Abstract
Background
Long‐term prognosis of acute pulmonary edema (APE) remains ill defined.
Methods and Results
We evaluated demographic, echocardiographic, and angiographic data of 806 consecutive patients with APE with (CAD) and without coronary artery disease (non‐CAD) admitted from 2000 to 2010. Differences between hospital and long‐term mortality and its predictors were also assessed. CAD patients (n=638) were older and had higher incidence of diabetes and peripheral vascular disease than non‐CAD (n=168), and lower ejection fraction. Hospital mortality was similar in both groups (26.5% vs 31.5%; P=0.169) but APE recurrence was higher in CAD patients (17.3% vs 6.5%; P<0.001). Age, admission systolic blood pressure, recurrence of APE, and need for inotropics or endotracheal intubation were the main independent predictors of hospital mortality. In contrast, overall mortality (70.0% vs 57.1%; P=0.002) and readmission for nonfatal heart failure after a 45‐month follow‐up (10–140; 17.3% vs 7.6%; P=0.009) were higher in CAD than in non‐CAD patients. Age, peripheral vascular disease, and peak creatine kinase MB during index hospitalization, but not ejection fraction, were the main independent predictors of overall mortality, whereas coronary revascularization or valvular surgery were protective. These interventions were mostly performed during hospitalization index (294 of 307; 96%) and not intervened patients showed a higher risk profile.
Conclusions
Long‐term mortality in APE is high and higher in CAD than in non‐CAD patients. Considering the different in‐hospital and long‐term mortality predictors herein described, which do not necessarily involve systolic function, it is conceivable that a more aggressive interventional program might improve survival in high‐risk patients.
Keywords: acute pulmonary edema, coronary artery disease, long‐term mortality
Subject Categories: Heart Failure
Introduction
Acute heart failure, which includes a variety of cardiac conditions such as cardiogenic shock, acute decompensation of chronic heart failure, right ventricular failure, and acute pulmonary edema (APE), accounts for an increasing number of deaths and hospital admissions.1, 2, 3, 4, 5 Although these presentations have their own profile, reports generally pool data from the different subsets and outcomes are presented as from a single entity.1, 2, 3, 5, 6 APE, however, may be considered a distinct condition because it develops abruptly, most often within the first hour from symptom onset, and it is often triggered by elements different from those causing a gradual decompensation of chronic heart failure.1, 7, 8, 9, 10 Hence, it is suspected that mechanisms of APE may vary from those of decompensated heart failure.1, 10
Several recent studies have reported on the 1‐year prognosis of patients with acute heart failure4, 11, 12, 13, 14, 15, 16 and 3 have analyzed longer follow‐up,15, 16, 17 one of them with decompensated heart failure and pulmonary edema.17 In contrast, long‐term follow‐up in patients with well‐defined APE has been limited to 1 year and has been described in only 2 old reports with a reduced number of patients.7, 18 Moreover, causes of death in the follow‐up were not investigated.
Thus, in the present study, we analyzed the in‐hospital and long‐term follow‐up events in patients with APE. We also analyzed the causes of death, the prognostic predictors, and the possible differences between patients with and patients without CAD as the main underlying heart disease.
Methods
Patients
From January 2000 to December 2010, 806 consecutive patients with APE admitted to our acute cardiac care unit were included. APE was defined as orthopnea of ≤6 hours with bilateral rales, hypoxemia (arterial oxygen saturation <90%), and radiographic evidence of alveolar and/or interstitial pulmonary edema. Oxygen saturation was assessed on admission and blood pressure, by cuff, and heart rate were measured at first medical attention. Also, a chest X‐ray and a standard 12‐lead electrocardiogram (ECG) were performed on hospital arrival. Serial blood samples for myocardial necrosis markers (creatine kinase MB [CK‐MB] and troponin I) were drawn every 4 to 6 hours during at least the first 24 hours. In view of the frequent presence of renal insufficiency in these patients, however, myocardial necrosis was assessed by levels of CK‐MB. Initial treatment included oxygen by mask, intravenous morphine sulphate, intravenous infusion of nitroglycerin, and intravenous furosemide. Sodium nitroprusside was added whenever needed, whereas hypotension was initially treated with dobutamine and/or noradrenaline. Patients with persistent respiratory insufficiency underwent noninvasive ventilatory support, whereas oral intubation and mechanical ventilation were instituted in cases of refractory hypoventilation. Angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptors antagonists and beta‐blockers were added in the subacute phase of the disease. These drugs along with diuretics were the main line of therapy for heart failure at hospital discharge. In recent years, aldosterone antagonists were also recommended. Additional medications were added according to the underlying heart disease. Also, coronary revascularization procedures, surgical treatment of valvular heart disease, and follow‐up management were dictated by the attending physicians that were largely within the recommendations of appropriate international guidelines.
Echocardiographic Study and Coronary Angiography
A two‐dimensional echocardiogram Doppler (Vivid 3 or Vivid i with harmonic imaging; General Electric, Fairfield, CT) was performed within 6 hours from admission. Left ventricular end‐diastolic and end‐systolic diameters were measured, and ejection fraction was calculated by the Simpson method; thickness of septal and posterior walls was measured in the long parasternal views. The presence of valvular disease was also investigated and the existence of mitral regurgitation was assessed by a semiquantitative approach by Doppler flow mapping (color). It was judged to be mild, moderate, or severe when the regurgitant jet occupied 5% to 19%, 20% to 39%, or ≥40% of the left atrial area, respectively. To assess diastolic function, diastolic transmitral flow velocities were recorded in the standard apical 4‐chamber view with the sample volume positioned at the mitral leaflet tips. The ratio between peak velocities of the E and A waves and the deceleration time were evaluated. The flow at the pulmonary veins was also analyzed, and diastolic inflow was categorized as normal, impaired relaxation, pseudonormalization, or restrictive patterns.
Coronary angiography was intended to be performed in most patients and was interpreted by 2 observers who visually evaluated by consensus the number of main coronary vessels with ≥70% stenosis. The protocol complied with the Declaration of Helsinki and was approved by the hospital ethics committee, and informed consent was obtained from patients before entering the study.
