Abstract
Background
Existing equations for prediction of atrial fibrillation (AF) have been developed and validated in white and African‐American populations. Whether these models adequately predict AF in more racially and ethnically diverse populations is unknown.
Methods and Results
We studied 6663 men and women 45 to 84 years of age without AF at baseline (2000–2002) enrolled in the Multi‐Ethnic Study of Atherosclerosis (MESA). Of these, 38% were non‐Hispanic whites, 28% non‐Hispanic African Americans, 22% Hispanics, and 12% Chinese Americans. AF during follow‐up was ascertained from hospitalization discharge codes through 2012. Information collected at baseline was used to calculate predicted 5‐year risk of AF using the previously published simple CHARGE‐AF model, which only includes clinical variables, and a biomarker‐enriched CHARGE‐AF model, which also considers levels of circulating N‐terminal of the prohormone B‐type natriuretic peptide and C‐reactive protein. For comparison purposes, we also assessed performance of the 10‐year Framingham AF model. During a mean follow‐up of 10.2 years, 351 cases of AF were identified. The C‐statistic of the CHARGE‐AF models were 0.779 (95% CI, 0.744–0.814) for the simple model and 0.825 (95% CI, 0.791–0.860) for the biomarker‐enriched model. Calibration was adequate in the biomarker‐enriched model (χ2=7.9; P=0.55), but suboptimal in the simple model (χ2=25.6; P=0.002). In contrast, the 10‐year Framingham score had a C‐statistic (95% CI) of 0.746 (0.720–0.771) and showed poor calibration (χ2=57.4; P<0.0001).
Conclusion
The CHARGE‐AF risk models adequately predicted 5‐year AF risk in a large multiethnic cohort. These models could be useful to select high‐risk individuals for AF screening programs or for primary prevention trials in diverse populations.
Keywords: atrial fibrillation, epidemiology, risk prediction
Subject Categories: Atrial Fibrillation, Epidemiology
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia associated with an increased risk of stroke, heart failure (HF), myocardial infarction, dementia, and mortality.1, 2 Interest in building predictive models that can identify individuals at higher risk of developing AF has increased in parallel with the growing prevalence of this arrhythmia.3 Starting with a risk score created by the Framingham Heart Study (FHS) investigators,4 and validated in separate cohorts,5 other models have been developed in single cohorts, such as the Atherosclerosis Risk in Communities (ARIC) study.6 More recently, the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE)‐AF consortium derived a new prediction model pooling data from several large prospective studies (FHS, Cardiovascular Health Study, and ARIC).7 This model, based on easily measured clinical variables, had adequate discrimination in the Age, Gene and Environment‐Reykjavik study (AGES), the Rotterdam study, and the EPIC‐Norfolk cohort.7, 8 An extension of the CHARGE‐AF model demonstrated the added benefit of selected biomarkers in AF prediction.9 A potential limitation of the CHARGE‐AF risk model, however, is that it was developed in a mostly biracial (white and African‐American) population and validated in predominantly white cohorts. Whether the model would adequately predict AF in more racially and ethnically diverse populations is not known. This is particularly relevant given the observed lower risk of AF in nonwhites (including Hispanics and Asian Americans) compared to whites.10, 11 Therefore, we assessed the predictive ability (discrimination and calibration) of the CHARGE‐AF risk model in the Multi‐Ethnic Study of Atherosclerosis (MESA), a community‐based, racially and ethnically diverse prospective cohort in the United States. For comparison purposes, we also determined the predictive ability of the FHS risk score for AF as well as that of scores for stroke prediction in AF, given their extensive use in the management of AF patients12, 13, 14 and attempts to extend them to the prediction of AF itself.15, 16, 17, 18
Methods
Study Population
A detailed description of the MESA cohort has been published elsewhere.19 Briefly, in 2000–2002, MESA recruited 6814 men and women, 45 to 84 years of age, free of clinical cardiovascular disease from 6 communities across the United States: Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; New York City, NY; and Saint Paul, MN. The main aims of MESA are to investigate the prevalence, progression, and risk factors of subclinical cardiovascular disease in the general population. For the present analysis, we excluded individuals with evidence of AF at baseline (n=70), those who did not have follow‐up beyond the baseline exam (n=33), and those with missing values in any of the variables contributing to the CHARGE‐AF model (n=48), leaving 6663 eligible participants. Analyses using the biomarker‐enriched model were performed in 5477 participants with available N‐terminal of the prohormone B‐type natriuretic peptide (NT‐proBNP) and high‐sensitivity C‐reactive protein (hsCRP) measurements. The study protocols have been approved by institutional review boards at all participant institutions. Participants provided written informed consent at baseline.
