Abstract
Background
Associations of atherosclerosis risk factors with unrecognized myocardial infarction (UMI) are unclear. We investigated associations of midlife risk factors with UMI and recognized MI (RMI) detected 31 years later by cardiac magnetic resonance.
Methods and Results
The Reykjavik Study (1967–1991) collected serial risk factors in subjects, mean (SD) age 48 (7) years. In ICELAND‐MI (2004–2007), 936 survivors (76 (5) years) were evaluated by cardiac magnetic resonance. Analysis included logistic regression and random effects modeling. Comparisons are relative to subjects without MI. At baseline midlife evaluation, a modified Framingham risk score was significantly higher in RMI and in UMI versus no MI (7.4 (6.3)%; 7.1 (6.2)% versus 5.4 (5.8)%, P<0.001). RMI and UMI were more frequent in men (65%, 64% versus 43%; P<0.0001). Baseline systolic and diastolic blood pressure were significantly higher in UMI (138 (17) mm Hg versus 133 (17) mm Hg; P<0.006; 87 (10) mm Hg versus 84 (10) mm Hg; P<0.02). Diastolic BP was significantly higher in RMI (88 (10) mm Hg versus 84 (10) mm Hg; P<0.02). Cholesterol and triglycerides were significantly higher in RMI (6.7 (1.1) mmol/L versus 6.2 (1.1) mmol/L; P=0.0005; and 1.4 (0.7) mmol/L versus 1.1 (0.7) mmol/L; P<0.003). Cholesterol trended higher in UMI (P=0.08). Serial midlife systolic BP was significantly higher in UMI versus no MI (β [SE] = 2.69 [1.28] mm Hg, P=0.04). Serial systolic and diastolic BP were significantly higher in RMI versus no MI (4.12 [1.60] mm Hg, P=0.01 and 2.05 [0.91] mm Hg, P=0.03) as were cholesterol (0.43 [0.11] mmol/L, P=0.0001) and triglycerides (0.3 [0.06] mmol/L, P<0.0001).
Conclusions
Midlife vascular risk factors are associated with UMI and RMI detected by cardiac magnetic resonance 31 years later. Systolic blood pressure was the most significant modifiable risk factor associated with later UMI.
Keywords: epidemiology, hypertension, magnetic resonance imaging, myocardial infarction, risk factors
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors, Magnetic Resonance Imaging (MRI)
Introduction
Epidemiologic studies describe associations between baseline cardiovascular risk factors and subsequent clinically recognized myocardial infarction (RMI), typically reported as mortality from ischemic heart disease or composite cardiovascular end points that include MI.1, 2, 3, 4, 5, 6, 7 It is uncertain whether traditional risk factors for RMI also account fully for unrecognized MI (UMI) or whether UMI has additional risk factors.8, 9, 10, 11 By comparison with RMI, risk factors for development of UMI are less clearly defined because of inability to detect UMI accurately. Using usual diagnostic clinical criteria, there can be underreporting of UMI for several reasons including missed diagnosis, lack of symptoms, insensitivity of 12‐lead ECG, or in some cases, regression of q waves with time.4, 12, 13, 14, 15 Therefore, UMI, which carries a significant risk of subsequent adverse cardiac events,16, 17 may be underestimated for prospective modification of risk.11, 17, 18
Advances in imaging by cardiac magnetic resonance (CMR) with late gadolinium enhancement allow more accurate detection of MI, including asymptomatic or unrecognized silent events.16, 19, 20 Indeed, CMR is the most accurate method available to detect myocardial scar and reports a higher prevalence of UMI16, 17, 21 than other technologies, making it more precise for population‐based studies.22 The association of UMI detected by CMR with specific risk factors measured decades earlier has not been reported previously.
Between 1967–1991, the Icelandic Heart Association Reykjavik Study (Reykjavik) assessed vascular risk factors in a cohort of then middle‐aged subjects born 1907–1935 and living in the Reykjavik region.23 The study was extended in the Age Gene/Environment Susceptibility‐Reykjavik Study (AGES 2002–2006) to evaluate phenotypes of aging in organ systems, including the cardiovascular system, in surviving cohorts of Reykjavik.24 ICELAND‐MI, a substudy of AGES, used CMR to detect MI scarring with a high degree of accuracy (2004–2007).16
The specific aim of this study was to investigate the association of cardiovascular risk factors measured at midlife with presence of UMI and RMI detected several decades later by CMR in ICELAND‐MI. We hypothesized that (1) baseline midlife cardiovascular risk factors that are important for clinically RMI would also be associated with UMI detected at late life by accurate CMR methodology, and (2) serial midlife measures of cardiovascular risk factors would be associated with both UMI and RMI at late life.
Methods
The Icelandic Reykjavik Study was a cohort of 30 795 randomly selected subjects born between 1907 and 1935 living in the Reykjavik area in 1967. Serial cardiovascular measures and covariates were collected from the Reykjavik cohort between 1967 and 1996 to provide cross‐sectional and longitudinal data. The AGES‐Reykjavik Study (2002–2006) was designed to study 5764 subjects aged 66 to 98 years old randomly selected from the surviving Reykjavik cohort.24 All participants signed a written informed consent and the study was approved by the National Bioethics Committee in Iceland and the Institutional Review Board of the Intramural Research Program of the National Institute on Aging.
In 2004, ICELAND‐MI was initiated to investigate prevalence of vascular risk factors and the presence of RMI and UMI identified by late gadolinium enhancement CMR. The sample size for this cohort has been previously described (n=936).16 Recruitment occurred in 2 phases. Phase 1 was a random sample of the AGES‐Reykjavik cohort, which enrolled 702 subjects. Phase 2 enrollment specifically recruited subjects with diabetes and enrolled another 290 subjects. After exclusion of subjects who did not undergo the CMR and subjects with technical problems that prevented image analysis, the final sample size was 936 subjects for the current study.
