Abstract
Background
Patients with congenital heart disease (CHD) may be at increased risk of ischemic stroke due to residual shunts, arrhythmias, and other cardiovascular abnormalities. We studied the relative risk and potential factors for developing ischemic stroke in children and young adults with CHD in Sweden.
Methods and Results
All patients in the Swedish Patient Register with a diagnosis of CHD, born between 1970 and 1993, were identified and compared with 10 controls for each patient, matched for age, sex, and county and randomly selected from the general population. Follow‐up data through 2011 were collected for both groups. Of 25 985 children and young adults with CHD (51.5% male, 48.5% female), 140 (0.5%) developed ischemic stroke. The hazard ratio for CHD patients developing ischemic stroke was 10.8 (95% CI, 8.5–13.6) versus controls. All major Marelli groups had significantly increased risk, but because of small CHD‐group sizes, only atrial septal defect/patent foramen ovale, double‐inlet ventricle, and aortic coarctation displayed significantly increased risk. In multivariate analysis of CHD patients, congestive heart failure carried the highest risk for developing ischemic stroke (hazard ratio 6.9 [95% CI, 4.7–10.3]), followed by hypertension and atrial fibrillation, which were also significantly associated with increased risk of ischemic stroke.
Conclusions
The risk of developing ischemic stroke was almost 11 times higher in young patients with CHD than in the general population, although absolute risk is low. Cardiovascular comorbidities were strongly associated with the development of ischemic stroke in young CHD patients.
Keywords: epidemiology; heart defects, congenital; registry; stroke
Subject Categories: Epidemiology, Congenital Heart Disease, Ischemic Stroke
Introduction
Stroke is a leading cause of death and a major cause of adult disability in developed countries.1 Ischemic stroke has been shown to be relatively uncommon in children and young adults compared with older adults.2 However, recent studies have shown that the incidence of stroke has decreased among the elderly but increased in the young population.3, 4 Traditional risk factors for ischemic stroke, such as hypertension, diabetes mellitus, and atrial fibrillation, are usually absent in children and young adults.5
Congenital heart disease (CHD) is present in about 1% of live births and is the most common congenital malformation.6 CHD is a major risk factor for ischemic stroke and stroke recurrence in children.7, 8, 9 Patients with CHD represent about 20% of all strokes among children,10 and cardiac surgery in children with CHD has been shown to be associated with increased stroke risk.9, 11 Previous studies have identified a strong association between cyanotic CHD and cerebral lesions,12, 13 whereas a later report has failed to demonstrate any correlation.14 These conflicting results may be the result of more frequent surgical repair, less use of potentially harmful treatments such as phlebotomy, or use of better thromboprophylaxis.15
Interestingly, a recent study showed a relatively low incidence of stroke in adults with CHD, but the risk was still estimated to be 10 to 100 times greater in CHD patients than in a healthy general population.16 Furthermore, a case–control study from Taiwan showed a 2.2 times increased risk of developing stroke in adults with 5 of the most common types of CHD compared to controls.17
Given the increasing number of patients with CHD who are now reaching adulthood, a large number of patients will now live with their condition for a long period. Thus, even a small relative increase in risk of stroke may, if present for a long time, result in significant total lifetime risk. Furthermore, CHD patients represent a conglomerate of patients with very mild residual abnormalities, moderate but well‐corrected disease, and patients with residual shunts or complex morphology. Each of these groups will be limited in number, highlighting the need for very large studies to identify risks that may be severely underestimated in a smaller study over a limited period of time.
Sweden (current population 9.7 million) has an inpatient register with almost complete coverage of thoracic procedures since 1970. The primary objective of the present study was to investigate the absolute and relative risks of ischemic stroke in children and young adults with CHD in Sweden. We also investigated possible risk factors and comorbidities associated with the development of ischemic stroke in young CHD patients.
Methods
Study Population
Residents in Sweden have a unique national 10‐digit registration number that includes their sex and date of birth. Since 1987 it has been mandatory for all hospital physicians to deliver data to the Swedish National Inpatient register. The 6 Swedish cardiothoracic surgery clinics have registered all procedures and hospitalizations since 1970, and data from the Hospital Outpatient register from 2000 are available. In addition, all deaths have been reported to the national register of Cause‐Specific Deaths since 1961. All diagnoses are coded according to the International Classification of Disease (ICD) 8th, 9th, and 10th editions. Swedish versions of the ICD were used, and diagnoses in the present study that were originally coded using ICD‐8 were re‐coded to ICD‐9 to reflect the 1987 revision.
We identified 26 976 patients born between January 1970 and December 1993 who had a diagnosis of CHD at any time during this period registered in the National Hospital Inpatient register, Outpatient Register, or the National Cause‐of‐Death register. Follow‐up and comorbidity data through December 2011 were collected for all patients. Patients were included in the study at the date of their first registration with a diagnosis of CHD and were followed until a first diagnosis of ischemic stroke, until death, or until the end of the study, December 31, 2011.
