Abstract
Background
Biomarkers of myocardial injury increase frequently during transcatheter aortic valve implantation (TAVI). The impact of postprocedural cardiac troponin (cTn) elevation on short‐term outcomes remains controversial, and the association with long‐term prognosis is unknown.
Methods and Results
We evaluated 577 consecutive patients with severe aortic stenosis treated with TAVI between 2007 and 2012. Myocardial injury, defined according to the Valve Academic Research Consortium (VARC)‐2 as post‐TAVI cardiac troponin T (cTnT) >15× the upper limit of normal, occurred in 338 patients (58.1%). In multivariate analyses, myocardial injury was associated with higher risk of all‐cause mortality at 30 days (adjusted hazard ratio [HR], 8.77; 95% CI, 2.07–37.12; P=0.003) and remained a significant predictor at 2 years (adjusted HR, 1.98; 95% CI, 1.36–2.88; P<0.001). Higher cTnT cutoffs did not add incremental predictive value compared with the VARC‐2–defined cutoff. Whereas myocardial injury occurred more frequently in patients with versus without coronary artery disease (CAD), the relative impact of cTnT elevation on 2‐year mortality did not differ between patients without CAD (adjusted HR, 2.59; 95% CI, 1.27–5.26; P=0.009) and those with CAD (adjusted HR, 1.71; 95% CI, 1.10–2.65; P=0.018; P for interaction=0.24). Mortality rates at 2 years were lowest in patients without CAD and no myocardial injury (11.6%) and highest in patients with complex CAD (SYNTAX score >22) and myocardial injury (41.1%).
Conclusions
VARC‐2–defined cTnT elevation emerged as a strong, independent predictor of 30‐day mortality and remained a modest, but significant, predictor throughout 2 years post‐TAVI. The prognostic value of cTnT elevation was modified by the presence and complexity of underlying CAD with highest mortality risk observed in patients combining SYNTAX score >22 and evidence of myocardial injury.
Keywords: aortic stenosis, prognosis, transcatheter aortic valve implantation, troponin
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Biomarkers, Catheter-Based Coronary and Valvular Interventions
Introduction
Transcatheter aortic valve implantation (TAVI) has evolved into a valuable treatment modality in high‐risk and inoperable patients with severe aortic stenosis (AS)1, 2, 3; however, individual risk stratification and long‐term prognostication remain challenging. While biomarkers of myocardial necrosis increase and predict adverse outcomes after coronary interventions,4 growing interest has focused on the clinical significance of myocardial injury that frequently occurs during TAVI. Previous studies have yielded conflicting results, with some investigations showing an impact of cardiac troponin (cTn) elevation on post‐TAVI outcomes5, 6, 7 and others dissociating postprocedural cTn levels from subsequent adverse events.8, 9 Previous reports were limited by smaller patient numbers and shorter duration of follow‐up and did not assess prognostic implications of cTn elevation according to the presence and complexity of coronary artery disease (CAD), despite the fact that coexisting coronary atherosclerosis varies substantially among patients with AS10 and affects the magnitude of myocardial necrosis during TAVI interventions.8 Most notably, earlier investigations applied distinctly different cutoffs of cTn to define myocardial injury, ranging from any increase,7, 9 5× increase,6 and up to 15× the upper limit of normal (ULN),5, 8 such that the proportion of patients with reported cTn elevation ranged from 17% to 99%. Using the current Valve Academic Research Consortium (VARC)‐2 cTn cutoff of 15× ULN to define myocardial injury,11 2 studies have reported discordant results regarding the predictive merit of cTnT elevation post‐TAVI.5, 8
Currently, the association of TAVI‐related cTn increase with short‐term outcomes remains controversial; the effect of the VARC‐2–defined threshold of myocardial injury on long‐term survival and the interrelation with the presence and severity of underlying CAD are unknown, and the relative predictive value of pre‐ versus post‐TAVI cTn measurement has only undergone limited investigation. Against this background, this study sought to determine the impact of VARC‐2–defined myocardial injury on clinical outcomes in a sizeable cohort of consecutive patients with severe AS undergoing TAVI.
Methods
Patient Population
This is a retrospective analysis of prospectively collected data. All patients with severe native‐valve AS (defined as indexed aortic valve area [AVA] ≤0.6 cm2/m2 or mean gradient >40 mm Hg) who underwent TAVI at our institution between August 2007 and December 2012 were entered into a dedicated database (Bern TAVI Registry). Patients were deemed inoperable or at high surgical risk by a multidisciplinary Heart Team. Of 606 consecutive patients, 577 (95.2%) had measurements of cTnT after the intervention and are included in the present analysis. The study was approved by the local Ethics Committee, and all patients provided written informed consent for prospective follow‐up.
Hemodynamic and Echocardiographic Assessment
Patients underwent coronary angiography as well as left and right heart catheterization for hemodynamic assessment before TAVI. Aortic valve gradients were measured by the pull‐back technique from the left ventricle to the ascending aorta. Calculation of AVA was derived from the Gorlin equation.12, 13 Transthoracic echocardiography (TEE) was performed before TAVI, and severity of aortic and mitral valve regurgitation was semiquantitatively graded as mild, moderate, and severe according to guidelines.14
TAVI Procedure
TAVI was performed using standard techniques.12, 13 Patients underwent implantation of the self‐expanding Medtronic CoreValve (Medtronic, Minneapolis, MN) or the balloon‐expandable Edwards Sapien XT prosthesis (Edwards LifeSciences, Irvine, CA), using the transfemoral, transapical, or subclavian access route, and the self‐expanding Symetis Acurate TA prosthesis (Symetis SA, Ecublens VD, Switzerland) using the transapical route. Selection of access route and valve type was based on anatomical characteristics determined by contrast‐enhanced computed tomography, angiography, and TEE. Among patients with CAD, revascularization of coronary artery segments with diameter stenosis ≥70% was attempted by percutaneous coronary intervention (PCI) either in a scheduled session before TAVI or at the time of TAVI (concomitant PCI).
Measurement of Troponin
Levels of cardiac troponin T (cTnT) were determined within 12 hours post‐TAVI; in case of increased levels (>1× ULN), repetitive measurements were obtained at 24 hours and later to assess maximal (peak) post‐TAVI levels. In a subgroup of 256 patients (44.4%), levels of cTnT were available at baseline before the procedure. cTnT measurements were performed up to August 2010 by means of the fourth‐generation Elecsys cTnT assay and thereafter by means of the high‐sensitivity Elecsys cTnT assay (both Roche Diagnostics, Mannheim, Germany). Based on the 99th percentile in a healthy population and the requirement of a ≤10% coefficient variation, the upper reference limits for cTnT levels were 0.010 and 0.014 μg/L, respectively, for the 2 assays.
Clinical Follow‐up
Demographic, clinical, and procedural characteristics were systematically collected. Laboratory values during the index hospitalization were retrieved from the central hospital laboratory. Patients were followed throughout 2 years. Adverse cardiac and cerebrovascular events were assessed in‐hospital, and regular follow‐up was performed at 30 days, 12 months, and 2 years by means of a clinical visit or a standardized telephone interview. All events were adjudicated by an independent clinical event committee consisting of invasive cardiologists and cardiac surgeons. All data were entered into a dedicated database and held at an academic clinical trials unit (CTU Bern, Bern University Hospital, Bern, Switzerland) responsible for central data audits and maintenance of the database.
