Abstract
Background
Animal and human studies indicate that ABCA1‐mediated cholesterol transport is important in Alzheimer's disease (AD). We hypothesized that the efficiency of cerebrospinal fluid (CSF) to facilitate ABCA1‐mediated cholesterol efflux would be reduced in participants with mild cognitive impairment (MCI) or AD compared with cognitively healthy participants.
Methods and Results
CSF was collected from a cross‐sectional study of cognitively healthy participants (n=47) and participants with MCI (n=35) or probable AD (n=26).The capacity of CSF to mediate cholesterol transport was assessed using a BHK cell line that can be induced to express the ABCA1 transporter. ABCA1‐mediated cholesterol efflux capacity was 30% less in participants with MCI or AD compared with cognitively healthy participants (P<0.001 for both). Cholesterol efflux capacity correlated with CSF cholesterol content (r=0.37, P<0.001). CSF phosphatidylcholine decreased in participants with MCI and AD compared with cognitively healthy participants (9% less in MCI and 27% less in AD compared with cognitively healthy participants, P=0.01) and correlated with CSF efflux capacity (r=0.3, P=0.001). CSF sphingomyelin also correlated with the efflux capacity (r=0.24, P=0.02). Concentrations of CSF apoA‐I and apoE did not significantly correlate with measures of efflux capacity.
Conclusions
In people with MCI and AD, the capacity of CSF to facilitate ABCA1‐mediated cholesterol efflux is impaired. This lesser cholesterol efflux in MCI supports a pathophysiological role for ABCA1‐mediated cholesterol transport in early neurodegeneration.
Keywords: ABCA1 transporters, Alzheimer's disease, cerebrospinal fluid, cholesterol efflux
Subject Categories: Lipids and Cholesterol
Introduction
The human brain is the largest repository of cholesterol in the body,1 so, not surprisingly, cholesterol homeostasis altered in the pathology of Alzheimer's disease (AD).2 Adding to this unique lipid environment is the synthesis of cholesterol within the brain, predominantly in glial cells, without significant import from the liver.3 Neuronal cholesterol is dysregulated in AD, and persistent neuronal damage response may increase cholesterol biosynthesis.4 Genomewide association studies in AD have implicated genes in cholesterol metabolism.5 The many roles of cholesterol, especially when altered in the neuronal membranes, would affect brain function widely. The mechanisms and priority of when cholesterol is altered in relation to early AD pathology are not fully understood.2
Cholesterol efflux is an essential part of its transport and metabolism, mediated when cholesterol acceptors (apolipoproteins) interact with membrane transporters (eg, ABCA1, ABCG1, SRB1) or through passive mechanisms to export cholesterol from cells.6 Cerebrospinal fluid (CSF) is enriched with high‐density lipoproteins7 (containing apoE and apoA‐I) that can maintain cholesterol homeostasis in brain cells. A previous study suggested that CSF lipoproteins participate in cholesterol efflux and that this function might be impaired with cognitive decline8; however, there is conflicting evidence about whether levels of the CSF lipoproteins significantly differ between participants with and without AD.8, 9, 10, 11 CSF lipoproteins are highly enriched with phosphatidylcholine (PC) and sphingomyelin (SM), and both PC and SM participate in cholesterol efflux. Experiments by Stein et al demonstrated that the removal of cholesterol from cells could be enhanced by addition of sonicated suspensions of PC or SM to human high‐density apolipoproteins.12 Using reconstituted discoidal high‐density lipoprotein particles prepared with apoA‐I, it was shown that increasing the content of SM up to 20 mol per particle was associated with significantly increased ability of high‐density lipoprotein to promote cholesterol efflux from human skin fibroblasts,13 erythrocyte ghost membranes,14 or Fu5AH cells that overexpress SRB1.15 SM promoted cholesterol efflux in control, familial high‐density lipoprotein deficiency, or Tangier disease fibroblasts that lack ABCA1 receptors.16 We recently reported alterations in CSF phospholipids and sphingolipids in both the supernatant and brain‐derived nanoparticles from CSF of participants with late‐onset AD.17, 18 The functional relevance of these compartmentalized alterations in CSF phospholipids on cholesterol efflux capacity is not known. Cholesterol efflux capacity has not been well defined in AD brain because fresh tissue is limited; however, the readily available brain‐derived CSF19 offers a sample source that can contribute to cholesterol efflux studies.
Although there are several pathways for cholesterol transport in the brain, cholesterol efflux through the ABCA1 transporter is particularly important. First, facilitating ABCA1 cholesterol efflux decreased amyloid plaques and improved cognition in mouse models.20 Second, the lack of the ABCA1 gene decreased brain apoE levels and increased amyloid deposition in APP23 mice.21 Third, loss‐of‐function mutations in the ABCA1 gene in humans are associated with AD.22, 23 The goal of this study was to test whether the efficiency of CSF to facilitate ABCA1 cholesterol efflux is reduced in participants with mild cognitive impairment (MCI) or AD compared with cognitively healthy (CH) participants and to define determinants of this efflux capacity.
Methods
Clinical Samples
The institutional review board of Huntington Memorial Hospital approved the study (HMH‐99‐09), and all study participants gave written informed consent. Participants were classified as CH, MCI, or probable AD, as described.24
Biochemical Measurements
ApoE and apoA‐I were measured by an enzyme‐linked immunosorbent assay (Academy Biomedical) with intraplate and interplate coefficients of variation <10%. Fasting plasma total cholesterol was measured in the routine clinical laboratory; CSF total cholesterol was measured using the Amplex Red cholesterol kit, as described by the manufacturer (Life Technologies). ApoE was genotyped, as reported.24 A dot blot performed on nitrocellulose membrane was used to assess apoE or apoA‐I content of nanoparticles with antibodies from Academy Biomedical. After the protein samples were spotted onto the membrane, the membrane was placed in a plastic container and sequentially incubated in blocking buffer, antibody solutions, and enzyme substrate and imaged by chemiluminescence.