Magnetic Resonance Imaging
All CMR studies were performed on a 1.5‐T Signa Twinspeed scanner using a 4‐element cardiac phased–array coil (General Electric Medical Systems, Waukesha, WI) as previously described.16, 25 Images were acquired during breath‐hold and triggered to the ECG or to pulse oximetry if ECG gating was suboptimal.
Late gadolinium enhancement imaging has been validated in both small and large animals, and in an international randomized controlled trial.26, 27, 28, 29 Imaging was performed to evaluate MI scar, typically 6 to 15 minutes after injection of gadopentetate dimeglumine at low dose (0.1 mmol/kg; Magnevist, Schering AG, Berlin, Germany), using a phase‐sensitive segmentation gradient echo inversion recovery sequence.25 Calculated median glomerular filtration rate was 69 (interquartile range 59–82) mL/min per 1.73 m2 and no subject had significant renal failure.16, 30
Using the 17‐segment standardized model of the American Heart Association,31 the diagnosis of MI was based on consensus of 2 cardiologists experienced in CMR and blinded to subject clinical characteristics. For each segment, MI scar was considered present if the detected lesion had endocardial involvement and followed a coronary distribution. Scar patterns considered atypical for MI were not designated as MI. The size of left ventricular infarct was expressed as a percentage of total left ventricle.
Modifiable Risk Factors and Modified Framingham Score at Midlife
By design, a portion of the Reykjavik cohort were examined up to 5 times between 1967 and 199124 to document modifiable risk factors including lipid levels, blood pressure (BP), smoking, diabetes, obesity measured as body mass index, and physical activity. Other reported modifiable risk factors32 including psychosocial factors, nutrition, and alcohol use were not assessed in this study.
Serum cholesterol and triglycerides were measured after an overnight fast using a chemical colorimetric method.3, 33, 34
Supine BP was measured twice in fasting subjects by a nurse after 5 minutes of rest between 8:30 am and 10:30 am. The mercury “Erkameter” wall‐model sphygmomanometer (Erka, Germany) with cuff including a rubber bladder 15×32 cm, and total length 66 cm was used. BP was the average of the 2 measures.35
Body mass index was calculated as weight (kilograms) divided by height2 (meters). Physical activity was assessed using a questionnaire that asked about physical activity assessed as 1=never, 2=rarely, 3=occasionally, 4=moderate, 5=high.
A modified Framingham percentage risk was calculated using Framingham score variables that included age, gender, total cholesterol, smoking status, systolic BP, diabetes, and use of antihypertensive treatment, but excluded high‐density lipoprotein cholesterol, which was not estimated in the Reykjavik Study.36, 37
Covariates
Covariates included demographic information (age, sex), and risk factors associated with cardiovascular events assessed at midlife included smoking history, glucose level, and use of antihypertensive drugs.
Statistical Analysis
An adjudication committee determined whether or not subjects had a clinically recognized MI (RMI) event based on subject history supported by hospital or surveillance records. Unrecognized MI (UMI) was defined as MI detected by CMR but without evidence of clinical RMI from hospital or surveillance records.16 The group without MI was the remainder of subjects who did not have a clinical event attributed to RMI by the adjudication committee or UMI detected by CMR.
Baseline characteristics at midlife were compared between groups first with and without MI, and then for RMI, UMI, or no MI using logistic regression adjusted for sex and midlife age, and then further adjusted for use of antihypertensive drugs, blood glucose, smoking, all at midlife, and age at late life. Multivariate analysis was performed by logistic regression to compare all MI versus no MI and by polynomial logistic regression to compare RMI and UMI with no MI.
Serial systolic and diastolic BP, serum cholesterol, and triglyceride courses were estimated visually by plotting mean values at each midlife measure, stratified by presence of RMI or UMI or no MI.
A random‐effects model (with random intercept and slope function) was used to model the trends in midlife cardiovascular measures by presence of recognized, unrecognized, or no MI. This approach takes into account the correlation of within‐individual measures, and the fact that there are unequal numbers of observations per individual. The random intercept and slope allow the estimates to vary on an individual level. Each model was tested for a significant interaction term between RMI or UMI and age to test whether the slopes of the cardiovascular risk factors differ between the 3 groups.
The models were first adjusted for sex, and age at midlife and late life, and then further adjusted for midlife variables including smoking history (never versus ever), use of BP‐lowering medication, and blood glucose.
Statistical analysis was performed using SAS 9.3 (SAS Institute Inc, Cary, NC). Data were expressed as mean (standard deviation) or percentage (number). In all analyses, the conventional α‐level of 0.05 was used for significance testing. The mean per time point for each variable was plotted according to the presence or absence of MI using R software version 2.13.0 (R Foundation for Statistical Computing, Vienna, Austria).38
The analytic sample consisted of 936 subjects described previously who completed the CMR examination on average 31 (3) years after the baseline Reykjavik visit.16 There were 1 or more assessments of risk factors in the subjects at midlife; 460 had 1 assessment, 185 had 2 assessments, 42 had 3 assessments, 93 had 4 assessments, and 156 had 5 assessments between 1967 and 1991. The subjects who came for 1 rather than for multiple visits were older (49.4 (6.4) versus 46.2 (7.9) years; P<0.001), and less likely to be men (43.1% versus 53.2%, P=0.04), but more likely to smoke (35.1% versus 8.8%, P<0.001), be diabetic (1.7% versus 0.2%, P=0.05), and to take antihypertensive agents (7.6% versus 1.0%, P<0.001; Table 1).
Table 1.
