Abstract
Background
We determined whether vascular and valvular calcification predicted incident major coronary heart disease, cardiovascular disease (CVD), and all‐cause mortality independent of Framingham risk factors in the community‐based Framingham Heart Study.
Methods and Results
Coronary artery calcium (CAC), thoracic and abdominal aortic calcium, and mitral or aortic valve calcium were measured by cardiac computed tomography in participants free of CVD. Participants were followed for a median of 8 years. Multivariate Cox proportional hazards models were used to determine association of CAC, thoracic and abdominal aortic calcium, and mitral and aortic valve calcium with end points. Improvement in discrimination beyond risk factors was tested via the C‐statistic and net reclassification index. In this cohort of 3486 participants (mean age 50±10 years; 51% female), CAC was most strongly associated with major coronary heart disease, followed by major CVD, and all‐cause mortality independent of Framingham risk factors. Among noncoronary calcifications, mitral valve calcium was associated with major CVD and all‐cause mortality independent of Framingham risk factors and CAC. CAC significantly improved discriminatory value beyond risk factors for coronary heart disease (area under the curve 0.78–0.82; net reclassification index 32%, 95% CI 11–53) but not for CVD. CAC accurately reclassified 85% of the 261 patients who were at intermediate (5–10%) 10‐year risk for coronary heart disease based on Framingham risk factors to either low risk (n=172; no events observed) or high risk (n=53; observed event rate 8%).
Conclusions
CAC improves discrimination and risk reclassification for major coronary heart disease and CVD beyond risk factors in asymptomatic community‐dwelling persons and accurately reclassifies two‐thirds of the intermediate‐risk population.
Keywords: coronary disease, prognosis, risk factors
Subject Categories: Computerized Tomography (CT), Cardiovascular Disease, Prognosis
Introduction
Cardiovascular disease (CVD), the leading cause of death in men and women,1 is preceded by calcified atherosclerotic plaques in multiple major vascular beds and calcification in the heart valves. Atherosclerosis is systemic disease of the arterial vascular wall. Asymptomatic forms of “subclinical” atherosclerosis, including calcified plaques, can be detected as early as adolescence and progress with age.2 Development and progression of atherosclerosis are strongly associated with major cardiovascular risk factors. Subclinical atherosclerosis manifesting as coronary artery calcification (CAC) is associated with future coronary heart disease (CHD), independent of traditional risk factors, and CAC improves discrimination and classification of CHD risk overall and in persons at intermediate risk by placing more persons in the most extreme risk categories.3, 4 Similar associations with incident CHD and/or CVD have been reported for noncoronary calcifications including the thoracic and abdominal aorta using either plain radiographs5, 6, 7, 8, 9 or computed tomography (CT) scans10, 11, 12 and for aortic sclerosis using echocardiography.13 Nevertheless, it remains largely unclear whether calcium measures obtained in multiple vascular beds and the major left heart valves are incremental to CAC for reclassification of risk for cardiovascular events.
The primary objective of the current study, in a large prospective community‐based white cohort, was to determine prediction, discrimination, and reclassification of risk for cardiovascular events by CAC and by noncoronary aortic and valvular calcifications, reflecting the systemic nature of atherosclerotic disease, and to determine whether associations seen for CHD can be extended to CVD end points and overall mortality.
Methods
Details regarding the Framingham Heart Study population, the selection criteria and design of the Framingham multidetector CT (MDCT) imaging study, and the method of calcium measurements have been published and described elsewhere.14, 15, 16, 17
Study Population
Participants for this study were drawn from the Offspring and Third Generation cohorts of the community‐based Framingham Heart Study. Participants in the analysis attended the Offspring seventh examination cycle (1998–2001) or the Third Generation first examination cycle (2002–2005) and had complete risk factor information. Inclusion in the Framingham MDCT study was weighted toward participants from larger Framingham Heart Study families and those who resided in the Greater New England area. Participating men were aged ≥35 years, and women were aged ≥40 years. In addition, women were not pregnant (confirmed by a urine pregnancy test), and all participants weighed <350 lb according to MDCT scanner specifications. The institutional review boards of the Boston University Medical Center and Massachusetts General Hospital approved the study. All participants provided written consent.
CT Imaging of the Chest and Abdomen
Participants were imaged on an 8‐slice MDCT scanner (LightSpeed Ultra; General Electric) using established noncontrast imaging protocols for the heart (slice thickness 1.25 mm) and the abdomen (slice thickness 2.5 mm), aided by landmarks identified in an initial scout film.14 Cardiac CT scanning was performed during breath hold with the acquisition triggered to the cardiac cycle, resulting in diastolic images.18 Immediately after chest imaging was completed, the participant was repositioned, and abdominal imaging was performed in spiral CT mode covering 15 cm cranial from the top of the S1 vertebral body. The effective radiation exposure was 1.0 to 1.25 mSv imaging of the heart and 2.7 mSv for the abdomen.
Measurements of Vascular and Valvular Calcifications
All MDCT scans were read independently by experienced readers using a dedicated offline workstation (Aquarius; Terarecon). All calcifications were measured using published, highly reproducible methods, as described previously, based on a modified Agatston score (AS).19 CAC was defined as calcification along the course of the coronary arteries.14 Thoracic aortic calcification was defined as calcification within the aortic wall above the diaphragm, and abdominal aortic calcification (AAC) was defined as calcification above the iliac bifurcation and below the diaphragm.15 Aortic valve calcium was defined as calcium deposits of the aortic cusps or nodular deposits at the coaptation points of the aortic cusps. Mitral valve calcium (MVC) was defined as calcium deposits in the region of the annulus and/or the mitral valve leaflets.17 Participants and their care providers were informed of the findings only for CAC >90th percentile.
