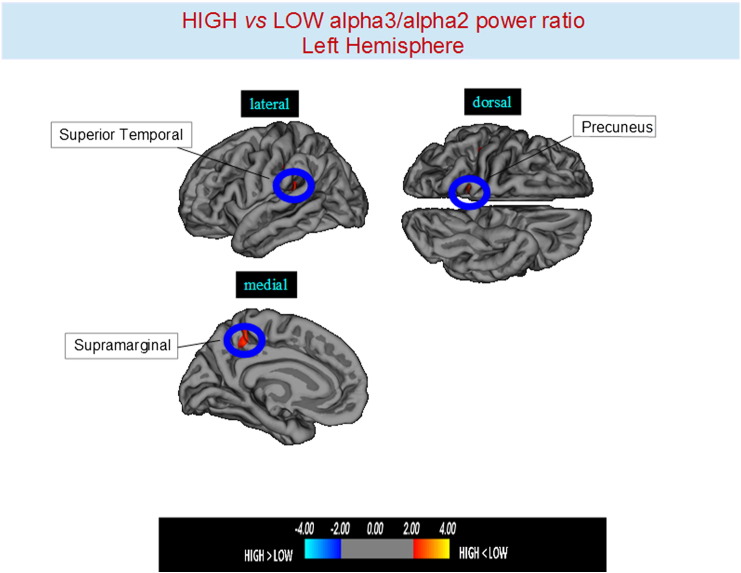
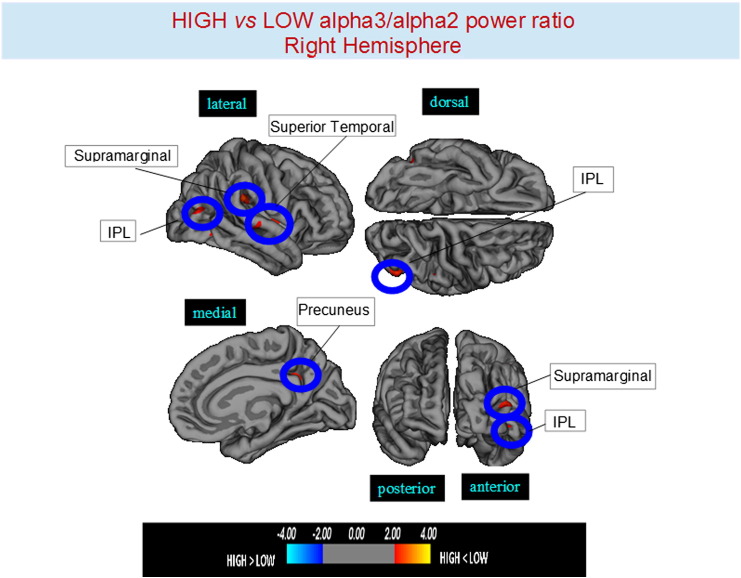
Higher vs lower: when compared to subjects with low a3/a2, patients with high a3/a2 frequency power ratio show greater thinning in the bilateral superior temporal gyrus, SG, precuneus and in the right inferior parietal cortex. The total cortical gray matter (CGM) reduction in high a3/2 group than low a3/a2 group was 471 mm2 (Fig. 1, Fig. 2, Table 3).
Fig. 1.
Brain regions with significant regional cortical thickness differences in MCI with high a3/a2 ratio compared to MCI with low a3/a2 ratio in the left hemisphere (p < 0.01 uncorrected). The color-coding for p values is on a logarithmic scale. Warmer color represents cortical thinning, cooler color represents cortical thickening. Results are presented on the pial cortical surface of brain: dark gray regions represent sulci and light gray regions represent gyri.
Fig. 2.
Brain regions with significant regional cortical thickness differences in MCI with high a3/a2 ratio compared to MCI with low a3/a2 ratio in the right hemisphere (p < 0.01 uncorrected). The color-coding for p values is on a logarithmic scale. Warmer color represents cortical thinning, cooler color represents cortical thickening. Results are presented on the pial cortical surface of brain: dark gray regions represent sulci and light gray regions represent gyri.
