Abstract
Introduction
We conducted a follow-on study to a phase I randomized, controlled trial conducted in Cuba, 2012, to assess the persistence of poliovirus antibodies at 21–22 months following booster dose of Sabin-IPV compared to Salk-IPV in adults who had received multiple doses of oral poliovirus vaccine (OPV) during childhood.
Methods
In 2012, 60 healthy adult males aged 19–23 were randomized to receive one booster dose, of either Sabin-inactivated poliovirus vaccine (Sabin-IPV), adjuvanted Sabin-IPV (aSabin-IPV), or conventional Salk-IPV. In the original study, blood was collected at days 0 (before) and 28 (after vaccination), respectively. In this study, an additional blood sample was collected 21–22 months after vaccination, and tested for neutralizing antibodies to Sabin poliovirus types 1, 2 and 3.
Results
We collected sera from 59/60 (98.3%) subjects; 59/59 (100%) remained seropositive to all poliovirus types, 21–22 months after vaccination. The decay curves were very similar among the study groups. Between day 28 and 21–22 months, there was a reduction of ⩾87.4% in median antibody levels for all poliovirus types in all study groups, with no significant differences between the study groups.
Conclusion
The decay of poliovirus antibodies over a 21–22-month period was similar regardless of the type of booster vaccine used, suggesting the scientific data of Salk IPV long-term persistence and decay may be broadly applicable to Sabin IPV.
Keywords: Adjuvant, Aluminum hydroxide, Inactivated poliovirus vaccine, Phase I trial, Sabin strains, Duration of antibodies
1. Introduction
In 2008, the WHA recommended the WHO develop safer inactivated poliovirus vaccine (IPV) production technology using attenuated seed strains, such as Sabin polioviruses (Sabin-IPV) [1]. Sabin-IPV technology would partly address the biosafety risks associated with Sabin-IPV production, therefore allowing for production in developing countries [2], [3].
The immunogenicity of Sabin-IPV administered in the primary series has been well-established in different clinical studies in China, Japan, Poland, and Cuba [4], [5], [6], [7], some of which demonstrated that Sabin-IPV induced adequate neutralizing antibodies to both Sabin and wild poliovirus [6], [8]. Sabin-IPV products are currently licensed in Japan and China, and are under development in many other countries [9]. As Sabin-IPV and adjuvanted Sabin-IPV (aSabin-IPV) are expected to be widely used in the near future, it is important to assess the medium and long-term persistence of Sabin-IPV boosted antibody response.
Several studies have demonstrated the long-term presence of neutralizing antibodies, induced by Salk IPV [10], [11], [12], [13]. To date however, only one study has assessed the duration of immunity induced by Sabin-IPV. This was a phase III trial conducted in Japan, using tetravalent diphtheria-tetanus-acellular pertussis-Sabin-IPV vaccine (DTaP-Sabin-IPV), which demonstrated comparable immunity between Salk-IPV and DTaP-Sabin-IPV, 6–18 months after vaccination [5].
We conducted a follow-on study to the phase I Cuba study conducted in 2012. This study is the first to assess and compare the decay of neutralizing antibodies to poliovirus, between day 28 and 21–22 months, in adults who received multiple doses of oral poliovirus vaccine (OPV) during childhood, following a booster dose of either Sabin-IPV, aSabin-IPV, or Salk-IPV, in a tropical setting.
2. Methods
In the phase I trial in Cuba conducted in 2012, sixty healthy male subjects aged 19–23 years, who had received polio vaccination with multiple doses of OPV during childhood, in accordance with the Cuban national immunisation program, and with no history of receiving poliovirus vaccine since the age of 9 years, were enrolled and randomized, to receive a booster dose of either conventional Salk-IPV, or Sabin-IPV, or aSabin-IPV (adjuvanted with Aluminum hydroxide), with the following formulations: Salk-IPV 40:8:32 D-antigen Units per dose (DU/dose), Sabin-IPV 20:32:64 DU/dose, and aSabin-IPV 10:16:32 DU/dose and 0.5 mg aluminum hydroxide, respectively [14]. All Sabin-IPV and Salk-IPV vaccines were provided by the Netherlands Vaccine Institute (NVI) (currently called Intravacc) [7].
In our follow-on study, all subjects were contacted at 21–22 months after initial vaccination, for blood collection. Sera were tested at the Institute Pedro Kouri, for neutralizing antibodies to Sabin poliovirus types 1–3, using standard micro-neutralization assay. The antibody titers were diluted to 1:65,536, above the standard 1:1024 because high titers were expected with a boosting dose of IPV. Seroconversion was defined as a ⩾fourfold increase in reciprocal antibody titers.