Risk Factor Measurements
The standard clinic examination at the Offspring seventh cycle or the Third Generation first examination cycle included a physician interview, a physical examination, and laboratory tests. Body mass index was defined as weight (in kilograms) divided by the square of height (in meters) and was measured at each index examination. Adult‐onset diabetes was defined as fasting glucose ≥126 mg/dL at a Framingham examination or treatment with either insulin or a hypoglycemic agent. Participants were considered to be current smokers if they smoked at least 1 cigarette per day for the previous year. Blood pressure was measured in the left arm of the seated participant using a standardized protocol. Systolic and diastolic blood pressures were obtained using standardized protocols, as reported previously.20 Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive drug treatment. Total and high‐density lipoprotein cholesterol measurements were obtained using standardized protocols, as reported previously.20 Hypercholesterolemia was defined as total cholesterol ≥240 mg/dL or use of lipid‐lowering drug treatment.
Patients with prior stroke, congestive heart failure, myocardial infarction, coronary insufficiency, coronary artery bypass grafting, valve replacement, or percutaneous coronary stent placement were excluded from the analyses.
CVD Outcomes
In the Framingham Heart Study, CVD in previous risk algorithms was defined as CHD (ie, a fatal coronary event, myocardial infarction) or a cerebrovascular event (ie, ischemic stroke, hemorrhagic stroke).21 For the purpose of this study, major CHD events included recognized myocardial infarction and death from CHD, and major CVD events included major CHD events and ischemic stroke, in accordance with atherosclerotic CVD end points defined in the 2013 American College of Cardiology/American Heart Association risk assessment guidelines.22 In addition, we determined all‐cause mortality. All study participants were under continuous surveillance for the development of CVD events and death, and information about CVD events on follow‐up was obtained with the aid of medical histories, physical examinations at the study clinic, hospitalization records, and communication with personal physicians. All suspected new events were reviewed by a panel of 3 experienced investigators who evaluated all pertinent medical records. A separate review committee that included a neurologist adjudicated cerebrovascular events, and a heart study neurologist examined most participants with suspected stroke.
Statistical Analysis
The primary outcome of interest was major CHD. Additional outcomes were major CVD and all‐cause mortality.
CAC, AAC, thoracic aortic calcification, MVC, and aortic valve calcium were the primary exposures. CAC was stratified into categories of 0, 1 to 100, 101 to 300, and >300. All other measures of calcification were treated as continuous variables with natural logarithmic transformation of the modified AS. Log‐transformed continuous CAC was a secondary exposure.
For each outcome analysis, participants for whom the outcome was already prevalent at baseline were excluded.
After checking the assumption of proportional hazards for both calcium measures and outcomes (all P>0.10), we used Cox proportional hazards regression models to relate each calcification measure to time to event. Multivariable models were initially adjusted for Framingham risk factors (FRFs), as reported by D'Agostino et al21 (age, sex, systolic blood pressure, prevalence of antihypertensive treatment, prevalence of diabetes, total cholesterol, high‐density lipoprotein cholesterol, and current smoking status), followed by a second model for extracoronary calcifications, which was also adjusted for the natural logarithm of CAC.
The discriminatory ability of the Cox models associated with primary outcomes after multivariable adjustment models was assessed with the use of the C‐statistic.23 Specifically, the C‐statistic from FRF‐adjusted multivariate models were compared with risk factors plus the log of coronary calcium–adjusted models.
We further calculated the net reclassification index (NRI) and its 2‐sided 95% CI, as done by Pencina et al,24 to assess the incremental discriminatory ability of calcium measures above traditional FRFs. To determine NRI, we used the cut points of 2.5%, 5%, and 10% for major CHD and 2.5%, 6.5%, and 10% for major CVD.
Analyses were performed with the use of SAS software, version 9.2 (SAS Institute). P values were considered significant using a 2‐sided 0.05 level of significance and were 2‐sided. There was no adjustment for multiple comparisons.
Results
Study Population
Of the 3529 participants undergoing MDCT, 3505 attended Offspring examination 7 or Third Generation examination 1. Of these, 3486 had an evaluable result for at least 1 of the 5 calcium measures of interest, had a complete risk factor profile, and were available for analysis. Table 1 displays descriptive statistics for the cohort that was free of any CVD (major or otherwise; n=3217). Because participants with CHD or CVD outcomes at baseline were excluded from the analyses, there were slightly different sample sizes for major CHD and CVD analyses. Participants had a mean age of 50 years, half were women, and nearly two‐thirds were at low risk based on FRFs (<6%) (Table 1). Abdominal aorta calcification was most prevalent (51.2%), followed by CAC (42.5%), whereas calcifications of the thoracic aorta (20.8%) and the valves (7.0–14.5%) were less frequent. All calcium measures were significantly correlated with each other, with the highest correlation observed between CAC and AAC (r=0.10–0.37; all P<0.0001 after adjustment for age and sex).
Table 1.
Baseline Characteristics Including Demographics; Framingham Risk Factors; and Measures of Coronary, Aortic, and Valvular Calcification
| Imaging Cohort | |
|---|---|
| Participants, na | 3217 |
| Age, y, | 50±10 |
| Female, n (%) | 1639 (50.9) |
| Hypercholesterolemia, n (%) | 21.4% |
| On statin treatment, n (%) | 11.5% |
| Total cholesterol, mg/dL | 196.7±35.0 |
| HDL cholesterol, mg/dL | 53.8±16.6 |
| Hypertension, n (%) | 42.9 |
| On hypertensive treatment, n (%) | 522 (16.2) |
| Systolic blood pressure, mm Hg | 122±18 |
| Diastolic blood pressure, mm Hg | 77±20 |
| Diabetes mellitus, n (%) | 169 (5.3) |
| Body mass index, kg/m2 (mean) | 27.7±5.9 |
| Obese (>30 kg/m2) | 861 (26.8) |
| Smoking, n (%) | |
| Current, n (%) | 401 (12.5) |
| Former, n (%) | 1219 (37.9) |
| Never, n (%) | 1597 (49.6) |
| Framingham Risk Score | |
| Mean | 6.6±6 |
| Low/intermediate/high, n (%)b | 1926 (59.9)/1166 (36.2)/124 (3.9) |
| Vascular and valvular calcifications | |
| CAC | |
| Prevalence, n (%) | 1351 (42.5) |
| 25th/50th/75th/95th percentile | 0/0/32.3/502.9 |
| 0, 1 to 100, 101 to 300, >300, n (%) | 1831 (57.5), 822 (25.8), 270 (8.5), 259 (8.1) |
| AAC | |
| Prevalence, n (%) | 1637 (51.2) |
| 25th/50th/75th/95th percentile | 0/1.2/368/4099 |
| TAC | |
| Prevalence, n (%) | 660 (20.8) |
| 25th/50th/75th/95th percentile | 0/0/0/559 |
| AVC | |
| Prevalence, n (%) | 475 (14.9) |
| 25th/50th/75th/95th percentile | 0/0/0/75 |
| MVC | |
| Prevalence, n (%) | 225 (7.0) |
| 25th/50th/75th/95th percentile | 0/0/0/18 |
All values shown are mean±SD except when otherwise specified. AAC indicates abdominal aorta calcification; AVC, aortic valve calcification; CAC, coronary artery calcification; HDL, high‐density lipoprotein; MVC, mitral valve calcium; TAC, thoracic aorta calcification.