For each CHD patient, 10 control individuals without a diagnosis of CHD or previous ischemic stroke were selected from the Total Population Register in Sweden, matched by age (calendar year of diagnosis), sex, and county. However, for 14 CHD patients only 9 controls could be matched.
Atrial shunts such as atrial septal defect (ASD) and patent foramen ovale (PFO) have the same ICD diagnosis code; however, PFO is commonly diagnosed at the time of ischemic stroke during adulthood.18, 19 To avoid overestimation of ischemic stroke in this group of patients, where a PFO might have gone undiagnosed if a stroke had not occurred, we excluded all patients who were diagnosed with ASD and PFO (n=554) after the age of 18 years as well as their matched controls. Patients diagnosed with ischemic stroke before or on the same day as the first registration with a CHD diagnosis (n=118) and patients who died the same day they were registered with CHD (n=319) as well as their controls were also excluded from the present study. Controls with prior ischemic stroke were also excluded (n=75).
To characterize our CHD cohort further, we used the hierarchic CHD categorization recently published by Marelli et al, which has been widely accepted.20
Educational levels of the patients and controls were extracted from the Longitudinal Integration Database for Health Insurance and Labor Market Studies.21, 22 Education was classified as low (compulsory only), medium, or high (university level or similar).
Definitions
CHD was defined as any patient with at least one outpatient visit, or a hospital discharge, or a death certificate with any ICD code, as shown in Table 1. Ischemic stroke was defined as codes 434 or 436 (ICD‐8 and ICD‐9), or I63 or I64 (ICD‐10). Atrial fibrillation was defined as codes 427.92 (ICD‐8), 427D (ICD‐9), or I48 (ICD‐10). Hypertension was defined as codes 401‐405 (ICD‐8 and ICD‐9) or I10‐I15 (ICD‐10). Congestive heart failure was defined as codes 427.00 (ICD‐8) or 428 (ICD‐9) or I50 (ICD‐10). Diabetes mellitus was defined as codes 250 (ICD‐8 and ICD‐9) or E10‐14 (ICD‐10). Operations on the cardiovascular system were classified as codes 30–32 (Classification of Operations, Swedish 6th version), or F codes (Classification of Surgical Procedures version 1.9). The perioperative period was defined as the interval between the date of operation or intervention and 3 months later.
Table 1.
International Classification of Disease (ICD) Diagnosis
| Diagnosis | ICD 8 | ICD 9a | ICD 10 |
|---|---|---|---|
| Common arterial trunk | 745.0 | 745A | Q20.0 |
| Transposition of great vessels | 745.1 | 745B | Q20.3 |
| Tetralogy of Fallot | 745.2 | 745C | Q21.3 |
| Ventricular septal defect | 745.4 | 745E | Q21.0 |
| Atrial septal defect or patent foramen ovale | 745.5 | 745F | Q21.1 |
| Congenital tricuspid stenosis or atresia | 746.1 | 746B | Q22.4 |
| Ebstein's anomaly | 746.2 | 746C | Q22.5 |
| Congenital stenosis of aortic valve | 746.3 | 746D | Q23.0 |
| Congenital insufficiency of aortic valve | 746.4 | 746E | Q23.1 |
| Congenital mitral stenosis | 746.5 | 746F | Q23.2 |
| Congenital mitral insufficiency | 746.6 | 746G | Q23.3 |
| Hypoplastic left heart syndrome | 746.7 | 746H | Q23.4 |
| Congenital subaortic stenosis | 746.81 | 746W | Q24.4 |
| Cor triatriatum | 746.82 | 746W | Q24.2 |
| Infundibular pulmonic stenosis | 746.83 | 746W | Q24.3 |
| Congenital coronary vessel anomalies | 746.85 | 746W | Q24.5 |
| Congenital heart block | 746.86 | 746W | Q24.6 |
| Coarctation of aorta | 747.1 | 747B | Q25.1 |
| Interruption of aortic arch (atresia or stenosis of aorta) | 747.11 | 747B | Q25.2, Q25.3 |
| Other unspecified congenital malformations of aorta | 747.2 | 747C | Q25.4, Q25.8, Q25.9 |
| Congenital malformations of pulmonary artery | 747.3 | 747D | Q25.5 to Q25.7 |
| Congenital malformations of great veins | 747.4 | 747E | Q26 |
| Cor biloculare | 745.7 | 745H | Q20.8 |
| Double outlet right ventricle | 745.11 | 745B | Q20.1 |
| Double outlet left ventricle | 745.19 | 745B | Q20.2 |
| Double inlet ventricle | 745.3 | 745D | Q20.4 |
| Discordant atrioventricular connection | 745.12 | 745B | Q20.5 |
| Isomerism of atrial appendages | 745.8 | 745W | Q20.6 |
| Unspecified congenital malformations of cardiac chambers | 746.9 | 746X | Q20.8, Q20.9 |
| Atrioventricular septal defect | 745.6 | 745G | Q21.2 |