Table 3.
Brain regions with significant regional cortical thickness differences in MCI with high a3/a2 ratio compared to MCI with low a3/a2 ratio (high a3/a2 < low a3/a2) and MCI with middle a3/a2 ratio (high a3/a2 < middle a3/a2). Cluster size represents the extension of contiguous significant voxels in the cluster obtained at p < 0.01 uncorrected (cluster size > 30 mm2). Stereotaxic coordinates reveal the position of the most significant voxel of the cluster, and side denotes its localization on the left (L) or right (R) brain hemisphere. Thickness denotes the average cortical thickness and standard deviation values within the cluster in high and middle a3/a2 groups. P denotes the significance level of the differences in thickness between groups.
| High a3/a2 < low a3/a2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cluster size (mm2) | Region | Side | Stereotaxic Coordinate |
p | Thickness (mm2) |
|||
| x | y | z | High | Low | ||||
| 60 | Supramarginal | L | − 40 | − 36 | 18 | 0.0008 | 1.95 ± 0.18 | 2.08 ± 0.19 |
| 35 | Precuneus | L | − 14 | − 48 | 58 | 0.0002 | 1.87 ± 0.14 | 1.98 ± 0.19 |
| 59 | Supramarginal | R | 49 | − 29 | 27 | < 0.0001 | 1.94 ± 0.18 | 2.08 ± 0.20 |
| 52 | Precuneus | R | 11 | − 49 | 30 | 0.0001 | 1.81 ± 0.17 | 1.93 ± 0.15 |
| 85 | Inferior parietal | R | 46 | − 75 | 10 | 0.0001 | 1.94 ± 0.22 | 2.07 ± 0.23 |
| 59 | Inferior parietal | L | − 57 | − 18 | 18 | 0.0002 | 1.51 ± 0.15 | 1.62 ± 0.17 |
| 71 | Supramarginal | L | − 53 | − 42 | 46 | 0001 | 1.95 ± 0.18 | 2.11 ± 023 |
| 33 | Precuneus | L | − 16 | − 43 | 60 | 0001 | 1.87 ± 0.14 | 1.94 ± 0.17 |
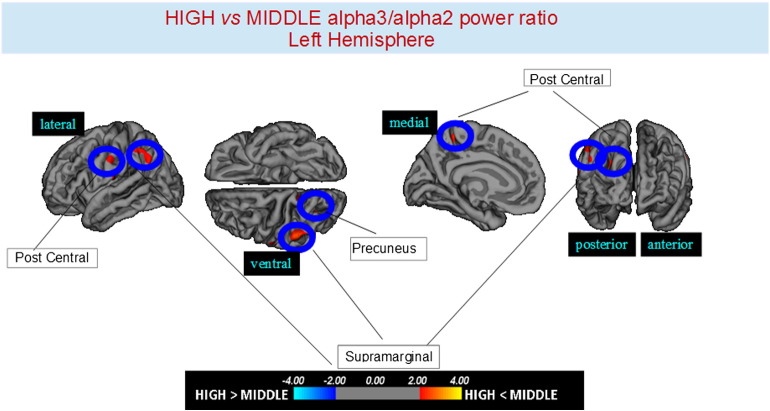
Higher vs. middle: the same group showed a similar but less wide pattern of cortical thinning when compared to middle a3/a2 frequency power group: the regions of atrophy were located in the left SG, left precuneus and left inferior parietal cortex. The total CGM reduction in higher a3/2 compared to middle a3/a2 frequency power ratio group was 160 mm2 (Fig. 3, Table 2). When higher a3/2 was compared to lower a3/2 frequency power ratio group the total extension of cortical thinning (471 mm2) was 34% wider than the comparison between higher and middle frequency power ratio group (160 mm2). No regions of major cortical atrophy were found in groups with middle or lower a3/a2 frequency power ratio when compared to higher a3/a2 group. No significant cortical thickness differences were found between middle and lower a3/a2 frequency power ratio groups.