Ascertainment of Atrial Fibrillation
MESA participants or a proxy were contacted by phone every 9 to 12 months to identify all new hospitalizations. Trained staff abstracted discharge diagnostic and procedure codes from these hospitalizations. Using discharge diagnostic codes, we defined incident AF if an International Classification of Diseases, Ninth Revision, Clinical Modification code 427.31 (AF) or 427.32 (atrial flutter) was present in any position. AF hospitalizations associated with open cardiac surgery were ignored in the definition. Previous studies have demonstrated the adequate validity of hospital discharge codes for ascertainment of AF in large cohort studies.20, 21 For this analysis, cases identified through the end of 2012 were considered.
Assessment of Baseline Covariates
Age, sex, race, ethnicity, smoking status, and medication use were self‐reported at baseline. Race/ethnicity was classified in 4 categories: non‐Hispanic white; non‐Hispanic African American; Hispanic; and Chinese American. Height and weight were measured with the participant wearing a light gown. Systolic and diastolic blood pressures (BPs) were measured 3 times after the participant rested for 5 minutes; the mean of the last 2 measurements was used for analysis. Diabetes was defined as having a fasting blood glucose ≥126 mg/dL or self‐reported use of antidiabetic medications. Standard 10‐second 12‐lead electrocardiograms (ECGs) were obtained in all participants using a Marquette MAC 1200 electrocardiograph (GE Healthcare, Little Chalfont, UK). Study ECGs were transmitted to a central reading center (EPICARE, Wake Forest University, Winston‐Salem, NC), where they were processed and analyzed. P‐wave durations and amplitudes were automatically measured with the GE Marquette 12‐SL program (2001 version; GE Marquette, Milwaukee, WI). PR interval was defined as the median PR interval in all leads. By design, MESA excluded individuals who had a past history of coronary heart disease or HF. NT‐proBNP concentration was measured in serum using a commercially available immunoassay (Roche Diagnostic Elecsys proBNP Assay; Roche Corporation, Indianapolis, IN). hsCRP was measured using a BNII nephelometer (N High‐Sensitivity CRP; Dade Behring, Deerfield, IL).
Statistical Analysis
We used Cox proportional hazards models to determine the association between baseline characteristics and incident AF. We defined time of follow‐up as time from the baseline exam to incident AF, death, or last available follow‐up contact, whichever occurred first. To assess the performance of the CHARGE‐AF model in MESA, we calculated 5‐year predicted risk of AF using baseline covariates and the CHARGE‐AF risk function.7 We assessed model discrimination by estimating the C‐statistic in a Cox model, including the predicted risk as the only covariate,22 and calibration by comparing the observed and predicted 5‐year event rates across deciles of predicted risk using a Hosmer–Lemeshow test modified for survival analysis.23 For this analysis, we combined Hispanics and Chinese Americans with non‐Hispanic African Americans in a “nonwhite” category given their similar AF rates in the MESA cohort.11 We repeated the analysis using the biomarker‐enriched CHARGE‐AF model, which includes information on circulating NT‐proBNP and hsCRP, as well as using separate models that include each biomarker individually.9 To further determine the benefit of adding biomarker information to the simple CHARGE‐AF model, we calculated the categorical net reclassification index (NRI) using 2.5% and 5% as cut‐off points and also the continuous NRI (>0).24, 25 The cut‐off points for the categorical NRI were chosen to be consistent with previous CHARGE‐AF publications.7, 9 For comparison purposes, we evaluated discrimination and calibration of a Cox model including all the components of the CHARGE‐AF models as separate covariates. This model provides an upper limit of the best prediction provided by the CHARGE‐AF variables in the MESA cohort.
We conducted similar analyses for the Framingham model, which predicts 10‐year AF risk.4 We also examined the usefulness of the CHADS2 and CHA2DS2‐VASc scores, originally developed for prediction of stroke in AF patients,12, 13 to predict incident AF in MESA. Information on the presence of significant cardiac murmur, included in the Framingham model, was not available. However, we assumed it to be absent given that, by study design, MESA participants were free of cardiovascular disease at baseline. A complete list of variables included in each score is provided in Table S1. All analyses were performed using SAS software (9.3 for Windows; SAS Institute Inc., Cary, NC).
Results
During a mean follow‐up of 10.2 years, we identified 351 cases of AF among 6663 eligible MESA participants (incident rate, 5.2 per 1000 person‐years) and 296 cases of AF among 5477 participants with information on NT‐proBNP and hsCRP. The incidence rate of AF was highest among non‐Hispanic whites (7.0 per 1000 person‐years) and was similar among African Americans (4.0 per 1000 person‐years), Hispanics (4.4 per 1000 person‐years), and Chinese Americans (3.3 per 1000 person‐years). Table 1 shows selected baseline characteristics overall and by incident AF status. As expected, those who developed AF were older, had higher body mass index and systolic BP and longer PR interval, and were more likely to be male, white, diabetic, and on antihypertensive medications. Baseline characteristics of study participants by race/ethnicity are presented in Table S2. Table 2 presents results from a multivariable Cox model including simultaneously the components of the CHARGE‐AF models as separate covariates. In the simple model, all the covariates were significantly associated with risk of AF with the exception of diabetes, diastolic BP, and smoking. NT‐proBNP was strongly associated with AF risk, whereas associations of race, smoking, and blood pressure with AF were attenuated in the biomarker‐enriched model. No significant association was observed between hsCRP and AF risk.