Without prevalent cardiovascular disease.
Framingham Heart Study: low 0% to 6%, intermediate 6% to 20%, high >20%.
Outcomes
During a mean follow‐up of 8 years, we observed 59 major CHD events (1.7%; 55 myocardial infarction and 4 CHD death); 107 major CVD events (3.1%; 59 CHD events and 48 stroke); and 152 deaths (4.4%), including 74 deaths from cancer, in participants with at least 1 calcium measure.
Prediction of Major CHD, CVD, and Mortality by Calcifications
Major CHD
Figure 1 demonstrates the unadjusted Kaplan–Meier curves by CAC categories for 8 years of follow‐up for participants without prevalent major CHD and with an available value for coronary artery calcium (n=3340). Event rates increased with category of CAC (P<0.001), from 0.5% in participants with AS of 0 (n=1884, 56.4%) to 0.9% in participants with an AS of 1 to 100 (n=851, 25.5%), 4.5% in participants with an AS of 101 to 300 (n=287, 8.6%), and 8.5% in participants with an AS >300 (n=318, 9.5%). In both unadjusted and multivariate‐adjusted analysis, an AS of 1 to 100 did not carry a significantly elevated event risk (multivariate‐adjusted hazard ratio [HR] 1.46, 95% CI 0.57–3.75; P=0.43), whereas AS of both 101 to 300 and >300 carried significantly elevated risk for CHD (HR 4.63, 95% CI 1.73–12.40, and HR 9.36, 95% CI 3.60–24.40, respectively; P≤0.002). Log‐continuous AS was also significantly associated with an increased risk for major CHD (multivariate‐adjusted HR per 1‐SD log [AS]: 2.46, 95% CI 1.75–3.48; P<0.001). Notably, all noncoronary calcifications also predicted major CHD events independent of age, sex, and traditional FRFs (Table 2); however, after further adjustment for CAC, the HR for each noncoronary measure was attenuated and was not statistically significant for prediction of CHD events.
Figure 1.
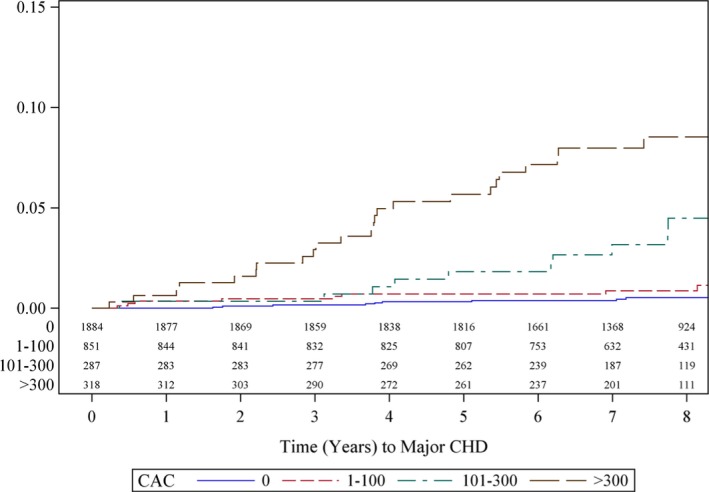
Kaplan–Meier estimates of CHD events by CAC burden in the Framingham population. Kaplan–Meier curve demonstrating a significantly increased rate of CHD events in patients with >100 Agatston score. CAC indicates coronary artery calcium; CHD, coronary heart disease.
Table 2.
