Abstract
Background
Stroke associated with acute carotid occlusion is associated with poor effectiveness of tissue plasminogen activator (tPA) thrombolysis and poor prognosis. Rupture of atherosclerotic plaques resulting in vascular occlusions may occur on plaques, causing variable stenosis. We hypothesized that degree of stenosis may affect recanalization rates with tPA. Ultrasound+tPA (sonothrombolysis) has been shown to improve recanalization for intracranial occlusions but has not been tested for carotid occlusion. Our primary aim was to determine thrombolytic recanalization rates in a model of occlusion with variable stenosis, with a secondary aim to investigate sonothrombolysis in this model.
Methods and Results
Rat carotid arteries were crushed and focal stenosis created (25% baseline Doppler flow) with a silk‐suture tie invoking thrombosis and occlusion. To model mild or severe stenosis, the tie was released pretreatment or left in place. Animals were treated with tPA (10 mg/kg) or tPA+ultrasound (2‐MHz) in each stenosis model (n=7/group). Recanalization was assessed by Doppler flow. Thrombolytic recanalization rates were significantly higher in mild stenosis groups (71% versus 0% with severe stenosis; P<0.0001). Recanalization rates were not significantly higher with additional ultrasound in either model.
Conclusions
In this model, the degree of carotid stenosis had a large effect on thrombolytic recanalization. Sonothrombolysis using standard parameters for intracranial sonothrombolysis did not increase recanalization. Further testing is warranted. The degree of underlying stenosis may be an important predictor of thrombolytic recanalization, and clinical correlation of these findings may provide new approaches to treatment selection for patients with carotid occlusion.
Keywords: carotid arteries, rats, sonothrombolysis, stenosis, thrombolysis
Subject Categories: Ultrasound, Stenosis, Thrombosis, Cerebrovascular Disease/Stroke
Introduction
Carotid artery occlusion can lead to devastating consequences such as stroke. Patients with carotid occlusion–associated stroke have high rates of death and disability but treatments are limited. Successful recanalization with intravenous tissue plasminogen activator (tPA), the current stroke thrombolytic, is only achieved in 10% to 30% of patients with carotid occlusion.1, 2, 3 Although a severe carotid stenosis predicts a greater risk of stroke for any individual,4 the population frequency of mild stenosis is greater. Hence, acute occlusion of the carotid artery that leads to stroke may occur with varying degrees of carotid stenosis. We hypothesized that the degree of underlying stenosis of carotid artery occlusion may be an important predictor of successful thrombolytic recanalization. Given the low efficacy of tPA in carotid athero‐thrombotic occlusion, approaches to enhance its effect are required. Ultrasound+tPA (sonothrombolysis) appears promising for improving intracranial recanalization,5 but has not been tested in extracranial carotid artery occlusion. Therefore, our primary aim was to determine whether the degree of carotid stenosis affected rates of sustained recanalization with thrombolysis at 4.5 hours postocclusion. Our secondary aim was to determine whether ultrasound enhances sustained tPA recanalization rates in carotid occlusion.
Methods
General Animal Details
Experiments were approved by the Animal Care and Ethics Committee of the University of Newcastle, Australia (Approval No. A‐2010‐128) and performed in compliance with requirements of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.6 Male Wistar rats (n=28; 311–417 g; Animal Resources Centre, Perth, Australia) were anesthetized with 5% isoflurane and maintained with 1.5% to 2% in 30/70% O2/N2. Rectal temperature was maintained at 37°C with a feedback‐controlled heat mat (Faculty of Health Workshop, University of Newcastle, Australia). Heart rate, respiration, and oxygen saturation were monitored throughout surgery.