Table 1.
Selected Baseline Characteristics of Study Participants Overall and by AF Status During Follow‐up, Multi‐Ethnic Study of Atherosclerosis
| Overall (N=6663) | No AF (N=6312) | AF (N=351) | |
|---|---|---|---|
| Age, y | 62 (10) | 62 (10) | 69 (8) |
| Women, N (%) | 3517 (53) | 3369 (53) | 148 (42) |
| Race/ethnicity, N (%) | |||
| Non‐Hispanic white | 2559 (38) | 2372 (38) | 187 (53) |
| Non‐Hispanic African American | 1835 (28) | 1763 (28) | 72 (21) |
| Hispanic | 1477 (22) | 1412 (22) | 65 (19) |
| Chinese American | 792 (12) | 765 (12) | 27 (8) |
| Height, cm | 166 (10) | 166 (10) | 168 (11) |
| Weight, kg | 79 (17) | 78 (17) | 82 (18) |
| Systolic BP, mm Hg | 126 (21) | 126 (21) | 135 (23) |
| Diastolic BP, mm Hg | 72 (10) | 72 (10) | 72 (11) |
| Current smoking, N (%) | 870 (13) | 833 (13) | 37 (11) |
| Use of antihypertensive medication, N (%) | 2457 (37) | 2270 (36) | 187 (53) |
| Diabetes, N (%) | 833 (13) | 777 (12) | 56 (16) |
| PR interval, ms | 166 (25) | 165 (24) | 173 (32) |
| NT‐proBNP, pg/mL | 101 (249) | 93 (225) | 247 (491) |
| hsCRP, mg/L | 3.8 (5.9) | 3.8 (5.9) | 3.9 (5.7) |
Values correspond to mean (SD) or N (%).AF indicates atrial fibrillation; BP, blood pressure; hsCRP, high‐sensitivity C‐reactive protein; NT‐proBNP, N‐terminal of the prohormone B‐type natriuretic peptide.
Table 2.
HRs (95% CIs) of AF for Variables Included in the Simple and Biomarker‐Enriched CHARGE‐AF Risk Models, Multi‐Ethnic Study of Atherosclerosis, 2000–2012
| Simple Model | Biomarker‐Enriched Model | |
|---|---|---|
| N | 6663 | 5477 |
| HR (95% CI) | ||
| Age, per 5 y | 1.5 (1.4, 1.6) | 1.4 (1.3, 1.5) |
| White race, vs nonwhite | 1.6 (1.3, 2.0) | 1.2 (1.0, 1.6) |
| Height, per 10 cm | 1.3 (1.1, 1.4) | 1.2 (1.0, 1.4) |
| Weight, per 15 kg | 1.2 (1.1, 1.3) | 1.3 (1.2, 1.5) |
| Systolic BP, per 20 mm Hg | 1.3 (1.1, 1.4) | 1.0 (0.9, 1.2) |
| Diastolic BP, per 10 mm Hg | 0.9 (0.8, 1.0) | 1.1 (0.9, 1.2) |
| Current smoking | 1.3 (0.9, 1.8) | 1.2 (0.8, 1.8) |
| Use of antihypertensive medication | 1.4 (1.1, 1.7) | 1.3 (1.0, 1.6) |
| Diabetes | 1.1 (0.8, 1.5) | 1.2 (0.9, 1.7) |
| Log (NT‐proBNP), per 1 unit | — | 2.0 (1.7, 2.2) |
| Log (hsCRP), per 1 unit | — | 0.9 (0.8, 1.0) |
Results from a single Cox proportional hazards model including all the covariates simultaneously. AF indicates atrial fibrillation; BP, blood pressure; CHARGE, the Cohorts for Heart and Aging Research in Genomic Epidemiology; HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; NT‐proBNP, N‐terminal of the prohormone B‐type natriuretic peptide.
