Abstract
Background
Black US residents experience higher rates of ischemic stroke than white residents but have lower rates of clinically apparent atrial fibrillation (AF), a strong risk factor for stroke. It is unclear whether black persons truly have less AF or simply more undiagnosed AF.
Methods and Results
We obtained administrative claims data from state health agencies regarding all emergency department visits and hospitalizations in California, Florida, and New York. We identified a cohort of patients with pacemakers, the regular interrogation of which reduces the likelihood of undiagnosed AF. We compared rates of documented AF or atrial flutter at follow‐up visits using Kaplan–Meier survival statistics and Cox proportional hazards models adjusted for demographic characteristics and vascular risk factors. We identified 10 393 black and 91 380 white patients without documented AF or atrial flutter before or at the index visit for pacemaker implantation. During 3.7 (±1.8) years of follow‐up, black patients had a significantly lower rate of AF (21.4%; 95% CI 19.8–23.2) than white patients (25.5%; 95% CI 24.9–26.0). After adjustment for demographic characteristics and comorbidities, black patients had a lower hazard of AF (hazard ratio 0.91; 95% CI 0.86–0.96), a higher hazard of atrial flutter (hazard ratio 1.29; 95% CI 1.11–1.49), and a lower hazard of the composite of AF or atrial flutter (hazard ratio 0.94; 95% CI 0.88–99).
Conclusions
In a population‐based sample of patients with pacemakers, black patients had a lower rate of AF compared with white patients. These findings indicate that the persistent racial disparities in rates of ischemic stroke are likely to be related to factors other than undiagnosed AF.
Keywords: atrial fibrillation, atrial flutter, epidemiology, risk factors, stroke
Subject Categories: Arrhythmias, Atrial Fibrillation, Epidemiology, Race and Ethnicity, Risk Factors
Introduction
Black US residents experience ischemic stroke at a 1.5‐fold higher rate than white residents.1 Conversely, studies consistently report that black patients have lower rates of atrial fibrillation (AF)—one of the strongest risk factors for stroke2—than white patients.3, 4, 5, 6, 7, 8, 9, 10, 11 It is unclear whether the lower reported rate of AF in black persons represents a truly lower burden of AF or more frequent underdiagnosis of this often paroxysmal dysrhythmia in black persons.12, 13 Better understanding of the true relative burden of AF in black persons may shed light on reasons for racial disparities in stroke rates that cannot be entirely accounted for by differences in demographic and socioeconomic characteristics or the prevalence of established stroke risk factors.14 To better compare the true burden of AF in black and white persons, we examined rates of AF diagnosis in a population‐based sample of patients with indwelling pacemakers, which allow regular interrogation of patients’ heart rhythms.
Methods
Design
We performed a retrospective cohort study using administrative claims data on emergency department visits and acute care hospitalizations in California, Florida, and New York. We chose these states because they comprise demographically heterogeneous populations that account for ≈20% of the total US population15 and offer deidentified longitudinal data on all emergency department and hospital discharges at all nonfederal health care facilities.16 State health agencies collect discharge information from health care facilities using a standardized format and perform quality checks before making these data available in a deidentified form via the Healthcare Cost and Utilization Project. All visit records contain information on demographic characteristics and up to 25 International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes and up to 21 ICD‐9‐CM procedure codes. Multiple visits for a given patient can be linked together using an anonymous linkage identifier.17 The Weill Cornell Medicine institutional review board approved our analysis of these deidentified, publicly available data.
Patient Population
We identified patients at the time of their first hospitalization for implantation of a cardiac pacemaker, identified using ICD‐9‐CM procedure codes 37.80 through 37.83. To define reasons for pacemaker implantation, we examined the primary discharge diagnosis at the time of the index hospitalization for pacemaker implantation. To focus on incident cases, we excluded patients with a recorded diagnosis of AF (ICD‐9‐CM diagnosis code 427.31) or atrial flutter (ICD‐9‐CM code 427.32) prior to or at the time of their index hospitalization for pacemaker placement. Because we were interested in differences in AF and stroke rates in black and white patients, we excluded persons of other races and ethnicities. Race and ethnicity were determined from the documented self‐report of race or ethnicity by patients or their surrogates. We included patients with an index hospitalization on or after January 1, 2005, in California or Florida and January 1, 2006, in New York because these were the first available years with longitudinal linkage variables. We included patients with index hospitalizations through December 31, 2010, in California or New York and December 31, 2011, in Florida because these were the last available data that allowed us at least 1 year of follow‐up.
