Abstract
Background
Off‐pump coronary artery bypass (OPCAB) has been shown to reduce the risk of neurologic complications as compared to coronary artery bypass grafting performed with cardiopulmonary bypass. Side‐clamping of the aorta while constructing proximal anastomoses, however, still carries substantial risk of cerebral embolization. We aimed to perform a comprehensive meta‐analysis of studies assessing 2 clampless techniques: aortic “no‐touch” and proximal anastomosis devices (PAD) for OPCAB.
Methods and Results
PubMed, CINAHL, CENTRAL, and Google Scholar databases were screened for randomized controlled trials and observational studies comparing “no‐touch” and/or PAD with side‐clamp OPCAB and reporting short‐term (≤30 days) outcomes: cerebrovascular accident and all‐cause mortality. A total of 18 studies (3 randomized controlled trials) enrolling 25 163 patients were included. Aortic “no‐touch” was associated with statistically lower risk of cerebrovascular accident as compared to side‐clamp OPCAB: risk ratio 95% CI: 0.41 (0.27–0.61); P<0.01; I2=0%. Event rates were 0.36% and 1.28% for “no‐touch” and side‐clamp OPCAB, respectively. No difference was seen between PAD and side‐clamp OPCAB: 0.71 (0.33–1.55); P=0.39; I2=39%. A trend towards increased 30‐day all‐cause mortality with PAD and no difference with “no‐touch” were observed when compared to side‐clamp OPCAB. In a subset analysis, “no‐touch” consistently reduced the risk of cerebrovascular accident regardless of patients’ baseline risk characteristics. A benefit with PAD was observed in low‐risk patients.
Conclusions
Aortic “no‐touch” technique was associated with nearly 60% lower risk of postoperative cerebrovascular events as compared to conventional side‐clamp OPCAB with effect consistent across patients at different risk.
Keywords: bypass surgery, coronary circulation, meta‐analysis, revascularization
Subject Categories: Cardiovascular Surgery, Meta Analysis, Revascularization
Introduction
Coronary artery bypass grafting (CABG) is associated with reduction of mortality and remains a standard of care in patients with extensive coronary artery disease as compared to percutaneous coronary intervention (PCI) and medical treatment alone.1, 2, 3 CABG with the use of cardiopulmonary bypass is recognized as the “gold standard” technique in terms of safety and effectives for surgical myocardial revascularization. A further effort in minimizing the occurrence of some complications related to conventional CABG has led to the development of off‐pump coronary artery bypass (OPCAB) technique in which the anastomoses are performed on the beating heart.4 The benefit of OPCAB to reduce the risk for stroke is controversial, with single randomized controlled trials (RCTs) showing no difference as compared to on‐pump CABG surgery. In a recently available meta‐analysis of 100 RCTs, however, OPCAB was demonstrated to be associated with statistically lower risk of stroke as compared to conventional CABG with cardiopulmonary bypass (odds ratio [95% CI]: 0.72 [0.56–0.92]; P=0.009). While pooled results showed no differences between 2 approaches in regard to all‐cause mortality and myocardial infarction, by meta‐regression, the benefits of OPCAB were significantly related to patients risk profile, with advantage arising in a high‐risk population.5
In the majority of cases, OPCAB still requires partial clamping of the aorta in order to construct proximal anastomoses (ie, using saphenous vein grafts). In patients with heavily calcified or “porcelain” aorta, however, this approach is contraindicated and other measures are sought for complete myocardial revascularization. Two techniques of OPCAB that obviate the need for partial clamping have been extensively tested in a clinical setting: (1) Aortic “no‐touch,” in which double internal mammary artery (in situ grafts) and/or composite grafts (saphenous vein or free radial artery anastomosed end‐to‐side [in “T” or “Y” fashion] to internal mammary artery bypass graft) are used for revascularization. (2) Proximal anastomotic devices (PAD) that allow construction of anastomosis (either automatic or hand‐sewn) to the ascending aorta with a graft vessel “loaded” on the device's delivery system. Because of the limited number of RCTs that are underpowered for hard clinical end points, we aimed to perform a most updated and comprehensive meta‐analysis of RCTs and observational data to further corroborate the obtained results.
Methods
Data Sources and Search Strategy
Established methods were used in compliance with the PRISMA statement for reporting systematic reviews and meta‐analyses in healthcare interventions.6 PubMed, CINAHL, the Cochrane Register of Controlled Clinical Trials (CENTRAL), and Google Scholar databases were screened for published randomized and observational studies comparing aortic “no‐touch” OPCAB and/or OPCAB with PAD against conventional OPCAB employing partial clamping of the aorta. Search terms were: “OPCAB,” “OPCABG,” “no‐touch,” “anaortic,” “clampless”, “in‐situ graft*”, “with* aortic manipulation”, “proximal anastomosis/anastomotic device”, “facilitated anastom*”, “Heartstring”, “Enclose”, “Symmetry”, “PAS‐Port”, “random*”, “trial”, “study”. No language restrictions were imposed. Both blinded and open‐label trials were considered eligible. Databases were searched until July 2015. The most updated or inclusive data for each study were used for abstraction. References of original and review articles were cross‐checked.
Selection Criteria and Quality Assessment
Citations were screened at title/abstract level and retrieved as full reports if they fulfilled the inclusion criteria: (1) human studies; (2) RCTs or observational studies with control group; (3) studies comparing clampless “no‐touch”and/or OPCAB with PAD versus conventional side‐clamp OPCAB. Exclusion criteria were (1) cohort studies without control group; (2) studies (or arms) comparing OPCAB versus CABG with cardiopulmonary bypass; (3) studies comparing alone clampless “no‐touch” versus OPCAB with PAD; (4) follow‐up of the study not pertinent to the design of the meta‐analysis. Two independent reviewers (M.K. and W.P.) selected the studies for the inclusion, extracted studies and patients characteristics of interest and relevant outcomes; divergences were resolved by consensus after discussion with a third reviewer (L.A.). Three authors (M.K., W.P., and G.M.R.) independently assessed the trials’ eligibility and risk of bias. The bias risk for RCTs was assessed using the components recommended by the Cochrane Collaboration (ie, random sequence generation and random allocation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting, and other sources of bias).7 Quality of observational studies was appraised with Newcastle–Ottawa Scale, a tool used for assessing the bias (the selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure or outcome of interest) in case–control and cohort studies included in a systematic review and/or meta‐analyses.8
Outcome Measure
Primary end point was the incidence of cerebrovascular accident (CVA) at <30 days after CABG, analysis of all‐cause mortality was performed as well. Study definitions as per CVAs were applied.