Fig. 3.
Brain regions with significant regional cortical thickness differences in MCI with high a3/a2 ratio compared to MCI with middle a3/a2 ratio in the right hemisphere (p < 0.01 uncorrected). The color-coding for p values is on a logarithmic scale. Warmer color represents cortical thinning, cooler color represents cortical thickening. Results are presented on the pial cortical surface of brain: dark gray regions represent sulci and light gray regions represent gyri.
4. Discussion
4.1. Preliminary remarks
This suggests a clinical–theoretical assumption useful as a proposal to stimulate a discussion in the broad field of Alzheimer's disease. A similar method has been used by a previous work in which a hereditary deficiency of language (induced by a mutation in the Forkhead box P2 (FOXP2) gene on chromosome 7) has been associated by fMRI scans to Broca's area, homologous of the area F5, in which mirror neurons have been first located in the monkeys. The authors speculated that at the basis of this defect of language there could be an involvement of the anterior part of the mirror system in humans [40]. The recent evidence that a possible pathological uncoupling of the mirror system could be claimed to explain the cognitive deficits in prodromal AD [8], [9], with a major involvement of the posterior part of the mirror network, moved the working hypothesis of the present research. Our results suggested an association between a marker EEG and atrophy in the IPL, where it was previously individuated the posterior part of the mirror system, in subjects with prodromal AD. Of course, the specific involvement of mirror neurons in the IPL could not be stated. In the IPL neurons may be present not belonging to the mirror system. For example, it has recently been revealed as the inferior parietal cortex is part of a network that allows processing of faces without awareness. In the same way, there is no doubt that Alzheimer's disease is a complex pathology that cannot be reduced to the impairment of the mirror neurons system and much less it could be a primary disorder of the mirror neurons system itself. However, the involvement of the mirror system in prodromal AD cannot be excluded, supported by clinical and neurophysiological considerations [41], [42]. Whether these anomalous neural coding mechanisms of higher alpha generation in MCI due to AD patients represent a dysfunction of the mirror system is a topic for future research. Findings of the current study need further empirical confirmation revealing the correlation with cognitive deficits in larger samples of prodromal AD patients and also the investigation of other neurodegenerative disorders is needed.
4.2. Mirror system and prodromal Alzheimer's disease
The cortical thickness analysis is a very sensitive measure of brain atrophy resulting from a loss of neurons by computing the distance between the white matter surface and the gray matter/cerebrospinal fluid boundary over the entire cortex and allowing a precise matching of cortical anatomy between subjects. Of note, increasing thinning in specific cortical areas has been demonstrated a cortical signature of prodromal AD. The abnormal thinning of cortical areas in AD parallels known regional vulnerability to AD neuropathology and relates to symptom severity even in the earliest stages of clinical symptoms. Furthermore, subtle thinning is present in asymptomatic older controls with brain amyloid binding as detected with amyloid imaging [43]. On the whole, this kind of computation of cortical morphometry is a reliable measure of cortical mantle atrophy and loss of neurons. Our results show a specific atrophy of cortical regions belonging to the posterior mirror neurons network, namely the IPL, in subjects with MCI due to prodromal AD. In particular, the SG, a large subdivision of the IPL, is significantly more atrophic in MCI subjects at major risk to develop AD. These results confirm the outcomes of a recent fMRI study, showing that, when specifically taxing cognitive skills related to the mirror neurons system, the posterior part is specifically impaired as compared to the relatively spared anterior part, located in the frontal lobe [44]. Furthermore, a recent meta-analysis confirmed that in humans an important part of the mirror neuron systems is localized in IPL [45] and an fMRI study has demonstrated that bilateral activation pattern including the inferior parietal cortex is crucial to imitate gesture that is an essential feature of praxic skills [46]. The location of the IPL at the junction of the parietal, temporal and occipital lobes makes it ideally situated to perform the kinds of cross-modal abstraction (proprioceptive/hearing/vision) required for motor praxis and mirror neuron-like computation. Recently it has been suggested that the mirror neuron system within the IPL (originally evolved for the kind of cross-modal abstraction required for prehension and praxis) was subsequently adapted to also perform the more abstract types of re-conceptualisation, such as metaphor and arithmetic [47]. Multimodal functions of the IPL lobe play a critical role in the processing of visuo-spatial informations. Injury of AG and SG result in typical neuropsychological syndromes. The AG syndrome encompasses wide neuropsychological symptoms: anomia, alexia, acalculia, disgrafia, right–left disorientation, finger agnosia, and ideomotor apraxia. The right parietal lobe syndrome includes visuospatial deficit, unilateral spatial neglect, constructional apraxia, aprosodia deficiency navigation and topographical disorientation. Moreover, IPL takes place fundamental linguistic elaboration. In particular, the transition of graphemic symbols in phonological expression seems to be typical of the SG. Impaired naming was also associated with cortical thinning of the SG (BA 40) [48].