ABCA1‐Mediated Cholesterol Efflux
The protocol to measure ABCA1‐mediated cholesterol efflux was published25 and modified for using CSF as the cholesterol acceptor. Briefly, BHK cell lines transfected with a mifepristone switch to express ABCA1 was used.26 BHK cell lines were plated at 20 000 per well with high‐glucose DMEM plus 10% FBS in 96‐well plates. On the second day, cells were labeled with 1 μCi/mL (3H) cholesterol (Moravek) using serum‐free high‐glucose DMEM, 2 mg/mL fatty acid–free albumin (Sigma‐Aldrich), and 2 μg/mL acyl‐coenzyme A:cholesterol acyltransferase inhibitor (Sigma‐Aldrich). On the third day, ABCA1 gene expression was induced with 10 nmol/L mifepristone overnight. On the following day, cholesterol acceptors or media alone were added to each well, with efflux assessed by the ratio of cholesterol in the media by total cholesterol (cells and media) 4 hours after incubation with acceptor or media. To test the efficiency of ABCA1 expression, purified apoE and apoA‐1 (Academy Biomedical) were used in increasing doses as cholesterol acceptors with and without mifepristone. As demonstrated in Figure S1A, apoA‐I and apoE efficiently accepted labeled (3H) cholesterol and did so in a dose‐dependent manner (Figure S1B). Control CSF between 0% and 100% of media was used to optimize the concentration of the CSF used in this assay. CSF was an efficient acceptor of cholesterol, and increasing CSF volume from 25 to 100 μL demonstrated a linear cholesterol efflux response (Figure S1C). Cholesterol efflux from the individual samples was then assessed using 35 μL CSF per well. Samples were run in triplicate, and the intraplate coefficient of variation for CSF samples was <10%. Furthermore, 5 μg/mL of purified apoE was run as a control in every plate. The interplate coefficient of variation was 11%.
CSF Fractionation
CSF was fractionated into supernatant fluid and nanoparticle fractions, as described previously.19 Nanoparticles are not just debris, rather they include important structures: intact synaptic vesicles, large dense‐core vesicles, and nanoparticle‐specific enzymes with neuromodulators such as cyclooxygenases that are absent from supernatant.19 Briefly, 4 mL CSF per person was centrifuged at 17 000g, the supernatant was centrifuged again at 200 000g, and the supernatant (supernatant fluid [SF]) was stored at −80°C. The final pellet containing CSF nanoparticles was washed with 4 mL PBS and repelleted at 200 000g, and the final pellet (nanoparticles) was resuspended in 50 μL PBS. The supernatant and nanoparticle fractions reported in this study are both from this final fractionation step. Electron microscopy of nanoparticles with negative staining was performed using a Morgagni 268D transmission electron microscope (FEI), as described previously.19 Examples of nanoparticles revealed by transmission electron microscopy from a control participant are shown in Figure S2A, which demonstrates their high abundance, heterogeneity, and prominent membranes. Both apoA‐1 and apoE were assessed in nanoparticles and SF using a dot blot assay that confirmed nanoparticles carry these 2 apolipoproteins (Figure S2B). In an enzyme‐linked immunosorbent assay, apoE concentrations were 30‐fold less in nanoparticles compared with SF (SF 3.59 versus nanoparticles 0.12 μg/mL). Total protein concentrations were less in the nanoparticles compared with the SF (SF 561 versus nanoparticles 49 μg/mL). In addition, 35 μL nanoparticle and SF were added to BHK cells to assess their capacity to facilitate ABCA1‐mediated efflux (Figure S2C). The nanoparticles were able to facilitate ABCA1‐mediated efflux capacity; however, their efflux capacity was much lower than that of the SF and proportional to the lower concentrations of apolipoproteins in these nanoparticles. These results indicated that the majority of lipoproteins were in the SF of CSF.
CSF Lipids
Total lipids were extracted from each CSF fraction using a modified Bligh and Dyer method.27 PC and SM in each fraction were measured using liquid chromatography–tandem mass spectrometry and parent ion monitoring of the phosphocholine head group (m/z=184).17
Phospholipase A2 and Sphingomyelinase Activities
CSF phospholipase A2 activity was measured using a fluorescent assay with a substrate cocktail, as described previously.17 Sphingomyelinase activity was measured using a fluorescent assay kit following the instructions of the manufacturer (Molecular Probes). Enzyme assays were normalized to CSF total protein concentration and expressed as relative fluorescent units per minute per microgram of protein.
Statistics
Mean (standard deviation) or median (25th, 75th percentile) for nonnormally distributed data were computed. Pearson or Spearman coefficients for nonnormally distributed data were used to correlate lipoprotein levels, CSF lipids, cholesterol, and efflux. The groups were compared using linear regression to allow adjustment for CSF lipids or apolipoproteins. The association between APOE genotype and efflux and the interaction between disease groups and APOE genotype on efflux were also modeled using linear regression. The statistical program R version 3.2.3 (R Foundation for Statistical Computing) was used. Significance was defined as P<0.05.
Results
We examined the capacity of CSF to facilitate ABCA1 efflux capacity in 3 groups of participants: CH, MCI, and AD. We previously reported the criteria used to classify the 3 clinical groups using a comprehensive medical and neuropsychological examination and consensus diagnosis and conferencing.24 A summary of participant demographics is presented in Table 1. Our findings demonstrate that CSF efflux capacity was 30% less in those with MCI and AD compared with CH participants (P<0.001 for both) (Figure 1A and Table 2). CSF PC and SM levels in the SF were also lower in those with MCI and AD compared with CH participants (Figure 1B and 1C). The difference in CSF cholesterol efflux capacity among the 3 groups was attenuated after adjusting for CSF PC in the supernatant fraction but remained significant (P=0.04). The concentrations of apoA‐I and apoE in the CSF were significantly correlated with the concentrations of SM and PC in the CSF SF (Figure 2) but not in the nanoparticles (data not shown). CSF efflux capacity was correlated with SM levels (r=0.23, P=0.02) (Figure 3A) and PC levels (r=0.3, P=0.001) in the SF (Figure 3B). A weak correlation between cholesterol efflux capacity and CSF apoA‐I concentrations (r=0.16, P=0.09) (Figure 3C) and no correlation between cholesterol efflux capacity and apoE concentrations (r=0.06, P=0.4) (Figure 3D) was observed. CSF apoA‐I levels did not significantly differ by group. In contrast, apoE concentrations were significantly less in the AD group compared with the CH and MCI groups (Table 2). The relationship of apoE with cholesterol efflux capacity differed in the 3 groups of participants (Figure 4). A significant correlation between CSF cholesterol efflux capacity and apoE was observed in the AD group (r=0.62, P<0.001) but not in the MCI or CH groups. Consequently, the reduced efflux in AD can be explained in part by the lower CSF apoE concentrations; however, reductions of cholesterol efflux capacity early in the disease process (MCI) were not reflected by measures of apoE in the CSF. In contrast, CSF SM and PC in the supernatant were less in MCI and AD, paralleling the differences observed with cholesterol efflux capacity.
