Abstract
Background
Studies have suggested that patients with atrial fibrillation (AF) have impairment in the baroreflex. It is not clear whether these findings are the result of the associated comorbid conditions or the arrhythmia itself. We hypothesized that AF is associated with impairment in baroreflex function and that the arrhythmia itself is a contributing factor.
Methods and Results
Twenty‐four patients with persistent AF referred for cardioversion were enrolled. A second group of patients with no history of AF matched for age and left ventricular ejection fraction was identified and served as the control group. In the AF group, baroreflex gain (BRG) was measured on the day of cardioversion (Day 1) and again at 30 days post‐cardioversion (Day 30) in patients who remained in sinus rhythm (SR). The clinical characteristics of patients with AF were not different than those of the control group. The mean BRG in the AF group on Day 1 was significantly lower than the mean BRG of the control group (5.2±3.6 versus 10.8±5.5 ms/mm Hg, P<0.05). Ten patients experienced AF recurrence before the 30‐day follow‐up and 14 patients remained in SR. In the group that remained in SR, BRG increased from 4.1±3.7 ms/mm Hg on Day 1 to 7.0±6.0 ms/mm Hg on Day 30 (P<0.01).
Conclusion
We have shown that AF is associated with impairment of the baroreflex and that restoration of SR improves BRG. Our data suggest that AF might be a contributing factor to the observed impairment in BRG and that restoring SR might help improve baroreflex function.
Keywords: atrial fibrillation, baroreflex gain, hypertension
Subject Categories: Atrial Fibrillation, Autonomic Nervous System
Introduction
The relationship between atrial fibrillation (AF) and the autonomic nervous system is complex. On the one hand, changes in autonomic tone have been shown to play a major role in the genesis of AF.1, 2 On the other hand, AF has been shown to have an effect on autonomic function through its hemodynamic changes.3, 4 While several studies have suggested that patients with AF have impairment in the baroreflex, it is not clear whether these findings are the result of the associated comorbid conditions or the arrhythmia itself.
The purpose of this study was to evaluate the effects of persistent AF on the baroreflex. We hypothesized that AF is associated with impairment in baroreflex function and that the arrhythmia itself is a contributing factor. Because it is hard to predict AF onset and, thus, the long‐term effects of AF on the baroreflex, we evaluated the effects of restoring sinus rhythm (SR) on baroreflex gain (BRG) in patients with persistent AF. Assessing the effect of restoring SR on the baroreflex should improve our understanding of the effects of AF on blood pressure (BP) regulation, orthostatic tolerance, and mortality.
Methods
Patient Population
The study was conducted at the University of Utah Health Science Center in Salt Lake City and the University of Wisconsin Hospitals and Clinics in Madison and was approved by the institutional review boards at the respective institutions. Informed consent was obtained from all patients, and all procedures were conducted in accordance with institutional guidelines. All patients older than 18 years who had persistent AF (>30 days) and an indication for DC cardioversion were asked to enroll. Exclusion criteria included the presence of a pacemaker or an implantable cardioverter defibrillator with pacing programming different than VVI at 40 bpm, inability to measure BRG during SR because of frequent ectopy, and changes in cardiac medications during enrollment. A second group of patients with no history of AF matched for age and left ventricular ejection fraction (LVEF) was identified and served as the control group.
Experimental Protocol
Day of cardioversion
On the day of cardioversion, baseline heart rate (HR) and BP were obtained while the patient was in AF. Propofol was administered for sedation and DC cardioversion was performed according to the hospital protocol. At 2 to 4 hours after cardioversion, HR and beat‐to‐beat BP were recorded and arterial BRG was calculated as described later. Data obtained on the day of cardioversion were referred to as “Day 1 post‐cardioversion.”
Thirty days post‐cardioversion
At 30 days post‐cardioversion, subjects returned to undergo 12‐lead electrocardiography (ECG). If the patient remained in SR, he or she underwent collection of BP and HR data with repeat assessment of BRG. If the patient had evidence of AF recurrence during the 30 days of monitoring or was not in SR during the follow‐up visit, no additional data were collected. Data obtained 30 days after cardioversion were referred to as “Day 30 post‐cardioversion.”
A total of 24 patients with persistent AF were successfully enrolled in the study (AF group). Ten patients experienced AF recurrence before the 30‐day follow‐up (AF‐recurrence group), and 14 patients remained in SR (AF‐no recurrence group).