Statistical Analysis
Data were analyzed according to the intention‐to‐treat principle. Risk ratios (RRs) and 95% CIs were used as summary statistics. Heterogeneity was assessed by Cochran Q test. Potential publication bias was examined for the primary end point by constructing a “funnel plot” in which the SE of the log RR was plotted against the RR. The asymmetry of the plot was estimated both visually and by a linear regression approach.9 The influence of each study and potential publication bias were addressed by testing whether deleting each study in turn would have changed significantly the pooled results of the meta‐analysis for the primary end point. Studies were analyzed separately in 2 subsets: (1) aortic “no‐touch” versus conventional side‐clamp OPCAB, and (2) OPCAB with PAD versus conventional side‐clamp OPCAB in a random‐effects model as a more conservative approach for observational data accounting for between‐ and within‐study variability.10 Furthermore, an attempt was made to explore the possible relationship between age, sex, history of cerebrovascular accident (CVA), type 2 diabetes, kidney disease, urgency of the surgery and baseline left ventricular ejection fraction, and occurrence of primary end point. Depending on availability of the data, studies in the “no‐touch” versus conventional OPCAB and the PAD versus conventional OPCAB analyses were separately dichotomized by these characteristics. The cutoff points were made so as to have equal, or nearly equal, numbers of studies on each side of the dichotomy: mean age (cutoff 65 years of age); percentage of men (cutoff 73–75%); history of CVA (cutoff 8%); type 2 diabetes mellitus (cutoff 40%); chronic kidney disease (cutoff 4–5%); nonelective cases (cutoff 20–25%); and left ventricular ejection fraction (cutoff 55%). Pooled RRs were obtained for each subset of studies, and combined in a random‐effect meta‐analysis. Finally, in a sensitivity analysis for primary end point, we excluded studies not reporting diagnostic criteria for CVA. Review Manager V.5.1 (The Nordic Cochrane Centre, Kobenhavn, Denmark) was used for statistical computations. A P value <0.05 was considered statistically significant, and reported as 2‐sided.
Results
The process of study selection is shown in the analysis flow diagram (Figure 1). All published studies were retrieved as full texts. Baseline characteristics of included studies, patient demographics, number of distal anastomoses, and PADs used are listed in Table 1. A total of 18 studies11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 (among them 3 RCTs24, 26, 27) comprising 25 163 patients met the inclusion criteria and entered the final analysis. Patients were divided into 3 groups: (1) aortic “no‐touch”—8291 patients; (2) PAD—3192 patients; and (3) side‐clamp OPCAB—13 680 patients, respectively. Three of the included studies12, 17, 20 compared all 3 OPCAB techniques. Analysis of potential sources of bias is available in Table 2.
Figure 1.
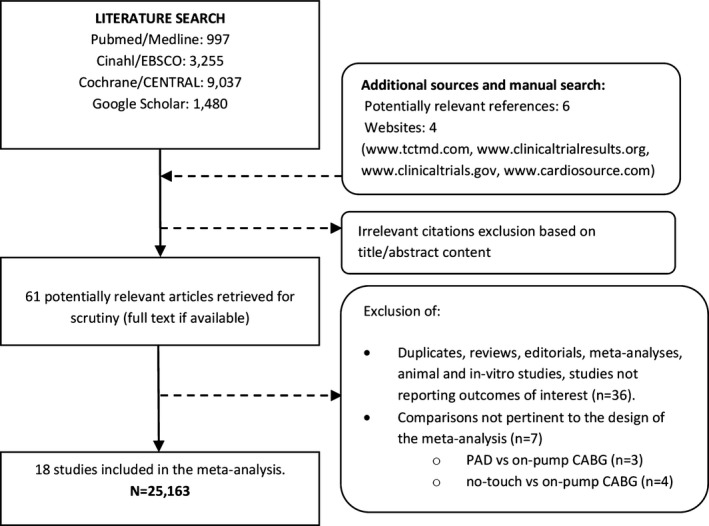
Flow diagram of the review process according to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) statement. CABG indicates coronary artery bypass grafting; PAD, proximal anastomosis device.
Table 1.
Characteristics of Included Studies
| Study (Reference) | Year | Design | Comparison | No. of Pts. | Mean Age (y) | Sex (% Male) | Arterial Grafts | PAD Used | Follow‐Up | |
|---|---|---|---|---|---|---|---|---|---|---|
| Calafiore11 | 2002 | Retrospective | “No‐touch” | 1533 |
64±9 with CVA 62.3±9.6 without CVA |
NA | NA | — | 30 days | |
| Side‐clamp | 460 | |||||||||
| Emmert12 | 2011 | Prospective, propensity scoring | “No‐touch” | 271 | NA | NA | 1.76±0.87 | Heartstring® | In‐hospital | |
| Side‐clamp | 567 | 63±10 | 79.0 | 1.60±0.91 | ||||||
| PAD | 1365 | 66±10 | 79.4 | 1.49±0.80 | ||||||
| Kapetanakis13 | 2004 | Retrospective | “No‐touch” | 476 | 61.2±11.3 | 65.1 | NA | — | 30 days | |
| Side‐clamp | 2527 | 66.2±10.7 | 68.6 | |||||||
| Kim14 | 2002 | Matched cohort | “No‐touch” | 222 | 61±9 | 73.4 |
Bilateral ITA RA RGEA |
95.5% 2.3% 50.0% |
— | 30 days |
| Side‐clamp | 123 | 63±9 | 66.7 |
Bilateral ITA RA RGEA |
22.0% 5.7% 7.3% |
|||||
| Leacche15 | 2003 | Retrospective | “No‐touch” | 84 | 62±13 | 83.3 |
Bilateral ITA BITA+RA RGEA |
38.7% 7.6% 2.5% |
— | 30 days |
| Side‐clamp | 556 | 64±10 | 78.1 | NA | ||||||
| Lev‐Ran16 | 2004 | Retrospective | “No‐touch” | 429 | 67.4±11.5 | 72.8 |
Bilateral ITA RA RGEA |
55.3% 40% 6.3% |
— | In‐hospital |
| Side‐clamp | 271 | 68.4±10.9 | 70.0 |
Bilateral ITA BITA +RA RGEA |
30.6% 2.9% 0.4% |
|||||
| Manabe17 | 2009 | Retrospective | “No‐touch” | 185 | 68.4±8.8 | 90.3 |
RA RGEA |
55.4% 60.8% |
Heartstring®
Enclose II® |
In‐hospital |
| Side‐clamp | 241 | 68.1±9.1 | 73.0 |
RA RGEA |
60.5% 9.2% |
|||||
| PAD | 109 | 70.9±8.4 | 83.5 |
RA RGEA |
48.3% 3.4% |
|||||
| Matsuura18 | 2013 | Retrospective | “No‐touch” | 264 | 67.3±8.0 | 84.5 |
