Abstract
In the human body, glycogen is a branched polymer of glucose stored mainly in the liver and the skeletal muscle that supplies glucose to the blood stream during fasting periods and to the muscle cells during muscle contraction. Glycogen has been identified in other tissues such as brain, heart, kidney, adipose tissue, and erythrocytes, but glycogen function in these tissues is mostly unknown. Glycogen synthesis requires a series of reactions that include glucose entrance into the cell through transporters, phosphorylation of glucose to glucose 6-phosphate, isomerization to glucose 1-phosphate, and formation of uridine 5ʹ-diphosphate-glucose, which is the direct glucose donor for glycogen synthesis. Glycogenin catalyzes the formation of a short glucose polymer that is extended by the action of glycogen synthase. Glycogen branching enzyme introduces branch points in the glycogen particle at even intervals. Laforin and malin are proteins involved in glycogen assembly but their specific function remains elusive in humans. Glycogen is accumulated in the liver primarily during the postprandial period and in the skeletal muscle predominantly after exercise. In the cytosol, glycogen breakdown or glycogenolysis is carried out by two enzymes, glycogen phosphorylase which releases glucose 1-phosphate from the linear chains of glycogen, and glycogen debranching enzyme which untangles the branch points. In the lysosomes, glycogen degradation is catalyzed by α-glucosidase. The glucose 6-phosphatase system catalyzes the dephosphorylation of glucose 6-phosphate to glucose, a necessary step for free glucose to leave the cell. Mutations in the genes encoding the enzymes involved in glycogen metabolism cause glycogen storage diseases.
Keywords: Glucose, Glucokinase, Phosphoglucomutases, Glycogen synthase, Glycogen phosphorylase, α-Glucosidase, Glycogen storage diseases
Highlights
-
•
Glycogen is a branched polymer of glucose that permits glucose storage in humans.
-
•
Glycogen assembly and breakdown are complex processes that require several enzymes.
-
•
Deficit of the enzymes involved in glycogen metabolism causes various glycogenoses.
-
•
Effective glycogen metabolism is important during fasting and muscle contraction.
-
•
In addition to be used as a fuel, glycogen-derived glucose serves other functions.
1. Introduction
Glycogen is a branched polymer of glucose that contains a minor amount of phosphate and glucosamine. In the linear chains, the glucose residues are connected by α-1,4-glycosidic linkages while α-1,6-glycosidic bonds create the branch points. Branches within normal glycogen are distributed at even intervals resulting in a structure with spherical shape. The source and function of phosphate and glucosamine in human glycogen are unclear. The glycogen particle consists of up to 55.000 glucose residues. In skeletal muscle, glycogen particles have a size of 10–44 nm in diameter while in the liver measure approximately 110–290 nm. Glycogen can be identified by electron microscopy inside the cells [1].
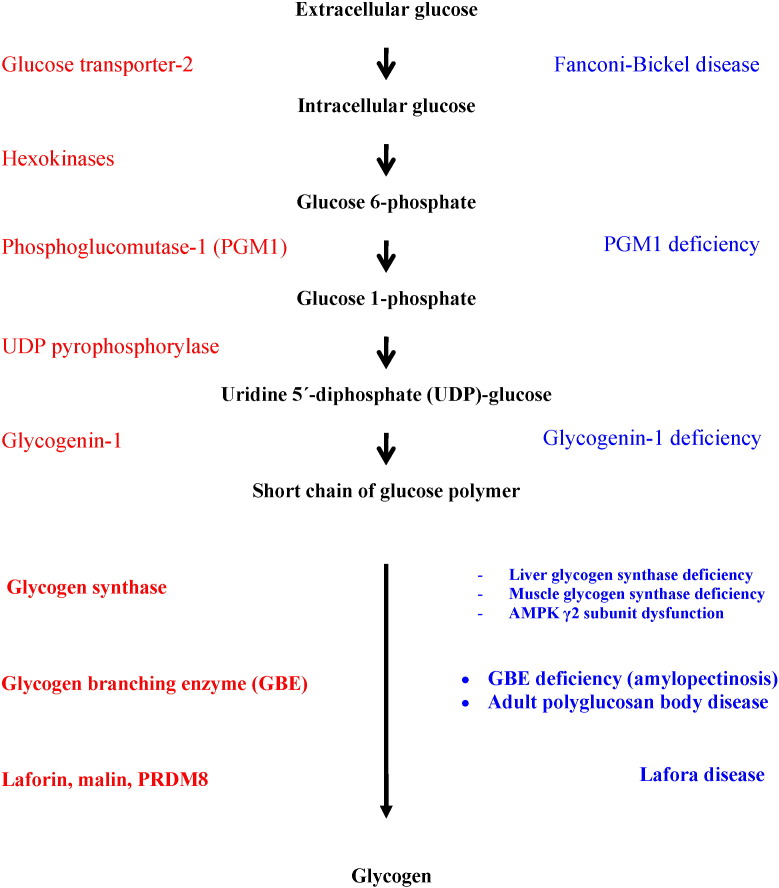
The synthesis of glycogen requires the coordinated action of a number of enzymes (Fig. 1). Glucose enters the cells via glucose transporters, being phosphorylated to glucose 6-phosphate by hexokinase isoenzymes. The next step is the isomerization of glucose 6-phosphate into glucose 1-phosphate by phosphoglucomutase-1. Then, uridine 5ʹ-diphosphate (UDP)-glucose pyrophosphorylase catalyzes the formation of UDP-glucose from glucose 1-phosphate. UDP-glucose is the immediate glucose donor for glycogen construction. Glycogenin initiates the synthesis of glycogen by autoglycosylation transporting glucose from UDP-glucose to itself and forming a short linear chain of about 10–20 glucose moieties. The elongation of this initial glycogen sequence is catalyzed by glycogen synthase that transfers a glycosyl moiety from UDP-glucose to the growing glycogen strand, providing the α-1,4-glycosidic linkages between glucose residues. The branching enzyme introduces branch points in the glycogen particle, by creating α-1,6 glycosidic bonds at regular intervals. Laforin and malin are proteins of undefined function in humans that influence glycogen assembly.
Fig. 1.
Glycogen synthesis and glycogen storage diseases.
The source of the glucose residues that form the glycogen particle is either the ingested food (direct pathway of glycogen synthesis) or the gluconeogenesis route (indirect pathway), in which gluconeogenic precursors such as lactate and alanine produce glucose 6-phosphate that may be used to synthesize glycogen.
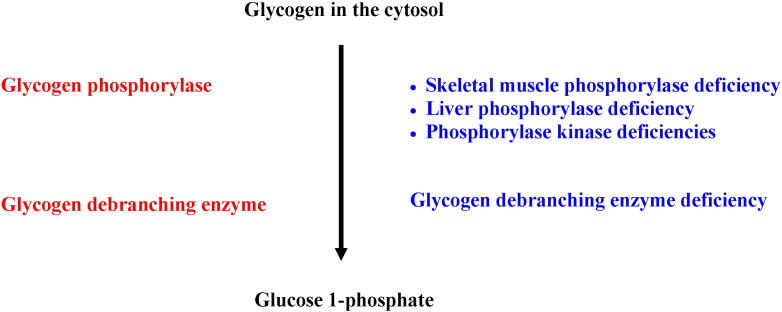
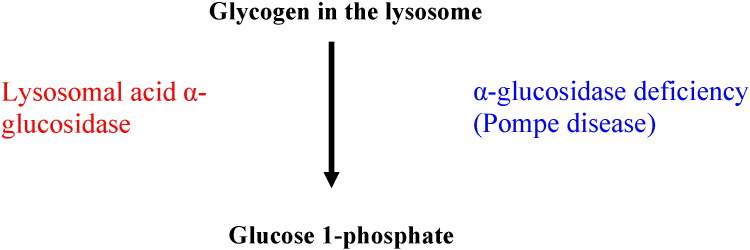
Glycogen degradation takes place both in the cytoplasm and inside the lysosomes. In the cytosol, glycogen breakdown is accomplished by the coordinated action of two enzymes, glycogen phosphorylase, which releases glucose 1-phosphate by untangling the α-1,4-glycosidic linkages, and glycogen debranching enzyme that unfastens the branch points releasing free glucose (Fig. 2). Glucose 1-phosphate derived from glycogen in the cytosol may be isomerized into glucose 6-phosphate which is dephosphorylated to free glucose by glucose 6-phosphatase (Fig. 3) in order for glucose to leave the cell via glucose transporters. In the lysosomes, the breakdown of glycogen is accomplished by the lysosomal enzyme acid α-glucosidase or acid maltase (Fig. 4).
Fig. 2.
Glycogen degradation in the cytosol and glycogen storage diseases.
Fig. 3.
Glucose 6-phosphatase system and glycogen storage disease type I.
Fig. 4.
Glycogen breakdown in the lysosomes and α-glucosidase deficiency.
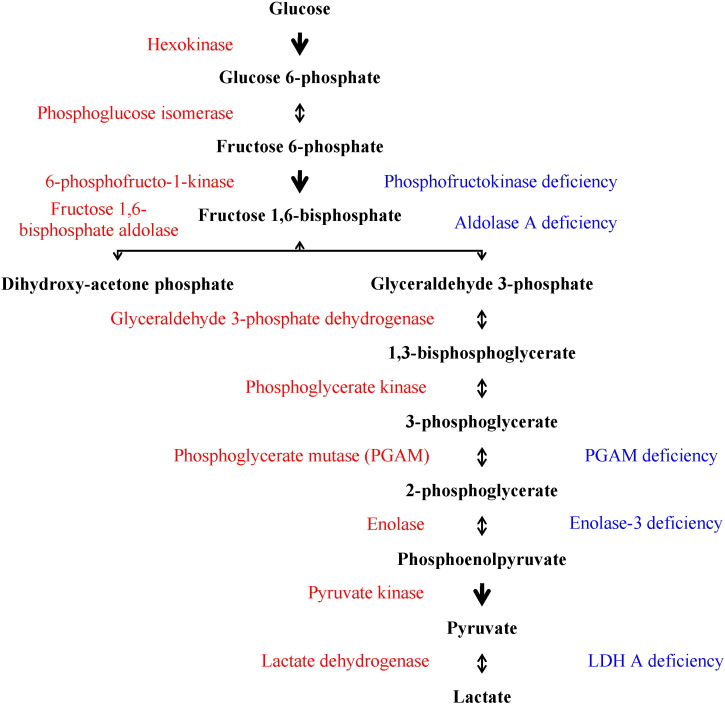
Molecular changes in the genes that encode enzymes involved in glycogen metabolism may cause glycogen storage diseases (GSDs) by interfering either with glycogen synthesis or with glycogen degradation (Table 1). In addition, some mutations in genes that code enzymes implicated in the glycolytic pathway have been labeled as glycogen storage diseases (Fig. 5).
Table 1.
Congenital disorders of glycogen metabolism.
| Glycogen storage disease | Protein | Gene | Location | Inheritance | Main consequence |
|---|---|---|---|---|---|
| 0 (Hepatic glycogen synthase deficiency) | Liver isoenzyme of glycogen synthase | GYS2 | 12p12.2 | Autosomal recessive | Reduction of glycogen synthesis in the liver |
| 0 (Muscle glycogen synthase deficiency) | Muscle isoenzyme of glycogen synthase | GYS1 | 19q13.3 | Undefined | Reduction of glycogen synthesis in muscle |
| I (Glucose 6-phosphatase system deficiency or von Gierke disease): Ia and Ib | |||||
| Ia | Glucose 6-phosphatase catalytic-1 (G6PC1) | G6PC1 | 17q21 | Autosomal recessive | Glycogen accumulation in liver and kidney |
| Ib | Glucose 6-phosphate translocase (G6PT) | SLC37A4 | 11q23 | Autosomal recessive | Neutropenia and glycogen accumulation in liver and kidney |
| Glucose 6-phosphatase catalytic-3 deficiency is a glycosylation disorder. | Glucose 6-phosphatase catalytic-3 (G6PC3) | G6PC3 | 17q21.31 | Autosomal recessive | Congenital neutropenia type 4 with no glycogen accumulation |
| II (Lysosomal acid α-glucosidase or Pompe disease) | Lysosomal acid α-glucosidase or acid maltase (GAA) | GAA | 17q25.2–q25.3 | Autosomal recessive | Glycogen accumulation in lysosomes |
| Danon disease | Lysosome-associated membrane protein-2 | LAMP2 | Xq24 | X-linked dominant | Similar to Pompe disease with normal GAA activity |
| III (Glycogen debranching enzyme deficiency or Cori–Forbes disease) | Glycogen debranching enzyme (AGL) | AGL | 1p21 | Autosomal recessive | Accumulation of abnormal glycogen in liver, heart, and skeletal muscle |
| IV (Glycogen branching enzyme deficiency or Anderson disease) | Glycogen branching enzyme | GBE1 | 3p14 | Autosomal recessive | Polyglucosan accumulation |
| V (Skeletal muscle glycogen phosphorylase deficiency or McArdle disease) | Skeletal muscle isoenzyme of glycogen phosphorylase (PYGM) | PYGM | 11q13 | Autosomal recessive | Defective glycogenolysis in skeletal muscle. |
| VI (Liver glycogen phosphorylase deficiency or Hers disease) | Liver isoenzyme of glycogen phosphorylase (PYGL) | PYGL | 14q21–22 | Autosomal recessive | Defective glycogenolysis in the liver. |
| VII (Phosphofructokinase-1 deficiency or Tarui disease) | Phosphofructokinase-1 | PFKM | 12q13.3 | Autosomal recessive | Glycogen accumulation in skeletal muscle |
| Glycogen phosphorylase kinase deficiency: GSD VIII, GSD IXa, GSD IXb, GSD IXc and cardiac phosphorylase deficiency | |||||
| VIII (Skeletal muscle glycogen phosphorylase kinase deficiency) | Skeletal muscle isoform of the α-subunit of glycogen phosphorylase kinase (PHKA1) | PHKA1 | Xq12–q13 | X-linked recessive | Defective glycogenolysis in skeletal muscle. |
| IXa (Liver glycogen phosphorylase kinase deficiency) | Liver isoform of the α-subunit of glycogen phosphorylase kinase (PHKA2) | PHKA2 | Xp22.13 | X-linked recessive | Defective glycogenolysis in the liver. |
| IXb (β-Subunit of glycogen phosphorylase kinase deficiency) | β-subunit of glycogen phosphorylase kinase (PHKB) | PHKB | 16q12–q13 | Autosomal recessive | Defective glycogenolysis in liver and skeletal muscle. |
| IXc (Liver isoform of the γ-subunit of glycogen phosphorylase kinase deficiency) | Liver isoform of the γ-subunit of glycogen phosphorylase kinase (PHKG2) | PHKG2 | 16p12.1–p11.2 | Autosomal recessive | Defective glycogenolysis in the liver. |
| Cardiac glycogen phosphorylase kinase deficiency | Cardiac glycogen phosphorylase kinase | Defective glycogenolysis in the heart. | |||
| X (Skeletal muscle phosphoglycerate mutase deficiency) | Skeletal muscle phosphoglycerate mutase | PGAM2 | 7p13–p12 | Autosomal recessive | Exercise intolerance with near-normal glycogen content in skeletal muscle |
| XI (Fanconi–Bickel disease) | Glucose transporter-2 | GLUT2 | 3q26.1–q26.3 | Autosomal recessive | Altered glucose entry and exit from cells |
| XI Lactate dehydrogenase (LDH) A deficiency | Isoenzyme A of LDH | LDHA | 11p15.4 | Autosomal recessive | Exercise intolerance |
| XII (Aldolase A deficiency) | Aldolase A | ALDOA | 16p11.2 | Undefined | Exercise intolerance with no glycogen accumulation in skeletal muscle |
| XIII (β-enolase or enolase-3 deficiency) | Enolase-3 (β-Enolase) | ENO3 | 17p13.2 | Undefined | Exercise intolerance with near-normal glycogen in skeletal muscle |
| XIV (Phosphoglucomutase-1 deficiency) | Phosphoglucomutase-1 | PGM1 | 1p31 | Likely autosomal recessive | Impairment of liver and muscle glucose utilization |
| Glucokinase deficiency (Maturity-onset diabetes type II) | Glucokinase | GCK | 7p15.3–p15.1 | Autosomal dominant | Reduction of glycogen synthesis in the liver |
| Glycogenin-1 deficiency | Glycogenin-1 | GYG1 | 3q25.1 | Likely autosomal recessive | Reduction of glycogen synthesis in muscle |
| γ-2 subunit of 5'adenosine monophosphate-activated protein kinase (AMPK) deficiency | γ-2 subunit of AMPK (PRKAG2) | PRKAG2 | 7q36.1 | Undefined | Non-physiological AMPK activation and secondary polyglucosan accumulation |
| Lafora disease | Laforin | EPM2A | 6q24 | Autosomal recessive | Polyglucosan accumulation |
| Malin | EPM2B | 6p22.3 | |||
| PRDM8 | PRDM8 | 4q21.21 | |||
Fig. 5.