During the first 5 years of follow‐up, 129 AF cases occurred (109 in the subset with available biomarkers). The C‐statistics of the simple and biomarker‐enriched CHARGE‐AF models were 0.779 and 0.825, respectively (Table 3). A model adding NT‐proBNP alone to the simple CHARGE‐AF model had similar discrimination and calibration to the biomarker‐enriched model including both biomarkers, whereas addition of hsCRP alone did not improve prediction (Table 3). Calibration of the simple model was suboptimal (P=0.002), whereas the biomarker‐enriched model showed adequate calibration (P=0.55). Calculating the NRI provided similar information, with improved reclassification after adding NT‐proBNP, but not hsCRP, to the simple CHARGE‐AF model: The continuous NRI (>0) (95% CI) for the NT‐proBNP, hsCRP, and joint biomarker models were 0.431 (0.242, 0.619), 0.120 (−0.063, 0.309), and 0.344 (0.143, 0.537), whereas the categorical NRI (2.5%, 5%) were 0.045 (−0.049, 0.147), −0.037 (−0.102, 0.020), and 0.041 (−0.055, 0.147), respectively. Reclassification tables are provided in Table S3. Discrimination and calibration of the CHARGE‐AF models was similar across sex and race (white and nonwhite) subgroups (Table 4).
Table 3.
Discrimination and Calibration of Risk Prediction Models, Multi‐Ethnic Study of Atherosclerosis, 2000–2012
| C‐Statistic, 95% CI | Calibration χ2 (P Value) | |
|---|---|---|
| Best MESA simple modela | 0.780 (0.745, 0.815) | 6.4 (P=0.70) |
| Best MESA biomarker modela | 0.834 (0.800, 0.868) | 14.9 (P=0.09) |
| Simple CHARGE‐AFa | 0.779 (0.744, 0.814) | 25.6 (0.002) |
| hsCRP‐enriched CHARGE‐AFa | 0.784 (0.747, 0.821) | 15.2 (0.08) |
| NT‐proBNP‐enriched CHARGE‐AFa | 0.825 (0.791, 0.859) | 9.9 (0.36) |
| Biomarker‐enriched CHARGE‐AFa | 0.825 (0.791, 0.860) | 7.9 (0.55) |
| Framingham modelb | 0.746 (0.720, 0.771) | 57.4 (<0.0001) |
| CHADS2 a | 0.671 (0.628, 0.714) | NA |
| CHA2DS2‐VASca | 0.695 (0.654, 0.735) | NA |
AF indicates atrial fibrillation; BP, blood pressure; CHARGE, the Cohorts for Heart and Aging Research in Genomic Epidemiology; hsCRP, high‐sensitivity C‐reactive protein; MESA, Multi‐Ethnic Study of Atherosclerosis; NA, not applicable; NT‐proBNP, N‐terminal of the prohormone B‐type natriuretic peptide.
5‐year prediction.
10‐year prediction.
Table 4.
Discrimination and Calibration of Risk Prediction Models by Race and Sex, Multi‐Ethnic Study of Atherosclerosis, 2000–2012
| Whites | Nonwhites | Women | Men | |
|---|---|---|---|---|
| AF cases | 187 | 164 | 148 | 203 |
| Person‐time | 26 723 | 41 115 | 36 333 | 31 505 |
| AF rate (per 1000 person‐years) | 7.0 | 4.0 | 4.1 | 6.4 |
| Simple CHARGE‐AF | ||||
| C‐statistic (95% CI) | 0.764 (0.718, 0.810) | 0.776 (0.724, 0.829) | 0.775 (0.721, 0.830) | 0.772 (0.727, 0.818) |
| Calibration χ2 (P value) | 14.6 (0.10) | 12.3 (0.20) | 13.3 (0.15) | 14.9 (0.09) |
| Biomarker‐enriched CHARGE‐AF | ||||
| C‐statistic (95% CI) | 0.811 (0.764, 0.859) | 0.825 (0.773, 0.877) | 0.821 (0.766, 0.877) | 0.823 (0.779, 0.867) |
| Calibration χ2 (P value) | 8.9 (0.44) | 7.2 (0.62) | 9.2 (0.42) | 4.4 (0.88) |
| Framingham | ||||
| C‐statistic (95% CI) | 0.750 (0.716, 0.784) | 0.743 (0.704, 0.781) | 0.741 (0.704, 0.778) | 0.748 (0.714, 0.781) |
| Calibration χ2 (P value) | 8.1 (0.53) | 73.9 (<0.0001) | 32.1 (0.0002) | 36.4 (<0.0001) |
AF indicates atrial fibrillation; CHARGE, the Cohorts for Heart and Aging Research in Genomic Epidemiology.
Models including individual CHARGE‐AF covariates (“best MESA model”) provided only a small improvement in discrimination (C‐statistics: 0.780 for simple model and 0.834 for biomarker model), though calibration of the simple model was notably enhanced. Figure shows the predicted and observed risk of AF by deciles of predicted risk for the CHARGE‐AF models and the best MESA models. Overall, the simple CHARGE‐AF model slightly overestimated AF risk, particularly in the top decile of risk. Notably, the top decile of predicted risk in the biomarker CHARGE‐AF model identified a subset with a 5‐year AF risk of ≈10%, with 48 of the 109 AF cases (44%) occurring in this high‐risk group.
