Abstract
The co-occurrence of the three disease entities, inflammatory bowel disease (IBD), colorectal cancer (CRC), type 2diabetes mellitus (T2DM) along with inflammation and dismicrobism has been frequently reported. Some authors have even suggested that dysbiosis could be the link through a molecular crosstalk of multiple inflammatory loops including TGFβ, NFKB, TNFα and ROS among others.
This review focuses on the inflammatory process along with the role of microbiota in the pathophysiology of the three diseases.
The etiology of IBD is multifactorial, and like CRC and T2DM, it is associated with a widespread and sustained GI inflammation and dismicrobism, whereby an array of pro-inflammatory mediators and other related biomolecules are up-regulated, both locally and systematically. Such a persistent or an inadequately resolved chronic inflammation may be a causative agent, in the presence other factors, leading to several pathologies such as IBD, CRC and T2DM.
TGFβ plays a crucial role in pancreatic β cell malfunctioning as glucotoxicity stimulates its signaling cascade through smad 3, IL-6 and epithelial to mesenchymal transition. Such a cascade could lead to macrophages and other cells recruitment, inflammation, then IBD and CRC.
NFkB is also another key regulator in the crosstalk among the pathways leading to the three disease entities. It plays a major role in linking inflammation to cancer development through its ability to up regulate several inflammatory and tumor promoting cytokines like: IL-6, IL-1 α and TNF α, as well as genes like BCL2 and BCLXL. It activates JAK/STAT signaling network via STAT3 transcription factors and promotes epithelial to mesenchymal transition. It also increases the risk for T2DM in obese people. In brief, NFKB is a matchmaker between inflammation, IBD, cancer and diabetes.
In addition, TNFα plays a pivotal role in systemic inflammation. It is increased in the mucosa of IBD patients and has a central role in its pathogenesis. It also activates other signaling pathways like NFKB and MAPK leading to CRC. It is also overexpressed in the adipose tissues of obese patients thus linking it to T2DM, chronic inflammation and consequently CRC.
On the other hand, increasing evidence suggests that dysbiosis plays a role in initiating, maintaining and determining the severity of IBD. Actually, among its functions, it modulates genotoxic metabolites which are able to induce CRC, a fact proven to be sustained by stool transfer from patients with CRC. Probiotics, however, may actively prevent CRC as well as IBD and results in a significant decrease in fasting glycemia in T2DM patients.
In conclusion, IBD, CRC and T2DM are commonly occurring interrelated clinical problems. They share a common basis influenced by an inflammatory process, an imbalance in intestinal microbiota, and a crosstalk between various signaling pathways. Would probiotics interrupt the crosstalk or orient it in the physiological direction?
Keywords: IBD, CRC, T2DM, Probiotic, Dysbiosis, Inflammation
1. Introduction
The medical literature is depleting in publications reporting the co-occurrence of the three disease entities: inflammatory bowel disease (IBD), colorectal cancer (CRC) and type 2 diabetes mellitus (T2DM), along with inflammation and dismicrobism [8], [17], [21], [41], [51]. Actually, the body microbiota represents a complex ecosystem with enormous microbial diversity. Trillions of bacterial cells colonize the GI surface within the human body and can reach 10 times the number of the cells forming the whole organism. In addition; the genes of these microorganisms are 150 times higher in number than those in the human body. Most of the commensal bacteria are symbiotic; however, they could cause pathology after their translocation to the mucosa or under specific conditions. The largest number of bacterial cells is found in the large intestine (1011 per gram of intestinal content), the main geography of IBD and CRC [19], [41].
In general, intestinal dysbiosis is associated with an undesirable qualitative and quantitative alteration in the balance between beneficial and harmful bacteria in the gut [17]. It is also well documented that changes in the homeostasis of the intestinal microbiota have far-reaching effects on local and systemic immunity and contribute to the pathogenesis of gastrointestinal diseases like inflammatory bowel disease, irritable bowel syndrome and colorectal cancer, as well as extra intestinal systemic diseases like obesity, diabetes and atherosclerosis among others [57]. Some authors have even suggested that dysbiosis could be the link between chronic inflammation, IBD, CRC and T2DM trough a crosstalk between several molecular pathways, in particular, TGFβ, NFKB, TNFα and ROS among others [20]. The mechanisms forming the basis of such a phenomenon were referred to a series of complex interactions between the intestinal epithelium, microbiota, genetic factors, and the host immune system, which modulate the intestinal equilibrium.
Normally, the intestinal epithelium has a critical function as a protective barrier against luminal antigens. It is covered by a single layer of intestinal epithelial cells (IECs) distinguished by a fast renewal rate. They are circumferentially tied by intracellular tight junctions and coated by a thick adherent mucus gel, thus forming a dynamic physical interface between the host and its environment [35], [62]. Despite its robust and multi-faced nature, this barrier can be breached, hence disrupting the intestinal homeostasis, and thus promoting a state of chronic inflammation in all these three disease entities: IBD, T2DM and CRC [29].
Normally, inflammation is basically a beneficial activity that rejuvenates injured tissue and removes the foreign agents disturbing homeostasis, thus leading to recovery of the equilibrium state. This task is achieved through a complex inflammatory response which may involve a balance between a huge panel of bioactive molecules, pro and anti-inflammatory (IL-6, NFKB, TNFα and TGFβ, among others …), provided from resident or infiltrating inflammatory cells. However, a persistent or an inadequately resolved chronic inflammation, due to the tilting of the balance in favor of pro-inflammatory agents, may increase the risk of several pathologies such as IBD, CRC and T2DM. How inflammation lies on the causative pathway mechanistically linking these three chronic clinical problems remains unclear [43], [13].
In this review, the focus is on the inflammation process as constituting the basis in the development of CRC, IBD and T2DM. Several molecular signaling pathways involved in such inflammation like TGFβ, JAK/STAT, TNFα, NFkB and ROS production mechanisms will be discussed, along with the role of microbiota in the physiopathology of these three diseases. Collectively, the molecular crosstalk between all of these pathways could probably define the nature of the link or links between all three disease entities.