We calculated the decay in antibody titers at day 28 and at 21–22 months, and the overall increase in antibody titers during day 0 and 21–22 months, by poliovirus type and study arm with 95% confidence intervals using bootstrapping sampling and estimation with 10,000 replications. We tested differences in antibody titers between the study groups, with Salk-IPV as the reference group, by poliovirus type, using Wilcoxon rank sum test with significance indicated by p ⩽ 0.05. All analyses were conducted using statistical application “R 3.1.2” [15].
3. Results
3.1. Study population
In the previous study, there were no significant differences between the three groups, in baseline characteristics of age, height, weight, time since receiving last OPV dose, or, baseline titer of neutralizing antibodies to Sabin poliovirus types 1–3 [7].
In our study, a total of 59/60 (93.1%) subjects were followed-up at 21–22 months (654–675 days). One subject in the aSabin-IPV arm was lost to follow-up, and one subject in the aSabin-IPV group had moved to Havana, where their blood was collected.
3.2. Antibody decay (day 28 to 21–22 months)
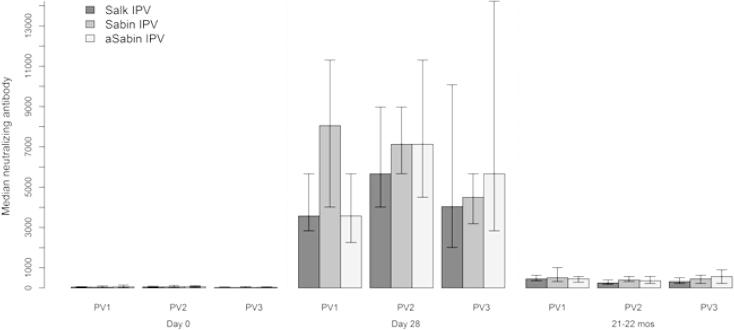
In the previous study, there were no differences in immunogenicity to Sabin poliovirus types 1–3 between the study groups during day 0 and day 28, with all subjects seroconverting or boosted by day 28 [7]. At day 28, median antibody titers were highest for poliovirus type 1 in the Sabin-IPV study group, and for poliovirus types 2 and 3 in the aSabin-IPV group (Fig. 1).
Fig. 1.
Median antibody titers (log2) to poliovirus types 1–3, on day 0, day 28, and 21–22 months, by study group; 95% confidence intervals calculated using bootstrapping with 10,000 replications.
In our study, at 21–22 months, all subjects had detectable antibody titers for all Sabin poliovirus types, with median antibody titers highest for poliovirus types 1 and 2 in the Sabin-IPV study group, and for poliovirus type 3, in aSabin-IPV group. Median titers were lowest for all poliovirus types in the Salk-IPV group. We did not find any significant differences in median antibody titers between the study groups for all poliovirus types (Fig. 1, Table 1).
Table 1.
Median antibody titers (log2) to poliovirus types 1–3, on day 0, day 28, and 21–22 months, by study group with 95% confidence intervals calculated using bootstrapping with 10,000 replications.
| Day 0 |
Day 28 |
21–22 months |
|||||
|---|---|---|---|---|---|---|---|
| Median titer (95% CI) | p-Value | Median titer (95% CI) | p-Value | Median titer (95% CI) | p-Value | ||
| Type 1 | Salk IPV | 45.0 (18.0–71.0) | Ref | 3573.0 (2839.0–5664.0) | Ref | 450.0 (357.0–635.3) | Ref |
| Sabin IPV | 36.0 (25.4–100.8) | 0.322 | 8053.0 (4009.4–11300.0) | 0.271 | 508.0 (318.4–1007.3) | 0.693 | |
| aSabin IPV | 57.0 (14.0–142.0) | 0.473 | 3573.0 (2255.0–5664.0) | 0.365 | 450.0 (284.0–566.0) | 0.64 | |
| Type 2 | Salk IPV | 51.0 (28.0–90.0) | Ref | 5664.0 (4009.4–8976.0) | Ref | 254.5 (179.0–400.8) | Ref |
| Sabin IPV | 57.0 (36.0–126.9) | 0.957 | 7130.0 (5664.0–8976.0) | 0.225 | 403.5 (318.4–566.0) | 0.177 | |
| aSabin IPV | 71.0 (36.0–113.0) | 0.693 | 7130.0 (4499.0–11300.0) | 0.365 | 357.0 (225.0–566.0) | 0.352 | |
| Type 3 | Salk IPV | 25.5 (15.9–50.6) | Ref | 4036.0 (2009.7–10071.2) | Ref | 320.5 (225.0–504.7) | Ref |
| Sabin IPV | 25.5 (14.1–71.0) | 0.755 | 4499.0 (3184.9–5664.0) | 0.946 | 450.0 (225.0–635.3) | 0.724 | |
| aSabin IPV | 28.0 (14.0–57.0) | 0.921 | 5664.0 (2839.0–14226.0) | 0.248 | 566.0 (225.0–898.0) | 0.535 | |
Note: excluded 1 subject missing data for 21–22 months.