Clinical Endpoints and Definitions
Clinical endpoints were defined according to VARC‐2 criteria.11 Myocardial injury was defined using the VARC‐2 cutoff for postprocedure cTnT (>15× ULN). The study's primary endpoint was all‐cause mortality within 30 days. Secondary endpoints were cardiovascular death, cerebrovascular events (stroke, transient ischemic attack), and myocardial infarction (MI) at 30 days and 2 years. An exploratory analysis assessed clinical outcomes in relation to elevated (>1× ULN) baseline, preintervention cTnT in the subset of patients with available measurements. Renal dysfunction was defined as estimated glomerular filtration rate (GFR) <60 mL/min per 1.73 m2. CAD was defined as the presence of 1 or more lesions of the epicardial coronary arteries with ≥50% diameter stenosis in vessels ≥1.5 mm in diameter. For patients with CAD, the SYNTAX score at baseline (preceding any PCI) was calculated using the respective algorithm15 (available at: www.syntaxscore.com).
Statistical Analyses
Continuous variables are summarized as mean±SD: categorical ones, as actual numbers, and percentages. Baseline and procedural characteristics were compared using Fisher's tests for binary responses, chi‐square tests for more than 2 responses, and unpaired t tests for means. Median post‐TAVI troponin levels were compared using the Kruskall–Wallis test or Mann–Whitney U test, as appropriate. Predictors of post‐TAVI cTnT levels were assessed using univariate regression and retained in the multivariate regression if they had a significant effect of P<0.2. Clinical outcomes were analyzed using Cox regression analysis (time to first event) and graphically presented using Kaplan–Meier curves with incidence rates calculated from life tables. The independent association between cTnT elevation and subsequent mortality was examined using Cox proportional hazards models. Hazard ratios (HRs) with 95% CIs, and P values were derived from the Wald chi‐square test. Adjusted HRs were calculated using baseline variables with a uni‐ and/or multivariate effect (P<0.2) on all‐cause mortality at 30 days: age, renal dysfunction, and Society of Thoracic Surgeons (STS) score. Prognostic discrimination of post‐TAVI and pre‐TAVI cTnT levels was assessed by receiver operating characteristic (ROC) curves and quantified by areas under the curve (AUCs). Findings were considered statistically significant at the 0.05 level. All analyses were performed with Stata software (version 13.1; StataCorp LP, College Station, TX).
Results
Baseline and Procedural Characteristics
Levels of cTnT were measured at 12 hours in all patients and at 24 hours and later in 96.2% of patients. Multivariate predictors of peak troponin levels included body weight (P<0.001), history of MI (P=0.041), STS score (P<0.0001), and transapical access (P<0.001; Table S1). Distribution of post‐TAVI cTnT peak levels is shown in Figure 1. VARC‐2–defined myocardial injury was observed in 338 of 577 patients (58.1%). At baseline, patients with versus without myocardial injury more frequently had renal dysfunction, CAD, and multi vessel disease and had higher STS scores (Table 1). Procedural characteristics of TAVI interventions are summarized in Table 2. The majority of interventions (80%) were performed by means of the femoral route; myocardial injury occurred in 49.1% of transfemoral and 100% of transapical interventions (P<0.001). The Metronic Corevalve was implanted in 52%, the Edwards Sapien XT in 46%, and the Symetis Acurate TA prosthesis in 2% of patients. The Edwards Sapien valve was used more commonly in patients with myocardial injury (Table 2) and was associated with higher peak cTnT levels overall (Table 3), whereas there was no difference in a subanalysis excluding transapical interventions (Table 4). Concomitant PCI was performed in 16% of patients and was not associated with frequency of myocardial injury (P=0.91; Table 2) or with peak cTnT levels (P=0.71; Table 3). Invasive hemodynamic measures are summarized in Table S2 and were generally not related to cTnT elevation, with the exception of higher left ventricular ejection fraction in patients with versus without myocardial injury (54.6±14.1% vs 52.1±15.5%; P=0.045).
Figure 1.
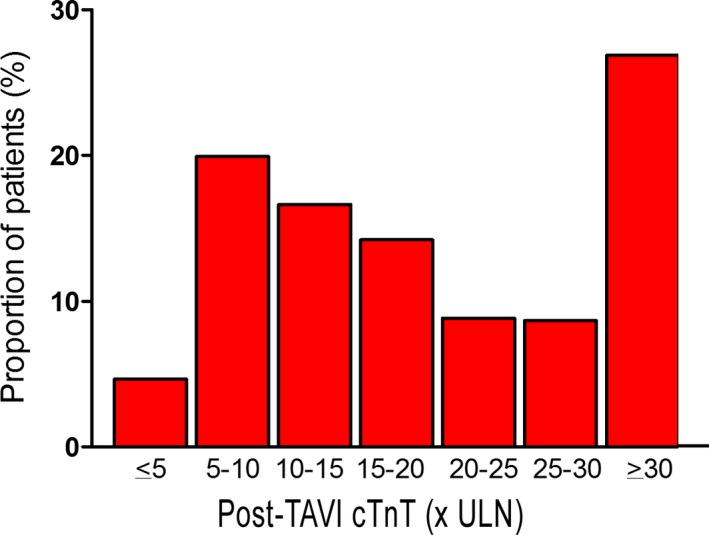
Proportion of patients (n=577) across increments of post‐TAVI peak cTnT levels expressed as multiples of the upper limit of normal (ULN). cTnT indicates cardiac troponin T; TAVI, transcatheter aortic valve implantation.
Table 1.
Baseline Characteristics of the Study Population
| Variable | cTnT ≤15× ULN n=239 | cTnT >15× ULN n=338 | P Value |
|---|---|---|---|
| Demographics | |||
| Age, y | 82.4±5.1 | 82.4±5.8 | 0.99 |
| Female sex, n (%) | 135 (56) | 179 (53) | 0.44 |
| Body mass index, kg/m2 | 26.7±5.2 | 26.0±4.7 | 0.07 |
| Cardiac risk factors | |||
| Diabetes mellitus, n (%) | 67 (28) | 90 (27) | 0.70 |
| Hypercholesterolemia, n (%) | 143 (60) | 225 (67) | 0.11 |
| Hypertension, n (%) | 199 (83) | 289 (86) | 0.48 |
| Current smoker, n (%) | 17 (8) | 35 (11) | 0.23 |
| Coronary artery disease, n (%) | 140 (59) | 227 (67) | 0.036 |
| SYNTAX score | 15.5±11.0 | 17.0±13.2 | 0.28 |
| Multivessel disease, n (%) | 42 (18) | 85 (25) | 0.03 |
| Previous myocardial infarction, n (%) | 34 (14) | 53 (16) | 0.72 |
| Previous CABG, n (%) | 20 (9) | 27 (9) | 1.000 |
| Previous PCI, n (%) | 56 (23) | 91 (27) | 0.38 |
| Chronic obstructive pulmonary disease, n (%) | 35 (15) | 64 (19) | 0.18 |
| Renal dusfunction (GFR <60 mL/min per 1.73 m2) | 145 (61) | 250 (74) | 0.001 |
| Severity of aortic regurgitation, n (%) | 0.31 | ||
| None/mild | 215 (93) | 291 (91) | 0.35 |
| Moderate/severe | 16 (7) | 30 (9) | 0.35 |
| Severity mitral regurgitation, n (%) | 0.75 | ||
| None/mild | 181 (76) | 252 (75) | 0.77 |
| Moderate/severe | 58 (24) | 86 (25) | 0.77 |
| Left ventricular mass index, g/m2 | 149.1±46.0 | 142.0±41.9 | 0.11 |
| Atrial fibrillation, n (%) | 63/204 (31) | 76/268 (28) | 0.61 |
| NYHA III/IV, n (%) | 181 (76) | 223 (66) | 0.016 |
| CCS III/IV, n (%) | 32 (13) | 46 (14) | 1.000 |
| Logistic EuroScore (%) | 22.1±14.0 | 23.4±13.6 | 0.25 |
| STS score (%) | 6.1±3.6 | 7.6±5.9 | 0.001 |
| Post‐TAVI CK‐MB, μg/L | 5.3±2.5 | 17.8±40.3 | <0.001 |
CABG indicates coronary artery bypass graft; CCS, Canadian Cardiovascular Society; CK‐MB, creatine phosphokinase‐MB; cTnT, cardiac troponin T; GFR, glomerular filtration rate; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation; ULN, upper limit of normal.