Glycolytic pathway and glycogen storage diseases.
2. Glycogen synthesis
2.1. Glucose uptake: glucose transporters
In most human tissues glucose crosses the plasma membrane and enters into the cells through glucose transporters via facilitated transport.
2.1.1. Glucose uptake into the brain
Glucose transporter-1 (GLUT1) is the primary mediator of glucose transport across the endothelium of the blood–brain barrier. GLUT1 deficiency syndrome is the result of impaired glucose transport into the brain. This syndrome has a broad clinical spectrum characterized by infantile seizures, acquired microcephaly, movement disorder, intellectual impairment and low glucose concentration in the cerebrospinal fluid [2]. Ketogenic diets to promote the synthesis of ketone bodies have been used in patients affected with this disease, but not all of them experience resolution of symptoms [3]. In healthy subjects, glucose uptake by the brain is not mediated by insulin and accounts for most of glucose taken up by the whole body during the post-absorptive period [4]. GLUT1 is also operative in the red blood cells and skeletal muscle [2].
2.1.2. Glucose uptake in skeletal muscle
In human skeletal muscle, glucose uptake is carried out predominantly by two transporters, GLUT1 and GLUT4. GLUT1 lies on the plasma membrane likely facilitating basal glucose transportation into the muscle fiber. By contrast, GLUT4 resides inside intracellular storage vesicles under basal conditions, being translocated to the plasma membrane upon stimulation by muscle contraction or insulin [5]. Glucose uptake by human skeletal muscle is stimulated by endurance training and insulin at least in part via an increase in the level of GLUT4 on the plasma membrane [6].
2.1.3. Glucose uptake in liver and pancreas
Glucose uptake into human hepatocytes and pancreatic β-cells is performed by GLUT2 (solute carrier family 2, member A2 or SLC2A2), a transporter that allows glucose entry down the concentration gradient between the blood and the tissue. GLUT2 protein is encoded by the GLUT2 gene, mapped to chromosome 3q26.1–q26.3 and expressed in human liver, kidney, and pancreas [7].
2.1.4. Glucose transporter-2 deficiency or Fanconi–Bickel disease (GSD type XI)
Fanconi–Bickel disease is an autosomal recessive disorder caused by mutations in the GLUT2 gene and characterized by impaired utilization of glucose and galactose that leads to hypergalactosemia and hyperglycemia in the postprandial period and ketotic hypoglycemia in the fasting state. Typical features of the disease are accumulation of glycogen in the liver and kidney involvement with proximal tubular nephropathy including glucosuria, aminoaciduria, and hyperphosphaturia. Dietary therapy includes galactose restriction and small and frequent meals with uncooked cornstarch at bedtime to avoid fasting hypoglycemia and ketogenesis [8].
2.2. Glucose phosphorylation: hexokinases
Once inside the cell, hexokinase isoenzymes phosphorylate free glucose to glucose 6-phosphate, ensuring the permanence of this metabolite inside the cell. In humans, there are four isoenzymes of hexokinase, termed hexokinases I, II, III, and IV (glucokinase). The isoenzyme of hexokinase expressed in skeletal muscle is hexokinase II and the human gene encoding this isoenzyme has been mapped to chromosome 2p13.1 [9].
The gene encoding human glucokinase (GCK) is located on chromosome 7p15.3–p15.1. Glucokinase has been identified in human liver and pancreatic β- and α-cells, but it is not found in the exocrine pancreas [10].
It is generally accepted that human hexokinases I to III show a high affinity for glucose and are inhibited by their product, glucose 6-phosphate. In contrast, glucokinase (hexokinase IV) shows lower affinity for glucose and is not inhibited by glucose 6-phosphate [11].
Glucokinase action is modulated by the glucokinase regulatory protein, encoded by the gene glucokinase regulator (GCKR). Human GCKR is expressed in the liver but it does not appear to be appreciably expressed in pancreatic β-cells. In the fasting state, hepatic glucokinase regulatory protein inhibits glucokinase action limiting glucose utilization. By contrast, in the postprandial state, the intracellular glucose concentration increases in the hepatocyte and glucokinase remains unrestrained in the cytosol, generating glucose 6-phosphate and promoting glucose metabolism [12].
Glucose transport inside the cell does not appear to be rate-limiting for glucose uptake by the pancreatic β-cells and the hepatocytes, as glucose entry into these cells is driven by the concentration gradient between the blood and the intracellular concentration via the GLUT2 transporter. Glucose phosphorylation by glucokinase is likely a major factor in the regulation of glucose utilization in pancreatic β-cells and hepatocytes [11].
The importance of glucokinase action is highlighted by the fact that mutations in the glucokinase gene lead to disorders of glucose metabolism. Gain of function mutations in the glucokinase gene result in persistent hyperinsulinemic hypoglycemia of infancy, as glucokinase activation facilitates glucose metabolism in the pancreatic β-cells, enhancing insulin secretion and leading to hypoglycemia. Inactivating mutations in the GCK locus cause permanent neonatal diabetes mellitus and maturity onset diabetes of the young (GK-MODY or MODY 2), an autosomal dominant form of monogenic diabetes [13].
Glucokinase deficiency or inactivation hinders glucose metabolism in the pancreatic β-cells and the hepatocyte, leading to impaired insulin secretion in the pancreas and reduced glycogen synthesis in the liver during the postprandial period [14]. The net increment in hepatic glycogen content after a meal is lower in glucokinase-deficient patients compared to controls. These patients also show enhanced hepatic gluconeogenesis after meals and the gluconeogenic pathway is relatively more important for synthesizing hepatic glycogen than the direct pathway for glycogen synthesis [15].
2.3. Glucose isomerization: phosphoglucomutases
In order to synthesize glycogen, glucose 6-phosphate undergoes isomerization into glucose 1-phosphate by some isoenzymes of phosphoglucomutase. Five phosphoglucomutase isoenzymes are known to exist in humans (PGM1, PGM2, PGM3, PGM4 and PGM5). Human genes encoding PGM1 (PGM1), PGM3 (PGM3), and PGM5 (PGM5) isoforms have been identified, being located on chromosomes 1p31, 6q, and 9, respectively [16], [17].
2.3.1. Phosphoglucomutase-1 (PGM1)
Phosphoglucomutase-1 is a phosphotransferase that catalyzes the reversible transfer of phosphate between the 1- and 6-positions of glucose and therefore the interconversion of glucose 6-phosphate and glucose 1-phosphate. This isoenzyme requires a divalent metal ion (predominantly Mg2 +) for activity. Human PGM1 is a highly polymorphic protein. The locus encoding this isoenzyme exhibits a very high incidence of allelic variation in all populations and has been an important anchor point for linkage analysis and for positioning markers on human chromosome 1p [16].
2.3.1.1. Phosphoglucomutase-1 deficiency (GSD XIV)
In 2009, congenital deficiency of PGM1 was identified in a 35-year-old man who presented with exercise intolerance including cramps and rhabdomyolysis, basal elevated creatine kinase level, and hyperammonemia with normal lactate elevation on a forearm exercise test. Molecular analysis of the PGM1 gene revealed two heterozygous mutations and it was proposed that PGM1 deficiency should be designated glycogenosis type XIV [18]. Fasting intolerance with hypoglycemia also occurs in patients with PGM1 deficiency [19]. More recently, congenital deficiency of PGM1 due to recessive mutations in the PGM1 gene has been associated with a congenital disorder of glycosylation with a broad range of clinical manifestations that include hepatopathy, dilated cardiomyopathy, and cardiac arrest, in addition to fasting hypoglycemia and myopathy. The mechanism underlying defective glycosylation due to PGM1 deficiency is poorly understood [20]. A prospective multicenter trial is ongoing to evaluate the efficacy of D-galactose intake in PGM1 deficiency, since a few patients with this disease have shown some improvement after D-galactose supplementation [21].
2.3.2. Phosphoglucomutase-3 (PGM3)
In 2002, human genes AGM1 and PGM3, encoding N-acetylglucosamine phosphate mutase-1 (AGM1) and phosphoglucomutase-3, respectively, were found to be identical, both mapping to chromosome 6. AGM1 (PGM3) catalyzes the reversible conversion between N-acetylglucosamine 6-phosphate and N-acetylglucosamine 1-phosphate. N-acetylglucosamine 1-phosphate is required for the biosynthesis of UDP-N-acetylglucosamine, an essential precursor for glycosylation of proteins and lipids [22]. Autosomal recessive mutations in the PGM3 locus have been associated with a congenital disorder of glycosylation characterized by widespread clinical manifestations, including atopy with increased serum IgE levels, immune deficiency with recurrent bacterial and viral infections, immune-mediated disorders, and motor and neurocognitive impairment likely associated with hypomyelination [23], [24].
2.4. Formation of uridine 5ʹ-diphosphate-glucose: UDP-glucose pyrophosphorylase or glucose 1-phosphate uridyltransferase
The next step in the synthesis of glycogen is the formation of UDP-glucose from glucose 1-phosphate, catalyzed by the enzyme UDP-glucose pyrophosphorylase (UGP). This enzyme catalyzes the reversible formation of UDP-glucose and pyrophosphate from uridine 5ʹ-triphosphate (UTP) and glucose 1-phosphate in the presence of Mg2 +. UDP-glucose is the immediate glucose donor for the synthesis of glycogen, supplying glucose residues for the initiation and the elongation of the glycogen particle. In addition, UDP-glucose is involved in the synthesis of UDP glucuronides, which facilitates the excretion of endogenous compounds such as bilirubin and foreign molecules such as acetaminophen by converting them into more polar metabolites. The crystal structure of the human UGP has been determined, revealing that this enzyme adopts an octameric structure [25].
In human tissues, the enzyme UGP has been identified in all organs tested, with the highest levels in skeletal muscle, followed by liver, heart, and kidney [26]. Two isoenzymes of UGP are known in humans, UGP1 and UGP2. UGP1 is coded by a gene located on chromosome 1q21–q23 [27]. The human gene coding for UGP2 has been assigned to chromosome 2p13–p14 [28].
2.5. Initiation of glycogen synthesis: glycogenin
Glycogenin is a glycosyltransferase that catalyzes the transfer of glucose residues from UDP-glucose to itself, forming α-1,4-glycosidic linkages to create a linear glucose polymer of approximately 10–20 glucose moieties. This oligosaccharide chain is the base for the subsequent synthesis of glycogen that proceeds by the combined action of glycogen synthase and glycogen branching enzyme [29]. In humans, there are two isoforms of glycogenin, glycogenin-1 and glycogenin-2. Glycogenin-1 is encoded by the GYG1 gene, located to chromosome 3q25.1. This gene is predominantly expressed in skeletal muscle and heart and to a lesser extent in lung, kidney, brain, pancreas, and placenta. Glycogenin-1 has not been identified in the liver [30]. GYG2 gene encodes glycogenin-2 and is primarily expressed in the human liver [31].
In 2010 congenital deficiency of glycogenin-1 due to biallelic mutations in the GYG1 gene was reported in one patient affected with cardiomyopathy, cardiac arrhythmia, and muscle weakness. Echocardiography showed increased posterior-wall thickness and cardiac magnetic resonance imaging revealed increased left ventricular mass. Muscle biopsy showed deficit of glycogen in the muscle fibers with mitochondrial proliferation. Endomyocardial biopsy revealed hypertrophic cardiomyocytes containing large vacuoles filled with an unidentified periodic acid-Schiff (PAS)-positive material that was removed by treatment with α-amylase. Electron microscopy examination showed glycogen depletion in the cytoplasm. There was accumulation of unglycosylated glycogenin-1 protein in the skeletal muscle and the heart, but glycosylated glycogenin was not detected. Congenital inactivation of muscle glycogenin-1 in this patient resulted in impaired priming of glycogen synthesis and secondary glycogen depletion in heart and skeletal muscle, with a clinical phenotype of cardiomyopathy and muscle weakness [32]. In 2014, congenital deficiency of glycogenin-1 due to homozygous or compound heterozygous deleterious variants in the glycogenin-1 gene was reported in 7 adult patients affected with myopathy. In skeletal muscle, 30–40% of the fibers showed inclusions filled with PAS-positive material that had a variable degree of digestion by α-amylase treatment [33].
2.6. Elongation of the linear glycogen chain: glycogen synthase
Glycogen synthase is a glycosyltransferase that catalyzes the elongation of the glycogen chain by incorporating glycosyl residues from UDP-glucose to the growing glycogen strand, forming α-1,4-glycosidic linkages with the release of UDP. This enzyme connects the carbon-1 of the donor glucose from UDP-glucose to the carbon-4 of glycogen [34].
There are two isoenzymes of glycogen synthase in humans, GYS1 and GYS2, encoded by the genes GYS1 and GYS2, respectively. GYS1 is the isoform present in skeletal muscle and heart while GYS2 is the liver isoenzyme. Human GYS1 has been assigned to chromosome 19q13.3 [35] while human GYS2 is located to 12p12.2 [36].