2. Inflammatory bowel disease
IBD is an idiopathic, chronic, inflammatory disease that affects the colon and other parts of the gastrointestinal tract. It may also have extra-intestinal manifestations [20]. The exact etiology of IBD is unrevealed yet, but it is generally known to be multifactorial, with the possible leading trigger as a disbalance of the luminal mucosal homeostasis in genetically susceptible organisms [29], [44]. Consequently, an altered balance between regulatory and inflammatory mediators could contribute to an inappropriate and sustained inflammatory response probably caused by a dysfunction of the microfloral integrity [17]. IBD encompasses a range of intestinal pathologies, the 2 major clinical entities (ulcerative colitis (UC) and Crohn's disease (CD)) show overlapping features and a similar symptom profile which can include; diarrhea, rectal bleeding, abdominal pain and weight loss [34], [23].
2.1. Crohn's disease (CD)
It is often associated with a widespread inflammation that can affect any region of the intestine from the mouth to the anal canal but mostly involves the ileum and the colon [59].
At the histological level, CD is characterized by the spread of lymphoid aggregates in the mucosa and submucosa layers of the bowel. At the beginning of the disease, neutrophil infiltrates are observed in the epithelial layer. As the disease progresses, neutrophils infiltrate into the crypts. They form crypt abscesses, hence destroying the crypt leading to colonic atrophy. Another observed feature resulting from this chronic inflammation is the formation of non-necrotizing granulomas. Ulcers constitute also a main feature of CD which enlarge and may then develop into a network of long perforating ulcerations [46]. These microscopic changes ultimately lead to a series of complications including: abdominal pain, diarrhea which is a common presentation of CD, rectal bleeding, fistula and perianal disease which are often very difficult to treat, and malabsorption often due to the inflammation of the terminal ileum (59).
The profile of cytokines ruling the inflammation of Crohn's disease, while well defined, continues to be a work in progress. A Th17 response is the main cause of Crohn's disease inflammation through the production of IL-17 and IFN-γ. These are not, of course, the only cytokines taking part in the CD inflammation, their downstream cytokines: TNF-α, IL-6 and IL-1 play pivotal roles as the immediate effectors of inflammation, in addition to several others including IL-22 [60].
2.2. Ulcerative colitis
UC is also an inflammatory disease featuring recurrent and chronic inflammation of the colonic mucosa and submucosa [25]. It usually begins in the rectum. Depending on the severity and the course of the disease; it could extend to the proximal parts in a continuous fashion [58]. In severe cases, acute dilatation of the colon, necrosis, perforation and massive hemorrhage with the formation of pseudopolyps are clinically manifested [19]. Histologically, UC can result in crypt abscesses with neutrophils infiltrating the crypts. Moreover, in severe cases, hypertrophy of the muscularis mucosa can occur which may lead to a narrowing and shortening of the colon [46].
Typical symptoms of UC include rectal bleeding or bloody diarrhea associated with abundant mucus discharge, abdominal pain, fever, weight loss and malaise [19]. The presence of these symptoms and their severity correlate with the activity of the disease [59]. The predominant cytokines in UC are mainly from the Th2 pattern of the adaptive immune response which produces several pro-inflammatory cytokines such as IL-4, IL-5 and IL-13 [41].
3. Diabetes mellitus
Diabetes mellitus (DM) is a chronic disease that is classified into two major types: type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM). T2DM constitutes 90% of all diabetes and is a common endocrine disorder. On the other hand, T1DM is an immunologically mediated disorder. It is insulin-dependent diabetes whereby the body's immune system destroys the βeta cells that release insulin and eventually eliminating insulin production from the body. However, in T2DM, the body cannot use the insulin leading to insulin resistance [8].
Insulin resistance and β-cell dysfunction are considered as early and critical components in the pathophysiology of T2DM. In addition, a timely inflammatory process has been described with the up regulation of growth factors like TGFβ and pro-inflammatory cytokines like IL-1β and other biological molecules like ROS [18], [50], [51].
4. Colorectal cancer
It is well established that CRC originates from the epithelial cells lining the colon or rectum of the gastrointestinal tract and represents the third most common form of cancer worldwide [22]. Recently, CRC incidence has been increasing along with its mortality rates. Multiple molecular pathways intersect, thus probably promoting its occurrence. However, there is constantly an inflammatory reaction persisting and maintained. Whereby, an array of pro-inflammatory mediators is elevated both locally and systemically, such as; cyclooxygenase 2 (COX-2), prostaglandin E2 (PGE2), TNF α, NFkB, TGFβ among others …. Despite the multiple factors responsible for its onset, its incidence and prevalence increase more in patients with IBD and/or T2DM [13], [17], [21].
5. TGFβ
TGF-β constitutes a common factor in all the three disease entities. It is a multifunctional cytokine that plays an important role in the regulation of many cellular pathways including cell growth, apoptosis, differentiation and immune reactions. Under normal conditions, TGF-β is a strong anti-inflammatory cytokine exerting a suppressive effect on carcinogenesis by inhibiting abnormal cell growth. The uncontrolled cell growth and differentiation in many GI cancers are attributed to a genetic loss of TGF-β signaling molecules and an alteration of TGF-β receptors. Once a tumor has developed, TGF-β will promote tumor cell growth, enhance invasiveness and metastasis and inhibit immune surveillance [51], [53].