There were no statistically significant differences in the decay of antibody titers during day 28 and 21–22 months, between Salk-IPV, Sabin-IPV and aSabin-IPV groups, with relative reduction as a percentage decline in median antibody titers by poliovirus type: 92.1%, 92.1%, 87.4% for poliovirus type 1 (p = 0.54; p = 0.61, respectively); 96.0%, 95.0%, 95.0% for poliovirus type 2 (p = 0.66; p = 0.93, respectively); 93.7%, 92.1%, 93.7% for poliovirus type 3 (p = 0.67; p = 0.50, respectively) (Table 2).
Table 2.
Relative increase in median antibody titers (%) between day 0 and 21–22 months; relative reduction in median antibody titers during day 28 and 21–22 months, to poliovirus types 1–3, by study group with 95% confidence intervals calculated using bootstrapping with 10,000 replications.
| Day 0 and 21–22 months comparison |
Day 28 and 21–22 months comparison |
||||
|---|---|---|---|---|---|
| Median % relative increase (95% CI) | p-Value | Median % relative reduction (95% CI) | p-Value | ||
| Type 1 | Salk IPV | 1121.3 (896.4–2126.5) | Ref | 92.1 (84.2–92.9) | Ref |
| Sabin IPV | 1028.5 (535.1–1714.3) | 0.534 | 92.1 (90.0–93.7) | 0.543 | |
| aSabin IPV | 893.0 (151.4–1904.2) | 0.399 | 87.4 (80.1–92.1) | 0.613 | |
| Type 2 | Salk IPV | 458.7 (147.3–1032.1) | Ref | 96.0 (92.9–96.8) | Ref |
| Sabin IPV | 694.7 (124.4–1305.0) | 0.705 | 95.0 (93.7–96.0) | 0.655 | |
| aSabin IPV | 402.8 (100.0–893.0) | 0.945 | 95.0 (93.7–96.8) | 0.933 | |
| Type 3 | Salk IPV | 1043.9 (685.8–1670.5) | Ref | 93.7 (91.0–95.5) | Ref |
| Sabin IPV | 1276.9 (250.1–2727.8) | 0.892 | 92.1 (91.0–95.0) | 0.665 | |
| aSabin IPV | 1150.0 (694.7–2396.5) | 0.768 | 93.7 (92.1–96.8) | 0.500 | |
Note: excluded 1 subject missing data for 21–22 months.
3.3. Antibody increase (day 0 to 21–22 months)
There were no statistically significant differences in the relative increase in antibodies from day 0 to 21–22 months between Salk-IPV, Sabin-IPV and aSabin-IPV study groups by poliovirus type: 1121.3%, 1028.5%, 893.0%, for poliovirus type 1 (p = 0.53; p = 0.40, respectively); 458.7%, 694.7%, 402.8% for poliovirus type 2 (p = 0.71; p = 0.95, respectively); 320.5, 450.0, 566.0, for poliovirus type 3 (p = 0.72; p = 0.54, respectively) (Table 2).
4. Discussion
This study is the first to assess the decay of neutralizing antibodies to Sabin poliovirus beyond 28 days after a booster dose of Salk-IPV, Sabin-IPV, and aSabin-IPV, and found no differences between the study groups in adults who received multiple doses of OPV during childhood.
The only other study assessing the decay of neutralizing antibodies to Sabin poliovirus beyond 28 days after vaccination, was conducted by Okada in Japan, however this assessed antibody titers induced in infants, 6–18 months following a primary series administration with three doses of combination DTaP-Sabin-IPV; the relative percentage decline in median antibody titers during day 28 and 6–18 months was 75.3%, 63.9%, 86.3%, for poliovirus types 1–3, respectively [8].