Model of Carotid Artery Occlusion With Stenosis
To create carotid artery occlusion with variable degrees of stenosis, a modification of the Folt's model was used.7 A timeline and surgical schematic are presented in Figure 1. The right carotid arteries were exposed and a 20‐MHz, 0.8‐mm Doppler flow probe (Iowa Doppler Products, USA) was positioned over the external carotid artery, distal to the intended injury/stenosis site. Flow was monitored audibly through the Doppler instrumentation and visually via LabChart software (ADInstruments, Australia). Since the primary outcome was recanalization of the occluded common carotid artery, the internal carotid artery was ligated. This was done to avoid the potential confounding effects of variable strokes that may result from thromboembolism. A 5‐0 silk suture with a double throw tie was placed around the common carotid artery at the intended stenosis site, but not tightened. Using hemostats guarded with tape, the common carotid artery was crushed 3 times (30‐s crush, 30‐s rest) to disrupt the endothelium, exposing prothrombotic factors. Crushes were made over and directly adjacent to the suture. Following the third crush, a stenosis was created by tightening the suture over the site of injury, reducing blood flow by 75% of precrush baseline flow. Flow was monitored for cyclic patterns until complete occlusion was achieved. Complete occlusion was confirmed by cessation of audible flow and irregular flow trace (Figure 2A and 2B). Up to 2 additional crushes were made if: (1) continuous flow was maintained for 10 minutes after the crush cycle, or (2) if cyclic flow patterns including occlusion/recanalization events were observed for 30 minutes postcrush with no complete occlusion.
Figure 1.
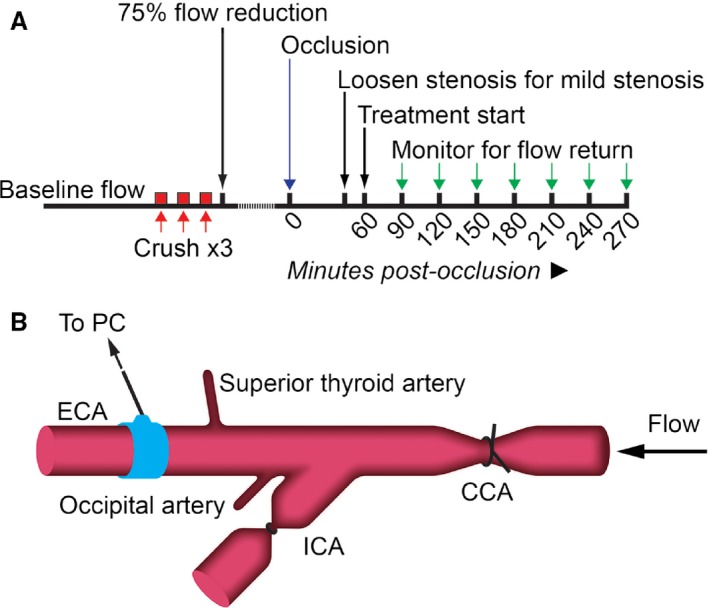
Experimental timeline (A) and surgery schematic (B) of carotid artery occlusion with stenosis. Branching arteries were cauterized (superior thyroid and occipital arteries), and the internal carotid artery (ICA) was ligated to prevent embolism to the brain. A flow probe was placed over the external carotid artery (ECA) and baseline flow monitored for 5 minutes (depicted in blue in [B]). The stenosis suture was placed around the common carotid artery (CCA) but not tightened. Three 30‐s crushes ([A] red) were made over and immediately adjacent to the suture with 30 second rest periods between crushes. The suture was tightened to reduce flow by 75% and flow was monitored for occlusion. Following 45 minutes of stable occlusion, the stenosis suture was either loosened to create mild stenosis or left in place for a severe stenosis. Treatment began 60 minutes postocclusion. Flow was monitored every 30 minutes post–treatment onset for any signs of recanalization ([A] green arrows).
Figure 2.