Underlying heart disease
Two cardiac conditions were recognized: CAD and non‐CAD. Diagnosis of CAD was based on ≥1 of the following criteria: (1) previous myocardial infarction (pathological Q waves in ≥2 contiguous leads, enzyme rise, or a fixed perfusion defect in myocardial scintigraphic studies); (2) acute coronary syndromes: acute myocardial infarction with increased levels of CK‐MB mass >10 μg/L (upper normal limit: 6 μg/L) with or without chest pain or ECG changes, or unstable angina, also with or without chest pain but with transient ECG changes and CKMB mass ≤10 μg/L; or (3) coronary stenosis ≥70% in ≥1 major epicardial vessel. The non‐CAD group included patients with a primary moderate‐severe valvular heart disease, those with dilated (left ventricular end‐diastolic diameter ≥65 mm) or hypertrophic (wall thickness ≥15 mm) cardiomyopathy and those with no apparent heart disease. Patients with myocardial infarction and valvular heart disease, however, were categorized as CAD, whereas those with severe valvular heart disease (severe aortic stenosis, mitral stenosis, or aortic regurgitation) and small myocardial infarction (CK‐MB <20 μg/L) were classified as non‐CAD.
Statistical Analysis
We compared clinical, electrocardiographic, and echocardiographic and clinical outcome between CAD and non‐CAD patients. We also compared the profile of hospital survivors and nonsurvivors and that of long‐term follow‐up survivors and nonsurvivors. We used the Student t test for comparison of 2 continuous variables with normal distribution, the Mann–Whitney U test for variables with abnormal distribution, and the chi‐square or the Fisher exact test to compare categorical variables. A multivariable logistic regression analysis examined the predictive value of variables associated with in‐hospital mortality in a univariate analysis, whereas a Cox regression analysis was used for predictors of overall mortality. Long‐term survival was estimated by the Kaplan‐Meier method. The analysis was performed with SPSS software (version 15.0; SPSS, Inc., Chicago, IL), data expressed as mean±SD, and differences considered significant at P<0.05.
Results
There were 638 patients with CAD and 168 without, of whom 111 had valvular heart disease, 18 a cardiomyopathy, and 39 no apparent heart disease. Moderate‐to‐severe mitral insufficiency, aortic stenosis, and mitral stenosis were judged to be the main valvular disorders in 68, 26, and 17 non‐CAD patients, respectively.
Demographic, Echocardiographic, and Angiographic Data
In most patients, APE was a de novo presentation since 85% of CAD and 84% of non‐CAD cases had no previous episodes. CAD patients were older (73±10 vs 67±13 years; P<0.001) and had a higher rate of diabetes (50% vs 35%; P=0.001) and peripheral vascular disease (30% vs 14%; P<0.001) than non‐CAD patients, but showed a lower incidence of chronic atrial fibrillation (15% vs 30%; P<0.001). They also presented a lower ejection fraction (40.7±12.4% vs 50.2±17.0%; P<0.001). Coronary angiography was performed in 506 patients (63%), 69% from the CAD group, and 43% from the non‐CAD group. Significant coronary disease was present in 94% and 32%, respectively, with a predominance of multivessel disease in the former (80%).
In‐Hospital Outcome
In‐hospital mortality occurred in 222 patients (27.5%) and tended to be lower in the CAD (169, 26.5%) than in the non‐CAD group (53; 31.5%; P=0.169; Figure 1). The cause of death was identified in 221 patients and was strictly cardiac in 110 cases (50%), mostly cardiogenic shock or recurrence of APE, whereas in 95 (42.8%) it was associated with complications of the pulmonary edema or a transiently impaired hemodynamic condition. The proportion of cardiac deaths in CAD and non‐CAD patients was similar (49.4% vs 38.5%; P=0.200). Nonsurvivors were older than survivors in the 2 groups and had a lower admission blood pressure and lower heart rate. They also had greater impairment of left ventricular diastolic function. Ejection fraction was also lower in nonsurvivors than in survivors in the CAD group, but not in the non‐CAD group where it was higher in nonsurvivors (Table 1). In both groups, nonsurvivors were less frequently treated with ACE inhibitors, mostly because they had continued with nitroglycerin infusion, and were more frequently intubated or treated with dobutamine. Recurrence of APE was more often observed in nonsurvivors of the CAD group and in 46 patients (41.1%) it was associated with in‐hospital angina or myocardial infarction. Moreover, the incidence of in‐hospital myocardial infarction or reinfarction was also higher in nonsurvivors than in survivors (Table 2). Of the 113 CAD patients with either in‐hospital angina, myocardial infarction, or recurrent APE, nonrevascularized patients showed a strong trend toward a higher mortality than the revascularized ones (31 of 52 [59.6%] vs 25 of 61 [41%]; P=0.060).
Figure 1.
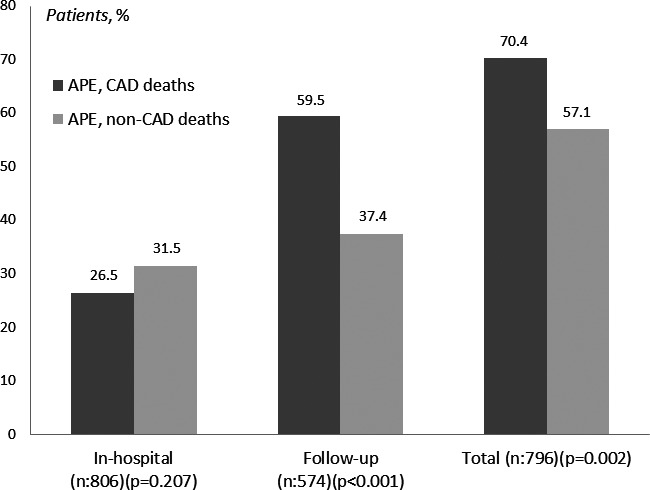
Among patients with APE, those with CAD presented a higher follow‐up and total mortality than those with non‐CAD, whereas in‐hospital mortality was similar. APE indicates acute pulmonary edema; CAD, coronary artery disease.