Figure 1.
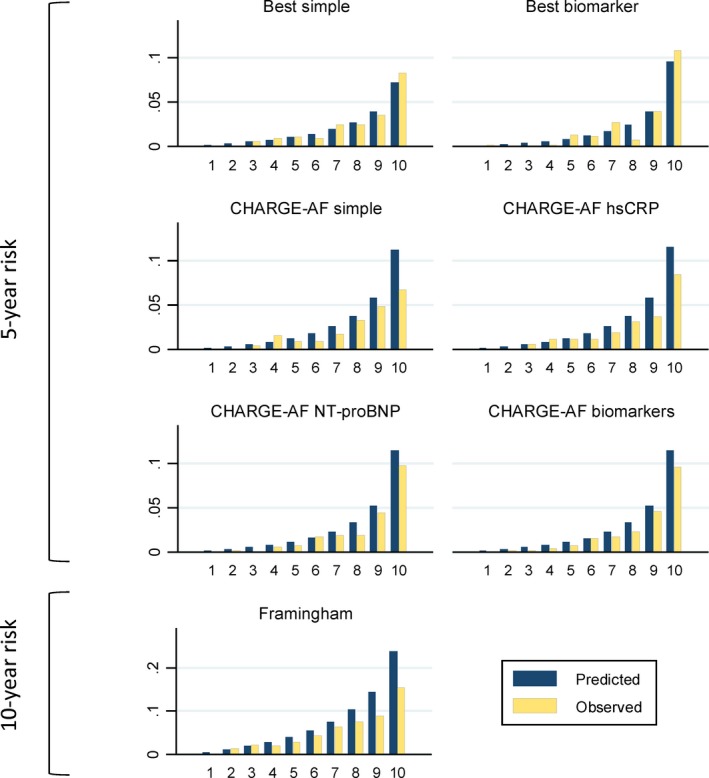
Mean predicted and observed risk of atrial fibrillation by deciles of predicted risk derived from MESA best models (5‐year risk), CHARGE‐AF models (5‐year risk), and Framingham model (10‐year risk), Multi‐Ethnic Study of Atherosclerosis, 2000–2012. AF indicates atrial fibrillation; CHARGE, the Cohorts for Heart and Aging Research in Genomic Epidemiology; hsCRP, high‐sensitivity C‐reactive protein; MESA, Multi‐Ethnic Study of Atherosclerosis; NT‐proBNP, N‐terminal of the prohormone B‐type natriuretic peptide.
Discrimination of the 10‐year Framingham model was somewhat lower than that of the CHARGE‐AF model (C‐statistic: 0.746) and had worse calibration (P<0.001), substantially overestimating the risk of AF across all deciles of risk (Table 3 and Figure). Because the Framingham model was originally developed in a predominantly white population, we explored discrimination and calibration of the model separately in whites and nonwhites. In whites, both discrimination and calibration of the model were good, whereas among nonwhites discrimination of the model was adequate but calibration was poor (Table 4 and Figure S1). Similar results were observed in men and women separately.
Finally, we assessed the discrimination of the CHADS2 and CHA2DS2‐VASc scores as potential simpler alternatives to the CHARGE‐AF and Framingham models. In both scoring systems, discrimination, as assessed by the C‐statistics, was poor (<0.7) and considerably lower than for the AF‐specific models (Table 3).
Discussion
In a racially and ethnically diverse cohort, we found that the previously developed CHARGE‐AF models, particularly the models including NT‐proBNP, showed excellent ability to predict 5‐year risk of AF and adequate calibration. In contrast, the 10‐year Framingham model had slightly worse predictive ability and very poor calibration in the entire cohort (though calibration in whites was good). Finally, models originally developed for stroke prediction in AF, such as CHADS2 and CHA2DS2‐VASc, were clearly inferior and should not be used for prediction of AF given their limited discrimination.
The demonstrated ability of the CHARGE‐AF models to predict AF in diverse populations could meaningfully impact 3 areas. First, the CHARGE‐AF models may be employed in the identification of individuals more likely to benefit from AF screening. Recent studies have demonstrated the feasibility and efficacy of implementing programs for AF screening in undiagnosed individuals.26, 27 Cost‐effectiveness of those programs will hinge heavily on selecting high‐risk individuals, in which any screening strategy will have a higher yield and subsequent treatment with oral anticoagulants for stroke prevention will provide the most benefit.28 Second, the CHARGE‐AF models could be helpful in selecting participants for AF primary prevention trials. Secondary analyses of completed trials suggest that statins,29 angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers,30 or some lifestyle interventions31 may reduce risk of AF onset. However, adequately powered trials specifically designed to test primary prevention approaches for AF are needed; inclusion of individuals at higher risk of developing AF will make those trials more efficient. Finally, the NT‐proBNP or joint biomarker‐enriched CHARGE‐AF models could be used as benchmarks against which any potential novel biomarker for AF prediction should be compared. New biomarkers for AF are being continuously proposed,32 but they need to prove their added value against predictive models including established risk factors and biomarkers. The CHARGE‐AF models, having demonstrated good discrimination ability across different populations, are excellent candidates to be effective standards.