Measurements
Our main outcome was a diagnosis of AF. The ICD‐9‐CM code for AF has an excellent positive predictive value and good sensitivity in administrative claims data.18 Secondary outcomes were atrial flutter and a composite of AF or atrial flutter. In our primary analysis, we used data from all emergency department visits and hospitalizations to identify clinically documented diagnoses of AF and atrial flutter. In a sensitivity analysis, we limited our ascertainment of AF and flutter only to periods when patients underwent a documented pacemaker interrogation. We ascertained pacemaker interrogations using ICD‐9‐CM procedure codes 89.45 through 89.48. We also assessed rates of ischemic stroke, defined using a previously validated ICD‐9‐CM diagnosis code algorithm.19 In a sensitivity analysis, we limited stroke cases to ICD‐9‐CM code 434.11, which has a 73% specificity for embolic strokes.20
To account for imbalances in vascular risk factors that may explain racial differences in AF and stroke rates, we used ICD‐9‐CM codes from the index hospitalization to define the following comorbidities that have been reported as risk factors for AF and stroke2, 21: hypertension, diabetes mellitus, coronary heart disease, congestive heart failure, lung disease, chronic kidney disease, valvular disease, obesity, alcohol abuse, and tobacco use. To compare the burden of stroke risk factors between groups, we also used comorbidity data to calculate the CHA2DS2‐VASc score.22
Statistical Analysis
Kaplan–Meier survival statistics were used to calculate cumulative rates of outcomes. Patients entered observation at the time of their index hospitalization and were censored at the time of an outcome, in‐hospital death, or last available follow‐up. For analyses of AF and atrial flutter, patients were censored at the time of the composite diagnosis of AF or flutter. The log‐rank test was used to compare cumulative rates of outcomes between white and black patients. Cox proportional hazards models were used to determine the association between black race and outcomes while adjusting for age, sex, insurance type, income, and the comorbidities defined above. We used a stepwise approach to developing our Cox models: an unadjusted model, a model adjusted for demographic characteristics only, and a model adjusted for demographic characteristics and vascular risk factors. In a sensitivity analysis, we treated all baseline covariates as time varying and updated them at the time of subsequent visits. Given the strong effect of age on AF risk,23 we performed additional sensitivity analyses using the fully adjusted model within strata defined by quintiles of age. Because the frequency of visits and pacemaker interrogations may have influenced rates of AF diagnosis, we performed a sensitivity analysis adjusting for these 2 factors. In analyses of stroke, we also included a time‐varying covariate for incident diagnoses of AF or atrial flutter. In a sensitivity analysis, we matched black patients with white patients at a 1:1 ratio using all baseline covariates above and performed a matched Cox analysis of stroke risk. The proportional hazards assumption was verified by visual inspection of log‐log plots. All statistical analyses were performed using Stata/MP (version 13; StataCorp). The threshold of statistical significance was set at α=0.05.
Results
We identified 10 393 black patients and 91 380 white patients without a diagnosis of AF or atrial flutter at the time of a first‐recorded pacemaker implantation. Black patients were younger; more often were female; more often had Medicaid insurance; had greater prevalence of hypertension, diabetes, heart failure, chronic kidney disease, and prior stroke or transient ischemic attack; and had a lower prevalence of coronary heart disease, peripheral vascular disease, and chronic obstructive pulmonary disease (Table 1). Black patients had a slightly higher mean CHA2DS2‐VASc score at baseline (3.5±1.4) than white patients (3.2±1.4). Indications for pacemaker implantation were comparable between groups (Table 1).
Table 1.