| Aortopulmonary septum defect | 745.8 | 745W | Q21.4 |
| Other congenital malformations of cardiac septum | 745.8 | 745W | Q21.8 |
| Unspecified congenital malformations of cardiac septum | 745.9 | 745X | Q21.9 |
| Pulmonary valve atresia | 746.01 | 746A | Q22.0 |
| Congenital stenosis of pulmonary valve | 746.02 | 746A | Q22.1 |
| Congenital pulmonary valve insufficiency | 746.09 | 746A | Q22.2 |
| Other congenital malformations of pulmonary valve | 746.00 | 746A | Q22.3 |
| Hypoplastic right heart syndrome | 746.1 | 746B | Q22.6 |
| Other congenital malformations of tricuspid valve | 746.1 | 746B | Q22.8, Q22.9 |
| Other congenital malformations of aortic and mitral valves | 746.89 | 746W | Q23.8, Q23.9 |
| Congenital phlebectasia | 747.89 | 747G | Q27.4 |
| Other specified congenital malformations of the heart | 746.89 | 746W | Q24.8 |
| Unspecified congenital malformations of the heart | 746.9 | 746X | Q24.9 |
| Patent ductus arteriosus | 747.0 | 747A | Q25.0 |
| Unspecified congenital malformations of circulation | 747.9 | 747X | Q28.9 |
| Sequestration of lung | 748.5 | 748F | Q33.2 |
| Secondary hypertension | 405 | 405 | I15.8, I15.9 |
| Vitium organicum cordis (VOC) | 421, 424 | 421, 424 | I33 to 37 |
Swedish version of ICD 9.
Ethics
All national registration numbers were removed and replaced with a code in the final data set by the National Board of Health and Welfare in Sweden. Accordingly, informed consent for this investigation could not be provided and was therefore waived. The study complied with the Declaration of Helsinki and was approved by the Gothenburg Regional Research Ethics Board.
Statistics
We used SAS® software, Version 9.4 (SAS Institute, Inc, Cary, NC) and R software, Version 3.1 (R Foundation for Statistical Computing, Vienna, Austria) to perform all statistical analyses. Hazard ratios (HRs) and 95% CIs estimated from the fitted regression models are presented. Only 2‐sided P‐values were used. A P value <0.05 was considered statistically significant.
To estimate the HR of experiencing ischemic stroke during follow‐up in patients versus matched controls, we used a stratified version of Cox regression. The proportional hazard assumption was checked through the inspection of Schoenfeld residuals. The strata in the analyses were defined by age, sex, and the year of diagnosis. These criteria were also used to match 10 random controls from the population with each patient. The time scale in our analyses was age of the first registration with a CHD diagnosis, and the time zero was not the same for all participants; as such, it was left truncated—not all patients start their follow‐up at birth—a fact we corrected for in our models. Cox regression was also used when estimating HRs for factors such as sex, educational level, and comorbidities with ischemic stroke as an end point. The incidence rates of ischemic stroke were estimated as the count of the first case of ischemic stroke divided by the total person‐time at risk. The comorbidities were measured at any time prior to CHD and including at the baseline visit or hospitalization (Table 2).
Table 2.
Sociodemographic Data and Comorbidities of the Study Population
| Characteristic | Patients With Congenital Heart Disease n=25 985 | Controls n=259 750 |
|---|---|---|
| Women, n (%) | 12 602 (48.5) | 125 946 (48.5) |
| Median age at index registration (IQR), y | 5.7 | 5.7 |
| Median age at end of study period (IQR), years | 28.6 | 29.5 |
| Born in Sweden, n (%) | 24 006 (92.4) | 241 169 (92.9) |
| Education category at last registration a | ||
| Low | 5190 (22.8) | 49 586 (19.6) |
| Mid | 10 685 (46.9) | 117 343 (46.4) |
| High | 6885 (30.3) | 86 075 (34.0) |
| Registration at the baseline (any diagnostic position), n (%) | ||
| Hypertension | 1836 (7.1) | 1412 (0.5) |
| Atrial fibrillation | 635 (2.4) | 431 (0.2) |
| Congestive heart failure | 1325 (5.1) | 160 (0.06) |
| Diabetes | 384 (1.5) | 2065 (0.8) |
IQR indicates interquartile range.
Highest attained education level during the study.
Figures 1 and 2 and Figure S1 show the cumulative incidence of ischemic stroke in the study population, respectively, for every Marelli group and was based on the Nelson‐Aalen estimator of the cumulative hazard. The time of censoring is December 31, 2011, and death was the competing event.
Figure 1.