Bilateral ITA RA |
68.1% 53.8% |
— | In‐hospital |
| Side‐clamp | 72 | 68.9±9.1 | 77.8 |
Bilateral ITA RA |
50.0% 44.4% |
|||||
| Misfeld19 | 2010 | Retrospective | “No‐touch” | 1346 | 67.2±10.7 | 76.3 |
Bilateral ITA RA |
24.7% 44.4% |
— | 30 days |
| Side‐clamp | 600 | 67.2±10.7 | 73.3 |
Bilateral ITA RA |
13.7% 27.8% |
|||||
| Moss20 | 2015 | Retrospective, adjusted in a logistic regression | “No‐touch” | 1550 | 62.1±12.2 | NA | NA | Heartstring® | In‐hospital | |
| Side‐clamp | 6449 | 62.9±10.7 | ||||||||
| PAD | 1551 | 66.2±10.5 | ||||||||
| Patel21 | 2002 | Prospective, adjusted in a logistic regression | “No‐touch” | 597 | 61 (55–68) | 79.6 | NA | — | In‐hospital | |
| Side‐clamp | 520 | 63 (55–69) | 72.9 | |||||||
| Pawlaczyk22 | 2011 | Retrospective | “No‐touch” | 133 | 65.8 | 69.9 | RA | 5.3% | — | In‐hospital |
| Side‐clamp | 499 | 67.0 | 73.3 | RA | 1.2% | |||||
| Vallely23 | 2008 | Prospective, adjusted in a logistic regression | “No‐touch” | 1201 | 67.6 (30.7–91.1) | 75.7 |
Bilateral ITA RA |
26.5% 77.1% |
— | 30 days |
| Side‐clamp | 557 | 67.6 (22.1–90.7) | 71.8 |
Bilateral ITA RA |
14.5% 22.9% |
|||||
| Biancari24 | 2007 | RCT | PAD | 11 | 63.9±7.8 | 73 | RA | 18% | Spyder® | In‐hospital |
| Side‐clamp | 9 | 71.4±4.6 | 78 | RA | 11% | |||||
| Boova25 | 2006 | Retrospective | PAD | 60 | 69.4 | 65 | 13% | Enclose® | In‐hospital | |
| Side‐clamp | 137 | 68.9 | 70 | 27% | ||||||
| El Zayat26 | 2011 | RCT | PAD | 29* |
S 63.7±9.9 B 60.9±13.5 |
65.5 | NA | Heartstring® | In‐hospital | |
| Side‐clamp | 28* |
S 64.7±10.3 B 61.2±12.0 |
75 | |||||||
| Kempfert27 | 2008 | RCT | PAD | 51 | 74.5±0.6 | 80.4 | NA | PAS‐Port® | In‐hospital | |
| Side‐clamp | 48 | 75.5±0.5 | 77.1 | |||||||
| Skjelland28 | 2005 | Prospective | PAD | 16 | 66.4 (43–85) | 87.5 | NA | Symmetry® | In‐hospital | |
| Side‐clamp | 16 | 64.9 (53–77) | 87.5 | |||||||
CVA indicates cerebrovascular accident; ITA, internal thoracic artery; NA, not available; PAD, proximal anastomosis device; RA, radial artery; RCT, randomized controlled trial; RGEA, right gastroepiploic artery.
*Nested randomization to S (sucker) and B (blower) for clearing the anastomotic site from blood.
Table 2.
Bias Assessment
| Cochrane Tool for Bias Assessment in Randomized Controlled Trials | |||||||
|---|---|---|---|---|---|---|---|
| Study | Random Sequence | Allocation Concealment | Participants and Personnel Blinding | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Biases |
| Biancari24 | + | + | ± | ± | ± | + | + |
| El Zayat26 | + | + | ± | ± | + | + | + |
| Kempfert27 | + | ± | ± | ± | + | + | + |
| Newcastle–Ottawa Scale of Bias Risk for Observational Studies | |||||||
|---|---|---|---|---|---|---|---|
| Study | Adequacy of Selection | Comparability | Outcomes Assessment | ||||
| Representativeness of the Exposed Cohort | Selection of the Nonexposed Cohort | Ascertainment of Exposure | Assessment of Outcomes | Follow‐Up Period Long Enough for Outcome to Occur | Adequacy of Follow‐Up Period Among Cohorts | ||
| Calafiore11 | ** | ** | ** | * | *** | *** | *** |
| Emmert12 | *** | ** | ** | *** | *** | ** | *** |
| Kapetanakis13 | *** | ** | *** | * | *** | *** | *** |
| Kim14 | *** | ** | *** | *** | *** | *** | *** |
| Leacche15 | * | ** | ** | ** | *** | *** | *** |
| Lev‐Ran16 | ** | *** | *** | *** | *** | ** | ** |
| Manabe17 | ** | * | *** | * | *** | ** | *** |
| Matsuura18 | *** | * | ** | * | *** | ** | *** |
| Misfeld19 | *** | ** | *** | *** | *** | *** | *** |
| Moss20 | * | ** | *** | * | *** | ** | *** |
| Patel21 | ** | *** | ** | ** | *** | ** | *** |
| Pawlaczyk22 | * | ** | *** | * | *** | ** | *** |
| Vallely23 | *** | ** | ** | *** | *** | *** | *** |
| Boova25 | *** | ** | ** | *** | *** | ** | *** |
| Skjelland28 | * | ** | *** | ** | *** | ** | *** |
+ indicates low risk of bias, ±, unclear risk of bias.
Asterisks are the star rating as per the Newcastle–Ottawa scale; ** and *** indicate highest ratings for these categories, while * suggests low rating.
Primary End Point
All 18 studies reported the incidence of primary end point of 30‐day CVA. The definitions applied varied across included studies and are outlined in Table 3. No signs of publication bias, as examined by visual inspection of the “funnel plot,” were observed (Figure 2). Aortic no‐touch technique was associated with a significant, nearly 60% CVA risk reduction as compared to side‐clamp OPCAB: RR (95% CI): 0.41 (0.27–0.61); P<0.01; I2=0%. The corresponding event rates were 0.36% (30/8291) and 1.28% (172/13 442) for “no‐touch” and side‐clamp OPCAB, respectively. No significant differences were seen between PAD and side‐clamp OPCAB with respect to CVA: RR (95% CI): 0.71 (0.33–1.55); P=0.39; I2=39%. The respective event rates were 1.00% (32/3192) and 1.41% (106/7495). Pooled incidence of 30‐day CVA in the clampless‐ and side‐clamp OPCAB was 0.54% (62/11 483) and 1.30% (178/13 680), respectively (Figure 3).
Table 3.