2.6.1. Muscle glycogen synthase deficiency (muscle GSD 0)
Loss of function mutations in the GYS1 gene has been described in two families and cause inherited deficiency of the muscle isoenzyme of glycogen synthase, leading to glycogen depletion in skeletal muscle and the heart resulting in myopathy and cardiomyopathy with exercise intolerance, hypertrophic cardiomyopathy, and sudden cardiac arrest. Some patients with muscle GSD 0 have long QT. Glycogen synthase activity in cultured skin fibroblasts is reduced in the affected patients compared to controls and skeletal muscles show depletion of glycogen stores and increased number of mitochondria [37].
2.6.2. Liver glycogen synthase deficiency (liver GSD 0)
Mutations in the GYS2 gene cause an autosomal recessive disease due to hepatic glycogen synthase deficiency. After meals, the inability to store glucose as glycogen in the liver leads to postprandial hyperglycemia, glucosuria, and hyperlactatemia. In addition, the inability to store glycogen in the liver leads to fasting hypoglycemia and ketone formation. Frequent measurements of blood glucose, lactate, and ketones in both the fed and fasting states (24-h metabolic profile) show the characteristic biochemical disturbances. In liver biopsy samples, hepatocytes contain small amounts of glycogen and show moderate steatosis. Symptoms of GSD type 0 might be ameliorated with frequent (every 4 h) protein-rich meals and bedtime feeding of uncooked cornstarch. Protein-rich meals may provide the substrate for gluconeogenesis and a lower content of dietary carbohydrate reduces postprandial hyperglycemia, glycosuria, and lactic acidemia [38].
2.6.2.1. Glycogen synthase regulation
The regulatory mechanisms that modulate the activity of glycogen synthase in humans differ partially in liver and muscle, as these two glycogen depots carry out different functions. Reversible phosphorylation and dephosphorylation of glycogen synthase is a major determinant of its activity. Phosphorylation is catalyzed by kinases and leads to inactivation of the enzyme. Dephosphorylation of glycogen synthase is catalyzed by phosphatases and results in activation of the enzyme. In addition, glycogen synthase activity is stimulated by glucose 6-phosphate and inhibited by increased glycogen concentration. Glycogen synthase activity is also coordinated with glycogen phosphorylase activity, the enzyme that releases glucose 1-phosphate residues from the linear chain of glycogen, in order to achieve either glycogen synthesis or glycogen degradation.
2.6.3. Glycogen synthase kinases
Glycogen synthase phosphorylation and subsequent inactivation by kinases is a complex and insufficiently characterized process in humans involving multiple phosphorylation sites and several kinases, including 5ʹAMP-activated protein kinase (AMPK) [39].
2.6.3.1. Congenital deficiency of the γ-2 subunit of AMPK
AMPK consists of three subunits, α, β, and γ. The α-subunit contains the catalytic site, the β-subunit binds glycogen, and the γ-subunit is composed of two regulatory components (γ-1 and γ-2) that bind AMP and ATP. AMPK is activated by an increase in the intracellular concentration of AMP and calcium (Ca2 +). The activation of this kinase enhances oxidative metabolism to produce ATP while inhibiting biosynthetic pathways such as glycogen synthesis (via phosphorylation and subsequent inactivation of glycogen synthase) [39].
Mutations in the PRKAG2 gene, that encodes the γ-2 subunit of AMPK, induce accumulation of abnormal glycogen in the heart, underlining the relationship between AMPK and glycogen metabolism in humans. In 2001, mutations in the PRKAG2 gene, located to chromosome 7q36.1, were described in two families with severe familial hypertrophic cardiomyopathy and conduction system abnormalities in the heart [40].
Histopathology of cardiac specimens from affected patients demonstrated large cytosolic vacuoles inside the cardiomyocytes filled with polyglucosan material, which is an amylopectin-like material composed of PAS-positive glucose polymers variably resistant to digestion by α-amylase that evokes abnormally structured glycogen. The biochemical mechanism underlying the formation of this material remains undefined [41]. Ablation of atrioventricular accessory pathways caused by mutations in the PRKAG2 gene is not usually effective to correct conduction system abnormalities [42].
2.6.4. Glycogen synthase phosphatases: protein phosphatase-1
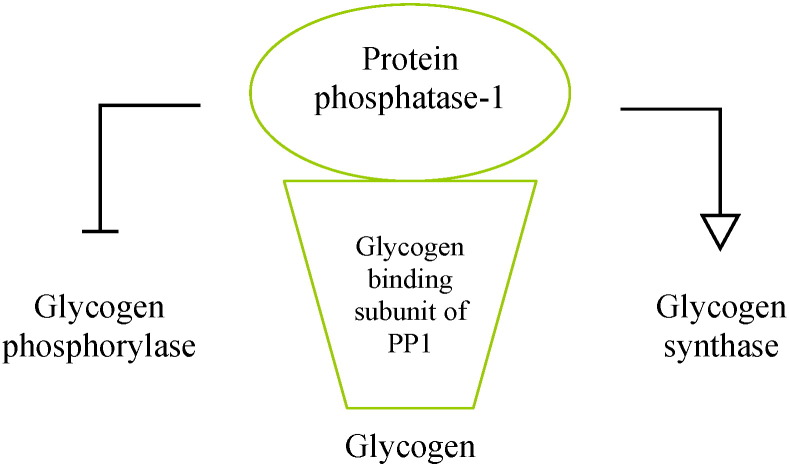
Protein phosphatase-1 (PP1) catalyzes the dephosphorylation and subsequent activation of glycogen synthase, promoting glycogen synthesis. Insulin activates PP1 and this could be at least in part the mechanism by which this hormone enhances glycogen synthesis in liver and skeletal muscle. PP1 is composed of a catalytic subunit bound to a regulatory subunit that modulates the catalytic action of PP1 by targeting the protein to particular subcellular locations and specific substrates. The glycogen targeting subunits of PP1 are regulatory components of the phosphatase that steer the protein to glycogen. Several glycogen binding subunits of PP1 have been reported in humans, including PPP1R3, PPP1R4, PPP1R5, and PPP1R6, encoded by the genes PPP1R3A, PPP1R3B, PPP1R3C, and PPP1R3D, respectively [43] (Table 2).
Table 2.
Glycogen targeting subunits of protein phosphatase-1 (PP1).
| Glycogen binding subunit of PP1 | Gene | Human tissue distribution |
|---|---|---|
| PPP1R3 | PPP1R3A | Skeletal muscle |
| PPP1R4 | PPP1R3B | Skeletal muscle, liver |
| PPP1R5 | PPP1R3C | Skeletal muscle, liver, heart |
| PPP1R6 | PPP1R3D | Skeletal muscle, heart, other tissues |
2.6.5. Effect of glucose 6-phosphate on hepatic glycogen synthase activity
Glucose 6-phosphate is an allosteric activator of glycogen synthase. Simultaneously, glucose 6-phosphate converts the enzyme into a better substrate for protein phosphatases, which lead to the covalent activation of glycogen synthase [34].
2.6.6. Balanced control of glycogen phosphorylase and glycogen synthase activities
The reciprocal control between glycogen synthase and glycogen phosphorylase is mainly accomplished by PP1 which activates glycogen synthase (by dephosphorylation of the enzyme) and simultaneously inactivates glycogen phosphorylase (Fig. 6). Similarly, insulin inhibits glycogen phosphorylase inducing a simultaneous activation of glycogen synthase and this may be a component of the mechanism by which insulin promotes hepatic glycogen synthesis [43].
Fig. 6.
Protein phosphatase-1 (PP1).
2.7. Branching of the glycogen particle: glycogen branching enzyme
Glycogen branching enzyme catalyzes the transfer of a glycosyl chain of 6 to 8 units to the glycogen thread forming an α-1,6 linkage and making glycogen a multi-branched polymer. The human gene that encodes the glycogen branching enzyme (GBE1) has been mapped to chromosome 3p14 [44].
2.7.1. Glycogen branching enzyme deficiency or Andersen disease (GSD IV)
Mutations in the gene that encodes the glycogen branching enzyme cause amylopectinosis, Andersen disease or GSD type IV [44]. Congenital deficiency of glycogen branching enzyme is an autosomal recessive disorder that leads to intracytoplasmatic accumulation of abnormally branched glycogen resembling amylopectin (polyglucosan) in multiple tissues, including liver, heart, skeletal muscle, and the nervous system. Intracytoplasmic polyglucosan deposits in spleen, bone marrow, and lymph nodes have been observed in one female infant patient [45]. Amylopectinosis may result in a variable clinical phenotype, including neurological, hepatic, musculoskeletal, and cardiac manifestations such as dilated cardiomyopathy and hypertrophic cardiomyopathy [46]. Rarely, mild proteinuria, stroke-like episodes and hypohydrosis may suggest Fabry's disease diagnosis in patients with GSD type IV [47]. Prenatal history includes polyhydramnios, fetal hydrops, and reduced fetal movements during pregnancy. The newborn usually presents with cardiomyopathy and profound muscular hypotonia leading to respiratory distress that requires mechanical ventilation. In early childhood, patients with glycogen branching enzyme deficiency usually develop liver dysfunction and progressive liver cirrhosis that may require liver transplantation. Some patients experience muscle weakness and dilated cardiomyopathy. The late-onset variant of glycogen branching enzyme deficiency is known as adult polyglucosan body disease (APBD). Most APBD patients with glycogen branching enzyme deficiency are of Askenazi Jewish ancestry [48]. APBD is a condition that affects predominantly the nervous system. A multinational study of the natural history of APBD shows that the most common clinical findings are neurogenic bladder (100%), spastic paraplegia with vibration loss (90%), and axonal neuropathy (90%). As the disease progresses, mild cognitive decline may affect up to half of the patients. APBD is characterized by leukodystrophy on brain MRI. Atrophy of the medulla and spine is universal [49]. APBD may produce acute neurological symptoms mimicking stroke in the absence of cardiovascular risk factors [50].
2.8. Laforin, malin, and PRDM8
Laforin is a phosphatase that belongs to the dual-specificity phosphatase family, which is able to dephosphorylate serine and threonine residues in addition to tyrosine [51]. Human laforin is a dimeric structure with a carbohydrate binding subunit and a phosphatase domain. A cleft formed between these two components allows the binding of the substrate and the subsequent catalytic activity of the enzyme [52]. Laforin is encoded by the gene EPM2A located on chromosome 6q24 [53]. Malin is a protein encoded by the EPM2B (NHLRC1) gene, located on chromosome 6p22.3 [54]. It has been reported that malin is an E3 ubiquitin ligase that ubiquitinates laforin to be degraded [51]. The gene PRDM8 on chromosome 4q21.21 encodes a protein named PRDM8 that translocates laforin and malin to the nucleus, leading to deficiency of their activity in the cytoplasm [55].
2.8.1. Lafora disease
The function of laforin, malin, and PRDM8 remains elusive, but their link to human glycogen metabolism is highlighted by Lafora disease, an autosomal recessive disorder characterized by progressive myoclonus epilepsy caused by mutations in the genes EPM2A, EPM2B, or PRDM8. Patients with Lafora disease show cytoplasmic accumulation of polyglucosan in several tissues, including liver, skeletal muscle, heart, skin, kidney, and brain (neurons), although clinical manifestations are restricted to the nervous system [52].
3. Glycogen degradation
Glycogen breakdown may occur both in the cytoplasm and inside the lysosomes. Mutations in the genes coding the enzymes involved in either of the two pathways of degradation may cause congenital disorders of glycogen metabolism.
3.1. Glycogen degradation in the cytosol
Glycogen breakdown in the cytosol is catalyzed by the coordinated action of two enzymes, glycogen phosphorylase and glycogen debranching enzyme. Glycogen phosphorylase releases glucose 1-phosphate from a linear glycogen chain, but the action of this enzyme is blocked when it reaches four glucose residues from the glycogen branch point (called the limit dextrin) and complete glycogen degradation requires the activity of the glycogen debranching enzyme to untie the branch points [56].
3.1.1. Glycogen phosphorylase
Glycogen phosphorylase liberates glucose 1-phosphate from a linear glycogen chain by unfastening the α-1,4 glycosidic bonds. There are three isoenzymes of glycogen phosphorylase in humans, muscle, liver, and brain isoforms, encoded by separate genes, named PYGM, PYGL, and PYGB respectively [56]. The brain isoform is present in brain, heart and liver. In the nervous system, this isoenzyme is found predominantly in astrocytes and weakly in neurons [57]. The locus encoding the brain isoenzyme has been mapped to chromosome 20 [58]. Congenital deficiency of the skeletal muscle and liver isoforms of glycogen phosphorylase cause defective glycogenolysis in skeletal muscle and liver, respectively.
3.1.1.1. Skeletal muscle glycogen phosphorylase deficiency or McArdle's disease (GSD V)
GSD type V (McArdle disease) is an autosomal recessive disorder caused by diminished glycogen phosphorylase activity in skeletal muscle due to mutations in the gene encoding the skeletal muscle isoform of glycogen phosphorylase (PYGM) on chromosome 11q13. In patients with McArdle disease defective glycogenolysis in skeletal muscle limits glucose availability when muscle contraction is initiated, inducing fatigue, cramps, and a rise in heart rate out of proportion of the work load. After a few minutes, fatigue and heart rate spontaneously subside and McArdle patients experience an improvement in exercise tolerance called the second wind phenomenon that has been attributed to the utilization by active muscle of other substrates, such as blood-borne glucose, lactate [59], free fatty acids [60], and branched-chain amino acids [61]. In addition to exercise intolerance, patients with McArdle disease may experience resting elevation of serum creatine kinase concentration and recurrent episodes of rhabdomyolysis and myoglobinuria induced by exercise [62].
A Cochrane review finds no conclusive evidence of clinical benefit associated with the use of a carbohydrate-rich diet in patients with GSD type V [63]. A ketogenic diet instituted in one patient improved exercise tolerance [3]. Supplements of branched-chain keto acids have been suggested while branched-chain amino acids deteriorate exercise performance [61].
3.1.1.2. Liver glycogen phosphorylase deficiency or Hers disease (GSD VI)
GSD type VI (Hers disease) is an autosomal recessive disorder caused by loss of glycogen phosphorylase activity in the liver owing to mutations in the gene that encodes the liver isoform of glycogen phosphorylase (PYGL) on chromosome 14q21–22 [64].
Hers disease is an uncommon disorder that usually leads to a mild clinical phenotype including fasting ketotic hypoglycemia, lactic acidemia, hepatomegaly, and growth retardation. Elevation of serum glucose in response to glucagon is absent. Hepatic nodular hyperplasia and left ventricular and septal hypertrophy occur rarely in patients with Hers disease. Treatment is directed toward avoiding prolonged fasting and ingestion of a bedtime snack to avoid early morning hypoglycemia [65].