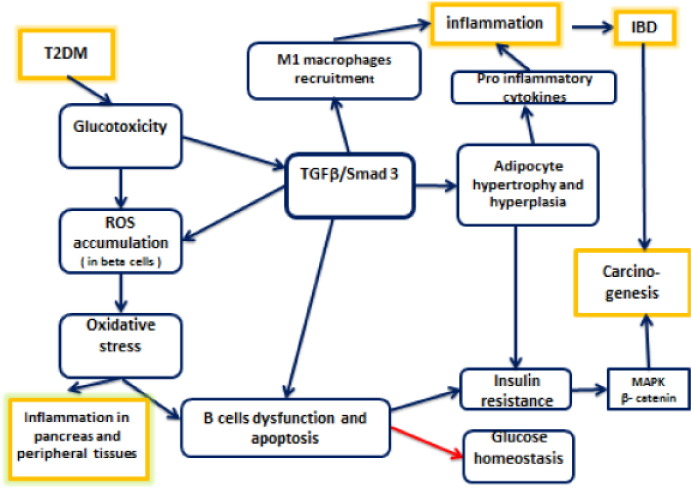
During the pathogenesis of T2DM, TGFβ plays a crucial role in β cell malfunctioning, as glucotoxicity stimulates TGFβ signaling cascade, moreover, glucotoxicity, which is the chronic exposure to hyperglycemia, leads to reactive oxygen species (ROS) accumulation in pancreatic β islets. As anti-oxidative enzymes are not sufficiently present in β cells, ROS accumulation may result in oxidative stress, a well-known trigger for β cells apoptosis [51] (Fig. 1). This oxidative stress, along with Free Fatty Acids—FFAs—(secreted by stimulated adipocytes) abolishes the normal function of β cells and induces the generation of pro-inflammatory mediators, such as IL-1 β, TNFα and IL-6, thus causing inflammation in pancreatic islets and peripheral tissues [36]. Consequently, β cell dysfunction may lead to insulin resistance and disruption of glucose homeostasis. Pro-inflammatory mediators, such as IL-1 β binds IL-1 receptor (IL-1 R) and leads to NFkB activation, which will interact with multiple signaling pathways, as will be depicted later on [36], [51].
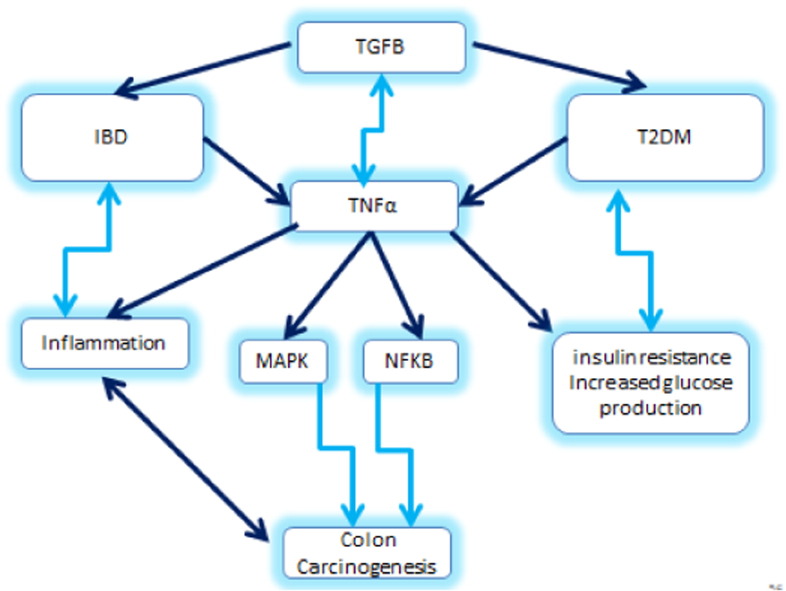
Fig. 1.
The various interactions between TGFβ, Smad 3, IBD, T2DM and colon carcinogenesis are illustrated. Note that blue arrows indicate induction and stimulation, while red arrow means disruption or inhibition.
TGFβ and its related factors control the development, growth and function of diverse cell types. It transmits its signals via dual serine/threonine kinase receptors and transcription factors called smads, with smad 3 acting as the main facilitator of TGFβ signaling [61]. Several studies illustrate the role of TGFβ/smad3 signaling in the pathogenesis of T2DM. In a study conducted by Hariom et al. in 2011, smad 3 deficient mice were protected from diet-induced obesity and diabetes, while systemic blockage of TGFβ signaling protects mice from diabetes and hepatic steatosis [18]. TGFβ also induces adipocytes hypertrophy and adipocytes produce FFAs. A prolonged exposure of FFAs to β cells inhibits insulin synthesis and promotes oxidative stress, leading eventually to insulin resistance [51] (Fig. 1).
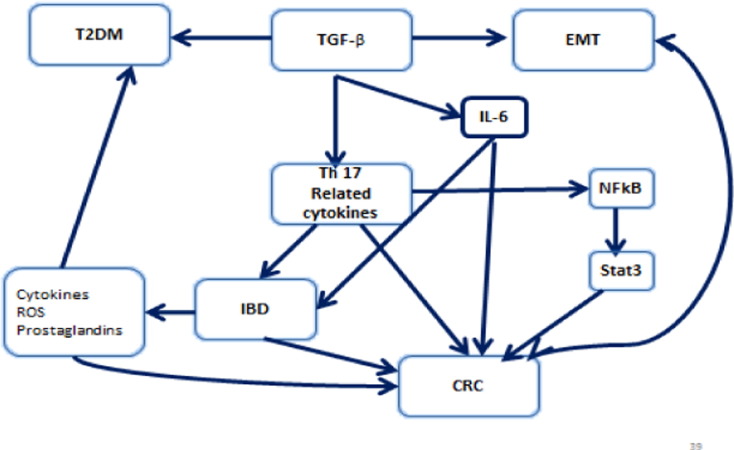
Furthermore, the GI tract which is constantly exposed to various pathogens and food antigens can induce a series of host defensive reactions such as recruitment of immune cells, activation of anti-inflammatory enzymes and secretion of anti-inflammatory cytokines. Regulatory T (Treg) cells are among the various regulatory mechanisms whose function is partly to maintain the gut barrier and other immune reactions in order to prevent a pathological inflammation and maintain GI tract homeostasis through tolerance. In addition IL-6, plays an important role in inflammation, a key field of action of IL-6 is the regulation of T cell differentiation and activation. Numerous studies provided evidence that IL-6 is a critical regulator of murine models of experimental colitis. It was proven that IL-6 inhibition, decreases the multiplication and size of colonic adenomas in Azoxymethane (AOM) and Dextran sodium sulfate (DSS) mice models [52]. The key factor and regulator of IL-6 in chronic intestinal inflammation works via th1 and th17 cells [14]. IL-6, through its trans-signaling can also modulate epithelial cells, neutrophils, macrophages and T cells. In addition, IL-6 promotes the production of th17 cells and TGF-β and induces the maintenance of IL-17 signaling T lymphocytes in inflamed tissues while it exerts a suppressive effect on the generation of regulatory T cells (Treg). Moreover, IL-6 is an important factor for the synthesis of C-reactive protein whose level is found to be elevated in acute and chronic inflammation. Furthermore, IL-6 plays a potential role in the pathogenesis of diabetes through mediating local inflammation and tissue destruction in a NOD mouse model. It was also reported that IL-6R signaling blockage and STAT-3 activation lead to a T cell apoptosis in lamina propria cells in culture [42]. Fig. 2 demonstrates that TGF β has a role in the pathogenesis of IBD, and it also may contribute to the cancer risk associated with chronic inflammation of the gut. TGF β in the presence of IL-6, which is produced during active infections or other inflammatory states, leads to the differentiation of naïve T cells into Th-17 cells, a set of T helper cells secreting IL-17, a potent inflammatory cytokine. The production of IL-17 leads to chemokine expression such as IL-6 and TNFα [30]. Th-17 appears to play an important role in IBD, and drives a pro-carcinogenic process under poorly regulated pro-inflammatory conditions [49] (Fig. 2).