Primary End Point Definitions
| Study | Primary End Point Definition |
|---|---|
| Calafiore11 | Cerebrovascular accident was defined as global or focal neurologic deficit that could be evident after emergence from anesthesia (early CVA) or after first awaking without any neurologic deficits (delayed CVA). CVA was diagnosed by a neurologist and confirmed by a brain CT scan or nuclear MRI. |
| Emmert12 | Stroke was defined as a new neurologic deficit that appears and remains at least partially evident for more than 24 h after its onset and occurs during or after the CABG procedure; moreover, strokes needed to be diagnosed before discharge. Other than by clinical symptoms, diagnosis was confirmed by a neurologist and brain imaging. Transient ischemic attacks, intellectual impairment, confusion, or irritation were excluded. |
| Kapetanakis13 | New CVA was defined as a postoperatively occurring new focal neurologic deficit, persisting for longer than 72 h after onset, diagnosed by clinical findings, confirmed by a neurologist or brain imaging (head CT or MRI), and noted before discharge or death. Transient neurologic events, intellectual impairment, and confusional or irritable states were not included. |
| Kim14 | Stroke was defined as a new and sudden onset of neurologic deficits lasting more than 24 h with no apparent nonvascular causes. |
| Leacche15 | Stroke was defined as the development of a new focal neurologic deficit confirmed by clinical findings and CT scan. |
| Lev‐Ran16 | Major neurologic complications were defined as any global or focal neurologic deficit that was evident after emergence from anesthesia. All neurologic events were evaluated by a neurologist and further assessed by CT scan. |
| Manabe17 | Stroke was suspected from any new global or focal neurological deficit and was confirmed by CT or MRI. It was diagnosed definitively by an attending neurologist. Reversible cerebral ischemic events were not considered as stroke. |
| Matsuura18 | Neurological event was defined by neurologists and radiologists as a neurological deficit confirmed by brain MRI or CT findings. |
| Misfeld19 | Neurological complications were defined as focal or global neurological deficits that were evident after emergence from anesthesia and diagnosed by a neurologist and confirmed by CT or MRI. Neurological complications also included any deterioration of a previous neurological deficit that was diagnosed preoperatively. |
| Moss20 | Stroke was defined as any confirmed neurologic deficit of abrupt onset caused by a disturbance in blood supply to the brain that did not resolve within 24 h. |
| Patel21 | Focal neurologic deficit was defined as a new focal neurologic deficit or a comatose state occurring postoperatively that persisted for more than 24 h after onset and was noted before discharge or death. Transient neurologic events, confusional states, or intellectual impairment were not included. |
| Pawlaczyk22 | Focal neurologic deficit was defined as a new focal neurologic deficit, which was assessed by attending neurologist and confirmed by CT. |
| Vallely23 | Neurological complications were defined as a new global or focal neurological deficit that was evident after the operation and categorized as either permanent or reversible. Permanent stroke was defined as a new central neurological deficit that persisted for more than 72 h. A transient neurological deficit was defined as a new central neurological deficit that had resolved completely within 72 h. |
| Biancari24 | Not defined; transient ischemic attack excluded. |
| Boova25 | Not defined; 3 patients sustained permanent neurologic deficits. |
| El‐Zayat26 | Not defined. |
| Kempfert27 | Stroke was defined as prolonged (>72 h) or permanent neurologic deficit that was usually associated with abnormal results of MRI or CT scans43 |
| Skjelland28 | Not defined; one patient experienced perioperative cardiac arrest and postoperative tamponade. This patient had cognitive impairment with reduced memory and impaired orientation for time and situation on clinical neurologic evaluation 3 months postoperatively. |
CABG indicates coronary artery bypass grafting; CT, computed tomography; CVA; cerebrovascular accident; MRI, magnetic resonance imaging.
Figure 2.
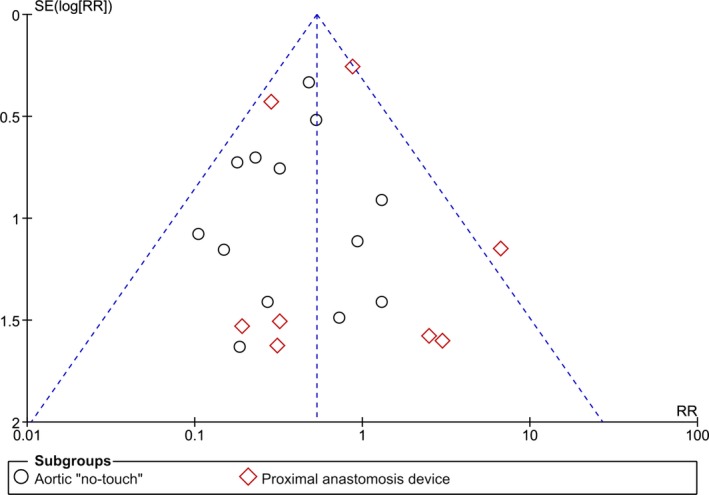
Publication bias analysis—Funnel plot of constructed for studies included in the meta‐analysis for the risk of 30‐day cerebrovascular accident stratified by off‐pump coronary artery bypass technique. No funnel plot asymmetry was apparent by visual inspection between effect estimates and the study precision, suggesting absence of small study effect. RR indicates risk ratio.
Figure 3.
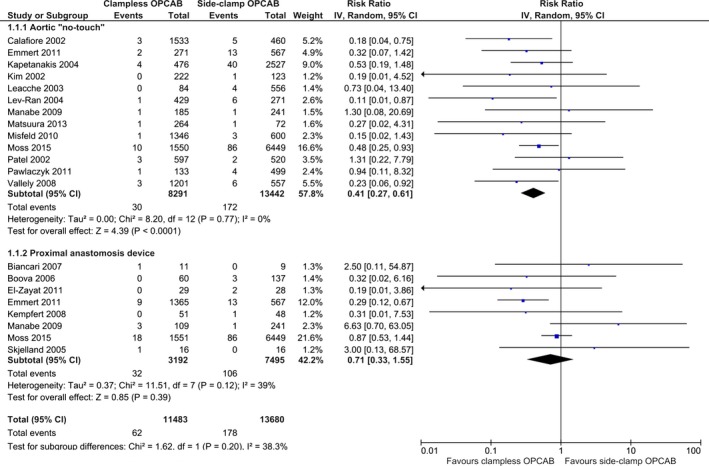
Summary analysis of primary end point—30‐day cerebrovascular accident stratified by the off‐pump coronary artery bypass (OPCAB) technique. Each square denotes the RR (risk ratio) for the within‐study comparison with the horizontal lines showing the 95% CI (confidence interval). The size of the square is directly proportional to the statistical weight of each study. The black diamond shapes give the pooled RR from the random‐effects model; the center of the diamond denotes the RR and the extremities the 95% CIs. IV indicate inverse variance.
All‐Cause Mortality
Thirteen studies (N=22 725) reported the incidence of 30‐day all‐cause mortality. No difference between the 2 techniques was observed in the comparison: “no‐touch” versus side‐clamp OPCAB: RR (95% CI): 0.90 (0.66–1.22); P=0.49; I2=0%. The respective event rates were 1.19% (77/6494) for “no‐touch”‐ and 1.29% (166/12 910) for side‐clamp OPCAB. A borderline significant increase of the risk of all‐cause mortality was observed with PAD as compared to side‐clamp OPCAB: RR (95% CI): 1.46 (1.00–2.13); P=0.05; I2=0%. The corresponding mortality rates ranged from 1.03% (77/7442) in the side‐clamp‐ to 1.69% (53/3136) in the PAD OPCAB subgroups. Pooled rates of all‐cause mortality in clampless‐ and side‐clamp OPCAB were 1.35% (130/9630) and 1.29% (169/13 095), respectively (Figure 4).
Figure 4.