Assessment of BRG
Arterial BRG was assessed by using 2 different methods during the course of this study because measurements were conducted at 2 different institutions. The methods used were the modified Oxford technique5 at the University of Utah and the sequence method6 at the University of Wisconsin. With the modified Oxford technique, nitroprusside was administered in 50‐μg boluses until a 20– to 30–mm Hg drop in systolic BP (SBP) was noted, followed by phenylephrine administration in 50‐μg boluses until a 20– to 30–mm Hg increase in SBP above baseline was noted. Arterial BRG was determined as changes in RR interval/SBP. Beat‐to‐beat noninvasive BP and ECG were monitored by using a Colin Monitor (Colin Medical Instruments), and all data were recorded to the Windaq data acquisition system (DATAQ Instruments). With the sequence method, the analysis involved identification of spontaneous changes in BP and HR that are mediated by the arterial baroreflex. Unlike with the drug‐induced changes, the computer identifies spontaneously occurring sequences of consecutive beats in which progressive increases in SBP of ≥1 mm Hg/beat for ≥3 consecutive heartbeats are followed by a progressive lengthening in RR intervals of ≥4 ms/beat. Similarly, the computer seeks to identify progressive decreases in SBP followed by progressive shortening in RR intervals.7, 8 The slope of the regression line between SBP and RR changes for all identified sequences is averaged and used as an index of the sensitivity of arterial baroreflex modulation of HR. The sequence method has been shown to correlate with the Oxford technique9 and to have high intrasubject reproducibility.10 For the sequence method, beat‐to‐beat noninvasive BP monitoring was performed by using the Finometer Midi (Finapres Medical Systems). ECG of 2 surface leads and all data were recorded through a PowerLab system (ADInstruments) for subsequent analysis.
In the AF group (n=24), all patients had their BRG measured on Day 1 post‐cardioversion. The modified Oxford technique was used in 9 patients, and the sequence method was used in the remaining 15 patients. In the AF‐no recurrence group (n=14), all patients had their BRG measured on both Day 1 and Day 30 post‐cardioversion. The modified Oxford technique was used in 7 patients, and the sequence method was used in the remaining 7 patients. The same method was used for both measurements. In the control group (n=24), all patients had their BRG measured by using the sequence method.
Statistics Analysis
All data are reported as mean±SD. Comparisons were made by using paired Student t tests for normally distributed data and the Wilcoxon signed rank test for non–normally distributed data. Two‐sample t tests and Mann–Whitney rank sum tests for non–normally distributed data were used for comparisons between groups. Repeated‐measures ANOVA was used for comparison of BP and HR during AF and Day 1 and Day 30 post‐cardioversion, with Holm–Sidak or the Dunn method applied for posthoc comparisons. The χ2 or Fisher exact tests were used to compare proportions between groups. Statistical analysis was performed with SigmaStat (Systat Software Inc).
Results
Patients’ Clinical Characteristics
The mean age for the AF group was 67±11 years, with the majority of patients being male (n=18/24). LVEF was 55±13% in the AF group and 57±6% in the control group (P=NS). The prevalence of diabetes, hypertension, and heart failure was not significantly different between the groups; however, the use of β‐blockers, Ca2+ channel blockers, and antiarrhythmic agents was significantly higher in the AF group compared with the control group.
The clinical characteristics of patients with AF recurrence and no recurrence were not significantly different. The mean LVEF and use of antiarrhythmic agents were lower in the AF‐recurrence group compared with the AF‐no recurrence group; however, the differences were not statistically significant (51±9% versus 58±13% for LVEF; 40% versus 64% for antiarrhythmic agent use). A summary of the patients’ characteristics including medication use at the time of enrollment is provided in Table 1.
Table 1.
Patients’ Characteristics
| Control (n=24) | All AF (n=24) | AF‐Recurrence (n=10) | AF‐No Recurrence (n=14) | P Value | |
|---|---|---|---|---|---|
| Mean age, y | 62±14 | 67±11 | 65±11 | 69±11 | 0.15 |
| Sex, n M:F | 13:11 | 18:6 | 7:3 | 11:3 | 0.89 |
| Mean EF (%) | 57±6 | 55±13 | 51±9 | 58±13 | 0.06 |
| Diabetes | 17% | 13% | 30% | 0% | 0.10 |
| Hypertension | 63% | 63% | 50% | 71% | 0.90 |
| Heart failure | 4% | 17% | 30% | 7% | 0.30 |
| Medications | |||||
| β‐Blockers | 29%a | 63% | 70% | 57% | 0.98 |
| Ca2+ channel blockers | 0%a | 29% | 60% | 7% | 0.08 |
| Antiarrhythmic agents | 8%a | 54% | 40% | 64% | 0.72 |
AF indicates atrial fibrillation; EF, ejection fraction.