Fig. 2.
TGF β creates a cancer-promoting feedback loop and is related through various pathways to IBD, CRC and T2DM. Note that blue arrows mean induction or stimulation.
TGFβ also contributes to epithelial to mesenchymal transition (EMT), a hallmark that facilitates metastasis and invasion through multiple signaling mechanisms, leading eventually to tight junctions (TJ) and adherent junctions (AJ) dissolution. Another mechanism, by which TGFβ facilitates EMT, is through the up regulation of metalloproteinases (MMPs), contributing to colon carcinogenesis and metastasis [24] (Fig. 2).
In a study conducted by Hawinkels et al. in 2014, a strong activation of TGF-β/Smad signaling pathway was observed in CRC. TGF-β1 stimulation caused a remarkable up regulation of MMPs, plasminogen activator inhibitor-1 (PAI-1) and TGF-β1 itself, thus, leading to cumulative production of TGF-β and proteinases within the tumor microenvironment and consequently creating a cancer-promoting feedback loop [32] (Fig. 2).
Actually, in IBD a battery of cytokines were described as being secreted (TNFα, IL-6, IL-17, IL-27 among others), which contribute to the formation of a tumor supporting microenvironment.
Macrophages, phagocytic cells of the innate immune system, are essential not only for engulfing foreign agents that enter the body, but also for eliminating apoptotic cells and digesting wastes from tissues. The activity of macrophages can be increased by cytokines secreted by helper T cells, especially IFN-γ. During inflammation, macrophages release a wide range of pro-inflammatory cytokines such as TNF-α, IL-6, IL-8 and IL-12, in addition to the release of chemokines, prostaglandins and complement. The interactions of all of these mediators could lead to an increased vascular permeability and recruitment of inflammatory cytokines [26]. Consequently, environmental triggers like microbiota and inflammation together can result in damage to the epithelial cells. The increase in inflammatory cells and the factors they produce result in oxidative stress, which can induce DNA damage resulting in genetic and epigenetic alterations to the epithelial cells, thus causing dysplasia, cancer and clonal expansion of these cells. The net result is disruption of the balance of mediators with increase in TGFβ, IL-6 and TNFα among others leading to IBD, CRC and consequently T2DM.
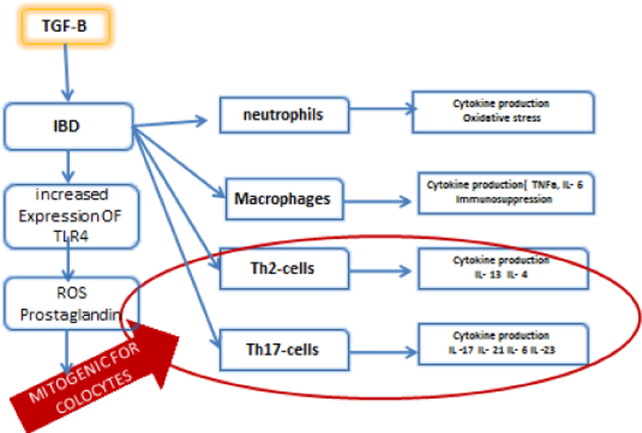
Among the different cytokines, IL-13 and IL-4 secreted by Th2 cells have been shown to increase the colonic mutations in epithelial cells, to support angiogenesis and to produce TGFβ. In addition, cytokines secreted by Th-17 are proven to be mitogenic. Fig. 3 shows the different effector cells recruited in IBD and their associated cytokines [16].
Fig. 3.
The different effector cells recruited in IBD and their associated cytokines leading to a disbalance in pro and anti-inflammatory agents. Note that blue arrows indicate induction, or stimulation and red color (circle and arrow) means mitogenic stimulation.
6. NFKB
There are several lines of evidence posing NFKB as a key regulator in the crosstalk among the pathways leading to T2DM, CRC, and IBD. Actually, various carcinogens, growth factors, inflammatory stimuli including microbiota and pro-oxidants activate the transcription factor NFkB which plays a central role in inflammation and is mostly expressed in cancers [37]. It was documented that NFkB is activated via phosphorylation of inhibitor of kappa B (IKB) leading to its ubiquitination and proteasomal degradation. Such a reaction will unmask the nuclear localization signal of NF-KB, and once in the nucleus, it will activate several genes that regulate proliferation, apoptosis, angiogenesis, invasion, inflammation and metastasis [63], [47] (Fig. 4).
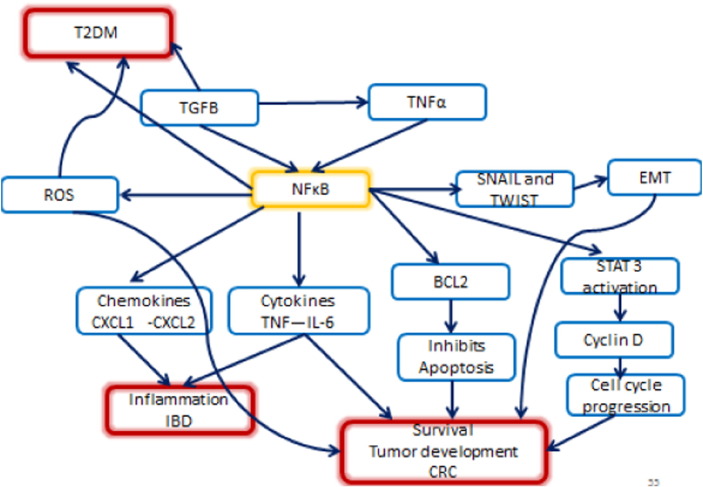
Fig. 4.