Characteristics of Subjects With One Versus Multiple Visits at Baseline Midlife Assessment in the Reykjavik Study
| Characteristics at First BP Measurement, M (SD) | One Visit | Multiple Visits | P Valuea |
|---|---|---|---|
| n=459 | n=477 | ||
| Age | 49.4 (6.4) | 46.2 (7.9) | <0.0001 |
| Men, % (n) | 43.1 (198) | 53.2 (254) | 0.04 |
| Height, cm | 170.4 (8.6) | 171.9 (9.1) | 0.26 |
| Weight, kg | 74.1 (12.6) | 75.3 (13.1) | 0.80 |
| BMI, kg/m2 | 25.4 (3.5) | 25.4 (3.4) | 0.41 |
| Tobacco use, % (n) | |||
| Ever smoker | 58.8 (270) | 14.3 (68) | <0.0001 |
| Current smoker | 35.1 (161) | 8.8 (42) | <0.0001 |
| Antihypertensive medication, % (n) | 7.6 (35) | 1.0 (5) | 0.0003 |
| Diabetes mellitus, % (n) | 1.7 (8) | 0.2 (1) | 0.05 |
| Blood pressure, mm Hgb | |||
| Systolic | 133.8 (17.2) | 134.1 (16.8) | 0.25 |
| Diastolic | 84.5 (10.2) | 84.9 (10.1) | 0.38 |
| Blood sample | |||
| Cholesterol, mmol/L | 6.34 (1.14) | 6.29 (1.06) | 0.57 |
| Triglycerides, mmol/Lc | 1.18 (0.69) | 1.16 (0.63) | 0.84 |
| Fasting glucose, mmol/L | 80.3 (10.9) | 80.5 (11.2) | 0.54 |
| Creatinine, μmol/L | 85.4 (13.8) | 85.5 (14.7) | 0.08 |
BMI indicates body mass index; BP, blood pressure; M (SD), mean (standard deviation).
Logistic regression to compare subjects with one or more than one visit, adjusted for age and sex.
Adjusted for antihypertensive medications.
Adjusted for cholesterol.
Results
Baseline Midlife Cardiovascular Risk Factors and Subsequent MI in Late Life
At late‐life ICELAND‐MI follow‐up, the mean age of the group was 76.7 (5.3) years. Twenty‐six percent (248/936) of subjects had MI when assessed by CMR in late life and of these, the majority were unrecognized (63%; 157/248), whereas 37% (91/248) of subjects had a clinically recognized event.16 Among clinically RMI, 22 subjects (24% RMI or 9% all MI) did not have evidence of myocardial scar by CMR, and 10/22 (45%) of these subjects had undergone a revascularization procedure.
At the baseline midlife assessment, the modified Framingham risk score was significantly higher in the entire MI group (7.2 [6.2]% versus 5.4 [5.8]%, P<0.001; Table 2). There were significantly more men in the MI group compared with the no MI group (64.1% versus 42.6%; P<0.001; Table 2). The MI group had a significantly higher systolic and diastolic BP (138 [16] versus 133 [17] mm Hg; P=0.002 and 87 [10] versus 84 [10] mm Hg; P=0.002), and serum cholesterol (6.52 [1.15] versus 6.24 [1.07] mmol/L; P=0.001). Serum triglycerides were significantly higher only after adjustment for sex and age at mid‐ and late life, and midlife covariates (1.31 [0.68] versus 1.12 [0.64] mmol/L, P=0.03).
Table 2.
Baseline Characteristics of Population at Midlife by Presence of Late‐Life Myocardial Infarction Detected by Cardiac MR at ICELAND_MI
| General Characteristics, M (SD) | MI | No MI | P Valuea |
|---|---|---|---|
| N=248 | N=688 | ||
| Age | 48.1 (7.0) | 47.7 (7.5) | <0.06 |
| Men, % (n) | 64.1 (159) | 42.6 (293) | <0.001 |
| Height, cm | 172.7 (8.6) | 170.6 (8.9) | 0.08 |
| Weight, kg | 76.7 (12.8) | 74.0 (12.8) | 0.55 |
| BMI, kg/m2 | 25.6 (3.4) | 25.4 (3.5) | 0.84 |
| Tobacco use, % (n) | |||
| Ever smoker | 35.5 (88) | 36.3 (250) | 0.38 |
| Current smoker | 23.8 (59) | 20.9 (144) | 0.44 |
| Antihypertensive medication, % (n) | 5.2 (13) | 3.9 (27) | 0.45 |
| Diabetes mellitus, % (n) | 0.4 (1) | 1.2 (8) | 0.37 |
| Blood pressure, mm Hgb | |||
| Systolic | 138 (16) | 133 (17) | 0.002 |
| Diastolic | 87 (10) | 84 (10) | 0.002 |
| Blood sample | |||
| Cholesterol, mmol/L | 6.52 (1.15) | 6.24 (1.07) | 0.001 |
| Triglycerides, mmol/Lc | 1.31 (0.68) | 1.12 (0.64) | 0.09 |
| Fasting glucose, mmol/L | 4.5 (0.5) | 4.5 (0.6) | 0.25 |
| Creatinine, μmol/L | 88.0 (17.6) | 88.0 (17.6) | 0.56 |
| Midlife physical activityd | 2.8 (1.3) | 2.9 (1.3) | 0.23 |
| Modified Framingham % riske | 7.2 (6.2) | 5.4 (5.8) | <0.0001 |
BMI indicates body mass index; M (SD), mean (standard deviation); MI, myocardial infarction; MR, magnetic resonance imaging.
Logistic models to compare the overall difference between the groups adjusted for age and sex.
Adjusted for antihypertensive medications.
Adjusted for cholesterol.
Exercise: 1=never, 2=rarely, 3=occasionally, 4=moderate, 5=high.
Model not corrected because age and sex are included in risk score.
The modified Framingham risk score was significantly higher in RMI and in UMI compared with no MI (7.4 [6.3]% and 7.1 [6.2]% versus 5.4 [5.8]%, P=0.0003; Table 3). The percentage of men was higher in RMI and in UMI than in no MI (Table 3). Baseline systolic BP was significantly higher in UMI, and trended higher in RMI (P=0.06). Baseline diastolic BP was elevated significantly in both RMI and UMI (Table 3). Serum cholesterol and triglycerides were significantly higher in RMI (Table 3). Serum cholesterol trended higher in UMI (P=0.08). When risk factors for RMI were compared with risk factors for UMI, there were not significant differences.