3.1.1.3. Regulation of glycogen phosphorylase
The activity of glycogen phosphorylase is controlled by allosteric effectors and by reversible phosphorylation and dephosphorylation of the enzyme. Glucose 6-phosphate and UDP-glucose inhibit the enzyme [66]. Liver and muscle glycogen phosphorylase isoenzymes differ in their responsiveness to regulatory mechanisms. Muscle glycogen phosphorylase is strongly activated by AMP [67] and by an increase in glycogen concentration in muscle [68].
In the human liver, glucagon is an effective activator of glycogen phosphorylase likely via an increase in the level of 3ʹ5ʹ-adenosine monophosphate or cyclic-AMP (cAMP) [69].
Dephosphorylation of glycogen phosphorylase inactivates the enzyme and is catalyzed by PP1 whereas phosphorylation activates the enzyme and is catalyzed by glycogen phosphorylase kinase (PHK). PHK is composed of four subunits (α, β, γ, and δ) with stoichiometry α4–β4–γ4–δ4. The γ-subunit contains the catalytic site while the α- and β-subunits have a regulatory role in the activation of glycogen phosphorylase. The δ-subunit is calmodulin and confers calcium sensitivity to the kinase. PHK promotes glycogen breakdown releasing glucose in response to increases in cellular calcium and cAMP [70], [71]. The α-subunit of glycogen phosphorylase kinase (PHKA) has two isoforms, muscle (PHKA1) and liver (PHKA2), encoded by two separate genes, PHKA1 and PHKA2, respectively, which are located on Xq12–q13 and Xp22.13, respectively. The β-subunit (PHKB) is encoded by a single gene, PHKB, located on chromosome 16q12–q13. Alternative splicing generates muscle, liver, and brain tissue specific transcripts.
The γ-subunit of glycogen phosphorylase kinase (PHKG) has two isoforms, muscle (PHKG1) and liver (PHKG2), encoded by two different genes, PHKG1 and PHKG2, respectively, which are located to 7p11.2 and 16p12.1–p11.2, respectively. The δ-subunit of PHK or calmodulin is ubiquitously expressed, being encoded by three independent genes, CALM1, CALM2, and CALM3. Unlike α, β, and γ-subunits, deficiency of PHK has not been associated with mutations in the genes encoding calmodulin [71], [72].
3.1.1.4. Glycogen phosphorylase kinase deficiency (GSD VIII and GSD IX)
Molecular changes in most subunits of PHK cause defective activity of the kinase in the specific tissues where the subunit is distributed, impairing the release of glucose from glycogen in those tissues. Mutations in the PHKA1 gene that codes the skeletal muscle isoform of the α-subunit of PHK cause phosphorylase kinase deficiency in the skeletal muscle (GSD type VIII). Mutations in the PHKA2, PHKB, and PHKG2 genes cause GSD IXa, GSD IXb, and GSD IXc, respectively. Mutations in the PHKA2 gene that encodes the liver isoform of the α-subunit of PHK cause phosphorylase kinase deficiency in the liver (GSD IXa). Mutations in the PHKB gene that encodes the β-subunit of PHK cause deficiency of the kinase in both liver and skeletal muscle (GSD IXb). Mutations in the PHKG2 gene that encodes the liver isoform of the γ-subunit of PHK induce deficiency of the enzyme in the liver (GSD IXc) [70], [71] (Table 3). Mutations in the PHKG1 gene encoding the muscle isoform of the γ-subunit of PHK have not been reported.
Table 3.
Glycogen phosphorylase kinase (PHK) subunits.
| PHK subunit | Gene | Glycogen storage disease | Glycogenolysis defect |
|---|---|---|---|
| α-1 (Muscle isoform) | PHKA1 | VIII | Skeletal muscle |
| α-2 (Liver isoform) | PHKA2 | IXa | Liver |
| β | PHKB | IXb | Liver and muscle |
| γ-1 (Muscle isoform) | PHKG1 | No mutations reported | |
| γ-2 (Liver isoform) | PHKG2 | IXc | Liver |
3.1.1.5. Glycogen phosphorylase kinase deficiency due to mutations in the PHKA1 gene (GSD VIII)
Congenital deficiency of the skeletal muscle isoform of the α-subunit of PHK (PHKA1) is an X-linked disorder that leads to an impairment of glycogen breakdown in skeletal muscle by reducing glycogen phosphorylase activation. GSD VIII is usually a mild myopathy with slight elevation of plasma creatine kinase concentration and muscle glycogen content [72]. Mutations in the PHKA1 gene may also result in cognitive impairment with no apparent myopathy [73].
3.1.1.6. Glycogen phosphorylase kinase deficiency due to mutations in the PHKA2 gene (GSD IXa)
Congenital deficiency of the liver isoform of the α-subunit of PHK (PHKA2) is an X-linked disorder that results in defective glycogenolysis in the liver clinically characterized by fasting intolerance with ketotic hypoglycemia, hepatomegaly, chronic liver disease, and growth retardation. Patients harboring mutations in the PHKA2 gene display a wide spectrum of clinical severity. Frequent doses of uncooked cornstarch and protein supplementation have been suggested to treat patients with GSD type IXa [71].
3.1.1.7. Glycogen phosphorylase kinase deficiency due to mutations in the PHKB gene (GSD IXb)
Mutations in the PHKB gene that encodes the β-subunit of PHK cause an autosomal recessive disease characterized by deficiency of the enzyme in both liver and skeletal muscle leading to defective glycogenolysis in both tissues [70]. Patients with mutations in the PHKB gene usually exhibit a mild clinical phenotype, including hypoglycemia after prolonged fasting, hepatomegaly, and mild muscle hypotonia [71].
3.1.1.8. Glycogen phosphorylase kinase deficiency due to mutations in the PHKG2 gene (GSD IXc)
Congenital deficiency of the liver isoform of the γ-subunit of PHK (PHKG2) cause an autosomal recessive disease with variable clinical phenotype ranging from mild disease characterized by tendency to hypoglycemia to liver fibrosis that may progress to liver cirrhosis during childhood. Hepatic adenomas have been occasionally reported [70], [71].
3.1.1.9. Congenital deficiency of cardiac glycogen phosphorylase kinase
Congenital deficiency of PHK in the heart has been rarely reported, likely because affected patients usually die of progressive hypertrophic cardiomyopathy during early infancy. Accumulation of normally structured glycogen is identified in the heart due to defective glycogenolysis. PHK activity is reduced in the heart while near-normal activity is usually observed in liver and skeletal muscle [74], [75].
3.1.2. Glycogen debranching enzyme
After glycogen phosphorylase has released the outer glucose 1-phosphate moieties from the glycogen chain, four glycosyl residues remain attached to the branch point and the action of glycogen debranching enzyme (AGL) is required to untangle the branch points. AGL is a monomeric protein that possesses two catalytic activities on a single polypeptide chain, α-1,4-glucanotransferase and amylo-α-1,6-glucosidase, in order to catalyze the removal of branching from glycogen via a two-step process. First, the transferase activity relocates three glucose residues from the lateral thread that contains four glucose moieties to another linear strand. Second, the glucosidase activity hydrolyzes the α-1-6-glycosidic bond of the branch-point, liberating glucose and permitting the access of glycogen phosphorylase to the α-1,4 linkages [76].
The human gene encoding AGL has been mapped to chromosome 1p21 and its structural organization has been reported [77]. Mutations in the AGL gene that affect the carbohydrate binding domain of the enzyme impair its ability to bind glycogen and induce loss of both transferase and glucosidase activities. Mutations in other locations of the AGL gene may deteriorate either glucosidase or transferase activities [78]. Glycogen debranching enzyme has been identified as a prognostic marker in patients with bladder cancer, low AGL expression indicating aggressive disease [79].
3.1.2.1. Glycogen debranching enzyme deficiency or Cori–Forbes disease (GSD III)
Congenital deficiency of glycogen debrancher is an autosomal recessive disorder characterized by defective debranching of glycogen that results in accumulation of an abnormally structured glycogen in affected tissues, including liver, skeletal muscle, and heart [76]. Clinical manifestations of GSD type III affect predominantly to the liver, skeletal muscle, and heart. Most patients with GSD type III show both myopathy and hepatopathy (GSD type IIIa) whereas approximately 15% have only liver involvement without evidence of cardiomyopathy or myopathy (GSD type IIIb). In rare cases, there is selective loss of only one of the two enzyme activities. When the glucosidase activity is lacking, the disease is called GSD type IIIc whereas patients with GSD type IIId display AGL transferase deficiency with normal glucosidase activity [80]. Liver involvement is characterized by hepatomegaly and liver cirrhosis. Hepatic adenoma may develop and hepatocellular carcinoma may be a complication. Intolerance to fasting with ketotic hypoglycemia also occurs and may resemble the clinical phenotype of fatty acid oxidation disorders with increased plasma concentration of medium-chain fatty acids, predominantly C8 and C10 [81].
In patients with GSD type III, defective glycogen debranching in skeletal muscle leads to muscle weakness and reduced exercise capacity [82]. During exercise, the oxidation of glucose in skeletal muscle is lower while oxidation of fatty acids is higher in these patients compared to healthy subjects [83]. Deficiency of AGL in the heart produces cardiomyopathy that echocardiographically mimics idiopathic hypertrophic cardiomyopathy [84]. In order to avoid hypoglycemia, frequent meals high in carbohydrate with uncooked cornstarch supplements may be implemented. A ketogenic diet has been used in some patients with GSD type III [3]. Fructose ingestion improves exercise tolerance in these patients [85].
3.2. Glycogen degradation in the lysosomes: lysosomal acid α-glucosidase (GAA)
Glycogen may be deposited inside the lysosomes via autophagic vacuoles that enclose a fraction of the cytoplasm and fuse with these organelles in order to process their content. Inside the lysosomes glycogen is hydrolyzed to glucose by the enzyme acid α-1,4-glucosidase, 1,4-α-glucan hydrolase or acid maltase (GAA). It is generally accepted that this enzyme primarily hydrolyzes 1,4-linked α-glucose polymers, being unclear the way in which the branch points of lysosomal glycogen are untangled. Human GAA undergoes post-translational processing to become the functional enzyme present inside the lysosomes, a glycoprotein containing N-linked carbohydrate chains [86]. The enzyme is initially translated as a precursor polypeptide of 110 kDa that displays seven potential glycosylation sites in its primary structure. N-linked glycosylation of GAA occurs in the endoplasmic reticulum by attachment of carbohydrate side chains to asparagine residues in the protein precursor. Some of the carbohydrate chains attached to the GAA precursor are phosphorylated. The phosphorylation of mannose residues to form mannose 6-phosphate targets the GAA precursor to the lysosomes. The maturation of GAA involves additional proteolytic processing at both the amino- and carboxyl-terminal ends, resulting in a 95 kDa intermediate form and finally in the formation of two lysosomal species of 76 and 70 kDa which are considered to represent the mature species of the enzyme [87].
3.2.1. Lysosomal α-glucosidase (acid maltase) deficiency or Pompe disease (GSD II)
The gene encoding human GAA (GAA) is located on chromosome 17q25.2-q25.3. Mutations in the GAA locus cause GSD type II or Pompe disease, an autosomal recessive disorder due to congenital deficiency of functional GAA characterized by accumulation of glycogen in the lysosomes primarily of muscle tissue, including skeletal muscle, cardiac muscle, and smooth muscle [88]. Clinical manifestations of Pompe disease involve predominantly skeletal muscle and heart. The infantile form usually appears in the first month of life and progresses rapidly, being characterized by severe cardiac involvement. The late-onset (adult form) form is characterized by progressive muscle weakness that may result in respiratory failure due to involvement of respiratory muscles [89]. Adult patients with Pompe disease show reduced peak oxidative capacity, but fat and carbohydrate oxidation in skeletal muscle during exercise is similar to healthy subjects, suggesting that acid maltase does not play a significant role in the production of energy during exercise. Intravenous glucose infusion failed to improve exercise tolerance in patients with Pompe disease [90]. Histopathologic findings in muscle biopsies obtained from late-onset patients reveal vacuolar degeneration and glycogen granules in lysosomes of muscle cells. Autophagic vacuoles are visible occasionally. Excess glycogen is also present in the capillary wall of muscle and skin [91]. Adult patients with Pompe disease typically exhibit milder clinical course compared to infantile patients [92]. Enzyme replacement therapy with human recombinant and transgenic acid α-glucosidase is used in patients with Pompe disease, although this therapy has some limitations, including the variability of response among different tissues and the formation of neutralizing antibodies. Gene therapy might be of benefit in the future [93]. l-alanine has been assessed in a double blind placebo-controlled crossover study in one patient with GSD type II. The patient showed no improvement [94].
Exercise training has a positive effect on muscular strength and functional capacity in patients with late-onset Pompe disease [95].
3.2.1.1. Danon disease
Danon disease is an X-linked dominant condition caused by mutations in the gene that encodes lysosome-associated membrane protein-2 (LAMP2 gene), mapped to Xq24. Clinical manifestations include hypertrophic cardiomyopathy or dilated cardiomyopathy and myopathy. Some patients also show intellectual disability. In most cases, males experience earlier and more severe symptoms than females. The pathogenesis of this disorder is mostly unknown but the LAMP-2 protein may be involved in the fusion between autophagic vacuoles and lysosomes and mutations in the LAMP2 gene may impair the process of transporting cellular material into the lysosome [96].
4. Glucose dephosphorylation: glucose 6-phosphatase system
Glucose 1-phosphate released from the glycogen chain undergoes isomerization to glucose 6-phosphate by phosphoglucomutases. Glucose 6-phosphate is then dephosphorylated to free glucose in order to leave the cell. This reaction takes place in the endoplasmic reticulum and is accomplished by the coordinated action of glucose 6-phosphate translocase, that transports glucose 6-phosphate from the cytosol into the endoplasmic reticulum, and glucose 6-phosphatase isoenzymes, that catalyze the dephosphorylation of glucose 6-phosphate to free glucose and inorganic phosphate [97].
4.1. Glucose 6-phosphate translocase or glucose 6-phosphate transporter
The transport of glucose 6-phosphate from the cytosol to the endoplasmic reticulum is accomplished by glucose 6-phosphate translocase or glucose 6-phosphate transporter (G6PT), encoded by the gene SLC37A4, mapped to human chromosome 11q23.
Human G6PT is an integral membrane protein that exchanges cytoplasmic glucose 6-phosphate for inorganic phosphate stored in the lumen of endoplasmic reticulum. In human tissues, the G6PT transcript is ubiquitously expressed, being detected in brain, heart, skeletal muscle, placenta, spleen, liver, kidney, adrenal gland, lymph node, neutrophils, monocytes, intestine, and lung. Alternative splicing produces a variant of G6PT specifically expressed in the brain, heart, and skeletal muscle [98].