Baseline Characteristics of Black Versus White Patients With Pacemakers
| Characteristica | Blackb (n=10 393) | White (n=91 380) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 72.8 (13.3) | 76.6 (12.0) | <0.001 |
| Female | 5891 (56.7) | 41 367 (45.3) | <0.001 |
| Payment sourcec | <0.001 | ||
| Medicare | 7666 (73.8) | 75 005 (82.1) | |
| Medicaid | 790 (7.6) | 1754 (1.9) | |
| Private | 1416 (13.6) | 12 469 (13.7) | |
| Self‐pay | 238 (2.3) | 783 (0.9) | |
| Other | 281 (2.7) | 1368 (1.5) | |
| Primary diagnosis at device implantationd | <0.001 | ||
| Sinoatrial node dysfunction | 3360 (32.3) | 32 268 (35.3) | |
| Unspecified conduction disorder | 2084 (20.1) | 20 105 (22.0) | |
| Other specified cardiac dysrhythmia | 871 (8.4) | 6365 (7.0) | |
| Other second degree atrioventricular block | 694 (6.7) | 5861 (6.4) | |
| Mobitz type II atrioventricular block | 435 (4.2) | 3060 (3.4) | |
| Hypertension | 8804 (84.7) | 65 888 (72.1) | <0.001 |
| Diabetes | 4222 (40.6) | 23 916 (26.2) | <0.001 |
| Coronary heart disease | 3684 (35.5) | 38 639 (42.3) | <0.001 |
| Congestive heart failure | 2324 (22.4) | 15 628 (17.1) | <0.001 |
| Peripheral vascular disease | 775 (7.5) | 7666 (8.4) | 0.001 |
| Chronic obstructive pulmonary disease | 945 (9.1) | 11 463 (12.5) | <0.001 |
| Chronic kidney disease | 2011 (19.4) | 10 256 (11.2) | <0.001 |
| Prior transient ischemic attack | 113 (1.1) | 812 (0.9) | 0.04 |
| Prior ischemic stroke | 242 (2.3) | 1190 (1.3) | <0.001 |
| CHA2DS2‐VASc score, mean (SD) | 3.5 (1.4) | 3.2 (1.4) | <0.001 |
Data are presented as number (%) unless otherwise specified.
Race was self‐reported by patients or their surrogates.
Numbers do not sum to group totals because of missing payment‐source data for <0.01% of patients.
Due to the large number of individual diagnoses, only the 5 most common diagnoses (representing >70% of cases) are listed.
During a mean follow‐up period of 3.7 (±1.8) years, the cumulative rate of AF diagnosis was significantly lower in black patients (21.4%; 95% CI 19.8–23.2) compared with white patients (25.5%; 95% CI 24.9–26.0; P<0.001 for the log‐rank test) (Figure 1). Black patients had a lower hazard of AF than white patients after adjustment for demographic characteristics and vascular risk factors (hazard ratio 0.91; 95% CI 0.86–0.96) (Table 2). These findings were similar in sensitivity analyses censoring patients at the time of their last documented pacemaker interrogation, stratifying patients by quintiles of age, adjusting for the frequency of visits or pacemaker interrogations, or treating baseline covariates as time varying.
Figure 1.
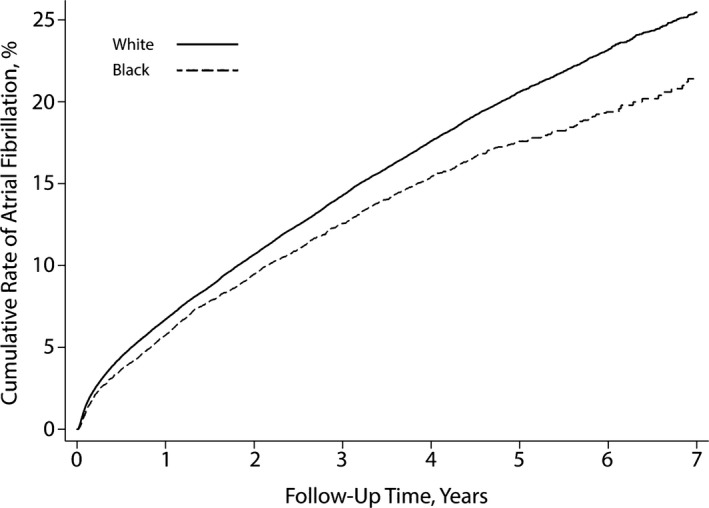
Cumulative rate of atrial fibrillation in patients with pacemakers, stratified by black vs white race. The difference between groups was significant (P<0.001 for the log‐rank test).
