Abstract
Background
Adherence to medication has been repeatedly proposed to represent a major cause of treatment‐resistant hypertension (TRH); however, treatment decisions such as treating TRH with renal denervation depend on accurate judgment of adherence. We carefully analyzed adherence rates to medication before and after renal denervation and its effect on blood pressure (BP) control.
Methods and Results
Eighty patients with TRH were included in 2 prospective observational studies that assessed the difference of potential antihypertensive and nephroprotective effects of renal denervation. To compare prescribed with actual medication intake (representing a measure of adherence), we analyzed urine samples collected at baseline and at 6 months after renal denervation for antihypertensive compounds or metabolites (by liquid chromatography–mass spectrometry). In addition to office BP, 24‐hour ambulatory BP and central hemodynamics (central systolic pressure, central pulse pressure) were assessed. Informed consent for analyses of urine metabolites was obtained from 79 of 80 patients. Actual intake of all antihypertensive drugs was detected at baseline and at 6 months after renal denervation in 44 (56%) and 52 (66%) patients, respectively; 1 drug was missing in 22 (28%) and 17 (22%) patients, respectively, and ≥2 drugs were missing in 13 (16%) and 10 (13%) patients, respectively. At baseline, 24‐hour ambulatory BP (P=0.049) and central systolic BP (P=0.012) were higher in nonadherent patients. Adherence did not significantly change overall (McNemar‐Bowker test, P=0.362). An increase in adherence was observed in 21 patients, and a decrease was observed in 11 patients. The decrease in 24‐hour ambulatory BP was not different in those with stable adherence 6 months after renal denervation (n=41, −7±13 mm Hg) compared with those with increased adherence (n=21, −10±13 mm Hg) and decreased adherence (n=11, −7±14 mm Hg) (P>0.20). Our study is limited by the relatively small sample size and potentially by the specific health environment of our university center (Northern Bavaria, Germany).
Conclusions
Nonadherence to medication among patients with TRH was relatively low: ≈1 of 6 patients with TRH did not take ≥2 of the prescribed drugs. Adherence pattern did not change significantly after renal denervation and had no impact on the overall observed BP changes, supporting the concept that renal denervation is an effective treatment in patients with TRH.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifiers: NCT00888433, NCT01442883 and NCT01687725.
Keywords: adherence, antihypertensive medication, renal denervation, resistant hypertension, treatment
Subject Categories: Cell Therapy, Translational Studies, Catheter-Based Coronary and Valvular Interventions, Myocardial Infarction
Introduction
Adherence to (or compliance with) an antihypertensive drug regimen is generally defined as the extent to which hypertensive patients take the medication as described by their individual physicians. It is well accepted that nonadherence to pharmacological therapy is the pivotal challenge of successful blood pressure (BP) control because antihypertensive therapy implies, in most cases, a lifelong treatment.1, 2, 3, 4 Several outcome research studies found that nonadherence to antihypertensive agents significantly increased cardiac and cerebrovascular risk.5, 6, 7 Furthermore, the choice of prescribed drug class affects adherence rates of antihypertensive therapies.8, 9 In patients with treatment‐resistant hypertension (TRH), who are treated on average with 5 to 6 antihypertensive agents, fixed‐dose combinations mitigate the problem of taking multiple pills per day, but in addition to the antihypertensive agents, these comorbid patients generally require additional cardioprotective drugs (eg, statins, antidiabetic agents).1, 10
Recommendations for the management of TRH emphasize the importance of judging the adherence behavior of each patient.10 The prevalence of nonadherence differs widely because of the inconsistency of study designs and the lack of objective measures to determine nonadherence to treatment. In clinical trials in which adherence is assessed by pill‐counting adherence rates ≥80% are accepted as complete adherence to medication.1, 11 Electronic methods of adherence monitoring (ie, computerized records of pharmacy prescription or electronic monitoring of pill box opening) may be considered reliable alternatives, but it remains subject to the patient's behavior whether the removed pills are indeed ingested.1, 12, 13 Recently, toxicological urine analysis of the compounds or their metabolites has gained increasing interest.14, 15, 16, 17 These biochemical analyses represent spot assessments of adherence but are subject to the “white coat adherence” effect.18, 19 The term white coat adherence describes that patients improve their medication‐taking behavior 5 days prior to and, in particular, on the appointment day with their physicians.1, 13
Triggered by 2 recent publications on nonadherence to antihypertensive treatment, as assessed by toxicological analysis,14, 15 we analyzed adherence rates in patients with TRH at baseline and at 6 months after renal denervation. The study included only those patients who successfully completed their 6‐month follow‐up period.20, 21 We report adherence rates at baseline and at 6 months after renal denervation and the relationship of adherence level with the corresponding BP measurements in 79 patients with resistant hypertension.
Methods
Study Cohort and Design
The study population consisted of patients with TRH who were included in studies analyzing the effects of renal denervation on office BP, 24‐hour ambulatory blood pressure (ABP), central hemodynamics, albuminuria, and renal function.20, 21 The studies were registered at the US National Institutes of Health ClinicalTrials.gov website (NCT00888433, NCT01442883, and NCT01687725). These investigator‐initiated prospective clinical trials were initiated in June 2009, September 2011, and September 2012, respectively, and results were published recently.20, 21, 22, 23 In July 13, 2013, we submitted an amendment to our local ethics committee to have all patients retrospectively consent in written form for the measurement of their prescribed antihypertensive drugs or their corresponding metabolites from urine samples that were stored at their baseline and 6‐month follow‐up visits. When the study proposal was approved on August 23, 2013, we could identify 80 patients with TRH who were included in 1 of the trials (NCT00888433, NCT01442883, and NCT01687725), and all patients had already completed 6‐month follow‐up. Informed consent was obtained from 79 of 80 patients; 1 patient could not be contacted because she had moved to Bosnia.