Table 1.
Demographic Data for Study Participants
| Parameters | CH (n=47) | MCI (n=35) | AD (n=26) | P Value |
|---|---|---|---|---|
| Age, mean (SD) | 78 (7) | 77 (7) | 77 (10) | 0.93 |
| Female (%) | 58 | 60 | 64 | 0.81 |
| Years of education, mean (SD) | 17 (3)a | 16 (3) | 14 (3) | <0.001 |
| MMSE, mean (SD) | 29 (1.2)b | 28 (1.4)c | 15 (8) | <0.001 |
| Race, white (%) | 98 | 97 | 92 | 0.58 |
| Ethnicity, Hispanic or Latino (%) | 2 | 3 | 8 |
The 3 groups were compared using a linear regression model. AD indicates Alzheimer's disease; CH, cognitively healthy; MCI, mild cognitive impairment; MMSE, mini–mental state examination score.
AD compared with CH, P=0.004.
AD compared with CH, P<0.001.
AD compared with MCI, P<0.001.
Figure 1.
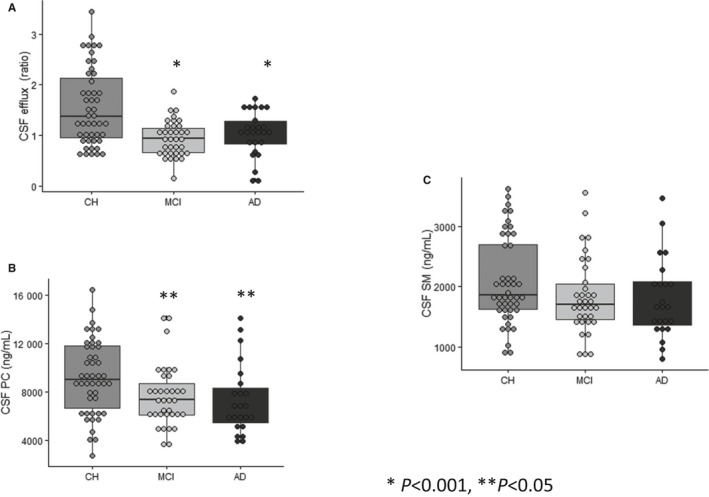
CSF cholesterol efflux capacity is reduced in MCI and AD. A, CSF cholesterol efflux capacity was measured in the 3 groups of participants: CH (n=47), MCI (n=35), and AD (n=26). CSF from CH participants demonstrated greater efflux capacity compared with participants with MCI or AD (*P<0.001). B, In agreement with cholesterol efflux capacity, PC levels were greater in CSF from CH participants compared with those with MCI and AD. C, The differences in CSF SM among the 3 groups were not significant. PC and SM were measured in the supernatant fraction of CSF. The 3 groups were compared using a linear regression model. The differences in cholesterol efflux among the 3 groups remained significant after adjusting for CSF PC (P=0.04). AD indicates Alzheimer's disease; CH, cognitively healthy; CSF, cerebrospinal fluid; MCI, mild cognitive impairment; PC, phosphatidylcholine; SM, sphingomyelin.
Table 2.
Biochemical Measurements for Study Participants
| Parameters | CH (n=47) | MCI (n=35) | AD (n=26) | P Value |
|---|---|---|---|---|
| Cholesterol efflux (ratio) | 1.56 (0.73)a, b | 0.94 (0.35) | 1.02 (0.43) | <0.001 |
| CSF supernatant PC, ng/mL | 9047 (6668, 11 802)a, b | 7382 (6108, 8717) | 6669 (5506, 8367) | 0.016 |
| CSF nanoparticle PC, ng/mL | 19 (14, 23) | 19 (14, 29) | 18 (14,22) | 0.80 |
| CSF supernatant SM, ng/mL | 2091 (718) | 1837 (643) | 1722 (561) | 0.14 |
| CSF nanoparticle SM, ng/mL | 102 (50) | 90 (35) | 93 (41) | 0.11 |
| Plasma cholesterol, mg/dL | 181 (31) | 180 (39) | 185 (33) | 0.99 |
| CSF cholesterol, μg/mL | 3.49 (0.84) | 3.21 (0.75) | 3.16 (0.56) | 0.04 |
| ApoE levels, μg/mL | 7.2 (2.0) | 7.5 (2.6) | 5.3 (2.1) c | <0.001 |
| ApoA‐I levels, μg/mL | 1.76 (1.3) | 1.74 (1.3) | 1.74 (1.1) | 0.99 |
| CSF PLA2 activity, RFU/min | 887 (233) | 917 (220) | 980 (269) | 0.32 |
| CSF acid sphingomyelinase, RFU/min | 25 (10) | 24 (8) | 15 (6)c | 0.001 |
| CSF neutral sphingomyelinase, RFU/min | 22 (17,26) | 19 (17,24) | 20 (18,24) | 0.38 |
| CSF tau, pg/mL | 275 (154) | 278 (175) | 489 (227)c | <0.001 |
| CSF β‐amyloid 42, pg/mL | 727 (288) | 737 (269) | 522 (223)c | <0.001 |
Data presented as mean (SD) or median (25%, 75% percentile) for nonnormally distributed data. Cholesterol efflux capacity of CSF was measured in all samples and normalized to efflux of 5 μg of purified apoE run in every plate. The 3 groups were compared using a linear regression model. The difference in cholesterol efflux capacity persisted after adjusting for CSF lipids or apolipoprotein levels. AD indicates Alzheimer's disease; CH, cognitively healthy; CSF, cerebrospinal fluid; MCI, mild cognitive impairment; PC, phosphatidylcholine; PLA2, phospholipase A2; RFU, relative fluorescence unit; SM, sphingomyelin.
CH compared with AD, P<0.001.
CH compared with MCI, P<0.001.
AD compared with CH and MCI, P<0.001.
Figure 2.