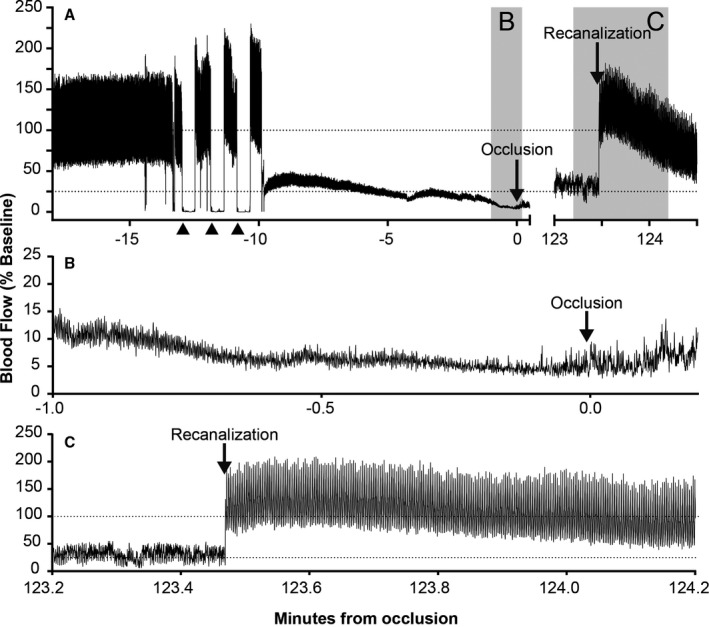
Example flow trace of crush, occlusion, and recanalization. A, Doppler Flow recorded via LabChart (ADInstruments, Australia) demonstrating crush injury (▲), stenosis (at −10 min), flow decrease to occlusion (gray shading, B), and recanalization (gray shading, C). B, Flow decrease to occlusion, correlating with gray shading labeled B in (A). At low flow rates, audible signal was a better indicator of flow due to “noise” of the flow trace, as can be seen around the point of occlusion in (B). C, Recanalization correlating with gray shading labeled C in (A).
To model mild or severe stenosis, the tie was loosened (mild) or left in place (severe) after 45 minutes of stable occlusion. If flow spontaneously returned pretreatment (n=1), the vessel was recrushed and reobserved for 60‐minute occlusion. For each stenosis protocol, animals were randomized to tPA, or tPA+ultrasound. tPA (10 mg/kg; Alteplase, Boehringer Ingelheim, Australia) was administered intravenously via the femoral vein at 60 minutes postocclusion (10% bolus over 1 minute, remainder over 1 hour). For ultrasound groups, the skin was sutured, covered with Tegaderm™ (3M, North Ryde, Australia), and the ultrasound probe was positioned over the occlusion using a laboratory‐manufactured spacer containing ultrasound gel. Insonation began with the start of tPA delivery, continuing for 2 hours (2‐MHz, 720 mW/cm2, 25‐mm depth, sample volume 10 mm; EZ‐Dop®, DWL Compumedics, Germany). Recanalization was monitored at 30‐minute intervals after tPA onset until 4.5 hours postocclusion. Recanalization was confirmed by audible flow return and a regular LabChart flow trace (Figure 2A and 2C).
Statistical Analysis
Baseline time for analysis was 60 minutes postocclusion, immediately prior to tPA administration. Recanalization was reported as sustained when a return of flow was observed and remained until the final observation time (4.5 hours postocclusion). Rates of sustained recanalization (number of animals with sustained recanalization from the total cohort) were compared using log‐rank test with differences considered significant if P<0.05 (Graphpad Prism 6). Investigation of degree of stenosis was performed by pooling tPA and tPA+ultrasound groups per stenosis model (Figure 3B). Effect of treatment on sustained recanalization was analyzed for each stenosis model.
Figure 3.