In all 79 patients, TRH was defined as office BP ≥140/90 mm Hg despite being treated with at least 3 antihypertensive drugs including a diuretic.10 All were on a stable drug regimen for at least 1 month prior to the baseline examination. In every patient, true resistant hypertension was confirmed by initial 24‐hour ABP monitoring (≥130/80 mm Hg), thereby excluding the white coat effect.24, 25 In line with recent position papers of the European Society of Hypertension (ESH) and European Society of Cardiology, the main exclusion criteria were renal artery anatomy and any secondary causes of hypertension (except treated sleep apnea syndrome and chronic kidney disease).24, 25 Only patients from the clinical research center at the Department of Nephrology and Hypertension, University Hospital Erlangen, Germany, were included. The study was performed according to the Declaration of Helsinki and good clinical practice guidelines.
Office BP and 24‐Hour ABP
Office BP was measured with an oscillometric device (Dinamap Pro 100V2; Criticon, Norderstedt, Germany) after 5 minutes of rest in a sitting position, and subsequent office BP measurements were performed on the arm with the higher BP readings; the average of 3 measurements was taken. ABP measurements were performed with automatic portable devices validated according to the ESH International Protocol (eg, Spacelab No.90207).26
Central Hemodynamics
Radial artery waveforms were sampled using a noninvasive technique, calibrated to the measured average brachial BP, using the Sphygmocor System (Atcor Medical, Sydney, Australia), as described previously.27 Corresponding central (aortic) waveforms were automatically generated from the arterial waveform by built‐in validated transfer function. Values were computed for central systolic BP, central diastolic BP, and central pulse pressure; in addition, augmentation index and pressure were derived. At least duplicate recordings were assessed, and all were of high quality, defined as a quality index >80% (based on an in‐device algorithm).
Measurement of Albuminuria and Renal Function
Urinary albumin/creatinine ratio was determined from early morning single‐spot urine by measuring urinary albumin and creatinine concentration (university hospital central laboratory).20 Estimated glomerular filtration rate was calculated according to the Chronic Kidney Disease Epidemiology Collaboration equation.28
Catheter‐Based Renal Denervation
To have access for the renal denervation catheter, the femoral artery was punctured with standard endovascular technique. A radiofrequency catheter (Symplicity Flex RDN System; Medtronic Inc) was used to apply at least 4 radiofrequency ablations (energy delivery for 120 seconds each, controlled and regulated by radiofrequency generator) longitudinally and rotationally within the lengths of each renal artery to cover a full 4‐quadrant ablation. The radiofrequency catheter was advanced in each renal artery under the control of angiographic images. Patients received 5000 IU of heparin, and visceral pain during the procedure was managed with anxiolytics and narcotics.
Urinary Toxicological Analysis
From the urine samples collected at baseline and 6‐month follow‐up visits, toxicological urine analysis were performed by high‐performance liquid chromatography–mass spectrometry (LC‐MS/MS). In brief, 50 μL of internal standard (methadone‐d9 0.1 ng/μL in acetonitrile) and 1 mL of ethylacetate were added to the urine samples (0.2 mL), followed by centrifugation for 10 minutes at 16 000g. The organic phase was then transferred to a clean vial. After evaporation for 10 minutes at 20°C, the dry residue was redissolved in 100 μL 0.1% formic acid/acetonitrile (80:20, vol/vol), of which 2 μL was used for further analysis. For LC‐MS/MS analysis, a system from Agilent was used consisting of a 1290 Infinity LC coupled via JetStream electrospray interface to a 6460 Triple Quadrupole mass spectrometer (MS/MS). Analytes were separated at 30°C on a Kinetex™ XB‐C18, 100 Å, 100×2.1‐mm ID column equipped with guard column (Kinetex™ XB‐C18) from Phenomenex. The mobile phase consisted of (1) 0.01% formic acid containing 5 mmol/L ammonium format and (2) acetonitrile containing 0.1% formic acid. The elution program increased from 5% to 95% acetonitrile containing 0.1% formic acid over 4 minutes, followed by 2 minutes at 95% acetonitrile containing 0.1% formic acid and 1.2 minutes of reequilibration at 5% at a flow rate of 0.5 mL/min. Electrospray interface parameters were gas flow 11 L/min (300°C), nebulizer 45 psi, sheath gas flow 12 L/min (400°C), and capillary voltage 3500 V. For hydrochlorothiazide, furosemide, and xipamide, negative electrospray interface mode was used; all other compounds were analyzed in positive electrospray interface mode.
The MS/MS was operated in dynamic multiple reaction monitoring mode with at least 2 transitions recorded for each of the following targeted analytes: alisikiren, amiloride, amlodipine, benazepril, benazeprilat, bisoprolol, candesartan, canrenone, carvedilol, chlorthalidone, clonidine, diltiazem, doxazosin, enalapril, enalaprilat, felodipine, furosemide, hydrochlorothiazide, irbesartan, lercanidipine, lisinopril, losartan, metoprolol, minoxidil, moxonidine, nebivolol, nifedipine, nitrendipine, olmesartan, perindopril, piretanide, prazosin, ramipril, ramiprilat, spironolactone, telmisartan, torasemide, triamterene, urapidil, valsartan, and verapamil.