In prodromal AD both visuo-spatial and language skills are impaired. The presence of melokinetic apraxia is an early sign of MCI due to AD, followed by typical disorientation or ideo-motor apraxia syndrome later in the course of disease [49]. These clinical manifestations are supported by morpho-structural and functional studies. For the discrimination of Alzheimer's disease, the decrease of cortical thickness in SG, together with hippocampal and entorinal cortex volume, has been demonstrated highly specific and sensible in identifying subjects with prodromal AD [50]. Furthermore, a resting PET study revealed hypoperfusion in bilateral inferior parietal lobule and posterior cingulate areas as typical of early AD covariance pattern [51]. As about language, the anomic aphasia can represent one of the initial symptoms of Alzheimer's disease, recently coded as logopenic [52]. Recent studies have demonstrated that during the successful encoding of new items there is a desynchronization in the IPL language and visuo-spatial skills related networks whereas a synchronization prevent a successful semantic encoding [53], [54]. It is of great interest that there is an overlapping between the brain regions associated with increase of EEG alpha3/alpha2 frequency power ratio (hypersynchronization of high alpha) in our study and the IPL nodes in fMRI studies related to semantic encoding [55], [56]. The deleterious role of synchronization has been recently demonstrated by an interesting study facing the intriguing relationship between the functional and structural neurodegeneration of temporo-parietal eteromodal associative regions in AD [57]. These regions were demonstrated to be selectively vulnerable in AD pathology, due to the damage of inhibitory interneurons providing a loss of inhibition at cellular level. According to the authors, the disinhibition provokes an increasing amount of neural activity at network level, giving as a final result an hypersynchronization of brain areas. Of note, this overactivity is excitotoxic and determines cellular apoptosis and brain atrophy [57]. Also, Palop and Mucke emphasize the role of inhibitory interneuron dysfunction, leading to hypersynchronization [58]. Our results are in line with these previous influential studies. A possible integrative view of all the results could be as follows: 1) the higher neuronal activity in the IPL hub region starts from a dysfunction of cellular inhibition; 2) the consequent disinhibition drives neural network to an oversynchronization; 3) this oversynchronization is peculiar of the hub regions of the IPL; 4) these overactivated regions are prone to neurodegeneration and atrophy; 5) a possible neurophysiological sign of this oversynchronization is the increase of the alpha3/alpha 2 power ratio we have found in the posterior part of mirror system network.
4.3. Clinical and translational perspective of mirror system impairment
According to the associative learning hypothesis, the mirror neuron system is a product of evolutive associative learning [59]. The associative hypothesis is supported by recent data showing that, even in adulthood, the sensorimotor learning can enhance [59], or abolish [60] mirror activation in human subjects [61]. The associative account implies that mirror neurons come from sensorimotor experience, and that much of this experience is obtained through process of social interaction, emotional elaboration and cognitive processing. In this view, it should be possible to train the mirror neurons in order to obtain a partial anatomical recover or, at least, a process of active cerebral plasticity inducing a functional improvement in patients with cognitive decline.