NFkB the matchmaker between, inflammation, IBD, CRC and T2DM and their wide mechanisms of action. Note that blue arrows mean induction or stimulation.
NFKB plays a major role in linking chronic inflammation to cancer development through its ability to up regulate several inflammatory, and tumor promoting cytokines such as IL-6, IL-1α and TNF-α, as well as survival genes such as BCL2 and BCLXL [63] (Fig. 4).
In a study conducted by Greten FR et al. in 2004, a crucial role for IKK beta was demonstrated, linking inflammation to cancer in a mouse model of colitis-associated cancer, deleting IKK beta in myeloid cells resulted in a significant decrease in tumor size. This deletion also diminished the expression of pro-inflammatory cytokines [12] (Fig. 4).
In addition, NFkB promotes EMT, through its activation of snail and twist [5] in the microenvironment, and the expression of inflammatory cytokines. Moreover, NFKB seems to be involved in the tumor associated macrophage (TAM) recruitment and acts in cancer-associated fibroblasts (CAF), by promoting the expression of a pro-inflammatory gene signature, which is important for macrophage recruitment, neovascularization and tumor growth that were abolished when NFKB was inhibited [1], [40] (Fig. 4).
Furthermore, NFKB plays an important role in T2DM, as obesity activates the transcription factor NFKB which increases the risk for T2DM. It has been shown that NFKB pathway inhibition exerts a beneficial effect on T2DM, salsalate a prodrug that reduces NFkB activity was shown to decrease HBA1C levels [38].
Collectively, NFKB could be considered as the matchmaker between inflammation, IBD, cancer and diabetes, and its wide mechanisms of action are summarized in Fig. 4.
7. TNFα
TNFα is a cytokine produced by activated macrophages as well as other cells. It is one of the first cytokines to be released during the acute inflammatory response. It plays a pivotal role in systemic inflammation as it induces the synthesis of C-reactive protein, vasodilatation, and vascular permeability and helps in the recruitment of inflammatory cells. In harmony with IL-17, TNF-α triggers the expression of neutrophil-attacking chemokines CXCL1, CXCL2 and CXCL5 which in turn increase CXCR2-dependent neutrophil migration to the inflammation site. Diapedisis is also facilitated through TNF-α's effect on cell adhesion molecules [15], [54] (Fig. 5).
Fig. 5.
TNFα relates to various molecules leading to IBD, CRC, and T2DM.
Among the immune-regulatory factors, TNF α is increased in the mucosa of IBD patients, and has been shown to play a central role in the pathogenesis of the disease, and anti TNF α therapy has proven to be beneficial in the treatment of IBD [28]. In addition, TNFα activates other signaling pathways such as NFkB and Mitogen-activated protein kinases (MAPK) pathway, resulting in Jun N-terminal kinase (JNK) and the activator protein 1 (AP-1) activity. Thus the sustained activity of both NFκB and AP-1 is known to be a mediator in distinct phases of colon carcinogenesis [11] (Fig. 5).
More than a decade ago, it was discovered that TNFα is overexpressed in the adipose tissues of obese mice, thereby establishing a clear link between obesity, T2DM and chronic inflammation [64]. Consequently, as represented in Fig. 5, TNF α is a key player in IBD, CRC and T2DM.
The association between T2DM and cancer is validated in several studies: Orsini and Wolk demonstrated from a meta-analysis of 15 studies including more than 2.5 million patients that diabetic people have 30% increased risk of developing CRC compared to non-diabetics [48]. 371 million people worldwide have DM [56]. Seshasai et al. stated in a review based on 97 prospective studies that 123,205 cases of mortality of cancer were linked to diabetes among a total mortality of 820,900 cases. The relative risk of diabetes associated to CRC is 1.40 with 95% confidence interval (CI) [10], [56].
On the other hand several studies investigated the correlation between T2DM and IBD in 2015, SY LEE et al. showed that metformin which is an effective widely used T2DM treatment was able to decrease the severity of IBD and diminishes inflammation [31].
8. The link of microbiota to IBD, T2DM and CRC
The role of dysbiosis in the pathogenesis of IBD, CRC, and T2DM is being strengthened by several studies and reports involving probiotics. The term “probiotics”, meaning “for life” in Greek, was first used in 1965 to describe any substance or organism that was beneficial in promoting microfloral balance in the intestinal system [9]. The Beneficial role of probiotics in IBD, CRC and T2DM was demonstrated in several studies [3]. XuZ et al. showed that the probiotic bifidobacterium could significantly ameliorate the disorder of glucose and lipid metabolism [65]. Furthermore a clinical study investigated the effect of milk fermented with Lactobacillus helveticus on blood pressure, glycemic control and cardiovascular risk factors in T2DM using a randomized, double-blinded, prospective, placebo-controlled study. The change in fasting blood glucose concentration differed significantly between the two groups with a larger increase in the placebo group [27]. It has been also reported that consumption of a symbiotic shake resulted in a significant decrease in fasting glycemia in T2DM patients [2] (Fig. 6).
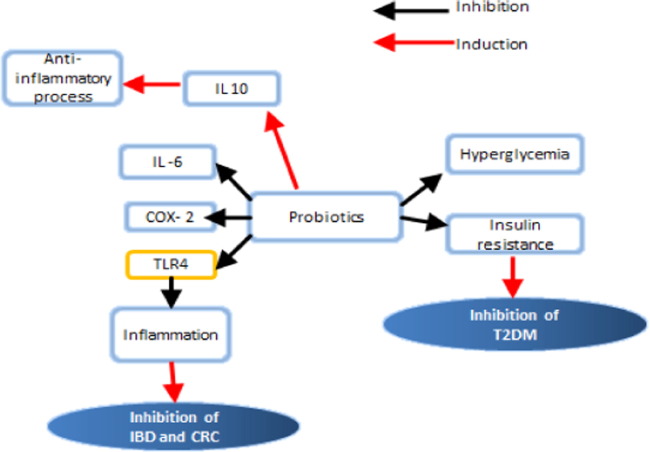
Fig. 6.