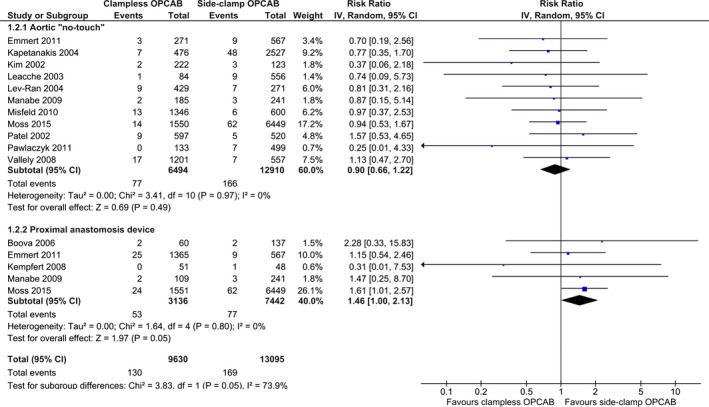
Summary analysis of postoperative all‐cause mortality stratified by the off‐pump coronary artery bypass (OPCAB) technique. Each square denotes the RR for the within‐study comparison with the horizontal lines showing the 95% CI. The size of the square is directly proportional to the statistical weight of each study. The black diamond shapes give the pooled RR from the random‐effects model; the center of the diamond denotes the RR and the extremities the 95% CIs. IV indicate inverse variance.
Sensitivity Analysis
In a prespecified sensitivity analysis, calculations repeated for the primary end point stratified by patients’ baseline characteristics revealed no signs that any of the chosen variables influenced the results for the comparison of “no‐touch” and side‐clamp OPCAB. P values for interaction between studies subsets ranged from 0.61 to 0.85. PAD‐facilitated OPCAB as compared to side‐clamp OPCAB reduced the risk of primary end point in studies enrolling patients as follows: (1) with lower prevalence of prior CVA (RR [95% CI]: 0.33 [0.16–0.72]; P<0.01; P int=0.07); (2) lower prevalence of diabetes (RR [95% CI]: 0.33 [0.16–0.71]; P<0.01; P int=0.10); (3) as elective cases (RR [95% CI]: 0.32 [0.15–0.69]; P<0.01; P int=0.04); and (4) with higher baseline left ventricular ejection fraction (RR [95% CI]: 0.33 [0.15–0.73]; P<0.01; P int=0.05) (Figure 5). Deleting each study and repeating the calculations did not alter the direction of the overall effect except for one instance: after omitting the study by Moss et al20 in analysis of 30‐day all‐cause mortality, there was no longer any difference between PAD and side‐clamp OPCAB: RR (95% CI): 1.22 (0.64–2.31); P=0.55; I2=0%. Exclusion of studies not reporting diagnostic criteria for postoperative cerebral stroke did not influence the estimates (Figure 6).
Figure 5.
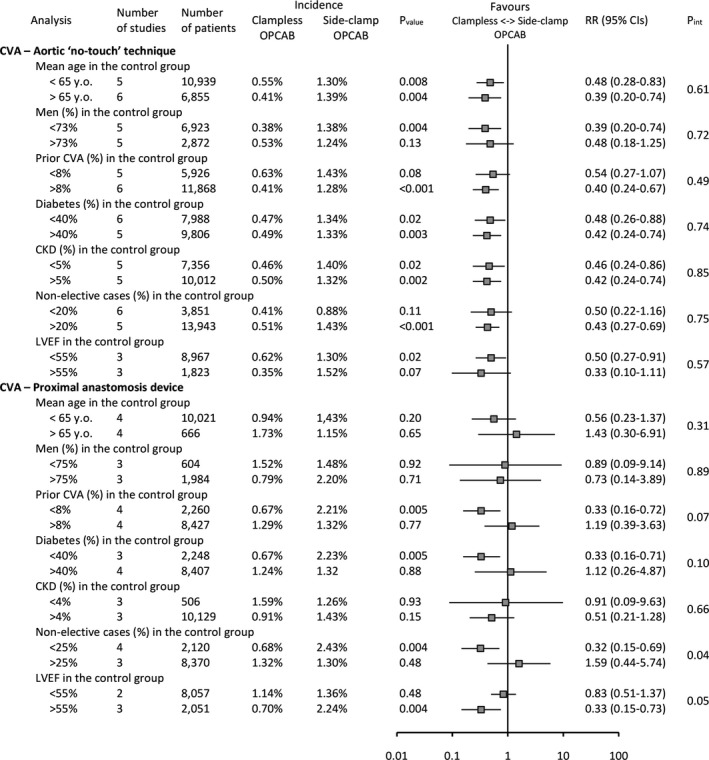
Sensitivity analysis for the primary end point—30‐day cerebrovascular accident stratified by patients’ baseline characteristics (age, sex, prior CVA, diabetes, CKD, nonelective cases, LVEF). CKD indicates chronic kidney disease; CVA, cerebrovascular accident; LVEF, left ventricular ejection fraction; OPCAB, off‐pump coronary artery bypass; RR, risk ratio.
Figure 6.
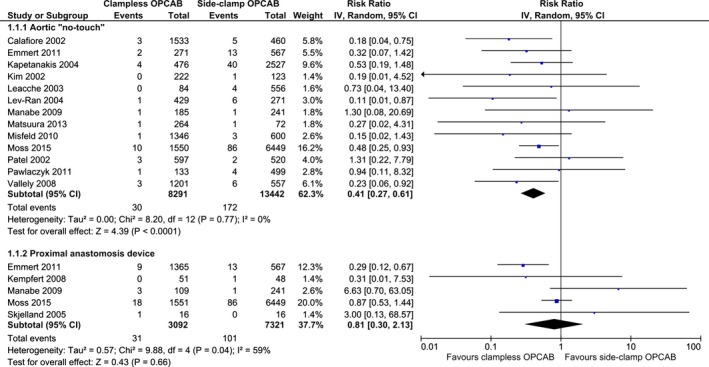
Sensitivity analysis of primary end point—30‐day cerebrovascular accident stratified by the OPCAB technique after exclusion of studies not reporting diagnostic criteria for primary end point. Each square denotes the RR for the within‐study comparison with the horizontal lines showing the 95% CI. The size of the square is directly proportional to the statistical weight of each study. The black diamond shapes give the pooled RR from the random‐effects model; the center of the diamond denotes the RR and the extremities the 95% CIs. IV indicate inverse variance.
Discussion
Results of the current comprehensive meta‐analysis are the first, to the authors’ knowledge, to address the safety of the 2 clampless approaches to OPCAB with regard to 30‐day postoperative risk of cerebral stroke. The main findings of the present analysis are the following: (1) aortic “no‐touch” technique was associated with an almost 60% statistically lower risk of postoperative CVA as compared to conventional partial‐clamp OPCAB; (2) no difference in CVAs was observed between conventional OPCAB versus OPCAB with use of PAD in pooled analysis; (3) 30‐day all‐cause mortality was unaltered with “no‐touch” and conventional OPCAB; (4) a trend towards increase in 30‐day all‐cause mortality was seen in the PAD group. Additionally, in a sensitivity analysis, the incidence of primary end point remained constantly reduced with “no‐touch” OPCAB; OPCAB with PAD was associated with significant reduction of CVA in patients at lower baseline risk. With over 25 000 patients, this meta‐analysis represents the largest to date database on OPCAB without aortic manipulation ever analyzed.