5. Conclusion
High EEG alpha3/alpha2 frequency power ratio in MCI subjects prone to convert in AD was associated with atrophy of IPL, where mirror neurons have been localized in humans. A link between AD and the uncoupling of the mirror neurons system has been hypothesized.
References
- 1.Di Pellegrino G., Fadiga L., Fogassi L., Gallese V., Rizzolatti G. Understanding motor events: a neurophysiological study. Exp. Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- 2.Rizzolatti G., Fogassi L., Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- 3.Parsons L.M., Fox P.T., Downs J.H. Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature. 1995;375:54–58. doi: 10.1038/375054a0. [DOI] [PubMed] [Google Scholar]
- 4.Buccino G., Binkofski F., Fink G.R. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur. J. Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- 5.Hyvaerinen J. Posterior parietal lobe of the primate brain. Physiol. Rev. 1982;62:1060–1129. doi: 10.1152/physrev.1982.62.3.1060. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran V.S., Oberman L.M. Broken mirrors: a theory of autism. Sci. Am. 2006;295:62–69. doi: 10.1038/scientificamerican1106-62. [DOI] [PubMed] [Google Scholar]
- 7.Rapoport S.I. Hypothesis: Alzheimer's disease is a phylogenetic disease. Med. Hypotheses. 1989;29(3):147–1504. doi: 10.1016/0306-9877(89)90185-0. [DOI] [PubMed] [Google Scholar]
- 8.Poletti M., U. B. J Neuropsychol. 2013 Mar;7(1):121–131. doi: 10.1111/j.1748-6653.2012.02040.x. (Epub 2012 Nov 5. Alteration of Affective Theory of Mind in Amnestic Mild Cognitive Impairment) [DOI] [PubMed] [Google Scholar]
- 9.Fontana A.P., Kilner J.M., Rodrigues E.C., Joffily M., Nighoghossian N., Vargas C.D., Sirigu A. Role of the parietal cortex in predicting incoming actions. NeuroImage. 2012 Jan 2;59(1):556–564. doi: 10.1016/j.neuroimage.2011.07.046. Epub 2011 Jul 23. [DOI] [PubMed] [Google Scholar]
- 10.Schroeter M.L., Neumann J. Combined imaging markers dissociate Alzheimer's disease and frontotemporal lobar degeneration — an ALE meta-analysis. Front. Aging Neurosci. 2011 Jul 19;3(10):1–6. doi: 10.3389/fnagi.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moretti D.V., Frisoni G.B., Fracassi C., Pievani M., Geroldi C., Binetti G., Rossini P.M., Zanetti O. MCI patients' EEGs show group differences between those who progress and those who do not progress to AD. Neurobiol. Aging. 2011;32(4):563–571. doi: 10.1016/j.neurobiolaging.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Folstein M.F., Folstein S.E., McHugh P.R. ‘Mini mental state’: a practical method for grading the cognitive state of patients for clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Hughes C.P., Berg L., Danziger W.L., Cohen L.A., Martin R.L. A new clinical rating scale for the staging of dementia. Br. J. Psychiatry. 1982;140:1225–1230. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 14.Rosen W.G., Terry R.D., Fuld P.A., Katzman R., Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann. Neurol. 1980;7(5):486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 15.Lawton M.P., Brodie E.M. Assessment of older people: self maintaining and instrumental activity of daily living. J. Gerontol. 1969;9:179–186. [PubMed] [Google Scholar]