Table 2.
Associations Between Black Versus White Race and Atrial Fibrillation or Flutter
| Outcomea | Model 1b | Model 2c | Model 3d |
|---|---|---|---|
| Atrial fibrillation | 0.85 (0.80–90) | 0.93 (0.87–0.98) | 0.91 (0.86–0.96) |
| Atrial fibrillation or flutter | 0.87 (0.83–0.92) | 0.95 (0.90–1.01) | 0.94 (0.88–0.99) |
| Atrial flutter | 1.13 (0.98–1.31) | 1.32 (1.14–1.52) | 1.29 (1.11–1.49) |
Results are reported as the hazard ratio (95% CI) for the outcome in black compared with white patients.
Unadjusted.
Adjusted for baseline age, sex, insurance status, and income.
Adjusted for model 2 covariates plus baseline hypertension, diabetes mellitus, coronary heart disease, congestive heart failure, lung disease, chronic kidney disease, valvular disease, obesity, alcohol abuse, and tobacco use.
An opposite pattern was found for the secondary end point of atrial flutter: The cumulative rate of atrial flutter was nonsignificantly higher in black patients (3.5%; 95% CI 2.9–4.3) than in white patients (3.3%; 95% CI 3.1–3.5; P=0.08 for the log‐rank test) (Figure 2). After adjustment for demographic characteristics and comorbidities, black patients had a significantly higher hazard of atrial flutter than white patients (hazard ratio 1.29; 95% CI 1.11–1.49); however, the secondary composite end point of AF or atrial flutter occurred less often in black than white patients. The cumulative rate throughout follow‐up was 22.7% (95% CI 21.0–24.4) in black patients versus 26.7% (95% CI 26.1–27.3) in white patients (P<0.001 for the log‐rank test) (Figure 3). This difference persisted after adjustment for demographic characteristics and vascular comorbidities (hazard ratio 0.94; 95% CI 0.88–99).
Figure 2.
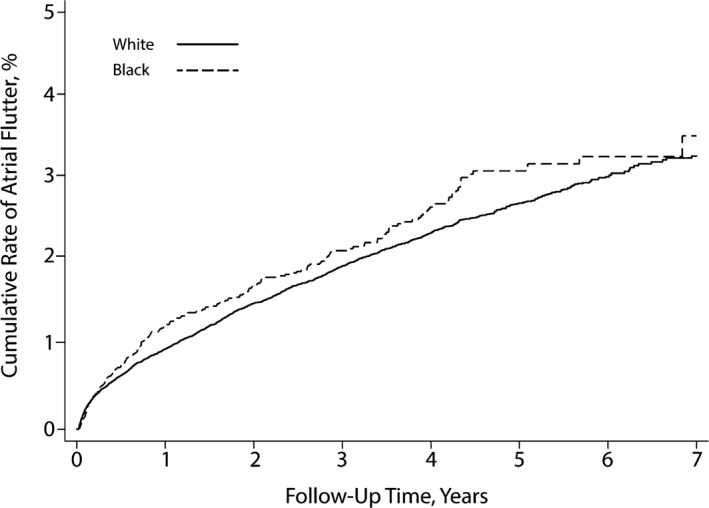
Cumulative rate of atrial flutter in patients with pacemakers, stratified by black vs white race. The difference between groups was not significant (P=0.08 for the log‐rank test).
Figure 3.