HRs Per SD of Log‐Calcium or Category of Calcium and 95% CIs for Regression of Major Cardiovascular Events and All‐Cause Mortality Associated With Coronary, Aortic, and Valvular Calcification
| End point | Major CHD | Major CVD | Mortality | |||
|---|---|---|---|---|---|---|
| No. events/sample size/% | 59/3399/1.7 | 103/3374/3.1 | 152/3486/4.4 | |||
| Years of follow‐up | 7.55 | 7.54 | 8.62 | |||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| CAC continuous | ||||||
| A/S adjusted | 2.89 (2.06–4.05) | <0.001 | 1.97 (1.54–2.51) | <0.001 | 1.34 (1.08–1.66) | 0.009 |
| RF adjusted | 2.46 (1.75–3.48) | <0.001 | 1.75 (1.37–2.44) | <0.001 | 1.26 (1.01–1.57) | 0.045 |
| CAC categorical | ||||||
| A/S adjusted | ||||||
| 0 | Ref | Ref | Ref | |||
| 1 to 100 | 1.91 (0.75–4.85) | 0.17 | 1.69 (0.87–3.25) | 0.12 | 0.80 (0.45–1.41) | 0.44 |
| 101 to 300 | 6.52 (2.46–17.2) | <0.001 | 4.77 (2.39–9.52) | <0.001 | 1.48 (0.81–2.70) | 0.21 |
| >300 | 15.3 (5.90–39.5) | <0.001 | 6.04 (2.93–12.5) | <0.001 | 1.52 (0.82–2.82) | 0.18 |
| RF adjusted | ||||||
| 0 | Ref | Ref | Ref | |||
| 1 to 100 | 1.46 (0.57–3.75) | 0.43 | 1.36 (0.70–2.63) | 0.36 | 0.71 (0.40–1.26) | 0.24 |
| 101 to 300 | 4.63 (1.73–12.4) | 0.002 | 3.73 (1.86–7.47) | <0.001 | 1.38 (0.75–2.54) | 0.30 |
| >300 | 9.36 (3.60–24.4) | <0.001 | 4.27 (2.08–8.78) | <0.001 | 1.26 (0.67–2.36) | 0.48 |
| AAC | ||||||
| A/S adjusted | 2.71 (1.76–4.11) | <0.001 | 1.95 (1.44–2.64) | <0.001 | 1.57 (1.18–2.08) | 0.002 |
| RF adjusted | 1.95 (1.27–3.00) | 0.002 | 1.50 (1.11–2.05) | 0.01 | 1.34 (1.01–1.79) | 0.046 |
| lgCAC adjusted | 1.21 (0.78–1.90) | 0.39 | 1.20 (0.86–1.67) | 0.29 | 1.26 (0.92–1.73) | 0.145 |
| TAC | ||||||
| A/S adjusted | 1.59 (1.21–2.09) | <0.001 | 1.31 (1.07–1.59) | 0.007 | 1.50 (1.26–1.78) | <0.001 |
| RF adjusted | 1.40 (1.06–1.83) | 0.02 | 1.18 (0.98–1.44) | 0.09 | 1.41 (1.18–1.68) | <0.001 |
| lgCAC adjusted | 1.11 (0.83–1.47) | 0.48 | 1.04 (0.86–1.27) | 0.67 | 1.33 (1.10–1.61) | 0.003 |
| AVC | ||||||
| A/S adjusted | 1.31 (1.06–1.62) | 0.013 | 1.17 (1.00–1.36) | 0.049 | 1.21 (1.06–1.38) | 0.003 |
| RF adjusted | 1.26 (1.01–1.57) | 0.037 | 1.13 (0.97–1.33) | 0.12 | 1.20 (1.05–1.37) | 0.006 |
| lgCAC adjusted | 1.07 (0.86–1.34) | 0.54 | 1.09 (0.93–1.28) | 0.29 | 1.12 (0.97–1.29) | 0.13 |
| MVC | ||||||
| A/S adjusted | 1.21 (1.04–1.24) | 0.014 | 1.24 (1.11–1.38) | <0.001 | 1.23 (1.13–1.34) | <0.001 |
| RF adjusted | 1.20 (1.02–1.41) | 0.03 | 1.23 (1.11–1.38) | <0.001 | 1.22 (1.11–1.33) | <0.001 |
| lgCAC adjusted | 1.11 (0.94–1.31) | 0.21 | 1.18 (1.06–1.32) | 0.003 | 1.20 (1.09–1.32) | <0.001 |
With the exception of CAC categorical, HRs are per 1‐SD increase in log‐transformed calcium measures. For CAC categorical, HRs are versus the CAC 0 group. No significant interaction of sex was present with of any of the calcium measurements to predict major CHD (P>0.10). For major CVD, the P values for the interaction of sex with measurements were between 0.05 and 0.10 for log‐transformed AAC, TAC, and MVC, but the direction of the effect was similar in men and women. A/S indicates adjusted for age and sex; AAC, abdominal aorta calcification; AVC, aortic valve calcification; CAC, coronary artery calcification; CHD, coronary heart disease; CVD, cardiovascular disease; HR, hazard ratio; lgCAC, adjusted for CAC; MVC, mitral valve calcification; Ref, reference group; RF, adjusted for Framingham risk factors; TAC, thoracic aorta calcification.
Major CVD
Associations for major CVD followed a similar pattern as for major CHD, but associations of CAC with major CVD were generally weaker than for CHD. Figure 2 demonstrates the unadjusted Kaplan–Meier curves by AS categories over 8 years of follow‐up. Event rates increased with category of CAC (P<0.001), from 1.1% in participants with CAC 0% to 2.5% in participants with an AS of 1–100, 8.8% in participants with an AS of 101–300, and 13.3% in participants with an AS >300. In both unadjusted and multivariate‐adjusted analysis, an AS of 1 to 100 did not carry a significantly elevated event risk (multivariate‐adjusted HR 1.36, 95% CI 0.70–2.63), whereas AS of both 101 to 300 and >300 carried significantly elevated risk for CVD (HR 3.73, 95% CI 1.86–7.47, and HR 4.27, 95% CI 2.08–8.78, respectively; P<0.001). Log‐continuous AS was also significantly associated with an increased risk for major CVD (multivariate HR 1.75, 95% CI 1.37–2.44; P<0.001). MVC is the only noncoronary calcium that predicted major CVD events independent of age and sex, FRFs, and CAC (HR 1.18, 95% CI 1.06–1.32; P=0.003). AAC predicted major CVD events independent of age and sex and FRFs but not independent of CAC, whereas thoracic aortic calcification and aortic valve calcium were not associated with major CVD, adjusting for FRFs and FRFs plus CAC.
Figure 2.
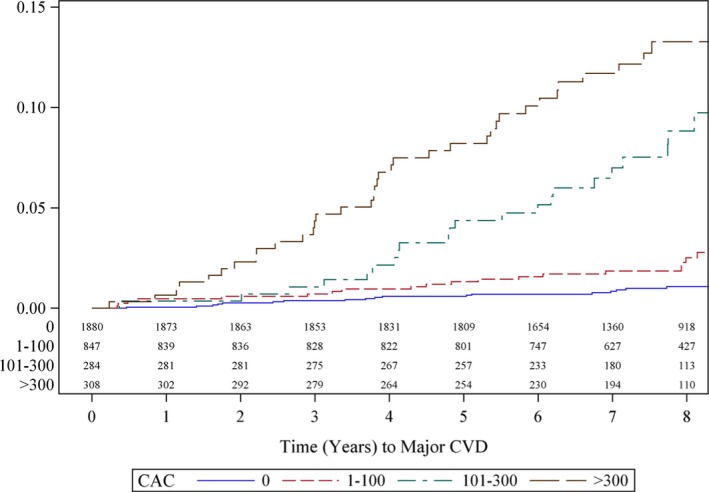
Kaplan–Meier estimates of CVD events by CAC burden in the Framingham population. Kaplan–Meier curve demonstrating a significantly increased rate of CVD events in patients with >100 Agatston score. CAC indicates coronary artery calcium; CVD, cardiovascular disease.