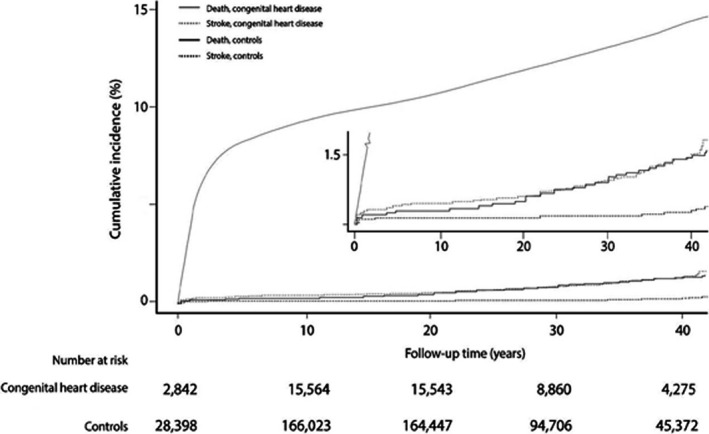
Cumulative incidence of ischemic stroke in patients with congenital heart disease and controls for up to 42 years of follow‐up.
Figure 2.
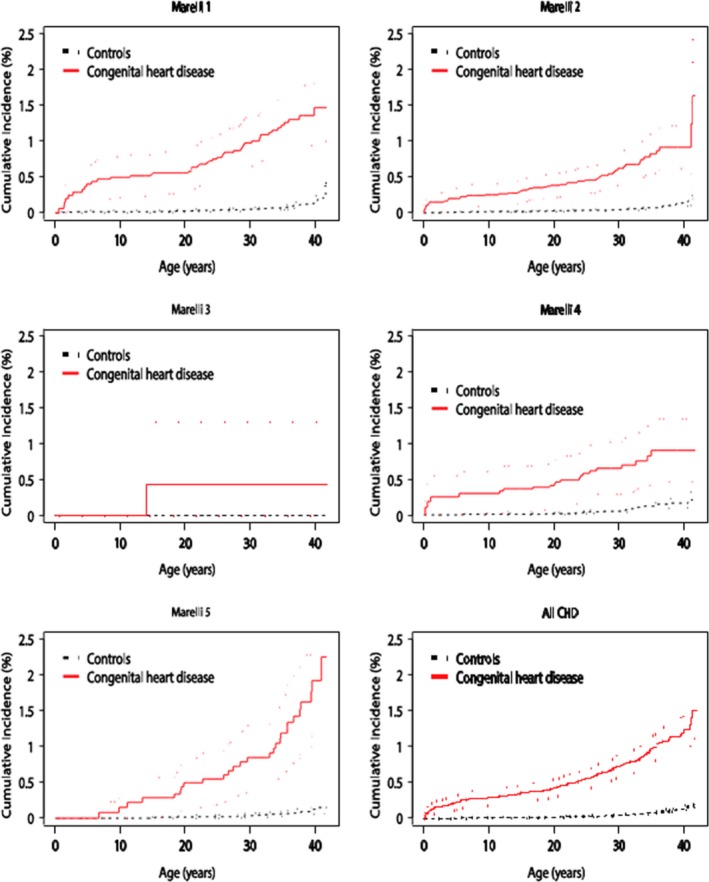
Cumulative incidence of ischemic stroke in overall and Marelli groups of patients with congenital heart disease (CHD) and controls for up to 42 years of age.
Results
A total of 25 985 CHD patients and 259 750 controls were included in the study (48.5% women). The characteristics of the study population are shown in Table 2. The mean and median ages at CHD diagnosis were 9.51 and 5.74 years, respectively; mean follow‐up was 20.1 years. Educational level and proportion of individuals born in Sweden were similar among CHD patients and controls. However, diabetes and cardiovascular diseases such as congestive heart failure, hypertension, and atrial fibrillation at any time point were more common in CHD patients than in controls.
One hundred forty CHD patients (0.54%) and 137 controls (0.05%) developed ischemic stroke during a maximum of 42 years of follow‐up. Median age at the time of ischemic stroke diagnosis was 23.1 years in CHD patients versus 30.5 years in controls. The HR of ischemic stroke was 10.76 (95% CI, 8.49–13.63) in CHD patients compared with controls.
Table 3 shows the HRs for ischemic stroke by Marelli classification. The first Marelli group, which represents patients with the most complex form of CHD, had an HR of 12.22 (95% CI, 7.93–18.85) for developing ischemic stroke compared with controls. However, similarly elevated HRs for developing ischemic stroke were observed in patients with less complex congenital heart malformations, such as in Marelli groups 2 and 4 (9.50 [95% CI, 6.35–14.21] and 7.12 [95% CI, 4.03–12.60], respectively).
Table 3.