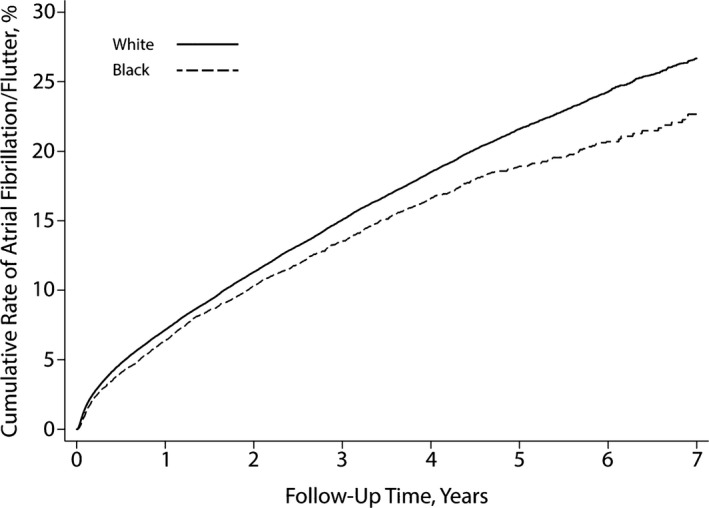
Cumulative rate of atrial fibrillation or flutter in patients with pacemakers, stratified by black vs white race. The difference between groups was significant (P<0.001 for the log‐rank test).
Despite their lower rate of AF or atrial flutter, black patients had a higher cumulative rate of ischemic stroke (7.3%; 95% CI 6.4–8.3) than white patients (5.1%; 95% CI 4.8–5.3; P<0.001 for the log‐rank test). The higher hazard of stroke in black patients persisted after adjustment for demographic characteristics and vascular risk factors, including AF and atrial flutter (hazard ratio 1.58; 95% CI 1.42–1.76). This association was not substantially different in a sensitivity analysis limited to embolic stroke or in a matched Cox analysis.
Discussion
In a large heterogeneous sample of patients with newly implanted pacemakers, we found a significantly lower rate of AF in black patients compared with white patients, even after adjustment for vascular risk factors. Conversely, black patients had a higher rate of atrial flutter than white patients, but atrial flutter composed a small proportion of cases compared with AF, and the composite outcome of AF or atrial flutter was less likely in black than white patients.
These findings are consistent with the results of several prior studies that found lower rates of AF in black compared with white patients.3, 4, 5, 6, 7, 8, 9, 10, 11 These studies involved analysis of administrative claims data in all patients presenting for acute care,3 prospective ascertainment of AF based on periodic ECG screening and hospitalization records,4, 5, 6, 7, 8 or examination of electronic medical records to identify clinical diagnoses of AF or ECGs showing AF.9, 10 Because none of these approaches involved long‐term continuous heart‐rhythm monitoring, their results may have reflected differences in AF symptomatology or patterns of care between white and black patients that resulted in differential ascertainment of AF12, 13 rather than true differences in the burden of AF. A prospective study assessed racial differences in AF detection using pacemakers or implanted defibrillators, but this study included a small number of black patients and was able to adjust for a limited number of potentially confounding factors.11 Our results build on these prior studies by focusing on a large population with indwelling pacemakers who thus underwent frequent interrogation of their heart rhythms. The lower rate of AF diagnosis that we found in black patients is unlikely to represent differences in intensity of screening for AF, especially because we found similar results in analyses censoring patients at the time of their last documented emergency department or inpatient pacemaker interrogation.
Several factors have been proposed to explain the previously reported lower rate of AF diagnosis in black than white patients24, 25: different paroxysmal patterns of AF in black patients, less intensive screening for AF among black persons, longer life expectancy and thus opportunities for AF to manifest in white persons, or an inheritable susceptibility to developing dysrhythmias. Our findings provide some support for the hypothesis that atrial dysrhythmia presents in different paroxysmal forms in black persons because we found a higher rate of atrial flutter in black compared with white patients. The lower rate of AF and the higher rate of atrial flutter in black patients may reflect racial differences in the interplay between atrial tissue substrate and electrical activity, but further research is required to understand this discordance. Atrial flutter was rare compared with AF, and the composite of AF or atrial flutter still occurred less often in black patients. Our results are more consistent with the hypothesis that racial differences in dysrhythmia susceptibility, and not just vascular risk factors, are important in explaining population patterns of this disease.6, 26 Even with this explanation, however, the topic of AF still presents a paradox in black persons because they experience significantly more ischemic stroke than white persons. A hypothesis that may explain this discordance is that abnormal atrial substrate predisposing to thromboembolism may manifest differently in black and white persons. Although black persons less often manifest AF, they have a significantly higher prevalence of other electrocardiographic signs of left atrial abnormality.4 This suggests that some of the observed racial disparities in stroke rates may be explained by cases of left atrial thromboembolism that currently go unrecognized because they occur in the absence of AF.27 Further research appears justified to explore the relationship among atrial abnormalities, thromboembolic risk, and racial disparities in stroke rates.