Table 2.
Procedural Characteristics
| Variable | cTnT ≤15× ULN n=239 | cTnT >15× ULN n=338 | P Value |
|---|---|---|---|
| Access route, n (%) | |||
| Femoral | 235 (98) | 227 (67) | <0.001 |
| Apical | 0 (0) | 106 (31) | <0.001 |
| Subclavian | 4 (2) | 5 (1) | 1.000 |
| Valve type, n (%) | <0.001 | ||
| Medtronic CoreValve | 151 (63) | 150 (44) | <0.001 |
| Edwards Sapien valve | 88 (37) | 177 (52) | <0.001 |
| Symetis acurate | 0 (0) | 11 (3) | 0.004 |
| Concomitant PCI, n (%) | 37 (15) | 54 (16) | 0.91 |
| Procedural specifications, n (%) | |||
| Device success VARC‐2 | 159 (67) | 228 (67) | 0.86 |
| Procedural mortality | 0 (0) | 1 (0) | 1.000 |
| TAV‐in‐TAV deployment | 1 (0) | 11 (3) | 0.02 |
| Repeat unplanned intervention within 30 days | 1 (0) | 3 (1) | 0.65 |
| Postimplantation mean aortic gradient ≥20 mm Hg | 5 (2) | 9 (3) | 0.78 |
| Moderate/severe patient prosthesis mismatch | 58 (24) | 60 (18) | 0.06 |
| Moderate/severe aortic regurgitation | 25 (10) | 45 (13) | 0.36 |
cTnT indicates cardiac troponin T; PCI, percutaneous coronary intervention; TAV: transcatheter aortic valve; ULN, upper limit of normal; VARC, Valve Academic Research Consortium.
Table 3.
Post‐TAVI Troponin T Levels in Relation to Access Route, Valve Type, and Concomitant PCI
| N | Median ×ULN (IQR) | P Value | |
|---|---|---|---|
| Overall | 577 | 17.7 (10.1–32.5) | |
| Procedural access | <0.001a | ||
| Femoral | 462 | 14.8 (9.3–24.3) | |
| Transapical | 106 | 43.7 (29.8–63.3) | |
| Subclavian | 9 | 20.2 (7.3–61.4) | |
| Device | <0.001a | ||
| Medtronic CoreValve | 301 | 15.0 (9.3–25.4) | |
| Edwards Sapien XT | 265 | 21.3 (12.0–38.8) | |
| Symetis Acurate | 11 | 49.1 (39.1–86.6) | |
| Concomitant PCI | 0.71b | ||
| Yes | 91 | 17.5 (9.8–36.2) | |
| No | 486 | 17.9 (10.1–31.7) |
IQR indicates interquartile range; PCI, percutaneous coronary intervention; TAVI; transcatheter aortic valve implantation; ULN, upper limit of normal.
Kruskall–Wallis tests.
Mann–Whitney U test.
Table 4.
Frequency of Myocardial Injury in Relation to Valve Type in the Subgroup of Patients Who Underwent Transfemoral or Subclavian TAVI (n=471)
| Valve Type | cTnT ≤15× ULN (No Myocardial Injury) | cTnT >15× ULN (Myocardial Injury) | P Value |
|---|---|---|---|
| Medtronic CoreValve, n (%) | 150 (49.8) | 151 (50.2) | 0.74 |
| Edwards Sapien XT, n (%) | 82 (48.2) | 88 (51.8) |
cTnT indicates cardiac troponin T; ULN, upper limit of normal.
Primary Endpoint: Post‐TAVI Troponin in Relation to 30‐Day Mortality
Follow‐up was complete by 100% at 30 days and 94.3% at 2 years. Mortality rates were 5.2% at 30 days and 24.8% at 2 years. Myocardial injury was associated with a higher risk of all‐cause death (HR, 10.33; 95% CI, 2.46–43.37; P<0.001) and cardiovascular death at 30 days (HR, 8.50; 95% CI, 2.00–36.07; P<0.001). Differences remained significant after adjusting for independent predictors of 30‐day mortality (Table S3), which included age, renal dysfunction, and STS score (Table 5).
Table 5.
Clinical Outcomes at 30 Days and 2 Years in Relation to Post‐TAVI Troponin T >15× ULN Versus ≤15× UNL
| Outcome | cTnT ≤15× ULN n=239 | cTnT >15× ULN n=338 | Hazard Ratio (95% CI) | P Value | Adjusted Hazard Ratio (95% CI) | Adjusted P Value |
|---|---|---|---|---|---|---|
| 30 days | ||||||
| Death, n (%) | 2 (0.8) | 28 (8.3) | 10.33 (2.46–43.37) | 0.001 | 8.77 (2.07–37.12) | 0.003 |
| Cardiovascular death, n (%) | 2 (0.8) | 23 (6.9) | 8.50 (2.00–36.07) | 0.004 | 6.95 (1.62–29.84) | 0.009 |
| Cerebrovascular events, n (%) | 6 (2.5) | 15 (4.5) | 1.79 (0.70–4.63) | 0.23 | 1.44 (0.55–3.77) | 0.45 |
| Major stroke, n (%) | 3 (1.3) | 15 (4.5) | 3.61 (1.05–12.47) | 0.04 | 2.99 (0.86–10.44) | 0.09 |
| Minor stroke, n (%) | 1 (0.4) | 0 (0.0) | 0.24 (0.01–5.87) | 0.41 | ||
| Transient ischemic attack, n (%) | 2 (0.8) | 0 (0.0) | 0.14 (0.01–2.90) | 0.17 | ||
| Myocardial infarction, n (%) | 0 (0.0) | 3 (0.9) | 4.95 (0.26–95.39) | 0.27 | ||
| Death or major stroke, n (%) | 4 (1.7) | 35 (10.4) | 6.42 (2.28–18.06) | <0.001 | 5.32 (1.87–15.11) | 0.002 |
| Death, major stroke, or MI, n (%) | 3 (1.3) | 37 (11.0) | 9.11 (2.81–29.53) | <0.001 | 7.56 (2.31–24.74) | 0.001 |
| Major or life‐threatening bleeding, n (%) | 55 (23.0) | 135 (40.0) | 1.85 (1.35–2.53) | <0.001 | 1.81 (1.31–2.48) | <0.001 |
| Major vascular complications, n (%) | 21 (8.8) | 32 (9.5) | 1.08 (0.62–1.87) | 0.781 | 1.00 (0.57–1.76) | 0.989 |
| 2 years | ||||||
| Death, n (%) | 39 (17.0) | 104 (31.9) | 2.13 (1.48–3.08) | <0.001 | 1.98 (1.36–2.88) | <0.001 |
| Cardiovascular death, n (%) | 25 (11.1) | 71 (22.6) | 2.26 (1.43–3.56) | <0.001 | 2.00 (1.25–3.18) | 0.004 |
| Cerebrovascular events, n (%) | 14 (6.3) | 24 (8.1) | 1.31 (0.68–2.54) | 0.42 | 1.21 (0.62–2.37) | 0.58 |
| Major stroke, n (%) | 8 (3.6) | 19 (6.0) | 1.80 (0.79–4.11) | 0.16 | 1.69 (0.73–3.90) | 0.22 |
| Minor stroke, n (%) | 2 (0.9) | 1 (0.4) | 0.39 (0.04–4.30) | 0.44 | 0.40 (0.04–4.50) | 0.46 |
| Transient ischemic attack, n (%) | 4 (1.8) | 3 (1.3) | 0.59 (0.13–2.65) | 0.49 | 0.51 (0.11–2.32) | 0.38 |
| Myocardial infarction, n (%) | 2 (0.9) | 10 (3.5) | 3.96 (0.87–18.06) | 0.08 | 3.62 (0.78–16.93) | 0.10 |
| Death or major stroke, n (%) | 44 (19.1) | 112 (34.2) | 2.04 (1.44–2.89) | <0.001 | 1.91 (1.34–2.72) | <0.001 |
| Death, major stroke, or MI, n (%) | 44 (19.1) | 117 (35.7) | 2.17 (1.53–3.07) | <0.001 | 2.04 (1.43–2.90) | <0.001 |
Depicted are number of first events (% cumulative incidence), hazard ratios (HRs) with respective 95% CI from Cox's regressions. Adjusted HRs were obtained after adjusting for age, renal dysfunction, and STS score. Age, renal dysfunction, and STS score had a P<0.2 univariate effect on the primary outcome all‐cause death at 30 days. Missing data were singly imputed (n=1 for renal dysfunction and n=1 for STS score). cTnT indicates cardiac troponin T; MI, myocardial infarction; STS, Society of Thoracic Surgeons; ULN, upper limit of normal.