Table 3.
Baseline Characteristics of Population at Midlife by Presence of Late‐Life Recognized or Unrecognized Myocardial Infarction Detected by Cardiac MR at ICELAND_MI
| Baseline Midlife Risk Factors, M (SD) | Recognized MI | Unrecognized MI | No MI | P Valuea |
|---|---|---|---|---|
| N | 91 | 157 | 688 | |
| Age | 47.9 (6.6) | 48.3 (7.2) | 47.7 (7.48) | 0.07 |
| Men, % (n) | 64.8 (59)¶ | 63.7 (100)¶ | 42.6 (293) | <0.0001 |
| Height, cm | 172.7 (8.9) | 172.7 (8.5) | 170.6 (8.9) | 0.20 |
| Weight, kg | 75.9 (12.9) | 77.1 (12.8) | 74.0 (12.8) | 0.49 |
| BMI, kg/m2 | 25.4 (3.5) | 25.8 (3.4) | 25.4 (3.5) | 0.63 |
| Tobacco use, % (n) | ||||
| Ever smoker | 38.5 (35) | 33.8 (53) | 36.3 (250) | 0.51 |
| Current smoker | 26.4 (24) | 22.3 (35) | 20.9 (144) | 0.59 |
| Antihypertensive medication, % (n) | 3.3 (5) | 5.1 (8) | 3.9 (27) | 0.68 |
| Diabetes mellitus, % (n) | 0 | 0.6 (1) | 1.2 (8) | 0.89 |
| Blood pressure, mm Hgb | ||||
| Systolic | 137.4 (15.1)# | 138.3 (16.9)** | 132.6 (17.0) | 0.01 |
| Diastolic | 87.7 (9.8)†† | 87.2 (10.3)†† | 83.7 (10.0) | 0.006 |
| Blood sample | ||||
| Cholesterol, mmol/L | 6.67 (1.14)‡ | 6.43 (1.15)§§ | 6.24 (1.07) | 0.001 |
| Triglycerides, mmol/Lc | 1.40 (0.72)∥∥ | 1.25 (0.66) | 1.12 (0.65) | 0.009 |
| Fasting glucose, mmol/L | 80.2 (8.6) | 80.6 (9.6) | 80.3 (11.6) | 0.48 |
| Creatinine, μmol/L | 89.2 (13.2) | 88.4 (14.9) | 84.3 (14.1) | 0.77 |
| Midlife physical activityd | 2.6 (1.3) | 2.9 (1.2) | 2.88 (1.25) | 0.18 |
| Modified Framingham % riske | 7.4 (6.3)¶¶ | 7.1 (6.2)¶¶ | 5.4 (5.8) | 0.0003 |
BMI indicates body mass index; M (SD), mean (standard deviation); MI, myocardial infarction; MR, magnetic resonance imaging.
ANOVA and logistic models to compare recognized MI and unrecognized MI with no MI adjusted for age and gender.
Adjusted for antihypertensive medications.
Adjusted for serum cholesterol.
Exercise: 1=never, 2=rarely, 3=occasionally, 4=moderate, 5=high.
Model not corrected for age and gender because these are included in the score.
¶ P≤0.0001; # P=0.06; **P≤0.006; †† P<0.02; ‡ P=0.0005; §§ P=0.08; ∥∥ P≤0.003; ¶¶ P<0.05.
In a multivariate analysis of baseline midlife risk factors, gender, systolic BP, and serum cholesterol were all significantly higher in all MI vs. no MI (Table 4). Similarly, sex, serum cholesterol, and triglycerides were significantly associated risk factors for RMI, whereas sex and systolic BP were the most significant factors for UMI (Table 4).
Table 4.
Multivariate Analysis of Baseline Midlife Risk Factors With Late‐Life Myocardial Infarction Using Logistic Regression
| Baseline Midlife Risk Factors | All MIa | Recognized MIa | Unrecognized MIa |
|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | |||
| Age | 1.02 (0.99, 1.04) | 1.00 (0.96, 1.03) | 1.03 (1.00, 1.05) |
| Sex | 2.91 (1.88, 4.51) | 2.69 (1.41, 5.12) | 3.08 (1.83, 5.19) |
| Cholesterol | 1.18 (1.01, 1.37) | 1.39 (1.12, 1.72) | 1.06 (0.89, 1.28) |
| Systolic BP | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.03) | 1.02 (1.00, 1.03) |
| BMI, kg/m2 | 0.98 (0.94, 1.04) | 0.95 (0.87, 1.02) | 1.01 (0.95, 1.07) |
| Glucose | 0.99 (0.97, 1.01) | 0.99 (0.97, 1.01) | 0.99 (0.97, 1.01) |
| Serum creatinine | 0.52 (0.14, 1.97) | 0.89 (0.13, 6.04) | 0.38 (0.08, 1.87) |
| Serum triglyceride | 1.27 (0.99, 1.62) | 1.40 (1.02, 1.93) | 1.17 (0.87, 1.59) |
| Physical activity | 1.09 (0.92, 1.29) | 0.99 (0.77, 1.28) | 1.15 (0.94, 1.41) |
| Hypertension medications | 0.82 (0.38, 1.74) | 0.76 (0.27, 2.26) | 0.84 (0.34, 2.03) |
| Smoking | 1.21 (0.87, 1.70) | 1.08 (0.66, 1.76) | 1.31 (0.87, 1.96) |
BMI indicates body mass index; BP, blood pressure; MI, myocardial infarction.
Compared with no MI group.
The percentage size of UMI was smaller than the percentage size of RMI (6 [7]% versus 14 [13]%, P<0.0001).