Our study should be interpreted in light of several limitations. First, we lacked data on outpatient visits and thus could not ascertain diagnoses of AF made purely in the outpatient setting; however, this is unlikely to have affected our results in this population. Because pacemaker implantation is an inpatient procedure, we were likely to have thoroughly captured the population of patients who received pacemakers. After pacemaker implantation, we found similar results in sensitivity analyses that censored patients at the last documented pacemaker interrogation, thereby ensuring evaluation of heart rhythm during the entire period of analysis because pacemakers would be expected to keep in memory any AF episodes throughout this interval. This makes it unlikely that differences in inpatient versus outpatient care were responsible for our results. Second, because we included only patients with pacemakers, we cannot be certain that our results are generalizable to the overall population. In support of the generalizability of our study, we found the same relative hazard for stroke in black versus white patients (≈1.5) as was found in prior population‐based studies.1 Third, we lacked data on antithrombotic medication use and thus cannot directly assess the degree to which the higher stroke risk in black patients is due to differences in anticoagulation use in patients with AF. Fourth, we lacked direct clinical data and relied on ICD‐9‐CM codes to identify outcomes. Distinctions between AF and atrial flutter based on claims data may not be reliable; therefore, further work will be needed to better understand racial differences in these dysrhythmias. We relied on AF diagnoses rather than direct assessment of rhythm, so we were unable to assess differences in the relative burden of AF in black and white patients. Because pacemakers in our cohort were not implanted primarily to screen for AF, some cases of brief AF captured on pacemaker interrogations may have been considered clinically insignificant and thus not documented. This is especially true given ongoing uncertainty about the pathogenic significance of brief AF episodes.28, 29 If failure to document AF occurred with similar frequency among black and white patients, nondifferential misclassification of diagnoses would have served to introduce a conservative bias by attenuating associations between race and outcomes and thereby reducing our ability to detect differences in AF rates between white and black patients. It is possible, however, that AF diagnoses captured by pacemakers were less frequently documented in black than white patients, although this scenario is not supported by the available evidence.11
In summary, we found that black patients with indwelling pacemakers had a lower rate of AF diagnosis than white patients. Despite this lower burden of AF, black patients experienced a higher rate of ischemic stroke than white patients, even after adjustment for known stroke risk factors. These findings indicate that the persistent racial disparities in rates of ischemic stroke are likely to be related to factors other than undiagnosed AF.
Sources of Funding
This research was supported by grant K23NS082367 to Dr Kamel from NIH/NINDS.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002492 doi: 10.1161/JAHA.115.002492)
This article was handled independently by Thomas Gerber, MD, PhD, as a guest editor. The editors had no role in the evaluation of the manuscript or in the decision about its acceptance.