Post‐TAVI Troponin in Relation to 2‐Year Outcomes
VARC‐2–defined myocardial injury had an AUC of 0.63 (0.56–0.67; P<0.001), sensitivity of 0.37, and specificity of 0.81 for prediction of 2‐year mortality. We did not identify a higher cTnT cutoff with higher AUC compared with the 15× ULN cutoff with respect to 2‐year mortality. Myocardial injury was associated with higher risk of all‐cause death (HR, 2.13; 95% CI, 1.48–3.08; P<0.001) and cardiovascular death at 2 years (HR, 2.26; 95% CI, 1.43–3.56; P<0.001). Differences remained significant after multivariate adjustment (adjusted HR, 1.98; 95% CI, 1.36–2.88; P<0.001 and adjusted HR, 2.00; 95% CI, 1.25–3.18; P=0.004, respectively; Figure 2 and Table 5), as well as in a landmark analysis between 30 days and 2 years (Figure 3). Myocardial injury was consistently related to higher 2‐year mortality in sensitivity analyses including patients without renal dysfunction (n=181; 31%: adjusted HR, 2.14; 95% CI, 1.10–4.16; P=0.026), those who did not undergo concomitant PCI (n=486; 84%: adjusted HR, 1.84; 95% CI, 1.23–2.74; P=0.003), and those without trans apical access (n=471; 82%: adjusted HR, 2.00; 95% CI, 1.34–2.98; P=0.001; Tables S4 through S6; Figure S1). These findings also held true when we excluded patients with serious complications resulting in procedure‐unrelated troponin elevation, including emergent cardiac surgery, pericadiotomy, or ventricular rupture (n=7, all with myocardial injury; adjusted HR, 2.01; 95% CI, 1.38–2.93; P<0.001).
Figure 2.
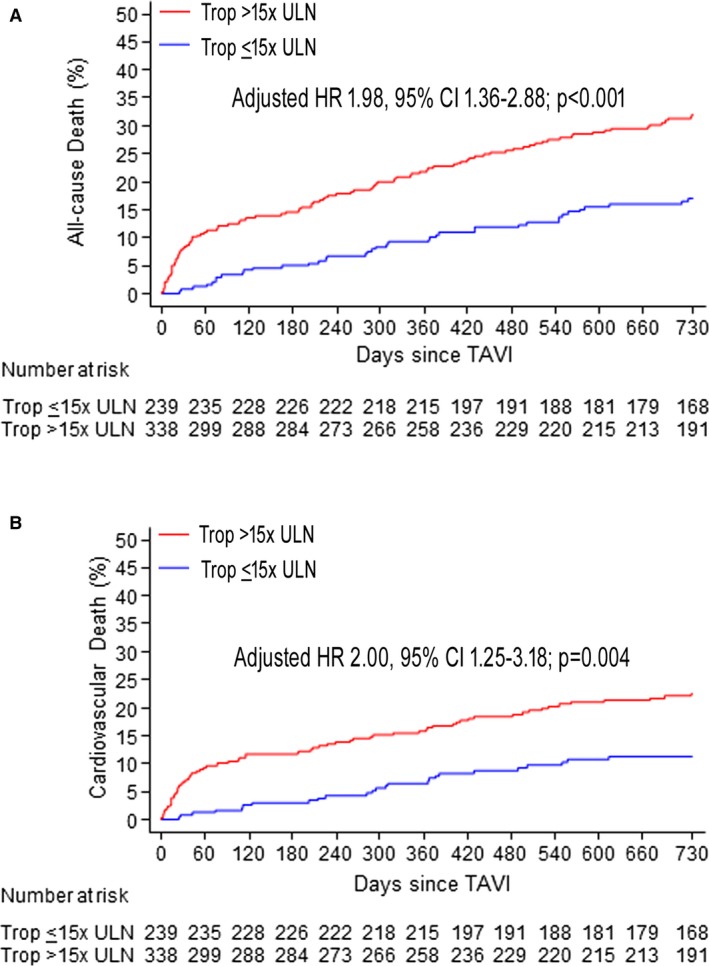
Cumulative event curves for all‐cause mortality (A) and cardiovascular death within 2‐year follow‐up (B) in patients with or without post‐TAVI cTnT elevation >15× ULN. cTnT indicates cardiac troponin T; HR, hazard ratio; TAVI, transcatheter aortic valve implantation; Trop, troponin; ULN, upper limit of normal.
Figure 3.
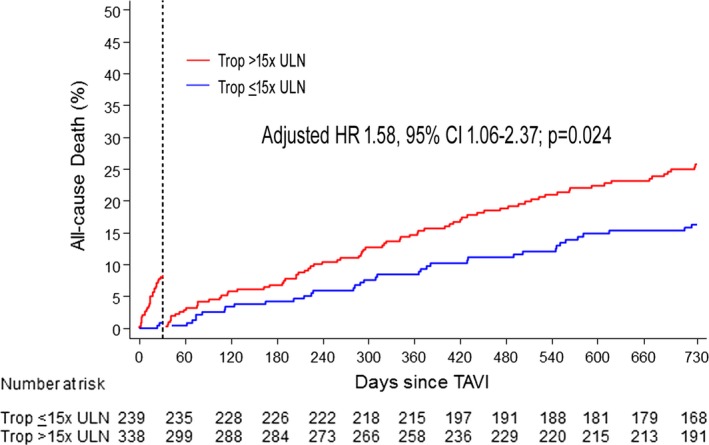
Landmark analysis of the cumulative incidence of all‐cause mortality between 30 days and 2 years in patients with or without post‐TAVI cTnT elevation >15× ULN. Adj. HR indicates adjusted hazard ratio; cTnT, cardiac troponin T; HR, hazard ratio; TAVI, transcatheter aortic valve implantation; Trop, troponin; ULN, upper limit of normal.
Prognostic Impact of Post‐TAVI Troponin in Relation to the Presence and Complexity of CAD
CAD was present in 367 patients (64%). Myocardial injury was more frequent in patients with versus without CAD (61.8% vs 52.8%; P=0.036). Event rates were lowest in patients without CAD and no myocardial injury and highest in those with CAD and myocardial injury (2‐year mortality 11.6% vs 33.1%, respectively; Figure 4 and Table 6). The association of myocardial injury with 2‐year mortality was consistent in patients without CAD (adjusted HR, 2.59; 95% CI, 1.27–5.26; P=0.009) and those with CAD (adjusted HR, 1.71; 95% CI, 1.10–2.65; P=0.018: P for interaction=0.24; Figure 4). When patients with CAD were further stratified according to CAD complexity, highest event rates were observed in patients with SYNTAX scores >22 and myocardial injury (n=64; 2‐year mortality, 41.1%).