The beneficial effect of probiotics on T2DM, IBD and CRC. Note that blue arrows mean induction while red arrows indicate the inhibition.
In addition to its inhibiting effect on T2DM, existing evidence clearly support an immunomodulatory role of probiotics in CRC, particularly its ability to modulate intestinal inflammation, a well-known risk factor for CRC. Other studies along this line showed that the administration of L. plantarum Lp91 significantly reduced COX-2 and TNFα while up regulated IL-10 levels in the colon tissues of mice with TNBS-induced colitis [45]. Another treatment involving the probiotic mixture VSL#3 led again to reduction in COX-2, NFkB, TNF-α, IL-6 and inducible nitric oxide synthase, while increasing IL-10 and TGFβ expression in colonic tissue of rats with dextran sulfate sodium-induced colitis [4].
Beneficial effects of probiotic treatment have been observed with the restoration of normal goblet cells numbers and stimulation of the mucosal immune system in the patients' intestinal tract to secrete protective immunoglobulins such as secretory IgA, protective defensins and bacteriocidins in the colonic lumen [39].
On the other hand, increasing evidence suggests that intestinal microbiota play a role in initiating, maintaining and determining the severity of IBD. Such effect is limited to a continuous antigenic stimulation causing chronic intestinal injury concurring to the typical alterations of the gut associated lymphoid tissue associated with IBD [33].
Furthermore, molecular dynamics of dysbiosis encompass modulation of genotoxic metabolites from different bacterial strains and are able to induce CRC formation in the presence of polymorphisms responsible of reduced activity of NOD2 [7]. Dysbiosis was proven to exert detrimental effects on the mechanisms responsible of cellular differentiation, apoptosis and proliferation control [6]. The great importance of colonic microbiome in CRC development is substantiated by experiments of stool transfer from individuals with colon cancer and healthy germ-free mice [55]. They showed that regular probiotics intake may actively prevent the initiation and development of CRC [17].
The common positive effect of probiotics on T2DM, IBD and CRC may help in clarifying the pathological link, or links between these 3 chronic diseases. Could it be dysbiosis?
9. Conclusion
Inflammation is a fundamental homeostatic response triggered by both endogenous and exogenous noxious stimuli. It ensures the integrity of the organisms. However, inflammation may be the mysterious cause of several diseases including, neurodegenerative disorders, atherosclerosis, T2DM, cancer and IBD among others. On the other hand, IBD, CRC and T2DM are commonly occurring interrelated clinical problems. They share a common basis influenced by disease-related inflammation, a process characterized by up regulation of expression of common inflammatory cytokines along with TGFβ, TNFα, NFKB, ROS and other signaling molecules, leading to an imbalance in the intestinal microbiota. The intersection between and the converging of all these molecular pathways constitute a crosstalk leading, most likely to these three interrelated chronic diseases. However, further clinical studies seem necessary to clarify the complex interplay between CRC, IBD and T2DM in addition to the role of microbiota in this loop.
A treatment that can interrupt the cross talk could probably be of interest in managing the three disease entities and form a novel therapeutic target. Would probiotics interrupt the crosstalk or orient it in the physiological direction?
References
- 1.Ahmed M.Z.D., Xu R. Nuclear factor-kappaB in inflammatory bowel disease and colorectal cancer. S –. Am. J. Dig. Dis. 2014 [Google Scholar]
- 2.Ostadrahimi A., Taghizadeh A., Mobasseri M., Farrin N., Payahoo L., Gheshlaghi Z.B., Vahedjabbari M. Effect of probiotic fermented milk (Kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Iranian J. Public Health. 2015 [PMC free article] [PubMed] [Google Scholar]
- 3.Bellavia M., Rappa F., Lo Bello M. Lactobacillus casei and Bifidobacterium lactis supplementation reduces tissue damage of intestinal mucosa and liver after 2,4,6-trinitrobenzenesulfonic acid treatment in mice. J. Biol. Regul. Homeost. Agents. 2014;28(2):251–261. [PubMed] [Google Scholar]
- 4.Dai C., Zheng C.Q., Meng F., Zhou Z., Sang L., Jiang M. Springer; 2013. VSL#3 Probiotics Exerts the Anti-Inflammatory Activity via PI3k/Akt and NF-κB Pathway in rat Model of DSS-Induced Colitis—Molecular and Cellular Biochemistry. [DOI] [PubMed] [Google Scholar]
- 5.Min C., Eddy S.F., Sherr D.H., Sonenshein G.E. Wiley Online Library; 2008. NF-κB and Epithelial to Mesenchymal Transition of Cancer. Journal of Cellular Biochemistry. [DOI] [PubMed] [Google Scholar]
- 6.Collins D., Hogan A.M., Winter D.C. Microbial and viral pathogens in colorectal cancer. Lancet Oncol. 2011;12(5):504–512. doi: 10.1016/S1470-2045(10)70186-8. [DOI] [PubMed] [Google Scholar]
- 7.Couturier-Maillard A., Secher T., Rehman A. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J. Clin. Invest. 2013;123(2):700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannata D., Fierz Y., Vijayakumar A., LeRoith D. Wiley Online Library; 2010. Type 2 Diabetes and Cancer: What is the Connection? Mount Sinai Journal of Medicine. [DOI] [PubMed] [Google Scholar]
- 9.DM Lilly, RH Stillwell. Probiotics: growth-promoting factors produced by microorganisms—Science, 1965. [DOI] [PubMed]
- 10.Whiting D.R., Guariguata L., Weil C., Shaw J. Elsevier; 2011. IDF Diabetes Atlas: Global Estimates of the Prevalence of Diabetes for 2011 and 2030—Diabetes Research and Clinical Practice. [DOI] [PubMed] [Google Scholar]