Table 1.
Clinical, Angiographic and Echocardiographic Characteristics of Patients With APE
| In‐Hospital Course (n=806) | Follow‐up (n=574) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non‐CAD (n=168) Survivors | P Value | CAD (n=638) Survivors | P Value | Non‐CAD (n=115) Survivors | P Value | CAD (n=459) Survivors | P Value | |||||
| Yes (115) | No (53) | Yes (469) | No (169) | Yes (72) | No (43) | Yes (186) | No (273) | |||||
| Age, y | 65.1±13.4 | 72.3±9.4 | 0.001 | 72.0±9.9 | 74.0±8.0 | 0.021 | 63.4±13.4 | 68.0±13.0 | 0.071 | 68.9±10.3 | 74.2±9.1 | <0.001 |
| Female | 38% | 53% | 0.076 | 35% | 40% | 0.256 | 39% | 37% | 0.858 | 30% | 38% | 0.073 |
| Hypertension | 71% | 62% | 0.241 | 75% | 76% | 0.782 | 71% | 72% | 0.885 | 77% | 75% | 0.910 |
| Diabetes mellitus | 33% | 40% | 0.406 | 50% | 50% | 0.910 | 24% | 49% | 0.005 | 49% | 51% | 0.584 |
| Active smoking | 33% | 11% | 0.017 | 24% | 27% | 0.684 | 38% | 24% | 0.042 | 27% | 22% | 0.524 |
| Chronic obstructive pulmonary disease | 29% | 43% | 0.060 | 31% | 35% | 0.333 | 25% | 35% | 0.257 | 31% | 31% | 0.962 |
| Previous cerebrovascular accident | 15% | 15% | 0.958 | 14% | 18% | 0.165 | 11% | 21% | 0.151 | 10% | 16% | 0.055 |
| Peripheral vascular disease | 14% | 15% | 0.839 | 30% | 30% | 0.873 | 6% | 28% | 0.001 | 21% | 36% | 0.001 |
| Chronic renal failure | 17% | 25% | 0.248 | 25% | 22% | 0.487 | 17% | 16% | 0.957 | 21% | 28% | 0.067 |
| Old myocardial infarction | 8% | 9% | 0.705 | 36% | 35% | 0.784 | 7% | 14% | 0.216 | 31% | 39% | 0.086 |
| Chronic atrial fibrillation | 27% | 36% | 0.241 | 15% | 17% | 0.432 | 26% | 28% | 0.859 | 11% | 18% | 0.038 |
| Previous heart failure | 37% | 40% | 0.782 | 20% | 24% | 0.365 | 36% | 40% | 0.714 | 19% | 21% | 0.534 |
| Previous APE | 13% | 23% | 0.115 | 15% | 15% | 0.847 | 13% | 14% | 0.823 | 10% | 19% | 0.009 |
| Angina during APE | 18% | 17% | 0.840 | 64% | 65% | 0.719 | 28% | 16% | 0.180 | 63% | 64% | 0.765 |
| Admission Killip Class IV | 6% | 8% | 0.748 | 5% | 16% | <0.001 | 6% | 2% | 0.649 | 6% | 5% | 0.736 |
| In‐hospital APE | 29% | 48% | 0.026 | 23% | 33% | 0.029 | 33% | 27% | 0.499 | 18% | 28% | 0.038 |
| Creatinine, mg/dL | 1.4±0.4 | 1.8±1.6 | 0.044 | 1.7±1.3 | 1.9±1.3 | 0.110 | 1.3±0.8 | 1.7±0.9 | 0.057 | 1.5±1.1 | 2.0±1.4 | 0.001 |
| CKMB, μg/L | 6±8 | 6±7 | 0.641 | 86±159 | 127±190 | 0.006 | 7±10 | 5±4 | 0.257 | 90±167 | 83±154 | 0.642 |
| Admission SBP, mm Hg (n=742) | 156±52 | 136±40 | 0.018 | 154±43 | 130±42 | <0.001 | 148±50 | 170±53 | 0.031 | 152±45 | 155±42 | 0.355 |
| Admission DBP, mm Hg (n=742) | 87±29 | 72±21 | 0.001 | 86±24 | 76±23 | <0.001 | 81±26 | 97±31 | 0.009 | 86±25 | 85±23 | 0.677 |
| Admission HR, beats/min (n=742) | 113±32 | 103±28 | 0.059 | 109±26 | 102±23 | 0.005 | 113±32 | 113±33 | 0.977 | 110±24 | 109±26 | 0.836 |
| Hemoglobin, g/dL | 12.7±2.4 | 11.2±2.2 | 0.001 | 12.6±2.3 | 12.3±2.3 | 0.271 | 13.1±2.3 | 12.0±2.5 | 0.823 | 13.0±2.5 | 12.2±2.1 | 0.003 |
| Left bundle branch block | 30% | 43% | 0.101 | 26% | 27% | 0.833 | 28% | 35% | 0.423 | 23% | 28% | 0.234 |
| Ejection fraction, % | 48.4±16.5 | 54.4±17.4 | 0.039 | 41.5±12.1 | 38.5±13.2 | 0.008 | 49.7±17.2 | 46.2±15.3 | 0.276 | 42.1±11.9 | 41.0±12.2 | 0.322 |
| LVEDD, mm | 56.1±9.3 | 52.6±10.2 | 0.047 | 52.6±7.2 | 51.0±7.1 | 0.053 | 56.0±8.9 | 56.4±10.3 | 0.874 | 52.5±7.2 | 52.7±7.3 | 0.745 |
| LVESD, mm | 40.6±10.5 | 35.5±11.2 | 0.019 | 39.6±8.6 | 38.4±8.0 | 0.267 | 40.1±10.3 | 41.9±11.0 | 0.492 | 39.4±8.5 | 39.7±8.8 | 0.810 |
| Septal thickness, mm | 13.2±3.4 | 12.6±2.4 | 0.255 | 12.6±2.7 | 12.5±2.7 | 0.591 | 12.8±2.9 | 13.7±4.1 | 0.185 | 12.3±2.4 | 12.8±2.8 | 0.041 |
| Posterior wall thickness, mm | 12.2±2.0 | 11.9±2.0 | 0.336 | 11.7±2.2 | 11.5±2.1 | 0.361 | 12.2±2.1 | 12.2±2.3 | 0.950 | 11.5±2.1 | 11.9±2.3 | 0.040 |
| Diastolic function | (n=45) | (n=22) | (n=265) | (n=73) | (n=29) | (n=18) | (n=97) | (n=168) | ||||