Figure 4.
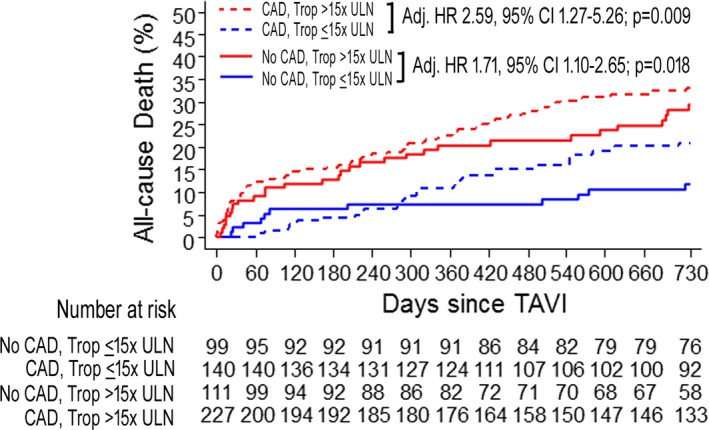
Kaplan–Meier graphs of the cumulative incidence of all‐cause mortality at 2 years in patients stratified according to presence of coronary artery disease (CAD) and according to post‐TAVI cTnT elevation >15× ULN. Adj. HR indicates adjusted hazard ratio; cTnT, cardiac troponin T; HR, hazard ratio; TAVI, transcatheter aortic valve implantation; Trop, troponin; ULN, upper limit of normal.
Table 6.
Clinical Outcomes at 30 Days and 2 Years in Relation to Presence of CAD and Post‐TAVI Troponin >15× ULN vs ≤15× ULN
| No CAD | Adjusted HR (95% CI) | Adjusted P Value | CAD | Adjusted HR (95% CI) | Adjusted P Value | |||
|---|---|---|---|---|---|---|---|---|
| cTnT ≤15× ULN n=99 | cTnT >15× ULN n=111 | cTnT ≤15× ULN n=140 | cTnT >15× ULN n=227 | |||||
| 30 days | ||||||||
| Death, n (%) | 2 (2.0) | 8 (7.2) | 2.42 (0.50–11.59) | 0.270 | 0 (0.0) | 20 (8.8) | ||
| Cardiovascular death, n (%) | 2 (2.0) | 6 (5.5) | 1.72 (0.34–8.76) | 0.512 | 0 (0.0) | 17 (7.5) | ||
| Cerebrovascular events | 3 (3.0) | 9 (8.1) | 2.41 (0.64–9.10) | 0.196 | 3 (2.1) | 6 (2.7) | 0.88 (0.21–3.70) | 0.857 |
| Death or major stroke, n (%) | 3 (3.0) | 12 (10.8) | 2.79 (0.77–10.09) | 0.118 | 1 (0.7) | 23 (10.1) | 12.70 (1.70–94.77) | 0.013 |
| Death, major stroke, or MI, n (%) | 2 (2.0) | 13 (11.7) | 4.59 (1.01–20.73) | 0.048 | 1 (0.7) | 24 (10.6) | 13.28 (1.78–98.87) | 0.012 |
| 2 years | ||||||||
| Death, n (%) | 11 (11.6) | 30 (29.5) | 2.59 (1.27–5.26) | 0.009 | 28 (20.8) | 74 (33.1) | 1.71 (1.10–2.65) | 0.018 |
| Cardiovascular death, n (%) | 6 (6.4) | 17 (17.5) | 2.39 (0.91–6.26) | 0.076 | 19 (14.4) | 54 (25.0) | 1.77 (1.04–3.01) | 0.035 |
| Cerebrovascular events | 5 (5.3) | 11 (10.8) | 1.89 (0.64–5.57) | 0.248 | 9 (7.0) | 13 (6.8) | 0.90 (0.38–2.14) | 0.818 |
| Death or major stroke, n (%) | 14 (14.8) | 35 (34.0) | 2.38 (1.26–4.50) | 0.008 | 30 (22.2) | 77 (34.4) | 1.67 (1.09–2.56) | 0.019 |
| Death, major stroke, or MI, n (%) | 12 (12.7) | 35 (33.9) | 2.81 (1.44–5.51) | 0.003 | 32 (23.7) | 82 (36.6) | 1.71 (1.13–2.58) | 0.011 |
Depicted are number of first events (% cumulative incidence), hazard ratios (HRs) with respective 95% CIs from Cox's regressions. Adjusted HRs were obtained after adjusting for age, renal dysfunctions and STS score. Age, renal dysfunctions and STS score had a P<0.2 univariable effect on the primary outcome all‐cause death at 30 days. Missing data were singly imputed (n=1 for renal dysfunction and n=1 for STS score). CAD indicates coronary artery disease; cTnT, cardiac troponin T; MI, myocardial infarction; STS, Society of Thoracic Surgeons; TAVI; transcatheter aortic valve implantation; ULN, upper limit of normal.
Exploratory Analysis: Pre‐TAVI Troponin in Relation to Outcomes
Among patients with pre‐TAVI cTnT measurement, 192 (75%) had preprocedure cTnT levels >1× ULN. Patients with versus without pre‐TAVI cTnT elevation had a higher risk of death at 30 days (HR, 9.05; 95% CI, 0.55–150.10; P=0.043) and at 2 years (HR, 2.86; 95% CI, 1.30–6.31; P=0.009; Table S7). Pre‐TAVI cTnT elevation (>1× ULN) compared with post‐TAVI myocardial injury (cTnT>15× ULN) showed inferior prognostic discrimination for 30‐day mortality (AUC, 0.71 [0.59–0.83] vs 0.79 [0.71–0.87], respectively; P=0.09 between curves), but similar performance for 2‐year mortality (AUC, 0.66 [0.58–0.74] vs 0.63 [0.56–0.67]; P=0.67 between curves).
Discussion
The main findings of the present analysis, the largest study to date assessing the prognostic implications of cTnT elevation post‐TAVI and the first to examine the impact of VARC‐2–defined myocardial injury on long‐term clinical outcomes, can be summarized as follows: (1) Postprocedural cTnT elevation above the VARC‐2 cutoff (>15× ULN) was observed in more than half of patients undergoing TAVI, it was a strong, independent predictor of 30‐day mortality, and remained a modest, but significant, predictor of mortality within 2 years after the intervention; (2) whereas myocardial injury was more common in patients with CAD, its predictive impact was consistent in patients with and without CAD; (3) occurrence of myocardial injury as well as presence and complexity of underlying CAD had a synergistic effect on prognosis, with 4‐fold higher 2‐year mortality risk in patients with SYNTAX scores >22 and post‐TAVI myocardial injury compared to those without CAD and no myocardial injury; and (4) preintervention cTnT levels above the ULN did not confer superior prognostic value compared with VARC‐2–defined postprocedure myocardial injury. Together, these findings advance previous conflicting evidence—derived from smaller studies with shorter follow‐up durations and heterogeneous cTn cutoffs—regarding the clinical significance of myocardial injury in patients undergoing TAVI.