4.2. Glucose 6-phosphatase isoenzymes
Three isoforms of glucose 6-phosphatase have been identified in humans, namely glucose 6-phosphatase-1 (G6PC1), glucose 6-phosphatase-2 (G6PC2), and glucose 6-phosphatase-3 (G6PC3). G6PC1 is encoded by the G6PC1 gene, located on chromosome 17q21. G6PC1 is a glycoprotein anchored in the membrane of the endoplasmic reticulum with the active center facing into the lumen. It is generally accepted that the expression of G6PC1 is restricted to liver, kidney, and intestine [97]. G6PC2 is encoded by the G6PC2 gene, located on human chromosome 2q28–q32. G6PC2 is the pancreatic islet-specific isoform of glucose 6-phosphatase, as the G6PC2 gene is expressed only in human pancreas, other tissues being negative. The function of G6PC2 is unknown [99]. G6PC3 is encoded by the G6PC3 gene, located on human chromosome 17q21.31. The G6PC3 gene is ubiquitously expressed, although predominantly in brain, muscle, and kidney. There is no expression of G6PC3 in the liver. The biologic role of G6PC3 is poorly defined [97], [100].
4.2.1. Glucose 6-phosphatase system deficiency or von Gierke disease (GSD I)
GSD type I may be caused by congenital deficiency of either glucose 6-phosphatase-1 which produce GSD type Ia or glucose 6-phosphate translocase that cause GSD type Ib. GSD type Ia represents approximately 80% of patients with GSD type I.
4.2.2. Mutations in the G6PC1 gene (GSD Ia)
Mutations in the G6PC1 gene that encodes glucose 6-phosphatase-1 result in GSD type Ia, an autosomal recessive disorder characterized by defective hydrolysis of glucose 6-phosphate to glucose which leads to intracellular elevation of glucose 6-phosphate and inadequate release of glucose to the blood stream. The inability to release glucose from the liver during fasting induces fasting hypoglycemia. The intracellular elevation of glucose 6-phosphate promotes alternative pathways of glucose metabolism inducing excess formation of triglycerides, lactic acid, and uric acid, leading to hypertriglyceridemia, lactic acidosis, and hyperuricemia. Glycogen is accumulated in the liver, kidney, and intestine. Hepatomegaly and hepatic adenomas that rarely evolve to hepatocarcinoma may be present. Kidney manifestations are prominent in this disease including kidney enlargement, glomerular hyperfiltration, and proximal tubular dysfunction with glucosuria, phosphaturia, aminoaciduria, hypokalemia, and β-2 microglobulinuria. Chronic kidney failure may develop as a late complication. Kidney stones composed of calcium oxalate or uric acid may occur. Some patients affected with GSD Ia suffer osteoporosis. Liver transplantation may be beneficial in selected patients with GSD Ia [100].
4.2.3. Mutations in the G6PT gene (GSD Ib)
Mutations in the G6PT gene that encodes glucose 6-phosphate translocase cause GSD Ib, an autosomal recessive disorder due to the failure to transport glucose 6-phosphate into the lumen of the endoplasmic reticulum to be dephosphorylated. The metabolic phenotype of GSD Ib is similar to that of GSD Ia. In addition, some patients with GSD Ib develop neutropenia and neutrophil dysfunction with tendency to bacterial infections. The underlying cause of these alterations in GSD Ib is unknown, but neutrophil functional impairment is reflected by reduced chemotaxis and respiratory burst [101]. Patients with GSD Ib are at risk for inflammatory bowel disease. Autoimmune endocrine disorders including thyroiditis and growth hormone deficiency may also occur [102].
To prevent fasting hypoglycemia in patients with GSD I and metabolic phenotype, frequent (every 3–4 h) oral uncooked cornstarch during the day and at night or a continuous nasogastric infusion of glucose is used [103]. A meta-analysis indicates that overnight intermittent administration of uncooked cornstarch prevents nocturnal hypoglycemia in GSD-Ia children more effectively than continuous nocturnal feeding of dextrose. Waxy maize extended release cornstarch at bedtime to preserve overnight glucose level has shown to improve the quality of life of patients with GSD I while maintaining adequate metabolic control [104].
4.2.4. Glucose 6-phosphatase catalytic-3 deficiency
Mutations in the G6PC3 locus cause an autosomal recessive disorder called severe congenital neutropenia type 4, characterized by congenital neutropenia and neutrophil dysfunction, recurrent bacterial infections, oral and intestinal mucosal ulcerations and inflammatory intestinal diseases, intermittent thrombocytopenia, facial dysmorphism, a prominent superficial venous pattern, congenital heart defects, and urogenital malformations. These patients do not suffer metabolic disturbance [105].
Patients with congenital deficiency of either G6PC3 or G6PT exhibit a severe defect in the synthesis of N- and O-glycans in the neutrophils, showing a profound alteration on the N- and O-glycosylation profiles of these cells. The mechanism of this anomalous glycosylation is unclear, but it is predicted to have a major negative effect on neutrophil function. It has been proposed that both severe congenital neutropenia type 4 (due to mutations in the G6PC3 gene) and GSD type Ib (due to mutations in the G6PT gene) should be designated as a new class of congenital disorders of glycosylation [106].
5. Glycogen storage diseases induced by congenital deficiency of glycolytic enzymes
The glycolytic pathway converts glucose into lactate being a major catabolic pathway in glucose metabolism (Fig. 5). Defective glycolysis might divert glucose utilization to other routes, including glycogen synthesis. Congenital deficiency of some enzymes involved in the glycolytic pathway has been categorized as GSDs, although some of them do not appear to induce a significant disturbance on glycogen metabolism.
5.1. Phosphofructokinase deficiency or Tarui disease (GSD VII)
GSD type VII (Tarui disease) is an autosomal recessive disorder due to congenital deficiency of phosphofructokinase-1 (PFK) in skeletal muscle. Phosphofructokinase-1 catalyzes the phosphorylation of fructose 6-phosphate to fructose 1,6-bisphosphate in the glycolytic pathway [107]. There are three isoenzymes of phosphofructokinase in humans, muscle (M), liver (L) and platelet or fibroblast, encoded by separate loci, mapping to 12q13.3, 21q22, and 10p15 respectively. Human skeletal muscle expresses only the M isoenzyme whereas erythrocytes express both the M and L isoforms [108]. The activity of phosphofructokinase-1 in skeletal muscle is absent in patients affected with Tarui disease while the activity of the enzyme in the liver is normal and the activity in the erythrocyte is reduced by about 50%. Muscle phosphofructokinase deficiency is clinically characterized by exercise intolerance associated with hemolytic anemia. Hypertrophic cardiomyopathy may occur. Muscle biopsy reveals lack of phosphofructokinase activity and excessive glycogen storage in muscle fibers [109]. A ketogenic diet produced clinical improvement in a patient with phosphofructokinase deficiency [3].
5.2. Phosphoglycerate mutase deficiency (GSD X)
Phosphoglycerate mutase (PGAM) catalyzes the interconversion of 3-phosphoglycerate and 2-phosphoglycerate in the glycolytic pathway. Two isoenzymes of phosphoglycerate mutase, brain (PGAM1) and muscle (PGAM2), are known to exist in humans, encoded by separate genes located to 7p13–p12 and 10q25.3, respectively [110]. Deficiency of the skeletal muscle isoenzyme is an autosomal recessive condition characterized by elevated plasma level of creatine kinase, intolerance to exercise and recurrent episodes of myoglobinuria after exercise. Forearm ischemic exercise causes abnormally low venous lactate responses. Glycogen content in muscle biopsy is normal or slightly increased [111].
5.3. Aldolase A deficiency (GSD XII)
Aldolase A catalyzes the interconversion of fructose 1,6-bisphosphate into glyceraldehyde 3-phosphate and dihydroxyacetone phosphate in the glycolytic pathway. This enzyme is encoded by the ALDOA gene located on chromosome 16p11.2. In 1996, one patient with intolerance to exercise due to congenital deficiency of aldolase A was reported. In this patient, PAS staining of muscle tissue was negative and electron microscopy failed to reveal glycogen accumulation [112]. Red cell aldolase deficiency has been associated with increased hepatic glycogen and hereditary hemolytic anemia [113]. Congenital deficiency of aldolase A has been termed GSD type XII [114].
5.3.1. β-Enolase (enolase-3) deficiency (GSD XIII)
Enolase catalyzes the interconversion of 2-phosphoglycerate to 2-phosphoenolpyruvate in the glycolytic pathway. There are three isoenzymes of enolase, namely α, β, and γ, encoded by different genes. The human β-enolase gene (ENO3) is expressed in skeletal muscle and maps to chromosome 17p13.2. α-Enolase is ubiquitously expressed while γ-enolase is neuron-specific [115]. Congenital deficiency of enolase-3 (β-enolase) has been reported in three patients and this disorder has been named GSD type XIII. Patients affected with congenital deficiency of β-enolase suffer from adult-onset intolerance to exercise and recurrent episodes of rhabdomyolysis and myogloburinuria induced by exercise. In one patient, ultrastructural analysis of muscle biopsy reveals scattered accumulation of glycogen while muscle biopsy shows no glycogen deposition in the other two patients [116].
5.4. Lactate dehydrogenase deficiency
Lactate dehydrogenase (LDH) catalyzes the final reaction of the glycolytic pathway, the reversible conversion between pyruvate and L-lactate. The two subunits A and B that compose the tetrameric LDH molecule are encoded by separate genes, LDHA, located on 11p15.4 and LDHB, sited on 12p12.2–p12.1. Inherited deficiency of the LDH A subunit (LDH A deficiency) is an autosomal recessive disease clinically characterized by exercise intolerance with recurrent rhabdomyolysis after strenuous exercise [117]. Both LDH A deficiency and Fanconi Bickel disease have been named GSD type XI.
6. Glycogen metabolism in liver and skeletal muscle of healthy humans
Glycogen is predominantly stored in liver and skeletal muscle. It has been identified in other human tissues such as brain, heart, kidney, adipose tissue, and erythrocytes, but its function in these tissues is mostly unknown. Glycogen depots in liver and skeletal muscle accomplish partially different functions and are regulated in a different manner. Liver glycogen predominantly supplies glucose to the blood stream during fasting periods whereas glycogen stored in skeletal muscle provides glucose to muscle fibers during muscle contraction. Consequently, liver glycogen content decreases during fasting and muscle glycogen content diminishes after exercise in the working muscles. There is no major decrease of muscle glycogen content during short periods of fasting. These two main glycogen depots are physiologically related to each other, as liver glycogen delivers glucose to the blood stream during exercise and lactate produced in the muscle during muscle contraction is converted to glucose in the liver contributing to hepatic glycogen replenishment. During physical exercise, glucose uptake by the contracting muscle increases despite low concentration of insulin.
6.1. Glycogen metabolism in the liver
Liver glycogen is predominantly restored during the postprandial period in healthy humans. The source of glucose moieties that form the glycogen particle in the liver is either the ingested food (direct pathway of glycogen synthesis) or the gluconeogenesis route (indirect pathway), that produces glucose 6-phosphate from precursors such as lactate and alanine [118]. Among patients with congenital glucokinase deficiency, the indirect pathway becomes more important than the direct pathway to synthesize glycogen after food ingestion, as phosphorylation of glucose to glucose 6-phosphate in the liver is impaired hindering the utilization of food-derived glucose [15]. The role of other monosaccharides such as fructose and galactose as sources of hepatic glycogen in humans is unclear. Dietary galactose has been estimated to contribute approximately 19% to liver glycogen synthesis in healthy individuals [119]. Galactose or fructose supplementation has been reported to be more effective than glucose in restoring liver glycogen after exercise in trained subjects [120].
6.2. Glycogen metabolism in the skeletal muscle
6.2.1. Exercise diminishes glycogen concentration in contracting skeletal muscle
Glucose released from glycogen is a major energy source for contracting muscles and high-intensity physical exercise depletes glycogen stores in the active skeletal muscle. Skeletal muscle biopsies obtained from healthy volunteers reveal that glycogen content is markedly reduced following cycling exercise in the working muscles while the glycogen level of the inactive muscles remains unchanged [121], [122]. The endurance capacity of skeletal muscle to exercise is associated with muscle glycogen content and fatigue develops when glycogen storage is exhausted in the active muscles [121]. The administration of fructose to spare muscle glycogen has been reported both similar to glucose or placebo [123] and better than glucose or placebo [124]. Elevated concentration of free fatty acids in plasma contributes to spare skeletal muscle glycogen during exercise [125].
6.2.2. Exercise promotes glycogen storage in the previously active skeletal muscle
Prior exercise enhances the capacity of skeletal muscle to store glycogen expanding the glycogen reserve in active muscles. Skeletal muscle biopsy samples from healthy volunteers demonstrate that, in the previously exercised muscles, the glycogen content during recovery increases rapidly reaching overtime a greater level compared to the pre-exercise level [121], [122]. In addition, training reduces muscle glycogen utilization during exercise in healthy subjects, enhancing the capacity of skeletal muscle to metabolize fat [125]. Consequently, muscle glycogen content is higher in endurance trained subjects compared to untrained subjects [126].
The increase of glycogen synthesis in response to previous exercise requires intact glycogenolysis. In patients with McArdle's disease, glycogenolysis is deficient in skeletal muscle due to congenital deficiency of glycogen phosphorylase. These patients show low basal levels of glycogen synthase activity and reduced activation of glycogen synthase following exercise compared to controls [39].
In addition to exercise, muscle glycogen content by itself influences glycogen synthesis in healthy humans. A high glycogen concentration inhibits the synthesis of glycogen in skeletal muscle [68].
The effectiveness of fructose, galactose, and amino acids to improve glycogen restoration has been investigated in a number of studies. The rate of glycogen synthesis in exercised muscles during the recovery period has been reported higher during glucose administration compared with fructose feeding. The addition of fructose to glucose does not improve the restoration capacity of glucose alone [127]. The co-ingestion of amino acids and carbohydrate does not increase the rate of glycogen synthesis in skeletal muscle after exercise compared with the same amount of carbohydrate in healthy subjects [128]. In trained subjects, the addition of amino acids to galactose supplementation does not improve muscle glycogen restoration during the recovery period [129].
6.2.3. Dietary modifications alone do not alter significantly glycogen storage capacity in resting muscles
In periods of muscle resting, neither fasting nor food ingestion largely change glycogen stores in skeletal muscle. The muscle glycogen content increases slightly by acute intake of large amount of carbohydrates. After an oral glucose tolerance test, approximately 15% of the ingested glucose is stored as muscle glycogen in healthy subjects. Prolonged intake of high amount of carbohydrates does not increase glycogen content in skeletal muscle in untrained subjects [129]. Glycogen level in inactive muscles is unmodified by high carbohydrate delivery [121], [122]. Skeletal muscle biopsies from healthy subjects show that glycogen concentration increases slightly in the inactive muscle whereas previously working muscles augment markedly their glycogen content after exercise when carbohydrate-rich food is ingested [121]. The glycogen concentration in skeletal muscle is not significantly altered in the unexercised muscle following either low-carbohydrate or high-carbohydrate diet [122]. Glycogen synthesis in cultured human skeletal muscle cells increases when leucine is added to insulin, as compared with insulin alone [131]. Elevated plasma concentration of free fatty acids does not change muscle glycogen content in healthy individuals, either basally or during hyperinsulinemia [132]. An increase in the l-carnitine content of skeletal muscle may contribute to augment muscle glycogen content in healthy subjects [133].