Data evaluation was performed using the Agilent MassHunter software (B 06.01). Identification was achieved based on comparison with the results from analysis of a blank urine and a urine extract containing reference substance of all target compounds in low concentrations. A deviation of ±0.1 minute of the expected retention time and a quantifier/qualifier ratio ±20% of the expected ratio were required.
Statistical Analysis
All statistical analyses were performed using IBM SPSS statistics for Windows, version 20.0 (IBM Corp). Normal distribution of data was confirmed by the Kolmogorov–Smirnov test before further analyses. Normally distributed data are expressed as mean±SD in the text and as mean±SEM in figures. Adherence data were categorized as complete adherence, indicating that all prescribed antihypertensive drugs were detected; partial adherence, indicating that, at maximum, 1 of the prescribed drugs was missing; and nonadherence, indicating that ≥2 of the prescribed drugs were not found in the toxicological analysis (“numeric adherence”). In a second set of analyses, we defined adherence as detection of ≥80% of the detectable drugs and nonadherence as detection of <80% measured in the urine analysis. These criteria are established for the judgment of adherence in pharmacological studies.1, 11
Nonnormally distributed data are presented as median and interquartile range.
Original data were analyzed by paired and unpaired Student t tests to compare before versus after and between‐groups adherence, respectively, if normally distributed and by Wilcoxon and Mann‐Whitney U test if not normally distributed. The McNemar‐Bowker test was applied to test the change in adherence rates before versus 6 months after renal denervation. A 2‐sided P value of <0.05 was considered statistically significant.
Results
Rates of Adherence to Antihypertensive Medication
The clinical characteristics of the study cohort are listed in Table 1. At 6 months after renal denervation, office systolic and diastolic BP decreased by 13±22 and 7±12 mm Hg, respectively (both P<0.001) and 24‐hour systolic and diastolic ABP decreased by 8±16 and 5±10 mm Hg, respectively (both P<0.001). Urinary albumin excretion tended to decrease after 6 months (P=0.097). The number of antihypertensive medications prescribed decreased significantly (P=0.042), but the number of detectable compounds remained unchanged (5 on average) (Table 1).
Table 1.
Clinical Characteristics of the Study Cohort
| Total Study Cohort (N=79) | Baseline | 6 Months | P Value |
|---|---|---|---|
| Age, y | 60.4±10 | — | |
| Sex (male/female) | 57/22 | — | |
| Ethnicity (white, %) | 76 (96.2) | — | |
| Type 2 diabetes, N (%) | 40 (50.6) | — | |
| Coronary heart disease, N (%) | 29 (36.7) | — | |
| Body mass index, kg/m2 a | 31.1±4.6 | 31.0±4.9 | 0.681 |
| Office BP systolic, mm Hga | 158±21 | 145±21 | <0.001 |
| Office BP diastolic, mm Hga | 88±16 | 81±13 | <0.001 |
| 24‐hour ABP systolic, mm Hga | 155±14 | 148±18 | <0.001 |
| 24‐hour ABP diastolic, mm Hga | 88±13 | 85±10 | <0.001 |
| Number of antihypertensive drugs prescribedb | 6.0 (5.0–7.0) | 5.0 (4.0–7.0) | 0.042 |
| Number of antihypertensive drugs detectedb | 5.0 (4.0–6.0) | 5.0 (4.0–7.0) | 0.480 |
| eGFR, mL/min per 1.73 m²a | 73.8±25 | 73.0±29 | 0.517 |
| UACR, mg/g creatinineb | 53.0 (7.75–239) | 35.0 (6.0–183.5) | 0.097 |
ABP indicates ambulatory blood pressure; eGFR, estimated glomerular filtration rate; UACR, urinary albumin/creatinine ratio.
Paired t test has been applied.
Wilcoxon test has been applied.
At baseline, complete adherence was found in 44 (56%) patients, partial adherence was found in 22 (28%), and nonadherence was found in 13 (16%). The respective numbers at the 6‐month follow‐up visit were 52 (66%) patients with complete adherence, 17 (22%) with partial adherence, and 10 (13%) with nonadherence (Table 2). In 3 patients at baseline and in 1 patient at follow‐up, none of the prescribed drugs could be detected. Nonadherence appeared to be similar among antihypertensive drug classes at baseline (Figure 1). In analyzing changes in adherence between baseline and 6‐month visits, no significant change in adherence to antihypertensive medication was noted (McNemar‐Bowker test, P=0.362) (Figure 2A through 2C); for example, of the 13 patients with TRH that were nonadherent at baseline, 3 patients became completely adherent and 4 became partially adherent.
Table 2.
Adherence to Antihypertensive Medication in the Total Study Cohort and the Severe Treatment‐Resistant Hypertension Subgroup
| Measure of Adherence At Baseline | Measure of Adherence At 6‐Month Follow‐up | |
|---|---|---|
| Number of patients, N (%) | 79 (100) | 79 (100) |
| Average number of drugs screened | 5.7 (±1.5) | 5.4 (±1.7) |
| Average number of drugs detectable | 5.0 (±1.9) | 5.1 (±1.9) |
| Complete adherence,a n (%) | 44 (56) | 52 (66) |
| Partial adherence,b n (%) | 22 (28) | 17 (22) |
| Nonadherence,c n (%) | 13 (16) | 10 (13) |
All detectable drugs detected.
One drug not detected.
Two or more drugs not detected.
Figure 1.