- 11.Balkwill F. Tumour necrosis factor and cancer. Nat. Rev. Cancer, nature. 2009 doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 12.Greten F.R., Eckmann L., Greten T.F., Park J.M., Li Z.W., Kagnoff M.F., Egan L.J. Elsevier; 2004. IKKβ Links Inflammation and Tumorigenesis in a Mouse Model of Colitis-Associated Cancer. Cell. [DOI] [PubMed] [Google Scholar]
- 13.Ganepola G.A.P., Nizin J., Rutledge J.R., Chang D.H. Use of blood-based biomarkers for early diagnosis and surveillance of colorectal cancer. J. Gastrointestinal Enterology. 2014 doi: 10.4251/wjgo.v6.i4.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hundorfean G., Neurath M.F., Mudter J. Wiley Online Library; 2012. Functional Relevance of T Helper 17 (Th17) Cells and the IL-17 Cytokine Family in Inflammatory Bowel Disease. Inflammatory Bowel Diseases. [DOI] [PubMed] [Google Scholar]
- 15.Griffin G.K., Newton G., Tarrio M.L., Bu D., Alcaide P., Azcutia V., Garcia E.M. IL-17 and TNF-α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J. Immunol. 2012 doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monteleone G., Pallone F., Stolfi C. The dual role of inflammation in colon carcinogenesis. Int. J. Mol. Sci. 2012 doi: 10.3390/ijms130911071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomasello G., Tralongo P., Damiani P., Sinagra E., Trapani B., Zeenny M.N., Hussein I.H., Jurjus A., Leone A. Dismicrobism in inflammatory bowel disease and colorectal cancer: changes in response of colocytes. World J. Gastroenterol. 2014 doi: 10.3748/wjg.v20.i48.18121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav H., Quijano C., Kamaraju A.K., Gavrilova O., Malek R., Chen W., Zerfas P. Elsevier; 2011. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling, Cell Metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajj Hussein I.A., Tohme R., Barada K., Mostafa M.H., Freund J.N., Jurjus R.A., Karam W., Jurjus A. Inflammatory bowel disease in rats: bacterial and chemical interaction. World J. Gastroenterol. 2008 doi: 10.3748/wjg.14.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andújar I., Recio M.C., Giner R.M., Jovellanos E., Laghi S., Muguerza B., Ríos J.L. Inhibition of ulcerative colitis in mice after oral administration of a polyphenol-enriched cocoa extract is mediated by the inhibition of STAT1 and STAT3 phosphorylation in colon cells. J. Agric. Food Chem. 2011 doi: 10.1021/jf2008925. [DOI] [PubMed] [Google Scholar]
- 21.Cheng I., Caberto C.P., Jones A.L., Seifried A., Wilkens L.R., Schumacher F.R., Monroe K.R. Type 2 diabetes risk variants and colorectal cancer risk: the multiethnic cohort and PAGE studies. Gut. 2011 doi: 10.1136/gut.2011.237727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crncec I., Pathria P., Svinka J., Eferl R. Springer; 2015. Induction of Colorectal Cancer in Mice and Histomorphometric Evaluation of Tumors, Mouse Models of Cancer: Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 23.Kim J.J., Shajib S., Manocha M.M., Khan W.I. Investigating intestinal inflammation in DSS-induced model of IBD. J. Vis. Exp. 2012 doi: 10.3791/3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massagué J. Elsevier; 2008. TGFβ in Cancer, Cell. [Google Scholar]
- 25.Meier J., ASturm Current treatment of ulcerative colitis. World J. Gastroenterol. 2011 doi: 10.3748/wjg.v17.i27.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rugtveit J., Nilsen E.M., Bakka A., Carlsen H., Brandtzaeg P., Scott H. Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease. Gastroenterology. 1997 doi: 10.1016/s0016-5085(97)70030-1. [DOI] [PubMed] [Google Scholar]
- 27.KD Hove, C Brøns, K Fae: rch, SS Lund, P Rossing, A Vaag Effects of 12 weeks of treatment with fermented milk on blood pressure, glucose metabolism and markers of cardiovascular risk in patients with type 2 diabetes: a randomised double-blind placebo-controlled study—Eur. J. Endocrinol., 2015. [DOI] [PubMed]
- 28.Papadakis K.A., Targan S.R. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 2000 doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 29.Maloy K.J., Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011 doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 30.Feagins L.A. Wiley Online Library; 2010. Role of Transforming Growth Factor-β in Inflammatory Bowel Disease and Colitis-Associated Colon Cancer—Inflammatory bowel diseases. [DOI] [PubMed] [Google Scholar]
- 31.Lee, Lee S.H., Kim E.K., Kim J.K., Shin D.Y., Cho M.L. Metformin ameliorates inflammatory bowel disease by suppression of STAT3 signaling pathway and regulation of the Th17/Treg balance (THER3P. 1001) J. Immunol. 2015 doi: 10.1371/journal.pone.0135858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.L Hawinkels, M Paauwe, HW Verspaget, E Wiercinska, JM van der Zon, K van der Ploeg, PJ Koelink, et al.. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts—Oncogene, 2014. [DOI] [PubMed]
- 33.Maynard C.L., Elson C.O., Hatton R.D. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattar M.C., Lough D., Pishvaian M.J., Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest. Cancer Res. 2011 [PMC free article] [PubMed] [Google Scholar]
- 35.Laukoetter M.G., Nava P., Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2008 doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akash M.S.H., Rehman K., Chen S. Wiley Online Library; 2013. Role of Inflammatory Mechanisms in Pathogenesis of Type 2 Diabetes Mellitus. Journal of Cellular Biochemistry. [DOI] [PubMed] [Google Scholar]
- 37.Hayden M.S., Ghosh S. Elsevier; 2008. Shared Principles in NF-κB Signaling. Cell. [DOI] [PubMed] [Google Scholar]
- 38.Rubin M.R., Goldfine A.B., McMahon D.J., Donovan D.S., Cremers S., Dworakowski E., Schaefer E.J. Springer; 2015. Effects of the Anti-Inflammatory Drug Salsalate on Bone Turnover in Type 2 Diabetes Mellitus—Endocrine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nami Y., Abdullah N., Haghshenas B. Assessment of probiotic potential and anticancer activity of newly isolated vaginal bacterium lactobacillus plantarum 5BL. Microbiol. Immunol. 2014;58(9):492–502. doi: 10.1111/1348-0421.12175. [DOI] [PubMed] [Google Scholar]