The greater decay for Sabin-IPV may have been due to higher median antibody titers boosted by Sabin-IPV at day 28 with baseline of multiple OPV doses in our study, compared to lower median antibody titers induced by DTaP-Sabin IPV at day 28 following primary schedule, in Okada’s study: 3565.8, 5792.6, 4096, for Sabin-IPV in our study, compared to 2076.6, 1428.2 and 1663.5, induced by DTaP-Sabin-IPV in Okada’s study for poliovirus types 1, 2 and 3, respectively. However, the greater decay observed may have been an artifact, due to the differences in the dilution of antibody titers; in our study antibody titers were diluted up to 1:65,536, however we were not able to verify the level of dilution in Okada’s study.
Previous boosting studies for Salk-IPV demonstrated greater antibody decay following booster dose in the developing country, compared to industrialized country setting. A study conducted in Oman by Sutter demonstrated a relative reduction of 72.4% in median antibody titers to poliovirus type 3, 6 months after administration of a booster dose of Salk-IPV, in children who received a primary schedule of OPV. Furthermore, the decay in median antibody titers may have been greater in this study, as antibody titers were only diluted up to the standard 1:1448 [15]. This contrasts with findings from the Netherlands, published by Rumke, which demonstrated a relative reduction in antibody geometric mean titers of 62.1%, 71.3%, 83.5% to poliovirus types 1, 2, and 3, respectively, 5 years after administration of a booster dose of DT-Salk-IPV, in children who received a primary schedule of DTP-IPV, and a booster dose of DT-IPV at 4 years [16], [17].
Our study had some limitations. The sample size was small, limited to healthy adults from a potentially socio-economically homogenous area. The study was conducted in a specific tropical developing country setting. While our study found similar rates of antibody decay in adults, at 21–22 months between Sabin-IPV, aSabin-IPV, and Salk-IPV groups, we cannot directly extrapolate this trend to longer term decay between the groups. Therefore similar studies are needed to assess and compare medium and long term antibody decay, particularly in naive children.
Our study only assessed antibodies against Sabin virus type 1, 2, 3. Previously a study conducted in infants in Poland using the same vaccine (produced by Intravacc), demonstrated that seroconversion rates against both Sabin and wild virus were equivalent between the Sabin-IPV and Salk-IPV groups; however, neutralizing antibody titers induced by Sabin-IPV were higher against Sabin strains compared to wild poliovirus strains, and similarly, titers induced by Salk-IPV were higher against wild poliovirus strains [18]. Therefore, although we assume the rate of decay of antibodies against Salk strains is similar between Salk and Sabin IPV, the cross neutralisation effect is worth assessing in future studies.
There may have been secondary exposure to OPV during the study period (10/2012–08/2014); however we concluded that secondary exposure of enrolled subjects was minimal, as although there were four national OPV campaigns targeting children under 3 years of age, two of which also included children 9 years of age, during this period, we did not find that any subjects demonstrated an increase in antibody titers between day 28 and 21–22 months.
This study demonstrates comparable immunogenicity and trends in antibody decay 21–22 months in adults who had received multiple doses of OPV, after booster dose Sabin-IPV and aSabin-IPV, to Salk-IPV. This should galvanize momentum for countries and vaccine producers to further develop Sabin-IPV and aSabin-IPV technology. Future research should be conducted to further assess, and build the evidence for the medium and long-term immunity of Sabin-IPV.
Role of the funding source
Funding for this study was provided by World Health Organization, Geneva and the Ministry of Health, Labour and Welfare, Japan. Intravacc provided inactivated poliovirus vaccines free of charge for this study.
Role of medical writer or editor
No medical writer or editor was engaged.
Ethics committee approval
This study was approved by the Ethics Review Committee of the World Health Organization in Geneva, by the Ethics Review Committees of the Pedro Kouri Institute in Havana, and the Provincial Center for Hygiene and Epidemiology, Camaguey.
Declaration of interests
Authors did not declare any conflict of interest.
Acknowledgements
The authors would like to acknowledge the Government of Cuba, its Ministry of Health, Health Authorities of Camaguey Province and the Cuban office of the Pan American Health Organization for their support of this study. We would also like to thank Natalie Molodecky for her robust support with statistical analysis; and the subjects who participated in the trial. The authors would like to acknowledge Intravacc for their donation of inactivated poliovirus vaccines.
Trial registration: The trial was registered with the Australian New Zealand Clinical Trials Registry with trial ID: ACTRN 12612000465853.
References
- 1.World Health Resolutions WHA 61.1, 2008: Poliomyelitis: mechanism for management of potential risks to eradication. Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA61-REC1/A61_Rec1-part2-en.pdf.