P<0.05 control group vs all AF. P values are provided comparing the AF‐recurrence and AF‐no recurrence groups.
BP and HR Changes
The average diastolic BP and mean BP were not significantly different in the AF group compared with the control group; however, the average SBP was higher in the AF group compared with the control group (137±30 versus 117±26 mm Hg, P<0.05). Similarly, the mean HR was significantly higher in the AF group compared with the control group (78±17 versus 69±11 bpm, P<0.05).
On Day 1 post‐cardioversion, no significant changes occurred in BP measurements compared with the measurements made during AF; however, a significant decrease in HR was noted in both AF groups. In patients who remained in SR at Day 30 (ie, AF‐no recurrence group), there was a trend for a decrease in diastolic BP and mean BP after restoring SR. Diastolic BP decreased from 73±18 mm Hg during AF to 70±14 mm Hg on Day 1 and to 66±11 mm Hg on Day 30 (P=0.36). Mean BP decreased from 95±21 mm Hg during AF to 93±15 mm Hg on Day 1 and to 92±12 mm Hg on Day 30 (P=0.85). SBP decreased from 136±27 mm Hg during AF to 134±16 mm Hg on Day 1 but increased to 144±9 mm Hg on Day 30 (P=0.14). A summary of all the baseline hemodynamic measurements and changes during Day 1 and Day 30 post‐cardioversion is provided in Table 2.
Table 2.
Heart Rate, BP, and BRG Measurements in the Control Group and AF Cardioversion Group
| Control (n=24) | All AF (n=24) | AF‐Recurrence (n=10) | AF‐No Recurrence (n=14) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D1 | AF | D1 | D30 | AF | D1 | D30 | AF | D1 | D30 | |
| SBP, mm Hg | 117±26a | 137±30 | 136±26 | N/A | 138±35 | 139±36 | N/A | 136±27 | 134±16 | 144±9 |
| DBP, mm Hg | 76±10 | 73±22 | 70±18 | N/A | 74±27 | 71±23 | N/A | 73±18 | 70±14 | 66±11 |
| MBP, mm Hg | 89±11 | 93±24 | 93±21 | N/A | 91±27 | 94±27 | N/A | 95±21 | 93±15 | 92±12 |
| HR, bpm | 69±11a | 78±17 | 62±11b | N/A | 81±19 | 62±9b | N/A | 75±15 | 62±12b | 60±12b |
| BRG, ms/mm Hg | 10.8±5.5a | N/A | 5.2±3.6 | N/A | N/A | 6.7±2.9 | N/A | N/A | 4.1±3.7 | 7±6c |
AF indicates atrial fibrillation; BRG, arterial baroreflex gain; D1, sinus rhythm Day 1; D30, sinus rhythm Day 30; HR, heart rate; N/A, not applicable; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure.
P<0.05 vs all AF.
P<0.05 vs AF within‐column grouping.
P<0.05 vs D1 in the AF‐no recurrence column.
Arterial BRG Measurements
The mean BRG in the AF group (n=24) on Day 1 post‐cardioversion was significantly lower than the mean BRG of the control group (5.2±3.6 versus 10.8±5.5 ms/mm Hg, P<0.05). There were no differences in the clinical characteristics, BP or HR measurements during AF or Day 1 post‐cardioversion in the AF‐recurrence group when compared with the AF‐no recurrence group. A summary of the BRG measurements in the control group and AF group including the AF‐recurrence and AF‐no recurrence groups is provided in Table 2.
In the AF‐no recurrence group (n=14), BRG increased from 4.1±3.7 ms/mm Hg on Day 1 to 7.0±6.0 ms/mm Hg on Day 30 post‐cardioversion (P<0.01). When looking at the subsets using the different methods of BRG assessment, the statistical significance was still present regardless of the method used (P=0.04 with the modified Oxford technique; P=0.03 with the sequence method technique). A representation of the individual BRG data points is shown in Figure.
Figure 1.