Myocardial biomarkers increase commonly post‐TAVI.5, 6, 7, 8 Whereas procedure‐related cTn elevation predicts adverse events after coronary revascularization4, 16 as well as surgical aortic valve replacement,17 the clinical significance of increased cTn post‐TAVI has been a matter of debate. Rodes‐Cabau et al. showed that the magnitude of cTnT elevation was related to cardiac, but not all‐cause, mortality in 101 patients followed for 9±10 months (with 46 of those patients having ≥6‐month follow‐up).5 Similarly, Yong et al. found that myocardial injury, defined as increase of creatine phosphokinase‐MB (CK‐MB) and/or cTnT >5× ULN, independently predicted 30‐day mortality.6 In sharp contrast, other studies did not confirm the predictive utility of any cTn elevation9 or of cTn increase >15× ULN.8 These conflicting outcomes may be related to small sample sizes, low numbers of events, use of different cTn isoforms, T5, 6 or I,8 which have shown dissimilar prognostic performance in different clinical settings,18 or heterogeneity of cTn cutoffs to define myocardial injury—although findings were discordant even between the 2 studies that applied the same VARC‐2–defined threshold.5, 8
Against this background, the present study of 577 consecutive patients demonstrates a significant association between VARC‐2–defined cTnT elevation and clinical outcomes. The mortality risk was particularly pronounced within the early postprocedural period, but persisted over time and remained significant over 2 years, including a landmark analysis between 30 days and 2 years. Importantly, these results held true in sensitivity analyses excluding potential confounders of cTnT elevation (ie, renal dysfunction), concomitant PCI, and transapical intervention. It is important to note that the predictive merit was only modest for 2‐year outcomes; hence, it needs to be viewed in conjunction with established, stronger risk predictors. The AUC for 2‐year mortality in this study is similar to that reported previously with regard to 9‐month survival.5 The reasonably high specificity, but low sensitivity, further indicate that the presence, but not the absence, of myocardial injury may be prognostically meaningful. Patients with evidence of periprocedural myocardial injury have considerable likelihood to experience adverse outcomes; in contrast, absence of myocardial injury does not preclude an unfavorable subsequent prognosis in a substantial proportion of such patients.
The cutoff to define procedural myocardial injury post‐TAVI increased form 10× ULN in the VARC consensus document19 to 15× ULN in the VARC‐2 definition,11 largely based on the study by Rodes‐Cabau et al., who found that this higher cutoff better predicted adverse outcomes.5 By associating cTnT elevation >15× ULN with 10‐fold higher mortality at 30 days and 2‐fold increased mortality at 2 years, the present study supports the current VARC‐2 definition as a clinically meaningful, prognostically relevant cutoff of post‐TAVI cTn increase. Unlike elective PCI procedures, where cTn cutoffs higher than the respective diagnostic thresholds for periprocedural MI enhance prognostication,20 in this cohort of TAVI patients, greater magnitudes of cTnT elevation did not provide incremental prognostic information compared with the VARC‐2–defined cutoff.
Coronary atherosclerosis frequently coexists in patients with AS and adversely affects TAVI outcomes.10, 21 This study is the first to assess the interrelated impact of myocardial injury and underlying CAD on patient prognosis. We found that the association of VARC‐2 myocardial injury with 2‐year outcomes was consistent, irrespective of coronary status. Moreover, by quantifying the complexity of CAD by means of the SYNTAX score—an established angiographic tool for this purpose—we were able to identify patients with lowest mortality post‐TAVI (ie, patients without CAD and no myocardial injury) versus 4‐fold higher risk (those with SYNTAX score >22 and myocardial injury). Previous analyses focusing solely on CAD complexity reported 1.6‐fold higher death and 2.4‐fold higher cardiac death rates post‐TAVI in patients with SYNTAX score >22 versus patients without CAD.10 Thereby, our findings advance previous evidence of an escalating risk of adverse TAVI outcomes across increasingly complex CAD10, 22 by indicating that detection of procedure‐related cTnT elevation substantially refines risk stratification in this setting.
The mechanisms of troponin release during TAVI are likely multifactorial. First, direct myocardial damage is induced by mechanical compression of the left ventricular outflow tract during balloon dilatation and valve implantation and—to a greater extent—by myocardial puncture during transapical interventions. Second, some,23 but not all, mechanistic studies employing magnetic resonance imaging24 pointed to the primary role of distal embolization of calcium microparticles into the coronaries during valvuloplasty and prosthesis implantation—a mechanism that is consistent with preclinical evidence25 as well as evidence of cerebrovascular microemboli post‐TAVI.26 Third, temporary hypotension during rapid pacing or balloon predilatation, excessive bradycardia because of conduction abnormalities, or, conversely, tachycardia in the setting of inotropic hemodynamic support can result in myocardial ischemia attributed to hypoperfusion of the typically hypertrophic myocardium. Along these lines, it is reasonable to assume that myocardial supply‐demand mismatch during the intervention is exacerbated, and thus cTn release is augmented in the presence of obstructive coronary lesions; this is supported by our findings of more‐frequent myocardial injury in patients with CAD, particularly multivessel disease. Previous studies have been discordant by showing no difference of CAD status5, 6 or higher prevalence of CAD in patients with TAVI‐related myocardial injury.8
Relevant to the aforementioned mechanisms, measures that could theoretically attenuate myocardial injury during TAVI include optimization of hemodynamic support to avoid prolonged episodes of hypotension, shortening of rapid pacing, cautious use of balloon dilatation (clearly to the extent that is anatomically and clinically feasible), appropriate balloon and device sizing to minimize direct myocardial injury, possibly revascularization before TAVI procedures, and ensuring that transapical interventions are reserved only for patients who absolutely cannot undergo transarterial TAVI. Dedicated studies would be required to assess the impact of beta‐blockers or medications with anti‐ischemic properties on the ensuing myocardial injury and to determine possible clinical benefits.
One can speculate on the mechanistic relationship between myocardial injury and adverse prognosis. The fact that CAD was more common in patients with versus without myocardial injury may, in part, be a link between more‐extensive troponin release in the acute phase and impaired survival in the long term. Moreover, microvascular dysfunction—which is common in the presence of long‐standing AS,27 is related to ischemia‐induced arrhythmias and ominous prognosis irrespective of epicardial CAD28 and is only in part reversible after replacement of the stenotic valve29—may have been more pronounced in patients (with or without CAD) with myocardial injury, but this remains purely speculative. Although this observational study cannot elucidate mechanisms responsible for unfavorable outcomes in patients with profound cTn elevation, our findings are consistent with previous investigations with short‐ and mid‐term follow‐up5, 6, 7 and are corroborated further by the large, consecutive sample size.
In the setting of PCI, preintervention cTn levels better predict outcomes compared with post‐PCI cTn rise.30 While minor cTn elevation provides prognostic information in patients with severe AS treated medically or with surgical valve replacement,31 the relative value of pre‐TAVI cTn elevation versus post‐TAVI myocardial injury is unknown. In this study, minor pre‐TAVI cTnT elevation above the ULN was observed in three quarters of patients and did not outperform the predictive merit of post‐TAVI increase >15× ULN. Although this exploratory analysis is limited by the availability of pre‐TAVI cTnT measurements in only half of all patients, it represents the largest cohort to date assessing the impact of baseline cTn levels on subsequent TAVI outcomes.
This study advances current evidence of the clinical significance of myocardial injury in TAVI in several ways. First, given the conflicting results of previous smaller studies regarding the presence5, 6 of absence8, 9 of a prognostic value of myocardial injury, this study—with a population larger than the sum of all previous reports—indicates that troponin elevation is a strong predictor of 30‐day mortality and remains a significant, albeit modest, predictor at 2 years. Second, against a background of discordant findings regarding the current VARC‐2 definition of myocardial injury,5, 8 this analysis provides validation of the VARC‐2 cutoff over other possible values as a meaningful threshold for patient prognostication. Third, given the established adverse impact of concomitant CAD on TAVI outcomes,10, 21 we demonstrate that risk stratification can be enhanced substantially by means of combined assessment of CAD complexity and periprocedural troponin elevation. Fourth, the observation that—unlike PCI30—preintervention troponin does not outperform the prognostic value of post‐TAVI troponin elevation is a novel finding that merits further investigation.