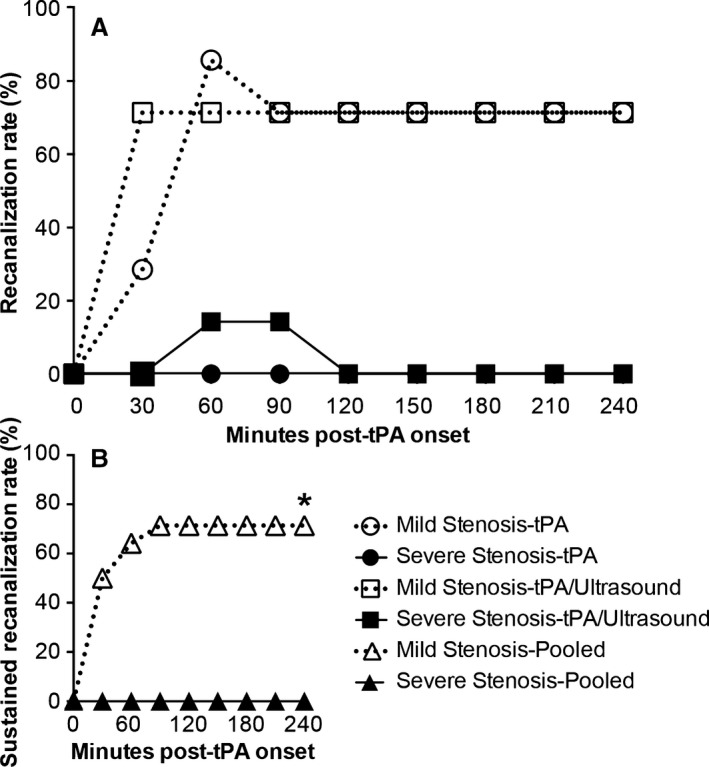
Carotid artery recanalization in the setting of mild or severe stenosis. Recanalization rates are expressed as the percentage of animals recanalized per group (n=7/group). Recanalization was measured every 30 minutes post–treatment onset for 240 minutes (4.5 hours postocclusion). A, Recanalization/reocclusion events for all animals with mild or severe stenosis treated with tissue plasminogen activator (tPA) alone or tPA+ultrasound (n=7/group). B, Sustained recanalization (recanalization at end point) in mild (open triangle) and severe stenosis models, pooled treatment (*P<0.0001, hazard ratio=16.7 [4.2–67.4, 95% CI]; n=14/group). Presence of ultrasound made no significant difference to the rates of sustained recanalization in either mild or severe stenosis models.
Results
No animals were excluded. One animal died prior to final observation (mild stenosis‐tPA group). This animal had fluctuating body temperatures throughout surgery and high temperatures (>39°C) leading up to its death. It was included in the primary analysis, and re‐analysis excluding this animal did not change the reported results.
Exact flow values relative to baseline are not presented because closing the surgical site for ultrasound insonation affected the Doppler flow trace relative to baseline, preventing accurate judgment of the degree of recanalization. However, flow return was clearly discernible irrespective of skin closure (Figure 2C). Therefore, in the interests of accuracy, we chose sustained recanalization as the marker of recanalization success, rather than attempting to quantify percentage recanalization.
The carotid artery of all animals had been occluded for 60 minutes at tPA onset. High rates of sustained recanalization were observed in the mild stenosis model compared to severe stenosis (P<0.0001; Figure 3A and 3B). In the mild stenosis‐tPA group, all animals exhibited recanalization. Sustained recanalization was observed in 5/7 rats, and the remaining 2 animals had recanalization/reocclusion. Reocclusion occurred within 30 minutes of recanalization onset in both cases. In all, initial recanalization occurred between 30 and 90 minutes post–treatment onset. In severe stenosis‐tPA, no recanalization was observed.
Sonothrombolysis had limited effects on recanalization in either mild or severe stenosis groups (Figure 3A). In mild stenosis‐tPA+ultrasound, sustained recanalization occurred within 30 minutes of treatment onset in 5/7 rats, as was also seen without the addition of ultrasound (P=0.67). Recanalization/reocclusion occurred in 2/7 of severe stenosis‐tPA+ultrasound rats, but none had sustained recanalization (Figure 3A).
Discussion
This study demonstrates a strong effect of the degree of carotid stenosis on thrombolytic recanalization after thrombotic carotid occlusion. Sustained recanalization rates were >70% with mild stenosis, compared to 0% with severe stenosis. These findings have potentially important clinical implications. If the degree of carotid stenosis has similar effects clinically and new imaging techniques enable degree of stenosis to be assessed, this could guide the choice of thrombolysis versus endovascular treatment for stroke patients with acute carotid occlusion. The conventional “rat dose” of tPA, 10 mg/kg, was chosen based on its common use in the literature. There is evidence that this dose exerts a maximum effect8 and further dosage increases do not enhance recanalization.9 This study did not demonstrate a benefit of sonothrombolysis over tPA alone for extracranial carotid occlusion.