- 16.Petersen R.C., Doody R., Kurz A., Mohs R.C., Morris J.C., Rabins P.V., Ritchie K., Rossor M., Thal L., Winblad B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 17.Portet F., Ousset P.J., Visser P.J., Frisoni G.B., Nobili F., Scheltens P., Vellas B., Touchon J., MCI Working Group of the European Consortium on Alzheimer's Disease (EADC) Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2006;77:714–718. doi: 10.1136/jnnp.2005.085332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubois B., Feldman H.H., Jacova C., Dekosky S.T., Barberger-Gateau P., Cummings J., Delacourte A., Galasko D., Gauthier S., Jicha G., Meguro K., O'brien J., Pasquier F., Robert P., Rossor M., Salloway S., Stern Y., Visser P.J., Scheltens P. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. Aug. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. (Review) [DOI] [PubMed] [Google Scholar]
- 19.Lezak M., Howieson D., Loring D.W. fourth ed. University Press; Oxford: 2004. Neuropsychological Assessment. [Google Scholar]
- 20.Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- 21.Moretti D.V., Babiloni F., Carducci F., Cincotti F., Remondini E., Rossini P.M., Salinari S., C. B. Computerized processing of EEG–EOG–EMG artifacts for multi-centric studies in EEG oscillations and event-related potentials. Int J Psychophysiol. 2003;47(3):199–216. doi: 10.1016/s0167-8760(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 22.Moretti D.V. Conversion of mild cognitive impairment patients in Alzheimer's disease: prognostic value of Alpha3/Alpha2 electroencephalographic rhythms power ratio. Alzheimers Res Ther. 2015 Dec 29;7(1):80. doi: 10.1186/s13195-015-0162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moretti D.V., Paternicò D., Binetti G., Zanetti O. Frisoni GB.EEG markers are associated to gray matter changes in thalamus and basal ganglia in subjects with mild cognitive impairment. NeuroImage. 2012 Mar;60(1):489–496. doi: 10.1016/j.neuroimage.2011.11.086. [DOI] [PubMed] [Google Scholar]
- 24.Moretti D.V., Pievani M., Geroldi C., Binetti G., Zanetti O., Cotelli M., PM R., GB F. Increasing of hippocampal atrophy and cerebrovascular damage is differently associated with functional cortical coupling in MCI patients. Alzheimer Dis. Assoc. Disord. 2009 doi: 10.1097/WAD.0b013e31819d4a9d. [DOI] [PubMed] [Google Scholar]
- 25.Moretti D.V., Pievani M., Fracassi C., Geroldi C., Calabria M., De Carli C.S., Rossini P.M., Frisoni G.B. Brain vascular damage of cholinergic pathways and EEG markers in mild cognitive impairment. J. Alzheimers Dis. 2008 Nov;15(3):357–372. doi: 10.3233/jad-2008-15302. [DOI] [PubMed] [Google Scholar]
- 26.Moretti D.V., Prestia A., Fracassi C., Binetti G., Zanetti O., Frisoni G.B. Specific EEG changes associated with atrophy of hippocampus in subjects with mild cognitive impairment and Alzheimer's disease. Int. J. Alzheimers Dis. 2012 doi: 10.1155/2012/253153. (2012:253153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moretti D.V., Prestia A., Fracassi C., Geroldi C., Binetti G., Rossini P.M., Zanetti O., Frisoni G.B. Volumetric differences in mapped hippocampal regions correlate with increase of high alpha rhythm in Alzheimer's disease. Int. J. Alzheimers Dis. 2011;2011:208218. doi: 10.4061/2011/208218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moretti D.V., Babiloni C., Binetti G., Cassetta E., Dal F.G., Ferreri F., Ferri R., Lanuzza B., Miniussi C., Nobili F., Rodriguez G., Salinari S., Rossini P.M. Individual analysis of EEG frequency and band power in mild Alzheimer's disease. Clin. Neurophysiol. 2004;115:299–308. doi: 10.1016/s1388-2457(03)00345-6. [DOI] [PubMed] [Google Scholar]
- 29.Moretti D.V., Miniussi C., Frisoni G., Zanetti O., Binetti G., Geroldi C., Galluzzi S., Rossini P.M. Vascular damage and EEG markers in subjects with mild cognitive impairment. Clin. Neurophysiol. 2007;118:1866–1876. doi: 10.1016/j.clinph.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Klimesch W. EEG-alpha rhythms and memory processes. Int. J. Psychophysiol. 1997;26:319–340. doi: 10.1016/s0167-8760(97)00773-3. [DOI] [PubMed] [Google Scholar]