References
- 1. Howard G, Peace F, Howard VJ. The contributions of selected diseases to disparities in death rates and years of life lost for racial/ethnic minorities in the United States, 1999–2010. Prev Chronic Dis. 2014;11:E129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, deFerranti S , Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics C and Stroke Statistics S . Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 3. Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation. 2013;128:2470–2477. [DOI] [PubMed] [Google Scholar]
- 4. Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC Jr. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, Fox ER, Levitzky YS, Mehra R, Kerr KF, Deo R, Sotoodehnia N, Akylbekova M, Ellinor PT, Paltoo DN, Soliman EZ, Benjamin EJ, Heckbert SR; Candidate‐Gene Association Resource S . European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122:2009–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marcus GM, Olgin JE, Whooley M, Vittinghoff E, Stone KL, Mehra R, Hulley SB, Schiller NB. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123:375.e1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jensen PN, Thacker EL, Dublin S, Psaty BM, Heckbert SR. Racial differences in the incidence of and risk factors for atrial fibrillation in older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 2013;61:276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 10. Ruo B, Capra AM, Jensvold NG, Go AS. Racial variation in the prevalence of atrial fibrillation among patients with heart failure: the Epidemiology, Practice, Outcomes, and Costs of Heart Failure (EPOCH) study. J Am Coll Cardiol. 2004;43:429–435. [DOI] [PubMed] [Google Scholar]
- 11. Lau CP, Gbadebo TD, Connolly SJ, Van Gelder IC, Capucci A, Gold MR, Israel CW, Morillo CA, Siu CW, Abe H, Carlson M, Tse HF, Hohnloser SH, Healey JS. Ethnic differences in atrial fibrillation identified using implanted cardiac devices. J Cardiovasc Electrophysiol. 2013;24:381–387. [DOI] [PubMed] [Google Scholar]
- 12. Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, Zakai NA, Kleindorfer D, Safford M, Howard G. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010;41:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prineas RJ, Soliman EZ, Howard G, Howard VJ, Cushman M, Zhang ZM, Moy CS. The sensitivity of the method used to detect atrial fibrillation in population studies affects group‐specific prevalence estimates: ethnic and regional distribution of atrial fibrillation in the REGARDS study. J Epidemiol. 2009;19:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howard VJ. Reasons underlying racial differences in stroke incidence and mortality. Stroke. 2013;44:S126–S128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. U.S. Census Bureau . Census QuickFacts. Available at: http://quickfacts.census.gov. Accessed September 21, 2015.
- 16. Agency for Healthcare Research and Quality . Healthcare Cost and Utilization Project. Available at: http://hcupnet.ahrq.gov. Accessed August 11, 2015.
- 17. Agency for Healthcare Research and Quality . HCUP methods series: methodological issues when studying readmissions and revisits using hospital administrative data. Available at: http://www.hcup-us.ahrq.gov/reports/methods/2011_01.pdf. Accessed June 29, 2015.
- 18. Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 20. Goldstein LB. Accuracy of ICD‐9‐CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29:1602–1604. [DOI] [PubMed] [Google Scholar]
- 21. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 22. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 23. Lloyd‐Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. [DOI] [PubMed] [Google Scholar]
- 24. Soliman EZ, Alonso A, Goff DC Jr. Atrial fibrillation and ethnicity: the known, the unknown and the paradox. Future Cardiol. 2009;5:547–556. [DOI] [PubMed] [Google Scholar]
- 25. Soliman EZ, Prineas RJ. The paradox of atrial fibrillation in African Americans. J Electrocardiol. 2014;47:804–808. [DOI] [PubMed] [Google Scholar]
- 26. Lubitz SA, Yin X, Fontes JD, Magnani JW, Rienstra M, Pai M, Villalon ML, Vasan RS, Pencina MJ, Levy D, Larson MG, Ellinor PT, Benjamin EJ. Association between familial atrial fibrillation and risk of new‐onset atrial fibrillation. JAMA. 2010;304:2263–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamel H, Okin PM, Longstreth WT Jr, Elkind MS, Soliman EZ. Atrial cardiopathy: a broadened concept of left atrial thromboembolism beyond atrial fibrillation. Future Cardiol. 2015;11:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, Lau CP, Van Gelder IC, Hohnloser SH, Carlson M, Fain E, Nakamya J, Mairesse GH, Halytska M, Deng WQ, Israel CW, Healey JS; Investigators A . Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. [DOI] [PubMed] [Google Scholar]
- 29. Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CT, Keung EK, Singer DE. Atrial fibrillation burden and short‐term risk of stroke: a case‐crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. 2015;8:1040–1047. [DOI] [PubMed] [Google Scholar]