The CHARGE‐AF models have previously demonstrated adequate predictive ability in other cohorts, all of them in Europe and including a majority of white participants.7, 8, 9 We show, for the first time, that the CHARGE‐AF models have excellent discrimination and fair calibration in a racially and ethnically diverse sample. Comparing the calibration of the CHARGE‐AF and Framingham AF models suggests that models for AF prediction need to incorporate race/ethnicity as an important covariate. This is consistent with the growing evidence pointing to a higher risk of AF in whites compared to nonwhites.10, 11, 21, 33 Moreover, not surprisingly, we demonstrate that use of scores for risk stratification of ischemic stroke in patients with AF, such as CHADS2 and CHA2DS2‐VASc, are not adequate for AF prediction.
Our results confirm the value of circulating NT‐proBNP in the prediction of AF. Also, consistent with the previous publication developing the biomarker‐enriched CHARGE‐AF model,9 information on circulating hsCRP in the MESA cohort provided little benefit for AF prediction, whether assessed by changes in the C‐statistic or by the NRI. Numerous epidemiological studies have found consistent associations of hsCRP, a biomarker of inflammation, and BNP or NT‐proBNP, a biomarker of left atrial overload, with incidence of AF.34, 35, 36, 37, 38 Those studies, however, showed stronger associations of AF with the natriuretic peptides.
Our analysis has some limitations. First, ascertainment of AF relied on hospital diagnoses. As a consequence, undiagnosed AF and patients with AF managed exclusively in the outpatient setting were likely missed. Additionally, AF provoked by other systemic or acute conditions may be over‐represented in this sample. The AF cases included in this analysis therefore may not be representative of the average AF patient. Previous studies, however, have demonstrated adequate validity of this method of AF ascertainment in large epidemiological studies.20, 21 Moreover, the good calibration of the Framingham model among MESA whites indicates that the observed risk of AF in the MESA cohort is not different from the predicted risk calculated from the Framingham model, derived using a more detailed ascertainment of AF (including outpatient diagnosis).4 This consistency between predicted and observed risk of AF indirectly supports the validity of our AF ascertainment, at least compared with the AF ascertainment procedures using in the Framingham study. Second, the CHARGE‐AF models have a limited (5‐year) time horizon. Nonetheless, short‐term prediction may be particularly useful in the setting of screening programs and primary prevention trials. Future work should extend the CHARGE‐AF models to longer‐term prediction, including estimation of lifetime risk of AF. Third, the CHARGE‐AF models did not consider other variables, such as genetic variants, in the predictive models. In the Women's Genome Health Study, which included over 20 000 women of European ancestry, a genetic risk score calculated from risk alleles known to be associated with AF improved prediction beyond clinical variables.39 Whether a similar genetic score could improve prediction in a racially diverse population remains to be determined. Finally, the number of AF cases among nonwhites in the MESA cohort was insufficient to determine predictive ability of the models across separate nonwhite racial/ethnic groups.
In conclusion, our analysis of a multiethnic cohort provides further evidence supporting the value of the CHARGE‐AF models for prediction of AF in diverse populations. The CHARGE‐AF models, as well as other models for AF prediction, may not be ready yet for implementation in usual clinical practice given the lack of demonstrated effective approaches for AF prevention. However, AF screening programs and primary prevention trials should consider using these models for identification of high‐risk individuals and therefore more likely to benefit from these interventions.
Sources of Funding
This research was supported by contracts N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 and grant R01‐HL‐127659 from the National Heart, Lung, and Blood Institute and by grants UL1‐TR‐000040 and UL1‐TR‐001079 from NCRR. This work was additionally supported by American Heart Association grant 16EIA26410001 (Alonso).
Disclosures
None.