Serial Midlife Cardiovascular Risk Factors and Subsequent MI
Midlife BP and MI
Figure shows the trend in midlife systolic and diastolic BP and MI. In random effects models I and II, systolic BP was consistently significantly higher by 3.2 mm Hg in the entire MI group (P=0.003; Table 5). Serial systolic BP was significantly higher for both RMI and UMI (P=0.01 and 0.04, respectively; Figure, Table 5). Diastolic BP was consistently significantly higher by 1.5 mm Hg in the entire MI group (P=0.02; Figure, Table 5), but only RMI, not UMI, was the significant contributor to this finding.
Figure 1.
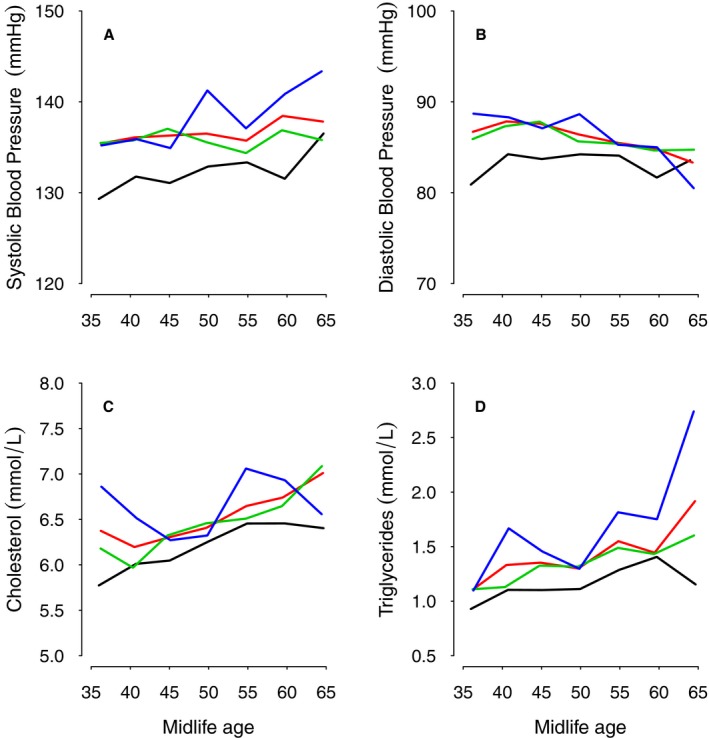
Five‐year trends in midlife systolic (A) and diastolic (B) blood pressure, cholesterol (C), and triglycerides (D) with increasing age related to all MI and recognized and unrecognized MI (red line=all MI; blue line=recognized MI; green line=unrecognized MI; black line=no MI). MI indicates myocardial infarction.
Table 5.
Association of Serial Midlife Risk Factors and Both Recognized and Unrecognized Myocardial Infarction Assessed by CMR in Later Life
| Risk Factors Comparing MI vs No MI | Model Ia | Model IIb | ||
|---|---|---|---|---|
| β (SE) | P Value | β (SE) | P Value | |
| Serial systolic blood pressure, mm Hgc | ||||
| All MI vs no MI | 3.20 (1.11) | 0.004 | 3.21 (1.08) | 0.003 |
| Unrecognized MI | 2.76 (1.31) | 0.04 | 2.69 (1.28) | 0.04 |
| Recognized MI | 4.03 (1.64) | 0.01 | 4.12 (1.60) | 0.010 |
| Serial diastolic blood pressure, mm Hgc | ||||
| All MI vs no MI | 1.50 (0.63) | 0.02 | 1.50 (0.62) | 0.02 |
| Unrecognized MI | 1.18 (0.73) | 0.11 | 1.18 (0.73) | 0.11 |
| Recognized MI | 2.05 (0.94) | 0.03 | 2.05 (0.91) | 0.03 |
| Serial cholesterol, mmol/L | ||||
| All MI vs no MI | 0.25 (0.08) | 0.001 | 0.25 (0.08) | 0.001 |
| Unrecognized MI | 0.14 (0.09) | 0.11 | 0.14 (0.09) | 0.11 |
| Recognized MI | 0.43 (0.11) | 0.0001 | 0.43 (0.11) | 0.0001 |
| Serial triglycerides, mmol/Ld | ||||
| All MI vs no MI | 0.15 (0.05) | 0.002 | 0.15 (0.05) | 0.002 |
| Unrecognized MI | 0.07 (0.06) | 0.25 | 0.07 (0.06) | 0.24 |
| Recognized MI | 0.31 (0.07) | <0.0001 | 0.30 (0.06) | <0.0001 |
| Serial glucose | ||||
| All MI vs no MI | 0.61 (0.58) | 0.60 | 0.60 (0.58) | 0.30 |
| Unrecognized MI | 0.48 (0.69) | 0.48 | 0.43 (0.69) | 0.53 |
| Recognized MI | 0.82 (0.85) | 0.33 | 0.88 (0.84) | 0.30 |
| Serial body mass index, kg/m2 | ||||
| All MI vs no MI | 0.13 (0.25) | 0.60 | 0.15 (0.25) | 0.54 |
| Unrecognized MI | 0.33 (0.30) | 0.30 | 0.36 (0.29) | 0.23 |
| Recognized MI | −0.22 (0.38) | 0.38 | −0.20 (0.37) | 0.60 |
| Midlife physical activitye | ||||
| All MI vs no MI | −0.10 (0.06) | 0.11 | 0.09 (0.06) | 0.17 |
| Unrecognized MI | −0.00 (0.08) | 0.96 | 0.01 (0.08) | 0.85 |
| Recognized MI | −0.26 (0.09) | 0.006 | −0.25 (0.09) | 0.007 |
CMR indicates cardiac magnetic resonance imaging; β (SE), coefficient (standard error); MI, myocardial infarction.
Model 1, mixed model adjusted for midlife age, late‐life age, and sex.