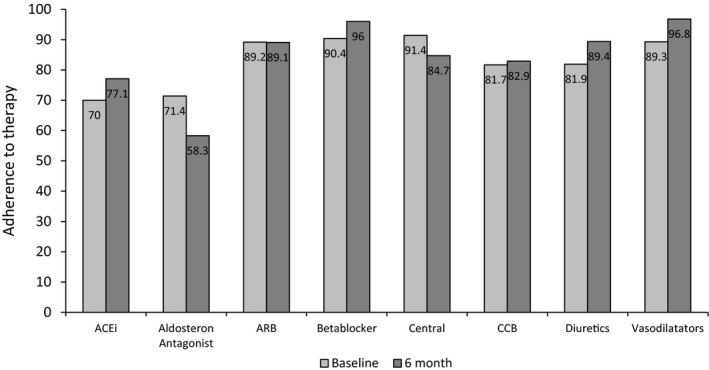
Adherence to therapy in different drug classes at baseline and 6‐month visits (descriptive illustration of percentages). ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; Central, Central sympatholytic agent; CCB, calcium channel blocker.
Figure 2.
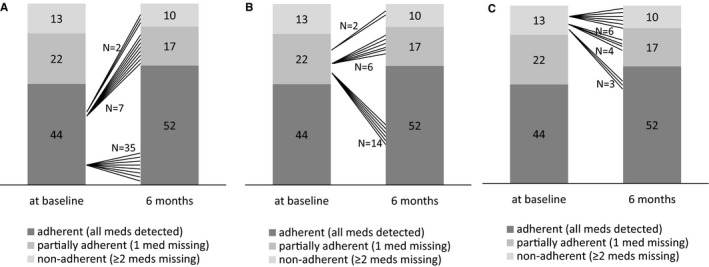
Shift analysis of adherence pattern from baseline to 6‐month visit in patients with complete adherence (A), partial adherence (B), and nonadherence (C) at baseline (based on numeric definition). Med indicates medication.
In applying the criterion of whether 80% of the detectable medication was detected, adherence was found in 59 of 79 (74.7%) patients with TRH at baseline and in 61 of 79 (77.2%) at 6‐month follow‐up. Figure 3A shows the distribution of percentage of adherence at baseline and after 6 months. The shift analysis revealed again that adherence remained the same in 53 of the 59 patients with TRH that were adherent at baseline and in 12 of the 20 patients that were nonadherent (full details in Figure 3B). Again, adherence rates did not change significantly (McNemar‐Bowker test, P=0.791).
Figure 3.
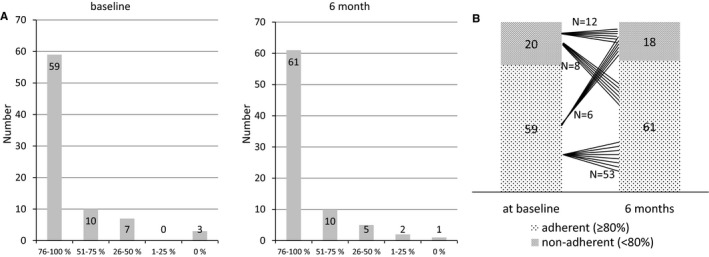
A, Distribution of adherence level at baseline according to the percentage of medication detected. B, Shift analysis of adherence pattern from baseline to 6‐month visit (based on percentage definition).
Adherence and BP
At baseline, office BP was not significantly different between the patients categorized according to adherence level (Figure 4A), but 24‐hour ABP was higher in nonadherent patients (P=0.317 for office BP and P=0.049 for ABP) (Figure 4C). Using the criteria of whether <80% and ≥80% of the medication was detected (Figure 4B and 4D), office BP and 24‐hour ABP tended to be higher in nonadherent patients (P=0.068 for office BP, P=0.099 for ABP).
Figure 4.
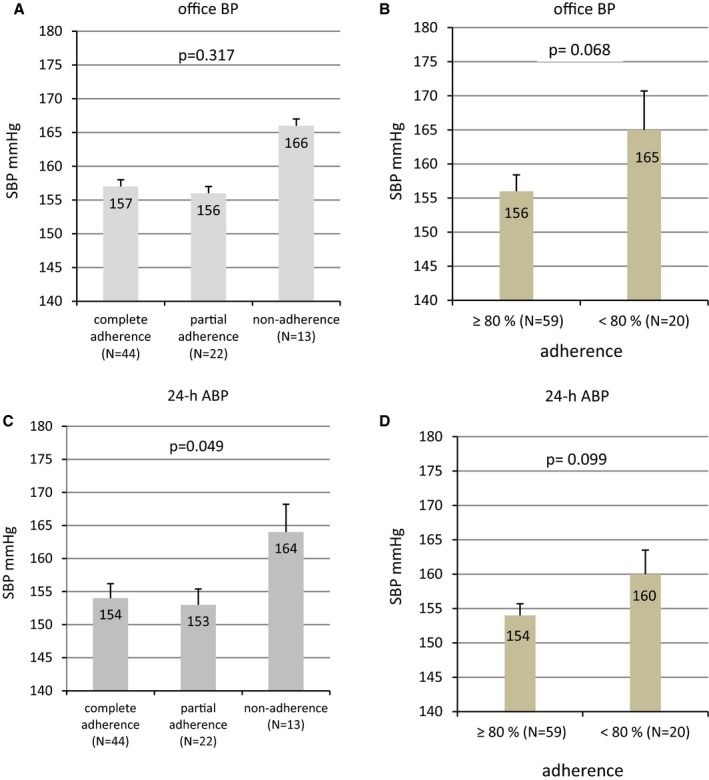
Office BP (A and B) and 24‐hour ABP (C and D) at baseline categorized according to the adherence level (numeric [left] and percentage [right] definitions), mean±SEM. ABP indicates ambulatory blood pressure; BP, blood pressure; h, hour; SBP, systolic blood pressure.