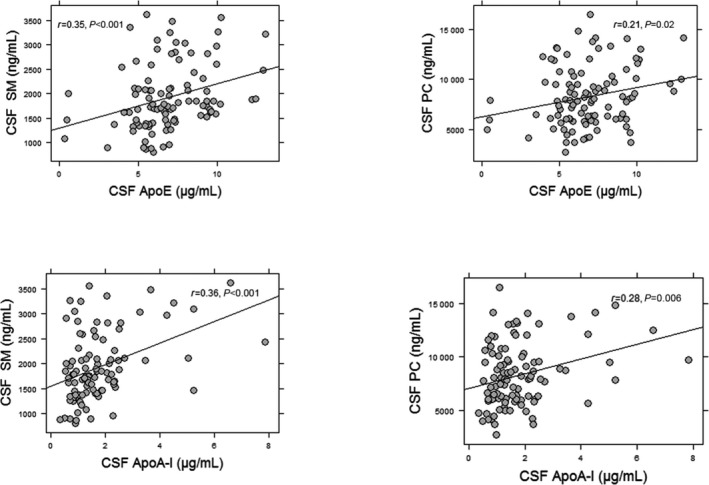
Association of CSF apoA‐I and apoE with CSF lipids. Greater concentrations of the apoE and apoA‐I were associated with greater CSF lipids SM and PC. The CSF lipids were measured in the supernatant fraction of CSF. The correlation coefficients and P values were obtained using Pearson correlations for normally distributed data or Spearman correlations for nonnormal distributions. CSF, cerebrospinal fluid; PC, phosphatidylcholine; SM, sphingomyelin.
Figure 3.
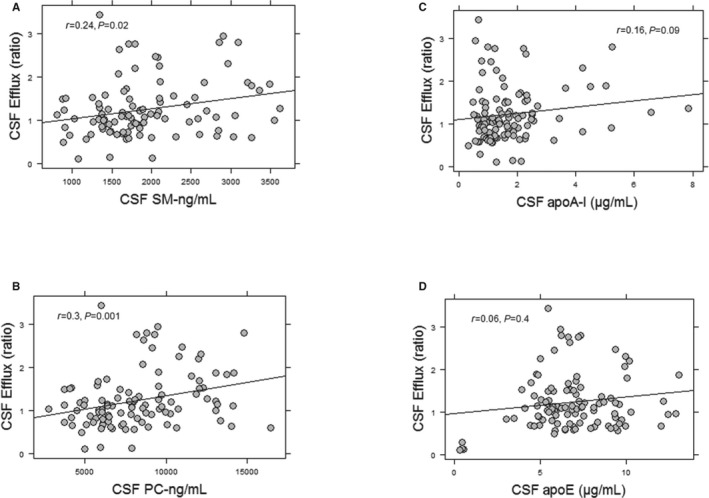
Association of CSF cholesterol efflux capacity with CSF lipid concentrations. CSF cholesterol efflux capacity correlated with CSF SM (A) and CSF PC levels (B). The correlation of CSF cholesterol efflux capacity with apoA‐I was weak (C). There was no significant correlation between CSF cholesterol efflux capacity and CSF apoE concentrations (D). PC and SM were measured in the supernatant fraction of CSF. The correlation coefficients and P values were obtained using Pearson correlations for normally distributed data or Spearman correlations for nonnormal distributions. CSF, cerebrospinal fluid; PC, phosphatidylcholine; SM, sphingomyelin.
Figure 4.
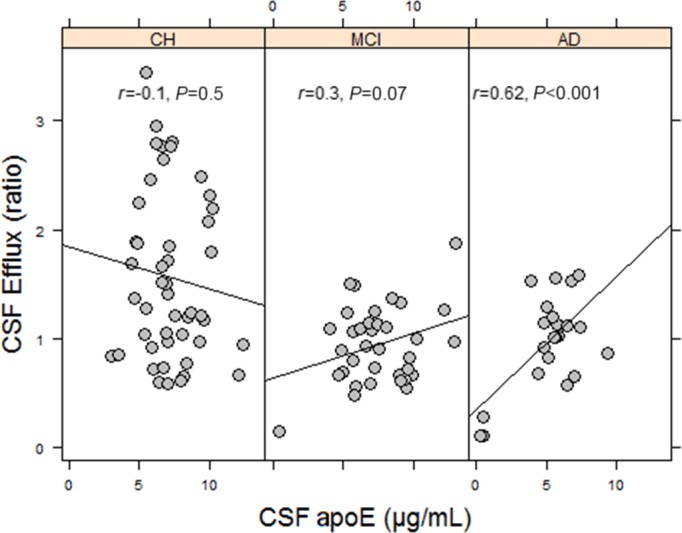
Association of CSF apoE concentrations and CSF cholesterol efflux capacity in AD. A significant correlation between CSF apoE concentrations and CSF cholesterol efflux capacity was observed only in participants with AD. This association was driven by lower apoE concentrations in CSF of AD participants. In contrast, this association was weak in participants with MCI and was not observed in CH participants. The correlation coefficients and P values were obtained using Pearson correlations for normally distributed data or Spearman correlations for nonnormal distributions. AD indicates Alzheimer's disease; CH, cognitively healthy; CSF, cerebrospinal fluid; MCI, mild cognitive impairment.
CSF‐mediated ABCA1 cholesterol efflux was significantly correlated with CSF cholesterol levels (r=0.37, P<0.001) (Figure 5A). The relationship, however, was driven by the CH subgroup (n=47, r=0.34, P=0.02) (Figure 5B). The correlation between cholesterol efflux and CSF cholesterol was weaker in participants with MCI (n=35, r=0.29, P=0.08) and with AD (n=26, r=0.22, P=0.27).
Figure 5.