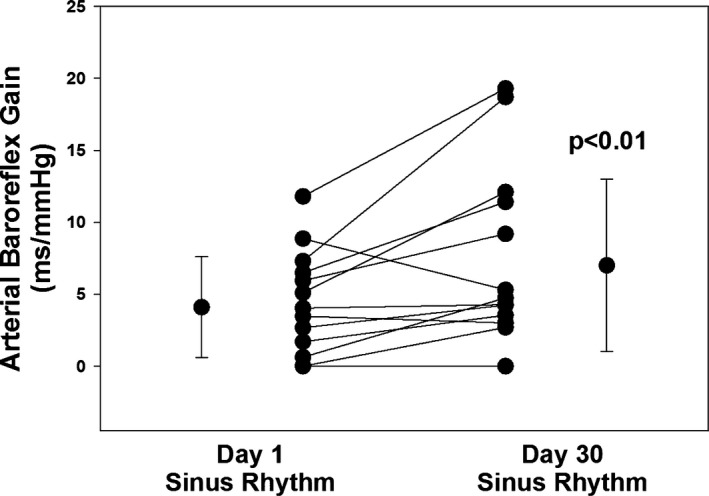
Arterial baroreflex gain values at Day 1 and Day 30 post‐cardioversion including averages in subjects who remained in sinus rhythm (ie, AF‐no recurrence group). There was a significant improvement in baroreflex gain on Day 30 compared with Day 1 post‐cardioversion (P<0.01). AF indicates atrial fibrillation.
Discussion
The main findings from this study were that AF was associated with an impairment of the baroreflex and that restoration of SR resulted in a significant improvement in arterial BRG at 30 days post‐cardioversion. The arterial baroreflex plays an important role in BP regulation in addition to its prognostic value in patients with heart disease. Our data provide evidence for the first time that AF might be a contributing factor to the observed impairment in BRG and that restoring SR could be another method for improving baroreflex function.
Effects of AF on Sympathetic Activity and Baroreflex Function
While there are several studies evaluating the role of the autonomic nervous system in the genesis of AF, there are only limited studies evaluating the effects of AF on sympathetic activity and baroreflex function.3, 11, 12, 13, 14 In the acute setting, we had previously shown that induced AF was associated with a 71% increase in sympathetic nerve activity (SNA) and that the variability in RR intervals played a major role in mediating the reflex sympathoexcitation.4 Interestingly, the increase in SNA was seen despite an increase in central venous pressure and absence of a significant decrease in BP, suggesting minimal contribution of the cardiopulmonary baroreflex and a presumed arterial baroreflex–mediated mechanism independent of mean BP. The postulated mechanism was that the long RR coupling intervals commonly observed in AF resulted in unloading of the arterial baroreceptors and reflex increase in SNA despite the absence of a significant decrease in mean BP. In a subsequent study, we demonstrated that the magnitude of sympathoexcitation correlated with the degree of variability in RR intervals.15 After controlling for the hemodynamic changes, there was a 6.1% increase in SNA for every 1% increase in irregularity. Ikeda et al13 found no difference in total multiunit muscle SNA in patients with heart failure and chronic AF compared with patients in SR; however, there was a significant increase in the single‐unit muscle SNA in the AF group compared with the SR group (62±9 versus 42±4 spikes/min, P<0.05). Similar to the previous studies, the authors attributed the increase in single‐unit muscle SNA to the prolonged RR intervals seen in AF.
In addition to the reported changes in SNA, some studies suggest that patients with AF have impairment of the baroreflex. In a study including 73 patients with paroxysmal AF,16 the authors found that mean baroreflex sensitivity was impaired in this population (7.8±5.8 ms/mm Hg) and that several autonomic variables including baroreflex sensitivity were predictive of quality of life scores. In another study that included patients with heart failure, the authors found that AF was associated with impaired sympathetic response to head‐up tilt. Transcardiac norepinephrine (NE) gradient and coronary sinus NE levels increased significantly from baseline during 30° head‐up tilt in patients with normal SR but not in patients with AF (P=0.014 for transcardiac NE; P<0.001 for coronary sinus NE).12 The authors concluded that AF was associated with impaired cardiac sympathetic response to baroreceptor unloading, possibly as a result of atrial fibrosis. What is unclear from these studies is whether the impairment in the baroreflex was a surrogate of the chronic illnesses commonly seen in patients with AF or the result of AF itself, or a combination of both factors.