Supporting information
Table S1. Variables Included in the Risk Prediction Models and Scores
Table S2. Selected Baseline Characteristics of Study Participants by Race/Ethnicity, Multi‐Ethnic Study of Atherosclerosis, 2000–2002
Table S3. Reclassification Tables Adding Biomarker Information to the Simple CHARGE‐AF Model, MESA 2000–2012. Cells Highlighted in Green Correspond to Correct Reclassification, Whereas Those in Red Represent Incorrect Reclassification
Figure S1. Mean predicted and observed 10‐year risk of atrial fibrillation by deciles of predicted risk derived from Framingham model separately for whites and nonwhites, Multi‐Ethnic Study of Atherosclerosis, 2000–2012.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
(J Am Heart Assoc. 2016;5:e003077 doi: 10.1161/JAHA.115.003077)
Accompanying Tables S1 through S3 and Figure S1 are available at http://jaha.ahajournals.org/content/5/2/e003077/suppl/DC1
References
- 1. Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, McManus DD, Dupuis J, Ellinor PT, Benjamin EJ. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011;124:1982–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soliman EZ, Lopez FL, O'Neal WT, Chen LY, Bengtson L, Zhang ZM, Loehr L, Cushman M, Alonso A. Atrial fibrillation and risk of ST‐segment elevation versus non‐ST segment elevation myocardial infarction: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2015;131:1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Wagoner DR, Piccini JP, Albert CM, Anderson ME, Benjamin EJ, Brundel B, Califf RM, Calkins H, Chen P‐S, Chiamvimonvat N, Darbar D, Eckhardt LL, Ellinor PT, Exner DV, Fogel RI, Gillis AM, Healey J, Hohnloser SH, Kamel H, Lathrop DA, Lip GYH, Mehra R, Narayan SM, Olgin J, Packer D, Peters NS, Roden DM, Ross HM, Sheldon R, Wehrens XHT. Progress toward the prevention and treatment of atrial fibrillation: a summary of the Heart Rhythm Society Research Forum on the Treatment and Prevention of Atrial Fibrillation, Washington, DC, December 9–10, 2013. Heart Rhythm. 2015;12:e5–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB Sr, Newton‐Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community‐based cohort study. Lancet. 2009;373:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schnabel RB, Aspelund T, Li G, Sullivan LM, Suchy‐Dicey A, Harris TB, Pencina MJ, D'Agostino RB Sr, Levy D, Kannel WB, Wang TJ, Kronmal RA, Wolf PA, Burke GL, Launer LJ, Vasan RS, Psaty BM, Benjamin EJ, Gudnason V, Heckbert SR. Validation of an atrial fibrillation risk algorithm in whites and African‐Americans. Arch Intern Med. 2010;170:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities (ARIC) Study). Am J Cardiol. 2011;107:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JoD, Janssens ACJW, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kääb S, Couper D, Harris TB, Soliman EZ, Stricker BHC, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF consortium. J Am Heart Assoc. 2013;2:e000102 doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pfister R, Brägelmann J, Michels G, Wareham NJ, Lubne R, Khaw K‐T. Performance of the CHARGE‐AF risk model for incident atrial fibrillation in the EPIC Norfolk cohort. Eur J Prev Cardiol. 2015;22:932–939. [DOI] [PubMed] [Google Scholar]
- 9. Sinner MF, Stepas KA, Moser CB, Krijthe BP, Aspelund T, Sotoodehnia N, Fontes JD, Janssens ACJW, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Vasan RS, Wang TJ, Agarwal SK, McManus DD, Franco OH, Yin X, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Astor BC, Ballantyne CM, Hoogeveen RC, Arai AE, Soliman EZ, Ellinor PT, Stricker BHC, Gudnason V, Heckbert SR, Pencina MJ, Benjamin EJ, Alonso A. B‐type natriuretic peptide and C‐reactive protein in the prediction of atrial fibrillation risk: the CHARGE‐AF Consortium of community‐based cohort studies. Europace. 2014;16:1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks and whites. Circulation. 2013;128:2470–2477. [DOI] [PubMed] [Google Scholar]
- 11. Rodriguez CJ, Soliman EZ, Alonso A, Swett K, Okin PM, Goff DC Jr, Heckbert SR. Atrial fibrillation incidence and risk factors in relation to race‐ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi‐Ethnic Study of Atherosclerosis. Ann Epidemiol. 2015;25:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 13. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 14. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suzuki S, Sagara K, Otsuka T, Kano H, Matsuno S, Takai H, Uejima T, Oikawa Y, Koike A, Nagashima K, Kirigaya H, Yajima J, Tanabe H, Sawada H, Aizawa T, Yamashita T. Usefulness of frequent supraventricular extrasistoles and a high CHADS2 score to predict first‐time appearance of atrial fibrillation. Am J Cardiol. 2013;111:1602–1607. [DOI] [PubMed] [Google Scholar]