| Normal | 2% | 0% | 0.059 | 2% | 0% | 0.027 | 3% | 0% | 0.190 | 0% | 2% | 0.001 |
| Reduced distensibility | 31% | 5% | 38% | 40% | 21% | 44% | 34% | 54% | ||||
| Pseudonormal pattern | 24% | 55% | 28% | 34% | 31% | 11% | 31% | 26% | ||||
| Restrictive pattern | 42% | 41% | 24% | 17% | 45% | 33% | 35% | 17% | ||||
| Moderate‐severe aortic stenosis | 15% | 25% | 0.346 | 6% | 11% | 0.047 | 18% | 9% | 0.198 | 4% | 8% | 0.176 |
| Moderate‐severe aortic insufficiency | 17% | 21% | 0.568 | 2% | 4% | 0.114 | 22% | 9% | 0.114 | 3% | 2% | 0.700 |
| Moderate‐severe mitral stenosis | 10% | 11% | 0.801 | 0.5% | 0.6% | 0.920 | 13% | 7% | 0.372 | 0.5% | 0.4% | 0.946 |
| Mitral insufficiency | ||||||||||||
| Mild | 31% | 26% | 0.380 | 39% | 25% | <0.001 | 31% | 33% | 0.714 | 41% | 38% | 0.897 |
| Moderate‐severe | 42% | 40% | 29% | 46% | 43% | 40% | 34% | 35% | ||||
| Coronary angiography, vessels with ≥70% stenosis | (n=334) | (n=103) | 0.206 | (n=159) | (n=175) | 0.172 | ||||||
| 0 | 7% | 3% | 9% | 4% | ||||||||
| 1 | 16% | 17% | 17% | 16% | ||||||||
| 2 | 24% | 17% | 26% | 22% | ||||||||
| 3 | 40% | 44% | 35% | 45% | ||||||||
| Left main ≥50% | 13% | 19% | 13% | 13% | ||||||||
Comparison between survivors and nonsurvivors in the CAD and non‐CAD groups during the in‐hospital course and the follow‐up (%, mean±SD). APE indicates acute pulmonary edema; CAD, coronary artery disease; DBP, diastolic blood pressure; HR, heart rate; LVEDD, left ventricular end‐diastolic diameter; LVESD, left ventricular end‐systolic diameter; SBP, systolic blood pressure.
Table 2.
Hospital and Follow‐up Management and Events in Patients With APE
| In‐Hospital Course (n=806) | Follow‐Up (n=574) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non‐CAD (n=169) Survivors | P Value | CAD (n=638) Survivors | P Value | Non‐CAD (n=115) Survivors | P Value | CAD (n=479) Survivors | P Value | |||||
| Yes (116) | No (53) | Yes (469) | No (169) | Yes (72) | No (43) | Yes (186) | No (273) | |||||
| Hospital treatment | ||||||||||||
| Beta‐blockers | 46% | 52% | 0.205 | 65% | 48% | 0.001 | 39% | 58% | 0.045 | 67% | 64% | 0.487 |
| Nitrates | 96% | 94% | 0.710 | 96% | 89% | <0.001 | 94% | 98% | 0.411 | 95% | 96% | 0.615 |
| ACE inhibitors | 71% | 45% | 0.001 | 95% | 62% | <0.001 | 75% | 65% | 0.257 | 93% | 97% | 0.092 |
| Diuretics | 100% | 100% | 1.0 | 99% | 100% | 0.297 | 100% | 100% | 1.0 | 99% | 100% | 0.381 |
| Aspirin | 11% | 13% | 0.723 | 99% | 99% | 0.367 | 8% | 16% | 0.193 | 97% | 99% | 0.109 |
| Dihydroperidines | 7% | 2% | 0.175 | 38% | 31% | 0.078 | 8% | 5% | 0.453 | 49% | 31% | <0.001 |
| Dobutamine | 24% | 49% | 0.001 | 18% | 59% | <0.001 | 24% | 26% | 0.812 | 22% | 15% | 0.054 |
| Noradrenaline | 17% | 46% | <0.001 | 17% | 53% | <0.001 | 14% | 24% | 0.233 | 18% | 17% | 0.794 |
| Endotracheal intubation | 20% | 47% | <0.001 | 16% | 54% | <0.001 | 18% | 23% | 0.500 | 17% | 15% | 0.695 |
| CPAP | 29% | 39% | 0.201 | 24% | 37% | 0.002 | 33% | 21% | 0.177 | 31% | 19% | 0.004 |
| Thrombolytics | 0% | 0% | 6% | 9% | 0.222 | 0% | 0% | 5% | 8% | 0.268 | ||
| Primary PCI | 0% | 0% | 7% | 13% | 0.075 | 0% | 0% | 11% | 6% | 0.053 | ||
| Interventions | 0.296 | 0.091 | 0.008 | <0.001 | ||||||||
| Late PCI | 4% | 0% | 24% | 18% | 0% | 0% | 27% | 22% | ||||
| CABG | 0% | 0% | 7% | 4% | 6% | 0% | 14% | 4% | ||||
| Valvular surgery | 23% | 23% | 3% | 7% | 33% | 11% | 20% | 2% | ||||
| Hospital events | ||||||||||||
| Angina | 2% | 9% | 0.074 | 10% | 18% | 0.020 | 0% | 0% | 6% | 14% | 0.009 | |
| Myocardial infarction | 0% | 0% | 1.5% | 13% | <0.001 | 0% | 0% | 1% | 2% | 0.593 | ||
| Recurrent APE | 6% | 16% | 0.086 | 10% | 32% | <0.001 | 5% | 10% | 0.304 | 6% | 14% | 0.024 |
| Follow‐up treatment | ||||||||||||
| Beta‐blockers | 50% | 59% | 0.384 | 70% | 74% | 0.329 | ||||||
| Nitrates | 3% | 10% | 0.121 | 30% | 18% | 0.003 | ||||||
| ACE inhibitors | 66% | 71% | 0.586 | 73% | 78% | 0.168 | ||||||