Study Limitations
This study has several limitations owing to its observational nature. First, although all data were prospectively collected, this is a retrospective, single‐center analysis and is subject to the limitations common to such analyses. Second, cTnT was not measured at prespecified time points postintervention; however, cTnT was measured in all patients at 12 hours and again at 24 hours and later in case of increased 12‐hour values (ie, in 96.2% of patients); hence, it is unlikely that peak cTnT values were not recorded in this population. Third, the mechanisms responsible for the association of cTnT elevation with subsequent outcomes cannot be fully elucidated in this clinical observational study; dedicated mechanistic studies are more suited to address this issue.23, 24, 32
Conclusions
VARC‐2–defined cTnT elevation emerged as an independent predictor of 30‐day mortality and remained a modest, but significant, predictor of 2‐year mortality post‐TAVI. Presence of troponin elevation appears to be prognostically relevant, whereas absence of myocardial injury does not preclude an adverse prognosis, as reflected by a reasonably high specificity but low sensitivity. Complexity of underlying CAD modified the prognostic value of myocardial injury, with highest mortality risk in patients combining SYNTAX score >22 and troponin elevation. These findings may have implications for refining risk stratification in patients treated with TAVI and for employing preventive measures to reduce procedural myocardial injury during TAVI interventions.
Disclosures
Stephan Windecker reports having received research grants to the institution from Abbott, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic, Medicines Company, and St Jude and speaker fees from Astra‐Zeneca, Eli Lilly, Abbott, Biotronik, Boston Scientific, Bayer, and Biosensors. Peter Wenaweser has received honoraria and lecture fees from Medtronic and Edwards Lifesciences. The other authors have nothing to report.
Supporting information
Table S1. Uni‐ and Multivariate Predictors of Post‐TAVI Peak Troponin Levels
Table S2. Invasive Hemodynamic Characteristics in Patients With or Without Periprocedural Myocardial Injury
Table S3. Predictors of 30‐Day Mortality
Table S4. Sensitivity Analysis of 2‐Year Clinical Outcomes in Relation to Post‐TAVI Troponin >15× ULN vs ≤15× ULN Including Patients Without Renal Dysfunction, Defined as Estimated Glomerular Filtration Rate <60 mL/min per 1.73 m2 (n=181)
Table S5. Sensitivity Analysis of 2‐Year Clinical Outcomes in Relation to Post‐TAVI Troponin >15× ULN vs ≤15× ULN Including Patients Who Did Not Undergo Concomitant PCI (n=486)
Table S6. Sensitivity Analysis of 2‐Year Clinical Outcomes in Relation to Post‐TAVI Troponin >15× ULN vs ≤15× ULN Including Patients Who Did Not Undergo TAVI by Trans‐Apical Route PCI (n=471)
Table S7. Clinical Outcomes at 30 Days and 2 Years in Relation to Pre‐TAVI Troponin >1× ULN vs ≤1× UNL
Figure S1. Cumulative event curves for all‐cause mortality within 2 years in patients with or without post‐TAVI troponin T elevation >15× ULN in the subgroups of patients without renal dysfunction (A) and without concomitant PCI during the TAVI procedure (B).
(J Am Heart Assoc. 2016;5:e002430 doi: 10.1161/JAHA.115.002430)
Accompanying Tables S1 through S7 and Figure S1 are available at http://jaha.ahajournals.org/content/5/2/e002430/suppl/DC1
References
- 1. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Jr , Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK; U.S. CoreValve Clinical Investigators . Transcatheter aortic‐valve replacement with a self‐expanding prosthesis. N Engl J Med. 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 2. Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB; PARTNER Trial Investigators . Two‐year outcomes after transcatheter or surgical aortic‐valve replacement. N Engl J Med. 2012;366:1686–1695. [DOI] [PubMed] [Google Scholar]
- 3. Xiong TY, Liao YB, Zhao ZG, Xu YN, Wei X, Zuo ZL, Li YJ, Cao JY, Tang H, Jilaihawi H, Feng Y, Chen M. Causes of death following transcatheter aortic valve replacement: a systematic review and meta‐analysis. J Am Heart Assoc. 2015;4:e002096 doi: 10.1161/JAHA.115.002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prasad A, Singh M, Lerman A, Lennon RJ, Holmes DR, Jr , Rihal CS. Isolated elevation in troponin T after percutaneous coronary intervention is associated with higher long‐term mortality. J Am Coll Cardiol. 2006;48:1765–1770. [DOI] [PubMed] [Google Scholar]
- 5. Rodes‐Cabau J, Gutiérrez M, Bagur R, De Larochellière R, Doyle D, Côté M, Villeneuve J, Bertran OF, Larose E, Manazzoni J, Pibarot P, Dumont E. Incidence, predictive factors, and prognostic value of myocardial injury following uncomplicated transcatheter aortic valve implantation. J Am Coll Cardiol. 2011;57:1988–1999. [DOI] [PubMed] [Google Scholar]
- 6. Yong ZY, Wiegerinck EM, Boerlage‐van Dijk K, Koch KT, Vis MM, Bouma BJ, Henriques JP, Cocchieri R, Piek JJ, de Mol BA, Baan J, Jr . Predictors and prognostic value of myocardial injury during transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2012;5:415–423. [DOI] [PubMed] [Google Scholar]
- 7. Barbash IM, Dvir D, Ben‐Dor I, Badr S, Okubagzi P, Torguson R, Corso PJ, Xue Z, Satler LF, Pichard AD, Waksman R. Prevalence and effect of myocardial injury after transcatheter aortic valve replacement. Am J Cardiol. 2013;111:1337–1343. [DOI] [PubMed] [Google Scholar]
- 8. Sinning JM, Hammerstingl C, Schueler R, Neugebauer A, Keul S, Ghanem A, Mellert F, Schiller W, Müller C, Vasa‐Nicotera M, Zur B, Welz A, Grube E, Nickenig G, Werner N. The prognostic value of acute and chronic troponin elevation after transcatheter aortic valve implantation. EuroIntervention. 2015; doi: 10.4244/EIJY15M02_02. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9. Carrabba N, Valenti R, Migliorini A, Vergara R, Parodi G, Antoniucci D. Prognostic value of myocardial injury following transcatheter aortic valve implantation. Am J Cardiol. 2013;111:1475–1481. [DOI] [PubMed] [Google Scholar]
- 10. Stefanini GG, Stortecky S, Cao D, Rat‐Wirtzler J, O'Sullivan CJ, Gloekler S, Buellesfeld L, Khattab AA, Nietlispach F, Pilgrim T, Huber C, Carrel T, Meier B, Jüni P, Wenaweser P, Windecker S. Coronary artery disease severity and aortic stenosis: clinical outcomes according to SYNTAX score in patients undergoing transcatheter aortic valve implantation. Eur Heart J. 2014;35:2530–2540. [DOI] [PubMed] [Google Scholar]
- 11. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés‐Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. [DOI] [PubMed] [Google Scholar]
- 12. O'Sullivan CJ, Stortecky S, Heg D, Pilgrim T, Hosek N, Buellesfeld L, Khattab AA, Nietlispach F, Moschovitis A, Zanchin T, Meier B, Windecker S, Wenaweser P. Clinical outcomes of patients with low‐flow, low‐gradient, severe aortic stenosis and either preserved or reduced ejection fraction undergoing transcatheter aortic valve implantation. Eur Heart J. 2013;34:3437–3450. [DOI] [PubMed] [Google Scholar]