6.2.4. Role of exercise in glycogen storage diseases
Exercise intolerance with cramps and episodes of rhabdomyolysis and myoglobinuria usually occur in GSDs that affect skeletal muscle, including deficiency of phosphoglucomutase-1, glycogenin-1, muscle glycogen synthase, muscle glycogen phosphorylase, muscle phosphorylase kinase, glycogen debranching enzyme, lysosomal acid α-glucosidase, muscle phosphofructokinase, phosphoglycerate mutase, aldolase A, enolase-3, and LDH A. A regular exercise training program may improve muscular performance in patients diagnosed with these diseases by enhancing the ability of muscle to oxidize fatty acids and by reducing the increased dependence on glycogen associated with prolonged muscle rest. Light intensity physical activity has improved exercise capacity in patients with McArdle disease (myophosphorylase deficiency) and Pompe disease (lysosomal acid α-glucosidase). The effect of regular exercise on muscle performance is not well known in other glycogenoses [134].
6.2.4.1. Concluding remarks
Glycogen metabolism is important to many physiological events in the human body. The synthesis of glycogen allows the storage of glucose to be used as fuel during periods of fasting or muscle contraction via glycogen breakdown. Both the assembly and degradation of glycogen are complex processes that require the coordinated action of a number of enzymes. Congenital deficiency of these enzymes typically results in fasting hypoglycemia, exercise intolerance or both. In addition to liver and skeletal muscle involvement, GSDs may display a broad clinical phenotype suggesting that glycogen depots in the human body may have other functions besides the release of glucose to be oxidized.
Some abnormalities of glycogen metabolism have a substantial impact on the nervous system. Congenital deficiency of glycogen branching enzyme (adult polyglucosan body disease) has devastating neurological effects, including cognitive impairment, neurogenic bladder, spastic paraplegia, and axonal neuropathy. Laforin and malin are proteins of elusive function in humans, whose influence on glycogen assembly is underlined by Lafora disease, characterized by progressive myoclonus epilepsy and cytoplasmic accumulation of polyglucosan in several tissues, including liver, skeletal muscle, heart, skin, kidney, and brain (neurons).
Altered glycogen metabolism may produce kidney dysfunction. Mutations in glucose 6-phosphatase gene result in proximal tubular dysfunction and kidney failure. Mutations in the glucose transporter GLUT2 also induce a proximal tubular nephropathy. Congenital deficiency of glycogen branching enzyme (GSD type IV) may rarely cause proteinuria, stroke-like episodes and hypohydrosis suggesting a diagnosis of Fabry's disease.
Glycogen is a vital molecule for healthy cardiac function. Hypertrophic or dilated cardiomyopathy and conduction system abnormalities are present in most GSDs. Mutations in the PRKAG2 gene that encodes the γ-2 regulatory subunit of 5'AMP-activated protein kinase cause familial hypertrophic cardiomyopathy and conduction system abnormalities in the heart with accumulation of amylopectin-like material in the heart.
Glycogen metabolism is impaired in patients with maturity onset diabetes of the young due to inactivating mutations in the glucokinase gene, underlining the role of glucose phosphorylation in the pathogenesis of diabetes mellitus. Hepatic replenishment of glycogen after a meal is impaired in glucokinase-deficient patients.
Similarly, patients with congenital deficiency of hepatic glycogen synthase are unable to store glycogen in the liver after meals, showing postprandial hyperglycemia, glucosuria, and hyperlactatemia. The scarcity of hepatic glycogen results in fasting intolerance with ketotic hypoglycemia.
Glycogen metabolism is linked to fatty acid metabolism. Congenital deficiency of glycogen debranching enzyme may clinically mimic fatty acid oxidation disorders with increased plasma concentration of medium-chain fatty acids, predominantly C8 and C10.
Lack of glycogen depot in liver, heart, and skeletal muscle has been reported in a case of carnitine acyl-carnitine translocase, the enzyme that transports acyl-carnitine esters across the inner mitochondrial membrane in exchange for free l-carnitine, underlining the relationship between glycogen metabolism and fatty acid metabolism.
In the skeletal muscle of healthy subjects a significant correlation has been found between l-carnitine concentration and glycogen content in skeletal muscle tissue biopsy samples. The increase in l-carnitine contributes to raise glycogen content in skeletal muscle of healthy individuals.
A model of the glycogen molecule has been developed that may contribute to understand the pathophysiology of GSDs. The crystal structure of some human enzymes involved in glycogen metabolism has been disclosed.
The relationship between glycogen metabolism and glycosylation processes is beginning to be outlined. Congenital deficiency of phosphoglucomutase-1 has been associated with a congenital disorder of glycosylation clinically characterized by fasting hypoglycemia, myopathy, dilated cardiomyopathy, and cardiac arrest, but the mechanism underlying defective glycosylation due to phosphoglucomutase-1 deficiency remains undefined.
Phosphoglucomutase-3 action is required for glycosylation of proteins and lipids. Congenital deficiency of phosphoglucomutase-3 is associated with a congenital disorder of glycosylation characterized by widespread clinical manifestations, including atopy with increased serum IgE levels, immune deficiency with recurrent bacterial and viral infections, autoimmunity, and motor and neurocognitive impairment. Mutations in the glucose 6-phosphate translocase gene impairs the transport of glucose 6-phosphate into the lumen of the endoplasmic reticulum to be dephosphorylated. Free glucose fails to be released from the liver during periods of fasting and glycogen is accumulated in some tissues including liver, kidney, and intestine. Some patients develop immune dysfunction owing to a marked defect in glycosylation processes in the neutrophils.
Therapy of GSDs is limited at present. Enzyme replacement therapy is available for lysosomal α-glucosidase deficiency but its efficacy is not universal. Gene therapy may be useful in the future. Nonviral gene vectors are being developed. The development of induced pluripotent stem cells from patients with GSD Ib may contribute to advances in the therapy of this disease. The design of therapeutic strategies for these diseases depends on the understanding of the structure of the genes, their transcriptional and epigenetic regulation, and the mechanisms of action and control of the enzymes involved in glycogen metabolism. In this review, we have tried to emphasized aspects of glycogen metabolism with translational potential.
Transparency document
Transparency document.
Acknowledgments
We are grateful to Ms. Gema Souto for her help during the writing of this manuscript.
Footnotes
The authors declare that there are no conflicts of interest.
There was no financial support for this work.
The Transparency document associated with this article can be found in the online version.
References
- 1.Chikwana V.M., Khanna M., Baskaran S. Structural basis for 2ʹ-phosphate incorporation into glycogen by glycogen synthase. Proc. Natl. Acad. Sci. U. S. A. 2013;110(52):20976–20981. doi: 10.1073/pnas.1310106111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Giorgis V., Veggiotti P. GLUT1 deficiency syndrome 2013: current state of the art. Seizure. 2013;22(10):803–811. doi: 10.1016/j.seizure.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Scholl-Bürgi S., Höller A., Pichler K., Michel M., Haberlandt E., Karall D. Ketogenic diets in patients with inherited metabolic disorders. J. Inherit. Metab. Dis. 2015;38(4):765–773. doi: 10.1007/s10545-015-9872-2. [DOI] [PubMed] [Google Scholar]
- 4.Gottesman I., Mandarino L., Gerich J. Estimation and kinetic analysis of insulin-independent glucose uptake in human subjects. Am. J. Physiol. 1983;244(6):E632–E635. doi: 10.1152/ajpendo.1983.244.6.E632. [DOI] [PubMed] [Google Scholar]
- 5.Lauritzen H.P., Schertzer J.D. Measuring GLUT4 translocation in mature muscle fibers. Am. J. Physiol. Endocrinol. Metab. 2010;299(2):E169–E179. doi: 10.1152/ajpendo.00066.2010. [DOI] [PubMed] [Google Scholar]
- 6.Jensen J., Rustad P.I., Kolnes A.J., Lai Y.C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front. Physiol. 2011;2:112. doi: 10.3389/fphys.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akagi M., Inui K., Nakajima S. Mutation analysis of two Japanese patients with Fanconi–Bickel syndrome. J. Hum. Genet. 2000;45(1):60–62. doi: 10.1007/s100380050013. [DOI] [PubMed] [Google Scholar]
- 8.Santer R., Schneppenheim R., Suter D., Schaub J., Steinmann B. Fanconi–Bickel syndrome—the original patient and his natural history, historical steps leading to the primary defect, and a review of the literature. Eur. J. Pediatr. 1998;157(10):783–797. doi: 10.1007/s004310050937. [DOI] [PubMed] [Google Scholar]
- 9.Lehto M., Xiang K., Stoffel M. Human hexokinase II: localization of the polymorphic gene to chromosome 2. Diabetologia. 1993;36(12):1299–1302. doi: 10.1007/BF00400809. [DOI] [PubMed] [Google Scholar]
- 10.Bedoya F.J., Wilson J.M., Ghosh A.K., Finegold D., Matschinsky F.M. The glucokinase glucose sensor in human pancreatic islet tissue. Diabetes. 1986;35(1):61–67. doi: 10.2337/diab.35.1.61. [DOI] [PubMed] [Google Scholar]
- 11.Pilkis S.J., Weber I.T., Harrison R.W., Bell G.I. Glucokinase: structural analysis of a protein involved in susceptibility to diabetes. J. Biol. Chem. 1994;269(35):21925–21928. [PubMed] [Google Scholar]
- 12.Lenzen S. A fresh view of glycolysis and glucokinase regulation: history and current status. J. Biol. Chem. 2014;289(18):12189–12194. doi: 10.1074/jbc.R114.557314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck T., Miller B.G. Structural basis for regulation of human glucokinase by glucokinase regulatory protein. Biochemistry. 2013;52(36):6232–6239. doi: 10.1021/bi400838t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raimondo A., Rees M.G., Gloyn A.L. Glucokinase regulatory protein: complexity at the crossroads of triglyceride and glucose metabolism. Curr. Opin. Lipidol. 2015;26(2):88–95. doi: 10.1097/MOL.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velho G., Petersen K.F., Perseghin G. Impaired hepatic glycogen synthesis in glucokinase-deficient (MODY-2) subjects. J. Clin. Invest. 1996;98(8):1755–1761. doi: 10.1172/JCI118974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putt W., Ives J.H., Hollyoake M., Hopkinson D.A., Whitehouse D.B., Edwards Y.H. Phosphoglucomutase 1: a gene with two promoters and a duplicated first exon. Biochem. J. 1993;296(Pt 2):417–422. doi: 10.1042/bj2960417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards Y.H., Putt W., Fox M., Ives J.H. A novel human phosphoglucomutase (PGM5) maps to the centromeric region of chromosome 9. Genomics. 1995;30(2):350–353. doi: 10.1006/geno.1995.9866. [DOI] [PubMed] [Google Scholar]
- 18.Stojkovic T., Vissing J., Petit F. Muscle glycogenosis due to phosphoglucomutase 1 deficiency. N. Engl. J. Med. 2009;361(4):425–427. doi: 10.1056/NEJMc0901158. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y., Stiers K.M., Kain B.N., Beamer L.J. Compromised catalysis and potential folding defects in in vitro studies of missense mutants associated with hereditary phosphoglucomutase 1 deficiency. J. Biol. Chem. 2014;289(46):32010–32019. doi: 10.1074/jbc.M114.597914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tegtmeyer L.C., Rust S., van Scherpenzeel M. Multiple phenotypes in phosphoglucomutase 1 deficiency. N. Engl. J. Med. 2014;370(6):533–542. doi: 10.1056/NEJMoa1206605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morava E. Galactose supplementation in phosphoglucomutase-1 deficiency; review and outlook for a novel treatable CDG. Mol. Genet. Metab. 2014;112(4):275–279. doi: 10.1016/j.ymgme.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pang H., Koda Y., Soejima M., Kimura H. Identification of human phosphoglucomutase 3 (PGM3) as N-acetylglucosamine-phosphate mutase (AGM1) Ann. Hum. Genet. 2002;66(Pt 2):139–144. doi: 10.1017/S0003480002001033. [DOI] [PubMed] [Google Scholar]
- 23.Sassi A., Lazaroski S., Wu G. Hypomorphic homozygous mutations in phosphoglucomutase 3 (PGM3) impair immunity and increase serum IgE levels. J. Allergy Clin. Immunol. 2014;133(5):1410–1419. doi: 10.1016/j.jaci.2014.02.025. (1419.e1411–1413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Yu X., Ichikawa M. Autosomal recessive phosphoglucomutase 3 (PGM3) mutations link glycosylation defects to atopy, immune deficiency, autoimmunity, and neurocognitive impairment. J. Allergy Clin. Immunol. 2014;133(5):1400–1409. doi: 10.1016/j.jaci.2014.02.013. (1409.e1401–1405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Q., Zheng X. The crystal structure of human UDP-glucose pyrophosphorylase reveals a latch effect that influences enzymatic activity. Biochem. J. 2012;442(2):283–291. doi: 10.1042/BJ20111598. [DOI] [PubMed] [Google Scholar]
- 26.Peng H.L., Chang H.Y. Cloning of a human liver UDP-glucose pyrophosphorylase cDNA by complementation of the bacterial galU mutation. FEBS Lett. 1993;329(1-2):153–158. doi: 10.1016/0014-5793(93)80213-e. [DOI] [PubMed] [Google Scholar]