| Diuretics | 61% | 8% | 0.467 | 61% | 57% | 0.359 | ||||||
| Aldosterone antagonists | 20% | 2% | 0.307 | 19% | 9% | 0.002 | ||||||
| Aspirin | 24% | 0% | 0.561 | 71% | 86% | <0.001 | ||||||
| Dihydroperidines | 3% | 2% | 0.896 | 16% | 7% | 0.001 | ||||||
| Calcium antagonists | 14% | 27% | 0.103 | 23% | 14% | 0.016 | ||||||
| Statins | 49% | 27% | 0.024 | 73% | 68% | 0.232 | ||||||
| Oral anticoagulants | 66% | 41% | 0.013 | 29% | 23% | 0.126 | ||||||
| Interventions | 0.090 | 0.024 | ||||||||||
| PCI | 2.8% | 7% | 5.4% | 1.5% | ||||||||
| CABG | 0% | 1% | 5.4% | 2.2% | ||||||||
| Valvular surgery | 8.3% | 0% | 3.2% | 0% | ||||||||
Comparison between survivors and nonsurvivors in the CAD and non‐CAD groups during the in‐hospital course and the follow‐up (%, mean±SD). ACE indicates angiotensin‐converting enzyme; APE, acute pulmonary edema; CABG, coronary artery bypass surgery; CPAP, continuous positive airway pressure, noninvasive ventilation; CVA cerebrovascular accident; PCI, percutaneous coronary intervention.
A multivariable analysis revealed that advanced age, admission systolic blood pressure, recurrence of APE, and need for inotropics or endotracheal intubation were the most significant independent markers of hospital mortality (Table 3).
Table 3.
Multivariable Predictors of In‐Hospital and Overall Mortality in Patients With APE
| OR | 95% CI | P Value | |
|---|---|---|---|
| In‐hospital mortality | |||
| Age (per year) | 1.062 | 1.031 to 1.092 | <0.001 |
| Endotracheal intubation | 2.733 | 1.573 to 4.772 | <0.001 |
| Dobutamine | 2.910 | 1.642 to 5.711 | <0.001 |
| Recurrent APE | 2.414 | 1.362 to 4.273 | 0.003 |
| Admission systolic blood pressure (per mm Hg) | 0.993 | 0.984 to 0.988 | 0.008 |
| CAD | 1.842 | 1.102 to 3.081 | 0.020 |
| Overall mortality | |||
| Age (per year) | 1.028 | 1.021 to 1.073 | <0.001 |
| Peripheral vascular disease | 1.672 | 1.683 to 4.532 | <0.001 |
| Peak CKMB, per μg/L | 1.001 | 1.000 to 1.002 | 0.001 |
| Coronary revascularization—valvular surgery | 0.883 | 0.785 to 0.988 | 0.001 |
| Diabetes | 1.353 | 1.059 to 1.719 | 0.015 |
| Mitral insufficiency | 1.164 | 1.034 to 1.303 | 0.015 |
| Chronic obstructive pulmonary disease | 1.392 | 1.066 to 1.807 | 0.015 |
| Creatinine, per mg/dL | 1.113 | 1.020 to 1.217 | 0.016 |
| Aortic stenosis | 1.201 | 1.026 to 1.391 | 0.022 |
| Dobutamine | 1.741 | 1.269 to 2.389 | 0.031 |
| Female sex | 1.323 | 1.008 to 1.737 | 0.043 |
Main hospital and overall mortality predictors using a single model with multiple predictors are listed with the corresponding units in continuous variables or as the presence of categorical variables. CAD indicates coronary artery disease; CK‐MB, creatine kinase MB; OR, odds ratio.
Follow‐up Events
Of the 584 hospital survivors, there were 10 with a missing follow‐up (1.2%), all from the CAD group, and median follow‐up for the 258 total survivors was 45 months (range, 10–40). There were 316 deaths that resulted in a follow‐up mortality of 55.1% (316 of 574) that was higher in CAD than in non‐CAD patients (273 of 459 [59.5%] vs 43 of 115 [37.4%]; P<0.001; Table 2; Figure 1). In the 2 groups, nonsurvivors were older than survivors and showed a higher rate of peripheral vascular disease, a higher creatinine, and lower hemoglobin levels. Nonsurvivors underwent less frequently coronary revascularization procedures or valve replacement than survivors, although this was often attributable to notable comorbidities (Table 2). Among survivors, hospital readmissions for nonfatal heart failure were higher in CAD than in non‐CAD patients (17.3% vs 7.6%; P=0.009). Cause of death in the follow‐up could be determined in 226 of 316 patients (72%), 192 of 273 with CAD (70%), and 34 of 43 with non‐CAD (79%). Death was of cardiac origin in 85 of 226 patients (38%), 76 of 192 of those with CAD (40%), and 9 of 34 of those with non‐CAD (27%; P=0.180).