Relative Risk of Ischemic Stroke in Patients With Congenital Heart Disease Compared With Matched Control Groups by Marelli Classification
| Categorical Hierarchy Block | Cases (N) | Controls (N) | IS Per 100 000 Person Years, Cases (N) | IS Per 100 000 Person Years, Controls (N) | HR for IS (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Marelli 1 | 5126 | 51 240 | 35.0 (45) | 2.9 (40) | 12.22 (7.93–18.85) | <0.001 |
| Marelli 2 | 11 286 | 112 810 | 21.0 (46) | 2.2 (50) | 9.50 (6.35–14.21) | <0.001 |
| Marelli 3 | 249 | 2490 | 15.9 (1) | — | 1.0 | — |
| Marelli 4 | 4749 | 47 450 | 24.3 (20) | 3.4 (31) | 7.12 (4.03–12.60) | <0.001 |
| Marelli 5 | 4513 | 45 096 | 47.8 (27) | 2.7 (16) | 17.16 (9.24–31.87) | <0.001 |
| All CHD | 25 985 | 259 750 | 28.4 (140) | 2.6 (137) | 10.76 (8.49–13.63) | <0.001 |
Marelli group 1 defined as diagnosis of common arterial trunk, transposition of great vessels, double‐inlet ventricle, hypoplastic left heart syndrome, tetralogy of Fallot, or atrioventricular septal defect. Marelli group 2 defined as atrial septal defect, ventricular septal defect, patent ductus arteriosus, coarctation of aorta, or Ebstein anomaly. Marelli group 3 defined as diagnosis of unspecified congenital malformations of cardiac septum. Marelli group 4 defined as diagnosis of congenital malformations of pulmonary artery, congenital malformations of pulmonary valve, congenital malformations of tricuspid valve, congenital malformations of aortic valve, congenital malformations of mitral valve, or congenital malformations of great veins. Marelli group 5 defined as diagnosis of other unspecified congenital malformations of aorta, other specified and unspecified congenital malformations of the heart, or unspecified congenital malformations of circulation. CHD indicates congenital heart disease; HR, hazard ratio (by measurement unit and defined by age, sex, and year of diagnosis); IS, ischemic stroke.
The risks for developing ischemic stroke in patients with isolated CHD diagnoses are shown in Table 4. Patients with coarctation of the aorta had the highest risk of developing ischemic stroke, with an HR of 12.86 (95% CI, 4.79–34.56). Diagnoses of isolated double‐inlet ventricle and atrial shunts (ASD or PFO) also carried a significantly elevated risk (HRs 4.49 [95% CI, 1.56–12.93] and 10.00 [95% CI, 2.02–49.55], respectively).
Table 4.
Relative Risk of Ischemic Stroke in Patients With Various Isolated Congenital Heart Diseases Compared With Controls
| CHD Diagnosis | Ischemic Stroke in CHD Patients/Total CHD Patients | Ischemic Stroke in Controls/Total Controls | HR (95% CI) | P Value |
|---|---|---|---|---|
| Total CHD | 140/25 985 | 137/259 750 | 10.76 (8.49–13.63) | <0.001 |
| Atrial septal defect or patent foramen ovale | 3/879 | 3/8790 | 10.00 (2.02–49.55) | 0.004 |
| Ventricular septal defect | 5/2924 | 20/29 215 | 2.50 (0.94–6.65) | 0.068 |
| Tetralogy of Fallota | 2/699 | 9/6687 | 2.20 (0.47–10.16) | 0.315 |
| Atrioventricular septal defect | 0/162 | 0/1619 | — | — |
| Common arterial trunk | 1/200 | 4/2000 | 3.33 (0.35–32.05) | 0.297 |
| Ebstein's anomalyb | 0/108 | 0/1079 | — | — |
| Transposition of great vesselsc | 1/238 | 2/2379 | 4.85 (0.14–11.87) | 0.997 |
| Double inlet ventricle | 5/1326 | 11/13 258 | 4.49 (1.56–12.93) | <0.001 |
| Patent ductus arteriosus | 1/2377 | 11/23 766 | 0.93 (0.17–7.23) | 0.947 |
| Hypoplastic left heart syndrome | 0/54 | 0/540 | — | — |
| Coarctation of aortad | 9/1823 | 6/18 204 | 12.86 (4.79–34.56) | <0.001 |
CHD indicates congenital heart disease; HR, hazard ratio.
Defined as tetralogy of Fallot diagnosis even if ventricular septal defect and pulmonary valve stenosis exist.
Defined as Ebstein's diagnosis even if ventricular septal defect or atrial septal defect or patent foramen ovale or patent ductus arteriosus diagnosis exists.
Defined as transposition of great vessels diagnosis even if ventricular septal defect or atrial septal defect or patent foramen ovale or patent ductus arteriosus diagnosis or pulmonary valve stenosis or tricuspid valve stenosis/atresia diagnosis exists.
Defined as coarctation of the aorta even if secondary hypertension exists.