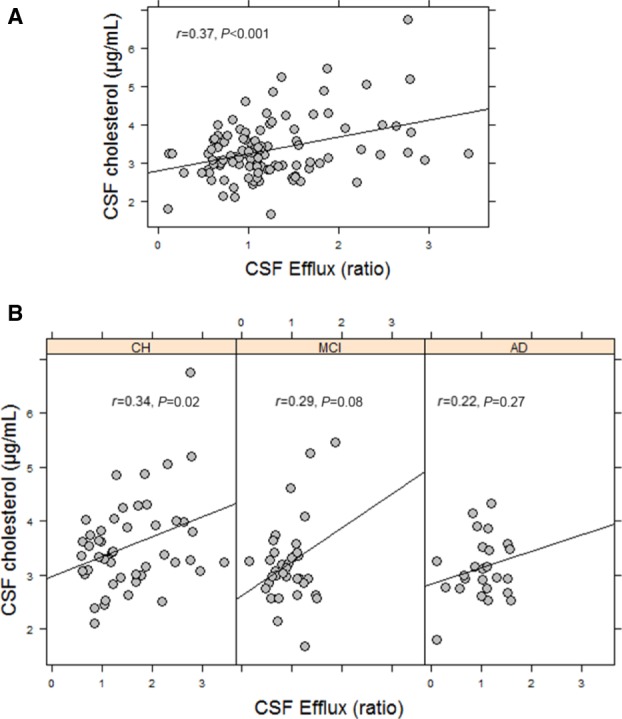
Capacity of CSF to facilitate ABCA1‐mediated cholesterol efflux correlates with CSF cholesterol content. A, Correlation between CSF efflux and CSF cholesterol concentrations in all the participants. Greater efflux of cholesterol through the ABCA1 transporter was associated with greater cholesterol in the CSF. B, When divided by the 3 subgroups, the correlation of CSF efflux and CSF cholesterol was significant only in CH participants. The correlation coefficients and P values were obtained using Pearson correlations for normally distributed data or Spearman correlations for nonnormal distributions. AD indicates Alzheimer's disease; CH, cognitively healthy; CSF, cerebrospinal fluid; MCI, mild cognitive impairment.
We previously reported that decreased CSF PC was associated with changes in phospholipase A2 activity.17 Changes in phospholipase A2 activity did not correlate with changes in CSF efflux (r=−0.1, P=0.3). In addition, CSF efflux did not correlate with the activities of acid (r=0.12, P=0.2) or neutral sphingomyelinase (r=−0.06, P=0.5). Our findings demonstrated 3 biochemical changes in the CSF of AD participants compared with CH participants or those with MCI: decreased apoE, decreased β‐amyloid (Aβ) 42, and elevated tau concentrations (Table 2). These results indicate that these biochemical changes appear later in the course of the disease, in contrast with CSF‐mediated cholesterol efflux and decreases in phospho‐ and sphingolipids that appeared earlier in participants with MCI.
Given that the homozygous APOE e4 genotype imparts a 12‐fold risk for AD,28 we examined whether differences in CSF‐mediated cholesterol efflux capacity were mediated in part by APOE genotype (Table 3). APOE genotype was available for 87 of the 108 participants. Among the 87 participants, 14 were heterozygous for the e4 allele and 2 were e4 homozygotes. There was no significant correlation between APOE genotype and CSF efflux in this study. Using a linear regression model, the interaction between APOE e4 genotype and disease group on efflux was not significant; however, CSF efflux capacity from 2 e4 homozygotes was less than half of the mean cholesterol efflux of the group. Consequently, it is possible that there is an interaction between carrying the e4 allele and the ability of CSF to facilitate ABCA1‐mediated cholesterol efflux in the CSF from homozygous e4 participants.
Table 3.
APOE Genotypes and CSF Cholesterol Efflux
| APOE Genotype | 2/3 | 2/4 | 3/3 | 3/4 | 4/4 |
|---|---|---|---|---|---|
| CH | |||||
| n | 6 | 2 | 25 | 9 | |
| Mean (SD) | 1.38 (0.77) | 2.33 (0.62) | 1.58 (0.77) | 1.61 (0.89) | |
| MCI | |||||
| n | 5 | 2 | 21 | 1 | 2 |
| Mean (SD) | 0.87 (0.27) | 0.90 (0.23) | 1.0 (0.36) | 0.94 | 0.68 (0.02) |
| AD | |||||
| n | 10 | 4 | |||
| Mean (SD) | 1.07 (0.49) | 1.31 (0.27) | |||
AD indicates Alzheimer's disease; CH, cognitively healthy; MCI, mild cognitive impairment.
Discussion
Our results indicate that CSF capacity to facilitate ABCA1 efflux is less in those with AD and MCI compared with CH persons. The normal cholesterol efflux in the asymptomatic stage differs at the first detectable clinical deterioration, the MCI stage. This reduced ability to transport cholesterol in MCI supports a pathophysiological role for reduced ABCA1‐mediated cholesterol efflux in early AD‐associated neurodegeneration. We found that changes in CSF PC and SM paralleled the changes of CSF cholesterol efflux capacity. CSF is highly enriched with phospholipids, and our findings link alterations in CSF lipids with changes in CSF cholesterol homeostasis.
Several studies imply a role for deviation from cholesterol homeostasis in the development of AD. A hallmark for diagnosing AD is the formation and deposition of amyloidogenic Aβ peptides. Variants in the cholesterol 24‐hydroxylase gene (which mediates removal of cholesterol from brain) were associated with amyloid deposition and the risk of AD in several populations.29, 30, 31 Because cholesterol concentration in neuronal membranes affects amyloid precursor protein processing, CSF lipoproteins may have an indirect role in the production of Aβ.32 Cholesterol efflux mediated by CSF lipoproteins reduces membrane cholesterol levels and thus alters the formation of Aβ peptides. Previous groups demonstrated that reduced plasma cholesterol in neuronal membranes favors the production of nonamyloidogenic Aβ peptides by α‐secretase and lysosomal degradation of amyloidogenic Aβ peptides in microglia. In contrast, increased cholesterol levels in neuronal membranes increased presenilin and BACE activities favoring amyloidogenic Aβ production and inhibited microglial lysosomal degradation of amyloidogenic Aβ peptides.33, 34 It is also possible that increased cholesterol accumulation in brain endothelial cells due to reduced efflux can impair the flux of Aβ into and from the nervous system; however, this pathway has not been investigated.
Cholesterol efflux through the ABCA1 transporter is of particular importance in AD. Loss‐of‐function ABCA1 gene variants were associated with an increased risk for AD.22, 23 Rodent studies confirmed that loss‐of‐function mutations in the ABCA1 gene increased amyloid deposition in APP23 mice.21 In one study, activation of ABCA1‐mediated efflux by an apoE‐mediated therapeutic (RXR agonist bexarotene) ameliorated AD pathology in mice.20 Attempts to reproduce this work confirmed some aspects of the original report but failed to replicate others.35, 36, 37, 38 A challenge for using such therapeutics is the lack of a biomarker that can assess the efficacy of treatment. Our results suggest that homozygosity for the e4 allele may be associated with lower CSF efflux; therefore, an apoE‐directed therapeutic may not induce cholesterol efflux capacity equally in carriers and noncarriers of this allele.