Although there were subgroups that carried higher risk for major CHD and CVD within this population (ie, male compared with female and Offspring compared with Third Generation), the associations between calcifications and events were similar, and there was no interaction with sex or age by cohort (detailed results are presented in the supplement).
All‐cause mortality
There was no increased risk for all‐cause mortality between those with CAC of 0 and categorical CAC; however, continuous CAC score was significantly associated with an increased risk for death independent of age, sex, and FRFs, albeit with a lower HR compared with cardiac‐specific outcomes. Moreover, the multivariate‐adjusted HR per 1‐SD increase in log‐CAC for CVD mortality was 2.43 (95% CI 1.30–4.56; P=0.006). In addition, both MVC and thoracic aortic calcification remained significantly associated with all‐cause mortality even after adjustment for age and sex, FRFs, and CAC (Table 2).
Sensitivity Analyses
If results were adjusted simply for the global Framingham risk score instead of individual risk factors contributing to the Framingham risk score, associations between calcification measures and Framingham risk score were even stronger. Results remained unchanged if additional adjustments for cholesterol medication use or body mass index were performed. In addition, smoking status was associated with CAC because former smokers had more CAC than those who never smoked and those who currently smoke. In further analyses, however, there were no significant interactions between smoking status and CAC with respect to outcomes.
Discrimination and Reclassification for Major CHD and CVD
Major CHD risk prediction
Adding log‐CAC to FRFs significantly increased discriminatory ability for major CHD over 8 years of follow‐up (C‐statistic for FRFs only: 0.78; C‐statistic after addition of CAC: 0.82; P value for difference between models <0.05) (Table 3).
Table 3.
Discrimination and Reclassification of Coronary, Aortic, and Valvular Calcification for Major CHD Events
| Major CHD Model: NRI Using Risk Categories of <0.025, 0.025 to <0.05, 0.05 to <0.10, ≥0.10 | C‐Statistic | NRI (95% CI) | Proportion Events/Nonevents Classified Correctly |
|---|---|---|---|
| RF only | 0.78 | — | — |
| RF+log CAC | 0.82 | 0.32 (0.11–0.53) | 0.33/−0.02 |
| RF+CAC cat | 0.83 | 0.22 (0.01–0.42) | 0.24/−0.02 |
| RF+log AAC | 0.79 | 0.12 (0.01–0.24) | 0.14/−0.02 |
| RF+log TAC | 0.80 | 0.11 (−0.03 to 0.24) | 0.11/−0.002 |
| RF+log MVC | 0.79 | 0.11 (−0.04 to 0.26) | 0.11/−0.003 |
| RF+log AVC | 0.79 | −0.03 (−0.19 to 0.11) | −0.03/0.0003 |
AAC indicates abdominal aorta calcification; AVC, aortic valve calcification; CAC, coronary artery calcification; cat, categorical; CHD, coronary heart disease; MVC, mitral valve calcification; NRI, net reclassification index; RF, adjusted for Framingham risk factors; TAC, thoracic aorta calcification.
The highest net reclassification of events and nonevents occurred in the model of log‐continuous CAC and FRFs (NRI 32%, 95% CI 11–53%). In addition, significant reclassification was conferred by categorical CAC (NRI 22%, 95% CI 1–42%]) and log‐AAC (NRI 12%, 95% CI 1–24%).
Major CVD risk prediction
Adding CAC to FRFs did not significantly increase discriminatory ability for major CVD over 8 years of follow‐up (C‐statistic FRFs only: 0.80; C‐statistic after addition of CAC: 0.82 for continuous and 0.82 for categorical CAC; P value for difference between models >0.05). The highest net reclassification of events and nonevents occurred in the model of log‐continuous CAC and FRF (NRI 25%, 95% CI 8–41%). In addition, there was significant reclassification conferred by log‐continuous CAC (NRI 25%, 95% CI 8–41%) and log‐AAC (NRI 12%, 95% CI 2–22) (Table 4).
Table 4.
Discrimination and Reclassification of Coronary, Aortic, and Valvular Calcification for Major CVD Events
| Major CVD Model: NRI Using Risk Categories of <0.025, 0.025 to <0.065, 0.065 to <0.10, and ≥0.10 | Major CVD C‐Statistic | Major CVD NRI (95% CI) | Proportion of Events/Nonevents Classified Correctly |
|---|---|---|---|
| RF only | 0.80 | — | — |
| RF+log CAC | 0.82 | 0.25 (0.08–0.41) | 0.27/−0.02 |
| RF+CAC cat | 0.82 | 0.20 (0.03–0.37) | 0.21/−0.01 |
| RF+log AAC | 0.80 | 0.12 (0.02–0.22) | 0.14/−0.02 |
| RF+log TAC | 0.81 | 0.04 (−0.06 to 0.15) | 0.04/0.001 |
| RF+log MVC | 0.80 | −0.05 (−0.20 to 0.07) | −0.06/0.01 |
| RF+log AVC | 0.80 | −0.02 (−0.13 to 0.10) | −0.01/−0.003 |
| RF+log CAC+log MVC | 0.82 | 0.19 (0.04–0.36) | 0.20/−0.003 |
| RF+CAC cat+log MVC | 0.82 | 0.13 (−0.02 to 0.29) | 0.12/0.01 |
| RF+all calcifications | 0.82 | 0.21 (0.05–0.38) | 0.22/−0.01 |
| RF+CAC cat+all noncoronary | 0.82 | 0.16 (−0.004 to 0.32) | 0.16/−0.002 |
AAC indicates abdominal aorta calcification; AVC, aortic valve calcification; CAC, coronary artery calcification; cat, categorical; CVD, cardiovascular disease; MVC, mitral valve calcification; NRI, net reclassification index; RF, adjusted for Framingham risk factors; TAC, thoracic aorta calcification.