- 31.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 32.Moretti D.V., Pievani M., Fracassi C., Binetti G., Rosini S., Geroldi C., Zanetti O., Rossini P.M., Frisoni G.B. Increase of theta/gamma and alpha3/alpha2 ratio is associated with amygdalo-hippocampal complex atrophy. J. Alzheimers Dis. 2009;120(2):295–303. doi: 10.3233/JAD-2009-1059. [DOI] [PubMed] [Google Scholar]
- 33.Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999 Feb;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 34.Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis I: segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 35.Ségonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K., Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004 Jul;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 36.Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex using magnetic resonance images. PNAS. 2000;97:11044–11049. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S., Busa E., Pacheco J., Albert M., Killiany R., Maguire P., Rosas D., Makris N., Dale A., Dickerson B., Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 38.Gronenschild E.H., Habets P., Jacobs H.I., Mengelers R., Rozendaal N., van Os J., Marcelis M. The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0038234. (238–234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuperberg G.R., Broome M.R., McGuire P.K., David A.S., Eddy M., Ozawa F., Goff D., West W.C., Williams S.C., van der Kouwe A.J., Salat D.H., Dale A.M., Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch. Gen. Psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 40.Watkins K.E., Vargha-Khadem F., Ashburner J., Passingham R.E., Connelly A., Friston K.J., Frackowiak R.S., Mishkin M., Gadian D.G. MRI analysis of an inherited speech and language disorder: structural brain abnormalities. Brain. 2002 Mar;125(Pt 3):465–478. doi: 10.1093/brain/awf057. [DOI] [PubMed] [Google Scholar]
- 41.Corballis M.C. FOXP2 and the mirror system. Trends Cogn. Sci. 2004 Mar;8(3):95–96. doi: 10.1016/j.tics.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Ghika J. Paleoneurology: neurodegenerative diseases are age-related diseases of specific brain regions recently developed by Homo sapiens. Med. Hypotheses. 2008;71:788–801. doi: 10.1016/j.mehy.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 43.Bakkour A., Morris J.C., Dickerson B.C. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72:1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baglio F., Castelli I., Alberoni M., Blasi V., Griffanti L., Falini A., Nemni R., Marchetti A. Theory of mind in amnestic mild cognitive impairment: an FMRI study. J. Alzheimers Dis. 2012;29(1):25–37. doi: 10.3233/JAD-2011-111256. [DOI] [PubMed] [Google Scholar]
- 45.Molenberghs P1., Cunnington R., Mattingley J.B. Is the mirror neuron system involved in imitation? A short review and meta-analysis. Neurosci. Biobehav. Rev. 2009 Jul;33(7):975–980. doi: 10.1016/j.neubiorev.2009.03.010. Epub 2009 Apr 1. [DOI] [PubMed] [Google Scholar]
- 46.Mühlau M1., Hermsdörfer J., Goldenberg G., Wohlschläger A.M., Castrop F., Stahl R., Röttinger M., Erhard P., Haslinger B., Ceballos-Baumann A.O., Conrad B., Boecker H. Left inferior parietal dominance in gesture imitation: an fMRI study. Neuropsychologia. 2005;43(7):1086–1098. doi: 10.1016/j.neuropsychologia.2004.10.004. Epub 2005 Jan 5. [DOI] [PubMed] [Google Scholar]
- 47.McGeoch P.D., Brang D., Ramachandran V.S. Apraxia, metaphor and mirror neurons. Med. Hypotheses. 2007;69(6):1165–1168. doi: 10.1016/j.mehy.2007.05.017. Epub 2007 Jun 28. [DOI] [PubMed] [Google Scholar]