- 13. Wenaweser P, Pilgrim T, Kadner A, Huber C, Stortecky S, Buellesfeld L, Khattab AA, Meuli F, Roth N, Eberle B, Erdös G, Brinks H, Kalesan B, Meier B, Jüni P, Carrel T, Windecker S. Clinical outcomes of patients with severe aortic stenosis at increased surgical risk according to treatment modality. J Am Coll Cardiol. 2011;58:2151–2162. [DOI] [PubMed] [Google Scholar]
- 14. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. [DOI] [PubMed] [Google Scholar]
- 15. Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, Serruys PW. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 16. Eigel P, van Ingen G, Wagenpfeil S. Predictive value of perioperative cardiac troponin I for adverse outcome in coronary artery bypass surgery. Eur J Cardiothorac Surg. 2001;20:544–549. [DOI] [PubMed] [Google Scholar]
- 17. Dehoux M, Provenchere S, Benessiano J, Lasocki S, Lecharny JB, Bronchard R, Dilly MP, Philip I. Utility of cardiac troponin measurement after cardiac surgery. Clin Chim Acta. 2001;311:41–44. [DOI] [PubMed] [Google Scholar]
- 18. Haaf P, Reichlin T, Twerenbold R, Hoeller R, Rubini Gimenez M, Zellweger C, Moehring B, Fischer C, Meller B, Wildi K, Freese M, Stelzig C, Mosimann T, Reiter M, Mueller M, Hochgruber T, Sou SM, Murray K, Minners J, Freidank H, Osswald S, Mueller C. Risk stratification in patients with acute chest pain using three high‐sensitivity cardiac troponin assays. Eur Heart J. 2014;35:365–375. [DOI] [PubMed] [Google Scholar]
- 19. Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Krucoff MW, Mack M, Mehran R, Miller C, Morel MA, Petersen J, Popma JJ, Takkenberg JJ, Vahanian A, van Es GA, Vranckx P, Webb JG, Windecker S, Serruys PW. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–269. [DOI] [PubMed] [Google Scholar]
- 20. Herrmann J, Lennon RJ, Jaffe AS, Holmes DR, Jr , Rihal CS, Prasad A. Defining the optimal cardiac troponin T threshold for predicting death caused by periprocedural myocardial infarction after percutaneous coronary intervention. Circ Cardiovasc Interv. 2014;7:533–542. [DOI] [PubMed] [Google Scholar]
- 21. Wenaweser P, Pilgrim T, Guerios E, Stortecky S, Huber C, Khattab AA, Kadner A, Buellesfeld L, Gloekler S, Meier B, Carrel T, Windecker S. Impact of coronary artery disease and percutaneous coronary intervention on outcomes in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. EuroIntervention. 2011;7:541–548. [DOI] [PubMed] [Google Scholar]
- 22. Khawaja MZ, Asrress KN, Haran H, Arri S, Nadra I, Bolter K, Wilson K, Clack L, Hancock J, Young CP, Bapat V, Thomas M, Redwood S. The effect of coronary artery disease defined by quantitative coronary angiography and SYNTAX score upon outcome after transcatheter aortic valve implantation (TAVI) using the Edwards bioprosthesis. EuroIntervention. 2014;11:450–455. [DOI] [PubMed] [Google Scholar]
- 23. Kim WK, Rolf A, Liebetrau C, Van Linden A, Blumenstein J, Kempfert J, Bachmann G, Nef H, Hamm C, Walther T, Möllmann H. Detection of myocardial injury by CMR after transcatheter aortic valve replacement. J Am Coll Cardiol. 2014;64:349–357. [DOI] [PubMed] [Google Scholar]
- 24. Ribeiro HB, Larose É, de la Paz Ricapito M, Le Ven F, Nombela‐Franco L, Urena M, Allende R, Amat‐Santos I, Dahou A, Capoulade R, Clavel MA, Mohammadi S, Paradis JM, De Larochellière R, Doyle D, Dumont É, Pibarot P, Rodés‐Cabau J. Myocardial injury following transcatheter aortic valve implantation: insights from delayed‐enhancement cardiovascular magnetic resonance. EuroIntervention. 2015;11:205–213. [DOI] [PubMed] [Google Scholar]
- 25. Haberthür D, Lutter G, Appel M, Attmann T, Schramm R, Schmitz C, Quaden RB. Percutaneous aortic valve replacement: valvuloplasty studies in vitro. Eur J Cardiothorac Surg. 2011;39:631–634. [DOI] [PubMed] [Google Scholar]
- 26. Ghanem A, Müller A, Nähle CP, Kocurek J, Werner N, Hammerstingl C, Schild HH, Schwab JO, Mellert F, Fimmers R, Nickenig G, Thomas D. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion‐weighted magnetic resonance imaging. J Am Coll Cardiol. 2010;55:1427–1432. [DOI] [PubMed] [Google Scholar]
- 27. Rajappan K, Rimoldi OE, Dutka DP, Ariff B, Pennell DJ, Sheridan DJ, Camici PG. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation. 2002;105:470–476. [DOI] [PubMed] [Google Scholar]
- 28. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 29. Rajappan K, Rimoldi OE, Camici PG, Bellenger NG, Pennell DJ, Sheridan DJ. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis. Circulation. 2003;107:3170–3175. [DOI] [PubMed] [Google Scholar]
- 30. Prasad A, Rihal CS, Lennon RJ, Singh M, Jaffe AS, Holmes DR, Jr . Significance of periprocedural myonecrosis on outcomes after percutaneous coronary intervention: an analysis of preintervention and postintervention troponin T levels in 5487 patients. Circ Cardiovasc Interv. 2008;1:10–19. [DOI] [PubMed] [Google Scholar]
- 31. Røsjø H, Andreassen J, Edvardsen T, Omland T. Prognostic usefulness of circulating high‐sensitivity troponin T in aortic stenosis and relation to echocardiographic indexes of cardiac function and anatomy. Am J Cardiol. 2011;108:88–91. [DOI] [PubMed] [Google Scholar]
- 32. Kahlert P, Al‐Rashid F, Plicht B, Wild C, Westhölter D, Hildebrandt H, Baars T, Neumann T, Nensa F, Nassenstein K, Wendt D, Thielmann M, Jakob H, Kottenberg E, Peters J, Erbel R, Heusch G. Myocardial injury during transfemoral transcatheter aortic valve implantation: an intracoronary Doppler and cardiac magnetic resonance imaging study. EuroIntervention. 2015; doi: 10.4244/EIJY15M05_10. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Uni‐ and Multivariate Predictors of Post‐TAVI Peak Troponin Levels
Table S2. Invasive Hemodynamic Characteristics in Patients With or Without Periprocedural Myocardial Injury
Table S3. Predictors of 30‐Day Mortality
Table S4. Sensitivity Analysis of 2‐Year Clinical Outcomes in Relation to Post‐TAVI Troponin >15× ULN vs ≤15× ULN Including Patients Without Renal Dysfunction, Defined as Estimated Glomerular Filtration Rate <60 mL/min per 1.73 m2 (n=181)
Table S5. Sensitivity Analysis of 2‐Year Clinical Outcomes in Relation to Post‐TAVI Troponin >15× ULN vs ≤15× ULN Including Patients Who Did Not Undergo Concomitant PCI (n=486)
Table S6. Sensitivity Analysis of 2‐Year Clinical Outcomes in Relation to Post‐TAVI Troponin >15× ULN vs ≤15× ULN Including Patients Who Did Not Undergo TAVI by Trans‐Apical Route PCI (n=471)
Table S7. Clinical Outcomes at 30 Days and 2 Years in Relation to Pre‐TAVI Troponin >1× ULN vs ≤1× UNL
Figure S1. Cumulative event curves for all‐cause mortality within 2 years in patients with or without post‐TAVI troponin T elevation >15× ULN in the subgroups of patients without renal dysfunction (A) and without concomitant PCI during the TAVI procedure (B).