Extent and accuracy of reclassification for major CHD and major CVD
The extent and accuracy of reclassification for 8‐year risk for major CHD and CVD by log‐continuous CAC incremental to FRFs is shown in Tables 5 and 6, respectively. The addition of CAC score resulted in more accurate prediction of event rates across categories of CAC.
Table 5.
Accuracy of Reclassification of 5‐Year Risk for Major CHD by CAC: Predicted Versus Observed Outcomes
| 5‐Year Risk Model Without CAC | 5‐Year Risk Model With CAC | ||||||
|---|---|---|---|---|---|---|---|
| 0% to <2.5% | 2.5% to <5% | 5% to <10% | ≥10% | Overall | Reclassified as Higher Risk | Reclassified as Lower Risk | |
| 0% to <2.5% | |||||||
| No. of participants | 2817 | 138 | 22 | 0 | 2977 | 160 | — |
| No. of events | 22 | 11 | 0 | 0 | 33 | 11 | — |
| 5‐Year estimate (95% CI) | 0.5% (0.3–0.8%) | 4.5% (2.1–9.8%) | 0% | — | 0.7% (0.4–1.1%) | 3.9% (1.8–8.4%) | — |
| 2.5% to <5% | |||||||
| No. of participants | 119 | 89 | 46 | 7 | 261 | 53 | 119 |
| No. of events | 0 | 1 | 6 | 1 | 8 | 7 | 0 |
| 5‐Year estimate (95% CI) | 0% | 1.2% (0.2–8.0%) | 7.0% (2.3–20.3%) | 14.3% (2.1–66.6%) | 2.0% (0.8–4.7%) | 8.0% (3.1–20.1%) | 0% |
| 5% to <10% | |||||||
| No. of participants | 13 | 26 | 32 | 15 | 86 | 15 | 39 |
| No. of events | 2 | 1 | 6 | 3 | 12 | 3 | 3 |
| 5‐Year estimate (95% CI) | 7.7% (1.1–43.4%) | 4.0% (0.6–25.2%) | 10.7% (3.5–30.2%) | 21.4% (7.5–52.8%) | 10.1% (5.1–19.2%) | 21.4% (7.5–52.8%) | 5.2% (1.3–19.2%) |
| ≥10% | |||||||
| No. of participants | 1 | 2 | 2 | 11 | 16 | — | 5 |
| No. of events | 0 | 0 | 0 | 1 | 1 | — | 0 |
| 5‐Year estimate (95% CI) | 0% | 0% | 0% | 14.3% (2.1–66.6%) | 9.1% (1.3–49.2%) | — | 0% |
| Overall | |||||||
| No. of participants | 2950 | 255 | 102 | 33 | 3340 | 228 | 163 |
| No. of events | 24 | 13 | 12 | 5 | 54 | 21 | 3 |
| 5‐Year estimate (95% CI) | 0.5% (0.3–0.9%) | 3.3% (1.7–6.5%) | 6.3% (2.9–13.6%) | 18.0% (7.9–38.1%) | 1.0% (0.7–1.4%) | 5.9% (3.5–10.0%) | 1.3% (0.3–4.9%) |
CAC indicates coronary artery calcification; CHD, coronary heart disease.
Table 6.
Accuracy of Reclassification of 5‐Year Risk for Major CVD by CAC: Predicted Versus Observed Outcomes
| 0% to <2.5% | 2.5% to <6.5% | 6.5% to <10% | ≥10% | Overall | Reclassified as Higher Risk | Reclassified as Lower Risk | |
|---|---|---|---|---|---|---|---|
| 0% to <2.5% | |||||||
| No. of participants | 2512 | 146 | 0 | 0 | 2658 | 146 | — |
| No. of events | 28 | 15 | 0 | 0 | 43 | 15 | — |
| 5‐Year estimate (95% CI) | 0.7% (0.5–1.1%) | 7.0% (3.8–12.7%) | — | — | 1.1% (0.7–1.5%) | 7.0% (3.8–12.7%) | — |
| 2.5% to <6.5% | |||||||
| No. of participants | 127 | 270 | 76 | 8 | 481 | 84 | 127 |
| No. of events | 4 | 14 | 11 | 0 | 29 | 11 | 4 |
| 5‐Year estimate (95% CI) | 1.6% (0.4–6.3%) | 1.1% (0.4–3.5%) | 8.5% (3.9–17.9%) | 0% | 2.4% (1.3–4.2%) | 7.6% (3.5–16.2%) | 1.6% (0.4–6.3%) |
| 6.5% to <10% | |||||||
| No. of participants | 4 | 26 | 49 | 29 | 108 | 29 | 30 |
| No. of events | 0 | 1 | 8 | 5 | 14 | 8 | 1 |
| 5‐Year estimate (95% CI) | 0% | 3.8% (0.6–24.3%) | 8.5% (3.3–21.1%) | 15.6% (6.1–36.4%) | 8.7% (4.6–16.1%) | 15.6% (6.1–36.4%) | 3.3% (0.5–21.4%) |
| ≥10% | |||||||
| No. of participants | 0 | 6 | 10 | 56 | 72 | — | 16 |
| No. of events | 0 | 1 | 2 | 10 | 13 | — | 3 |
| 5‐Year estimate (95% CI) | — | 16.7% (2.5–72.7%) | 10.0% (1.5–52.7%) | 16.4% (8.5–30.4%) | 15.5% (8.6–26.9%) | — | 12.5% (3.3–41.4%) |
| Overall | |||||||
| No. of participants | 2643 | 448 | 135 | 93 | 3319 | 259 | 173 |
| No. of events | 32 | 31 | 19 | 17 | 99 | 31 | 8 |
| 5‐Year estimate (95% CI) | 0.8% (0.5–1.2%) | 3.4% (2.1–5.6%) | 8.6% (4.8–14.9%) | 14.8% (8.7–24.7%) | 1.8% (1.4–2.3%) | 8.1% (5.3–12.3%) | 2.9% (1.2–6.9%) |
CAC indicates coronary artery calcification; CVD, cardiovascular disease.