- 27.Van Someren H., Van Henegouwen H.B., Westerveld A., Bootsma D. Synteny of the human loci for fumarate hydratase and udpg pyrophosphorylase with chromosome 1 markers in somatic cell hybrids. Cytogenet. Cell Genet. 1974;13(6):551–557. doi: 10.1159/000130306. [DOI] [PubMed] [Google Scholar]
- 28.Cheng S.D., Peng H.L., Chang H.Y. Localization of the human UGP2 gene encoding the muscle isoform of UDPglucose pyrophosphorylase to 2p13–p14 by fluorescence in situ hybridization. Genomics. 1997;39(3):414–416. doi: 10.1006/geno.1996.4426. [DOI] [PubMed] [Google Scholar]
- 29.Gibbons B.J., Roach P.J., Hurley T.D. Crystal structure of the autocatalytic initiator of glycogen biosynthesis, glycogenin. J. Mol. Biol. 2002;319(2):463–477. doi: 10.1016/S0022-2836(02)00305-4. [DOI] [PubMed] [Google Scholar]
- 30.Barbetti F., Rocchi M., Bossolasco M. The human skeletal muscle glycogenin gene: cDNA, tissue expression and chromosomal localization. Biochem. Biophys. Res. Commun. 1996;220(1):72–77. doi: 10.1006/bbrc.1996.0359. [DOI] [PubMed] [Google Scholar]
- 31.Mu J., Roach P.J. Characterization of human glycogenin-2, a self-glucosylating initiator of liver glycogen metabolism. J. Biol. Chem. 1998;273(52):34850–34856. doi: 10.1074/jbc.273.52.34850. [DOI] [PubMed] [Google Scholar]
- 32.Moslemi A.R., Lindberg C., Nilsson J., Tajsharghi H., Andersson B., Oldfors A. Glycogenin-1 deficiency and inactivated priming of glycogen synthesis. N. Engl. J. Med. 2010;362(13):1203–1210. doi: 10.1056/NEJMoa0900661. [DOI] [PubMed] [Google Scholar]
- 33.Malfatti E., Nilsson J., Hedberg-Oldfors C. A new muscle glycogen storage disease associated with glycogenin-1 deficiency. Ann. Neurol. 2014;76(6):891–898. doi: 10.1002/ana.24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen J.N., Richter E.A. Regulation of glycogen synthase in skeletal muscle during exercise. Acta Physiol. Scand. 2003;178(4):309–319. doi: 10.1046/j.1365-201X.2003.01165.x. [DOI] [PubMed] [Google Scholar]
- 35.Lehto M., Stoffel M., Groop L., Espinosa R., 3rd, Le Beau M.M., Bell G.I. Assignment of the gene encoding glycogen synthase (GYS) to human chromosome 19, band q13.3. Genomics. 1993;15(2):460–461. doi: 10.1006/geno.1993.1092. [DOI] [PubMed] [Google Scholar]
- 36.Nuttall F.Q., Gannon M.C., Kubic V.L., Hoyt K.J. The human liver Glycogen synthase isozyme gene is located on the short arm of chromosome 12. Genomics. 1994;19(2):404–405. doi: 10.1006/geno.1994.1086. [DOI] [PubMed] [Google Scholar]
- 37.Cameron J.M., Levandovskiy V., MacKay N. Identification of a novel mutation in GYS1 (muscle-specific glycogen synthase) resulting in sudden cardiac death, that is diagnosable from skin fibroblasts. Mol. Genet. Metab. 2009;98(4):378–382. doi: 10.1016/j.ymgme.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Weinstein D.A., Correia C.E., Saunders A.C., Wolfsdorf J.I. Hepatic glycogen synthase deficiency: an infrequently recognized cause of ketotic hypoglycemia. Mol. Genet. Metab. 2006;87(4):284–288. doi: 10.1016/j.ymgme.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen J.N., Wojtaszewski J.F., Haller R.G. Role of 5ʹAMP-activated protein kinase in glycogen synthase activity and glucose utilization: insights from patients with McArdle's disease. J. Physiol. 2002;541(Pt 3):979–989. doi: 10.1113/jphysiol.2002.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blair E., Redwood C., Ashrafian H. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum. Mol. Genet. 2001;10(11):1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- 41.Arad M., Benson D.W., Perez-Atayde A.R. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J. Clin. Invest. 2002;109(3):357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aggarwal V., Dobrolet N., Fishberger S., Zablah J., Jayakar P., Ammous Z. PRKAG2 mutation: an easily missed cardiac specific non-lysosomal glycogenosis. Ann. Pediatr. Cardiol. 2015;8(2):153–156. doi: 10.4103/0974-2069.154149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ragolia L., Begum N. Protein phosphatase-1 and insulin action. Mol. Cell. Biochem. 1998;182(1-2):49–58. [PubMed] [Google Scholar]
- 44.Akman H.O., Kakhlon O., Coku J. Deep intronic GBE1 mutation in manifesting heterozygous patients with adult polyglucosan body disease. JAMA Neurol. 2015;72(4):441–445. doi: 10.1001/jamaneurol.2014.4496. [DOI] [PubMed] [Google Scholar]
- 45.Magoulas P.L., El-Hattab A.W., Roy A., Bali D.S., Finegold M.J., Craigen W.J. Diffuse reticuloendothelial system involvement in type IV glycogen storage disease with a novel GBE1 mutation: a case report and review. Hum. Pathol. 2012;43(6):943–951. doi: 10.1016/j.humpath.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Aksu T., Colak A., Tufekcioglu O. Cardiac involvement in glycogen storage disease type IV: two cases and the two ends of a spectrum. Case Rep. Med. 2012;2012:764286. doi: 10.1155/2012/764286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sagnelli A., Savoiardo M., Marchesi C. Adult polyglucosan body disease in a patient originally diagnosed with Fabry's disease. Neuromuscul. Disord. 2014;24(3):272–276. doi: 10.1016/j.nmd.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Bao Y., Kishnani P., Wu J.Y., Chen Y.T. Hepatic and neuromuscular forms of glycogen storage disease type IV caused by mutations in the same glycogen-branching enzyme gene. J. Clin. Invest. 1996;97(4):941–948. doi: 10.1172/JCI118517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mochel F., Schiffmann R., Steenweg M.E. Adult polyglucosan body disease: natural history and key magnetic resonance imaging findings. Ann. Neurol. 2012;72(3):433–441. doi: 10.1002/ana.23598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Billot S., Herve D., Akman H.O. Acute but transient neurological deterioration revealing adult polyglucosan body disease. J. Neurol. Sci. 2013;324(1-2):179–182. doi: 10.1016/j.jns.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 51.Gentry M.S., Worby C.A., Dixon J.E. Insights into Lafora disease: malin is an E3 ubiquitin ligase that ubiquitinates and promotes the degradation of laforin. Proc. Natl. Acad. Sci. U. S. A. 2005;102(24):8501–8506. doi: 10.1073/pnas.0503285102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sankhala R.S., Koksal A.C., Ho L., Nitschke F., Minassian B.A., Cingolani G. Dimeric quaternary structure of human laforin. J. Biol. Chem. 2015;290(8):4552–4559. doi: 10.1074/jbc.M114.627406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minassian B.A., Ianzano L., Meloche M. Mutation spectrum and predicted function of laforin in Lafora's progressive myoclonus epilepsy. Neurology. 2000;55(3):341–346. doi: 10.1212/wnl.55.3.341. [DOI] [PubMed] [Google Scholar]
- 54.Ianzano L., Zhang J., Chan E.M. Lafora progressive myoclonus epilepsy mutation database-EPM2A and NHLRC1 (EPM2B) genes. Hum. Mutat. 2005;26(4):397. doi: 10.1002/humu.9376. [DOI] [PubMed] [Google Scholar]
- 55.Turnbull J., Girard J.M., Lohi H. Early-onset Lafora body disease. Brain. 2012;135(Pt 9):2684–2698. doi: 10.1093/brain/aws205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burwinkel B., Bakker H.D., Herschkovitz E., Moses S.W., Shin Y.S., Kilimann M.W. Mutations in the liver glycogen phosphorylase gene (PYGL) underlying glycogenosis type VI. Am. J. Hum. Genet. 1998;62(4):785–791. doi: 10.1086/301790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kato K., Shimizu A., Kurobe N., Takashi M., Koshikawa T. Human brain-type glycogen phosphorylase: quantitative localization in human tissues determined with an immunoassay system. J. Neurochem. 1989;52(5):1425–1432. doi: 10.1111/j.1471-4159.1989.tb09189.x. [DOI] [PubMed] [Google Scholar]
- 58.Newgard C.B., Littman D.R., van Genderen C., Smith M., Fletterick R.J. Human brain glycogen phosphorylase. Cloning, sequence analysis, chromosomal mapping, tissue expression, and comparison with the human liver and muscle isozymes. J. Biol. Chem. 1988;263(8):3850–3857. [PubMed] [Google Scholar]
- 59.Orngreen M.C., Jeppesen T.D., Taivassalo T. Lactate and energy metabolism during exercise in patients with blocked glycogenolysis (McArdle disease) J. Clin. Endocrinol. Metab. 2015 doi: 10.1210/jc.2015-1339. [DOI] [PubMed] [Google Scholar]
- 60.Orngreen M.C., Jeppesen T.D., Andersen S.T. Fat metabolism during exercise in patients with McArdle disease. Neurology. 2009;72(8):718–724. doi: 10.1212/01.wnl.0000343002.74480.e4. [DOI] [PubMed] [Google Scholar]
- 61.Wagenmakers A.J., Coakley J.H., Edwards R.H. Metabolism of branched-chain amino acids and ammonia during exercise: clues from McArdle's disease. Int. J. Sports Med. 1990;11(Suppl. 2):S101–S113. doi: 10.1055/s-2007-1024861. [DOI] [PubMed] [Google Scholar]
- 62.Petrou P., Pantzaris M., Dionysiou M., Drousiotou A., Kyriakides T. Minimally symptomatic McArdle disease, expanding the genotype-phenotype spectrum. Muscle Nerve. 2015 doi: 10.1002/mus.24716. [DOI] [PubMed] [Google Scholar]
- 63.Quinlivan R., Martinuzzi A., Schoser B. Pharmacological and nutritional treatment for McArdle disease (glycogen storage disease type V) Cochrane Database Syst. Rev. 2014;11 doi: 10.1002/14651858.CD003458.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newgard C.B., Fletterick R.J., Anderson L.A., Lebo R.V. The polymorphic locus for glycogen storage disease VI (liver glycogen phosphorylase) maps to chromosome 14. Am. J. Hum. Genet. 1987;40(4):351–364. [PMC free article] [PubMed] [Google Scholar]
- 65.Roscher A., Patel J., Hewson S. The natural history of glycogen storage disease types VI and IX: Long-term outcome from the largest metabolic center in Canada. Mol. Genet. Metab. 2014;113(3):171–176. doi: 10.1016/j.ymgme.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 66.Ercan-Fang N., Gannon M.C., Rath V.L., Treadway J.L., Taylor M.R., Nuttall F.Q. Integrated effects of multiple modulators on human liver glycogen phosphorylase a. Am. J. Physiol. Endocrinol. Metab. 2002;283(1):E29–E37. doi: 10.1152/ajpendo.00425.2001. [DOI] [PubMed] [Google Scholar]
- 67.Rath V.L., Ammirati M., LeMotte P.K. Activation of human liver glycogen phosphorylase by alteration of the secondary structure and packing of the catalytic core. Mol. Cell. 2000;6(1):139–148. [PubMed] [Google Scholar]
- 68.Munger R., Temler E., Jallut D., Haesler E., Felber J.P. Correlations of glycogen synthase and phosphorylase activities with glycogen concentration in human muscle biopsies. Evidence for a double-feedback mechanism regulating glycogen synthesis and breakdown. Metabolism. 1993;42(1):36–43. doi: 10.1016/0026-0495(93)90169-o. [DOI] [PubMed] [Google Scholar]
- 69.Keppens S., Vandekerckhove A., Moshage H., Yap S.H., Aerts R., De Wulf H. Regulation of glycogen phosphorylase activity in isolated human hepatocytes. Hepatology. 1993;17(4):610–614. doi: 10.1002/hep.1840170414. [DOI] [PubMed] [Google Scholar]
- 70.Bali D.S., Goldstein J.L., Fredrickson K. Variability of disease spectrum in children with liver phosphorylase kinase deficiency caused by mutations in the PHKG2 gene. Mol. Genet. Metab. 2014;111(3):309–313. doi: 10.1016/j.ymgme.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beauchamp N.J., Dalton A., Ramaswami U. Glycogen storage disease type IX: high variability in clinical phenotype. Mol. Genet. Metab. 2007;92(1–2):88–99. doi: 10.1016/j.ymgme.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 72.Orngreen M.C., Schelhaas H.J., Jeppesen T.D. Is muscle glycogenolysis impaired in X-linked phosphorylase b kinase deficiency? Neurology. 2008;70(20):1876–1882. doi: 10.1212/01.wnl.0000289190.66955.67. [DOI] [PubMed] [Google Scholar]
- 73.Echaniz-Laguna A., Akman H.O., Mohr M. Muscle phosphorylase b kinase deficiency revisited. Neuromuscul. Disord. 2010;20(2):125–127. doi: 10.1016/j.nmd.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 74.Mizuta K., Hashimoto E., Tsutou A. A new type of glycogen storage disease caused by deficiency of cardiac phosphorylase kinase. Biochem. Biophys. Res. Commun. 1984;119(2):582–587. doi: 10.1016/s0006-291x(84)80288-0. [DOI] [PubMed] [Google Scholar]
- 75.Bührer C., van Landeghem F.K., Felderhoff-Mueser U., Stadelmann C., Obladen M. Fetal bradycardia at 28 weeks of gestation associated with cardiac glycogen phosphorylase b kinase deficiency. Acta Paediatr. 2003;92(11):1352–1353. doi: 10.1080/08035250310006458. [DOI] [PubMed] [Google Scholar]
- 76.Chen Y.T., He J.K., Ding J.H., Brown B.I. Glycogen debranching enzyme: purification, antibody characterization, and immunoblot analyses of type III glycogen storage disease. Am. J. Hum. Genet. 1987;41(6):1002–1015. [PMC free article] [PubMed] [Google Scholar]
- 77.Bao Y., Dawson T.L., Jr., Chen Y.T. Human glycogen debranching enzyme gene (AGL): complete structural organization and characterization of the 5' flanking region. Genomics. 1996;38(2):155–165. doi: 10.1006/geno.1996.0611. [DOI] [PubMed] [Google Scholar]
- 78.Cheng A., Zhang M., Okubo M., Omichi K., Saltiel A.R. Distinct mutations in the glycogen debranching enzyme found in glycogen storage disease type III lead to impairment in diverse cellular functions. Hum. Mol. Genet. 2009;18(11):2045–2052. doi: 10.1093/hmg/ddp128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guin S., Pollard C., Ru Y. Role in tumor growth of a glycogen debranching enzyme lost in glycogen storage disease. J. Natl. Cancer Inst. 2014;106(5) doi: 10.1093/jnci/dju062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen J., Bao Y., Liu H.M., Lee P., Leonard J.V., Chen Y.T. Mutations in exon 3 of the glycogen debranching enzyme gene are associated with glycogen storage disease type III that is differentially expressed in liver and muscle. J. Clin. Invest. 1996;98(2):352–357. doi: 10.1172/JCI118799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li X.H., Gong Q.M., Ling Y. Inherent lipid metabolic dysfunction in glycogen storage disease IIIa. Biochem. Biophys. Res. Commun. 2014;455(1–2):90–97. doi: 10.1016/j.bbrc.2014.10.096. [DOI] [PubMed] [Google Scholar]