In multivariate analysis restricted to the CHD group (Table 5), a diagnosis of congestive heart failure was associated with the highest risk of developing ischemic stroke (HR 6.94 [95% CI, 4.96–10.34; P<0.001]); CHD patients with hypertension (HR 3.89 [95% CI, 2.44–6.22; P<0.001]) or atrial fibrillation (HR 2.94 [95% CI, 1.78–4.83; P<0.001]) were also at significantly greater risk of developing ischemic stroke. Eleven of 140 CHD patients (7.9%) developed ischemic stroke perioperatively.
Table 5.
Univariate and Multivariable Analysis of Factors Associated With Ischemic Stroke in 25 985 Patients With Congenital Heart Disease
| Variables | Univariate Analysis | Multivariable Analysisa | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Female sex (yes/no) | 0.88 | 0.63 to 1.23 | 0.55 | 0.97 | 0.69 to 1.36 | 0.86 |
| Birth yearb | 1.16 | 0.72 to 1.89 | 0.52 | 1.12 | 0.95 to 1.32 | 0.18 |
| Atrial fibrillation (yes/no) | 6.94 | 4.39 to 10.95 | <0.001 | 2.93 | 1.78 to 4.83 | <0.001 |
| Diabetes (yes/no) | 3.03 | 1.33 to 6.86 | <0.001 | 1.30 | 0.55 to 3.06 | 0.55 |
| Hypertension (yes/no) | 4.65 | 3.02 to 7.16 | <0.001 | 3.89 | 2.44 to 6.22 | <0.001 |
| Congestive heart failure (yes/no) | 10.39 | 7.25 to 14.88 | <0.001 | 6.94 | 4.70 to 10.34 | <0.001 |
HR indicates hazard ratio by measurement unit.
Cox regression analysis was adjusted for age, Marelli classification (by stratification), and comorbidities at the baseline.
Range of birth year is years from the first study year (0–23 years).
Figure 1 shows the cumulative incidence of ischemic stroke in CHD patients compared with controls during the 42‐year follow‐up period. CHD patients older than 20 years of age showed a marked increase in the incidence of ischemic stroke, to ≈1.5% compared with 0.2% for the controls at the end of the study. Furthermore, the incidence of ischemic stroke was increased subsequently in all Marelli classifications; however, the highest incidence was noted in the first and the fifth Marelli patient group (Figure 2).
Discussion
In this large‐register study, we found that in children and young adults with CHD, the risk of developing ischemic stroke was low in absolute terms but still almost 11 times higher than in matched controls. The elevated risk was most marked in, but not restricted to, patients with the most complex heart malformations. We found that isolated diagnoses of coarctation of the aorta, double‐inlet ventricle, and ASD or PFO placed patients at significantly higher risk of developing ischemic stroke than controls. These findings, taking into account the complexity and heterogeneity of congenital heart lesions, are clinically important.
A previous study evaluated the risk of ischemic stroke in CHD patients, and while the results varied, the risk of ischemic stroke was estimated to be from 10 to 100 times greater in samples of CHD patients of European or Canadian origin compared with published incidence data for young adults in the United States.16 No previous study has compared the risk of ischemic stroke in CHD patients with that of controls from the same background population.
A report from the Euro Heart Survey showed that the incidence of cerebrovascular accidents (including transient ischemic attacks) was 4% in 4000 adults with CHD during 5 years of follow‐up.23 This rate was much higher than that in our study population, in which we recorded only a 0.5% incidence of ischemic stroke during a mean follow‐up of ≈20 years. However, the Euro Heart Survey was a cross‐sectional study that focused mainly on patients with more complex heart malformations and had a relatively short follow‐up period compared with the present study.
In a study of 3267 adults with 5 of the most common types of CHD compared with 6534 controls,17 the risk of developing ischemic stroke was 2.2 times higher in adults with CHD compared to controls. Furthermore, a recent study investigated 2.5 million children in a North California Integrated Healthcare Plan and CHD was identified in 15 of 412 total pediatric stroke cases. The risk of stroke in children with CHD was 19 times higher than in randomly selected controls and the risk in children who had cardiac surgery remained elevated (13‐fold) also after the perioperative period.9 These findings support our results demonstrating that CHD is a risk factor for ischemic stroke, with a low absolute risk and a high relative risk.
Cardiovascular factors other than congenital malformation per se may also influence the risk of stroke. The prevalence of atrial fibrillation has been reported to be as high as 15% of the total CHD population and associated with a more than doubled risk of morbidities such as stroke.24 We found that the risk of ischemic stroke in CHD patients with cardiovascular comorbidities such as atrial fibrillation, heart failure, and hypertension was up to 6‐fold greater than that of CHD patients without these comorbidities.