The changes in cholesterol efflux capacity were explained in part by changes in CSF PC and SM levels but were not explained by changes in the concentrations of CSF apoA‐I or apoE. It is possible that this study was underpowered to observe significant correlations between CSF apolipoproteins and cholesterol efflux capacity; however, lipoproteins exist in multiple forms and are susceptible to posttranslational modifications. Their levels may not necessarily reflect their functional properties. Indeed, oxidation, glycation, and glyco‐oxidation of CSF lipoproteins have been reported in AD,39, 40 and oxidized apoA‐I has a limited capacity to mediate cholesterol efflux in plasma.26 Moreover, the lipidation state of lipoproteins can alter their affinity for lipid transporters. Lipid‐poor apoA‐I, also known as pre‐β1 high‐density lipoprotein based on its electrophoretic mobility, is a major determinant of ABCA1‐mediated efflux in plasma.41 Detailed characterization of apolipoprotein forms and modifications in the CSF merit additional study.
Our study has several areas of strength. This study is the largest to date assessing the functional capacity of CSF lipoproteins to mediate cholesterol efflux in relation to cognitive decline. Our findings are in accordance with a previous smaller scale study8 that reported reduced cholesterol efflux to CSF in 3 participants with AD. In contrast to the number of study participants in the study by Demeester et al, our AD group size was substantially larger (n=26). In addition, Demeester et al used rat astrocytes as the donor of cholesterol. Rat astrocytes express multiple cholesterol transporters, making it difficult to isolate the contributions of ABCA1 versus the other transport pathways. An additional strength of our study is the measurement of CSF PC and SM by mass spectrometry in both the SF and nanoparticle fractions. Our findings suggest a role for CSF PC and SM in cholesterol efflux, consistent with in vitro studies demonstrating that PC or SM can facilitate cholesterol transport.12, 13, 14, 15 Recent studies have proposed the measurement of plasma phospholipids as biomarkers of AD.42, 43 Our group has extended these observations to the central nervous system, in which aberrations in CSF glycerophospholipids and sphingolipids appear early in cognitive decline.17, 18 Our results suggest that decreases in CSF lipids have functional relevance to CSF cholesterol homeostasis early in the process of neurodegeneration.
Our study has several limitations. We focused on ABCA1 efflux and did not assess cholesterol efflux by the other pathways (eg, SRB1, ABCG1, or ABCA7). ABCA7 has recently been identified in genomewide association studies of human AD, but its contributions to cholesterol efflux are not known.28 We did not assess ABCA1‐mediated phospho‐ or sphingolipid efflux. Decreased CSF phospho‐ and sphingolipids can contribute to decreased ABCA1 efflux or can result from decreased ABCA1 activity. Future studies need to clarify the role of the ABCA1 transporter on PC and SM efflux. Our study did not have sufficient power to detect differences in SM or apoA‐I among the 3 groups.
Diagnosis of MCI or AD is imperfect without neuropathology, but our consensus clinical diagnostic conferences with biochemical evaluations give the best degree of clinical confidence. More information is needed to understand lipid transport in health and AD, between CSF compartments and how they correlate with brain lipid transport, and in terms of temporal resolution with regard to disease progression. We are pursuing CSF cholesterol efflux in longitudinal studies and investigating brain lipid changes in autopsy tissues. The extensive heterogeneity of older study participants, the wide variety of lipolytic enzyme isoforms, and the varieties of subcellular compartmentalization of brain chemistry are such that only gross contributors will be detected, and their interpretation is consequently limited. Nevertheless, our finding that there is a significant change in CSF cholesterol efflux in those with MCI compared with CH participants and that this change is maintained in AD participants implies a cholesterol disorder early in the process of AD‐associated neurodegeneration.
In conclusion, we reported reduced ABCA1‐mediated cholesterol efflux capacity of CSF in MCI and AD compared with CH controls. Bexarotene and other agents that enhance ABCA1 efflux are in clinical trials for AD; we propose that the assessment of ABCA1 functional capacity of CSF can help select at‐risk persons and offers a mechanism‐based outcome measure for evaluating such therapies.
Author Contributions
Drs Yassine, Harrington, and Chui designed the study. Mrs Feng and Ms Chiang assisted with the efflux and lipoprotein studies. Dr Fonteh critically appraised the study design and the manuscript.
Sources of Funding
Yassine was supported by K23HL107389 from National Institute of Heart, Lung and Blood. Chiang, Fonteh, and Harrington were supported by the LK Whittier Foundation and HMRI. ADRC project P50‐AG05142‐31 (Chui) from the National Institute of Aging supported the studies.
Disclosures
None.
Supporting information
Figure S1. Capacity of CSF to facilitate ABCA‐1 mediated cholesterol efflux. (a): Purified ApoA‐I and ApoE apolipoproteins were used as acceptors of labeled (3H) cholesterol. Both purified ApoA‐1 and ApoE facilitated ABCA‐1 mediated cholesterol efflux after mifepristone induced expression of ABCA‐1. (b): Increasing doses of ApoA‐1 or ApoE were used to assess the dose response of this cell line to export labeled cholesterol. (c): Increasing doses of CSF demonstrated cholesterol efflux with a linear dose response.
Figure S2. Nanoparticles in the CSF. (a): Left: Transmission electron microscopy illustrating the heterogeneous structures present in CSF nanoparticles (A‐D), prepared as in (Harrington et al, 2009), scale bar 100 nm. A and B show abundant circular particles 30‐100 nm in diameter; C shows distinct membranes around a structure that resembles a large dense core vesicle; D represents uncommon but striking examples of debris in the form of cilia and a large club‐shaped ventricular fragment (arrowed). (b): A dot blot demonstrating Apo A‐I and Apo E in both supernatant fraction (SF) and nanoparticle fraction (NP). +C: positive control, pure protein 10?g/ml. A negative control was also used. (c): Cholesterol efflux capacity of NP and SF fractions. 35?L of media only, NP and SF fractions of a control participant CSF were tested for their capacity to facilitate ABCA‐1 efflux. The capacity of NP to facilitate efflux was much lower than that of the SF.
Acknowledgments
BHK cells were a gift from Dr Chogren Tang, University of Washington.