Model 2, mixed model adjusted for midlife age, late‐life age, sex, midlife smoking, midlife antihypertensive medication, and midlife serum glucose.
Adjusted for antihypertensive medications.
Adjusted for cholesterol.
Physical activity: 1=never, 2=rarely, 3=occasionally, 4=moderate, 5=high.
Midlife serum cholesterol and triglycerides and MI
In random effects models I and II, serial cholesterol was significantly higher by 0.25 mmol/L in the entire MI group (P=0.001; Figure, Table 5). Similarly triglycerides were significantly higher by a mean of 0.15 mmol/L in the MI group (P=0.02; Figure, Table 5). For both cholesterol and triglycerides, only RMI was significantly related to blood levels in the models (Figure, Table 5).
Other modifiable risk factors and MI
There was no significant trend in midlife body mass index or blood glucose level and later MI (Table 5). Physical activity was marginally lower in RMI.
Discussion
In this cohort, midlife atherosclerotic risk determined using a modified Framingham risk score was associated significantly with all late‐life MI. Importantly, the modified Framingham percentage risk score was significantly higher not only in RMI, but also in UMI. It is perhaps surprising that late‐life RMI and UMI are associated strongly with a midlife summary risk score obtained 3 decades earlier and derived from single baseline measures of traditional risk factors, which could be prone to measurement errors and any interim personal changes.39
CMR is the most accurate technique available to detect myocardial scar whether recognized or unrecognized, and the presence of scar is associated with a worse prognosis.16 In earlier studies in which detection of MI was based on clinical and electrocardiographic findings, large numbers of subjects were needed to detect relationships.5 In this study, CMR detected recognized and unrecognized MI in 26% of subjects in this cohort of 936 subjects. A small percentage of clinically RMI subjects had normal CMR. Subjects with clinically RMI and yet no myocardial scar by CMR could be explained by revascularization, reversible pathology such as myocarditis, or a clinical misdiagnosis.
Midlife measures of systolic and diastolic BP and cholesterol were associated significantly with the presence of all late‐life MI. Although many reports describe the association of hypertension with increased cardiovascular mortality,2, 4, 14, 40, 41, 42, 43 this unique data set links small increases in BP and cholesterol at midlife with UMI and RMI evaluated by CMR on average 31 years later.
In ICELAND‐MI, serial midlife measures of both systolic and diastolic BP remained consistently higher in the MI group without a change in trend with time. Multiple factors may have contributed to persistent elevation of BP in the MI group including increasing age, changes in vascular compliance, and patient compliance with therapy.44, 45, 46, 47 Although antihypertensive therapy reduces clinical MI in randomized controlled trials,48 translation of trial results into effective control of BP during 25 years or more is challenging, despite increased drug therapy with time.16 Our results indicate that even minor elevations of BP in the prehypertension range may be a risk for later MI, and are consistent with recent data from the Chicago Heart Association.49 These data raise questions about target pressures recommended by recent European guidelines and US reports.50, 51, 52 The data may also be complementary to the SPRINT trial in which preliminary reports suggest that aggressive lowering of BP below current thresholds may be beneficial.53
Both baseline and serial cholesterol levels were significantly associated with all later MI. There are no data on high‐density lipoprotein cholesterol or on use of statins at midlife because these drugs were licensed in the late 1980s toward the end of the Reykjavik study. The association between serum triglycerides and all MI, independent of serum cholesterol, is consistent with prior combined mortality and morbidity data.54, 55
In this study, systolic BP was the most significant modifiable risk factor for UMI. There was also a trend for higher serum cholesterol in the UMI group. Equal prevalence of risk factors has been reported for both recognized and unrecognized MI detected by electrocardiographic criteria,11 but our study differs from earlier reports because this is an older cohort of late‐life survivors of the original Reykjavik study in whom MI was detected by CMR. Prevalence and risk factors of UMI demonstrate substantial variability, depending on the population under study.8
The size of unrecognized MI was smaller than RMI, a finding that has been reported in other studies.21 UMI shares some characteristics with silent cerebral infarctions, which are of smaller size in general, increase in prevalence with age, have few identified risk factors except age and hypertension, and portend later adverse cerebral events.56, 57 Whether UMI differs from RMI by presence of small vessel disease or in terms of pathophysiologic mechanisms cannot be addressed in this study.
There are a number of limitations to this study. The modified Framingham risk score does not include measures of high‐density lipoprotein cholesterol, and yet is still associated with later RMI and UMI. Also, the subjects are survivors still alive 31 years after the Reykjavik study. The prevalence of RMI versus UMI may alter with time, and so the relation of risk factors such as diabetes or smoking to a lethal disease could be underestimated (ie, survivor bias). MI itself can alter BP or cholesterol through pathophysiologic change, or prescription of drugs or diet, but the baseline midlife Reykjavik data are unlikely to have this limitation because the mean age was 48 years and probably before onset of MI in most cases. In the 1970s and 1980s, cardiovascular risk factor management was not as well defined as in current practice guidelines, which have lower thresholds for intervention for prehypertension and hypercholesterolemia.50, 52, 58 In the Reykjavik study, the baseline mean systolic and diastolic BPs at midlife were in the prehypertension range and higher than mean BPs reported in a recent National Health and Nutrition Examination Survey.59
In this Icelandic cohort, atherosclerotic risk factors at midlife are associated with both UMI and RMI detected by CMR 31 years later. Among modifiable midlife risk factors studied, systolic BP was the most significant factor associated with UMI, although the impact of other important risk factors such as diabetes could not be addressed in this report. The paper highlights the finding that even minor elevations of mean levels of cardiovascular risk factors are associated with later RMI and UMI. From a public health perspective, it may be useful to test whether outcomes improve with early aggressive risk factor modification and use of CMR to detect UMI in asymptomatic high‐risk populations.