In our study, we may have underestimated the risk of ischemic stroke because of the exclusion of all patients who were diagnosed with ASD or PFO during adulthood; however, patients with isolated ASD or PFO diagnosis before the age of 18 were still at 10 times greater risk of developing ischemic stroke than controls. Even so, these young patients do not, as a rule, approach the risk level at which anticoagulation is indicated (eg, atrial fibrillation). However, as the CHD population increases and becomes older, more CHD patients will likely reach that point and develop an indication for preventive antithrombotic treatment.
Studies with CHD patients are frequently limited in size and the results may not be representative of or applicable to groups or subgroups of CHD patients. Thus, a strength of the present study is that we identified virtually all CHD patients and drew matched controls from the same background population. Because of the high coverage of the Swedish registers, there is very limited loss to follow‐up, but transient ischemic attacks and minor strokes may not have been recognized.
Limitations
One of the limitations is that the study is based solely on registers and that no echocardiographic, heart morphologic, or clinical data were available for further analysis. With respect to the validity of CHD diagnoses, including the Marelli classification, children and adults with CHD are generally managed in a few highly specialized units, which should help to minimize misclassification, even though, due to the legal requirement to use only coded data, we were unable to validate the CHD diagnoses.
With respect to ischemic stroke, computed tomographic scans have been available and routinely used since the early 1980s in Sweden, so the distinction between hemorrhagic and ischemic stroke is probably valid. Furthermore, major cardiovascular diseases have been validated externally and by individual researchers with the overall positive predictive value of diagnoses in the register of about 85% to 95%.25
Previous studies have reported that clinically silent ischemic strokes are quite common postoperatively in children as well as adult patients.8, 26, 27 Our study was limited to clinically evident ischemic strokes and clinically silent strokes were not included in the present study.
Table 3 shows the risk of patients with isolated CHD diagnoses of developing ischemic stroke in the present study; however, this analysis cannot be extrapolated to patients with multiple CHD diagnoses.
Another limitation is that outpatient data are available only from 2000 in Sweden; cases managed only on an outpatient basis before that time could not be identified. Also, data from the primary care were not available, but it is unlikely that young patients with CHD and even minor ischemic stroke were not hospitalized. Finally, coding as well as ascertainment bias may be present in the current national registry study.
Conclusions
The risk of ischemic stroke in children and young adults with CHD is significantly higher than in the general population. Cardiovascular comorbidities, such as congestive heart failure, atrial fibrillation, and hypertension, increased the risk of ischemic stroke; therefore, these patients should be targeted for intervention as they grow older. Further research is need to clarify when and how to institute prevention for ischemic stroke.
Sources of Funding
This work was supported by grants from the Sahlgrenska Academy, from the Health & Medical Care Committee of the Regional Executive Board Region Västra Götaland (VGRFOUREG‐224861), grants from the Swedish State, under the agreement between the Swedish government and the county councils concerning economic support of research and education of doctors (ALF‐agreement), the Swedish Heart and Lung Foundation, and the Swedish Research Council (grant numbers 2013‐5187 and 2013‐4236).
Disclosures
None.
Supporting information
Figure S1. Cumulative incidence of ischemic stroke and mortality in overall and Marelli groups of patients with congenital heart disease and controls for up to 42 years of age.
(J Am Heart Assoc. 2016;5:e003071 doi: 10.1161/JAHA.115.003071)
An accompanying Figure S1 is available at http://jaha.ahajournals.org/content/5/2/e003071/suppl/DC1
References
- 1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics C, Stroke Statistics S . Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biller J. Stroke: improving characterization of childhood cerebral arteriopathies. Nat Rev Cardiol. 2009;6:395–397. [DOI] [PubMed] [Google Scholar]
- 3. Rosengren A, Giang KW, Lappas G, Jern C, Toren K, Bjorck L. Twenty‐four‐year trends in the incidence of ischemic stroke in Sweden from 1987 to 2010. Stroke. 2013;44:2388–2393. [DOI] [PubMed] [Google Scholar]
- 4. George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann Neurol. 2011;70:713–721. [DOI] [PubMed] [Google Scholar]
- 5. Poisson SN, Schardt TQ, Dingman A, Bernard TJ. Etiology and treatment of arterial ischemic stroke in children and young adults. Curr Treat Options Neurol. 2014;16:315. [DOI] [PubMed] [Google Scholar]