Overall Mortality
Total mortality was high (538 of 796; 67.6%) and was higher in CAD (442 of 628; 70.4%) than in non‐CAD patients (96 of 168 [57.1%]; P<0.002; Figures 1, 2 through 3), and this difference remained apparent in subsets with different age (Figure 2). Cardiac mortality among patients with known cause of death tended to be higher in CAD (158 of 361 [43.8%] vs 29 of 87 [33.3%]; P=0.090). A Cox regression analysis disclosed that advanced age, peripheral vascular disease, and peak CK‐MB during hospitalization index were the main independent predictors of mortality, whereas coronary revascularization or valvular surgery were significantly protective (Table 3). Most of these interventions were performed during hospitalization index (294 of 307; 96%), and patients who did not undergo these procedures were older (73±10 vs 70±10 years; P<0.001) and more often female (42% vs 29%; P<0.001), and had a higher rate of peripheral vascular disease (32% vs 22%; P=0.004) and previous renal insufficiency (25% vs 19%; P=0.057) than those not intervened. Ejection fraction, however, was similar in survivors and nonsurvivors in the 2 groups.
Figure 2.
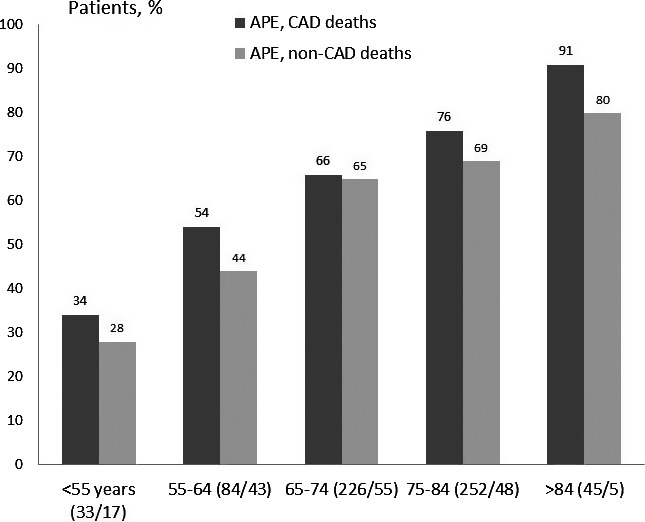
Total mortality in patients with APE and CAD was higher than in those with non‐CAD across the groups of different age (P=0.018) (numbers within brackets indicate CAD and non‐CAD cases). APE indicates acute pulmonary edema; CAD, coronary artery disease.
Figure 3.
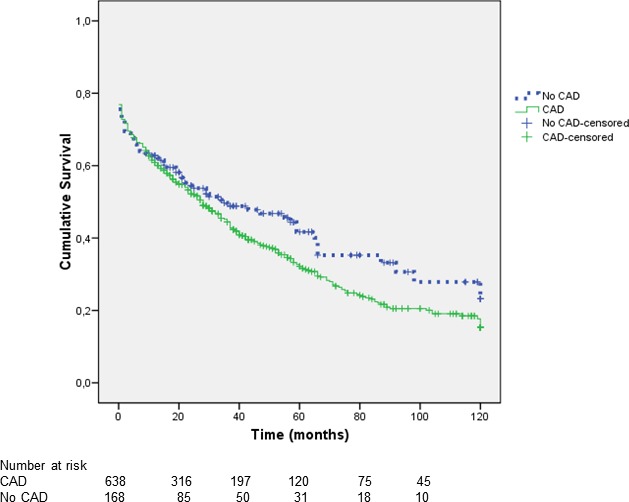
Kaplan–Meier survival curve showing a strong trend toward higher mortality in patients with APE and CAD that was mainly apparent during the follow‐up. APE indicates acute pulmonary edema; CAD, coronary artery disease.
Discussion
The principal findings of our study were: (1) a high hospital mortality that was similar in CAD and non‐CAD patients and that was mostly secondary to a cardiac cause or to complications derived from APE; (2) a higher 4‐year mortality and hospital readmission rate for nonfatal heart failure in CAD than in non‐CAD patients; and (3) the existence of different predictors of hospital and overall mortality.
In‐Hospital Outcome
Several investigators have reported on the in‐hospital mortality in patients with acute heart failure, which is an ample concept that includes acute decompensation of chronic heart failure, cardiogenic shock with or without pulmonary edema, right ventricular failure, and APE.3, 6, 14, 19, 20, 21 In these studies, mortality has varied from 6.4% to 17.2% and they have shown advanced age,6, 14, 19, 21 severe left ventricular dysfunction,14 acute coronary syndromes,14 admission blood pressure,6, 19, 21 renal failure,6, 19, 21 need for inotropics,19 and anemia19 as principal predictors.
In patients with APE, in‐hospital mortality seems comparable to patients with acute heart failure ranging also from 7.4% to 17%,7, 10, 18, 22, 23 although in small and earlier reports,7, 18 mortality was higher (12% and 17%) than in larger, more recent studies (7.4%–9.6%).10, 22, 23 Nevertheless, in patients admitted to critical care areas—such as in our study—hospital mortality is higher (32%), at least in the only reported series that involved 199 patients.24 In our work, hospital mortality was somewhat lower (25%), but it was higher than in patients not admitted to critical care areas,7, 10, 18, 22, 23 likely because of the more severe respiratory and/or hemodynamic condition, as indicated by the high need for endotracheal intubation or inotropic agents (26% and 29%), respectively. Another possible explanation was the high proportion of de novo cases of APE (>80%), a fact that has also been associated with higher hospital mortality.20 In comparison with existing series,7, 10, 18, 22, 23 we found similar major in‐hospital mortality markers, such as age, admission blood pressure, and the need for endotracheal intubation or inotropic agents. Nevertheless, we observed additional strong predictors not previously reported, such as moderate‐to‐severe mitral regurgitation and hospital recurrence of APE. The former was present in more than one third of patients and in a similar proportion of those with or without CAD, whereas the latter occurred more frequently in CAD patients and could be partly attributed to myocardial ischemia because in 46% it was associated with recurrence of angina or myocardial infarction.