We could not detect a clear relationship between the change of adherence level and BP changes (Figure 5A and 5B): The decrease in systolic office BP (and 24‐hour ABP) was not different in those who were completely or partially adherent at baseline and 6‐month follow‐up (stable adherence: −11±23 mm Hg for office BP, −8±17 mm Hg for ABP) compared with those who had an increased adherence level (−16±24 mm Hg for office BP, −10±13 mm Hg for ABP) and a decreased adherence level (−10±19 mm Hg for office BP, −7±14 mm Hg for ABP) (P=0.683 for office BP, P=0.869 for ABP). Changes in diastolic office BP and 24‐hour ABP also were not related to adherence level (data not shown). In analyzing the patient groups with increased versus decreased adherence, the decrease in office systolic BP (−16±24 versus −10±19 mm Hg, P=0.767) and 24‐hour ABP (−10±13 versus −7±14 mm Hg, P=0.891) was not significantly different.
Figure 5.
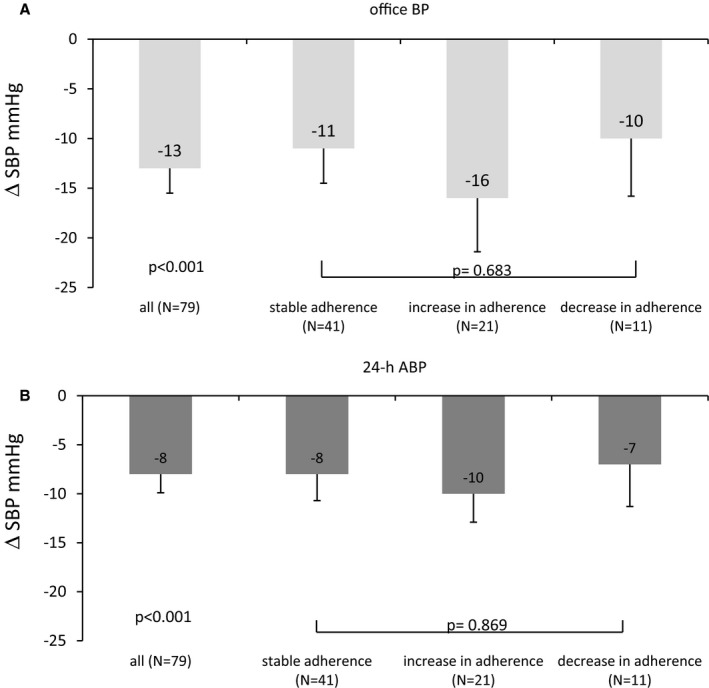
Decrease in office BP (A) and 24‐hour ABP (B) after renal denervation in all study patients (N=79) and subgroups (stable adherence, increase in adherence, and decrease in adherence at 6 months) based on numeric definition, mean±SEM. ABP indicates ambulatory blood pressure; BP, blood pressure; h, hour; SBP, systolic blood pressure.
Likewise, using the criteria for whether ≥80% and <80% of the medication was detected (Figure 6A and 6B), the reduction in systolic office BP (and 24‐hour ABP) was not different in those who were adherent at baseline and at 6‐month follow‐up (n=53: −10±22 mm Hg for office BP, −7±16 mm Hg for ABP) compared with those who had increased adherence (n=8: −31±23 mm Hg for office BP, −13±16 mm Hg for ABP) and decreased adherence (n=6: −17±15 mm Hg for office BP, −13±15 mm Hg for ABP) (P=0.101 for office BP, P=0.680 for ABP). Changes in diastolic office BP and 24‐hour ABP also were not related to adherence level (data not given). In analyzing the patient groups with increased versus decreased adherence, the decrease in office systolic BP (−31±23 versus −17±15 mm Hg, P=0.487) and 24‐hour ABP (−13±15 versus −13±15 mm Hg, P=0.998) was not significantly different.
Figure 6.
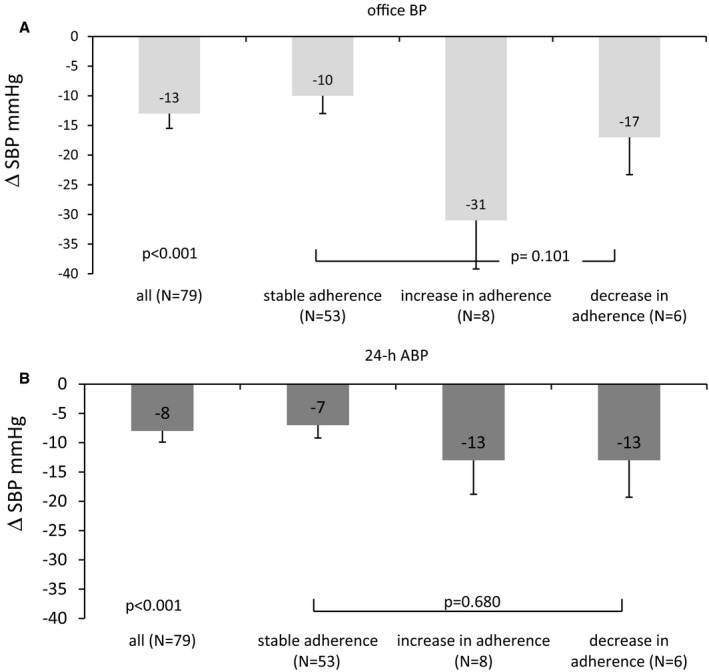
Decrease in office BP (A) and 24‐hour ABP (B) after renal denervation in all study patients (N=79) and subgroups (stable adherence, increase in adherence and decrease in adherence at 6 months) divided by whether ≥80% or <80% of the detectable drugs were detected (percentage definition), mean±SEM. ABP indicates ambulatory blood pressure; BP, blood pressure; SBP, systolic blood pressure.