Current international guidelines recommend CABG as the method of choice for patients with multivessel disease and diabetes.29 With 400 000 procedures performed annually in the United States, CABG remains the “gold standard” for surgical coronary revascularization that has been shown to be superior to PCI and medical treatment, with benefits pronounced over time.1, 2, 3 Despite technological improvements, and innovations in cardiovascular anesthesia, CABG performed “on‐pump” is still associated with substantial risk of postoperative morbidity. A further effort in minimizing the occurrence of some complications related to conventional CABG has led to the development of the off‐pump (OPCAB) technique in which the anastomoses are performed on the beating heart.4 Observational studies have suggested that, by avoiding the negative effects of CBP, OPCAB may substantially reduce the rate of mortality and morbidity when compared with conventional CABG 30, 31 and in particular in high‐risk patients.5, 32 Standard OPCAB, however, still requires application of a side biting clamp to complete proximal anastomoses, thus increasing the risk of dislodging fragile material from the aortic wall. It has been demonstrated that in the presence of a diffusely diseased aorta, off‐pump procedures performed “no‐touch” or with the use of proximal anastomotic devices indeed allow the avoidance of any type of clamping and may reduce the risk of postoperative neurologic events and cognitive functions impairment.33 However, data available so far, partially because of observational nature and partially because of small sample sizes, were inconclusive in drawing definite conclusions regarding these 2 techniques in surgical coronary revascularization.
Moderate‐to‐severe proximal aortic atherosclerosis has been long shown to be strongly associated with neurologic injury after CABG as strokes are mainly caused by large atherosclerotic emboli liberated by surgical manipulation of the aorta.34 Emerging from this picture is that with decreasing degree of aortic manipulation, the fewer strokes will occur. In line with our findings is a recent large cohort study, which demonstrated that the “no‐touch” technique has the lowest risk for postoperative stroke for patients undergoing CABG, while clamping the aorta during coronary artery bypass grafting increases this risk regardless of the severity of aortic disease.20 Proximal anastomosis devices reflect this concept, offering limited aortic manipulation without need for clamping, at the same time allowing complete revascularization with venous grafts. In a registry by Emmert et al,12 OPCAB patients undergoing revascularization with the HEARTSTRING® system had significantly fewer major adverse cardiac and cerebrovascular events (6.7% versus 10.8%; OR=0.59; 95% CI, 0.42–0.83; P=0.003) because of a significantly lower rate of stroke (0.7% versus 2.3%; odds ratio=0.28; 95% CI, 0.12–0.66; P=0.004). The authors concluded that stroke or other neurologic complications can be significantly minimized with such an anastomotic device when compared with the standard techniques, particularly in patients with a high atherosclerotic burden. On the other hand, proximal anastomosis with facilitating device still carries somewhat increased invasiveness, and probably during the puncturing of diseased aorta a certain amount of embolus material is detached regardless. Manabe et al17 suggested that during this procedure, solid atherosclerotic material might be fragmented when the connector penetrates the aorta, and an increased number of gas bubbles might be sucked into the bloodstream during connector attachment. Similarly to the largest available study by Moss et al,20 we were not able to show any benefit in terms of postoperative stroke with proximal anastomosis device as compared to standard OPCAB with partial clamping of the aorta in the pooled analysis (1.2% versus 1.3%; adjusted odds ratio [95% CI]: 1.41 [0.80–2.48]; P=0.23). On the other hand, we found that certain patients, especially those at lower risk (eg, no prior CVA, lower prevalence of diabetes, elective status, left ventricular ejection fraction >55%) may benefit from OPCAB with PAD as compared to conventional OPCAB with regard to neurologic complications. Certainly, another study, adequately powered for stroke comparing aortic “no‐touch” technique and proximal anastomosis devices in patients in whom partial clamping is not feasible, could better define the role of these devices in clinical practice.
A potentially most important finding of the current large‐scale analysis is an almost 60% statistically lower risk of postoperative cerebral stroke with the “no‐touch” approach. The extent and significance to which the incidence of stroke is reduced in the current analysis, coupled with no signs of heterogeneity, reject the hypothesis that the results are due to “play of chance” rather than depending on the real effect of the treatment. Indeed, the direction and magnitude of the estimates is sustained in the subsets analysis of the studies, further pointing to the effect of “no‐touch” technique on stroke, rather than baseline patients’ status. Surprisingly, no differences were seen between patients with higher prevalence of prior CVA as compared to patients in whom prior CVA occurred less often. This may in turn suggest the safety of “no‐touch” OPCAB in patients with cerebrovascular disease undergoing surgical coronary revascularization, and when confirmed in adequately powered study, “no‐touch” OPCAB should become the preferred approach in this setting.
In the present analysis, neurologic deficit occurred in 0.36% of patients in the “no‐touch” OPCAB that completely avoids any degree of aortic manipulation as compared to PAD and conventional OPCAB (1.00% and 1.30%, respectively). However, this extraordinary reduction should be viewed in wider perspective; indeed, although not directly the objective of the current investigation, “no‐touch” OPCAB might offer the long‐term survival benefit of CABG over PCI,2, 35 together with similar or lower than PCI 30‐day stroke rates.36, 37 The discussion about potential shortcomings of CABG as compared to PCI was fueled after the first large industry‐funded trial3 found significantly higher rates of major adverse cardiac and cerebrovascular events in the PCI group (17.8% versus 12.4% for CABG; P=0.002), in large part because of an increased rate of repeat revascularization (13.5% versus 5.9%); however, at 12 months, strokes were 4‐fold more likely to occur with CABG (2.2% versus 0.6% with PCI; P=0.003). None of the following studies comparing PCI with OPCAB38, 39, 40, 41 were powered for stroke, and none reports the extent of “no‐touch” technique in the CABG group. Conversely, one recent trial assessing midterm outcomes of 438 patients randomly assigned to the PCI with everolimus eluting stents and 442 randomly assigned to the CABG group42 found no difference in the risk of stroke between the 2 groups: hazard ratio 0.86 (0.39–1.93); P=0.72. Interestingly, OPCAB was performed in around two thirds of the cases, and arterial revascularization without manipulation of the aorta was encouraged by the study protocol.
We observed no differences in 30‐day all‐cause mortality between “no‐touch” and conventional OPCAB. In contrast, there was a borderline significant mortality increase in the PAD group. While unexpected, this finding might have been due to imbalance between 2 studied groups with regard to baseline risk profile. Indeed, in one study,20 patients at higher risk were included in the PAD group, they were older, more often urgent cases, and were more likely to have diabetes, renal insufficiency, moderate to severe COPD, and heart failure. This is further reflected by their atherosclerotic burden, precluding interventions on ascending aorta, both side‐ and cross clamping. Of note, after exclusion of this study, there was no longer any difference in mortality between PAD and conventional side‐clamp OPCAB.