Total Mortality
Most series that have analyzed total mortality in patients with acute heart failure have reported only a 1‐year follow‐up with mortality rates ranging from 17.4% to 34%5, 11, 12, 13, 14 and higher in patients admitted to a critical care area (46.5%).4 Predictors of 1‐year mortality appeared also to be age,12, 13, 14, 15 admission blood pressure,13, 15 anemia,13 renal failure,12, 13, 15 left ventricular dysfunction, and acute coronary syndromes.14 There are few studies (3), however, that have analyzed a longer follow‐up in patients with acutely decompensated heart failure,15, 16, 17 with mortality rates of 60.3%15 and 71%,16 with similar main predictors than in the 1‐year follow‐up series.15
In contrast to acute heart failure, only 2 studies have investigated the 1‐year mortality in patients with APE7, 18 and none has analyzed a longer follow‐up. The 2 studies were carried out nearly 2 decades ago and included 8618 and 150 patients7 with a mortality of 51.2% and 40%, respectively. In the present work, which is mostly an investigation of a de novo APE, the 4‐year mortality was 62%, and was higher in CAD than in non‐CAD patients. Moreover, death was more frequently of noncardiac origin, particularly in non‐CAD patients. Most significant independent markers were age, peripheral vascular disease, and peak CK‐MB during hospitalization index.
Hospital readmission rate for APE or acute decompensated heart failure was also higher in CAD than in non‐CAD patients. As expected, revascularized CAD patients and those with non‐CAD who had valvular replacement showed a lower hospital and long‐term mortality or recurrence of heart failure than those without these procedures. Indeed, the Cox regression analysis showed that practice of coronary revascularization or valvular surgery was significantly protective. Revascularization or valve replacement procedures, however, were less frequently performed in elderly patients mainly because of relevant comorbidities. Noteworthy, the more severe prognosis of CAD patients is likely multifactorial because they were older and had a higher rate of peripheral vascular disease, diabetes, and multivessel coronary disease conditions that portends a more severe arteriosclerotic profile. It is unclear why ejection fraction was not a marker of mortality, but, in part, this may relate to the rather high incidence of noncardiac deaths.
Strengths and Limitations
Relevant findings of our study and not previously reported are the 4‐year prognosis of patients with APE and the comparison of outcomes between patients with and without CAD. Also of importance is the fact that identification of CAD patients was based not only on clinical grounds, but also on coronary angiography data available in 69% of them, but also in 43% of those with non‐CAD. This is in contrast to most existing series of APE7, 10, 23 or acute heart failure,1, 2, 3, 4, 5, 13 where angiographic data are not provided. Of interest is the documentation of significant coronary artery stenosis in 32% of non‐CAD patients who underwent cardiac catheterization, pointing to the concomitant presence of CAD in patients with the primary diagnosis of valvular heart disease. In addition, all our patients had an echocardiogram during the first few hours from hospital admission to evaluate the underlying heart disease. Follow‐up was long and thorough given that only 1.7% of patients were lost, and in 73% the cause of death could be identified. As limitations, we recognized that our results may not be applicable to patients with previous admission with APE because in 85% it was a first event, or to those admitted to a regular ward rather than to a critical care area. Another drawback is the fact that only a minority of patients were treated with aldosterone antagonists. This, in part, may be explained by the years in which the study was carried out and also by the not infrequent presence of moderate renal failure.
Implications
Our results indicate that APE—at least in patients with a first episode admitted to an acute cardiac care unit—is associated with a high hospital and 4‐year mortality, particularly in those with CAD. In the latter subset, the role of advanced arteriosclerosis in their poor prognosis is underscored. On the other hand, coronary revascularization and valvular surgery significantly reduced overall mortality. Thus, identifying high‐risk patients for in‐hospital and long‐term mortality, in part, through the predictors herein described—which do not necessarily implicate the systolic function—we may speculate that a more aggressive interventional program might improve survival in high‐risk patients despite the frequent comorbidities. In keeping with this and in view of the fact that recurrence of in‐hospital APE—one of the markers of in‐hospital mortality—was often caused by recurrent symptomatic or silent myocardial ischemia, an increase in the frequency of coronary angiography and early revascularization might have improved their outcome.
Sources of Funding
This study was, in part, financially supported by a grant from the Fundació Recerca Biomèdica i Docència Hospital Vall d'Hebron (PR, HG 35/2000), Barcelona, Spain. This study was also supported by RETICS‐RIC, RD12/0042/0021.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002581 doi: 10.1161/JAHA.115.002581)
References
- 1. Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–2226. [DOI] [PubMed] [Google Scholar]
- 2. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. [DOI] [PubMed] [Google Scholar]
- 3. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola V, Hochadel M, Komajda M, Lassus J, Lopez‐Sendon JL, Ponikowski P, Tavazzi L. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. [DOI] [PubMed] [Google Scholar]
- 4. Zannad F, Mebazaa A, Juillière Y, Cohen‐Solal A, Guize L, Alla F, Rougé P, Blin P, Barlet MH, Paolozzi L, Vincent C, Desnos M, Samii K. Clinical profile, contemporary management and one‐year mortality in patients with severe acute heart failure syndromes: the EFICA study. Eur J Heart Fail. 2006;8:697–705. [DOI] [PubMed] [Google Scholar]