Adherence and Other Outcome Measurements
Patients with TRH categorized by adherence did not show any meaningful difference in albuminuria (Table 3), regardless of the definition of adherence. In analyzing the relationship of adherence with central hemodynamic parameters, central systolic pressure was greater in patients with nonadherence, regardless of the definition of adherence. Consistently, augmentation index was greater in patients with adherence <80% intake of medication.
Table 3.
Relationship Between Adherence and Albuminuria and Central Hemodynamics at Baseline
| Complete Adherencea | Partial Adherenceb | Nonadherencec | P Value | Adherenced | P Value | ||
|---|---|---|---|---|---|---|---|
| ≥80% | <80% | ||||||
| Albuminuria UACR, mg/gd | 47 (6.0–486) | 65 (7.0–195) | 44 (13.0–105) | 0.783 | 54 (7.0–406) | 44 (6.5–150) | 0.567 |
| Central systolic BP, mm Hgc | 138±18 | 147±28 | 159±25 | 0.012 | 141±22 | 153±24 | 0.048 |
| Central pulse pressure, mm Hgc | 56±17 | 61±25 | 64±21 | 0.401 | 58±21 | 62±19 | 0.445 |
| Augmentation index (–)c | 22.0±9 | 21.3±10 | 27.5±12 | 0.167 | 21.1±10 | 27.3±10 | 0.017 |
Discussion
In clinical practice, nonadherence is considered an important cause of uncontrolled hypertension, remains largely unrecognized, and is falsely interpreted as treatment resistance because it is difficult to diagnose or to exclude nonadherence objectively. In the current study, toxicological urine screening was applied as an objective measure to determine antihypertensive drug intake in patients with true resistant hypertension that underwent renal denervation. At baseline, adherence to all antihypertensive medications was found in 56% of the patients, and another 28% of patients were adherent with the exception of 1 antihypertensive drug. Thus, surprisingly at least 4 of 5 antihypertensive medications were detected in 84% of the study population. In contrast, ≥2 antihypertensive agents were missing in 16% of our patients, and none of the medication was detected in 3 of 79 patients (4%). Similar adherence rates were observed at the 6‐month follow‐up visit after catheter‐based renal denervation.
The described rates of adherence and nonadherence depend on the definition applied to the study population. Complete adherence has been found to range from 34% to 81%, and total nonadherence ranges from 9% to 35%.14, 15, 16, 17 In all of these studies carried out in TRH or hypertensive patients with insufficient BP control, LC‐MS/MS was used to measure the drug levels, allowing a comparison of adherence rates. The definition of partial nonadherence, however, varies widely from “at least one or more substances missing” to “fewer than prescribed” or is not described in detail at all.14, 15, 16, 17 In our study, we used only 1 antihypertensive substances missing for the definition of partial adherence. It might be argued whether missing the intake of 1 antihypertensive drug (in the face of 5.7 prescribed and 5.0 detectable drugs) is clinically relevant. In terms of achieved BP, we could not observe any significant or clinically meaningful difference in office BP and 24‐hour ABP between patients with complete adherence (all drugs detected) and partial adherence (only 1 drug missing). In contrast, nonadherence (defined in our trial by missing ≥2 antihypertensive compounds) was related to higher office BP and 24‐hour systolic ABP. When we categorized patients as adherent or nonadherent using the cutoff of 80% of the detectable drugs, patients with nonadherence (<80%) tended to have higher office BP and 24‐hour systolic ABP than patients with adherence (≥80%). Accordingly, central systolic pressure and augmentation index were higher in nonadherent patients. Similar to other reports, drug class did not appear to have any important impact on the adherence and nonadherence rates in patients with TRH; this result contrasts with findings in mild to moderate hypertension8, 9 (Figure 1).
To the best of our knowledge, we are the first to have analyzed adherence at baseline and at 6‐month follow‐up visit after interventional therapy (eg, renal denervation) with detailed informed consent obtained retrospectively from the patient, thereby avoiding the white coat adherence effect.18, 19 This approach represents a great advantage of our study. After 6 months, complete adherence and nonadherence rates were 66% and 13% (with 1 patient being completely nonadherent), respectively, and thus similar to the findings at baseline. Of the 44 of patients with complete adherence at baseline, 35 remained completely adherent, but 9 were less adherent. This may reflect the patients' (and primary care physicians') perceptions that after renal denervation, fewer antihypertensive agents might be required. Indeed, the number of prescribed medications decreased significantly. Adherence rates did not change significantly between baseline and 6‐month visits.