- 48.Leyton C.E., Piguet O., Savage S., Burrell J., Hodges J.R. The neural basis of logopenic progressive aphasia. J. Alzheimers Dis. 2012;32(4):1051–1059. doi: 10.3233/JAD-2012-121042. [DOI] [PubMed] [Google Scholar]
- 49.Ghika J. Paleoneurology: neurodegenerative diseases are age-related diseases of specific brain regions recently developed by Homo sapiens. Med. Hypotheses. 2008 Nov;71(5):788–801. doi: 10.1016/j.mehy.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 50.Devanand D.P., Habeck C.G., Tabert M.H., Scarmeas N., Pelton G.H., Moeller J.R., Mensh B.D., Tarabula T., Van Heertum R.L., Stern Y. PET network abnormalities and cognitive decline in patients with mild cognitive impairment. Neuropsychopharmacology. 2006 Jun;31(6):1327–1334. doi: 10.1038/sj.npp.1300942. [DOI] [PubMed] [Google Scholar]
- 51.Desikan R.S., Cabral H.J., Hess C.P., Dillon W.P., Glastonbury C.M., Weiner M.W., Schmansky N.J., Greve D.N., Salat D.H., Buckner R.L. Fischl B; Alzheimer’s disease neuroimaging initiative. Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease. Brain. 2009 Aug;132(Pt 8):2048–2057. doi: 10.1093/brain/awp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gorno-Tempini M.L., Brambati S.M., Ginex V., Ogar J., Dronkers N.F., Marcone A., Perani D., Garibotto V., Cappa S.F., Miller B.L. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008 Oct 14;71(16):1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sperling R.A., Dickerson B.C., Pihlajamaki M., Vannini P., LaViolette P.S., Vitolo O.V., Hedden T., Becker J.A., Rentz D.M., Selkoe D.J., Johnson K.A. Functional alterations in memory networks in early Alzheimer's disease. Neruomol. Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chatwal J.P., Sperling R.A. Functional MRI of mnemonic networks across the spectrum of normal aging, mild cognitive impairment, and Alzheimer's disease. J. Alzheimers Dis. 2012 Jan 1;31(0):S155–S167. doi: 10.3233/JAD-2012-120730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones D.T., Machulda M.M., Vemuri P., McDade E.M., Zeng G., Senjem M.L., Gunter J.L., Przybelski S.A., Avula R.T., Knopman D.S., Boeve B.F., Petersen R.C., Jack C.R., Jr. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology. 2011 Oct 18;77(16):1524–1531. doi: 10.1212/WNL.0b013e318233b33d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Haan W., Mott K., van Straaten E.C., Scheltens P., Stam C.J. Activity dependent degeneration explains hub vulnerability in Alzheimer's disease. PLoS Comput. Biol. 2012 Aug;8(8) doi: 10.1371/journal.pcbi.1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morris J.C., Ances B.M. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J. Neurosci. 2012 Jun 27;32(26):8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palop J.J., Mucke L. Synaptic depression and aberrant excitatory network activity in Alzheimer's disease: two faces of the same coin? Neruomol. Med. 2010 Mar;12(1):48–55. doi: 10.1007/s12017-009-8097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heyes C. Where do mirror neurons come from?Neuroscience and. Biobehav. Rev. 2010;34:575–583. doi: 10.1016/j.neubiorev.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Press C., Gillmeister H., Heyes C.M. Sensorimotor experience enhances automatic imitation of robotic action. Proc. R. Soc. Lond. Biol. Sci. 2007;274:2509–2514. doi: 10.1098/rspb.2007.0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heyes C., Bird G., Johnson H., Haggard P. Experience modulates automatic imitation. Cogn. Brain Res. 2005;22:233–240. doi: 10.1016/j.cogbrainres.2004.09.009. [DOI] [PubMed] [Google Scholar]