- 82.Gershen L.D., Prayson B.E., Prayson R.A. Pathological characteristics of glycogen storage disease III in skeletal muscle. J. Clin. Neurosci. 2015 doi: 10.1016/j.jocn.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 83.Preisler N., Pradel A., Husu E. Exercise intolerance in glycogen storage disease type III: weakness or energy deficiency? Mol. Genet. Metab. 2013;109(1):14–20. doi: 10.1016/j.ymgme.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 84.Mogahed E.A., Girgis M.Y., Sobhy R., Elhabashy H., Abdelaziz O.M., El-Karaksy H. Skeletal and cardiac muscle involvement in children with glycogen storage disease type III. Eur. J. Pediatr. 2015 doi: 10.1007/s00431-015-2546-0. [DOI] [PubMed] [Google Scholar]
- 85.Preisler N., Laforêt P., Madsen K.L. Skeletal muscle metabolism is impaired during exercise in glycogen storage disease type III. Neurology. 2015;84(17):1767–1771. doi: 10.1212/WNL.0000000000001518. [DOI] [PubMed] [Google Scholar]
- 86.Wisselaar H.A., Kroos M.A., Hermans M.M., van Beeumen J., Reuser A.J. Structural and functional changes of lysosomal acid alpha-glucosidase during intracellular transport and maturation. J. Biol. Chem. 1993;268(3):2223–2231. [PubMed] [Google Scholar]
- 87.Tsuji A., Omura K., Suzuki Y. Intracellular transport of acid alpha-glucosidase in human fibroblasts: evidence for involvement of phosphomannosyl receptor-independent system. J. Biochem. 1988;104(2):276–278. doi: 10.1093/oxfordjournals.jbchem.a122457. [DOI] [PubMed] [Google Scholar]
- 88.Remiche G., Ronchi D., Magri F. Extended phenotype description and new molecular findings in late onset glycogen storage disease type II: a northern Italy population study and review of the literature. J. Neurol. 2014;261(1):83–97. doi: 10.1007/s00415-013-7137-2. [DOI] [PubMed] [Google Scholar]
- 89.Bali D.S., Tolun A.A., Goldstein J.L., Dai J., Kishnani P.S. Molecular analysis and protein processing in late-onset Pompe disease patients with low levels of acid alpha-glucosidase activity. Muscle Nerve. 2011;43(5):665–670. doi: 10.1002/mus.21933. [DOI] [PubMed] [Google Scholar]
- 90.Preisler N., Laforet P., Madsen K.L. Fat and carbohydrate metabolism during exercise in late-onset Pompe disease. Mol. Genet. Metab. 2012;107(3):462–468. doi: 10.1016/j.ymgme.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 91.Katona I., Weis J., Hanisch F. Glycogenosome accumulation in the arrector pili muscle in Pompe disease. Orphanet J. Rare Dis. 2014;9:17. doi: 10.1186/1750-1172-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muraoka T., Murao K., Imachi H. Novel mutations in the gene encoding acid alpha-1,4-glucosidase in a patient with late-onset glycogen storage disease type II (Pompe disease) with impaired intelligence. Intern. Med. 2011;50(24):2987–2991. doi: 10.2169/internalmedicine.50.5563. [DOI] [PubMed] [Google Scholar]
- 93.Kishnani P.S., Beckemeyer A.A. New therapeutic approaches for Pompe disease: enzyme replacement therapy and beyond. Pediatr. Endocrinol. Rev. 2014;12(Suppl. 1):114–124. [PubMed] [Google Scholar]
- 94.Mundy H.R., Williams J.E., Cousins A.J., Lee P.J. The effect of l-alanine therapy in a patient with adult onset glycogen storage disease type II. J. Inherit. Metab. Dis. 2006;29(1):226–229. doi: 10.1007/s10545-006-0238-7. [DOI] [PubMed] [Google Scholar]
- 95.Terzis G., Dimopoulos F., Papadimas G.K. Effect of aerobic and resistance exercise training on late-onset Pompe disease patients receiving enzyme replacement therapy. Mol. Genet. Metab. 2011;104(3):279–283. doi: 10.1016/j.ymgme.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 96.Boucek D., Jirikowic J., Taylor M. Natural history of Danon disease. Genet. Med. 2011;13(6):563–568. doi: 10.1097/GIM.0b013e31820ad795. [DOI] [PubMed] [Google Scholar]
- 97.Martin C.C., Oeser J.K., Svitek C.A., Hunter S.I., Hutton J.C., O'Brien R.M. Identification and characterization of a human cDNA and gene encoding a ubiquitously expressed glucose-6-phosphatase catalytic subunit-related protein. J. Mol. Endocrinol. 2002;29(2):205–222. doi: 10.1677/jme.0.0290205. [DOI] [PubMed] [Google Scholar]
- 98.Lin B., Pan C.J., Chou J.Y. Human variant glucose-6-phosphate transporter is active in microsomal transport. Hum. Genet. 2000;107(5):526–529. doi: 10.1007/s004390000404. [DOI] [PubMed] [Google Scholar]
- 99.Martin C.C., Bischof L.J., Bergman B. Cloning and characterization of the human and rat islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) genes. J. Biol. Chem. 2001;276(27):25197–25207. doi: 10.1074/jbc.M101549200. [DOI] [PubMed] [Google Scholar]
- 100.Chou J.Y., Mansfield B.C. Mutations in the glucose-6-phosphatase-alpha (G6PC) gene that cause type Ia glycogen storage disease. Hum. Mutat. 2008;29(7):921–930. doi: 10.1002/humu.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jun H.S., Weinstein D.A., Lee Y.M., Mansfield B.C., Chou J.Y. Molecular mechanisms of neutrophil dysfunction in glycogen storage disease type Ib. Blood. 2014;123(18):2843–2853. doi: 10.1182/blood-2013-05-502435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Melis D., Della Casa R., Balivo F. Involvement of endocrine system in a patient affected by glycogen storage disease 1b: speculation on the role of autoimmunity. Ital. J. Pediatr. 2014;40(1):30. doi: 10.1186/1824-7288-40-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ross K.M., Brown L.M., Corrado M.M. Safety and efficacy of chronic extended release cornstarch therapy for glycogen storage disease type I. JIMD Rep. 2015 doi: 10.1007/8904_2015_488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shah K.K., O'Dell S.D. Effect of dietary interventions in the maintenance of normoglycaemia in glycogen storage disease type 1a: a systematic review and meta-analysis. J. Hum. Nutr. Diet. 2013;26(4):329–339. doi: 10.1111/jhn.12030. [DOI] [PubMed] [Google Scholar]
- 105.Banka S., Newman W.G. A clinical and molecular review of ubiquitous glucose-6-phosphatase deficiency caused by G6PC3 mutations. Orphanet J. Rare Dis. 2013;8(1):84. doi: 10.1186/1750-1172-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hayee B., Antonopoulos A., Murphy E.J. G6PC3 mutations are associated with a major defect of glycosylation: a novel mechanism for neutrophil dysfunction. Glycobiology. 2011;21(7):914–924. doi: 10.1093/glycob/cwr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Webb B.A., Forouhar F., Szu F.E., Seetharaman J., Tong L., Barber D.L. Structures of human phosphofructokinase-1 and atomic basis of cancer-associated mutations. Nature. 2015;523(7558):111–114. doi: 10.1038/nature14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Al Hasawi N., Alkandari M.F., Luqmani Y.A. Phosphofructokinase: a mediator of glycolytic flux in cancer progression. Crit. Rev. Oncol. Hematol. 2014;92(3):312–321. doi: 10.1016/j.critrevonc.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 109.Malfatti E., Birouk N., Romero N.B. Juvenile-onset permanent weakness in muscle phosphofructokinase deficiency. J. Neurol. Sci. 2012;316(1-2):173–177. doi: 10.1016/j.jns.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 110.Sakoda S., Shanske S., DiMauro S., Schon E.A. Isolation of a cDNA encoding the B isozyme of human phosphoglycerate mutase (PGAM) and characterization of the PGAM gene family. J. Biol. Chem. 1988;263(32):16899–16905. [PubMed] [Google Scholar]
- 111.Tsujino S., Shanske S., Sakoda S., Fenichel G., DiMauro S. The molecular genetic basis of muscle phosphoglycerate mutase (PGAM) deficiency. Am. J. Hum. Genet. 1993;52(3):472–477. [PMC free article] [PubMed] [Google Scholar]
- 112.Kreuder J., Borkhardt A., Repp R. Brief report: inherited metabolic myopathy and hemolysis due to a mutation in aldolase A. N. Engl. J. Med. 1996;334(17):1100–1104. doi: 10.1056/NEJM199604253341705. [DOI] [PubMed] [Google Scholar]
- 113.Beutler E., Scott S., Bishop A. Red cell aldolase deficiency and hemolytic anemia: a new syndrome. Trans. Assoc. Am. Phys. 1973;86:154–166. [PubMed] [Google Scholar]
- 114.Comi G.P., Fortunato F., Lucchiari S. Beta-enolase deficiency, a new metabolic myopathy of distal glycolysis. Ann. Neurol. 2001;50(2):202–207. doi: 10.1002/ana.1095. [DOI] [PubMed] [Google Scholar]
- 115.Peshavaria M., Hinks L.J., Day I.N. Structure of human muscle (beta) enolase mRNA and protein deduced from a genomic clone. Nucleic Acids Res. 1989;17(21):8862. doi: 10.1093/nar/17.21.8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Musumeci O., Brady S., Rodolico C. Recurrent rhabdomyolysis due to muscle beta-enolase deficiency: very rare or underestimated? J. Neurol. 2014;261(12):2424–2428. doi: 10.1007/s00415-014-7512-7. [DOI] [PubMed] [Google Scholar]
- 117.Miyajima H., Takahashi Y., Suzuki M., Shimizu T., Kaneko E. Molecular characterization of gene expression in human lactate dehydrogenase-A. Neurology. 1993;43(7):1414–1419. doi: 10.1212/wnl.43.7.1414. [DOI] [PubMed] [Google Scholar]
- 118.Woerle H.J., Meyer C., Dostou J.M. Pathways for glucose disposal after meal ingestion in humans. Am. J. Physiol. Endocrinol. Metab. 2003;284(4):E716–E725. doi: 10.1152/ajpendo.00365.2002. [DOI] [PubMed] [Google Scholar]
- 119.Barosa C., Silva C., Fagulha A. Sources of hepatic glycogen synthesis following a milk-containing breakfast meal in healthy subjects. Metabolism. 2012;61(2):250–254. doi: 10.1016/j.metabol.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 120.Décombaz J., Jentjens R., Ith M. Fructose and galactose enhance postexercise human liver glycogen synthesis. Med. Sci. Sports Exerc. 2011;43(10):1964–1971. doi: 10.1249/MSS.0b013e318218ca5a. [DOI] [PubMed] [Google Scholar]
- 121.Bergstrom J., Hultman E., Roch-Norlund A.E. Muscle glycogen synthetase in normal subjects. Basal values, effect of glycogen depletion by exercise and of a carbohydrate-rich diet following exercise. Scand. J. Clin. Lab. Invest. 1972;29(2):231–236. doi: 10.3109/00365517209081080. [DOI] [PubMed] [Google Scholar]
- 122.Kochan R.G., Lamb D.R., Lutz S.A., Perrill C.V., Reimann E.M., Schlender K.K. Glycogen synthase activation in human skeletal muscle: effects of diet and exercise. Am. J. Physiol. 1979;236(6):E660–E666. doi: 10.1152/ajpendo.1979.236.6.E660. [DOI] [PubMed] [Google Scholar]
- 123.Koivisto V.A., Härkönen M., Karonen S.L. Glycogen depletion during prolonged exercise: influence of glucose, fructose, or placebo. J. Appl. Physiol.(1985) 1985;58(3):731–737. doi: 10.1152/jappl.1985.58.3.731. [DOI] [PubMed] [Google Scholar]
- 124.Levine L., Evans W.J., Cadarette B.S., Fisher E.C., Bullen B.A. Fructose and glucose ingestion and muscle glycogen use during submaximal exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983;55(6):1767–1771. doi: 10.1152/jappl.1983.55.6.1767. [DOI] [PubMed] [Google Scholar]
- 125.Vukovich M.D., Costill D.L., Hickey M.S., Trappe S.W., Cole K.J., Fink W.J. Effect of fat emulsion infusion and fat feeding on muscle glycogen utilization during cycle exercise. J. Appl. Physiol.(1985) 1993;75(4):1513–1518. doi: 10.1152/jappl.1993.75.4.1513. [DOI] [PubMed] [Google Scholar]
- 126.Ryan A.S., Katzel L.I., Prior S.J., McLenithan J.C., Goldberg A.P., Ortmeyer H.K. Aerobic exercise plus weight loss improves insulin sensitivity and increases skeletal muscle glycogen synthase activity in older men. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69(7):790–798. doi: 10.1093/gerona/glt200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wallis G.A., Hulston C.J., Mann C.H., Roper H.P., Tipton K.D., Jeukendrup A.E. Postexercise muscle glycogen synthesis with combined glucose and fructose ingestion. Med. Sci. Sports Exerc. 2008;40(10):1789–1794. doi: 10.1249/MSS.0b013e31817e0f7e. [DOI] [PubMed] [Google Scholar]
- 128.Jentjens R.L., van Loon L.J., Mann C.H., Wagenmakers A.J., Jeukendrup A.E. Addition of protein and amino acids to carbohydrates does not enhance postexercise muscle glycogen synthesis. J. Appl. Physiol. (1985) 2001;91(2):839–846. doi: 10.1152/jappl.2001.91.2.839. [DOI] [PubMed] [Google Scholar]
- 129.Detko E., O'Hara J.P., Thelwall P.E. Liver and muscle glycogen repletion using 13C magnetic resonance spectroscopy following ingestion of maltodextrin, galactose, protein and amino acids. Br. J. Nutr. 2013;110(5):848–855. doi: 10.1017/S0007114512005818. [DOI] [PubMed] [Google Scholar]
- 131.Di Camillo B., Eduati F., Nair S.K., Avogaro A., Toffolo G.M. Leucine modulates dynamic phosphorylation events in insulin signaling pathway and enhances insulin-dependent glycogen synthesis in human skeletal muscle cells. BMC Cell Biol. 2014;15:9. doi: 10.1186/1471-2121-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Johnson A.B., Argyraki M., Thow J.C., Cooper B.G., Fulcher G., Taylor R. Effect of increased free fatty acid supply on glucose metabolism and skeletal muscle glycogen synthase activity in normal man. Clin. Sci. (Lond.) 1992;82(2):219–226. doi: 10.1042/cs0820219. [DOI] [PubMed] [Google Scholar]
- 133.Stephens F.B., Constantin-Teodosiu D., Laithwaite D., Simpson E.J., Greenhaff P.L. An acute increase in skeletal muscle carnitine content alters fuel metabolism in resting human skeletal muscle. J. Clin. Endocrinol. Metab. 2006;91(12):5013–5018. doi: 10.1210/jc.2006-1584. [DOI] [PubMed] [Google Scholar]
- 134.Preisler N., Haller R.G., Vissing J. Exercise in muscle glycogen storage diseases. J. Inherit. Metab. Dis. 2015;38(3):551–563. doi: 10.1007/s10545-014-9771-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.