- 40.Erez N., Truitt M., Olson P., Hanahan D. Elsevier; 2010. Cancer-Associated Fibroblasts are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-κB-Dependent Manner, Cancer Cell. [DOI] [PubMed] [Google Scholar]
- 41.Basso P.J., Fonseca M.T.C., Bonfá G., Alves V.B.F., Sales-Campos H., Nardini V., Cardoso C.R.B. Association among genetic predisposition, gut microbiota, and host immune response in the etiopathogenesis of inflammatory bowel disease. Braz. J. Med. Biol. Res. 2014 doi: 10.1590/1414-431X20143932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atreya R., Mudter J., Finotto S., Müllberg J., Jostock T., Wirtz S., Schütz M. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat. Med. 2010 doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 43.Sartor R.B. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am. J. Gastroenterol. 1997 [PubMed] [Google Scholar]
- 44.Xavier R.J., Podolsky D.K. The pathogenesis of inflammatory bowel disease. Nature. 2007 doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 45.Duary R.K., Bhausaheb M.A., Batish V.K., Grover S. Springer; 2012. Anti-Inflammatory and Immunomodulatory Efficacy of Indigenous Probiotic Lactobacillus Plantarum Lp91 in Colitis Mouse Model. Molecular Biology Reports. [DOI] [PubMed] [Google Scholar]
- 46.Thoreson R., Cullen J.J. Elsevier; 2007. Pathophysiology of Inflammatory Bowel Disease: An Overview. Surgical Clinics of North America. [DOI] [PubMed] [Google Scholar]
- 47.Gupta S.C., Kim J.H., Prasad S., Aggarwal B.B. Springer; 2010. Regulation of Survival, Proliferation, Invasion, Angiogenesis, and Metastasis of Tumor Cells Through Modulation of Inflammatory Pathways by Nutraceuticals. Cancer Metastasis Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.SC Larsson, N Orsini, A Wolk. Diabetes mellitus and risk of colorectal cancer: a meta-analysis—J. Natl. Cancer Inst. 2005. [DOI] [PubMed]
- 49.Erdman S.E., Poutahidis T. Roles for inflammation and regulatory T cells in colon cancer. Toxicol. Pathol. 2010 doi: 10.1177/0192623309354110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kahn S.E. Springer; 2003. The Relative Contributions of Insulin Resistance and Beta-Cell Dysfunction to the Pathophysiology of Type 2 Diabetes, Diabetologia. [DOI] [PubMed] [Google Scholar]
- 51.Fischbach S., Gittes G.K. The role of TGF-β signaling in β-cell dysfunction and type 2 diabetes: a review. J. Cytol. Histol. 2014 [Google Scholar]
- 52.Grivennikov S., Karin E., Terzi J., Mucida D., Yu G.Y., Vallabhapurapu S., Scheller J. Elsevier; 2009. IL-6 and Stat3 Are Required for Survival of intestinal epithelial cells and development of Colitis-Associated Cancer Cancer cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong S., Lee H.J., Kim S.J., Hahm K.B. Connection between inflammation and carcinogenesis in gastrointestinal tract: focus on TGF-β signaling. World J. Gastroenterol. 2010 doi: 10.3748/wjg.v16.i17.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vieira S.M., Lemos H.P., Grespan R., Napimoga M.H., Dal-Secco D., Freitas A., Cunha T.M. Wiley Online Library; 2009. A Crucial Role for TNF-α in Mediating Neutrophil Influx Induced by Endogenously Generated or Exogenous Chemokines, KC/CXCL1 and LIX/CXCL5—British journal of Pharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sobhani I., Jarrousse V., Guilemeau S. Colon cancer patients' microbiome induces intestinal precancerous change in germ-free mice. Gut. 2011;60(3):A1. [Google Scholar]
- 56.Seshasai S.R., Kaptoge S., Thompson A., Angelantonio E.D., Gao P., Sarwar N., Whincup P.H. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seo S.U., Chen G.Y., Núñez G., Kamada N. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013 doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 58.Kühbacher T., Schreiber S., Fölsch U.R. Springer; 2004. Ulcerative Colitis: Conservative Management and Long-Term Effects. Langenbeck's Archives of Surgery. [DOI] [PubMed] [Google Scholar]
- 59.Chandrasekar V.T., Venu N. Springer; 2015. So What Is Crohn's disease and Ulcerative Colitis? Pathophysiology of Crohn's disease and Ulcerative Colitis. Inflammatory Bowel Disease. [Google Scholar]
- 60.Strober W., Zhang F., Kitani A., Fuss I., Fichtner-Feigl S. Pro-inflammatory cytokines underlying the inflammation of Crohn's disease. Curr. Opin. Gastroenterol. 2010 doi: 10.1097/MOG.0b013e328339d099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng X.H., Derynck R. Specificity and versatility in TGF-β signaling through Smads. Rev. Cell Dev. Biol. 2005 doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 62.Wu X., Conlin V.S., Morampudi V., Ryz N.R., Nasser Y., Bhinder G., Bergstrom K.S. Vasoactive intestinal polypeptide promotes intestinal barrier homeostasis and protection against colitis in mice. PLoS One. 2015 doi: 10.1371/journal.pone.0125225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ben-Neriah Y., Karin M. 2011. Inflammation Meets Cancer, with NF-[Kappa] B as the Matchmaker—Nature Immunology. [DOI] [PubMed] [Google Scholar]
- 64.Liu Z., Brooks R.S., Ciappio E.D., Kim S.J., Crott J.W., Bennett G., Greenberg A.S. Elsevier; 2012. Diet-Induced Obesity Elevates Colonic TNF-α in Mice and is Accompanied by an Activation of Wnt Signaling: A Mechanism for Obesity-Associated Colorectal Cancer—The Journal of Nutritional Biochemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Z., Wang R., Yang Y., Dai R., Shang J., Yu Q. 2014. Intervention Effect of Probiotic Bifidobacterium on Type 2 Diabetes Mellitus Rats—Journal of Hygiene Research. [PubMed] [Google Scholar]