Limitations
Several shortcomings of the current analysis need to be acknowledged. First, there is a striking lack of randomized studies comparing “no‐touch” and conventional OPCAB. Absence of randomization weakens our conclusions regarding generalizability of the results to an all‐comers scenario. On the other hand, studies included in this meta‐analysis have generally selected higher‐risk patients for “no‐touch” OPCAB, making the lower rates of stroke in these patients even more relevant. Second, current meta‐analysis shares the limitations of included studies with their underlying biases we could not account for without individual patient‐level data.
Conclusions
Aortic “no‐touch” technique was associated with a statistically lower risk of postoperative cerebrovascular events as compared to conventional partial‐clamp OPCAB, with effect consistent in all strata of the patients. OPCAB with device‐facilitated proximal anastomoses may offer similar protection from CVAs, however, only in patients at lower risk.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002802 doi: 10.1161/JAHA.115.002802)
References
- 1. O'Connor CM, Velazquez EJ, Gardner LH, Smith PK, Newman MF, Landolfo KP, Lee KL, Califf RM, Jones RH. Comparison of coronary artery bypass grafting versus medical therapy on long‐term outcome in patients with ischemic cardiomyopathy (a 25‐year experience from the Duke Cardiovascular Disease Databank). Am J Cardiol. 2002;90:101–107. [DOI] [PubMed] [Google Scholar]
- 2. Sipahi I, Akay MH, Dagdelen S, Blitz A, Alhan C. Coronary artery bypass grafting vs percutaneous coronary intervention and long‐term mortality and morbidity in multivessel disease: meta‐analysis of randomized clinical trials of the arterial grafting and stenting era. JAMA Intern Med. 2014;174:223–230. [DOI] [PubMed] [Google Scholar]
- 3. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW; Investigators S . Percutaneous coronary intervention versus coronary‐artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. [DOI] [PubMed] [Google Scholar]
- 4. Hart JC, Puskas JD, Sabik JF III. Off‐pump coronary revascularization: current state of the art. Semin Thorac Cardiovasc Surg. 2002;14:70–81. [DOI] [PubMed] [Google Scholar]
- 5. Kowalewski M, Pawliszak W, Malvindi PG, Bokszanski MP, Perlinski D, Raffa GM, Kowalkowska ME, Zaborowska K, Navarese EP, Kolodziejczak M, Kowalewski J, Tarelli G, Taggart DP, Anisimowicz L. Off‐pump coronary artery bypass grafting improves short‐term outcomes in high‐risk patients compared with on‐pump coronary artery bypass grafting: meta‐analysis. J Thorac Cardiovasc Surg. 2016;151:60–77 e58. [DOI] [PubMed] [Google Scholar]
- 6. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati AD, Petticrew M, Shekelle P, Stewart LA; the P‐PG . Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- 7. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods G, Cochrane Statistical Methods G . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 9. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rucker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 10. Kuss O, Gummert JF, Borgermann J. Meta‐analyses with rare events should use adequate methods. J Thorac Cardiovasc Surg. 2008;136:241. [DOI] [PubMed] [Google Scholar]
- 11. Calafiore AM, Di Mauro M, Teodori G, Di Giammarco G, Cirmeni S, Contini M, Iaco AL, Pano M. Impact of aortic manipulation on incidence of cerebrovascular accidents after surgical myocardial revascularization. Ann Thorac Surg. 2002;73:1387–1393. [DOI] [PubMed] [Google Scholar]
- 12. Emmert MY, Seifert B, Wilhelm M, Grunenfelder J, Falk V, Salzberg SP. Aortic no‐touch technique makes the difference in off‐pump coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2011;142:1499–1506. [DOI] [PubMed] [Google Scholar]
- 13. Kapetanakis EI, Stamou SC, Dullum MK, Hill PC, Haile E, Boyce SW, Bafi AS, Petro KR, Corso PJ. The impact of aortic manipulation on neurologic outcomes after coronary artery bypass surgery: a risk‐adjusted study. Ann Thorac Surg. 2004;78:1564–1571. [DOI] [PubMed] [Google Scholar]
- 14. Kim KB, Kang CH, Chang WI, Lim C, Kim JH, Ham BM, Kim YL. Off‐pump coronary artery bypass with complete avoidance of aortic manipulation. Ann Thorac Surg. 2002;74:S1377–S1382. [DOI] [PubMed] [Google Scholar]
- 15. Leacche M, Carrier M, Bouchard D, Pellerin M, Perrault LP, Paga P, Hebert Y, Cartier R. Improving neurologic outcome in off‐pump surgery: the “no touch” technique. Heart Surg Forum. 2003;6:169–175. [PubMed] [Google Scholar]
- 16. Lev‐Ran O, Braunstein R, Sharony R, Kramer A, Paz Y, Mohr R, Uretzky G. No‐touch aorta off‐pump coronary surgery: the effect on stroke. J Thorac Cardiovasc Surg. 2005;129:307–313. [DOI] [PubMed] [Google Scholar]
- 17. Manabe S, Fukui T, Miyajima K, Watanabe Y, Matsuyama S, Shimokawa T, Takanashi S. Impact of proximal anastomosis procedures on stroke in off‐pump coronary artery bypass grafting. J Card Surg. 2009;24:644–649. [DOI] [PubMed] [Google Scholar]
- 18. Matsuura K, Mogi K, Sakurai M, Kawamura T, Takahara Y. Medium‐term neurological complications after off‐pump coronary artery bypass grafting with and without aortic manipulation. Coron Artery Dis. 2013;24:475–480. [DOI] [PubMed] [Google Scholar]
- 19. Misfeld M, Potger K, Ross DE, McMillan D, Brady PW, Marshman D, Mathur MN. “Anaortic” off‐pump coronary artery bypass grafting significantly reduces neurological complications compared to off‐pump and conventional on‐pump surgery with aortic manipulation. Thorac Cardiovasc Surg. 2010;58:408–414. [DOI] [PubMed] [Google Scholar]
- 20. Moss E, Puskas JD, Thourani VH, Kilgo P, Chen EP, Leshnower BG, Lattouf OM, Guyton RA, Glas KE, Halkos ME. Avoiding aortic clamping during coronary artery bypass grafting reduces postoperative stroke. J Thorac Cardiovasc Surg. 2015;149:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel NC, Deodhar AP, Grayson AD, Pullan DM, Keenan DJ, Hasan R, Fabri BM. Neurological outcomes in coronary surgery: independent effect of avoiding cardiopulmonary bypass. Ann Thorac Surg. 2002;74:400–405; discussion 405‐406. [DOI] [PubMed] [Google Scholar]
- 22. Pawlaczyk R, Szyndler K, Lango R, Jagielak D, Łoś A, Woś Ł, Bojar P, Rogowski J. Should we perform coronary artery revascularization without aortic manipulation more often? Kardiochir Torakochirurgia Pol. 2011;4:45–449. [Google Scholar]