Major CHD events
The clinically relevant group of participants at intermediate risk at 2.5% to <5% for major CHD by FRFs (n=261) had an observed event rate of 2.0%. CAC reclassified more than half of these patients (66%) to higher risk (n=53, observed event rate 8.0%) or lower risk (n=119; observed event rate 0.0%) (Table 5).
Major CVD events
The reclassification for major CVD was slightly less efficient than for major CHD. The addition of log‐CAC to FRFs reclassified 13% participants (432 of 3319) (Table 6).
Among the clinically relevant group of 481 participants at intermediate event risk based on FRFs (2.5% to <6.5% over 8 years; observed event rate 2.4%), CAC reclassified 26.4% to lower risk (n=127; observed event rate 1.6%) and 17.5% to higher risk (n=84; observed event rate 7.6%).
Discussion
In this large, community‐based, white cohort, CAC was associated with major CHD, major CVD, and all‐cause mortality independent of FRFs, with the strongest associations for CHD, followed by CVD and mortality. Among noncoronary calcifications, MVC was associated with major CVD and all‐cause mortality independent of FRFs and CAC. Using categories of CAC, more than half of participants at intermediate risk were correctly reclassified to higher or lower risk for both CHD and CVD, with an emphasis on correct reclassification of events.
Our results further strengthen and validate CAC as an independent and effective measure to reclassify risk for CHD in white US persons (area under the curve 0.80 versus 0.84, P<0.05) and are consistent with observations in white European participants in the Rotterdam Heart study3, 25 and the MESA study (area under the curve 0.76–0.79, P=0.11).4
Importantly, we demonstrated that CAC accurately reclassifies more than half of participants (58%) at intermediate risk correctly, based on the FRF score, to higher risk (n=39, observed event rate 16.6%) or lower risk (n=145; observed event rate0.0%). CAC was effective in both reclassification toward higher risk (1.2–6.7%) and lower risk (2.0–1.2%) across risk score categories for CHD nearly equally (41.5% and 54.5% being reclassified to lower risk for CHD and CVD, respectively), suggesting that most efficient use of CAC will occur if both scenarios will change patient management. These data are in accordance with reports from MESA26, 27 that demonstrate participants with CAC of 101–300 and >300 have event rates (8% and 12%, respectively, for CVD; 4% and 8%, respectively, for CHD; and 8% and 15%, respectively, for death after 8‐year follow‐up) close to a 10‐year risk of ≥20%, which is traditionally considered a “CHD risk equivalent” and thus may be eligible for risk factor modification to the same extent as secondary‐prevention patients.
Interestingly but not surprisingly, we found that associations of CAC were stronger for CHD than for CVD and all‐cause mortality. These findings are consistent with the systemic nature of atherosclerosis but also emphasize the preeminent importance of local findings, as reported from the Heinz Nixdorf Recall study28 and the MESA study.29 In comparison to MESA, the Framingham Heart Study cohort has a higher prevalence of participants with CAC >300, although this is particularly true for men because women also have lower prevalence of CAC >300 (14.2% versus 4.2%). This may be explained by the recruitment of participants from 2 Framingham Heart Study generations in the CT substudy; however, despite these differences, we found no interaction of the association of CAC with MACE by age and sex (Table S1).
Our study further confirms findings of studies that demonstrated that noncoronary calcifications predict CHD events independently and incrementally to FRFs.12 In particular, we reinforced the strong independent prediction of risk provided by AAC, thoracic aortic calcification, and MVC and suggest that reporting the extent of aortic calcifications in the absence of information on CAC may be helpful and clinically relevant. It is interesting that MVC emerged in our analyses as an independent risk factor for CVD and all‐cause mortality beyond FRFs and CAC. Prior evidence is sparse for MVC as an independent predictor of CVD risk in persons not selected for higher risk conditions such as atrial fibrillation or renal failure, so this finding warrants further research.
Strengths of our analysis include assessment of a community‐based cohort with standardized and highly reproducible physician‐based assessment of cardiovascular risk factors and prospectively determined clinical outcomes.
Our study has several limitations. The coverage of the thoracic aorta excluded the aortic arch and thus may underestimate the amount of calcification; however, our protocol reflects clinical practice. Consequently, our results are applicable to patients undergoing CT for coronary calcium. Results in white US persons may not be generalizable to other ethnic groups, although it should be noted that the reported significant differences in associations among risk factors, CAC, and outcomes in white compared with other ethnic groups suggest that ethnic group–specific prediction rules may be required.3, 30 Another limitation is that relatively few persons aged >60 years (16%) were included. We chose cut points for NRI and reclassification that were different than those in the Pooled Cohort Equations.
Conclusions
CAC predicted CHD, CVD, and death independent of FRFs in asymptomatic community‐dwelling white persons and accurately reclassified about half of the cohort, including the intermediate‐risk group, to either higher or lower risk for CHD events. Furthermore, CAC identified ≈20% of the cohort with a CAC score >100 with an event risk that may be eligible for risk factor modification to the same extent as secondary prevention. In the absence of information on CAC, the extent of noncoronary calcifications also identifies persons at higher risk for CHD and CVD independent of risk factors.
Sources of Funding
This work was supported by the NIH Heart, Lung, and Blood Institute's Framingham Heart Study (contract no. N01‐HC‐25195, HL076784, AG028321, HL070100, HL060040, HL080124, HL071039, HL077447, and HL107385). The study sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation o the data; and preparation, review, or approval of the manuscript.
Disclosures
None.