Focusing on changes in 24‐hour ABP, considered to be the gold standard for assessing the hemodynamic load on the cardiovascular system in hypertensive patients,10, 26 the decline in 24‐hour systolic (and diastolic) ABP was similar in patients with stable, increased, and decreased adherence (Figures 5 and 6). This observation was noted regardless of the definition of adherence applied to our study cohort. Analysis of the changes in office BP indicated that patients with increased adherence had a greater fall in BP than those with decreased adherence. We cannot rule out the possibility that such a numeric difference in office BP becomes significant with a larger study population; our study population was rather small to have enough statistical power to allow definite conclusions. Nevertheless, our results do not indicate any clinically meaningful effect in face of the corresponding differences in 24‐hour ABP between those who have an increase as opposed to those who have a decrease in adherence (3 and 0 mm Hg difference, respectively) (Figures 5 and 6). Our data indicate that adherence has not biased our published data after renal denervation.20, 21, 22, 23 Future trials need to address the adherence issue thoroughly.
Objective measures of adherence may provide a useful step in the management of patients with resistant hypertension. Previously, monitoring of adherence using electronic devices has been found to provide benefits in the management of patients with resistant hypertension.11 Notably, just the announcement of monitoring adherence resulted in significant improvement of BP.12 Although this approach can be used as a therapeutic tool, it distorts the true adherence rates in clinical practice. With the design of the current study, we can rule out such a bias because all BP data and urine samples were measured and database locked prior to addressing the objective of the current study.18, 19
Another advantage of our study is the use of toxicological urine screening as an objective measure to assess adherence rates. Toxicological screening using spot urine samples is noninvasive, reliable, and relatively inexpensive.14 The applied LC‐MS/MS technique has been refined and enabled us to measure antihypertensive drugs or their metabolites for 94.7% of the prescribed antihypertensive drugs. The method was unable to give us reliable results for only 5.3% of the prescribed drugs; however, we have to consider the possibility that we missed antihypertensive drugs with a short half‐life because, at each time point, we assessed only 1 urine sample. Nevertheless, because of the very low number of nondetectable drugs and the very high sensitivity and specificity of the method used, we believe that this inaccuracy is negligible and has not altered our results.
Several other limitations need to be addressed. We have not evaluated indirect measures of adherence at the same time. Only clinical judgment by our staff and the patients' primary care physicians' have been used to estimate patient adherence (in 13 of 79 patients, we may have missed adherence issues, and in 3 of 13 patients, none of the drugs were taken). It has been described previously that clinical judgment tends to overestimate the rate of nonadherence.29, 30 Another limitation is that we cannot extrapolate whether similar high adherence rates are evident in a wider hypertensive population or in other health care settings. Our results have been obtained in a single center, a specialized ESH hypertensive excellence university center in Northern Bavaria, Germany, and higher rates of nonadherence have been reported elsewhere.14, 15
Adherence to medication is a dynamic behavioral pattern, and different rates of adherence can occur within and among patients. Longitudinal assessment of patient adherence (eg, dosing history, refill pattern) has indicated more reliable capture of the dynamics, whereas our toxicological approach to adherence has been performed only twice.1, 12 Nevertheless, with a time difference of 6 months and despite changes in antihypertensive medication, the adherence pattern overall appeared to be quite stable, without significant changes between the 2 time points.
Conclusion and Perspective
Our data indicate that nonadherence is not as frequent as commonly thought in TRH. By applying an objective, sensitive, and specific method of drug screening, all antihypertensive drugs were detected in ≈6 of 10 patients, and all but 1 were detected in another 3 of 10 patients with TRH while being on 5 to 6 different antihypertensive drug classes on average. Nevertheless, a substantial portion of patients were found to be nonadherent by not taking ≥2 antihypertensive medications, and nonadherence was related to 24‐hour ABP and central systolic BP. For the first time, we analyzed adherence twice, and we observed similar adherence rates at the baseline and 6‐month visits. Intriguingly, nonadherence or change in adherence had no substantial impact on 24‐hour ABP changes following renal denervation.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002343 doi: 10.1161/JAHA.115.002343)
References
- 1. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. [DOI] [PubMed] [Google Scholar]
- 2. Hill MN, Miller NH, Degeest S; American Society of Hypertension Writing G , Materson BJ, Black HR, Izzo JL Jr, Oparil S, Weber MA. Adherence and persistence with taking medication to control high blood pressure. J Am Soc Hypertens. 2011;5:56–63. [DOI] [PubMed] [Google Scholar]
- 3. Burnier M. Managing ‘resistance’: is adherence a target for treatment? Curr Opin Nephrol Hypertens. 2014;23:439–443. [DOI] [PubMed] [Google Scholar]
- 4. Mancia G, Zambon A, Soranna D, Merlino L, Corrao G. Factors involved in the discontinuation of antihypertensive drug therapy: an analysis from real life data. J Hypertens. 2014;32:1708–1715; discussion 1716. [DOI] [PubMed] [Google Scholar]