- 5. Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Wendelboe Nielsen O, Zannad F, Tavazzi L. EURObservational Research Programme: regional differences and 1‐year follow‐up results of the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail. 2013;15:808–817. [DOI] [PubMed] [Google Scholar]
- 6. Logeart D, Isnard R, Resche‐Rigon M, Seronde MF, de Groote P, Jondeau G, Galinier M, Mulak G, Donal E, Delahaye F, Juilliere Y, Damy T, Jourdain P, Bauer F, Eicher JC, Neuder Y, Trochu JN. Current aspects of the spectrum of acute heart failure syndromes in a real‐life setting: the OFICA study. Eur J Heart Fail. 2013;15:465–476. [DOI] [PubMed] [Google Scholar]
- 7. Roguin A, Behar D, Ben Ami H, Reisner SA, Edelstein S, Linn S, Edoute Y. Long‐term prognosis of acute pulmonary oedema–an ominous outcome. Eur J Heart Fail. 2000;2:137–144. [DOI] [PubMed] [Google Scholar]
- 8. Peña Gil C, Figueras J, Soler Soler J. Acute cardiogenic pulmonary edema. Relevance of multivessel disease, conduction abnormalities and silent ischemia. Int J Cardiol. 2005;103:59–66. [DOI] [PubMed] [Google Scholar]
- 9. Rudiger A, Harjola VP, Muller A, Mattila E, Saila P, Nieminen M, Follath F. Acute heart failure: clinical presentation, one‐year mortality and prognostic factors. Eur J Heart Fail. 2005;7:662–670. [DOI] [PubMed] [Google Scholar]
- 10. Parissis JT, Nikolaou M, Mebazaa A, Ikonomidis I, Delgado J, Vilas‐Boas F, Paraskevaidis I, Mc Lean A, Kremastinos D, Follath F. Acute pulmonary oedema: clinical characteristics, prognostic factors, and in‐hospital management. Eur J Heart Fail. 2010;12:1193–1202. [DOI] [PubMed] [Google Scholar]
- 11. Siirila‐Waris K, Lassus J, Melin J, Peuhkurinen K, Nieminen MS, Harjola V. Characteristics, outcomes, and predictors of 1 year mortality in patients hospitalized for acute heart failure. Eur Heart J. 2006;27:3011–3017. [DOI] [PubMed] [Google Scholar]
- 12. Harjola VP, Follath F, Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Hochadel M, Komajda M, Lopez‐Sendon JL, Ponikowski P, Tavazzi L. Characteristics, outcomes, and predictors of mortality at 3 months and 1 year in patients hospitalized for acute heart failure. Eur J Heart Fail. 2010;12:239–248. [DOI] [PubMed] [Google Scholar]
- 13. Tavazzi L, Senni M, Metra M, Gorini M, Cacciatore G, Chinaglia A, Di Lenarda A, Mortara A, Oliva F, Maggioni AP. Multicenter prospective observational study on acute and chronic heart failure: one‐year follow‐up results of IN‐HF (Italian Network on Heart Failure) outcome registry. Circ Heart Fail. 2013;6:473–481. [DOI] [PubMed] [Google Scholar]
- 14. Parenica J, Spinar J, Vitovec J, Widimsky P, Linhart A, Fedorco M, Vaclavik J, Miklik R, Felsoci M, Horakova K, Cihalik C, Malek F, Spinarova L, Belohlavek J, Kettner J, Zeman K, Dušek L, Jarkovsky J. Long‐term survival following acute heart failure: the Acute Heart Failure Database Main registry (AHEAD Main). Eur J Intern Med. 2013;24:151–160. [DOI] [PubMed] [Google Scholar]
- 15. Lassus JP, Siirilä‐Waris K, Nieminen MS, Tolonen J, Tarvasmäki T, Peuhkurinen K, Melin J, Pulkki K, Harjola VP. Long‐term survival after hospitalization for acute heart failure—differences in prognosis of acutely decompensated chronic and new‐onset acute heart failure. Int J Cardiol. 2013;168:458–462. [DOI] [PubMed] [Google Scholar]
- 16. Joffe SW, Webster K, McManus DD, Kiernan MS, Lessard D, Yarzebski J, Darling C, Gore JM, Goldberg RJ. Improved survival after heart failure: a community‐based perspective. J Am Heart Assoc. 2013;2:e000053 doi: 10.1161/JAHA.113.000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grigorian Shamagian L, Roman AV, Ramos PM, Acuña JM, Veloso PR, Gonzalez‐Juanatey JR. Acute pulmonary edema in patients with decompensated heart failure. Role of underlying cardiopathy on the prognosis. Int J Cardiol. 2007;121:302–305. [DOI] [PubMed] [Google Scholar]
- 18. Goldberger JJ, Peled HB, Stroh JA, Cohen MN, Frishman WH. Prognostic factors in acute pulmonary edema. Arch Intern Med. 1986;146:489–493. [PubMed] [Google Scholar]
- 19. Tavazzi L, Maggioni AP, Lucci D, Cacciatore G, Ansalone G, Oliva F, Porcu M. Nationwide survey on acute heart failure in cardiology ward services in Italy. Eur Heart J. 2006;27:1207–1215. [DOI] [PubMed] [Google Scholar]
- 20. Follath F, Yilmaz MB, Delgado JF, Parissis JT, Porcher R, Gayat E, Burrows N, McLean A, Vilas‐Boas F, Mebazaa A. Clinical presentation, management and outcomes in the Acute Heart Failure Global Survey of Standard Treatment (ALARM‐HF). Intensive Care Med. 2011;37:619–626. [DOI] [PubMed] [Google Scholar]
- 21. Oliva F, Mortara A, Cacciatore G, Chinaglia A, Di Lenarda A, Gorini M, Metra M, Senni M, Maggioni AP, Tavazzi L. Acute heart failure patient profiles, management and in‐hospital outcome: results of the Italian Registry on Heart Failure Outcome. Eur J Heart Fail. 2012;14:1208–1217. [DOI] [PubMed] [Google Scholar]
- 22. Attias D, Mansencal N, Auvert B, Vieillard‐Baron A, Delos A, Lacombe P, N'Guetta R, Jardin F, Dubourg O. Prevalence, characteristics, and outcomes of patients presenting with cardiogenic unilateral pulmonary edema. Circulation. 2010;122:1109–1115. [DOI] [PubMed] [Google Scholar]
- 23. Gray A, Goodacre S, Nicholl J, Masson M, Sampson F, Elliott M, Crane S, Newby DE. The development of a simple risk score to predict early outcome in severe acute acidotic cardiogenic pulmonary edema: the 3CPO score. Circ Heart Fail. 2010;3:111–117. [DOI] [PubMed] [Google Scholar]
- 24. Rodríguez Mulero L, Carrillo Alcaraz A, Melgarejo Moreno A, Renedo Villarroya A, Párraga Ramírez M, Jara Pérez P, Millán MJ, González Díaz G. Predictive factors related to success of non invasive ventilation and mortality in the treatment of acute cardiogenic pulmonary edema. Med Clin (Barc). 2005;124:126–131. [DOI] [PubMed] [Google Scholar]