Present Study
In the present study, we have shown that patients with AF have a lower BRG compared with an age‐ and LVEF‐matched control group without AF. We do not believe the impairment in BRG was caused by comorbid conditions, because the prevalence of diabetes, hypertension, and heart failure was not significantly different between the groups. We have also shown that restoration of SR resulted in improvement in BRG, suggesting that AF itself was a contributing factor. Consistent with our hypothesis, mean BRG increased from 4.1±3.7 ms/mm Hg on Day 1 to 7.0±6.0 ms/mm Hg on Day 30 post‐cardioversion (P<0.01). Interestingly, despite the significant increase from baseline, mean BRG at Day 30 remained low compared with the control group (P<0.01), suggesting that there are several factors responsible for the impairment in the baroreflex, with the rhythm abnormality only being one of them. Alternatively, the process of recovery might be longer than 30 days. Of note, our results at Day 30 of a mean BRG equal to 7.0±6.0 ms/mm Hg were consistent with the results reported by van den Berg et al in patients with a paroxysmal AF in whom the mean baroreflex sensitivity was 7.8±5.8 ms/mm Hg.16 Another finding in our study was the presence of a higher mean BRG in patients who had AF recurrence compared with those with no recurrence. While the number of patients is small, this finding highlights again the role of role of the autonomic nervous system in the genesis of AF.
The mechanisms via which AF leads to impairment of the BRG are not clear. Persistent AF has been shown to be associated with atrial dilatation and fibrosis,17 which might lead to impairment of the cardiopulmonary baroreceptors. In addition, patients with AF have elevated inflammatory markers, endothelial dysfunction, and impaired vascular function,18, 19 which theoretically could alter the input to the arterial baroreceptors and, thus, modulate baroreflex function. It is important to note that while our data showed impairment in BRG in patients with AF, our assessment of baroreflex function was performed by measuring the HR limb of the baroreflex arc. Whether the same is true with baroreflex control of sympathetic nerve activity remains to be determined.
Limitations
BRG measurements obtained 2 to 4 hours post‐cardioversion were used as a surrogate of BRG during AF. AF‐induced changes in BRG are likely to be the result of structural changes that should still be present for several days post‐cardioversion. Therefore, it is reasonable to assume that our assessment of BRG at 2 to 4 hours post‐cardioversion was a reliable surrogate of the baroreflex during AF. The use of β‐blockers and antiarrhythmic agents was significantly lower in the control group compared with the AF group. Further, the 2 groups might have still differed in ways not captured by the data shown. While such differences might have played a role in our findings, the prevalence of major comorbid conditions known to affect BRG, such as diabetes, hypertension, and heart failure, was similar in the control group compared with the AF group. Day 1 BRG measurements were made after the patients received propofol. Propofol is known to cause bradycardia and hypotension; however, given its rapid onset and offset of actions and short half‐life, it is unlikely that it had an effect on our BRG measurements.20 The BRG measurements on Day 1 were performed under different conditions than on Day 30. Anxiety, stress, and changes in volume status were likely present on the day of cardioversion but not during follow‐up. All these factors could have played a role in the observed changes in BRG on Day 1 compared with Day 30. The improvement in BRG on Day 30 compared with Day 1 might be in part caused by “sinus node remodeling.”21 However, the absence of a difference in resting HR on Day 1 (62±12 bpm) compared with Day 30 (60±12 bpm) suggests that sinus node remodeling played a minimal role in the observed changes in BRG. Last, we used different methods for the assessment of the baroreflex, namely the modified Oxford technique and sequence method. It is important to note that both methods have been validated and shown to correlate with each other.9 In addition, subgroup analysis in patients who remained in SR showed significant improvement in BRG with both methods, thus further validating our hypothesis.
Conclusions
We have shown that patients with persistent AF have an impaired baroreflex and that restoration of SR resulted in an improvement at 30 days. Our data suggest that AF might be a contributing factor to the observed impairment in BRG and that rhythm control might be another mean of improving baroreflex function in patients with AF. The long‐term effects of rhythm control on BP regulation and orthostatic tolerance remain to be determined.