The results provide evidence of the influence of stenosis on thrombolysis as well as providing a preclinical model for testing thrombolytics in extracranial carotid occlusions. Correlation with clinical data is, however, needed to confirm this effect in patients. There are differences between our acute stenosis model and patients with carotid stenosis and/or occlusion. In patients, stenosis due to atherosclerosis is generally due to chronic plaque build‐up, while our model is an acute stenosis. Our model is not an absolute replicate of the human condition of atherothrombotic carotid stenosis and occlusion, yet it is an important step forward for experimental models of carotid occlusion. It mimics the stenotic narrowing of the artery, reduced blood flow, endothelial damage, and exposure of subendothelial matrix proteins that promote thrombosis and cause carotid occlusion. Previous models of carotid occlusion have used ex vivo prepared clots injected into the vessel,10 or other nonphysiological forms of thrombosis induction, such as FeCl3.11 Additionally, none of these models have investigated stenosis as a factor of thrombolytic efficacy. The results of our study indicate a clear difference in recanalization response to thrombolytic therapy with differing degrees of stenosis. It is this finding that should prompt further investigation of this effect clinically, and whether techniques can be developed to quantify the degree of underlying carotid stenosis. Our experimental data suggest that this may prove useful in the treatment allocation of patients with carotid occlusion‐associated stroke—particularly in centers that may need to transfer patients for endovascular therapies.
In this study, we used the standard ultrasound parameters used for transcranial sonothrombolysis;5, 12 however, even in the absence of skull attenuation, there did not appear to be a large additive benefit over tPA alone. Ultrasound power is attenuated through the skull by as much as 99%,13, 14 yet evidence that higher power increases clot lysis15 indicates that power attenuation likely limits the efficacy of intracranial sonothrombolysis. Additionally, there is evidence that low‐frequency ultrasound facilitates clot lysis.15, 16 However, for intracranial occlusion, low‐frequency ultrasound has the potential to cause the propagation of standing waves within the skull, causing intracerebral hemorrhage.14, 17 In the setting of carotid occlusion, where there is not the risk of standing waves and brain bleeding, lower frequency and/or higher ultrasound power may be more effective for thrombolysis. This proposition requires further testing.
Our data suggest that severe carotid stenosis is a likely explanation for at least some of the failed tPA recanalization in patients with carotid occlusion. In our model, as in human atherothrombotic carotid occlusion, the bulk of the thrombus forms distal to the stenosis, thereby limiting drug delivery to the thrombus. This is likely to be different from intracranial occlusions, which in Western populations are predominantly thromboembolic in nonstenosed vessels. Advanced computed tomography, magnetic resonance, and carotid duplex imaging can evaluate both intraluminal flow and vessel wall characteristics and are also now being used to evaluate patient suitability for reperfusion therapies in acute stroke. The ability to determine the degree of carotid stenosis in patients with carotid occlusion may help guide the choice of therapy for patients with this devastating form of stroke.