Supporting information
Table S1. Cox Proportional Hazards Regression of Atherosclerotic Cardiovascular Disease Versus Continuous and Stratified Calcification Measures—Interaction With Age, Sex, and Cohort
Acknowledgments
This research was conducted in part using data and resources from the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine. Dr Udo Hoffmann and Dr Christopher J. O'Donnell had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2016;5:e003144 doi: 10.1161/JAHA.115.003144)
An accompanying Table S1 is available at http://jaha.ahajournals.org/content/5/2/e003144/suppl/DC1
References
- 1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics C, Stroke Statistics S . Executive summary: Heart Disease and Stroke Statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. [DOI] [PubMed] [Google Scholar]
- 2. Webber BJ, Seguin PG, Burnett DG, Clark LL, Otto JL. Prevalence of and risk factors for autopsy‐determined atherosclerosis among US service members, 2001–2011. JAMA. 2012;308:2577–2583. [DOI] [PubMed] [Google Scholar]
- 3. Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 5. Wilson PW, Kauppila LI, O'Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. [DOI] [PubMed] [Google Scholar]
- 6. Levitzky YS, Cupples LA, Murabito JM, Kannel WB, Kiel DP, Wilson PW, Wolf PA, O'Donnell CJ. Prediction of intermittent claudication, ischemic stroke, and other cardiovascular disease by detection of abdominal aortic calcific deposits by plain lumbar radiographs. Am J Cardiol. 2008;101:326–331. [DOI] [PubMed] [Google Scholar]
- 7. Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283:2810–2815. [DOI] [PubMed] [Google Scholar]
- 8. Witteman JC, Kannel WB, Wolf PA, Grobbee DE, Hofman A, D'Agostino RB, Cobb JC. Aortic calcified plaques and cardiovascular disease (the Framingham Study). Am J Cardiol. 1990;66:1060–1064. [DOI] [PubMed] [Google Scholar]
- 9. Witteman JC, Kok FJ, van Saase JL, Valkenburg HA. Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986;2:1120–1122. [DOI] [PubMed] [Google Scholar]
- 10. Bastos Goncalves F, Voute MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, Verhagen HJ. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta‐analysis. Heart. 2012;98:988–994. [DOI] [PubMed] [Google Scholar]
- 11. Budoff MJ, Nasir K, Katz R, Takasu J, Carr JJ, Wong ND, Allison M, Lima JA, Detrano R, Blumenthal RS, Kronmal R. Thoracic aortic calcification and coronary heart disease events: the Multi‐Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2011;215:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eisen A, Tenenbaum A, Koren‐Morag N, Tanne D, Shemesh J, Imazio M, Fisman EZ, Motro M, Schwammenthal E, Adler Y. Calcification of the thoracic aorta as detected by spiral computed tomography among stable angina pectoris patients: association with cardiovascular events and death. Circulation. 2008;118:1328–1334. [DOI] [PubMed] [Google Scholar]
- 13. Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic‐valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. [DOI] [PubMed] [Google Scholar]
- 14. Hoffmann U, Massaro JM, Fox CS, Manders E, O'Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol. 2008;102:1136–1141, 1141.e1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lehman SJ, Massaro JM, Schlett CL, O'Donnell CJ, Hoffmann U, Fox CS. Peri‐aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis. 2010;210:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Preis SR, Hwang SJ, Fox CS, Massaro JM, Levy D, Hoffmann U, O'Donnell CJ. Eligibility of individuals with subclinical coronary artery calcium and intermediate coronary heart disease risk for reclassification (from the Framingham Heart Study). Am J Cardiol. 2009;103:1710–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thanassoulis G, Massaro JM, Cury R, Manders E, Benjamin EJ, Vasan RS, Cupple LA, Hoffmann U, O'Donnell CJ, Kathiresan S. Associations of long‐term and early adult atherosclerosis risk factors with aortic and mitral valve calcium. J Am Coll Cardiol. 2010;55:2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hong C, Bae KT, Pilgram TK. Coronary artery calcium: accuracy and reproducibility of measurements with multi‐detector row CT–assessment of effects of different thresholds and quantification methods. Radiology. 2003;227:795–801. [DOI] [PubMed] [Google Scholar]
- 19. Hoffmann U, Siebert U, Bull‐Stewart A, Achenbach S, Ferencik M, Moselewski F, Brady TJ, Massaro JM, O'Donnell CJ. Evidence for lower variability of coronary artery calcium mineral mass measurements by multi‐detector computed tomography in a community‐based cohort—consequences for progression studies. Eur J Radiol. 2006;57:396–402. [DOI] [PubMed] [Google Scholar]
- 20. Pursnani A, Massaro JM, D'Agostino RB Sr, O'Donnell CJ, Hoffmann U. Guideline‐based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 22. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW; American College of Cardiology/American Heart Association Task Force on Practice G . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. [DOI] [PubMed] [Google Scholar]
- 24. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207‐112. [DOI] [PubMed] [Google Scholar]
- 25. Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, Witteman JC. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–577. [DOI] [PubMed] [Google Scholar]
- 26. Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, O'Leary DH, Lima J, Blumenthal RS, Nasir K. Associations between C‐reactive protein, coronary artery calcium, and cardiovascular events: implications for the Jupiter population from MESA, a population‐based cohort study. Lancet. 2011;378:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin SS, Blaha MJ, Blankstein R, Agatston A, Rivera JJ, Virani SS, Ouyang P, Jones SR, Blumenthal RS, Budoff MJ, Nasir K. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2014;129:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalsch H, Lehmann N, Berg MH, Mahabadi AA, Mergen P, Mohlenkamp S, Bauer M, Kara K, Dragano N, Hoffmann B, Moebus S, Schmermund A, Stang A, Jockel KH, Erbel R. Coronary artery calcification outperforms thoracic aortic calcification for the prediction of myocardial infarction and all‐cause mortality: the Heinz Nixdorf Recall Study. Eur J Prev Cardiol. 2014;21:1163–1170. [DOI] [PubMed] [Google Scholar]
- 29. Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate‐risk individuals. JAMA. 2012;308:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G; Investigators R . Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cox Proportional Hazards Regression of Atherosclerotic Cardiovascular Disease Versus Continuous and Stratified Calcification Measures—Interaction With Age, Sex, and Cohort