- 16. Zuo ML, Liu S, Chan KH, Lau KK, Chong BH, Lam KF, Chan YH, Lau YF, Lip GY, Lau CP, Tse HF, Siu CW. The CHADS2 and CHA2DS2‐VASc scores predict new occurrence of atrial fibrillation and ischemic stroke. J Interv Card Electrophysiol. 2013;37:47–54. [DOI] [PubMed] [Google Scholar]
- 17. Baker WL, Coleman CI, White CM, Kluger J. Use of preoperative CHA2DS2‐VASc score to predict the risk of atrial fibrillation after cardiothoracic surgery: a nested case‐control study from the Atrial Fibrillation Suppression Trials (AFIST) I, II, and III. Pharmacotherapy. 2013;33:489–495. [DOI] [PubMed] [Google Scholar]
- 18. Fauchier L, Clementy N, Pelade C, Collignon C, Nicolle E, Lip GY. Patients with ischemic stroke and incident atrial fibrillation: a nationwide cohort study. Stroke. 2015;46:2432–2437. [DOI] [PubMed] [Google Scholar]
- 19. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 20. Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. [DOI] [PubMed] [Google Scholar]
- 23. D'Agostino RB, Nam BH. Evaluation of the performance of survival analysis models: discrimination and calibration measures In: Balakrishnan N, Rao CR, eds. Handbook of Statistics. Vol. 23 Amsterdam: Elsevier; 2004:1–25. [Google Scholar]
- 24. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. [DOI] [PubMed] [Google Scholar]
- 25. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Haemost. 2013;110:213–222. [DOI] [PubMed] [Google Scholar]
- 27. Svennberg E, Engdahl J, Al‐Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation. 2015;131:2176–2184. [DOI] [PubMed] [Google Scholar]
- 28. Healey JS, Sandhu RK. Are we ready for mass screening to detect atrial fibrillation? Circulation. 2015;131:2167–2168. [DOI] [PubMed] [Google Scholar]
- 29. Elgendy IY, Mahmoud A, Huo T, Beaver TM, Bavry AA. Meta‐analysis of 12 trials evaluating the effects of statins on decreasing atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2015;115:1523–1528. [DOI] [PubMed] [Google Scholar]
- 30. Khatib R, Joseph P, Briel M, Yusuf S, Healey J. Blockade of the renin–angiotensin–aldosterone system (RAAS) for primary prevention of non‐valvular atrial fibrillation: a systematic review and meta analysis of randomized controlled trials. Int J Cardiol. 2013;165:17–24. [DOI] [PubMed] [Google Scholar]
- 31. Martinez‐Gonzalez MA, Toledo E, Arós F, Fiol M, Corella D, Salas‐Salvadó J, Ros E, Covas MI, Fernández‐Crehuet J, Lapetra J, Muñoz MA, Fitó M, Serra‐Majem L, Pintó X, Lamuela‐Raventos RM, Sorlí JV, Babio N, Buil‐Cosiales P, Ruiz‐Gutiérrez V, Estruch R, Alonso A. Extra‐virgin olive oil consumption reduces risk of atrial fibrillation: the PREDIMED trial. Circulation. 2014;130:18–26.24787471 [Google Scholar]
- 32. Schnabel RB, Wild PS, Wilde S, Ojeda FM, Schulz A, Zeller T, Sinning CR, Kunde J, Lackner KJ, Munzel T, Blankenberg S. Multiple biomarkers and atrial fibrillation in the general population. PLoS One. 2014;9:e112486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jensen PN, Thacker EL, Dublin S, Psaty BM, Heckbert SR. Racial differences in the incidence of and risk factors for atrial fibrillation in older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 2013;61:276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. [DOI] [PubMed] [Google Scholar]
- 35. Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, Kronmal RA. N‐terminal pro‐B‐type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patton KK, Heckbert SR, Alonso A, Bahrami H, Lima JA, Burke G, Kronmal RA. N‐terminal pro‐B‐type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi‐Ethnic Study of Atherosclerosis: the effects of age, sex, and ethnicity. Heart. 2013;99:1832–1836. [DOI] [PubMed] [Google Scholar]
- 37. Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, Van Wagoner DR. C‐reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. [DOI] [PubMed] [Google Scholar]
- 38. Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, Tofler GH, Selhub J, Jacques PF, Wolf PA, Magnani JW, Ellinor PT, Wang TJ, Levy D, Vasan RS, Benjamin EJ. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Everett BM, Cook NR, Conen D, Chasman DI, Ridker PM, Albert CM. Novel genetic markers improve measures of atrial fibrillation risk prediction. Eur Heart J. 2013;34:2243–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Variables Included in the Risk Prediction Models and Scores
Table S2. Selected Baseline Characteristics of Study Participants by Race/Ethnicity, Multi‐Ethnic Study of Atherosclerosis, 2000–2002
Table S3. Reclassification Tables Adding Biomarker Information to the Simple CHARGE‐AF Model, MESA 2000–2012. Cells Highlighted in Green Correspond to Correct Reclassification, Whereas Those in Red Represent Incorrect Reclassification
Figure S1. Mean predicted and observed 10‐year risk of atrial fibrillation by deciles of predicted risk derived from Framingham model separately for whites and nonwhites, Multi‐Ethnic Study of Atherosclerosis, 2000–2012.