Sources of Funding
Tomkins was supported by a National Heart Foundation/National Stroke Foundation postgraduate scholarship. Spratt was supported by an NHMRC career development fellowship, #1035465.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002716 doi: 10.1161/JAHA.115.002716)
References
- 1. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta‐analysis. Stroke. 2007;38:967–973. [DOI] [PubMed] [Google Scholar]
- 2. Christou I, Felberg RA, Demchuk AM, Burgin WS, Malkoff M, Grotta JC, Alexandrov AV. Intravenous tissue plasminogen activator and flow improvement in acute ischemic stroke patients with internal carotid artery occlusion. J Neuroimaging. 2002;12:119–123. [DOI] [PubMed] [Google Scholar]
- 3. Pechlaner R, Knoflach M, Matosevic B, Ruecker M, Schmidauer C, Kiechl S, Willeit J. Recanalization of extracranial internal carotid artery occlusion after i.v. thrombolysis for acute ischemic stroke. PLoS One. 2013;8:e55318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, Hertzberg VS, McIff EB, Moore WS, Panagos PD, Riles TS, Rosenwasser RH, Taylor AJ. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Circulation. 2011;124:489–532. [DOI] [PubMed] [Google Scholar]
- 5. Tsivgoulis G, Eggers J, Ribo M, Perren F, Saqqur M, Rubiera M, Sergentanis TN, Vadikolias K, Larrue V, Molina CA, Alexandrov AV. Safety and efficacy of ultrasound‐enhanced thrombolysis: a comprehensive review and meta‐analysis of randomized and nonrandomized studies. Stroke. 2010;41:280–287. [DOI] [PubMed] [Google Scholar]
- 6. Council NHaMR . Australian Code for the Care and Use of Animals for Scientific Purposes. 8th ed Canberra: National health and medical research council; 2013. [Google Scholar]
- 7. Sturgeon SA, Jones C, Angus JA, Wright CE. Adaptation of the Folt's and electrolytic methods of arterial thrombosis for the study of anti‐thrombotic molecules in small animals. J Pharmacol Toxicol Methods. 2006;53:20–29. [DOI] [PubMed] [Google Scholar]
- 8. Tomkins AJ, Hood RJ, Levi CR, Spratt NJ. Tissue plasminogen activator for preclinical stroke research: neither “rat” nor “human” dose mimics clinical recanalization in a carotid occlusion model. Sci Rep. 2015;5:16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calandre L, Grau M, Cabello A. Lysis rates with rt‐PA vary between different human emboli in a rat model of cerebral embolism. Fibrinolysis Proteolysis. 1998;12:107–111. [Google Scholar]
- 10. Holscher T, Fisher DJ, Ahadi G, Voie A. Introduction of a rabbit carotid artery model for sonothrombolysis research. Transl Stroke Res. 2012;3:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Surin WR, Prakash P, Barthwal MK, Dikshit M. Optimization of ferric chloride induced thrombosis model in rats: effect of anti‐platelet and anti‐coagulant drugs. J Pharmacol Toxicol Methods. 2010;61:287–291. [DOI] [PubMed] [Google Scholar]
- 12. Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez‐Sabin J, Montaner J, Saqqur M, Demchuk AM, Moye LA, Hill MD, Wojner AW. Ultrasound‐enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. [DOI] [PubMed] [Google Scholar]
- 13. Pfaffenberger S, Devcic‐Kuhar B, Kollmann C, Kastl SP, Kaun C, Speidl WS, Weiss TW, Demyanets S, Ullrich R, Sochor H, Wober C, Zeitlhofer J, Huber K, Groschl M, Benes E, Maurer G, Wojta J, Gottsauner‐Wolf M. Can a commercial diagnostic ultrasound device accelerate thrombolysis? An in vitro skull model Stroke. 2005;36:124–128. [DOI] [PubMed] [Google Scholar]
- 14. Baron C, Aubry JF, Tanter M, Meairs S, Fink M. Simulation of intracranial acoustic fields in clinical trials of sonothrombolysis. Ultrasound Med Biol. 2009;35:1148–1158. [DOI] [PubMed] [Google Scholar]
- 15. Nedelmann M, Eicke BM, Lierke EG, Heimann A, Kempski O, Hopf HC. Low‐frequency ultrasound induces nonenzymatic thrombolysis in vitro. J Ultrasound Med. 2002;21:649–656. [DOI] [PubMed] [Google Scholar]
- 16. Akiyama M, Ishibashi T, Yamada T, Furuhata H. Low‐frequency ultrasound penetrates the cranium and enhances thrombolysis in vitro. Neurosurgery. 1998;43:828–832; discussion 832‐823. [DOI] [PubMed] [Google Scholar]
- 17. Daffertshofer M, Gass A, Ringleb P, Sitzer M, Sliwka U, Els T, Sedlaczek O, Koroshetz WJ, Hennerici MG. Transcranial low‐frequency ultrasound‐mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36:1441–1446. [DOI] [PubMed] [Google Scholar]