Sources of Funding
This study was supported by a contract from the National Institutes of Health (NO1‐AG‐1‐2100), the National Institute of Aging Intramural Research Program, the NHLBI Intramural Research Program (Contract 1 Z01 HL004607‐08CE), the NIH Clinical Center, all in Bethesda, MD and the Hjartavernd (the Icelandic Heart Association), Kopavogur, and the Althingi (the Icelandic Parliament), Reykjavik, both in Iceland. There were no relationships with industry.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002420 doi: 10.1161/JAHA.115.002420)
This article was handled independently by Christopher Kramer, MD, as a guest editor. The Editors had no role in the evaluation of this manuscript or in the decision about its acceptance.
References
- 1. Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ. 1980;58:113–130. [PMC free article] [PubMed] [Google Scholar]
- 2. Asia Pacific Cohort Studies C . Joint effects of systolic blood pressure and serum cholesterol on cardiovascular disease in the Asia Pacific region. Circulation. 2005;112:3384–3390. [DOI] [PubMed] [Google Scholar]
- 3. Jonsdottir LS, Sigfusson N, Gudnason V, Sigvaldason H, Thorgeirsson G. Do lipids, blood pressure, diabetes, and smoking confer equal risk of myocardial infarction in women as in men? The Reykjavik Study. J Cardiovasc Risk. 2002;9:67–76. [PubMed] [Google Scholar]
- 4. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 5. MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. [DOI] [PubMed] [Google Scholar]
- 6. Prospective Studies C , Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta‐analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. [DOI] [PubMed] [Google Scholar]
- 7. Salonen JT, Puska P, Tuomilehto J, Homan K. Relation of blood pressure, serum lipids, and smoking to the risk of cerebral stroke. A longitudinal study in Eastern Finland. Stroke. 1982;13:327–333. [DOI] [PubMed] [Google Scholar]
- 8. Valensi P, Lorgis L, Cottin Y. Prevalence, incidence, predictive factors and prognosis of silent myocardial infarction: a review of the literature. Arch Cardiovasc Dis. 2011;104:178–188. [DOI] [PubMed] [Google Scholar]
- 9. Rizk DV, Gutierrez O, Levitan EB, McClellan WM, Safford M, Soliman EZ, Warnock DG, Muntner P. Prevalence and prognosis of unrecognized myocardial infarctions in chronic kidney disease. Nephrol Dial Transplant. 2012;27:3482–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sheifer SE, Gersh BJ, Yanez ND III, Ades PA, Burke GL, Manolio TA. Prevalence, predisposing factors, and prognosis of clinically unrecognized myocardial infarction in the elderly. J Am Coll Cardiol. 2000;35:119–126. [DOI] [PubMed] [Google Scholar]
- 11. Sigurdsson E, Thorgeirsson G, Sigvaldason H, Sigfusson N. Unrecognized myocardial infarction: epidemiology, clinical characteristics, and the prognostic role of angina pectoris. The Reykjavik Study. Ann Intern Med. 1995;122:96–102. [DOI] [PubMed] [Google Scholar]
- 12. Lawes CM, Rodgers A, Bennett DA, Parag V, Suh I, Ueshima H, MacMahon S. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens. 2003;21:707–716. [DOI] [PubMed] [Google Scholar]
- 13. Miura K, Nakagawa H, Ohashi Y, Harada A, Taguri M, Kushiro T, Takahashi A, Nishinaga M, Soejima H, Ueshima H. Four blood pressure indexes and the risk of stroke and myocardial infarction in Japanese men and women: a meta‐analysis of 16 cohort studies. Circulation. 2009;119:1892–1898. [DOI] [PubMed] [Google Scholar]
- 14. Pencina MJ, D'Agostino RB Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30‐year risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tunstall‐Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case‐fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. [DOI] [PubMed] [Google Scholar]
- 16. Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ, Gudnason V, Harris TB, Arai AE. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308:890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event‐free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. [DOI] [PubMed] [Google Scholar]
- 18. Magnani JW, Wang N, Nelson KP, Connelly S, Deo R, Rodondi N, Schelbert EB, Garcia ME, Phillips CL, Shlipak MG, Harris TB, Ellinor PT, Benjamin EJ. Electrocardiographic PR interval and adverse outcomes in older adults: the Health, Aging, and Body Composition Study. Circ Arrhythm Electrophysiol. 2013;6:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast‐enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. [DOI] [PubMed] [Google Scholar]
- 20. Saraste A, Nekolla S, Schwaiger M. Contrast‐enhanced magnetic resonance imaging in the assessment of myocardial infarction and viability. J Nucl Cardiol. 2008;15:105–117. [DOI] [PubMed] [Google Scholar]
- 21. Barbier CE, Bjerner T, Johansson L, Lind L, Ahlstrom H. Myocardial scars more frequent than expected: magnetic resonance imaging detects potential risk group. J Am Coll Cardiol. 2006;48:765–771. [DOI] [PubMed] [Google Scholar]
- 22. Kokubo Y, Kamide K, Okamura T, Watanabe M, Higashiyama A, Kawanishi K, Okayama A, Kawano Y. Impact of high‐normal blood pressure on the risk of cardiovascular disease in a Japanese urban cohort: the Suita study. Hypertension. 2008;52:652–659. [DOI] [PubMed] [Google Scholar]
- 23. Sigurdsson E, Thorgeirsson G, Sigvaldason H, Sigfusson N. Prevalence of coronary heart disease in Icelandic men 1968–1986. The Reykjavik Study. Eur Heart J. 1993;14:584–591. [DOI] [PubMed] [Google Scholar]
- 24. Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V. Age, Gene/Environment Susceptibility‐Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase‐sensitive inversion recovery for detecting myocardial infarction using gadolinium‐delayed hyperenhancement. Magn Reson Med. 2002;47:372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsu LY, Natanzon A, Kellman P, Hirsch GA, Aletras AH, Arai AE. Quantitative myocardial infarction on delayed enhancement MRI. Part I: animal validation of an automated feature analysis and combined thresholding infarct sizing algorithm. J Magn Reson Imaging. 2006;23:298–308. [DOI] [PubMed] [Google Scholar]