- 2.Global Action Plan (GAP) III working draft 2014: WHO GAP III to minimize poliovirus facility-associated risk after type-specific eradication of wild polioviruses and sequential cessation of routine OPV use. Available from: http://www.who.int/immunization/sage/meetings/2014/october/3_GAP_III_Revision_10Oct14.pdf.
- 3.WHO Technical Working Group Meeting on Revision of the WHO Recommendations for the Production and Control of Inactivated Poliomyelitis Vaccines, May14-15, 2013: TRS No. 910, Annex 1. Available from: http://www.who.int/biologicals/areas/vaccines/IPV_Meeting_TRS_Revision_14-15_May_2013_Report_FINAL_22112013.pdf.
- 4.Liao G. Safety and immunogenicity of inactivated poliovirus vaccine made from Sabin strains: a phase II, randomized, positive-controlled trial. J. Infect. Dis. 2012;205(2):237–243. doi: 10.1093/infdis/jir723. [DOI] [PubMed] [Google Scholar]
- 5.Okada K. Phase II and III clinical studies of diphtheria-tetanus-acellular pertussis vaccine containing inactivated polio vaccine derived from Sabin strains (DTaP-sIPV) J. Infect. Dis. 2013;208(2):275–283. doi: 10.1093/infdis/jit155. [DOI] [PubMed] [Google Scholar]
- 6.Verdijk P. Safety and immunogenicity of inactivated poliovirus vaccine based on Sabin strains with and without aluminum hydroxide: a phase I trial in healthy adults. Vaccine. 2013;31(47):5531–5536. doi: 10.1016/j.vaccine.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Resik S. Reactogenicity and immunogenicity of inactivated poliovirus vaccine produced from Sabin strains: a phase I Trial in healthy adults in Cuba. Vaccine. 2014;32(42):5399–5404. doi: 10.1016/j.vaccine.2014.07.109. [DOI] [PubMed] [Google Scholar]
- 8.Pharmaceutical and Medical Devices Agency review of adsorbed diphtheria-purified pertussis-tetanus-inactivated polio (Sabin strain) combined vaccine. 2012. Quattrovac subcutaneous injection syringe. Available from: http://www.pmda.go.jp/files/000153291.pdf.
- 9.Sabin-IPV clinical studies: 12th OPV & IPV manufacturers consultation, WHO, Geneva, 10 October 2013. Available from: http://www.who.int/immunization_standards/vaccine_quality/bakker_wam_sabin_ipv_clinical_studies_manu_meeting_oct13.pdf.
- 10.Bottiger M. Polio immunity to killed vaccine: an 18-year follow-up. Vaccine. 1990;8(5):443–445. doi: 10.1016/0264-410x(90)90244-g. [DOI] [PubMed] [Google Scholar]
- 11.Faden H. Long-term immunity to poliovirus in children immunized with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines. J. Infect. Dis. 1993;168(2):452–454. doi: 10.1093/infdis/168.2.452. [DOI] [PubMed] [Google Scholar]
- 12.Swartz T.A. Use of a combined DTP-polio vaccine in a reduced schedule. Dev. Biol. Stand. 1986;65:159–166. [PubMed] [Google Scholar]
- 13.Murdin A.D., Barreto L., Plotkin S. Inactivated poliovirus vaccine: past and present experience. Vaccine. 1996;14(8):735–746. doi: 10.1016/0264-410x(95)00211-i. [DOI] [PubMed] [Google Scholar]
- 14.Westdijk J. Antigen sparing with adjuvanted inactivated polio vaccine based on Sabin strains. Vaccine. 2013;31(9):1298–1304. doi: 10.1016/j.vaccine.2012.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Team R.C. R Foundation for Statistical Computing. Vienna; Austria: 2014. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 16.Vidor E., Plotkin S. Poliovirus vaccine – inactivated. In: Plotkin S.A., Orenstein W.O., Offit P.A., editors. Vaccines. 6th ed. Elsevier; 2013. pp. 573–597. [Google Scholar]
- 17.Rumke H.C. Poliomyelitis in The Netherlands: a review of population immunity and exposure between the epidemics in 1978 and 1992. Epidemiol. Infect. 1995;115(2):289–298. doi: 10.1017/s0950268800058416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verdijk P. Safety and immunogenicity of a primary series of Sabin-IPV with and without aluminum hydroxide in infants. Vaccine. 2014;32(39):4938–4944. doi: 10.1016/j.vaccine.2014.07.029. [DOI] [PubMed] [Google Scholar]