(J Am Heart Assoc. 2016;5:e002886 doi: 10.1161/JAHA.115.002886)
An accompanying Figures S1 and S2 are available at http://jaha.ahajournals.org/content/5/2/e002886/suppl/DC1
References
- 1. Diestschy J, Turley S. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. [DOI] [PubMed] [Google Scholar]
- 2. Martín MG, Pfrieger F, Dotti CG. Cholesterol in brain disease: sometimes determinant and frequently implicated. EMBO Rep. 2014;15:1036–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004;24:806–815. [DOI] [PubMed] [Google Scholar]
- 4. Simpson JE, Ince PG, Minett T, Matthews FE, Heath PR, Shaw PJ, Goodall E, Garwood CJ, Ratcliffe LE, Brayne C, Rattray M, Wharton SB. Neuronal DNA damage response‐associated dysregulation of signalling pathways and cholesterol metabolism at the earliest stages of Alzheimer‐type pathology. Neuropathol Appl Neurobiol. 2015; doi: 10.1111/nan.12252. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gusareva ES, Carrasquillo MM, Bellenguez C, Cuyvers E, Colon S, Graff‐Radford NR, Petersen RC, Dickson DW, John JMM, Bessonov K. Genome‐wide association interaction analysis for Alzheimer's disease. Neurobiol Aging. 2014;35:2436–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yancey PG, Bortnick AE, Kellner‐Weibel G, de la Llera‐Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. [DOI] [PubMed] [Google Scholar]
- 7. Koch S, Donarski N, Goetze K, Kreckel M, Stuerenburg H‐J, Buhmann C, Beisiegel U. Characterization of four lipoprotein classes in human cerebrospinal fluid. J Lipid Res. 2001;42:1143–1151. [PubMed] [Google Scholar]
- 8. Demeester N, Castro G, Desrumaux C, De Geitere C, Fruchart J, Santens P, Mulleners E, Engelborghs S, De Deyn P, Vandekerckhove J. Characterization and functional studies of lipoproteins, lipid transfer proteins, and lecithin: cholesterol acyltransferase in CSF of normal individuals and patients with Alzheimer's disease. J Lipid Res. 2000;41:963–974. [PubMed] [Google Scholar]
- 9. Kandimalla RJ, Wani WY, Anand R, Kaushal A, Prabhakar S, Grover VK, Bharadwaj N, Jain K, Gill KD. Apolipoprotein E levels in the cerebrospinal fluid of north Indian patients with Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2013;28:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu Y, Malone JP, Fagan AM, Townsend RR, Holtzman DM. Comparative proteomic analysis of intra‐and interindividual variation in human cerebrospinal fluid. Mol Cell Proteomics. 2005;4:2000–2009. [DOI] [PubMed] [Google Scholar]
- 11. Cruchaga C, Kauwe JS, Nowotny P, Bales K, Pickering EH, Mayo K, Bertelsen S, Hinrichs A, Fagan AM, Holtzman DM. Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer's disease. Hum Mol Genet. 2012;21:4558–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stein O, Vanderhoek J, Stein Y. Cholesterol content and sterol synthesis in human skin fibroblasts and rat aortic smooth muscle cells exposed to lipoprotein‐depleted serum and high density apolipoprotein/phospholipid mixtures. Biochim Biophys Acta. 1976;431:347–358. [DOI] [PubMed] [Google Scholar]
- 13. Zhao Y, Sparks DL, Marcel YL. Effect of the apolipoprotein A‐I and surface lipid composition of reconstituted discoidal HDL on cholesterol efflux from cultured fibroblasts. Biochemistry. 1996;35:16510–16518. [DOI] [PubMed] [Google Scholar]
- 14. Swaney JB. Membrane cholesterol uptake by recombinant lipoproteins. Chem Phys Lipids. 1985;37:317–327. [DOI] [PubMed] [Google Scholar]
- 15. Jian B, de la Llera‐Moya M, Royer L, Rothblat G, Francone O, Swaney JB. Modification of the cholesterol efflux properties of human serum by enrichment with phospholipid. J Lipid Res. 1997;38:734–744. [PubMed] [Google Scholar]
- 16. Haidar B, Mott S, Boucher B, Lee CY, Marcil M, Genest J. Cellular cholesterol efflux is modulated by phospholipid‐derived signaling molecules in familial HDL deficiency/tangier disease fibroblasts. J Lipid Res. 2001;42:249–257. [PubMed] [Google Scholar]
- 17. Fonteh AN, Chiang J, Cipolla M, Hale J, Diallo F, Chirino A, Arakaki X, Harrington MG. Alterations in cerebrospinal fluid glycerophospholipids and phospholipase A2 activity in Alzheimer's disease. J Lipid Res. 2013;54:2884–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fonteh AN, Ormseth C, Chiang J, Cipolla M, Arakaki X, Harrington MG. Sphingolipid metabolism correlates with cerebrospinal fluid beta amyloid levels in Alzheimer's disease. PLoS One. 2015;10:e0125597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrington MG, Fonteh AN, Oborina E, Liao P, Cowan RP, McComb G, Chavez JN, Rush J, Biringer RG, Huhmer A. The morphology and biochemistry of nanostructures provide evidence for synthesis and signaling functions in human cerebrospinal fluid. Cerebrospinal Fluid Res. 2009;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cramer PE, Cirrito JR, Wesson DW, Lee CD, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ. ApoE‐directed therapeutics rapidly clear β‐amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koldamova R, Staufenbiel M, Lefterov I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem. 2005;280:43224–43235. [DOI] [PubMed] [Google Scholar]
- 22. Reynolds CA, Hong MG, Eriksson UK, Blennow K, Bennet AM, Johansson B, Malmberg B, Berg S, Wiklund F, Gatz M, Pedersen NL, Prince JA. A survey of ABCA1 sequence variation confirms association with dementia. Hum Mutat. 2009;30:1348–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nordestgaard LT, Tybjærg‐Hansen A, Nordestgaard BG, Frikke‐Schmidt R. Loss‐of‐function mutation in abca1 and risk of Alzheimer's disease and cerebrovascular disease. Alzheimers Dement. 2015;12:1430–1438. [DOI] [PubMed] [Google Scholar]