- 6. Khoshnood B, Lelong N, Houyel L, Thieulin AC, Jouannic JM, Magnier S, Delezoide AL, Magny JF, Rambaud C, Bonnet D, Goffinet F; Group ES . Prevalence, timing of diagnosis and mortality of newborns with congenital heart defects: a population‐based study. Heart. 2012;98:1667–1673. [DOI] [PubMed] [Google Scholar]
- 7. Mackay MT, Wiznitzer M, Benedict SL, Lee KJ, Deveber GA, Ganesan V; International Pediatric Stroke Study G . Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Ann Neurol. 2011;69:130–140. [DOI] [PubMed] [Google Scholar]
- 8. Rodan L, McCrindle BW, Manlhiot C, MacGregor DL, Askalan R, Moharir M, deVeber G. Stroke recurrence in children with congenital heart disease. Ann Neurol. 2012;72:103–111. [DOI] [PubMed] [Google Scholar]
- 9. Fox CK, Sidney S, Fullerton HJ. Community‐based case‐control study of childhood stroke risk associated with congenital heart disease. Stroke. 2015;46:336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dowling MM, Hynan LS, Lo W, Licht DJ, McClure C, Yager JY, Dlamini N, Kirkham FJ, Deveber G, Pavlakis S; International Paediatric Stroke Study G . International Paediatric Stroke Study: stroke associated with cardiac disorders. Int J Stroke. 2013;8(suppl A100):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Domi T, Edgell DS, McCrindle BW, Williams WG, Chan AK, MacGregor DL, Kirton A, deVeber GA. Frequency, predictors, and neurologic outcomes of vaso‐occlusive strokes associated with cardiac surgery in children. Pediatrics. 2008;122:1292–1298. [DOI] [PubMed] [Google Scholar]
- 12. Berthrong M, Sabiston DC Jr. Cerebral lesions in congenital heart disease, a review of autopsies on 162 cases. Bull Johns Hopkins Hosp. 1951;89:384–406. [PubMed] [Google Scholar]
- 13. Phornphutkul C, Rosenthal A, Nadas AS, Berenberg W. Cerebrovascular accidents in infants and children with cyanotic congenital heart disease. Am J Cardiol. 1973;32:329–334. [DOI] [PubMed] [Google Scholar]
- 14. Perloff JK, Marelli AJ, Miner PD. Risk of stroke in adults with cyanotic congenital heart disease. Circulation. 1993;87:1954–1959. [DOI] [PubMed] [Google Scholar]
- 15. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–1008. [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann A, Chockalingam P, Balint OH, Dadashev A, Dimopoulos K, Engel R, Schmid M, Schwerzmann M, Gatzoulis MA, Mulder B, Oechslin E. Cerebrovascular accidents in adult patients with congenital heart disease. Heart. 2010;96:1223–1226. [DOI] [PubMed] [Google Scholar]
- 17. Lin YS, Liu PH, Wu LS, Chen YM, Chang CJ, Chu PH. Major adverse cardiovascular events in adult congenital heart disease: a population‐based follow‐up study from Taiwan. BMC Cardiovasc Disord. 2014;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation. 2005;112:1063–1072. [DOI] [PubMed] [Google Scholar]
- 19. Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, Drobinski G, Thomas D, Grosgogeat Y. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–1152. [DOI] [PubMed] [Google Scholar]
- 20. Marelli AJ, Mackie AS, Ionescu‐Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. [DOI] [PubMed] [Google Scholar]
- 21. Olen O, Bihagen E, Rasmussen F, Ludvigsson JF. Socioeconomic position and education in patients with coeliac disease. Dig Liver Dis. 2012;44:471–476. [DOI] [PubMed] [Google Scholar]
- 22. Lind M, Svensson AM, Kosiborod M, Gudbjornsdottir S, Pivodic A, Wedel H, Dahlqvist S, Clements M, Rosengren A. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972–1982. [DOI] [PubMed] [Google Scholar]
- 23. Engelfriet P, Boersma E, Oechslin E, Tijssen J, Gatzoulis MA, Thilen U, Kaemmerer H, Moons P, Meijboom F, Popelova J, Laforest V, Hirsch R, Daliento L, Thaulow E, Mulder B. The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow‐up period. The Euro Heart Survey on adult congenital heart disease. Eur Heart J. 2005;26:2325–2333. [DOI] [PubMed] [Google Scholar]
- 24. Bouchardy J, Therrien J, Pilote L, Ionescu‐Ittu R, Martucci G, Bottega N, Marelli AJ. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. [DOI] [PubMed] [Google Scholar]
- 25. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Floyd TF, Shah PN, Price CC, Harris F, Ratcliffe SJ, Acker MA, Bavaria JE, Rahmouni H, Kuersten B, Wiegers S, McGarvey ML, Woo JY, Pochettino AA, Melhem ER. Clinically silent cerebral ischemic events after cardiac surgery: their incidence, regional vascular occurrence, and procedural dependence. Ann Thorac Surg. 2006;81:2160–2166. [DOI] [PubMed] [Google Scholar]
- 27. Chen J, Zimmerman RA, Jarvik GP, Nord AS, Clancy RR, Wernovsky G, Montenegro LM, Hartman DM, Nicolson SC, Spray TL, Gaynor JW, Ichord R. Perioperative stroke in infants undergoing open heart operations for congenital heart disease. Ann Thorac Surg. 2009;88:823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Cumulative incidence of ischemic stroke and mortality in overall and Marelli groups of patients with congenital heart disease and controls for up to 42 years of age.