- 27. Kim RJ, Albert TS, Wible JH, Elliott MD, Allen JC, Lee JC, Parker M, Napoli A, Judd RM; Gadoversetamide Myocardial Infarction Imaging I . Performance of delayed‐enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: an international, multicenter, double‐blinded, randomized trial. Circulation. 2008;117:629–637. [DOI] [PubMed] [Google Scholar]
- 28. Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. [DOI] [PubMed] [Google Scholar]
- 29. Schelbert EB, Hsu LY, Anderson SA, Mohanty BD, Karim SM, Kellman P, Aletras AH, Arai AE. Late gadolinium‐enhancement cardiac magnetic resonance identifies postinfarction myocardial fibrosis and the border zone at the near cellular level in ex vivo rat heart. Circ Cardiovasc Imaging. 2010;3:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 31. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 32. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, Investigators IS. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 33. Bjornsson OJ, Davidsson D, Olafsson O, Sigfusson N, Porsteinsson P. Survey of serum lipid levels in Icelandic men aged 34–61 years. An epidemiological and statistical evaluation. Acta Med Scand Suppl. 1977;616:1–150. [PubMed] [Google Scholar]
- 34. Sigfusson N, Sigvaldason H, Steingrimsdottir L, Gudmundsdottir II, Stefansdottir I, Thorsteinsson T, Sigurdsson G. Decline in ischaemic heart disease in Iceland and change in risk factor levels. BMJ. 1991;302:1371–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gudmundsson LS, Johannsson M, Thorgeirsson G, Sigfusson N, Sigvaldason H, Witteman JC. Risk profiles and prognosis of treated and untreated hypertensive men and women in a population‐based longitudinal study: the Reykjavik Study. J Hum Hypertens. 2004;18:615–622. [DOI] [PubMed] [Google Scholar]
- 36. Lloyd‐Jones DM, Wilson PW, Larson MG, Beiser A, Leip EP, D'Agostino RB, Levy D. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004;94:20–24. [DOI] [PubMed] [Google Scholar]
- 37. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 38. R: A Language and Environment for Statistical Computing . R Foundation for Statistical Computing. Vienna, Austria; 2010:520/id. [Google Scholar]
- 39. Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long‐term follow‐up of prospective studies. Am J Epidemiol. 1999;150:341–353. [DOI] [PubMed] [Google Scholar]
- 40. Gray L, Lee IM, Sesso HD, Batty GD. Blood pressure in early adulthood, hypertension in middle age, and future cardiovascular disease mortality: HAHS (Harvard Alumni Health Study). J Am Coll Cardiol. 2011;58:2396–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McCarron P, Okasha M, McEwen J, Davey Smith G. Blood pressure in early life and cardiovascular disease mortality. Arch Intern Med. 2002;162:610–611. [DOI] [PubMed] [Google Scholar]
- 42. Miura K, Daviglus ML, Dyer AR, Liu K, Garside DB, Stamler J, Greenland P. Relationship of blood pressure to 25‐year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago Heart Association Detection Project in Industry. Arch Intern Med. 2001;161:1501–1508. [DOI] [PubMed] [Google Scholar]
- 43. Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. [DOI] [PubMed] [Google Scholar]
- 44. Bitton A, Choudhry NK, Matlin OS, Swanton K, Shrank WH. The impact of medication adherence on coronary artery disease costs and outcomes: a systematic review. Am J Med. 2013;126:357.e7–357.e27. [DOI] [PubMed] [Google Scholar]
- 45. Blacher J, Safar ME. Large‐artery stiffness, hypertension and cardiovascular risk in older patients. Nat Clin Pract Cardiovasc Med. 2005;2:450–455. [DOI] [PubMed] [Google Scholar]
- 46. Fahey T, Schroeder K, Ebrahim S. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2006;2:CD005182. [DOI] [PubMed] [Google Scholar]
- 47. Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010;3:CD005182. [DOI] [PubMed] [Google Scholar]
- 48. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yano Y, Stamler J, Garside DB, Daviglus ML, Franklin SS, Carnethon MR, Liu K, Greenland P, Lloyd‐Jones DM. Isolated systolic hypertension in young and middle‐aged adults and 31‐year risk for cardiovascular mortality: the Chicago Heart Association Detection Project in Industry Study. J Am Coll Cardiol. 2015;65:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 Evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 51. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck‐Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker‐Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 52. Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499–503. [DOI] [PubMed] [Google Scholar]
- 53. The SPRINT Research Group . A Randomized Trial of Intensive versus Standard Blood‐Pressure Control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg‐Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. [DOI] [PubMed] [Google Scholar]
- 55. Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. [DOI] [PubMed] [Google Scholar]
- 56. Vermeer SE, Longstreth WT Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. [DOI] [PubMed] [Google Scholar]
- 57. Lee SC, Park SJ, Ki HK, Gwon HC, Chung CS, Byun HS, Shin KJ, Shin MH, Lee WR. Prevalence and risk factors of silent cerebral infarction in apparently normal adults. Hypertension. 2000;36:73–77. [DOI] [PubMed] [Google Scholar]
- 58. Stone NJ, Robinson JG, Lichtenstein AH, Goff DC Jr, Lloyd‐Jones DM, Smith SC Jr, Blum C, Schwartz JS; Panel AACG . Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 American College of Cardiology/American Heart Association cholesterol guideline. Ann Intern Med. 2014;160:339–343. [DOI] [PubMed] [Google Scholar]
- 59. Wright JD, Hughes JP, Ostchega Y, Yoon SS, Nwankwo T. Mean systolic and diastolic blood pressure in adults aged 18 and over in the United States, 2001–2008. Natl Health Stat Report. 2011;1–22:24. [PubMed] [Google Scholar]