- 23. Vallely MP, Potger K, McMillan D, Hemli JM, Brady PW, Brereton RJ, Marshman D, Mathur MN, Ross DE. Anaortic techniques reduce neurological morbidity after off‐pump coronary artery bypass surgery. Heart Lung Circ. 2008;17:299–304. [DOI] [PubMed] [Google Scholar]
- 24. Biancari F, Lahtinen J, Ojala R, Ahvenjarvi L, Jartti A, Mosorin M, Heikkinen J, Taskinen P, Lepojarvi M. Spyder aortic connector system in off‐pump coronary artery bypass surgery. Ann Thorac Surg. 2007;84:254–257. [DOI] [PubMed] [Google Scholar]
- 25. Boova RS, Trace C, Leshnower BG. Initial Experience with the Enclose Proximal Aortic Anastomosis Device during Off‐Pump Coronary Artery Bypass: An Alternative to Aortic Side Clamping. Heart Surg Forum. 2006;9:E607–E611. [PubMed] [Google Scholar]
- 26. El Zayat H, Puskas JD, Hwang S, Thourani VH, Lattouf OM, Kilgo P, Halkos ME. Avoiding the clamp during off‐pump coronary artery bypass reduces cerebral embolic events: results of a prospective randomized trial. Interact Cardiovasc Thorac Surg. 2012;14:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kempfert J, Opfermann UT, Richter M, Bossert T, Mohr FW, Gummert JF. Twelve‐month patency with the PAS‐port proximal connector device: a single center prospective randomized trial. Ann Thorac Surg. 2008;85:1579–1584. [DOI] [PubMed] [Google Scholar]
- 28. Skjelland M, Bergsland J, Lundblad R, Lingaas PS, Rein KA, Halvorsen S, Svennevig JL, Fosse E, Brucher R, Russell D. Cerebral microembolization during off‐pump coronary artery bypass surgery with the Symmetry aortic connector device. J Thorac Cardiovasc Surg. 2005;130:1581–1585. [DOI] [PubMed] [Google Scholar]
- 29. Authors/Task Force m , Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 30. Hannan EL, Wu C, Smith CR, Higgins RS, Carlson RE, Culliford AT, Gold JP, Jones RH. Off‐pump versus on‐pump coronary artery bypass graft surgery: differences in short‐term outcomes and in long‐term mortality and need for subsequent revascularization. Circulation. 2007;116:1145–1152. [DOI] [PubMed] [Google Scholar]
- 31. Puskas JD, Kilgo PD, Lattouf OM, Thourani VH, Cooper WA, Vassiliades TA, Chen EP, Vega JD, Guyton RA. Off‐pump coronary bypass provides reduced mortality and morbidity and equivalent 10‐year survival. Ann Thorac Surg. 2008;86:1139–1146; discussion 1146. [DOI] [PubMed] [Google Scholar]
- 32. Puskas JD, Thourani VH, Kilgo P, Cooper W, Vassiliades T, Vega JD, Morris C, Chen E, Schmotzer BJ, Guyton RA, Lattouf OM. Off‐pump coronary artery bypass disproportionately benefits high‐risk patients. Ann Thorac Surg. 2009;88:1142–1147. [DOI] [PubMed] [Google Scholar]
- 33. Szwed K, Pawliszak W, Anisimowicz L, Bucinski A, Borkowska A. Short‐term outcome of attention and executive functions from aorta no‐touch and traditional off‐pump coronary artery bypass surgery. World J Biol Psychiatry. 2014;15:397–403. [DOI] [PubMed] [Google Scholar]
- 34. Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, Aggarwal A, Marschall K, Graham SH, Ley C. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med. 1996;335:1857–1863. [DOI] [PubMed] [Google Scholar]
- 35. Smit Y, Vlayen J, Koppenaal H, Eefting F, Kappetein AP, Mariani MA. Percutaneous coronary intervention versus coronary artery bypass grafting: a meta‐analysis. J Thorac Cardiovasc Surg. 2015;149:831–838 e13. [DOI] [PubMed] [Google Scholar]
- 36. Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL. Everolimus‐eluting stents or bypass surgery for multivessel coronary disease. N Engl J Med. 2015;372:1213–1222. [DOI] [PubMed] [Google Scholar]
- 37. Palmerini T, Biondi‐Zoccai G, Reggiani LB, Sangiorgi D, Alessi L, De Servi S, Branzi A, Stone GW. Risk of stroke with coronary artery bypass graft surgery compared with percutaneous coronary intervention. J Am Coll Cardiol. 2012;60:798–805. [DOI] [PubMed] [Google Scholar]
- 38. Diegeler A, Thiele H, Falk V, Hambrecht R, Spyrantis N, Sick P, Diederich KW, Mohr FW, Schuler G. Comparison of stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery. N Engl J Med. 2002;347:561–566. [DOI] [PubMed] [Google Scholar]
- 39. Drenth DJ, Winter JB, Veeger NJ, Monnink SH, van Boven AJ, Grandjean JG, Mariani MA, Boonstra PW. Minimally invasive coronary artery bypass grafting versus percutaneous transluminal coronary angioplasty with stenting in isolated high‐grade stenosis of the proximal left anterior descending coronary artery: six months’ angiographic and clinical follow‐up of a prospective randomized study. J Thorac Cardiovasc Surg. 2002;124:130–135. [DOI] [PubMed] [Google Scholar]
- 40. Hong SJ, Lim DS, Seo HS, Kim YH, Shim WJ, Park CG, Oh DJ, Ro YM. Percutaneous coronary intervention with drug‐eluting stent implantation vs. minimally invasive direct coronary artery bypass (MIDCAB) in patients with left anterior descending coronary artery stenosis. Catheter Cardiovasc Interv. 2005;64:75–81. [DOI] [PubMed] [Google Scholar]
- 41. Eefting F, Nathoe H, van Dijk D, Jansen E, Lahpor J, Stella P, Suyker W, Diephuis J, Suryapranata H, Ernst S, Borst C, Buskens E, Grobbee D, de Jaegere P. Randomized comparison between stenting and off‐pump bypass surgery in patients referred for angioplasty. Circulation. 2003;108:2870–2876. [DOI] [PubMed] [Google Scholar]
- 42. Park SJ, Ahn JM, Kim YH, Park DW, Yun SC, Lee JY, Kang SJ, Lee SW, Lee CW, Park SW, Choo SJ, Chung CH, Lee JW, Cohen DJ, Yeung AC, Hur SH, Seung KB, Ahn TH, Kwon HM, Lim DS, Rha SW, Jeong MH, Lee BK, Tresukosol D, Fu GS, Ong TK; Investigators BT . Trial of everolimus‐eluting stents or bypass surgery for coronary disease. N Engl J Med. 2015;372:1204–1212. [DOI] [PubMed] [Google Scholar]
- 43. Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL, Takkenberg JJ, David TE, Butchart EG, Adams DH, Shahian DM, Hagl S, Mayer JE, Lytle BW; Councils of the American Association for Thoracic S, Society of Thoracic S, European Association for Cardio‐Thoracic S, Ad Hoc Liaison Committee for Standardizing Definitions of Prosthetic Heart Valve M . Guidelines for reporting mortality and morbidity after cardiac valve interventions. J Thorac Cardiovasc Surg. 2008;135:732–738. [DOI] [PubMed] [Google Scholar]