Sources of Funding
This work was supported in part by funds provided from the Mildred and Marv Conney Chair in Cardiology.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002997 doi: 10.1161/JAHA.115.002997)
References
- 1. Carpenter A, Frontera A, Bond R, Duncan E, Thomas G. Vagal atrial fibrillation: what is it and should we treat it? Int J Cardiol. 2015;201:415–421. [DOI] [PubMed] [Google Scholar]
- 2. Chen J, Wasmund SL, Hamdan MH. Back to the future: the role of the autonomic nervous system in atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:413–421. [DOI] [PubMed] [Google Scholar]
- 3. Lok NS, Lau CP. Abnormal vasovagal reaction, autonomic function, and heart rate variability in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 1998;21:386–395. [DOI] [PubMed] [Google Scholar]
- 4. Wasmund SL, Li JM, Page RL, Joglar JA, Kowal RC, Smith ML, Hamdan MH. Effect of atrial fibrillation and an irregular ventricular response on sympathetic nerve activity in human subjects. Circulation. 2003;107:2011–2015. [DOI] [PubMed] [Google Scholar]
- 5. Hunt BE, Fahy L, Farquhar WB, Taylor JA. Quantification of mechanical and neural components of vagal baroreflex in humans. Hypertension. 2001;37:1362–1368. [DOI] [PubMed] [Google Scholar]
- 6. Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl. 1985;3:S79–S81. [PubMed] [Google Scholar]
- 7. Fritsch JM, Eckberg DL, Graves LD, Wallin BG. Arterial pressure ramps provoke linear increases of heart period in humans. Am J Physiol. 1986;251:R1086–R1090. [DOI] [PubMed] [Google Scholar]
- 8. Hughson RL, Quintin L, Annat G, Yamamoto Y, Gharib C. Spontaneous baroreflex by sequence and power spectral methods in humans. Clin Physiol. 1993;13:663–676. [DOI] [PubMed] [Google Scholar]
- 9. Davies LC, Francis D, Jurak P, Kara T, Piepoli M, Coats AJ. Reproducibility of methods for assessing baroreflex sensitivity in normal controls and in patients with chronic heart failure. Clin Sci (Lond). 1999;97:515–522. [PubMed] [Google Scholar]
- 10. Johnson P, Shore A, Potter J, Panerai R, James M. Baroreflex sensitivity measured by spectral and sequence analysis in cerebrovascular disease: methodological considerations. Clin Auton Res. 2006;16:270–275. [DOI] [PubMed] [Google Scholar]
- 11. Bauernschmitt R, Malberg H, Wessel N, Brockmann G, Wildhirt SM, Kopp B, Kurths J, Bretthauer G, Lange R. Autonomic control in patients experiencing atrial fibrillation after cardiac surgery. Pacing Clin Electrophysiol. 2007;30:77–84. [DOI] [PubMed] [Google Scholar]
- 12. Gould PA, Yii M, Esler MD, Power JM, Kaye DM. Atrial fibrillation impairs cardiac sympathetic response to baroreceptor unloading in congestive heart failure. Eur Heart J. 2005;26:2562–2567. [DOI] [PubMed] [Google Scholar]
- 13. Ikeda T, Murai H, Kaneko S, Usui S, Kobayashi D, Nakano M, Ikeda K, Takashima S, Kato T, Okajima M, Furusho H, Takamura M. Augmented single‐unit muscle sympathetic nerve activity in heart failure with chronic atrial fibrillation. J Physiol. 2012;590:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mainardi L, Corino V, Belletti S, Terranova P, Lombardi F. Low frequency component in systolic arterial pressure variability in patients with persistent atrial fibrillation. Auton Neurosci. 2009;151:147–153. [DOI] [PubMed] [Google Scholar]
- 15. Segerson NM, Sharma N, Smith ML, Wasmund SL, Kowal RC, Abedin M, MacGregor JF, Pai RK, Freedman RA, Klein RC, Wall TS, Stoddard GJ, Hamdan MH. The effects of rate and irregularity on sympathetic nerve activity in human subjects. Heart Rhythm. 2007;4:20–26. [DOI] [PubMed] [Google Scholar]
- 16. van den Berg MP, Hassink RJ, Tuinenburg AE, van Sonderen EF, Lefrandt JD, de Kam PJ, van Gelder IC, Smit AJ, Sanderman R, Crijns HJ. Quality of life in patients with paroxysmal atrial fibrillation and its predictors: importance of the autonomic nervous system. Eur Heart J. 2001;22:247–253. [DOI] [PubMed] [Google Scholar]
- 17. Petersen P, Pedersen F, Madsen EB, Brun B, Gyldensted C, Boysen G. Echocardiography and cerebral computed tomography in chronic atrial fibrillation. Eur Heart J. 1989;10:1101–1104. [DOI] [PubMed] [Google Scholar]
- 18. Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wijesurendra RS, Casadei B. Atrial fibrillation: effects beyond the atrium? Cardiovasc Res. 2015;105:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu F, Lin J, Benditt DG. Conscious sedation and anesthesia in the cardiac electrophysiology laboratory. J Cardiovasc Electrophysiol. 2013;24:237–245. [DOI] [PubMed] [Google Scholar]
- 21. Lee JM, Kalman JM. Sinus node dysfunction and atrial fibrillation: two sides of the same coin? Europace. 2013;15:161–162. [DOI] [PubMed] [Google Scholar]