- 5. Corrao G, Parodi A, Nicotra F, Zambon A, Merlino L, Cesana G, Mancia G. Better compliance to antihypertensive medications reduces cardiovascular risk. J Hypertens. 2011;29:610–618. [DOI] [PubMed] [Google Scholar]
- 6. Mazzaglia G, Ambrosioni E, Alacqua M, Filippi A, Sessa E, Immordino V, Borghi C, Brignoli O, Caputi AP, Cricelli C, Mantovani LG. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120:1598–1605. [DOI] [PubMed] [Google Scholar]
- 7. Mathes J, Kostev K, Gabriel A, Pirk O, Schmieder RE. Relation of the first hypertension‐associated event with medication, compliance and persistence in naive hypertensive patients after initiating monotherapy. Int J Clin Pharmacol Ther. 2010;48:173–183. [DOI] [PubMed] [Google Scholar]
- 8. Mancia G, Parodi A, Merlino L, Corrao G. Heterogeneity in antihypertensive treatment discontinuation between drugs belonging to the same class. J Hypertens. 2011;29:1012–1018. [DOI] [PubMed] [Google Scholar]
- 9. Kronish IM, Woodward M, Sergie Z, Ogedegbe G, Falzon L, Mann DM. Meta‐analysis: impact of drug class on adherence to antihypertensives. Circulation. 2011;123:1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F; Task Force M . 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 11. De Geest S, Ruppar T, Berben L, Schonfeld S, Hill MN. Medication non‐adherence as a critical factor in the management of presumed resistant hypertension: a narrative review. EuroIntervention. 2014;9:1102–1109. [DOI] [PubMed] [Google Scholar]
- 12. Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burnier M, Schneider MP, Chiolero A, Stubi CL, Brunner HR. Electronic compliance monitoring in resistant hypertension: the basis for rational therapeutic decisions. J Hypertens. 2001;19:335–341. [DOI] [PubMed] [Google Scholar]
- 14. Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H, Toennes SW. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013;31:766–774. [DOI] [PubMed] [Google Scholar]
- 15. Tomaszewski M, White C, Patel P, Masca N, Damani R, Hepworth J, Samani NJ, Gupta P, Madira W, Stanley A, Williams B. High rates of non‐adherence to antihypertensive treatment revealed by high‐performance liquid chromatography‐tandem mass spectrometry (HP LC‐MS/MS) urine analysis. Heart. 2014;100:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strauch B, Petrak O, Zelinka T, Rosa J, Somloova Z, Indra T, Chytil L, Maresova V, Kurcova I, Holaj R, Wichterle D, Widimsky J Jr. Precise assessment of noncompliance with the antihypertensive therapy in patients with resistant hypertension using toxicological serum analysis. J Hypertens. 2013;31:2455–2461. [DOI] [PubMed] [Google Scholar]
- 17. Ceral J, Habrdova V, Vorisek V, Bima M, Pelouch R, Solar M. Difficult‐to‐control arterial hypertension or uncooperative patients? The assessment of serum antihypertensive drug levels to differentiate non‐responsiveness from non‐adherence to recommended therapy. Hypertens Res. 2011;34:87–90. [DOI] [PubMed] [Google Scholar]
- 18. Feinstein AR. On white‐coat effects and the electronic monitoring of compliance. Arch Intern Med. 1990;150:1377–1378. [PubMed] [Google Scholar]
- 19. Cramer JA, Scheyer RD, Mattson RH. Compliance declines between clinic visits. Arch Intern Med. 1990;150:1509–1510. [PubMed] [Google Scholar]
- 20. Ott C, Mahfoud F, Schmid A, Ditting T, Veelken R, Ewen S, Ukena C, Uder M, Bohm M, Schmieder RE. Improvement of albuminuria after renal denervation. Int J Cardiol. 2014;173:311–315. [DOI] [PubMed] [Google Scholar]
- 21. Ott C, Mahfoud F, Schmid A, Ditting T, Sobotka PA, Veelken R, Spies A, Ukena C, Laufs U, Uder M, Bohm M, Schmieder RE. Renal denervation in moderate treatment‐resistant hypertension. J Am Coll Cardiol. 2013;62:1880–1886. [DOI] [PubMed] [Google Scholar]
- 22. Ott C, Mahfoud F, Schmid A, Toennes SW, Ewen S, Ditting T, Veelken R, Ukena C, Uder M, Bohm M, Schmieder RE. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J Hypertens. 2015;33:1261–1266. [DOI] [PubMed] [Google Scholar]
- 23. Ott C, Schmid A, Toennes SW, Ditting T, Veelken R, Uder M, Schmieder RE. Central pulse pressure predicts BP reduction after renal denervation in patients with treatment‐resistant hypertension. EuroIntervention. 2015;11:110–116. [DOI] [PubMed] [Google Scholar]
- 24. Schmieder RE, Redon J, Grassi G, Kjeldsen SE, Mancia G, Narkiewicz K, Parati G, Ruilope L, van de Borne P, Tsioufis C. ESH position paper: renal denervation—an interventional therapy of resistant hypertension. J Hypertens. 2012;30:837–841. [DOI] [PubMed] [Google Scholar]
- 25. Mahfoud F, Luscher TF, Andersson B, Baumgartner I, Cifkova R, Dimario C, Doevendans P, Fagard R, Fajadet J, Komajda M, Lefevre T, Lotan C, Sievert H, Volpe M, Widimsky P, Wijns W, Williams B, Windecker S, Witkowski A, Zeller T, Bohm M; European Society of C . Expert consensus document from the European Society of Cardiology on catheter‐based renal denervation. Eur Heart J. 2013;34:2149–2157. [DOI] [PubMed] [Google Scholar]
- 26. Parati G, Stergiou G, O'Brien E, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y. European society of hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359–1366. [DOI] [PubMed] [Google Scholar]
- 27. Ott C, Raff U, Harazny JM, Michelson G, Schmieder RE. Central pulse pressure is an independent determinant of vascular remodeling in the retinal circulation. Hypertension. 2013;61:1340–1345. [DOI] [PubMed] [Google Scholar]
- 28. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chobanian AV. Impact of nonadherence to antihypertensive therapy. Circulation. 2009;120:1558–1560. [DOI] [PubMed] [Google Scholar]
- 30. Management of patient compliance in the treatment of hypertension. Report of the NHLBI Working Group. Hypertension. 1982;4:415–423. [DOI] [PubMed] [Google Scholar]