- 24. Harrington MG, Chiang J, Pogoda JM, Gomez M, Thomas K, Marion SD, Miller KJ, Siddarth P, Yi X, Zhou F, Lee S, Arakaki X, Cowan RP, Tran T, Charleswell C, Ross BD, Fonteh AN. Executive function changes before memory in preclinical Alzheimer's pathology: a prospective, cross‐sectional, case control study. PLoS One. 2013;8:e79378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yassine HN, Belopolskaya A, Schall C, Stump CS, Lau SS, Reaven PD. Enhanced cholesterol efflux to HDL through the ABCA1 transporter in hypertriglyceridemia of type 2 diabetes. Metabolism. 2014;63:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shao B, Tang C, Sinha A, Mayer PS, Davenport GD, Brot N, Oda MN, Zhao X‐Q, Heinecke JW. Humans with atherosclerosis have impaired abca1 cholesterol efflux and enhanced high‐density lipoprotein oxidation by myeloperoxidase. Circ Res. 2014;114:1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. [DOI] [PubMed] [Google Scholar]
- 28. Lambert J‐C, Ibrahim‐Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, Jun G, DeStefano AL, Bis JC, Beecham GW. Meta‐analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;12:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kolsch H, Lutjohann D, Ludwig M, Schulte A, Ptok U, Jessen F, von Bergmann K, Rao ML, Maier W, Heun R. Polymorphism in the cholesterol 24S‐hydroxylase gene is associated with Alzheimer's disease. Mol Psychiatry. 2002;7:899–902. [DOI] [PubMed] [Google Scholar]
- 30. Li L, Yin Z, Liu J, Li G, Wang Y, Yan J, Zhou H. CYP46A1 T/C polymorphism associated with the APOE epsilon4 allele increases the risk of Alzheimer's disease. J Neurol. 2013;260:1701–1708. [DOI] [PubMed] [Google Scholar]
- 31. Kolsch H, Lutjohann D, Jessen F, Popp J, Hentschel F, Kelemen P, Schmitz S, Maier W, Heun R. CYP46A1 variants influence Alzheimer's disease risk and brain cholesterol metabolism. Eur Psychiatry. 2009;24:183–190. [DOI] [PubMed] [Google Scholar]
- 32. Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of β‐amyloid in hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:6460–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolozin B. Cholesterol and the biology of Alzheimer's disease. Neuron. 2004;41:7–10. [DOI] [PubMed] [Google Scholar]
- 34. Lee CYD, Tse W, Smith JD, Landreth GE. Apolipoprotein E promotes β‐amyloid trafficking and degradation by modulating microglial cholesterol levels. J Biol Chem. 2012;287:2032–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fitz NF, Cronican AA, Lefterov I, Koldamova R. Comment on “ApoE‐directed therapeutics rapidly clear beta‐amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924‐c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Price AR, Xu G, Siemienski ZB, Smithson LA, Borchelt DR, Golde TE, Felsenstein KM. Comment on “ApoE‐directed therapeutics rapidly clear beta‐amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924‐d. [DOI] [PubMed] [Google Scholar]
- 37. Tesseur I, Lo AC, Roberfroid A, Dietvorst S, Van Broeck B, Borgers M, Gijsen H, Moechars D, Mercken M, Kemp J, D'Hooge R, De Strooper B. Comment on “ApoE‐directed therapeutics rapidly clear beta‐amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924‐e. [DOI] [PubMed] [Google Scholar]
- 38. Veeraraghavalu K, Zhang C, Miller S, Hefendehl JK, Rajapaksha TW, Ulrich J, Jucker M, Holtzman DM, Tanzi RE, Vassar R, Sisodia SS. Comment on “ApoE‐directed therapeutics rapidly clear beta‐amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924‐f. [DOI] [PubMed] [Google Scholar]
- 39. Bassett CN, Neely MD, Sidell KR, Markesbery WR, Switt LL, Montine TJ. Cerebrospinal fluid lipoproteins are more vulnerable to oxidation in Alzheimer's disease and are neurotoxic when oxidized ex vivo. Lipids. 1999;34:1273–1280. [DOI] [PubMed] [Google Scholar]
- 40. Smith MA, Taneda S, Richey PL, Miyata S, Yan S‐D, Stern D, Sayre LM, Monnier VM, Perry G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci USA. 1994;91:5710–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Asztalos BF, de la Llera‐Moya M, Dallal GE, Horvath KV, Schaefer EJ, Rothblat GH. Differential effects of HDL subpopulations on cellular ABCA1‐and SR‐BI‐mediated cholesterol efflux. J Lipid Res. 2005;46:2246–2253. [DOI] [PubMed] [Google Scholar]
- 42. Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, Hall WJ, Fisher SG, Peterson DR, Haley JM. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20:415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whiley L, Sen A, Heaton J, Proitsi P, García‐Gómez D, Leung R, Smith N, Thambisetty M, Kloszewska I, Mecocci P. Evidence of altered phosphatidylcholine metabolism in Alzheimer's disease. Neurobiol Aging. 2014;35:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Capacity of CSF to facilitate ABCA‐1 mediated cholesterol efflux. (a): Purified ApoA‐I and ApoE apolipoproteins were used as acceptors of labeled (3H) cholesterol. Both purified ApoA‐1 and ApoE facilitated ABCA‐1 mediated cholesterol efflux after mifepristone induced expression of ABCA‐1. (b): Increasing doses of ApoA‐1 or ApoE were used to assess the dose response of this cell line to export labeled cholesterol. (c): Increasing doses of CSF demonstrated cholesterol efflux with a linear dose response.
Figure S2. Nanoparticles in the CSF. (a): Left: Transmission electron microscopy illustrating the heterogeneous structures present in CSF nanoparticles (A‐D), prepared as in (Harrington et al, 2009), scale bar 100 nm. A and B show abundant circular particles 30‐100 nm in diameter; C shows distinct membranes around a structure that resembles a large dense core vesicle; D represents uncommon but striking examples of debris in the form of cilia and a large club‐shaped ventricular fragment (arrowed). (b): A dot blot demonstrating Apo A‐I and Apo E in both supernatant fraction (SF) and nanoparticle fraction (NP). +C: positive control, pure protein 10?g/ml. A negative control was also used. (c): Cholesterol efflux capacity of NP and SF fractions. 35?L of media only, NP and SF fractions of a control participant CSF were tested for their capacity to facilitate ABCA‐1 efflux. The capacity of NP to facilitate efflux was much lower than that of the SF.


